Abstract
Trichosporonosis is an emerging invasive fungal infection in immunosuppressed patients; a case of disseminated infection caused by Trichosporon loubieri presented confirms its role as a human pathogen.
CASE REPORT
A 56-year-old female was in good health until 2 months prior to admission when she developed progressive fatigue and bruised easily. Initial evaluation revealed marked pancytopenia. A bone marrow biopsy performed in November 2001 was diagnostic of pre-B-cell acute lymphoblastic leukemia. The patient had no significant past medical or family history. She was a resident of Connecticut and worked in an office setting. She was an avid gardener and swimmer. She denied international travel and gave no history of tobacco, alcohol, or drug use.
She was admitted to another institution for induction chemotherapy with cyclophosphamide, daunorubicin, vincristine, l-asparaginase, and prednisone. She became neutropenic and subsequently developed fevers to 39.4°C with no clinical response to empirical use of imipenem and vancomycin. A yeast grew on blood cultures 2 weeks after initiation of chemotherapy, and the patient was started on amphotericin B lipid complex therapy at 3 mg/kg of body weight/day. Blood cultures (BacT/Alert; bioMérieux, Durham, N.C.) remained positive for 5 days after initiation of antifungal therapy. She received granulocyte-macrophage colony-stimulating factor, and her indwelling catheter was removed. The catheter-tip culture showed no growth, and a cryptococcal antigen test was negative. Other diagnostic evaluations performed at the time were unrevealing and included transesophageal echocardiogram, lumbar puncture, and computed tomography of head, chest, abdomen, and pelvis. The patient developed bilateral upper abdominal pain and elevated liver function tests (LFTs), with the following peak values: alkaline phosphatase, 876 U/liter; aspartate aminotransferase, 276 U/liter; alanine aminotransferase, 369 U/liter; total bilirubin, 4.5 mg/dl; and direct bilirubin, 2.8 mg/dl. With recovery of her neutrophils, her fungemia cleared, her fever subsided, and her LFTs began to decline. The isolate was identified as a Trichosporon species by the referring institution. She received 2 more weeks of amphotericin B lipid complex at home and was referred to our institution for further management.
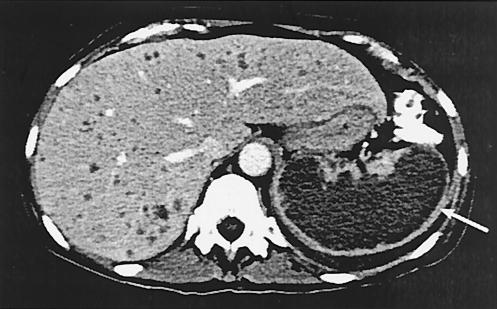
On admission to our institution, she reported worsening abdominal pain and low-grade fever. She appeared chronically ill and had tender hepatosplenomegaly with guarding. She had no skin lesions. Her white blood count was 17,200/μl (87% neutrophils), and her LFTs were as follows: alkaline phosphatase, 376 U/liter; aspartate aminotransferase, 27 U/liter; alanine aminotransferase, 89 U/liter; total bilirubin, 1.0 mg/dl; and direct bilirubin, 0.3 mg/dl. Blood cultures showed no growth. Initial chest radiograph demonstrated a left pleural effusion, and a contrast-enhanced abdominal computed tomography revealed multiple hypodense liver lesions and extensive coalescence of hypodensities in an enlarged spleen (Fig. 1). A bone marrow biopsy demonstrated remission of the leukemia. On admission, she was initially continued on liposomal amphotericin B therapy at a dose of 7.5 mg/kg/day.
FIG. 1.
Abdominal contrast-enhanced computed tomography demonstrates multiple hypodense lesions in the liver (left) and necrosis of the spleen (arrow).
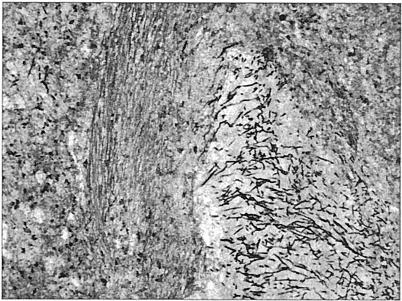
Given the need for further chemotherapy and the above radiological findings, the patient underwent splenectomy and a liver biopsy. Histopathological sections revealed extensive tissue necrosis with innumerable fungal elements (septate hyphae, pseudohyphae, and pleomorphic yeasts were delineated); angioinvasion was also noted (Fig. 2). There was no growth of the organism from the tissue specimens.
FIG. 2.
Spleen tissue slide. Multiple hyphal elements are observed in an intravascular space. Gomori's methenamine silver stain was used. Magnification, ×100. (Photomicrograph copyright Danny Milner.)
The blood isolate was obtained from the referring institution. Growth in potato-dextrose agar revealed white, dry colonies with a central depression and a radially furrowed outer zone; true and pseudohyphae, arthroconidia, and blastoconidia were observed microscopically. Identification was attempted with the use of the API 20C Aux yeast identification system (bioMérieux). The isolate was classified as Trichosporon mucoides with a 67% certainty (API 20C Aux 6745776). Antifungal susceptibilities were determined by using Sensititre YeastOne (TREK Diagnostic Systems, Cleveland, Ohio), and the following MICs were found: amphotericin B, 2 μg/ml; fluconazole, 4 μg/ml; itraconazole, 0.5 μg/ml; ketoconazole, 0.5 μg/ml; and flucytosine, 8 μg/ml.
The patient remained febrile with abdominal pain. A follow-up computed tomography revealed progression of the hepatic lesions and no other source of fever. Six days after admission, fluconazole at a dose of 800 mg/day was added to her therapy. She continued to be symptomatic, although abdominal computed tomography 2 weeks later demonstrated stabilization of the hepatic lesions. Given the severity of her illness and her decreased performance status, it was decided to not proceed with consolidation chemotherapy, and she was discharged and went home in January 2002. She continued to receive antifungal therapy at home and died in March 2002.
Given the uncertainty of identification of the isolate, PCR amplification and sequencing of gene segments of the small subunit of ribosomal DNA (18S rDNA), large subunit of rDNA (26S rDNA), and the internal transcriber spacer (ITS) region between these two genes were performed using well-described universal fungal primers (6, 22, 29). Briefly, fungal colonies were suspended in sterile water to a density of 0.5 McFarland standard. An aliquot was then flash-frozen by immersion into liquid nitrogen. DNA was isolated from 200 μl of the suspension utilizing the guanidium-based method common to Roche Amplicor system applications (Roche Diagnostics, Indianapolis, Ind.). Five microliters of the isolated DNA was amplified by using an AmpliTaq Gold PCR kit (Applied Biosystems, Foster City, Calif.), using 2.5 U of enzyme and 3 mM MgCl. All PCR amplifications were done for 35 cycles, with 1 cycle consisting of denaturation at 94°C for 45 s, annealing at 61°C for 60 s, and extension at 72°C for 90 s. The following primer pairs were used (Table 1). For the 18S rDNA amplicon, the primers described by Einsele (6) were used. For the ITS region amplicon, ITS1 and ITS4 primers were used (29). For the 26S rDNA amplicons, the P1 and P2 primers described by Sandhu (22) were used. PCR amplification was confirmed by electrophoresis on a 1% agarose gel containing ethidium bromide. PCR product was purified by use of Microcon YM-100 filters (Millipore, Billerica, Mass.), and sequencing was performed bidirectionally on an ABI 377 DNA sequencer (Applied Biosystems). Sequences were analyzed by using Lasergene software (Dnastar, Madison, Wis.).
TABLE 1.
T. loubieri isolate rDNA PCR products
| rDNA segment | Primersa | PCR product (bp) | GenBank accession no. | % Homologyb to:
|
|
|---|---|---|---|---|---|
| T. louberi CBS 7065 | T. mucoides CBS 7625 | ||||
| 18S (6) | ATT GGA GGG CAA GTC TGG TG | 462 | AY101606 | 100 (462/462) | 97.4 (450/462) |
| CCG ATC CCT AGT CGG CAT AG | AB001759 | AB001763 | |||
| ITS region (29) | TCC GTA GGT GAA CCT GCG G | 491 | AY101607 | 100 (491/491) | 84.9 (417/491) |
| TCC TCC GCT TAT TGA TAT GC | AB018027 | AB018030 | |||
| 26S (22) | ATC AAT AAG CGG AGG AAA AG | 727 | AY101608 | 99.8 (605/606) | 93.7 (568/606) |
| CTC TGG CTT CAC CCT ATT C | AF075522 | AF075515 | |||
The forward primer is shown on the first line, and the reverse primer is shown on the second line.
Percent homology to the T. louberi CBS 7065 or T. mucoides CBS 7625 sequence (CBS, Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands). The number of homologous base pairs to the total number of base pairs is shown in the parentheses. The GenBank accession number of the T. louberi CBS 7065 or T. mucoides CBS 7625 strain sequence is shown on the second line.
Sequences obtained from the three PCRs were compared to the nucleotide sequences in GenBank by using the on-line BLAST (1) program (National Center for Biotechnology Information, Bethesda, Md.) (Table 1). The isolate sequences for the ITS region and 18S rDNA amplicons were identical to those published for the CBS 7065 reference strain of Trichosporon loubieri (GenBank accession number AB001759 and AB018027, respectively) (24, 25). The 26S rDNA amplicon sequence differed in only one base pair to the published sequence of the same T. loubieri strain (C→T at position 490 of GenBank accession number AF075522) (9).
Further testing of the isolate demonstrated growth at 42°C. The carbohydrate assimilation profile provided by API 20C Aux was compatible with the T. loubieri profile published (13, 14), including growth with raffinose and with melibiose and lack of growth with melzitose. The ID 32C yeast identification system (bioMérieux) did not demonstrate growth with rhamnose or lactose (ID 32C 7577664725). The isolate has been deposited in the American Type Culture Collection (Manassas, Va.) under catalog number MYA-2615.
Since the reclassification of the genus Trichosporon in 1992 (14), there had been evidence that only six species were associated with human disease (5, 13). Trichosporon ovoides and Trichosporon inkin are agents typically associated with capital and pubic white piedra, respectively, which is a superficial hair shaft infection. These species as well as Trichosporon cutaneum and Trichosporon asteroides have been isolated in cases of superficial skin infections. Occasionally, these agents have been reported as causes of systemic infection, usually catheter related (13, 16, 17). On the other hand, trichosporonosis has been recognized as an emerging infection in immunosuppressed patients (2, 10). Trichosporon asahii is the most common isolate in these cases, followed by T. mucoides (10, 13). Trichosporon beigelii was commonly used in the literature prior to 1992, but that designation was rejected after reclassification of the genus (14).
T. loubieri type strain (CBS 7065) was isolated from a cow with mastitis (14), but T. loubieri had not been isolated in humans until recently (20), when infected cysts in a Nepalese patient with polycystic kidney disease were found after nephrectomy. No tissue invasion was seen on histological examination, no fungemia was documented, but the patient's fever subsided after nephrectomy. The patient reported here developed high-grade fungemia and went on to develop a severe hepatosplenic syndrome, described recently for T. asahii (19), after her chemotherapy-induced neutropenia resolved. The infection was finally controlled after a splenectomy and with the addition of high-dose fluconazole to amphotericin B. Histology demonstrated invasive disease and evidence of angioinvasion. Although trichosporonosis is known to cause false-positive cryptococcal antigen test results (18), this was not found in the present case. These findings confirm that T. loubieri is a human pathogen and that it is capable of causing invasive infection.
Gueho and colleagues mentioned the ability of T. loubieri to grow with rhamnose (14), but this is not an exclusive characteristic and only T. loubieri's ability to grow at 42°C is used in the physiological key for Trichosporon identification (14). In addition, the carbohydrate assimilation profiles of the two variants of T. loubieri from the patient with infected renal cysts (20) suggest that intraspecies variations exist. This underscores the relevance of molecular confirmation of Trichosporon clinical isolates for definitive identification (9, 25). Analysis of rDNA intergenic spacer regions may further aid in the classification of this genus and of species-specific strains (23).
The relative resistance of these organisms to amphotericin B is important to note. Clinical failures and the microbiologic resistance of Trichosporon spp. have been well-documented (26-28), and it has been shown that amphotericin B is fungistatic. This resistance explains in part the recalcitrant course of the patient presented despite the use of high doses of lipid formulations of amphotericin B. Interestingly, there was no growth of the organism from the surgical specimens obtained after several weeks of therapy. There is growing evidence that azole drugs have good activity against Trichosporon spp. (3, 8, 11, 21) and that combined administration of fluconazole and amphotericin B may be superior to either drug used alone against invasive infection (4, 15). Echinocandins have no activity against Trichosporon species (7), and a breakthrough infection in a patient receiving caspofungin was recently reported (12).
Given the robustness of the current classification of the genus Trichosporon (9, 14, 25), species-specific classification should be attempted on all relevant clinical isolates. Caution should be used when interpreting identification based solely on sugar assimilation profiles. Azole-based treatment regimens should be considered the first-line treatment of trichosporonosis.
(A preliminary report of this work was presented at the 40th Annual Meeting of the Infectious Diseases Society of America, Chicago, Ill., 25 October 2002.)
Nucleotide sequence accession numbers.
The rDNA sequences of the isolate have been deposited in GenBank (accession numbers AY101606, AY101607, and AY101608 for the 18S, ITS, and 26S rDNA gene segments, respectively).
Acknowledgments
We thank Akhtari Alam and Esperanza Albano, clinical mycology lab, Brigham & Women's Hospital, for valuable assistance and Takashi Sugita, Department of Microbiology, Meiji Pharmaceutical University, Tokyo, Japan, for insightful comments and for running the ID 32C carbohydrate assimilation test on the isolate.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anaissie, E., G. P. Bodey, H. Kantarjian, J. Ro, S. E. Vartivarian, R. Hopfer, J. Hoy, and K. Rolston. 1989. New spectrum of fungal infections in patients with cancer. Rev. Infect. Dis. 11:369-378. [DOI] [PubMed] [Google Scholar]
- 3.Anaissie, E., A. Gokaslan, R. Hachem, R. Rubin, G. Griffin, R. Robinson, J. Sobel, and G. Bodey. 1992. Azole therapy for trichosporonosis: clinical evaluation of eight patients, experimental therapy for murine infection, and review. Clin. Infect. Dis. 15:781-787. [DOI] [PubMed] [Google Scholar]
- 4.Anaissie, E. J., R. Hachem, N. C. Karyotakis, A. Gokaslan, M. C. Dignani, L. C. Stephens, and U. C. Tin. 1994. Comparative efficacies of amphotericin B, triazoles, and combination of both as experimental therapy for murine trichosporonosis. Antimicrob. Agents Chemother. 38:2541-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Atlas of clinical fungi, 2nd ed. Centraal Bureau voor Schimmelcultures, Utrecht, The Netherlands.
- 6.Einsele, H., H. Hebart, G. Roller, J. Loffler, I. Rothenhofer, C. A. Muller, R. A. Bowden, J. van Burik, D. Engelhard, L. Kanz, and U. Schumacher. 1997. Detection and identification of fungal pathogens in blood by using molecular probes. J. Clin. Microbiol. 35:1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espinel-Ingroff, A. 1998. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J. Clin. Microbiol. 36:2950-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A. 1998. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J. Clin. Microbiol. 36:198-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fell, J. W., H. Roeijmans, and T. Boekhout. 1999. Cystofilobasidiales, a new order of basidiomycetous yeasts. Int. J. Syst. Bacteriol. 49:907-913. [DOI] [PubMed] [Google Scholar]
- 10.Fleming, R. V., T. J. Walsh, and E. J. Anaissie. 2002. Emerging and less common fungal pathogens. Infect. Dis. Clin. N. Am. 16:915-933, vi-vii. [DOI] [PubMed] [Google Scholar]
- 11.Fournier, S., W. Pavageau, M. Feuillhade, S. Deplus, A. M. Zagdanski, O. Verola, H. Dombret, and J. M. Molina. 2002. Use of voriconazole to successfully treat disseminated Trichosporon asahii infection in a patient with acute myeloid leukaemia. Eur. J. Clin. Microbiol. Infect. Dis. 21:892-896. [DOI] [PubMed] [Google Scholar]
- 12.Goodman, D., E. Pamer, A. Jakubowski, C. Morris, and K. Sepkowitz. 2002. Breakthrough trichosporonosis in a bone marrow transplant recipient receiving caspofungin acetate. Clin. Infect. Dis. 35:E35-E36. [DOI] [PubMed] [Google Scholar]
- 13.Gueho, E., L. Improvisi, G. S. de Hoog, and B. Dupont. 1994. Trichosporon on humans: a practical account. Mycoses 37:3-10. [DOI] [PubMed] [Google Scholar]
- 14.Gueho, E., M. T. Smith, G. S. de Hoog, G. Billon-Grand, R. Christen, and W. H. Batenburg-van der Vegte. 1992. Contributions to a revision of the genus Trichosporon. Antonie Leeuwenhoek 61:289-316. [DOI] [PubMed] [Google Scholar]
- 15.Kamberi, P., H. Atsuro, T. Takayoshi, and N. Masaru. 1998. Efficacy of amphotericin B and azoles alone and in combination against disseminated trichosporonosis in neutropenic mice. Chemotherapy 44:55-62. [DOI] [PubMed] [Google Scholar]
- 16.Kustimur, S., A. Kalkanci, K. Caglar, M. Dizbay, F. Aktas, and T. Sugita. 2002. Nosocomial fungemia due to Trichosporon asteroides: firstly described bloodstream infection. Diagn. Microbiol. Infect. Dis. 43:167-170. [DOI] [PubMed] [Google Scholar]
- 17.Lopes, J. O., S. H. Alves, C. Klock, L. T. Oliveira, and N. R. Dal Forno. 1997. Trichosporon inkin peritonitis during continuous ambulatory peritoneal dialysis with bibliography review. Mycopathologia 139:15-18. [DOI] [PubMed] [Google Scholar]
- 18.McManus, E. J., M. J. Bozdech, and J. M. Jones. 1985. Role of the latex agglutination test for cryptococcal antigen in diagnosing disseminated infections with Trichosporon beigelii. J. Infect. Dis. 151:1167-1169. [DOI] [PubMed] [Google Scholar]
- 19.Meyer, M. H., V. Letscher-Bru, J. Waller, P. Lutz, L. Marcellin, and R. Herbrecht. 2002. Chronic disseminated Trichosporon asahii infection in a leukemic child. Clin. Infect. Dis. 35:E22-E25. [DOI] [PubMed] [Google Scholar]
- 20.Padhye, A. A., S. Verghese, P. Ravichandran, G. Balamurugan, L. Hall, P. Padmaja, and M. C. Fernandez. 2003. Trichosporon loubieri infection in a patient with adult polycystic kidney disease. J. Clin. Microbiol. 41:479-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2002. In vitro antifungal susceptibilities of Trichosporon species. Antimicrob. Agents Chemother. 46:1144-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandhu, G. S., B. C. Kline, L. Stockman, and G. D. Roberts. 1995. Molecular probes for diagnosis of fungal infections. J. Clin. Microbiol. 33:2913-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugita, T., M. Nakajima, R. Ikeda, T. Matsushima, and T. Shinoda. 2002. Sequence analysis of the ribosomal DNA intergenic spacer 1 regions of Trichosporon species. J. Clin. Microbiol. 40:1826-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugita, T., and T. Nakase. 1998. Molecular phylogenetic study of the basidiomycetous anamorphic yeast genus Trichosporon and related taxa based on small subunit ribosomal DNA sequences. Mycoscience 39:7-13. [Google Scholar]
- 25.Sugita, T., A. Nishikawa, R. Ikeda, and T. Shinoda. 1999. Identification of medically relevant Trichosporon species based on sequences of internal transcribed spacer regions and construction of a database for Trichosporon identification. J. Clin. Microbiol. 37:1985-1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toriumi, Y., T. Sugita, M. Nakajima, T. Matsushima, and T. Shinoda. 2002. Antifungal pharmacodynamic characteristics of amphotericin B against Trichosporon asahii, using time-kill methodology. Microbiol. Immunol. 46:89-93. [DOI] [PubMed] [Google Scholar]
- 27.Walsh, T. J., J. W. Lee, G. P. Melcher, E. Navarro, J. Bacher, D. Callender, K. D. Reed, T. Wu, G. Lopez-Berestein, and P. A. Pizzo. 1992. Experimental Trichosporon infection in persistently granulocytopenic rabbits: implications for pathogenesis, diagnosis, and treatment of an emerging opportunistic mycosis. J. Infect. Dis. 166:121-133. [DOI] [PubMed] [Google Scholar]
- 28.Walsh, T. J., G. P. Melcher, M. G. Rinaldi, J. Lecciones, D. A. McGough, P. Kelly, J. Lee, D. Callender, M. Rubin, and P. A. Pizzo. 1990. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J. Clin. Microbiol. 28:1616-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White, B. A. 1993. PCR protocols: current methods and applications. Humana Press, Totowa, N.J.