Abstract
Study Objectives:
To compare the craniofacial morphological phenotype of subjects with and without obstructive sleep apnea (OSA) using a quantitative photographic analysis technique.
Design:
Case-control study; subgroup matched for body mass index (BMI) and sex.
Setting:
Sleep investigation unit in a university teaching hospital.
Patients:
114 subjects (93% Caucasian) with OSA (apnea-hypopnea index [AHI] ≥ 10/h) and 66 controls (AHI < 10/h).
Interventions:
Standardized frontal-profile craniofacial photographic imaging performed prior to polysomnography. Photographs were analyzed for the computation of linear, angular, area and polyhedral volume measurements representing dimensions and relationships of the various craniofacial regions.
Measurements and Results:
Photographic craniofacial phenotypic differences were demonstrated between OSA and control subjects, including a range of measurements of the face, mandible, maxilla, eyes, nose, head and neck. After 1-for-1 subgroup matching for BMI and sex (51 subjects in each group), mandibular length 1 (6.21 ± 0.08 [mean ± SEM] vs 6.58 ± 0.08 cm, P = 0.006), mandibular-nasion angle 1 (35.0 ± 0.48 vs 36.7 ± 0.37 degrees, P = 0.006) and anterior neck space area (10.2 ± 0.53 vs 12.2 ± 0.52 cm2, P = 0.01) remained smaller in the OSA group. Mandibular width-length angle (88.0 ± 0.75 vs 85.3 ± 0.54 degrees, P = 0.005) and face width-midface depth angle (72.3 ± 0.44 vs 70.7 ± 0.39 degrees, P = 0.01) remained larger in the OSA group, whereas mandibular triangular area (39.2 ± 0.63 vs 41.7 ± 0.74 cm2, P = 0.01) was smaller.
Conclusions:
Craniofacial phenotypic differences in OSA in Caucasian subjects can be demonstrated using a photographic analysis technique.
Citation:
Lee RWW; Chan ASL; Grunstein RR; Cistulli PA. Craniofacial phenotyping in obstructive sleep apnea – a novel quantitative photographic approach. SLEEP 2009;32(1):37–45.
Keywords: obstructive sleep apnea, craniofacial abnormalities, photogrammetry, phenotype
OBSTRUCTIVE SLEEP APNEA (OSA) IS CHARACTERIZED BY REPETITIVE CLOSURE OF THE UPPER AIRWAY DURING SLEEP. ITS OCCURRENCE IS THE RESULT OF anatomic and functional abnormalities of the upper airway. While neuromuscular and respiratory control mechanisms play important roles in the maintenance of airway patency, an abnormal anatomic substrate is a key factor in the development of OSA.1,2 Craniofacial abnormalities, enlargement of upper airway soft tissue structures, central obesity, and an excess of regional adipose tissue are known anatomic risk factors for OSA.3–5 Although obesity is generally considered the major attributing risk factor for OSA,6 craniofacial morphology is increasingly recognized as an important interacting factor in OSA pathogenesis.
It is well recognized from studies using imaging techniques that craniofacial abnormalities are common in patients with OSA.7–9 Mandibular retrusion, maxillary deficiency, inferior displacement of the hyoid bone and cranial base abnormalities are amongst the most commonly reported findings.7,8,10,11 These abnormalities can result in a compromised airway space and an increase in upper airway collapsibility,12,13 thereby predisposing to OSA. It is thought that the interaction between craniofacial morphology and obesity determines the likelihood and severity of OSA in the majority of patients.14–16
While the available techniques (cephalometry, computed tomography [CT] and magnetic resonance imaging [MRI]) for craniofacial assessment allow detailed examination of bony and soft tissue structures, they are generally limited to research applications due to their expense, time-consuming analyses and/or radiation exposure. Craniofacial anthropometry and photogrammetry are alternative craniofacial assessment techniques that have the advantages of being noninvasive and readily accessible. Furthermore, they allow quantification of the surface morphology which is generally not achievable with the other imaging modalities. Craniofacial photogrammetry, involving measurements from photographs, has been applied in the assessment of subjects with craniofacial anomalies.17,18 Application of photogrammetry to examine subjects with OSA may reveal new insights in the craniofacial morphological phenotype of this condition.
The aims of this study were to compare the craniofacial morphological phenotype of subjects with and without OSA using a quantitative photographic analysis technique. Secondly, we aimed to determine whether these differences were present between subjects with similar degrees of obesity. Finally, we aimed to examine the relationships of these craniofacial photographic measurements to obesity and OSA severity.
METHODS
Subjects
Subjects referred for polysomnography to a university teaching hospital for the initial investigation of OSA were recruited consecutively. Exclusion criteria included those with a known history of syndromal craniofacial abnormalities (e.g., Down syndrome), previous craniofacial surgery, and excessive facial hair which significantly obscured facial landmarks. Subjects of all ethnicity (self-reported) were included. Anthropometry and the photographic procedure were performed on all subjects on the same day as the polysomnography. All data collection and photographic analyses were carried out by a single investigator (RL) who was blinded to the result of the polysomnography. Ethics approval was obtained from the institutional ethics committee, and written informed consent was obtained from all subjects.
Standardized Photographic Technique and Craniofacial Photogrammetry
Frontal and profile digital photographs of the head and neck were obtained with a standardized setup. A single-lens reflex digital camera (D70 with 18–70 mm lens and external flash unit SB-29s; Nikon Corp., Japan) was mounted on a tripod at a distance of 160 cm from the subject alignment plane. Standardized camera settings (focal length 70 mm, aperture 7.1, shutter speed 1/100th, ISO 400) were used to ensure consistency of the JPEG images (resolution 3008 by 2000 pixels). Subjects were photographed standing upright while assuming the natural head position. Prior to the photographs, certain bony and cartilaginous landmarks were pre-identified on the subjects by palpation and marked with a white tape (Figure 1). Standardized methods were used to align subjects for the photographs. For the frontal photograph, the subject's facial landmark nasion was aligned along the subject alignment plane while ensuring both ears were seen equally from the front. For the profile photograph, the subject was instructed to turn 90 degrees to the left after the frontal photograph was taken. This was aided by a laser pointer head-clip and calibrated markings on the side wall to ensure the profile views were perpendicular to the frontal views. The subject's mid-sagittal plane was aligned to the subject alignment plane.
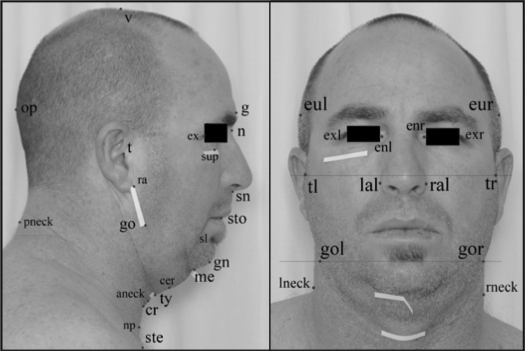
Figure 1.
Photographic Landmarks – Profile and Frontal View. Landmarks pre-identified on subject (marked with a white tape): sup – infraorbital rim; me – mentum; ty – thyroid; cr – cricoid; ste – sternal notch; gol – gonion (L); gor – gonion (R). Landmarks digitized on photographs: t – tragion; ex – exocanthion; sup – infraorbital rim; g – glabella; n – nasion; sn – subnasion; sto – stomion; sl – sublabiale; gn – gnathion; me – mentum; cer – cervical point; ty – thyroid; cr – cricoid; np; neck plane; ste – sternal notch; go – gonion; ra – ramus; op – opisthocranion; v – vertex; aneck – anterior neck; pneck – posterior neck; tl – tragion (L); tr – tragion (R); gol – gonion (L); gor – gonion (R); eul – euryon (L); eur – euryon (R); exl – exocanthion (L); exr – exocanthion (R); enl – endocanthion (L); enr – endocanthion (R); lal – alare (L); ral – alare (R); lneck – neck (L); rneck – neck (R); (L) = left side on the photograph, (R) = right side on the photograph.
Using image analysis software (Image J v1.36, NIH, Bethesda, MD), the photographs were examined for landmark digitization. Craniofacial landmarks of interest were captured as pixel coordinates (x, y) of the image which were then transferred to a custom-programmed spreadsheet for the computation of linear, angular, area, and polyhedral volume measurements. Pixel measurements were converted to metric dimensions (52 pixels/cm). In this study, a total of 71 craniofacial measurements were computed using this photogrammetry technique. These measurements represented the dimensions and relationships of the various craniofacial regions including the face, mandible, maxilla, eyes, nose, head, and neck. Technique validation (landmark digitization accuracy and test-retest reliability) was performed in a subgroup of subjects.
Anthropometry
Height was measured by a wall-mounted stadiometer (±0.1 cm). Subjects were weighed using an analogue scale (±0.5 kg) with minimal clothing. Neck circumference was measured with a tape measure (±0.5 cm) at the level of the cricothyroid membrane. Waist circumference was measured at the level of the ischial tuberosities with the subject in the standing position. Body mass index (BMI) was calculated with the formula of weight (kg) divided by height squared (m2).
Polysomnography
Diagnostic polysomnography (PSG) was performed in accordance with previous studies and recommendations.19,20 Sleep staging was determined using standardized definitions.21 Apnea was defined as complete airflow cessation ≥ 10 seconds with oxygen desaturation of at least 3% and/or associated with arousal. Hypopnea was defined as a reduction in amplitude of airflow or thoracoabdominal wall movement > 50% of the baseline measurement > 10 sec with an accompanying oxygen desaturation of at least 3%, and/or associated with arousals. Apnea-hypopnea index (AHI) was calculated as the total number of apneas and hypopneas per hour of sleep. Polysomnography scoring was performed by experienced accredited sleep technologists. The OSA cases were defined by an AHI ≥ 10 events per hour. The controls were defined by an AHI < 10 events per hour.
Data and Statistical Analysis
Statistical analysis was performed with SPSS (v13.0 for Windows, SPSS Inc., Chicago, IL, USA). The χ2 test was used for comparing categorical variables (gender and ethnicity). Fisher exact test was used if any of the cells in a categorical table have expected count less than 5. Student's t-test was used for comparing normally distributed continuous variables (craniofacial photogrammetry data). One-for-one matching for BMI (±0.5 kg/m2) and sex was performed in a subgroup of OSA and control subjects.
Analysis of the relationship between craniofacial photogrammetry and OSA severity, and obesity (BMI, neck circumference, waist circumference) were limited to those measurements which had a P value ≤ 0.10 in the primary case control analysis (to reduce the number of statistical comparisons). The AHI (with the addition of one) was logarithmic-transformed to obtain a normal distribution. Pearson correlations were used to examine the association between continuous variables. A partial correlation procedure was used to examine the linear relationship between OSA severity and craniofacial photographic measurements while controlling for the effect of BMI.
A P value ≤ 0.01 was considered statistically significant. All P values < 0.10 were shown in the results, those that were greater than or equal to 0.10 were shown as nonsignificant (NS). All data are expressed as mean ± SEM unless otherwise specified.
RESULTS
Subjects Characteristics
A total of 180 subjects were recruited and included in the analysis; this included 114 subjects in the OSA group (AHI ≥ 10) and 66 control subjects (AHI < 10). Three subjects were excluded from the analysis (2 subjects did not complete the PSG; 1 subject was found to have central sleep apnea). Twelve subjects (7 males, 5 females) declined study participation. Characteristics of the subjects in the control and OSA groups are summarized in Table 1. There was no significant difference in the proportion of males in the comparison groups. The mean age was higher in the OSA subjects (P = 0.003), as were various anthropometric measures of obesity (ranged from P = 0.002 to P < 0.001). The majority of subjects were of Caucasian background, with the exception of 5 Chinese and 3 Pacific Islander subjects.
Table 1.
Subject Characteristics
| Control (AHI < 10) n = 66 | OSA (AHI ≥ 10) n = 114 | P | |
|---|---|---|---|
| Males (%) | 46 (69.7%) | 91 (79.8%) | NS |
| Age (years) | 49.3 ± 14.9 | 55.8 ± 13.5 | 0.003 |
| Ethnicity (% Caucasians) | 100% | 93% | 0.02 |
| Anthropometry | |||
| BMI (kg/m2) | 27.1 ± 4.8 | 30.6 ± 4.9 | < 0.001 |
| Height (cm) | 173.7 ± 9.6 | 172.7 ± 9.3 | NS |
| Weight (kg) | 82.6 ± 19.1 | 91.2 ± 15.6 | 0.002 |
| Neck circumference (cm) | 39.5 ± 4.5 | 42.4 ± 4.1 | < 0.001 |
| Waist circumference (cm) | 97.5 ± 14.2 | 109.4 ± 12.7 | < 0.001 |
| Epworth sleepiness scale | 8.7 ± 0.61 | 8.9 ± 0.47 | NS |
| Polysomnography | |||
| Total AHI | 4.4 ± 3.1 | 33.4 ± 20.8 | - |
| AHI – NREM sleep | 3.5 ± 2.8 | 31.6 ± 22.6 | - |
| AHI – REM sleep | 8.3 ± 8.7 | 37.3 ± 22.8 | - |
| Minimum SaO2 (%) | 90.4 ± 4.8 | 79.5 ± 10.2 | - |
| Arousal index | 22.6 ± 11.5 | 40.6 ± 17.3 | - |
| Sleep efficiency (%) | 79.1 ± 14.6 | 78.6 ± 16.3 | - |
Mean ± SD; NS = nonsignificant; BMI = body mass index; AHI = apnea-hypopnea index
Craniofacial Photogrammetry
Results of the 71 craniofacial photographic measurements comparing OSA subjects and controls are presented in Table 2. These results are summarized below according to the various craniofacial regions.
Table 2.
Craniofacial Photogrammetry – Primary Analysis
| Craniofacial Landmarks | Control (AHI < 10) n = 66 | OSA (AHI ≥ 10) n = 114 | P | |
|---|---|---|---|---|
| Face* | ||||
| Upper face depth | t-n | 9.93 ± 0.08 | 10.0 ± 0.06 | NS |
| Mid face depth 1 | t-sn | 10.5 ± 0.08 | 10.5 ± 0.06 | NS |
| Mid face depth 2 | t-sl | 11.4 ± 0.09 | 11.5 ± 0.06 | NS |
| Lower face depth 1 | t-gn | 12.9 ± 0.10 | 13.0 ± 0.07 | NS |
| Lower face depth 2 | t-me | 12.4 ± 0.11 | 12.5 ± 0.07 | NS |
| Total face height | n-gn | 11.9 ± 0.09 | 12.1 ± 0.07 | 0.03 |
| Upper face height | n-sto | 7.72 ± 0.06 | 7.89 ± 0.05 | 0.03 |
| Lower face height 1 | sn-gn | 6.52 ± 0.08 | 6.65 ± 0.06 | NS |
| Lateral face height | ex-go | 10.3 ± 0.10 | 10.7 ± 0.07 | 0.005 |
| Face width | tl-tr | 15.0 ± 0.09 | 15.7 ± 0.08 | < 0.001 |
| Facial axis angle | n-t and go-gn | 36.2 ± 0.84 | 35.2 ± 0.64 | NS |
| Mandibular width-length angle | gor-me-gol | 84.4 ± 0.56 | 89.4 ± 0.58 | < 0.001 |
| Face width-midface depth angle | tr-sn-tl | 70.5 ± 0.37 | 72.7 ± 0.32 | < 0.001 |
| Face width-lower face depth angle | tr-me-tl | 61.9 ± 0.31 | 63.8 ± 0.28 | < 0.001 |
| Maxillary triangle area | t-sn-me | 39.0 ± 0.66 | 39.9 ± 0.42 | NS |
| Mandibular triangle area | t-go-me | 15.3 ± 0.57 | 15.3 ± 0.42 | NS |
| Maxillary-mandibular box area | t-sn-me-go | 54.4 ± 0.99 | 55.2 ± 0.66 | NS |
| Cranial base triangle area (ax) | tl-n-tr | 74.7 ± 0.87 | 78.6 ± 0.71 | 0.001 |
| Cranial base area 1 (ax) | tl-exl-exr-tr | 96.2 ± 1.04 | 102 ± 0.91 | < 0.001 |
| Cranial base area 2 (ax) | tl-exl-n-exr-tr | 104 ± 1.12 | 110 ± 0.97 | < 0.001 |
| Maxillary triangle area (ax) | tl-sn-tr | 78.9 ± 0.90 | 82.5 ± 0.77 | 0.003 |
| Middle cranial fossa volume | tl-tr-n-sn | 134 ± 2.21 | 143 ± 1.81 | 0.002 |
| Maxillary volume | tl-tr-sn-me | 196 ± 3.97 | 209 ± 2.84 | 0.007 |
| Mandibular volume | tl-tr-gol-gor-me | 141 ± 5.88 | 148 ± 4.49 | NS |
| Maxillary-mandibular volume 1 | tl-tr-gol-gor-sn-me | 337 ± 8.47 | 357 ± 6.31 | 0.06 |
| Mandible and Maxilla* | ||||
| Anterior mandibular height | sto-gn | 4.19 ± 0.05 | 4.27 ± 0.04 | NS |
| Mandibular length 1 | me-go | 6.54 ± 0.07 | 6.19 ± 0.06 | < 0.001 |
| Mandibular length 2 | gn-go | 7.57 ± 0.08 | 7.29 ± 0.06 | 0.008 |
| Posterior mandibular height | t-go | 6.86 ± 0.10 | 7.31 ± 0.07 | < 0.001 |
| Mandible width | gol-gor | 12.3 ± 0.12 | 13.0 ± 0.11 | < 0.001 |
| Maxillary depth angle | t-n-sn | 80.1 ± 0.41 | 79.2 ± 0.44 | NS |
| Mandibular depth angle 1 | t-n-sl | 72.3 ± 0.43 | 71.9 ± 0.41 | NS |
| Maxillary-mandibular relationship angle 1 | sn-n-sl | 7.87 ± 0.35 | 7.36 ± 0.28 | NS |
| Mandibular-nasion angle 1 | go-n-gn | 37.0 ± 0.37 | 34.8 ± 0.32 | < 0.001 |
| Mandibular-nasion angle 2 | go-n-me | 30.6 ± 0.34 | 28.2 ± 0.30 | < 0.001 |
| Mandibular-subnasion angle 1 | go-sn-gn | 55.9 ± 0.58 | 53.2 ± 0.52 | 0.001 |
| Mandibular-subnasion angle 2 | go-sn-me | 45.8 ± 0.48 | 42.7 ± 0.45 | < 0.001 |
| Mandibular plane angle 1 | go-me-TH | 29.1 ± 1.05 | 27.7 ± 0.94 | NS |
| Mandibular triangle area (ax) | gol-me-gor | 40.5 ± 0.71 | 40.3 ± 0.52 | NS |
| Eyes and Nose | ||||
| Nose height | n-sn | 5.47 ± 0.05 | 5.58 ± 0.04 | 0.09 |
| Eye width | exl-enl | 2.73 ± 0.03 | 2.68 ± 0.03 | NS |
| Intercanthal width | enl-enr | 3.27 ± 0.05 | 3.50 ± 0.04 | < 0.001 |
| Biocular width | exl-exr | 8.73 ± 0.05 | 8.87 ± 0.05 | 0.06 |
| Nose width | lal-ral | 3.72 ± 0.05 | 3.97 ± 0.04 | < 0.001 |
| Head | ||||
| Total craniofacial height | v-gn | 24.8 ± 0.18 | 24.8 ± 0.12 | NS |
| Maximum cranial length | g-op | 20.6 ± 0.17 | 20.7 ± 0.10 | NS |
| Maximum cranial width | eul-eur | 14.4 ± 0.12 | 14.7 ± 0.08 | 0.02 |
| Natural Head position angle | t-sup-TH | 3.68 ± 0.66 | 4.62 ± 0.61 | NS |
| Head base Inclination angle | t-n-TH | 16.5 ± 0.71 | 17.6 ± 0.63 | NS |
| Neck | ||||
| Thyromental distance – horizontal | ty-me (TH) | 4.09 ± 0.10 | 4.12 ± 0.08 | NS |
| Cricomental distance – horizontal | cr-me (TH) | 4.91 ± 0.11 | 5.04 ± 0.08 | NS |
| Sternomental distance | ste-me | 9.74 ± 0.16 | 9.42 ± 0.15 | NS |
| Sternomandibular distance – vertical | ste-go (TV) | 11.6 ± 0.14 | 10.8 ± 0.16 | < 0.001 |
| Sternotragion distance – vertical | ste-t (TV) | 18.1 ± 0.14 | 17.6 ± 0.15 | 0.05 |
| Cricomental space distance† | cer-cr-me | 0.65 ± 0.06 | 0.29 ± 0.05 | < 0.001 |
| Neck depth | aneck-pneck | 12.6 ± 0.21 | 13.9 ± 0.14 | < 0.001 |
| Neck width | lneck-rneck | 12.3 ± 0.16 | 13.1 ± 0.12 | < 0.001 |
| Neck perimeter | l-r-a-p-neck | 39.1 ± 0.55 | 42.4 ± 0.38 | < 0.001 |
| Cervicomental angle | np-cer-me | 154 ± 2.20 | 167 ± 1.64 | < 0.001 |
| Cricomental space area† | cer-cr-me | 2.15 ± 0.22 | 1.01 ± 0.16 | < 0.001 |
| Thyromental space area† | cer-ty-me | 1.02 ± 0.15 | 0.35 ± 0.10 | < 0.001 |
| Anterior neck space area† | ste-cr-ty-cer-me | 12.3 ± 0.47 | 10.2 ± 0.41 | 0.001 |
| Total anterior neck soft tissue area | go-me-cer-cr | 18.0 ± 0.43 | 18.2 ± 0.31 | NS |
| Posterior neck soft tissue area | cr-go-pneck | 39.9 ± 0.81 | 43.1 ± 0.73 | 0.006 |
| Total neck soft tissue area | go-me-cer-cr-pneck | 58.0 ± 1.13 | 61.3 ± 0.93 | 0.03 |
| Neck cross-sectional area | l-r-a-p-neck | 123 ± 3.38 | 144 ± 2.64 | < 0.001 |
| Mandibular cricoid area (ax) | gol-gor-cr | 47.2 ± 0.72 | 48.0 ± 0.69 | NS |
| Total anterior neck soft tissue volume 2‡ | gol-gor-me-cer-ty-cr | 74.0 ± 2.15 | 78.6 ± 1.59 | 0.08 |
| Total anterior neck space volume 2† | (gol-gor-me-cr) – ‡ | 9.27 ± 0.82 | 4.98 ± 0.72 | < 0.001 |
| Posterior neck soft tissue volume§ | pneck-cr-gol-gor | 494 ± 13.1 | 566 ± 12.9 | < 0.001 |
| Total neck soft tissue volume | ‡ + § | 567 ± 14.7 | 645 ± 14.0 | < 0.001 |
Mean ± SEM. NS = nonsignificant; TH = true horizontal distance between 2 landmarks; TV = true vertical distance between 2 landmarks; ax = axial measurement.
Photographic measurements of the face and maxillomandibular region can overlap;
Measurement can be of a negative value; Linear measurements are in cm, angles in degrees, areas in cm2, volumes in cm3.
Face
There were no significant differences in the vertical dimensions of the face (total, upper, or lower face heights). The mid and lower face widths were significantly greater in subjects with OSA (face width, mandibular width). In addition, the mid and lower face appeared to be wider and flatter (mandibular width-length angle, face width-midface depth angle, face width-lower face depth angle). The axial triangular or polygonal areas of the face at the level of the cranial base or maxilla were larger in the OSA subjects (cranial base triangle area (ax); cranial base area 1 (ax) and 2 (ax); maxillary triangle area (ax)). Similarly, the volume of the midface region was also larger in the OSA subjects (middle cranial fossa volume, maxillary volume). On the contrary, the areas and volume of the face in the mandibular region were similar between the 2 groups (mandibular triangle area and mandibular triangle area (ax); mandibular volume). The facial axis angle was similar between the 2 groups.
Mandible and Maxilla
The length of the mandible was shorter in the subjects with OSA (mandibular length 1 and 2). Correspondingly, a number of angular measurements also suggest the mandible was shorter in these subjects (mandibular-nasion angle 1 and 2; mandibular-subnasion angle 1 and 2). Maxillary deficiency was not detected in the OSA subjects (mid face depth 1 and 2; maxillary depth angle; maxillary-mandibular relationship angle 1). The inferior plane of the mandibular body relative to the horizontal plane was similar between the groups (mandibular plane angle 1).
Eyes and Nose
There was no difference in the horizontal size of the eyes between subjects with OSA and controls (eye width). However, the distance between the inner corners of the eyes was greater (intercanthal width) in subjects with OSA, but not between the outer corners of the eyes (biocular width). There was no difference in the nose height, but the nose width was greater in the OSA subjects.
Head
The vertical or anteroposterior lengths of the head were not different between subjects with OSA and controls (total craniofacial height; maximum cranial length). Neither was there any significant difference in the lateral dimension of the head (maximum cranial width). Head position relative to the horizontal plane was similar between the two groups (natural head position angle; head base inclination angle).
Neck
The vertical length of the neck tended to be shorter in the OSA group (sternomandibular distance – vertical; sternotragion distance – vertical). Measurements that relate to neck size were all greater in the OSA subjects (neck width; neck depth; neck perimeter; neck cross-sectional area; posterior and total neck soft tissue volume). While the anterior neck soft tissue area and volume were similar (total anterior neck soft tissue area; total anterior neck soft tissue volume 2), the area and volume of space in front of the neck and below the jaw were significantly smaller in the OSA subjects (anterior neck space area; total anterior neck space volume 2). Measurements that relate to the cricoid cartilage, thyroid cartilage and soft tissues on the anterior neck region were also different between the comparison groups (cricomental space distance; cervicomental angle; cricomental space area; thyromental space area).
Subgroup Analysis: Craniofacial Photogrammetry in Caucasian Men
Analysis was performed in the subgroup of 131 Caucasian men (85 OSA vs 46 controls). Differences in the craniofacial photographic measurements were consistent with those obtained in the primary analysis (data not shown).
Subgroup Analysis: Craniofacial Photogrammetry in BMI and Sex Matched Subjects
Subgroup analysis was performed after matching OSA and control subjects for BMI and sex. This procedure resulted in 51 subjects in each comparison group (Table 3). The mean age was higher in the OSA group (P = 0.01). No significant differences were seen in the neck circumference and waist circumference between the two groups. Compared to the primary analysis of the craniofacial photogrammetry data, a number of measurements were no longer different between the control and OSA groups after matching for BMI and sex (see Tables 2 and 3). However, mandibular length 1 (P = 0.001) and mandibular length 2 (P = 0.002) remained shorter; mandibular-nasion angle 1 (P = 0.006), mandibular-nasion angle 2 (P = 0.004) and anterior neck space area (P = 0.01) remained smaller, in the OSA group. The mandibular width-length angle (P = 0.005) and face width-mid face depth angle (P = 0.01) remained larger in the OSA group. In contrast to the primary analysis, the mandibular triangle area (ax) (P = 0.01) was smaller in the OSA group.
Table 3.
Craniofacial Photogrammetry – Subgroup Analysis 1-for-1 Matched for BMI and Sex
| Control (AHI < 10) n = 51 | OSA (AHI ≥ 10) n = 51 | P | |
|---|---|---|---|
| Total AHI* | 4.7 ± 3.1 | 29.0 ± 18.4 | - |
| Males (%) | 40 (78.4%) | 40 (78.4%) | - |
| Age (years)* | 49.5 ± 14.6 | 56.5 ± 13.0 | 0.01 |
| Anthropometry | |||
| BMI (kg/m2)* | 28.4 ± 4.62 | 28.4 ± 4.08 | - |
| Neck circumference (cm)* | 40.8 ± 4.09 | 40.9 ± 2.96 | NS |
| Waist circumference (cm)* | 102 ± 13.0 | 104 ± 9.40 | NS |
| Craniofacial Photogrammetry | |||
| Mandibular length 1 | 6.58 ± 0.08 | 6.21 ± 0.08 | 0.001 |
| Mandibular length 2 | 7.62 ± 0.08 | 7.23 ± 0.09 | 0.002 |
| Mandibular-nasion angle 1 | 36.7 ± 0.37 | 35.0 ± 0.48 | 0.006 |
| Mandibular-nasion angle 2 | 30.3 ± 0.34 | 28.6 ± 0.45 | 0.004 |
| Mandibular width-length angle | 85.3 ± 0.54 | 88.0 ± 0.75 | 0.005 |
| Face width-midface depth angle | 70.7 ± 0.39 | 72.3 ± 0.44 | 0.01 |
| Anterior neck space area† | 12.2 ± 0.52 | 10.2 ± 0.53 | 0.01 |
| Mandibular triangle area (ax) | 41.7 ± 0.74 | 39.2 ± 0.63 | 0.01 |
Mean ± SD; NS = nonsignificant; Craniofacial photogrammetry results with P ≤ 0.01 are shown; ax = axial measurement;
Measurement can be of a negative value; Linear measurements are in cm, angles in degrees, areas in cm2
Relationship to Obesity
Linear relationships between craniofacial photogrammetry and anthropometric measures of obesity were examined in the entire cohort of 180 subjects. Other than the photographic measurements relating directly to the neck (e.g., neck perimeter, neck cross-sectional area, etc.), the face width (r = 0.52, P < 0.001), mandible width (r = 0.58, P < 0.001) and cervicomental angle (r = 0.50, P < 0.001) had the strongest relationships with BMI. Similarly, the face width and mandible width had the strongest relationships with neck circumference (r[face width] = 0.76, P < 0.001; r[mandible width] = 0.76, P < 0.001) and waist circumference (r[face width] = 0.66, P < 0.001; r[mandible width] = 0.70, P < 0.001).
Relationship to OSA Severity
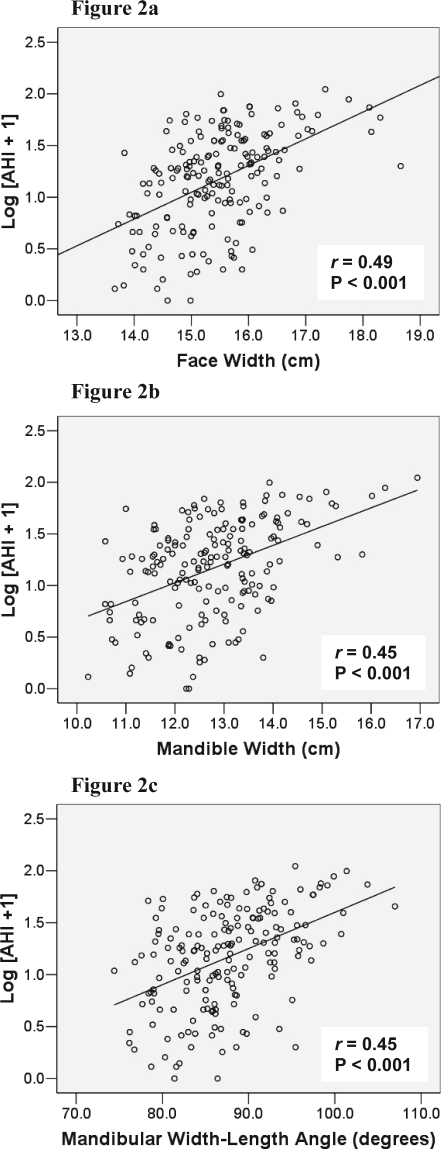
Linear relationships between craniofacial photogrammetry and OSA severity were also examined in the entire cohort of subjects. The strongest relationships were shown with the neck depth (r = 0.51, P < 0.001), neck perimeter (r = 0.50, P < 0.001), neck cross-sectional area (r = 0.49, P < 0.001), face width (Figure 2a; r = 0.49, P < 0.001), mandible width (Figure 2b; r = 0.45, P < 0.001) and mandibular width-length angle (Figure 2c; r = 0.45, P < 0.001). After controlling for BMI, these positive relationships remained (e.g., face width [r = 0.36, P < 0.001], mandibular width [r = 0.28, P < 0.001]).
Figure 2.
Relationships between OSA Severity (Log [AHI + 1]) and Craniofacial Photographic Measurements
Standardized Photographic Technique Validation
Landmark digitization accuracy and test-retest reliability were assessed in 20 subjects who completed the photographic imaging on two separate occasions with photogrammetry performed on separate days for each set of photographs. The overall mean coefficient of variation (CV) was 3.45% and intraclass correlation coefficient (ICC) was 0.96 for all the craniofacial measurements.
DISCUSSION
This study demonstrates that craniofacial differences between Caucasian subjects with and without OSA can be identified with a novel craniofacial photographic analysis technique. These differences include a range of measurements representing the morphological phenotype of the various craniofacial regions. Furthermore, these phenotypic differences were demonstrated independent of obesity. This study also suggests that some of these photographic measurements are related to measures of obesity and OSA severity.
Photographic differences were demonstrated across all the craniofacial regions including the face, mandible, maxilla, eyes, nose, head, and neck. Notably, most of these measurements appear to be capturing a combination of regional soft tissues and skeletal anatomy. For example, the face width, mandible width, intercanthal width, and nose width measurements were all larger in the OSA subjects in the primary analysis, whereas they were no longer significantly different in the BMI and sex matched subgroup analysis. Furthermore, there were moderate to strong linear relationships between some of these measurements and anthropometric measures of obesity. These data strongly support the notion that some craniofacial photographic measurements are capturing facial soft tissues or adiposity, which are in turn closely linked to general and regional obesity.
The BMI and sex-matched subgroup analysis demonstrated a number of craniofacial differences between subjects with and without OSA, independent of the effect of general obesity. Typically, subjects with OSA have a shorter and retruded jaw, smaller enclosed area within the mandible, wider and flatter mid and lower face, and more soft tissues or fat deposition on the anterior neck. Collectively, these findings demonstrate the average craniofacial phenotype of subjects with OSA, without the influence of obesity.
In general, these craniofacial phenotypic findings reflect those identified previously using standard imaging modalities. Retrusion or shortening of the mandible on cephalometry is one of the most consistent skeletal finding in OSA.7,8 A wider mandibular divergence (i.e., wider and flatter lower face) and smaller internal area bounded by the mandible have also been shown using MRI.9 Increased total neck size is well established as an independent risk factor for OSA, even after controlling for obesity.5,22 Specifically, distribution of fat within the neck seems to localize to the anterolateral aspects.23 This corresponds to the reduced area of space anterior to the neck found in the photographic analysis.
Craniofacial surface morphology assessment in OSA has mainly been restricted to using anthropometric techniques. Consequently, the range of craniofacial features examined has been very limited (e.g., retrognathia on clinical examination, cranial dimensions with calipers). While retrognathia may be a consistent finding in OSA subjects using cephalometry, it appears not to be detectable on clinical examination.4,24 This might suggest anthropometry and non-quantitative methods lack sensitivity in detecting craniofacial differences in OSA. Surface measurements of the anterior neck, such as the thyromental angle and cricomental space, have been examined and appear to be different between subjects with and without OSA.25,26 These findings are consistent with the photographic differences (e.g., cervicomental angle, cricomental space area) demonstrated in the primary analysis.
Limited work has been performed examining lateral dimensions of craniofacial structures. Cephalometry studies were generally limited to profile imaging, therefore only allowing assessment of anteroposterior (AP) dimensions and relationships. Similarly, MRI or CT imaging studies did not specifically examine for lateral craniofacial dimensions. Anthropometrics of the craniofacial form in subjects with OSA, however, do suggest that Caucasian OSA subjects have brachycephalic (wider laterally and shorter AP dimension) head form and euryprosopic (wider laterally and shorter vertically) facial form.3 The latter appears to be consistent with the photographic analysis which suggests that OSA subjects have a wider and flatter midface. Together with a wider and flatter face at the level of the mandible, we speculate these phenotypic findings could reflect the combined effects of excess facial fat/soft tissues and maxillary/mandibular deficiency.
Skeletal abnormalities, such as mandibular retrusion and maxillary deficiency, can result in a compromised airway space and an increase in upper airway collapsibility,12,13 thereby predisposing to OSA. While obesity might be strongly linked to OSA, this relationship is mediated through regional body fat distribution. Increased abdominal or visceral fat may cause upper airway compromise by reducing chest wall compliance, reducing lung volumes and hormonal factors.6 Increased neck fat might cause airway collapse by the direct mass loading effect in the supine position.27 However, facial fat deposition and OSA have not previously been examined. On the basis of the photographic phenotype findings, we speculate that facial adiposity may be an important additional factor in OSA pathogenesis. Preliminary data suggest that facial adiposity is strongly linked to central obesity28 and that it is equally metabolically active compared to visceral fat.29 Furthermore, excess facial soft tissues might be related to enlargement of oropharyngeal structures (such as tongue and uvula) that are known to be associated with OSA.4
Relationship between craniofacial measurements and OSA severity were demonstrated with most of the neck measurements, as expected. Face width and mandibular width were also related to OSA severity, even after controlling for BMI, suggesting these photographic craniofacial measurements capture elements (e.g., facial soft tissues, skeletal structures) which are important in OSA. Future studies examining the interrelationships between craniofacial measurements and OSA to identify phenotypic features might be useful in the prediction of OSA.
In addition to allowing detailed quantification of surface craniofacial structures and characterization of the craniofacial phenotype, photogrammetry has a number of clear advantages over conventional imaging methods. These include being safe, inexpensive, portable, quick in image acquisition, and widely available. These advantages make it an ideal technique for a number of clinical and research applications where high throughput is a requirement.
This study has a number of limitations. Subjects in this study were at higher risk for OSA as they were recruited from a sleep laboratory population. Therefore, the control subjects may be morphologically closer to OSA patients than would be expected if control subjects were selected from a nonclinical population. However, this suggests that the differences observed in our study are likely to be conservative estimates of the true differences. Ethnicity is an important consideration in the study of craniofacial anatomy. The majority of the subjects in this study were of Caucasian background. Hence, ethnic differences were not specifically examined. While differences between genders were also not examined, confounding due to gender differences was unlikely as indicated by the results of the subsample analyses of Caucasian men, and the BMI and sex-matched analysis. Although craniofacial structures may change with age, difference in mean age between the groups was small and unlikely have a major influence on the photographic measurements.
Limitations in regard to the craniofacial photographic analysis technique may include possible errors from subject alignment, camera lens distortion, or projection errors. While these issues may affect the accuracy of the technique, they are generally minor; however, precision of the technique remains excellent. While the internal craniofacial anatomy cannot be measured on the photographs, there might be surrogate photographic measurements that reflect the underlying skeletal structures or soft tissues oropharyngeal anatomy. These will need further investigation. Despite the ability to measure 3-dimensional polyhedral volumes, these remain derived measurements from 2-dimensional landmarks. The nonlinear nature of craniofacial anatomy cannot be captured by these measurements. In addition, measurements relate mainly to size rather than shapes. The latter may provide additional insights into the OSA craniofacial phenotype. Whilst we used a highly standardized approach for the acquisition of photographs, there may be scope to simplify the procedure to improve its functionality. Interobserver variability will need to be examined in future studies.
In summary, this study demonstrated a range of phenotypic craniofacial differences in Caucasian subjects with OSA using a photographic analysis technique. Furthermore, phenotypic differences were present between subjects with and without OSA, independent of obesity. Craniofacial photographic measurements appear to capture both soft tissues and skeletal anatomy. This novel technique for detailed craniofacial assessment may have potential as a clinical and research tool in the field of OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Lee is listed as an inventor on a patent application covering aspect of the material in this manuscript, the rights of which are owned by Dr. Lee's institution (Northern Sydney Central Coast Area Health Service, NSW, Australia). Dr. Cistulli is listed as co-inventor on a patent application covering aspect of the material in this manuscript, the rights of which are owned by Dr. Cistulli's institution (Northern Sydney Central Coast Area Health Service, NSW, Australia). Dr. Cistulli contributed to the development of an oral appliance for the treatment of OSA which is being commercialized by SomnoMed Ltd. Dr. Cistulli has consulted for and been on the advisory board of SomnoMed and has a financial interest in the company. Dr. Cistulli has received research support from ResMed and the use of equipment from ResMed and SomnoMed. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Staff of the Sleep Investigation Unit, Department of Respiratory Medicine, Royal North Shore Hospital.
This study was supported by the National Health and Medical Research Council (NHMRC) of Australia
REFERENCES
- 1.Fogel RB, Malhotra A, Dalagiorgou G, et al. Anatomic and physiologic predictors of apnea severity in morbidly obese subjects. Sleep. 2003;26:150–5. doi: 10.1093/sleep/26.2.150. [DOI] [PubMed] [Google Scholar]
- 2.Schwab RJ, Pasirstein M, Pierson R, et al. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–30. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 3.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163:947–50. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 4.Schellenberg JB, Maislin G, Schwab RJ. Physical findings and the risk for obstructive sleep apnea. The importance of oropharyngeal structures. Am J Respir Crit Care Med. 2000;162:740–8. doi: 10.1164/ajrccm.162.2.9908123. [DOI] [PubMed] [Google Scholar]
- 5.Stradling JR, Crosby JH. Predictors and prevalence of obstructive sleep apnoea and snoring in 1001 middle aged men. Thorax. 1991;46:85–90. doi: 10.1136/thx.46.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. doi: 10.1152/japplphysiol.00587.2005. [DOI] [PubMed] [Google Scholar]
- 7.Lowe AA, Fleetham JA, Adachi S, Ryan CF. Cephalometric and computed tomographic predictors of obstructive sleep apnea severity. Am J Orthod Dentofacial Orthop. 1995;107:589–95. doi: 10.1016/s0889-5406(95)70101-x. [DOI] [PubMed] [Google Scholar]
- 8.Miles PG, Vig PS, Weyant RJ, Forrest TD, Rockette HE., Jr Craniofacial structure and obstructive sleep apnea syndrome--a qualitative analysis and meta-analysis of the literature. Am J Orthod Dentofacial Orthop. 1996;109:163–72. doi: 10.1016/s0889-5406(96)70177-4. [DOI] [PubMed] [Google Scholar]
- 9.Okubo M, Suzuki M, Horiuchi A, et al. Morphologic analyses of mandible and upper airway soft tissue by MRI of patients with obstructive sleep apnea hypopnea syndrome. Sleep. 2006;29:909–15. doi: 10.1093/sleep/29.7.909. [DOI] [PubMed] [Google Scholar]
- 10.Guilleminault C, Riley R, Powell N. Obstructive sleep apnea and abnormal cephalometric measurements. Implications for treatment. Chest. 1984;86:793–4. doi: 10.1378/chest.86.5.793. [DOI] [PubMed] [Google Scholar]
- 11.Riha RL, Brander P, Vennelle M, Douglas NJ. A cephalometric comparison of patients with the sleep apnea/hypopnea syndrome and their siblings. Sleep. 2005;28:315–20. [PubMed] [Google Scholar]
- 12.Lyberg T, Krogstad O, Djupesland G. Cephalometric analysis in patients with obstructive sleep apnoea syndrome: II. Soft tissue morphology. J Laryngol Otol. 1989;103:293–7. doi: 10.1017/s0022215100108746. [DOI] [PubMed] [Google Scholar]
- 13.Sforza E, Bacon W, Weiss T, Thibault A, Petiau C, Krieger J. Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161:347–52. doi: 10.1164/ajrccm.161.2.9810091. [DOI] [PubMed] [Google Scholar]
- 14.Dempsey JA, Skatrud JB, Jacques AJ, et al. Anatomic determinants of sleep-disordered breathing across the spectrum of clinical and nonclinical male subjects. Chest. 2002;122:840–51. doi: 10.1378/chest.122.3.840. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson KA, Ono T, Lowe AA, Ryan CF, Fleetham JA. The relationship between obesity and craniofacial structure in obstructive sleep apnea. Chest. 1995;108:375–81. doi: 10.1378/chest.108.2.375. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:260–5. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 17.Kitano I, Park S, Kato K, Nitta N, Takato T, Susami T. Craniofacial morphology of conotruncal anomaly face syndrome. Cleft Palate Craniofac J. 1997;34:425–9. doi: 10.1597/1545-1569_1997_034_0425_cmocaf_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 18.Todd ES, Weinberg SM, Berry-Kravis EM, et al. Facial phenotype in children and young adults with PHOX2B-determined congenital central hypoventilation syndrome: quantitative pattern of dysmorphology. Pediatr Res. 2006;59:39–45. doi: 10.1203/01.pdr.0000191814.73340.1d. [DOI] [PubMed] [Google Scholar]
- 19.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163:1457–61. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 20.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 21.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles: Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 22.Hoffstein V, Mateika S. Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J. 1992;5:377–81. [PubMed] [Google Scholar]
- 23.Mortimore IL, Marshall I, Wraith PK, Sellar RJ, Douglas NJ. Neck and total body fat deposition in nonobese and obese patients with sleep apnea compared with that in control subjects. Am J Respir Crit Care Med. 1998;157:280–3. doi: 10.1164/ajrccm.157.1.9703018. [DOI] [PubMed] [Google Scholar]
- 24.Zonato AI, Bittencourt LR, Martinho FL, Junior JFS, Gregorio LC, Tufik S. Association of systematic head and neck physical examination with severity of obstructive sleep apnea-hypopnea syndrome. Laryngoscope. 2003;113:973–80. doi: 10.1097/00005537-200306000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Lam B, Ip MSM, Tench E, Ryan CF. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax. 2005;60:504–10. doi: 10.1136/thx.2004.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai WH, Remmers JE, Brant R, Flemons WW, Davies J, Macarthur C. A decision rule for diagnostic testing in obstructive sleep apnea. Am J Respir Crit Care Med. 2003;167:1427–32. doi: 10.1164/rccm.200112-110OC. [DOI] [PubMed] [Google Scholar]
- 27.Koenig JS, Thach BT. Effects of mass loading on the upper airway. J Appl Physiol. 1988;64:2294–9. doi: 10.1152/jappl.1988.64.6.2294. [DOI] [PubMed] [Google Scholar]
- 28.Levine JA, Ray A, Jensen MD. Relation between chubby cheeks and visceral fat. N Engl J Med. 1998;339:1946–7. doi: 10.1056/NEJM199812243392619. [DOI] [PubMed] [Google Scholar]
- 29.Sierra-Johnson J, Johnson BD, Bailey KR, Turner ST. Relationships between insulin sensitivity and measures of body fat in asymptomatic men and women. Obes Res. 2004;12:2070–7. doi: 10.1038/oby.2004.258. [DOI] [PubMed] [Google Scholar]