Abstract
Study Objectives:
Regularity of respiration is characteristic of stable sleep without sleep disordered breathing. Appearance of respiratory irregularity may indicate onset of wakefulness. The present study examines whether one can detect transitions from sleep to wakefulness using only the CPAP flow signal and automate this recognition.
Design:
Prospective study with blinded analysis
Setting:
Sleep disorder center, academic institution.
Participants:
74 subjects with obstructive sleep apnea/hypopnea syndrome (OSAHS)
Interventions:
n/a
Measurements and Results:
74 CPAP titration polysomnograms in patients with OSAHS were examined. First we visually identified characteristic patterns of ventilatory irregularity on the airflow signal and tested their relation to conventional detection of EEG defined wake or arousal. To automate recognition of sleep-wake transitions we then developed an artificial neural network (ANN) whose inputs were parameters derived exclusively from the airflow signal. This ANN was trained to identify the visually detected ventilatory irregularities. Finally, we prospectively determined the accuracy of the ANN detection of wake or arousal against EEG sleep/wake transitions. A visually identified irregular respiratory pattern (IrREG) was highly predictive of appearance of EEG wakefulness (Positive Predictive Value [PPV] = 0.89 to 0.98 across subjects). Furthermore, we were able to automate identification of this irregularity with an ANN which was highly predictive for wakefulness by EEG (PPV 0.66 to 0.86).
Conclusions:
Despite not detecting all wakefulness, the high positive predictive value suggests that analysis of the respiration signal alone may be a useful indicator of CNS state with potential utility in the control of CPAP in OSAHS. The present study demonstrates the feasibility of automating the detection of IrREG.
Citation:
Ayappa I; Norman RG; Whiting D; Tsai AHW; Anderson F; Donnely E; Silberstein DJ; Rapoport DM. Irregular respiration as a marker of wakefulness during titration of CPAP. SLEEP 2009;32(1):99-104.
Keywords: Sleep disordered breathing, artificial neural network, autotitration, arousal and wakefulness, obstructive sleep apnea
CONTINUOUS POSITIVE AIRWAY PRESSURE (CPAP) IS THE PRIMARY TREATMENT FOR PATIENTS WITH OBSTRUCTIVE SLEEP APNEA/HYPOPNEA SYNDROME (OSAHS).1,2 Despite the effectiveness of CPAP in abolishing upper airway obstruction, acceptance and compliance with therapy has been suboptimal.3,4 Attempts to improve compliance have addressed nasal symptoms by adding humidification,5,6 optimizing the interface,7,8 and minimizing expiratory pressure9,10 to reduce discomfort and anxiety associated with the treatment. Despite limited evidence, it is usually assumed that the optimal CPAP is the lowest pressure that is consistently therapeutic, and that pressures higher than this are undesirable as they may cause discomfort and decrease compliance.3
While the goal of CPAP is to treat sleep disordered breathing (SDB), positive pressure is not necessary during wakefulness. Conceptually, awareness of pressure occurs only during wakefulness. Thus, without compromising therapy, reducing pressure on arousal/awakening may minimize discomfort resulting from the sensation of pressure and thereby improve compliance. The first step in an algorithm to reduce pressure during wakefulness is detection; for chronic use with CPAP, this would have to be done without electroencephalography (EEG). NREM sleep (in the absence of SDB) has long been recognized as a period of very regular respiration.11 In addition, studies in anxious subjects have shown that irregular breathing may be associated with the anxious state.12 In a classic paper examining breathing in sleep and wakefulness in normal subjects, Bülow noted that there were characteristic changes in pattern and degree of ventilation during transitions between wake and sleep and that “it was always possible to recognize even transient periods of sleep from spirographic record alone.”13 We hypothesize that examination of the ventilatory pattern may provide the information necessary to identify wakefulness as we have also observed that transitions from sleep to wake are associated with changes in pattern (regularity) of respiration.
The purpose of this proof of concept study was twofold: (1) to examine the relationship between the onset of irregularity of breathing and the transition from sleep to either brief or sustained periods of wakefulness in patients undergoing CPAP treatment for SDB (2) to automate the detection of irregularity of breathing. This study was preliminary to the long-term goal of developing a device that provides CPAP only during sleep and withdraws application of pressure during wakefulness using this automated technology.
METHODS
The overall approach was to visually identify a ventilatory pattern of irregularity on the airflow signal and test its relation to EEG detection of wake or arousal using full nocturnal polysomnography (NPSG). In order to automate the recognition of sleep-wake transitions during CPAP from the flow signal, we developed an artificial neural network (ANN) whose inputs were simple parameters derived exclusively from this signal. Our ANN was first trained to identify the visually detected ventilatory irregularities. Subsequently, in a separate data set, we determined the ability of the automated ANN to detect EEG sleep/wake transitions.
All patients had been previously diagnosed with OSAHS (RDI between 13-119/hr) and were undergoing initial CPAP titration during full NPSG. The NPSG included recordings of central and occipital electroencephalogram (EEG), right and left electrooculograms (EOG), and submental electromyogram (EMG) which were used to monitor sleep. An anterior tibialis EMG was used to detect leg movements and a bipolar electrocardiogram (ECG) was used for cardiac monitoring. Oxygen saturation was monitored with a pulse oximeter (Masimo Radical, Irvine, CA). Chest wall and abdominal movement were monitored with piezoelectric strain gauges. Sleep position was monitored using a multiposition mercury switch (Biologic). Airflow and pressure signals were obtained from the CPAP generator and sampled at a frequency of 50 Hz. All signals were recorded on a digital polygraph (Sandman 7, Tyco), with analyses done offline using custom software. Sleep scoring was performed manually using the criteria of Rechtschaffen and Kales (R &K) using 30-second epochs.14 Arousal scoring was performed using American Academy of Sleep Medicine (AASM) criteria.15 Sleep disordered breathing events ( > 10 s) were scored using standard criteria: apneas were defined as airflow < 10% of baseline, hypopneas as airflow < 50% of baseline or airflow < 70% of baseline with a 4% oxygen desaturation, and respiratory effort related arousals (RERAs) as airflow < 70% of baseline followed by an arousal.
This protocol was granted exemption from full review and waiver of informed consent by the Institutional Review Board at the NYU School of Medicine, as data used were from previously performed and de-identified clinical CPAP studies. In addition, this study was reviewed and approved by the NYU School of Medicine Conflict of Interest committee.
1. Relationship Between Visual Onset of Irregularity of Breathing and EEG
NPSG data from 17 male and 3 female subjects with OSAHS during CPAP titration were first scored for sleep, microarousals, and sleep disordered breathing events. In a separate pass, scorers examined the flow signal alone while hiding the EEG signals and sleep stage information. Multiple scorers visually identified discrete periods of irregular/chaotic breathing (IrREG), defined as a sequence of ≥ 3 breaths with irregular (varying) amplitude and frequency. IrREG events were not marked during sleep disordered breathing events and the subsequent 3 breaths. As all studies were performed at or near therapeutic CPAP, only rare periods of SDB needed to be excluded. Each study was scored separately by at least 2 scorers, and differences were reconciled. Examples of these visually distinct patterns seen on the airflow signal are shown in Figures 1a–1c. In order to assess the interscorer agreement in identifying IrREG events, 9 studies were scored separately by 2 scorers blinded to each other's scores after which the proportion of specific agreement (PSA)16 was calculated. Agreement was defined as the simultaneous occurrence of an IrREG event scored by both scorers. Disagreement occurred whenever an IrREG event was detected by only 1 of the 2 scorers. The PSA describes the agreement between scorers; it is defined as the number of events with agreement between scorers divided by the average number of events scored by the 2 scorers.
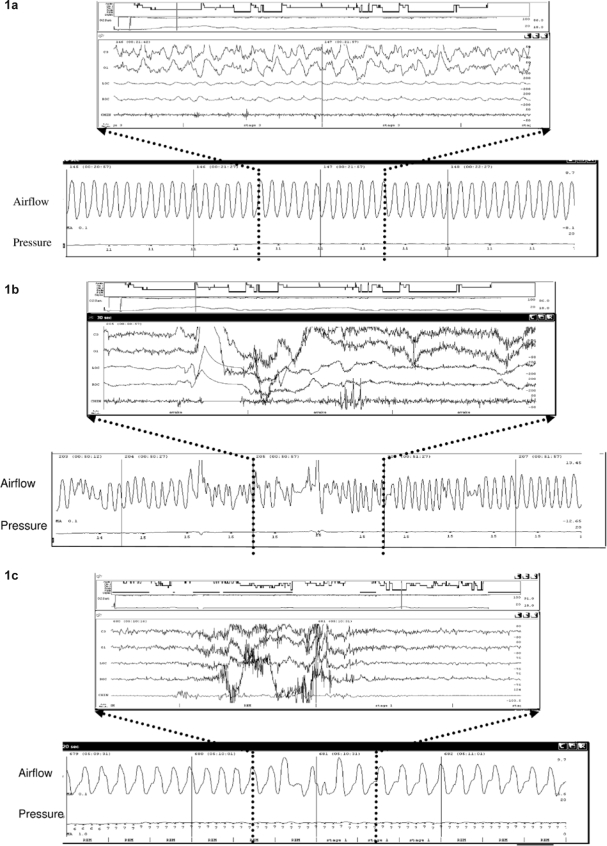
Figure 1.
Figure 1a shows the pattern of regular breathing usually seen during NREM sleep. The lower panel shows a 2-min window of the airflow signal from the CPAP machine. The upper panel shows the EEG corresponding the 30 sec highlighted by the dotted lines. In contrast, Figure 1b shows the irregularity in the airflow signal (both amplitude and frequency) seen during a transition from sleep to wake. Figure 1c shows a similar irregular pattern seen during a brief arousal.
The relationship between visually scored IrREG events and EEG was tabulated to determine both the prevalence of IrREG during sustained EEG defined wakefulness (runs) as well as examine the use of IrREG as surrogate for wakefulness using positive predictive values:
All runs of sustained wake (defined as ≥ 4 consecutive epochs scored as wake) were identified and tabulated for the presence or absence of IrREG within the first 2 minutes of the run. The percentage of runs of wake ( ≥ 4 consecutive epochs) associated with the presence of IrREG was calculated in each subject. In addition, to examine how often wakefulness was associated with irregular respiration, each epoch of wake was tabulated for the presence or absence of IrREG. If any part of the manually scored IrREG event was within the 30-sec wake epoch, it was tabulated as an agreement. The percentage of all wake epochs associated with IrREG was calculated in each subject to obtain the prevalence of IrREG.
Separately, visually scored IrREG events from the manual scoring were tabulated against both sleep stage (NREM, REM, and wake) and brief EEG arousal. An agreement for wake (true positive event) occurred if a majority of the breaths in the identified IrREG event occurred during an epoch scored as wake by EEG. An agreement for arousal occurred if the IrREG event occurred simultaneously with or was followed by an EEG arousal within 3 sec. A disagreement occurred (false positive) if the IrREG event occurred during an epoch of sleep (NREM or REM). Positive predictive values (PPV) were calculated in each subject for IrREG as a predictor of transition from sleep to wake, as well as for the combination of this and the presence of a brief arousal.
2A. Development of an Artificial Neural Network (ANN) for Automated Detection of Irregular Respiration
Data from 50 studies (20 used in the manual evaluation + 30 additional studies) previously diagnosed with OSAHS and undergoing in-lab PSG with CPAP titration were analyzed. Using the flow signal alone, these studies were manually scored and reconciled for IrREG as described in the previous section by at least 2 scorers. This data set formed the training set for the artificial neural network (ANN). An automated algorithm first identified individual breaths (∼300,000 breaths). All areas were analyzed by the ANN, whether or not sleep disordered breathing (apnea and hypopnea) was present. The temporal pattern of regularity of a sequence of 5 breaths was described by calculating 8 parameters measuring average breath timing and amplitude within the sequence and 17 parameters measuring variability of breath timing and amplitude between the 5 breaths. These 25 parameters formed the inputs into the ANN, which had 100 hidden nodes and one output (presence or absence of IrREG).The value (IrREG present/absent) was assigned to the last breath in the sequence and represents the presence of irregularity during the entire 5 preceding breaths. The next breath was evaluated by moving the entire 5-breath sequence by one breath.
Tabulation of data: For each breath the ANN classification (IrREG + or −) was compared to a classification derived from the manual scoring. The derivation of a breath classification based on manual scoring was obtained from examining the current breath and its 4 preceding breaths (the same window input into the ANN). If ≥ 3 of these 5 breaths fell within a manually marked IrREG event, then a manual value of IrREG+ was assigned to the breath. Otherwise it was classified as IrREG−.
2B. Validation of ANN
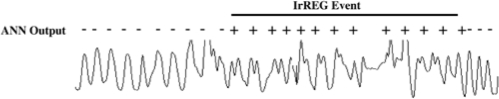
The validation of the ANN was performed using data from a separate set of PSGs in 24 subjects (20 M/4 F) undergoing CPAP titration. IrREG events were identified from the output of the ANN on ∼150,000 breaths on the flow signal. Each event consisted of one or more consecutive breaths which had been labeled IrREG by the ANN (each breath representing the irregularity of the 5 previous breaths). An example of the derivation of IrREG events is shown in Figure 2.
Figure 2.
Figure 2 shows the derivation of an IrREG event from individual breaths classified as IrREG+ or IrREG− by the ANN.
Tabulation of data: IrREG events identified from the output of the ANN were tabulated against both sleep stage and brief arousal as was done for the visual analysis in section 1. Positive predictive values (PPV) were calculated in each subject for IrREG as a predictor of transition from sleep to wake or the presence of a brief arousal. To evaluate the sensitivity of the ANN detected IrREG, each run of visually scored wake (defined as ≥ 4 consecutive epochs of wakefulness on EEG) was tabulated for the presence or absence of IrREG as described in Section 1.
RESULTS
1. Visual Identification of IrREG
In the 20 subjects a total of 315 (4–45 runs/subject) runs of wake lasting ≥ 2 min (4 consecutive epochs) were found. Of these, 164/315 (52%) were associated with visually detected IrREG within the first 2 min of the run. A total of 3116 wake epochs (including the runs) were scored (22–346 epochs/subject). IrREG occurred in 24% (7%% to 55%/subject) of these epochs with the lowest percentages occurring in the subjects with lowest sleep efficiency (r = 0.42, P = 0.06). This indicates that irregular respiration as defined in our analysis (IrREG) occurs in only a modest subset of wake epochs.
In these subjects, whose IrREG event scoring was the result of reconciliation of all scorers, 360 IrREG events were identified visually from respiratory pattern alone (3–48 events per subject). The distribution of the R&K scored sleep stage associated with events was: 321 (89%) of IrREG events occurred during wake; 32 (9%) during AASM arousal; 7 (2%) during NREM sleep, and 0 (0%) during REM sleep (Table 2). The mean positive predictive value of IrREG to identify wake on EEG was 0.89 (ranging from 0.33 to 1.0 across subjects). To identify either wake or brief arousal the PPV was 0.98 (ranging from 0.67 to 1.0 across subjects) (Table 3). Of note, visual detection of IrREG did not produce any false positive detections of REM.
Table 2.
Distribution of IrREG Events Identified Visually and by the ANN
| Visual (n = 360) | ANN (n = 524) | |
|---|---|---|
| IrREG events associated with | ||
| Wake | 321 (89%) | 346 (66%) |
| Arousal | 32 (9%) | 104 (20%) |
| NREM sleep | 7 (2%) | 39 (7%) |
| REM sleep | 0 (0%) | 35 (7%) |
Note: ANN = artificial neural network
Table 3.
PPV of IrREG for Transition from Sleep to Wake
| Visual |
ANN |
|||
|---|---|---|---|---|
| mean | range | mean | range | |
| For wake | 0.89 | 0.33–1.0 | 0.66 | 0.25–1.0 |
| For wake+arousal | 0.98 | 0.67–1.0 | 0.86 | 0.42–1.0 |
Note: ANN = artificial neural network
Reliability of scoring of IrREG events: A total of 149 IrREG events were scored by one or more raters. The PSA for visual identification of IrREG by the 2 scorers in 9 subjects was 76% (ranging from 42% to 91%). Of the 92 events scored by both scorers (agreement), 95% were associated with wake and 100% with either wake or arousal.
2A. ANN Training (data from 50 subjects)
The tabulation shown in Table 1 summarizes the results of comparing the ANN assignment and the classification derived from the manual scoring over all breaths in all subjects. The overall sensitivity of IrREG breath detection by ANN, compared to manually marked IrREG, was 5025/8337 = 60% and the specificity of IrREG breath detection by ANN to manually marked IrREG was 312,462/313,721 = 99.5%
Table 1.
Cross Tabulation of Breaths Based on their Manual and ANN Label as IrREG. (ANN Training Set)
| # of sequences | Identified by ANN |
||
|---|---|---|---|
| IrREG + | IrREG − (regular breathing) | Total | |
| Identified Visually | |||
| IrREG + | 5025 | 3312 | 8337 |
| IrREG − (regular breathing) | 1259 | 312462 | 313721 |
Note: ANN = artificial neural network
2B. ANN Validation (data from 24 subjects)
In the validation set, 524 IrREG events were identified from respiratory pattern alone by the ANN (mean: 22, range 5–56 events per subject). Of these, 346 (66%) were associated with EEG defined wake, 104 (20%) were associated with AASM arousal, 39 (7%) were associated with NREM sleep and 35 (7%) were associated with REM sleep (Table 2). The mean positive predictive value of IrREG for transition from sleep to wake was 0.66 for all subjects (ranging from 0.25 to 1.0 across all subjects) and 0.86 for transition from sleep to wake or arousal (ranging from 0.42 to 1.0 across all subjects) (Table 3). Seventy percent of the 158 runs of 4 or more consecutive EEG epochs of wake were associated with IrREG within the first 2 minutes.
DISCUSSION
Our data show that the presence of the irregular respiratory pattern we identified (IrREG) was highly predictive of the appearance of wakefulness defined by EEG and very rarely occurred during sleep (including REM). Furthermore, we were able to automate identification of this pattern of irregularity with an artificial neural network (ANN).
Despite the above, our data show that only a subset of individual epochs of EEG wakefulness is associated with ventilatory irregularity. However 52% of sustained periods of wakefulness (runs) were associated with an early period of ventilatory irregularity detected visually, and 70% of the runs of wakefulness were detected as IrREG by the ANN. We further suspect that these periods of sustained wakefulness associated with ventilatory irregularity on CPAP may be those associated with the greatest degree of arousal and possibly with the greatest discomfort. We postulate that identifying these periods and rapidly reducing CPAP when they occur might improve CPAP compliance. We note that this approach proposed is similar to that intuitively followed by many experienced sleep technologists, who, even when patients do not specifically complain about pressure, frequently lower CPAP during titration in the laboratory when patients are unable to fall asleep.
Prior studies have examined the variability of breathing during both wake and sleep. In general, these studies have analyzed the pattern of breathing only within each state.17 BuSha et al showed reduced respiratory variability in rats during sleep compared with wakefulness.18 Irregularity of the respiratory pattern during wakefulness has been shown to increase further during anxious states such as panic attacks,12,19 and some patients have a reduction in respiratory variability after treatment for panic attacks.20 Although aspects of the respiratory pattern (e.g., hyperventilation) may contribute to or even cause a panic attack, it has also been suggested that the irregularity of breathing, including the appearance of frequent sighs, may be a marker of anxiety and/or panic attack.21 There has been little study of the respiratory pattern at the transition between sleep and wake, or the sudden appearance of irregularity as a maker of transition from sleep to wake. Our data suggest that there may be frequent appearance of respiratory irregularity as a predictable correlate of arousal. Furthermore we speculate that even more dramatic deviation from the ventilatory regularity seen during sleep may occur in the presence of a potential irritant such as CPAP due to an associated period of anxiety and/or discomfort.
The visual recognition of IrREG was successfully automated using an artificial neural network with inputs solely from the respiratory signal. It is possible that additional sophistication in the analysis of the variability parameters might further improve detection of all wakefulness. Although a perfect arousal/wake detector using the flow signal alone might be desirable for other applications, this was not our primary goal for this particular application. If this algorithm was used in a CPAP device in order to lower pressure it would not be desirable to lower CPAP in response to a very brief arousal, as by definition the patient has rapidly returned to sleep and reduction in pressure in this setting would result in sleep disordered breathing. The high PPV and bias towards longer periods of wakefulness of the ANN as currently implemented suggests there will be a low rate of inappropriate reduction in CPAP. Lack of response of the ANN to all periods of EEG wake does not exclude the potential for enhanced patient comfort in those awakenings that are detected.
To automate the visual observation so that the human could identify a pattern of breathing that correlated with EEG wakefulness, we examined multiple statistical techniques (principal components analysis, discriminant analysis, tree classifiers). However, no simple combination of amplitude and frequency that was specific to this pattern of irregularity could be identified. Therefore, we used an ANN, as these algorithms are most often used to automate visual pattern recognition.
During manual scoring, we did not mark IrREG events during periods of clear sleep disordered breathing. However, we did not exclude these periods when the ANN was trained, thus presenting the full spectrum of respiratory patterns to the ANN. While this may seem counterintuitive, our goal was to develop an ANN which contrasted the irregular pattern seen at arousal with all other patterns of breathing including regularity seen during sleep and the pattern of SDB. In addition, robust automated methods, as used in many autotitrating CPAP units, exist to detect SDB itself.
REM sleep has been described as being associated with irregularity of breathing. However to the human eye the “pattern” of this irregularity is different from that at sleep/wake transitions. Our data showed that the human never misidentified REM as Wake (Table 2). The nature of this pattern difference appears to be a complex interaction of amplitude and frequency. The ANN was trained to mimic this human performance and ignore the occurrence of the irregularity of breathing associated with REM, thus minimizing the misidentification of REM as wake.
The present study suggests a new method of adjusting CPAP using a combination of detection of arousal and of SDB to control auto titration providing both adequate treatment during sleep and optimal comfort on arousal. Comfort and compliance may be improved if identification of ventilatory irregularity indicating wakefulness allows reduction of CPAP when it is not needed. This differs from current autoCPAP algorithms, which generally focus on raising pressure in response to SDB, and only incidentally lower pressure when no SDB is detected. This may take as much as 20 minutes after an arousal. In contrast, our approach is to identify arousal where CPAP is conceptually not needed. This would allow very rapid reduction of CPAP ( < 30 sec). Our bias is that subjects do not have pressure-related difficulties tolerating CPAP while asleep, but rather experience discomfort only while awake. Thus, if one can automatically detect the appearance of wake (or anxiety), it becomes possible to lower CPAP pressure rapidly when therapy is not needed. The present study provides an incentive to develop an automated CPAP device containing this algorithm so that the impact on comfort and/or CPAP compliance can be tested directly.
DISCLOSURE STATEMENT
This study was funded by Fisher and Paykel Healthcare. Dr. Ayappa has received research support from Fisher and Paykel Healthcare, Advanced Brain Monitoring, and Korosensor. Dr. Ayappa holds US patents and intellectual property rights covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Fisher and Paykel Healthcare and Advanced Brain Monitoring. Dr. Norman has received research support from Fisher and Paykel Healthcare, Advanced Brain Monitoring, and Korosensor. Dr. Norman holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Biologics, Fisher and Paykel Healthcare, Advanced Brain Monitoring, and Tyco. Dr. Rapoport has received research support from Fisher and Paykel Healthcare, Genzyme, Guidant, Korosensor, St. Jude Medical, and Advanced Brain Monitoring; has participated in speaking engagements for Genzyme, Guidant, Respironics, ResMed, and St. Jude Medical; has consulted for Invacare, Sanofi-Aventis, Boehringer Ingelheim, and Restore Medical; and holds multiple US and foreign patents covering techniques and analysis algorithms for the diagnosis of OSAHS and techniques for administering CPAP. Several of these have been licensed to Biologics, Fisher and Paykel Healthcare, Advanced Brain Monitoring, and Tyco. Mr. Whiting and Ms. Anderson are employees of Fisher and Paykel Healthcare. Ms. Donnely was employed by Fisher and Paykel Healthcare at the time of conducting the research for this study. Dr. Silberstein and Mr. Tsai have indicated no other financial conflicts of interest.
ACKNOWLEDGMENTS
Supported by grants from Fisher – Paykel Healthcare, Auckland, NZ, Foundation for Research in Sleep Disorders.
REFERENCES
- 1.Sullivan CE, Issa FG, Berthon-Jones M, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;1:862–5. doi: 10.1016/s0140-6736(81)92140-1. [DOI] [PubMed] [Google Scholar]
- 2.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. doi: 10.1093/sleep/29.3.381. [DOI] [PubMed] [Google Scholar]
- 3.Kribbs NB, Pack AI, Kline LR, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 1993;147:887–95. doi: 10.1164/ajrccm/147.4.887. [DOI] [PubMed] [Google Scholar]
- 4.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20:278–83. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 5.Massie CA, Hart RW, Peralez K, Richards GN. Effects of humidification on nasal symptoms and compliance in sleep apnea patients using continuous positive airway pressure. Chest. 1999;116:403–8. doi: 10.1378/chest.116.2.403. [DOI] [PubMed] [Google Scholar]
- 6.Neill AM, Wai HS, Bannan SP, Beasley CR, Weatherall M, Campbell AJ. Humidified nasal continuous positive airway pressure in obstructive sleep apnoea. Eur Respir J. 2003;22:258–62. doi: 10.1183/09031936.03.00035603. [DOI] [PubMed] [Google Scholar]
- 7.Massie CA, Hart RW. Clinical outcomes related to interface type in patients with obstructive sleep apnea/hypopnea syndrome who are using continuous positive airway pressure. Chest. 2003;123:1112–8. doi: 10.1378/chest.123.4.1112. [DOI] [PubMed] [Google Scholar]
- 8.Mortimore IL, Whittle AT, Douglas NJ. Comparison of nose and face mask CPAP therapy for sleep apnoea. Thorax. 1998;53:290–2. doi: 10.1136/thx.53.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nilius G, Happel A, Domanski U, Ruhle KH. Pressure-relief continuous positive airway pressure vs constant continuous positive airway pressure: a comparison of efficacy and compliance. Chest. 2006;130:1018–24. doi: 10.1378/chest.130.4.1018. [DOI] [PubMed] [Google Scholar]
- 10.Mulgrew AT, Cheema R, Fleetham J, Ryan CF, Ayas NT. Efficacy and patient satisfaction with autoadjusting CPAP with variable expiratory pressure vs standard CPAP: a two-night randomized crossover trial. Sleep Breath. 2007;11:31–7. doi: 10.1007/s11325-006-0078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reed CJ, Kleitman N. Effect of sleep on respiration. Am J Physiol. 1926;75:600–8. [Google Scholar]
- 12.Martinez JM, Kent JM, Coplan JD, et al. Respiratory variability in panic disorder. Depress Anxiety. 2001;14:232–7. doi: 10.1002/da.1072. [DOI] [PubMed] [Google Scholar]
- 13.Bülow K. Respiration and wakefulness in man. Acta Physiol Scand Suppl. 1963;209:1–110. [PubMed] [Google Scholar]
- 14.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques, and scoring system for sleep states of human subjects. Washington DC: US Government Printing Office; 1968. NIH Publication No. 204. [Google Scholar]
- 15.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 16.Fleiss JL. Statistical methods for rates and proportions. New York: Wiley and Sons; 1981. [Google Scholar]
- 17.Bruce EN. Chemoreflex and vagal afferent mechanisms enhance breath to breath variability of breathing. Respir Physiol. 1997;110:237–44. doi: 10.1016/s0034-5687(97)00088-1. [DOI] [PubMed] [Google Scholar]
- 18.BuSha BF, Stella MH. State and chemical drive modulate respiratory variability. J Appl Physiol. 2002;93:685–96. doi: 10.1152/japplphysiol.00951.2001. [DOI] [PubMed] [Google Scholar]
- 19.Papp LA, Martinez JM, Klein DF, et al. Respiratory psychophysiology of panic disorder: three respiratory challenges in 98 subjects. Am J Psychiatry. 1997;154:1557–65. doi: 10.1176/ajp.154.11.1557. [DOI] [PubMed] [Google Scholar]
- 20.Yeragani VK, Rao R, Tancer M, Uhde T. Paroxetine decreases respiratory irregularity of linear and nonlinear measures of respiration in patients with panic disorder. Neuropsychobiology. 2004;49:53–7. doi: 10.1159/000076410. A preliminary report. [DOI] [PubMed] [Google Scholar]
- 21.Abelson JL, Weg JG, Nesse RM, Curtis GC. Persistent respiratory irregularity in patients with panic disorder. Biol Psychiatry. 2001;49:588–95. doi: 10.1016/s0006-3223(00)01078-7. [DOI] [PubMed] [Google Scholar]