Abstract
Humoral and cellular immunity, associated with long-term protective immunological memory, defines the efficacy of a given vaccine formulation. However, few vaccines achieve this target without the aid of a suitable adjuvant. Molecular adjuvants in vaccination against infectious agents offer a noninvasive means of enhancing the immune response against target antigens. To examine the potency of two β-chemokines as immunomodulators, plasmid DNA encoding β-chemokines CCL19 and CCL21 (CCR7L) was codelivered intranasally with plasmid DNA or recombinant vaccinia virus encoding herpes simplex virus (HSV) gB (HSV-gB) in a prime-and-boost vaccination strategy. This vaccination regimen increased serum and vaginal immunoglobulin G (IgG) and IgA, respectively, as well as the numbers of HSV-gB498-505 peptide-specific gamma interferon-producing CD8+ T cells. Distinctively, a high number of cytotoxic T lymphocytes was achieved when pCCR7L was applied at both prime and boost as opposed to omission of pCCR7L. A rapid-recall response was induced in the genital tract upon challenge with the HSV McKrae strain, affording a high level of protection and survival of vaccinated mice. Our results demonstrate that high innate immune kinetics and distribution of adaptive response induced in the nasal mucosa appears to be key factors in generating protective memory responses against HSV. Thus CCR7L expressed ectopically may serve as a molecular adjuvant to boost the immune response to a codelivered antigen in mucosal surfaces.
Herpes simplex virus (HSV) is transmitted via mucosal surfaces, such as the genital and the oral mucosae (6, 19, 20). Accordingly, enhancement of immunity at such sites would protect against HSV infection. Therefore, development of a vaccine that would not just protect against but would prevent infection at mucosal surfaces remains a major goal in HSV vaccinology. The respiratory tract mucosa offers an attractive site for induction of mucosal immunity owing to the common-mucosa system concept (16, 41). However, a major problem with effecting protective mechanisms at mucosal surfaces is lack of appropriate immunomodulating systems to enhance a mucosal immune response. Encouraging results in the search for a suitable adjuvant have been reported recently (35): 3-O-deacylated monophosphoryl lipid A was used successfully with recombinant HSV glycoprotein D to induce immunity against HSV type 2 (HSV-2) when applied in aluminum hydroxide. However, the vaccine is effective only in women who are seronegative to both HSV-1 and -2. It is not known yet if 3-O-deacylated monophosphoryl lipid A can be applied to the respiratory mucosa to achieve a similar level of protection. Other immunomodulators with the potential to induce sufficient immune response include CpG containing unmethylated dideoxynucleotides. When coadministered with HSV antigen, CpG enhanced both systemic and mucosal immune responses, which provided protection upon challenge with HSV during the primary immune response (13, 14). However, the memory arising therefrom is not durable. Several studies have shown improvement in immune responses when cytokines are included as molecular adjuvants (34, 36). A more recent study demonstrated the efficacy of interleukin-15 (IL-15) in inducing a long-term memory when included in the vaccination regimen (33). Chemokines are proinflammatory molecules that play a major role in leukocyte migration, which allows the immune reaction to be focused on invading foreign antigens (reviewed in references 24 and 32). In particular, CCR7 ligands (CCR7L), i.e., CCL19 (Epstein-Barr virus-induced molecule 1 ligand chemokine) and CCL21 (secondary lymphoid tissue chemokine), participate in the interaction of dendritic cells (DCs) and T cells in secondary lymphoid tissue, which eventually gives rise to antigen-specific T cells capable of counteracting an infection (3, 10). CCR7L have also been reported to repair functional defects of CD8+ T cells in lymphotoxin-α-deficient mice (8) and to immunopotentiate DNA vaccination (9).
It is now becoming clear that the outcome of an immune response to a foreign antigen relies on the early innate environment established during the induction of that response. Consequently, this may require stimulation that can specifically alter the biology of antigen-presenting cells, leading to higher expression of costimulatory molecules, cytokines, and other auxiliary molecules, which in turn lower the threshold for stimulation of T-cell responses (4). In this study we hypothesized that if the kinetics of immune induction are combined with distribution of the response arising from a mucosal heterologous prime-and-boost (prime/boost) strategy of immunization, heightened immunity against HSV would result. In such a scenario plasmid DNA-encoded CCR7L would favorably influence the kinetics of T-cell priming by promoting immunocompetent cell migration to the site of immunization. Conversely, the intranasal route of immune induction would advantageously exploit the common-mucosa concept to distribute the response distally. To prove this hypothesis, first we explored the intranasal route of immunization and incorporation of plasmid DNA-encoded CCR7L at both prime and boost stages of immunization. Second, we examined the extent and duration of the immunity generated when this prime/boost strategy is used for vaccination against HSV. Indeed, using the mucosal route of immunization we have previously shown (7) that a robust mucosal and systemic immune response is achieved when mice are primed with recombinant vaccinia virus encoding glycoprotein B (rVVgB) of HSV and boosted with plasmid DNA encoding the same protein. Our results show that plasmid DNA-encoded chemokines may modulate the influx of immunocompetent cells to the site of immunization and therefore lead to enhanced induction of the immune response against HSV. When applied in a mucosal heterologous prime/boost strategy of immunization against HSV, pCCR7L was capable of directing a highly protective immune response at a distal mucosal site. Moreover, the protective efficacy of this vaccination approach appears to be long term.
MATERIALS AND METHODS
Mice and viruses.
Female C57BL/6 mice purchased from Harlan Sprague-Dawley (Indianapolis, Ind.) were used at age 5 to 6 weeks in all experiments described in this report. Animals were housed at the Laboratory Animal Facility of the University of Tennessee. All experimental procedures on mice adhered to the institutional and national guidelines on experimental animal care. HSV-1 KOS strain, rVVgB, and HSV McKrae strain (American Type Culture Collection, Manassas, Va.) were grown and titrated as PFU in Vero cells. Viruses were aliquoted and stored at −80°C until use.
Plasmid DNA.
CCL19- and CCL21-encoding cloning plasmids were a kind gift from A. Zlotnik (DNAX Research Institute, Palo Alto, Calif.) and J. G. Cyster (University of California, San Francisco). They were inserted into expression vector pCI-neo as described elsewhere (8). Plasmid DNA encoding gB of HSV-1 KOS has been described in detail elsewhere (25). Plasmid DNA was prepared as described by Kuklin et al. (21) with a slight modification. Briefly, following precipitation with polyethylene glycol the plasmid DNA was further subjected to endotoxin removal as follows. A 3 M solution of sodium acetate was added to plasmid DNA at a ratio of 1:10 and brought to a total volume of 1.0 ml with endotoxin-free water. The plasmid DNA was then incubated on ice for 5 min. Triton X-114 (0.03 volumes; Sigma, St. Louis, Mo.) was added to the samples, which were thoroughly mixed and then incubated at 50°C for 5 min. The aqueous phase containing DNA was removed following centrifugation at 14,000 rpm in an Eppendorf 5415c centrifuge. DNA samples were subjected to another round of Triton X-114 and finally precipitated with 96% ethanol. The result of the Limulus amebocyte lysate test (Charles River Endosafe, Charleston, S.C.) was 0.11 endotoxin unit/ml.
Peptide synthesis.
HSV-gB498-505 (SSIEFARL), specific for major histocompatibility complex class I (MHC-I) (H-2b)-restricted CD8+ T cells was synthesized by Genemed Synthesis Inc. (South San Francisco, Calif.). The peptide was dissolved in phosphate-buffered saline (PBS), aliquoted, and stored at −20°C until use. Unless otherwise stated the peptide was used at a concentration of 5 μg/2 × 106 cells.
Immunization.
Female C57BL/6 mice were divided into six groups. Group 1 was immunized intranasally at day 0 (prime) with 107 PFU of rVVgB/mouse and 100 μg of plasmid DNA encoding a CCR7L (pCCL19 or pCCL21), and then at day 14 (boost) mice were boosted with 100 μg of pCCR7L and 100 μg of plasmid DNA encoding glycoprotein B of HSV (pgB). Groups 2 to 6 were immunized intranasally with rVVgB (prime) and pgB (boost), β-galactosidase (β-Gal; both prime and boost); UV-inactivated HSV-1 KOS (107 PFU before inactivation) (prime only), and PBS (prime and boost), respectively. Primary responses were measured at day 14 postboost, and memory responses were measured at day 60 postboost. Serum samples and genital tract wash fluids were also collected at days 14 and 60 postboost for antibody analysis.
Preparation of spleen, LN, lung dendritic, and genital tract cell suspensions.
At various times lungs, spleens, lymph nodes (LN; appropriately peribronchial, cervical, mesenteric, and iliac), and genital tracts were removed. Spleens and LN were minced and passed through a metal sieve, and finally red blood cells (RBC) were lysed with RBC lysis buffer (Sigma) and washed three times in RPMI 1640 supplemented with 10% fetal calf serum. Genital tracts were surgically removed, minced, and digested with 1 mg of collagenase D (Roche, Penzberg, Germany)/ml for 45 min at 37°C with agitation. Finally the cells were washed and suspended in RPMI 1640. Lung DCs were prepared by digesting lungs with 0.5 mg of collagenase D/ml. CD11c+ DCs were positively selected with anti-CD11c+ magnetic microbeads by magnetic activated-cell sorting (MACS) (Miltenyi Biotec, Auburn, Calif.).
IFN-γ enzyme-linked immunospot (ELISPOT) assay.
MultiScreen HA sterile plates (Millipore, Bedford, Mass.) were coated overnight with a capture anti-gamma interferon (IFN-γ) antibody (BD Biosciences Pharmingen, San Diego, Calif.) in carbonate buffer, pH 9.6. Before plating the cells, the plates were washed with PBS three times and blocked with RPMI 1640 (Sigma) supplemented with 10% fetal calf serum. Responder cells (106) from spleens, LN, or lungs of immunized and control mice were added to each first well and further serially diluted twofold. A constant number (2.5 × 105 cells) of stimulator cells was added to each well, followed by 20 U of IL-2 (Hemagen, Columbia, Md.). Stimulator cells were prepared from naive mouse spleens and pulsed with HSV-gB498-505 for 1.5 h at 37°C and finally X-irradiated (3,000 rads). Plates were placed at 37°C in an incubator for 48 to 72 h. Subsequently, plates were washed with PBS-Tween, followed by addition of biotinylated IFN-γ (BD Biosciences Pharmingen) and incubation at 4°C overnight. Afterwards plates were washed in PBS-Tween, followed by addition of peroxidase-conjugated streptavidin (Jackson Immunoresearch, San Francisco, Calif.) and incubation at 37°C for 1.5 h. Finally plates were washed and incubated with 9-amino-9-ethylcarbazole (Sigma) for 10 to 20 min or until color developed. Spots were enumerated under dissecting microscope.
Intracellular cytokine staining.
Spleen or LN cells from vaccinated and control mice were added to a 96-well plate at a concentration of 106 cells per well and stimulated with 2.5 μg of HSVgB498-505 in the presence of GolgiPlug (BD Biosciences Pharmingen) and 50 U of IL-2 (Hemagen) for 5 h at 37°C. Further, the cell samples were processed basically as described by Kumaraguru and Rouse (22). The anti-CD8 fluorescein isothiocyanate (FITC) antibody was purchased from Caltag Laboratories, Burlingame, Calif. All other antibodies were purchased from BD Biosciences Pharmingen.
Antibody ELISA.
A standard enzyme-linked immunosorbent assay (ELISA) was done to quantitate the gB-specific antibody in the serum and genital wash fluids as described previously (7). Briefly, ELISA plates were coated overnight at 4°C with gB protein (Chiron, Emeryville, Calif.). For standards goat anti-mouse IgG or rabbit anti-mouse IgA was used for coating plates. Subsequently, the plates were washed with PBS containing Tween 20 three times and blocked with 3% skim milk. Mouse IgG was added to standards, and test samples were serially diluted twofold, incubated for 2 h at 37°C, and then incubated with goat anti-mouse IgG-conjugated horseradish peroxidase (HRP) (IgG-HRP) for 1 h. All antibodies were purchased from Southern Biotechnology Associates, Birmingham, Ala. For measurement of IgA levels in genital tract lavage fluid, biotinylated goat anti-mouse IgA was first added for 2 h at 37°C and then peroxidase-conjugated streptavidin (Jackson ImmunoResearch, West Grove, Pa.) was added. Finally, ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] was added, and plates were incubated at room temperature for 10 min. After color development antibody concentrations were calculated with an automated ELISA reader (Spectra MAX340; Molecular Devices, Sunnyvale, Calif.).
Cytotoxic T-lymphocyte (CTL) assay.
A standard 4-h 51Cr release assay was performed to assess cytolytic activity of the CD8+ T cells isolated from immunized and control mice as described elsewhere (8). Briefly, splenocytes or LN cells from immune or control mice were expanded in vitro for 5 days by restimulation with irradiated syngeneic HSV-gB498-505-pulsed (5 μg/2 × 106 cells) splenocytes. After expansion effector cells were then incubated with MHC-matched target cells (MC38; mouse colon adenocarcinoma) pulsed with HSV-gB498-505 at various effector-to-target cell ratios for 4 h. Total release was determined by adding 5% Triton X-100 (Sigma) to target cells. 51Cr release was assessed in a gamma counter (LKB-Wallac, Turku, Finland), and data were corrected by the formula [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100.
Virus challenge.
Mice are irregularly susceptible to genital infection with HSV unless synchronized into diestrus (11, 18). To synchronize the ovarian cycle, mice were injected subcutaneously with 2 mg of medroxyprogesterone (Pharmacia & Upjohn, Kalamazoo, Mich.)/mouse. Five days later each mouse was anesthetized with avertin and infected intravaginally with 107 PFU of HSV MacKrae. Every day a specimen of the genital tract lavage was collected for virus titration and antibody assay. Mice were monitored daily for clinical illness and pathology, scored according to criteria reported by Gallichan et al. (13) as follows: 0, no apparent infection; 1, mild inflammation of the external genitals, redness, and moderate swelling of external genitals; 3, severe redness and inflammation; 4, genital ulceration and severe inflammation; 5, hind limb paralysis and death.
Statistics.
Appropriate significant differences were calculated with Student's t test. P values ≤0.05 were considered to be statistically significant.
RESULTS
To investigate the potential of pCCR7L as an immunomodulator, we designed a prime/boost strategy of immunization similar to the one reported earlier (7) in which mucosal priming with rVVgB and mucosal boosting with pgB produced a considerably robust immune response. Mice were immunized intranasally with codelivery of plasmid DNA encoding either CCL19 or CCL21 at prime and boost stages.
Lung DCs increase in number upon administration of pCCR7L.
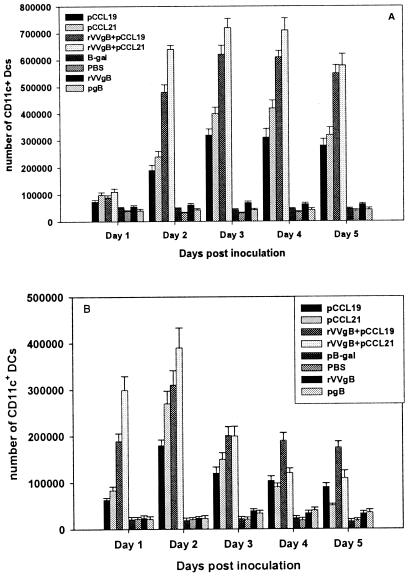
DCs highly express CCR7 upon activation and migrate to the peripheral LN, where they participate in naive-T-cell priming. Hence, coadministration of pCCR7L intranasally was primarily targeted at manipulating respiratory tract DCs so as to enhance the antigen uptake and presumably augment priming of T cells. We first assessed the numbers of DCs in the lung upon administration of pCCR7L, this being the primary site, among others, at which immune induction initiates after intranasal immunization. Mice were instilled plasmid DNA encoding CCR7L alone or together with rVVgB or rVVgB and pgB separately in both nares. Lungs and peribronchial LN (PBLN) were removed for assessment each day until 5 days to show the effect of ectopic expression of pCCR7L in the respiratory tract. Indeed there was a notable increase in the CD11c+ DCs, with numbers rising approximately 3.5-fold for pCCL19 and pCCL21 individually and 7-fold for mice given pCCR7L and rVVgB on days 3 and 4 (Fig. 1A). More CD11c+ DCs accumulated in the lungs when pCCR7L was codelivered with rVVgB than when pCCR7L was delivered alone. pCCL21, in comparison to pCCL19 induced slightly larger mobilization of DCs, as was evident from day 2 after administration, although the differences between means were insignificant (P ≥ 0.05). However, the increase in number of CD11c+ DCs in all groups treated with pCCR7L was significantly higher (P ≤ 0.05) than that for control animals which received the plasmid vector encoding the irrelevant protein β-Gal or naive mice that were given PBS. The numbers of CD11c+ DCs in PBLN (Fig. 1B) were higher in the first 48 h following immunization but decreased thereafter. This coincided with high accumulation of CD11c+ DCs in the lungs on the third day onwards. The accumulation of CD11c+ DCs observed in our study is therefore attributed to the expression of pCCR7L in the lung environment.
FIG. 1.
Administration of pCCR7L i.n. increases the numbers of lung DCs. C57BL/6 mice 5 to 6 weeks of age were given i.n. 100 μg of plasmid DNA encoding CCR7L (pCCL19 or pCCL21) with or without rVVgB, vector plasmid DNA encoding β-Gal, or PBS. Lungs and PBLN were removed on days 1 to 5, and DCs were isolated by positive selection with CD11c+ microbeads by MACS, stained with a monoclonal antibody against CD11c FITC, and analyzed by flow cytometry. (A) Comparison of lung CD11c+ DCs from various treatment groups on days 1 to 5; (B) CD11c+ DCs in PBLN following i.n. codelivery of pCCR7L.
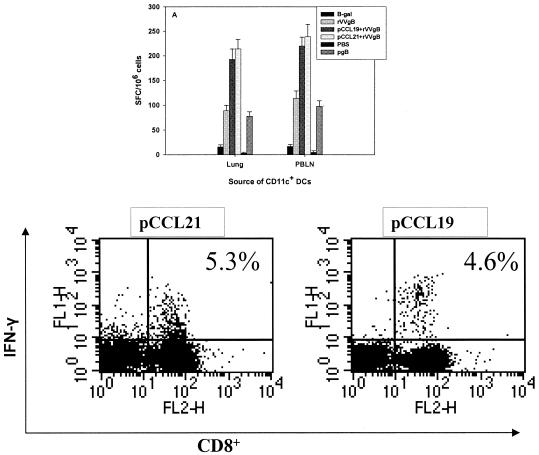
Consequently, we tested whether the DCs isolated from the respiratory tract were capable of presenting antigen administered intranasally (i.n.). Mice were first administered pCCR7L and 3 days later were immunized with rVVgB i.n. Lungs were removed after 7 days for isolation of lung DCs by MACS. The DCs were then incubated with splenocytes isolated from HSV-primed mice in an ELISPOT assay. The DCs from mice administered pCCR7L and later immunized with rVVgB were capable of stimulating T cells from HSV-primed mice (Fig. 2A). The numbers of spot-forming cells in splenocytes incubated with DCs from lungs and PBLN were comparable, suggesting that the functional status of CD11c+ DCs from PBLN was not altered despite reduced total cell numbers. Furthermore, the CD8+ T cells isolated from lungs of pCCR7L-treated mice and restimulated in vitro with HSV-gB498-505 produced IFN-γ (Fig. 2B). This observation suggests that additional antigen presentation upon codelivery of pCCR7L may take place in the lungs. However, additional data are required to support this observation. Collectively, these data show that ectopic expression of pCCR7L affects the mobility and function of DCs in the respiratory tract, more so because the DCs isolated from lungs of mice immunized with rVVgB and pCCR7L were functional antigen-presenting cells, as demonstrated by their ability to activate the splenocytes from HSV-primed mice.
FIG. 2.
CD11c+ DCs isolated from lungs and PBLN of pCCR7L-treated mice are capable of activating CD8+ T cells from mice previously primed with HSV. Mice were treated with pCCR7L, β-Gal, or PBS i.n., and 3 days later pCCR7L-treated mice were immunized i.n. with rVVgB. One group was infected with rVVgB only. An IFN-γ ELISPOT assay was performed to quantitate the functional capability of CD11c+ DCs originating from lungs and PBLN of pCCR7L-treated and control mice. Seven days following immunization lungs and PBLN were removed and CD11c+ DCs were prepared, purified by MACS, and incubated with splenocytes from mice primed earlier with HSV. CD11c+ DCs (2 × 104) were added to 105 splenocytes per well. (A) Spot-forming cells after stimulation with lung or PBLN CD11c+ DCs; (B) IFN-γ secretion by CD8+ T cells isolated from lungs of pCCR7L-treated mice upon restimulation in vitro with HSV-gB498-505. Intracellular staining for IFN-γ was performed as described in Materials and Methods.
Coadministration of pCCR7L increases antibody levels both in serum and genital tract lavage fluid.
Experiments were performed to compare the serum antibody levels in mice immunized with rVVgB and pgB to those of mice immunized with coadministration of pCCR7L. Serum samples were collected at 14 days postboost for primary response and at 60 days postboost for memory response and analyzed by ELISA for reactivity to HSV-gB antigen. In the primary immune response a clear difference between mice coadministered pCCR7L and mice not treated with pCCR7L was observed. Preliminary experiments showed higher responses when pCCR7L was applied at both prime and boost. This option was adopted throughout the vaccinations described in subsequent sections. The primary IgG response in serum in all vaccinated mice, irrespective of the protocol of immunization, differed significantly (P ≤ 0.05) from those in nonimmunized mice, which did not generate antibody levels above background (Table 1). The highest IgG concentration was observed in serum of mice immunized with inactivated HSV. We particularly focused on the defense mechanisms engaged at distal mucosal sites following i.n. immunization. Accordingly, we examined the antibody levels in the genital tract of pCCR7L-treated and non-pCCR7L-treated mice. Codelivery of pCCR7L at both prime and boost led to a clear increase in the genital tract IgA and IgG (Table 1) compared to no pCCR7L treatment. However, the genital tract antibody levels were lower than serum antibody levels. Because the vaccinated animals did not develop overt pathological lesions, we assumed that the antibody may have contributed to virus resolution. This suggests that Ig found in the genital tract after the primary immune response to vaccination may participate in neutralizing infecting virus. However, IgA levels in serum measured at the memory phase decreased about threefold in mice immunized with rVVgB and pgB and those coadministered pCCR7L. Even then the pCCR7L-treated mice had at least twofold-higher levels of serum IgG than non-pCCR7L-treated animals. Surprisingly, very little or no antigen-specific IgA was found in the genital tract in the memory phase. It appears that pCCR7L influenced the IgG and IgA levels in both mucosal and systemic compartments in the primary responses. In the studies described above we did not directly examine the neutralizing capacity of the antibodies generated against HSV using the described vaccination protocol.
TABLE 1.
pCCR7L codelivery with rVVgB and pgB influences serum and genital tract wash fluid antibody levelsa
| Group | Response (ng/ml)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Primary in:
|
Memory in:
|
|||||||
| Serum
|
Vaginal tract wash fluid
|
Serum
|
Vaginal tract wash fluid
|
|||||
| IgA | IgG | IgA | IgG | IgA | IgG | IgA | IgG | |
| rVVgB + pgB | 47 ± 12 | 29,138 ± 1,002 | 24 ± 2 | 1,869 ± 213 | 15 ± 7 | 3,896 ± 256 | NDb | 569 ± 49 |
| pCCL21 + rVVgB + pgB | 60 ± 8 | 48,883 ± 1,201† | 57 ± 12 | 2,188 ± 121 | 21 ± 9 | 5,986 ± 564 | ND | 745 ± 213 |
| pCCL19 + rVVgB + pgB | 58 ± 6 | 54,765 ± 7,753†† | 87 ± 16 | 2,371 ± 446 | 22 ± 8 | 5,265 ± 143 | ND | 629 ± 146 |
| pβ-Galc | 19 ± 1 | 103 ± 11 | 13 ± 5 | 24 ± 21 | 0 | 0 | ND | 0 |
| UV-HSVd | 201 ± 23 | 73,872 ± 14,036††† | 146 ± 18 | 2,972 ± 602 | 34 ± 6 | 13,003 ± 123 | ND | 1,092 ± 97 |
| PBS | 0 | 28 ± 19 | 0 | 29 ± 18 | 0 | 0 | ND | 0 |
Mice were immunized with rVVgB and pgB with or without codelivery of pCCR7L. Serum samples and genital tract wash fluid were collected at 14 days after boost (primary response) and 60 days after boost (memory response). ELISA for antibody detection was performed as described in Materials and Methods. Data represent means ± standard deviations for four animals per group in one experiment from two performed. †, P ≤ 0.05 in comparison to rVVgB-, β-Gal-, and PBS-treated mice; ††, P ≤ 0.05 in comparison to rVVgB-, β-Gal-, and PBS-treated mice; †††, P ≤ 0.05 in comparison to all groups.
ND, not detected.
pβ-Gal, plasmid DNA encoding β-Gal.
UV-HSV, UV-inactivated HSV.
pCCR7L treatment modulates cellular immune responses against HSV-1 antigen.
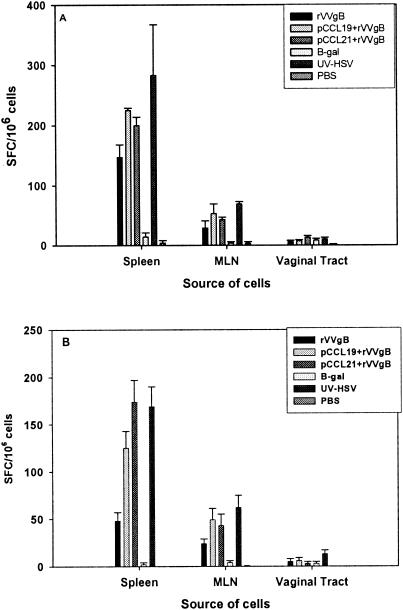
To determine whether pCCR7L coadministration affected the virus-specific CD8+ T-cell function, we investigated the capability of CD8+ T cells generated in this vaccination strategy to secrete IFN-γ and their potential to lyse HSV-gB498-505-pulsed targets. At 14 days after boosting with pgB or pgB and pCCR7L, splenocytes and mesenteric LN (MLN) cells were isolated and stimulated in vitro with HSV-gB498-505 for 5 h and then stained intracellularly for IFN-γ or were stimulated with syngeneic irradiated splenocytes pulsed with HSV-gB498-505 for 72 h in an IFN-γ ELISPOT assay. Both assays demonstrated that the T-cell population isolated from pCCR7L-treated mice contained CD8+ T cells that were capable of secreting IFN-γ upon restimulation in vitro (Fig. 3). However, in the acute immune response (Fig. 3A) the difference between mice primed with rVVgB and boosted with pgB only and those that were coadministered pCCR7L was not as clear as the difference in serum Ig levels, on which pCCR7L boost codelivery appeared to have exerted a synergistic effect. A possible explanation could be that rVVgB itself induces a strong immune response and as such appears to overshadow the effect of pCCR7L, at least in the primary phase. However, the responses measured in the memory phase (Fig. 3B) differed significantly between the groups that were given pCCR7L and the mice treated with rVVgB and pgB only (Fig. 3B). There was at least a threefold reduction in the number of IFN-γ-producing cells among immune cells isolated from mice immunized with rVVgB and pgB only compared to the number among cells isolated from mice in the same group but analyzed in the primary phase. The pCCL19-coadministered mice showed nearly a twofold reduction, and the pCCL21-coadministered mice showed only a slight decrease compared to similar response in the same group analyzed in the primary phase. Interestingly, memory CD8+ T cells from pCCR7L-treated mice rapidly responded to short-term ex vivo restimulation (5-h intracellular cytokine staining assay) with HSV-gB498-505 by secreting IFN-γ (Fig. 4). Therefore, it appears that pCCR7L codelivery may give rise to functional memory CD8+ T cells.
FIG. 3.
CD8+ T cells isolated from mice treated with pCCR7L have the capacity to produce more IFN-γ than CD8+ T cells from non-pCCR7L-treated mice. IFN-γ secretion by CD8+ T cells isolated from spleen, MLN, and genital tract was assessed by in vitro stimulation with splenocytes pulsed with gB498-505 in ELISPOT assays. CD8+ T cells were examined at 14 (A; acute phase) and 60 days (B; memory phase) after boost. Data are from a representative experiment of two performed.
FIG. 4.
Spleen memory CD8+ T cells from pCCR7L-treated mice secreted IFN-γ upon restimulation ex vivo more rapidly than CD8+ T cells from non-pCCR7L-treated mice. The cells were isolated at 60 days postboost and assayed for IFN-γ production ex vivo by intracellular cytokine staining. Spleen cells (106) were incubated in the presence of 2.5 μg of HSV-gB498-505, 50 U of IL-2, and GolgiPlug for 5 h and subsequently stained with anti-CD8+-FITC and anti-IFN-γ-phycoerythrin (PE) antibodies (except for groups treated with UV-inactivated HSV and PBS, for which IFN-γ-FITC and CD8+-PE were used). FITC-conjugated rat anti-IgG was used for the isotype control (data not shown). Cytometry and data analysis were performed with FACScan and Cell Quest, respectively. Figures show representative data from two independent experiments.
CTLs derived from pCCR7L-treated mice efficiently lyse antigen-specific targets.
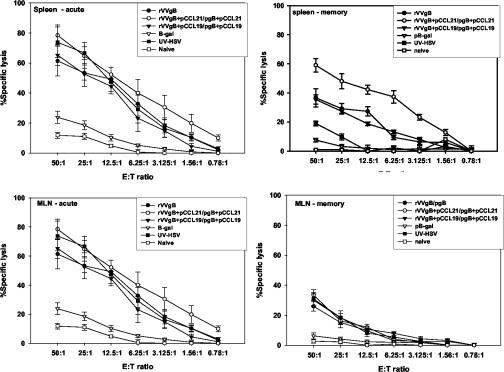
The hallmark of an enhanced immune response to an antigen is its impact on the functionality of the effector cell population generated, e.g., the capability of CTLs to efficiently kill the target. Accordingly, we investigated how incorporation of pCCR7L in the immunization protocol influences the function of CTLs against targets pulsed with the HSV immunodominant epitope. CTL activity was directed against HSV-gB498-505, and this effector activity was associated with the CD8+ T-cell population. The CTL activity was detected in cells isolated from the spleen and LN draining the gastrointestinal tract in both the primary and memory phases (Fig. 5). Although no ex vivo cytolytic activity could be demonstrated, this function of CD8+ T cells was more pronounced in the in vitro expanded population of splenocytes isolated in the primary phase than in that isolated in the memory phase. We noted that in the memory phase immune responses in mice treated with pCCL21 were slightly higher than those in mice treated with pCCL19. However, there was a degree of variability from animal to animal as concerns reactivity to the plasmid-encoded chemokines. Again, the capacity to lyse targets of CD8+ T cells from mice immunized with rVVgB and pgB only was lower than that of CD8+ T cells derived from mice vaccinated with codelivery of pCCR7L at both prime and boost stages. This indicates an immune-enhancing effect of pCCR7L when coexpressed locally with antigen.
FIG. 5.
Induction of SSIEFARL-specific cytolytic activity in mice vaccinated intranasally with rVVgB with or without codelivery of pCCR7L. Splenocytes and MLN were isolated on the 14th (primary phase) and 60th (memory phase) days postboost from vaccinated and control mice and expanded in vitro with syngeneic irradiated splenocytes pulsed with gB498-505 (specific for MHC-I-restricted CD8+ T cells) for 5 days, followed by a 51Cr release assay using MHC-matched MC38 (mouse colon adenocarcinoma) cells pulsed with gB498-505 as the targets. Data were corrected with the formula described in Materials and Methods. E:T ratio, effector-to-target cell ratio.
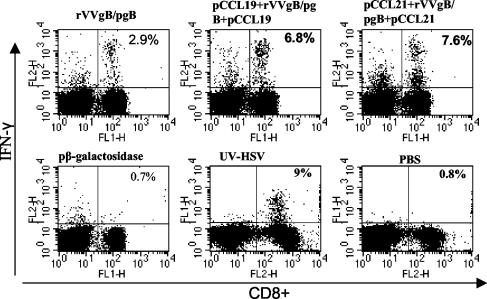
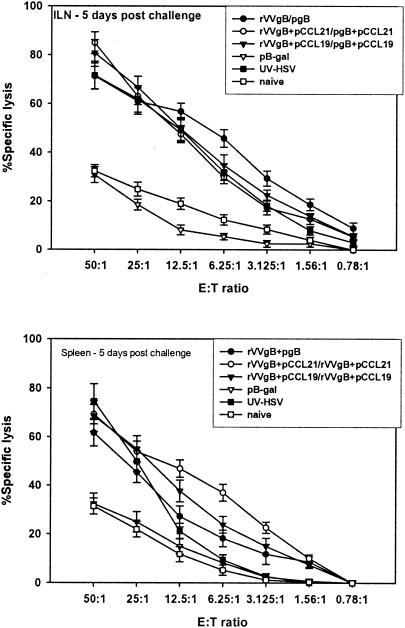
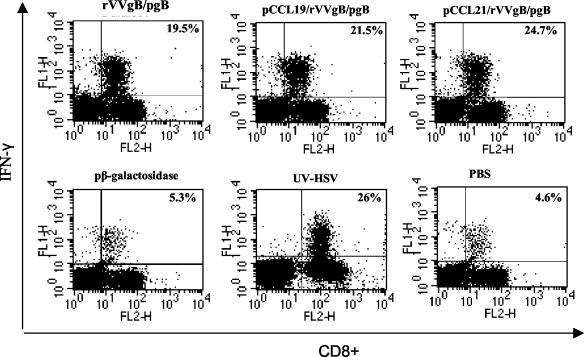
We next evaluated the CTL responses in vaccinated mice after challenge with a lethal dose of HSV McKrae. Sixty days after the boosting dose the female C57BL/6 mice were challenged genitally with 107 PFU of HSV McKrae/ml. This virus dose was preliminarily determined to be lethal for C57BL/6 mice. Five days following challenge, iliac LN and spleens were removed and T cells were isolated for CTL analysis. Whereas the memory levels of CD8+ CTL activity were lower, within 5 days of challenge high cytolytic activity was observed in mice that received pCCRL7 coadministration (Fig. 6) compared to that in mice vaccinated with rVVgB and pgB and controls. This illustrates the efficacy of pCCR7L codelivery in generating a CTL population that is capable of rapid expansion following infection with HSV. However, the capability of CTLs to expand after infection with HSV was also a characteristic possessed by CD8+ T cells from animals that were not given pCCR7L, although these T cells had less capability than those from pCCR7L-treated mice. Besides having high cytolytic activity, restimulated populations of CD8+ T cells isolated from iliac LN after challenge harbored a large population of IFN-γ-secreting CD8+ T cells that could rapidly respond upon peptide stimulation at short term (Fig. 7). The IFN-γ levels observed in these CD8+ T cells may suggest that the enhanced potential to secrete IFN-γ has a notable protective role against mucosal challenge with a highly pathogenic strain of HSV.
FIG. 6.
Postchallenge cytolytic potential of CD8+ T cells isolated from pCCR7L-immunized mice. The CTL assay was performed as described in Materials and Methods. Cells were isolated from mice at 5 days postchallenge. This is a representative experiment of two performed. E:T ratio, effector-to-target cell ratio.
FIG. 7.
Postchallenge IFN-γ secretion by CD8+ T cells. Sixty days after the boosting dose (memory phase) mice were synchronized with 2 mg of medroxyprogesterone/mouse and 5 days later were infected with McKrae at 107 PFU/mouse intravaginally. Five days later mice were sacrificed and CD8+ T cells were isolated from the iliac LN and spleen. To assess IFN-γ production by intracellular cytokine staining, 106 cells were stimulated with 2.5 μg of HSV-gB498-505 for 5 h in the presence of brefeldin A and IL-2 and stained with anti-IFN-γ-FITC and anti-CD8+-phycoerythrin and then analyzed by flow cytometry. Data are representative of two independent experiments performed.
Protection against mucosal challenge.
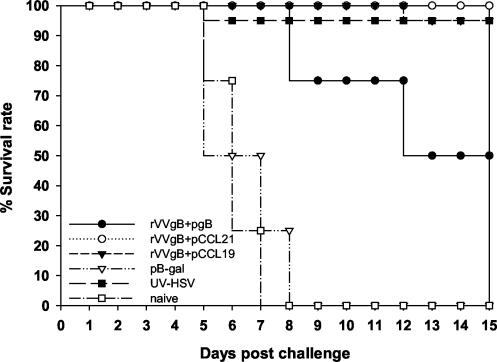
The levels of protective immunity resulting from immunization with rVVgB and pgB and coadministration of pCCR7L were compared to those for a well-established approach involving immunization of live or UV-inactivated HSV-1. Mice were immunized as described earlier and subsequently challenged with 107 PFU of HSV McKrae. The virologic course of primary infection was monitored by isolation of virus from the genital tract wash fluids collected at days 1 to 9 postinfection. Mean log10 titers of virus are shown in Table 2. Nonimmunized mice displayed symptoms of clinical illness beginning on day 5 or 6 and usually died by day 8. As expected no signs of illness were evident in mice immunized with inactivated virus. Death was mainly due to encephalitis (hunched posture and hind limb paralysis). In such mice virus was not cleared at all. High titers were observed on the third day after infection, especially in nonimmunized groups. Pathological lesions were more severe in nonimmunized mice than in mice immunized with rVVgB and pgB, but no visible lesions could be detected in mice immunized with inactivated virus as well as in those coadministered pCCR7L. The survival rate (Fig. 8) was higher for the groups receiving pCCR7L and inactivated virus than for the groups not coadministered pCCR7L. Postchallenge anti-HSV CTL activity (Fig. 6) was highest in mice coadministered pCCR7L. Correspondingly, large numbers of IFN-γ-producing CD8+ T cells (both in the ELISPOT assay [data not shown] and intracellular cytokine staining) were detected in genital tract cell suspension, as well as the iliac LN and spleen (Fig. 7). However, irrespective of the vaccine combination assessed, virus still replicated in the genital tract epithelium, indicating that no complete prevention of infection was achieved by the vaccination, although the titers were drastically reduced in pCCR7L-coadministered animals and those that were given inactivated virus.
TABLE 2.
Mean log10 titers of virus in the genital tract wash fluid collected each day for 9 days following challenge with 107 PFU of the McKrae strain of HSV/mouse
| Groupa | Mean log10 titer on day:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 3.30 ± 0.19 | 3.40 ± 0.21 | 2.78 ± 0.11 | 2.18 ± 0.32 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2 | 3.08 ± 0.09 | 3.86 ± 0.22 | 2.90 ± 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 3 | 3.26 ± 0.12 | 4.15 ± 0.11 | 4.18 ± 0.98 | 3.70 ± 0.23 | 3.24 ± 0.29 | 2.30 ± 0.03 | 1.70 ± 0.02 | 0.00 | 0.00 |
| 4 | 4.48 ± 0.32 | 5.18 ± 1.21 | 5.00 ± 0.99 | 4.85 ± 1.07 | 4.34 ± 0.92 | —b | — | — | — |
| 5 | 2.48 ± 0.41 | 3.00 ± 1.02 | 2.11 ± 0.7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 6 | 4.61 ± 0.31 | 5.04 ± 0.20 | 4.95 ± 1.1 | 4.63 ± 1.2 | 4.54 ± 0.68 | — | — | — | — |
1, rVVgB-pCCL19 plus pgB-pCCL19 at boost; 2, rVVgB-pCCL21 plus pgB-pCCL21; 3, rVVgB plus pgB; 4, vector encoding β-Gal; 5, UV-inactivated HSV-1; 6, naive PBS.
—, death.
FIG. 8.
Survival rates for pCCR7L-vaccinated and non-pCCR7L-vaccinated mice after intravaginal challenge at 60 days after boost immunization. Four mice were used per group and were synchronized by injecting 2 mg of medroxyprogesterone per mouse subcutaneously. Five days later each mouse was anaesthetized with avertin and infected intravaginally with 107 PFU of HSV MacKrae. Each day a specimen of the genital tract wash fluid was collected for virus titration. Mice were monitored daily for clinical illness and pathology, scored according to criteria reported by Gallichan et al. (13) as follows: 0, no apparent infection; 1, mild inflammation of the external genitals and redness and moderate swelling of external genitals; 3, severe redness and inflammation; 4, genital ulceration and severe inflammation; 5, hind limb paralysis and death.
When Ig levels were assessed postchallenge, both IgA and IgG were found to be lowest at initiation of infection, but levels gradually increased coincident with virus resolution. This indicates that gB-specific antibody-secreting cells were rapidly mobilized to the genital tract following infection and possibly participated in virus clearance. Similar data on the postchallenge levels of IgA and IgG have been reported previously by Gallichan and Rosenthal (12).
DISCUSSION
A number of studies has shown the potential of recombinant vaccinia virus and plasmid DNA encoding various viral proteins as vaccines (26, 39, 40). Appropriate combination of the two into what is termed the heterologous prime/boost strategy of immunization more than likely may emerge as a potential vaccination strategy capable of controlling HSV infection. In this study we have attempted a mucosal heterologous prime/boost vaccination against HSV infection in mice, using plasmids expressing β-chemokines as molecular adjuvants. The responses generated were protective in both primary and memory exposure to HSV. The mechanism of immune enhancement appeared to be mediated by the large number of DCs and T cells in the local environment, i.e., the respiratory tract in particular, caused by pCCR7L overexpression. Concerning the numbers of DCs our results are in line with a recent report (23) in which it was shown that, upon intranasal infection of mice with influenza virus, there is a rapid migration of CD11c+ DCs to the PBLN lasting for only 2 days. However, this report does not indicate whether there is accumulation of CD11c+ DCs in the lungs thereafter or not. It is highly probable that, upon instillation of plasmid DNA encoding the CC chemokines, there is an interaction between the CpG motif likely constituted in the plasmid backbone and the lung DCs through Toll-like receptor 9, facilitating high expression of CCR7 on the DCs, which in turn respond to the present CCR7L. The effect reported might also result from induction of other β chemokines. Jones et al. (17) have reported increased expression of macrophage inflammatory protein 1α (MIP-1α) and MIP-1β mRNA in lungs of mice following infection with influenza virus. Therefore, the efficient induction of cellular immune responses observed was presumably orchestrated by the strong interaction of the lung DCs and T cells.
It is required that DCs migrate to T-cell areas of secondary lymphoid organs in order to present antigen and prime naive T cells, resulting in development of an antigen-specific adaptive immunity (5). However, our studies show that this might not be an absolute prerequisite since antigen presentation can take place in nonlymphoid tissue too (2). A recent report (37) shows that exogenously applied CCL21 and CCL19 were capable of recruiting green fluorescent protein+ CD44low T cells to nonlymphoid tissue. In the LN the stromal cells create an environment where CCR7+ naive T cells and CCR7+ DCs are brought together at local high concentration of CCL21, thus establishing a physical interaction leading to T-cell activation (3). DCs isolated from the lungs of pCCR7L-treated mice were capable of activating CD8+ T cells to produce IFN-γ just as CD8+ T cells isolated from the same lungs were capable of responding to specific peptide stimulus. This effect was observed relatively early in the immune response, suggesting that these immune cells acquired these capabilities within the site of immune induction. However, irrespective of the protocol used, vaccination resulted in detectable response in the distal target mucosa, since antigen-specific responses could be detected in the distal mucosa during the primary phase. It is surprising that antigen-specific CD8+ T cells appeared in the genital mucosa at considerable levels relatively quickly after exposure to virus challenge, i.e., 3 to 5 days postexposure, compared to their appearance in naive animals, in which the immune response resembled that to a primary infection. Most likely the memory CD8+ T cells do not reside exclusively in the genital mucosa but are rapidly recruited to this site since we did not detect these cells in the prechallenge genital tracts, i.e., 60 days postboost. A similar suggestion has been reported before (28, 30). When the cellular responses in the prechallenge phase, i.e., at 60 days, were measured, cytolytic and IFN-γ-producing CD8+ T cells could be detected in lymphoid tissue, spleen, and MLN. The cell-mediated immune response generated against antigen-specific targets is the most efficient form of immunity. The fact that highly cytolytic CD8+ T cells could be detected in the spleen indicates the potential of mucosal vaccination to induce systemic responses as well. A recent report (1, 38) shows that central memory CD8+ T cells that develop after antigen has disappeared regain the capability to home to secondary lymphoid tissue. These memory cells are characterized by reexpression of CD62L and CCR7 and are highly proliferative upon reencounter with antigen. This could explain the rapid recall of CD8+ T cells to the genital tract mucosa after challenge with HSV McKrae. What remains unexplained is the lack of these cells within the genital mucosa in the absence of infection. It is clear from these results that upon virus challenge immunized mice are capable of mobilizing the CD8+ T cells to the genital tract to combat the infection, indicating an unconditional requirement for T cells in mediating protection of the genital mucosa. Although data indicating the CD4+ T-cell contribution to protection were not included in this report, the protective role of CD8+ T cells only should not be overstated since this activity of T cells is shared between CD4+ and CD8+ T cells in HSV infection. T-cell depletion studies (31) showed that both CD4+ and CD8+ T cells are involved in vaginal mucosa immunity against HSV-2, but CD4+ T cells were more important in protection against primary infection while CD8+ T cells appeared to be of significance largely during the memory response (27, 29). On the other hand Harandi et al. (15) showed that in CD4−/− mice virus-specific IFN-γ production and delayed-type hypersensitivity responses were impaired, which led to rapid death of CD4−/− mice upon challenge with HSV-2. The observed effect of vaccinating with rVVgB and pgB and codelivery of pCCR7L is consequently the interplay between the kinetics of the resulting immune response provided for by the enhancing effect of pCCR7L expression and the distribution accounted for by the common-mucosa system concept, allowing homing of responding immune cells to distal mucosa surfaces.
Our results show that ectopic expression of CCR7L at the site of immunization enhances the immune response against HSV, with CCR7L acting as a molecular adjuvant. This is characterized by a high frequency of functional CD8+ T cells. Although the levels of acute immune response in both pCCR7L-coadministered and non-pCCR7L-treated mice are comparable, the memory responses are different, bringing into question what CD8+ T-cell-priming mechanism is engaged upon pCCR7L coadministration. While previous work (9) showed the efficacy of pCCR7L in studies involving DNA-only immunization, we have extended those studies in a more robust heterologous prime/boost vaccination strategy and show that a mucosal approach with incorporation of pCCR7L elicits a distal mucosal memory response that is long term and capable of protecting the mice during a vaginal challenge with a lethal dose of HSV McKrae. Also what remains to be assessed is the precise role in this vaccination setup of CD4+ T cells, which appear to be involved in the immune response (not shown). Whatever the mechanism employed, it appeared to favor the development of a memory CD8+ T-cell pool that is capable of protecting the mice upon challenge with infectious HSV.
Acknowledgments
This work was supported by NIH grant AI 14981 to B.T.R.
REFERENCES
- 1.Badovinac, V. P., and J. T. Harty. 2003. Memory lanes. Nat. Immunol. 4:212-213. [DOI] [PubMed] [Google Scholar]
- 2.Constant, S. L., J. L. Brogdon, D. A. Piggott, C. A. Herrick, I. Visintin, N. H. Ruddle, and K. Bottomly. 2002. Resident lung antigen-presenting cells have the capacity to promote Th2 T cell differentiation in situ. J. Clin. Investig. 110:1441-1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cyster, J. G. 1999. Chemokines and cell migration in secondary lymphoid organs. Science 286:2098-2102. [DOI] [PubMed] [Google Scholar]
- 4.DiPaolo, R. J., and E. R. Unanue. 2002. Cutting edge: chemical dominance does not relate to immunodominance: studies of the CD4+ T cell response to a model antigen. J. Immunol. 169:1-4. [DOI] [PubMed] [Google Scholar]
- 5.Dodge, I. L., M. W. Carr, M. Cernadas, and M. B. Brenner. 2003. IL-6 production by pulmonary dendritic cells impedes Th1 immune responses. J. Immunol. 170:4457-4464. [DOI] [PubMed] [Google Scholar]
- 6.Edwards, S., and C. Carne. 1998. Oral sex and the transmission of viral STIs. Sex. Transm. Infect. 74:6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eo, S. K., M. Gierynska, A. A. Kamar, and B. T. Rouse. 2001. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J. Immunol. 166:5473-5479. [DOI] [PubMed] [Google Scholar]
- 8.Eo, S. K., U. Kumaraguru, and B. T. Rouse. 2001. Plasmid DNA encoding CCR7 ligands compensates for dysfunctional CD8+ T cell responses by effects on dendritic cells. J. Immunol. 167:3592-3599. [DOI] [PubMed] [Google Scholar]
- 9.Eo, S. K., S. Lee, U. Kumaraguru, and B. T. Rouse. 2001. Immunopotentiation of DNA vaccine against herpes simplex virus via co-delivery of plasmid DNA expressing CCR7 ligands. Vaccine 19:4685-4693. [DOI] [PubMed] [Google Scholar]
- 10.Forster, R., A. Schubel, D. Breitfeld, E. Kremmer, I. Renner-Muller, E. Wolf, and M. Lipp. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99:23-33. [DOI] [PubMed] [Google Scholar]
- 11.Gallichan, W. S., and K. L. Rosenthal. 1996. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology 224:487-497. [DOI] [PubMed] [Google Scholar]
- 12.Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. [DOI] [PubMed] [Google Scholar]
- 13.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 14.Gierynska, M., U. Kumaraguru, S. K. Eo, S. Lee, A. Krieg, and B. T. Rouse. 2002. Induction of CD8 T-cell-specific systemic and mucosal immunity against herpes simplex virus with CpG-peptide complexes. J. Virol. 76:6568-6576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845-853. [DOI] [PubMed] [Google Scholar]
- 16.Hiroi, T., M. Yanagita, N. Ohta, G. Sakaue, and H. Kiyono. 2000. IL-15 and IL-15 receptor selectively regulate differentiation of common mucosal immune system-independent B-1 cells for IgA responses. J. Immunol. 165:4329-4337. [DOI] [PubMed] [Google Scholar]
- 17.Jones, H. P., L. M. Hodge, K. Fujihashi, H. Kiyono, J. R. McGhee, and J. W. Simecka. 2001. The pulmonary environment promotes Th2 cell responses after nasal-pulmonary immunization with antigen alone, but Th1 responses are induced during instances of intense immune stimulation. J. Immunol. 167:4518-4526. [DOI] [PubMed] [Google Scholar]
- 18.Kaushic, C., A. A. Ashkar, L. A. Reid, and K. L. Rosenthal. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 77:4558-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knaup, B., S. Schunemann, and M. H. Wolff. 2000. Subclinical reactivation of herpes simplex virus type 1 in the oral cavity. Oral Microbiol. Immunol. 15:281-283. [DOI] [PubMed] [Google Scholar]
- 20.Koelle, D. M., and A. Wald. 2000. Herpes simplex virus: the importance of asymptomatic shedding. J. Antimicrob. Chemother. 45:1-8. [DOI] [PubMed] [Google Scholar]
- 21.Kuklin, N., M. Daheshia, K. Karem, E. Manickan, and B. Rouse. 1997. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J. Virol. 71:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumaraguru, U., and B. T. Rouse. 2000. Application of the intracellular gamma interferon assay to recalculate the potency of CD8+ T-cell responses to herpes simplex virus. J. Virol. 74:5709-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Legge, K. L., and T. J. Braciale. 2003. Accelerated migration of respiratory dendritic cells to the regional lymph nodes is limited to the early phase of pulmonary infection. Immunity 18:265-277. [DOI] [PubMed] [Google Scholar]
- 24.Mahalingam, S., K. Clark, K. I. Matthaei, and P. S. Foster. 2001. Antiviral potential of chemokines. Bioessays 23:428-435. [DOI] [PubMed] [Google Scholar]
- 25.Manickan, E., R. Rouse, Z. Yu, W. Wire, and B. Rouse. 1995. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J. Immunol. 155:259-265. [PubMed] [Google Scholar]
- 26.Meseda, C. A., K. L. Elkins, M. J. Merchlinsky, and J. P. Weir. 2002. Prime-boost immunization with DNA and modified vaccinia virus Ankara vectors expressing herpes simplex virus-2 glycoprotein D elicits greater specific antibody and cytokine responses than DNA vaccine alone. J. Infect. Dis. 186:1065-1073. [DOI] [PubMed] [Google Scholar]
- 27.Milligan, G. N., and D. I. Bernstein. 1995. Analysis of herpes simplex virus-specific T cells in the murine female genital tract following genital infection with herpes simplex virus type 2. Virology 212:481-489. [DOI] [PubMed] [Google Scholar]
- 28.Milligan, G. N., D. I. Bernstein, and N. Bourne. 1998. T lymphocytes are required for protection of the vaginal mucosae and sensory ganglia of immune mice against reinfection with herpes simplex virus type 2. J. Immunol. 160:6093-6100. [PubMed] [Google Scholar]
- 29.Morrison, L. A., and D. M. Knipe. 1997. Contributions of antibody and T cell subsets to protection elicited by immunization with a replication-defective mutant of herpes simplex virus type 1. Virology 239:315-326. [DOI] [PubMed] [Google Scholar]
- 30.Parr, E. L., and M. B. Parr. 1999. Immune responses and protection against vaginal infection after nasal or vaginal immunization with attenuated herpes simplex virus type-2. Immunology 98:639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parr, M. B., and E. L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price, D. A., P. Klenerman, B. L. Booth, R. E. Phillips, and A. K. Sewell. 1999. Cytotoxic T lymphocytes, chemokines and antiviral immunity. Immunol. Today 20:212-216. [DOI] [PubMed] [Google Scholar]
- 33.SangKon, O., J. Berzofsky, D. Burke, T. Waldmann, and L. Perera. 2003. Coadministration of HIV vaccine vector with vaccinia virus expressing IL-15 but not IL-2 induces long-lasting cellular immunity. Proc. Natl. Acad. Sci. USA 100:3392-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Somani, J., S. Lonial, H. Rosenthal, S. Resnick, I. Kakhniashvili, and E. K. Waller. 2002. A randomized, placebo-controlled trial of subcutaneous administration of GM-CSF as a vaccine adjuvant: effect on cellular and humoral immune responses. Vaccine 21:221-230. [DOI] [PubMed] [Google Scholar]
- 35.Stanberry, L. R., S. L. Spruance, A. L. Cunningham, D. I. Bernstein, A. Mindel, S. Sacks, S. Tyring, F. Y. Aoki, M. Slaoui, M. Denis, P. Vandepapeliere, and G. Dubin. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652-1661. [DOI] [PubMed] [Google Scholar]
- 36.Stritzke, J., T. Zunkel, J. Steinmann, N. Schmitz, L. Uharek, and M. Zeis. 2003. Therapeutic effects of idiotype vaccination can be enhanced by the combination of granulocyte-macrophage colony-stimulating factor and interleukin 2 in a myeloma model. Br. J. Haematol. 120:27-35. [DOI] [PubMed] [Google Scholar]
- 37.Weninger, W., H. S. Carlsen, M. Goodarzi, F. Moazed, M. A. Crowley, E. S. Baekkevold, L. L. Cavanagh, and U. H. von Andrian. 2003. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J. Immunol. 170:4638-4648. [DOI] [PubMed] [Google Scholar]
- 38.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. Von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225-234. [DOI] [PubMed] [Google Scholar]
- 39.Wierzbicki, A., I. Kiszka, H. Kaneko, D. Kmieciak, T. J. Wasik, J. Gzyl, Y. Kaneko, and D. Kozbor. 2002. Immunization strategies to augment oral vaccination with DNA and viral vectors expressing HIV envelope glycoprotein. Vaccine 20:1295-1307. [DOI] [PubMed] [Google Scholar]
- 40.Woodberry, T., J. Gardner, S. L. Elliott, S. Leyrer, D. M. Purdie, P. Chaplin, and A. Suhrbier. 2003. Prime boost vaccination strategies: CD8 T cell numbers, protection, and Th1 bias. J. Immunol. 170:2599-2604. [DOI] [PubMed] [Google Scholar]
- 41.Wu, H. Y., and M. W. Russell. 1997. Nasal lymphoid tissue, intranasal immunization, and compartmentalization of the common mucosal immune system. Immunol. Res. 16:187-201. [DOI] [PubMed] [Google Scholar]