Abstract
The biological activities of the papillomavirus E2 protein in transcription, replication, and maintenance of the papillomavirus genome rely on the E2 protein's ability to bind that genome specifically. The E2 binding sites (E2BSs), located within the long control region (LCR) of human papillomavirus (HPV) genomes, contain potential sites for 5′methylation at cytosine (CpG) residues. The E2 protein's capacity to bind E2BS in vitro is inhibited by methylation of these cytosines (59). Herein, we describe experiments to assess the influence of methylation on E2 function in cells. E2's ability to activate transcription was inhibited by the global methylation of CpG dinucleotides in E2-responsive transcriptional templates or when only the CpG dinucleotides within the E2BSs of a transcriptional template were methylated. Thus at least one biological activity of E2 that is dependent on its ability to bind DNA in a site-specific manner is influenced by the methylation status of its cognate binding site. The activity of DNA methylases is influenced by the differentiation status of mammalian cells. The life cycle of HPVs is tied to the differentiation of its host cells within stratified squamous epithelia. To investigate whether methylation of the papillomavirus genomes is influenced by the differentiation status of host epithelial cells, we analyzed HPV16 DNA harvested from a cervical epithelial cell line that was isolated from an HPV16-infected patient. We found, using bisulfite treatment to discriminate between methylated and unmethylated cytosines, that the HPV16 LCR was selectively hypomethylated in highly differentiated cell populations. In contrast, the HPV16 LCR from poorly differentiated, basal cell-like cells contained multiple methylated cytosines and were often methylated at E2BSs, particularly E2BS2. These experiments indicate that the methylation state of the viral genome, and particular that of E2BSs, may vary during the viral life cycle, providing a novel means for modulating E2 function. These studies also uncovered an extensive pattern of methylation at non-CpG dinucleotides indicative of de novo methylation. The potential implications of this de novo methylation pattern are discussed.
Cytosine 5′ methylation at CpG dinucleotides is thought to influence both transcription and replication of DNA in eukaryotic organisms. DNA methyltransferases are the enzymes responsible for methylating cytosines in the context of CpG dinucleotides. DNA methyltransferase 1 (DNMT1) is found at sites of DNA replication in association with proliferating nuclear antigen (PCNA) and is thought responsible for methylating nascent, hemimethylated DNA (10). DNMT3a and -b, which are highly expressed in embryonic stem cells and which are implicated in global changes in chromosomal DNA methylation during development, methylate DNA de novo (41, 45). DNA methylation is closely tied to chromatin remodeling. Methyl CpG binding proteins bind to methylated CpG dinucleotides and recruit associated histone deacetylases (HDACs) (39). Alternatively, at least one DNA methyltransferase, DNMT1, can recruit HDACs directly to chromatin (21). Deacetylation of histones leads to assembly of chromatin in a tightly packed configuration that is refractory to transcription. Coordinate DNA methylation and histone deacetylation are thought to contribute to transcriptional silencing as it relates to genomic imprinting and X-chromosome inactivation as well as to the regulation of DNA replication. It has been hypothesized that CpG methylation represents a vertebrate genome defense mechanism by which foreign “parasitic” DNAs, such as retrotransposons, that are comparatively rich in CpG dinucleotides are transcriptionally silenced (51). Deregulation of normal DNA methylation patterns in mammalian chromosomes and/or defects in DNA methyltransferases or methyl CpG binding proteins contribute to the development of cancer, to aging, and to certain neurodevelopmental disorders such as intravascular coagulation and fibrinolysis, Rett, and fragile X syndromes (65).
Papillomaviruses are small double-stranded DNA viruses that infect stratified squamous epithelia and induce warts. A subset of mucosotropic human papillomaviruses (HPVs) termed the high-risk HPVs (e.g., HPV type 16 [HPV16]) are causally associated with human cancers, including cervical and other anogenital cancers and some oral cancers (68). Papillomaviruses encode protein E2, the full-length translation product of the E2 open reading frame (ORF), which contributes to multiple biological processes including viral transcription, viral DNA replication, and, at least for bovine papillomavirus type 1 (BPV1), the stable inheritance of viral plasmid DNA during cell division (5, 9, 24, 66). All three activities of E2 are dependent on its ability to bind to the viral DNA genomes as a homodimer at specific, partially palindromic sequences (5′-ACCGN4CGGT-3′) called E2 binding sites (E2BSs). In the high-risk HPV genomes, four highly conserved E2BSs are located in the viral long control region (LCR), a noncoding region rich in cis elements. These E2BSs are located near DNA binding sites for various cellular transcription factors, such as TATA-binding protein, Sp1, Gps2/AMF-1, TopBP1, CDP, and YY1, as well as the DNA binding site for the virally encoded DNA helicase E1, which mediates viral DNA replication (24). The interaction of E2 with various E2BSs mediates repression or activation of transcription and, through its interaction with E1, viral DNA replication. Viral gene expression is affected by the occupancy status of the E2 protein at the different E2BSs, which differ in their binding affinities for E2. Their occupancy is determined at least in part by the level of E2 protein expressed during the different stages of the viral life cycles (52). In addition, the E2 ORFs of multiple papillomaviruses encode additional translation products arising from translation initiating from internal ATGs (e.g., E2TR/E2C) and/or products of fusions with upstream ORFs (e.g., E8/E2TR) arising from differentially spliced mRNAs (8, 62). These alternative E2 ORF gene products can compete for binding to E2BS as homodimers and/or form heterodimers with the full-length E2 gene product and attenuate the latter's activity (31). In addition, it has recently been argued that one of these alternative E2 ORF gene products, E8/E2, can itself confer transcriptional and replication silencing, as in the case of HPV31 (53). E2BSs, because they contain CpG dinucleotides within the conserved palindromic sequences, are potential sites for DNA methylation in the mammalian host cell. The capacity of the full-length E2 ORF gene product E2 to bind E2BSs in vitro is inhibited by methylation of cytosines within its binding site (59). Thus, in addition to levels of E2 protein and the relative abundances of other E2 ORF gene products, the methylation status of the individual E2BSs might represent a determinant that influences their occupancy by E2 and therefore its function.
In this study, we investigated the potential role of DNA methylation in modulating E2 protein function in cells. We provide evidence that methylation of cytosines at CpG dinucleotides within E2BSs inhibits transcriptional activation by the E2 protein. We also evaluated the methylation status of cytosines at E2BSs and elsewhere within the LCR of the high-risk HPV16 genome in the context of a naturally infected human cervical epithelial cell line that supports the complete viral life cycle. We found that the methylation status in particular of the E2BSs and in general of the LCR, which contains many cis elements critical for the replication and transcription of the viral genome, varied significantly depending on the differentiation status of the cells. This finding has potential implications for the viral life cycle, which is tied to the differentiation of the host cell. In addition, the pattern of methylation on the HPV16 genome implies a role for de novo methylation in modifying HPV DNA in cervical epithelia.
MATERIALS AND METHODS
Construction of plasmids.
Plasmid pKT266 contains HPV16 nucleotides (nt) 7009 to 103 cloned into the BamHI and AflIII site of Bluescript KS(−) (Stratagene). The AvrII-to-BamHI DNA fragment from plasmid pBS1033, also referred to as oriP-BamH1 C-Luc' (29), containing the “basic” BamC promoter from Epstein Barr virus (EBV) fused to the firefly luciferase gene, was cloned into the SpeI and BamHI restriction enzyme sites of pKT266 to generate pKT267. BPV1 nt 7914 to 27 were cloned into XbaI and HindIII restriction enzyme sites of the pUC19 vector (New England Biolabs) to generate pBPV1ori (so-called because the LCR fragment cloned contains the minimal E1-dependent origin of replication). The AvrII-to-BamHI DNA fragment from plasmid pBS1033 containing the basic BamC promoter from EBV fused to the firefly luciferase gene was cloned into the XbaI and BamHI restriction enzyme sites of pBPV1ori to generate pKT260. The plasmid pBS1013, also referred to as the thymidine kinase (tk)-luciferase plasmid, was previously described (38). The plasmid p1073 is closely derived from pBS1013 and has four consensus BPV1 E2 binding sites positioned upstream of the tk promoter (T. Gahn, unpublished data). HPV16 E2 expression vector pCMV4E2 (13) and BPV1 E2 expression vector pCGE2 (61) were used to express E2 in the transcriptional activity assays.
In vitro DNA methylation.
In vitro DNA methylation was accomplished with CpG methylase (SssI methyltransferase), by following the procedure recommended by New England Biolabs, the commercial provider of SssI. Completion of DNA methylation was assessed by digestion with the BstUI restriction enzyme, which cleaves at its recognition sequence only if the DNA is not methylated at the cytosine residue within it. For the generation of methylated and unmethylated E2BSs containing DNA fragments, complementary oligonucleotides (5′-TCGAC ATGTA TCTAA GGGTA ACCGA AAACG GT TAG TATAA AGCAG AGG-3′ and 5′-TCGAC CTCTG CTTTA TACTA ACCGT TTTCG GT TAC CCTTA GATAC ATG-3′) were annealed and the double-stranded oligonucleotide was purified on polyacrylamide gel. This double-stranded DNA fragment contains a single E2BS flanked by random nucleotide sequences to extend the length of the double-stranded DNA and thereby reduce possible constraints on the efficiency of in vitro methylation. Present at both ends of the double-stranded DNA fragment are SalI 3′ overhangs. The double-stranded DNA fragment, which was or was not subjected to in vitro methylation with the SssI CpG methylase, was cloned into the XholI-linearized reporter plasmid pGL2-promoter (Promega). Ligated DNAs were ethanol precipitated and digested with XhoI to linearize any self-ligated vectors, and a fraction of the ligation product was transformed into Escherichia coli. Plasmid DNAs from 20 individual transformants derived from the ligations with methylated or unmethylated oligonucleotides were sequenced to determine the frequency of oligonucleotide inserts per clone. Two micrograms of the ligated products generated with methylated or unmethylated double-stranded DNA fragments was transfected into 293 cells to monitor repression of the E2-dependent transcriptional activity by the E2BS-specific DNA methylation.
Cell culture, transfections, and luciferase assays.
The human cervical cancer derived cell line C33A and adenovirus-transformed human kidney epithelial cell line 293 were obtained from the American Type Culture Collection and grown in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum. W12E cells (clone 20850) were grown as previously described (26). C33A cells were electroporated as previously described (36). 293 cells were transfected by either the calcium phosphate precipitation method (23) or by a method using fuGENE 6 (Roche) liposomes as specified by the manufacturer. The amounts of plasmid DNAs used in transfections are specified in the figure legends. Luciferase activity was measured in a luminometer (Analytical Luminescence Laboratory; model 3010) by using the luciferase assay system supplied by Promega according to the manufacturer's protocol. Amounts of lysates used in the luciferase assays were normalized to the total concentration of protein as measured by the Bradford assay.
Elutriation-based size fractionation of W12E cells.
W12E cells were subjected to centrifugal elutriation, fractions containing small (16 to 28 ml/min) or large (38 to 46 ml/min) cells were pooled, and low-molecular-weight (Hirt) DNAs were extracted as previously described (22). Those DNAs were subjected to HPV-specific Southern analysis using a full-length 32P-labeled random primer labeled HPV-16 genomic DNA as the HPV16 DNA probe and sodium bisulfite modification as described below.
Bisulfite modification.
The cytosine methylation status of the HPV16 DNA was determined by bisulfite modification of the target DNAs (11) with the CpGemone DNA modification kit (Intergen) according to the manufacturer's instructions. For PCR amplification of bisulfite-treated HPV16 DNAs, primers F1 (5′-CATAC ATACA TTCTA TAAAT T CCAC-3′) and F2 (5′-GGAAA TTGAT TTAGG TTTGT AG-3′) were used to amplify the region from HPV16 nt 6833 to 6921, and primers F3 (5′-CACCA ACAAC CATTT TATAA CTTC-3′) and F4 (5′-GTGGG TTTTG AAATA TTGTA G-3′) were used to amplify the region from HPV16 nt 7450 to 100. PCR amplimers were cloned wth the pGEM-T vector system (Promega), and multiple individual clones were sequenced to identify the presence and patterns of methylated cytosine residues within HPV16 DNA.
RESULTS
Effect of cytosine methylation at CpG dinucleotides on the activation of transcription by papillomavirus E2 proteins.
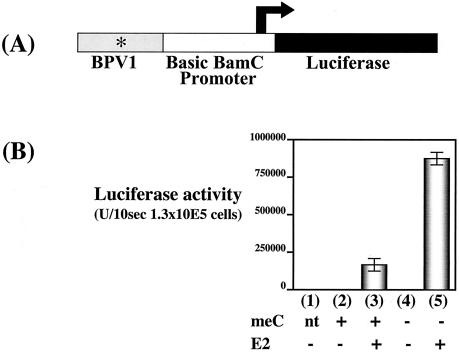
The papillomavirus E2 protein activates transcription in a DNA sequence-specific manner that requires E2BSs present in cis to the transcriptional promoter. These E2BSs are potential sites for 5′methylation at cytosine residues within CpG dinucleotides (Fig. 1). The HPV16 E2 protein's capacity in vitro to bind E2BSs is inhibited by methylation of cytosines within the E2BSs (59). Given E2's dependence on its ability to bind E2BSs to regulate viral transcription, we investigated the influence of CpG methylation on E2-dependent transcription in cells. Using the CpG methylase SssI methyltransferase, we globally methylated cytosines at CpG dinucleotides in the bacterially synthesized, E2-responsive transcriptional reporter plasmid pKT267 in vitro. This E2-responsive transcriptional reporter plasmid (pKT267) contains the HPV16 LCR positioned upstream of the minimal EBV BamC promoter, which in turn directs the transcription of the firefly luciferase gene. The HPV16 LCR contains four E2 binding sites that mediate E2-dependent transcriptional regulation as well as DNA binding sites for various cellular transcriptional regulatory factors that control cell type-specific expression of the papillomavirus genes (19, 54, 57, 63). In these experiments as well as those reported in Fig. 3 and 4, we verified the completeness of in vitro methylation of pKT267 by assessing the complete resistance of the methylated DNA to digestion with the restriction enzyme BstU1 (data not shown). The ability of E2 to activate transcription of the luciferase gene present in methylated or unmethylated pKT267 was monitored in short-term transfection experiments with the HPV-negative cervical epithelial cell line C33A (Fig. 2). The HPV16 E2 protein was provided in trans via the plasmid pCMV4E2. Unmethylated pKT267 supported a sevenfold induction of luciferase expression by HPV16 E2. In contrast, the methylated pKT267 transcription template supported less than a twofold induction of luciferase by HPV16 E2. This result indicates that methylation at CpG dinucleotides in an E2-responsive transcriptional template represses HPV16 E2-dependent transcription. In addition, we noted less than a twofold effect of methylation on the transcriptional activity of the luciferase reporter plasmid in the absence of E2, confirming that methylation of the basic BamC C promoter, which is missing two methylation-hypersensitive regions that mapped near this promoter, minimally affects its activity upon its introduction into cells by transfection (48a).
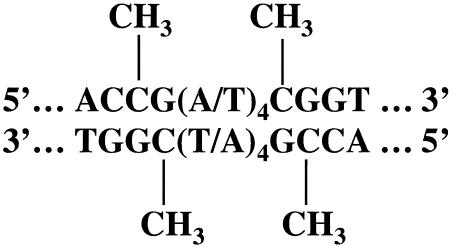
FIG. 1.
E2BS and potential sites of cytosine methylation. Shown is the schematic location of the potential CpG methylation sites on E2BS. The HPV16 genome contains four E2BSs in LCR, located at nt 7451 to 7462 (E2BS1), 7858 to 7869 (E2BS2), 35 to 46 (E2BS3), and 50 to 61(E2BS4).
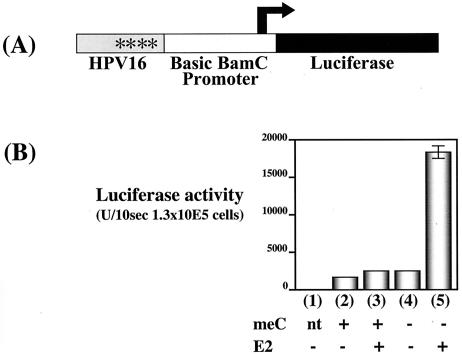
FIG. 3.
Effect of the global CpG methylation on BPV1 E2-dependent transcriptional activation. (A) Schematic of BPV1 LCR, the BamC promoter, and luciferase reporter plasmid pKT260, illustrating the relative positions of the BPV1 sequences, the basic BamC promoter from EBV, and the firefly luciferase translational ORF. Asterisk, relative position of the E2BS. (B) Bar graph summarizing the luciferase activities measured in lysates from cell populations transfected with methylated or unmethylated pKT260. Eight micrograms of the plasmid pKT260 that had or had not been treated in vitro with CpG methylase was transfected into C33A cells by electroporation in the presence or absence of the BPV1 E2 expression vector pCGE2 (10 μg). Luciferase activities were measured 48 h posttransfection. All experiments were repeated at least three times. Bars (error bars, standard deviations): 1, no DNA transfection control; 2, methylated pKT260 alone; 3 methylated pKT260 plus pCGE2; 4, unmethylated pKT260 alone; 5, unmethylated pKT260 plus pCGE2.
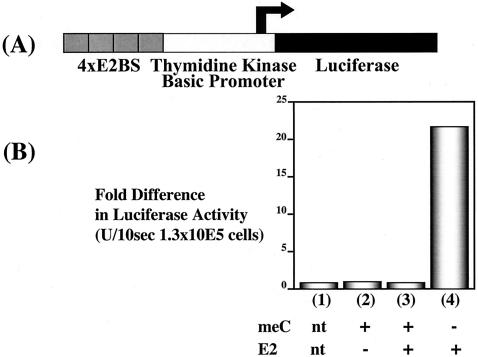
FIG. 4.
Effect of the global CpG methylation on HPV16 E2-dependent transcriptional activation. (A) Schematic of four E2BSs, the basic BamC promoter, and luciferase reporter plasmid pBS1073, illustrating the relative positions of the four E2BSs, the basic BamC promoter from EBV, and the firefly luciferase translational ORF. (B) Bar graph summarizing the luciferase activities measured in lysates from cell populations transfected with methylated or unmethylated pBS1073. Eight micrograms of the plasmid pBS1073 that had or had not been treated in vitro with CpG methylase was transfected into 293 cells by calcium phosphate precipitation in the presence or absence of the HPV16 E2 expression vector (5 μg) pCMV4E2. Luciferase activities were measured 48 h posttransfection. All experiments were repeated at least three times. Bars: 1, no DNA transfection control; 2, methylated pBS1073 alone; 3, methylated pBS1073 plus pCMV4E2; 4, unmethylated pBS1073 alone; 5, unmethylated pBS1073 plus pCMV4E2.
FIG. 2.
Effect of global CpG methylation on HPV16 E2-dependent transcriptional activation. (A) Schematic of HPV16 LCR, BamC promoter, and luciferase reporter plasmid pKT267, illustrating the relative positions of the HPV16 LCR, the basic BamC promoter from EBV, and the firefly luciferase translational ORF. Asterisks, relative positions of E2BSs. (B) Bar graph summarizing the luciferase activities measured in lysates from cell populations transfected with methylated or unmethylated pKT267. Three micrograms of the plasmid pKT267 that had or had not been treated in vitro with CpG methylase was transfected into C33A cells by electroporation in the presence or absence of 9 μg of the HPV16 E2 expression vector pCMV4E2. Luciferase activities were measured 48 h posttransfection. All experiments were repeated at least three times. Bars: 1, no DNA transfection control; 2 methylated pKT267 alone; 3 methylated pKT267 plus pCMV4E2; 4 unmethylated pKT267 alone; 5 unmethylated pKT267 plus pCMV4E2 (error bar, standard deviation).
To assess whether the ability of another E2 protein, that of BPV1, to activate transcription is likewise influenced by methylation of its cognate binding sites, we cloned a portion of the BPV1 LCR (nt 7914 to 27) containing one of the E2BSs with highest affinity (35) into the above-described BamC promoter-driven luciferase reporter plasmid (pBS1033) to generate the plasmid pKT260. Methylated and unmethylated pKT260 DNAs were transfected into C33A cells with or without a BPV1 E2 expression plasmid (pCGE2), and luciferase activity at 48 h posttransfection was measured. There was a 320-fold induction of luciferase expression from the unmethylated pKT260 template DNA by BPV1 E2 but only a 44-fold induction from the methylated pKT260 template (Fig. 3). Thus the abilities of both HPV16 and BPV1 E2 proteins to activate transcription are impaired by global methylation of cytosines at CpG dinucleotides on E2-responsive transcriptional templates.
The LCR regions from HPV16 and BPV1 present in pKT267 and pKT260, respectively, contain not only E2BSs but also potential binding sites for other transcription factors. Furthermore, we observed some small effect of methylation (less than twofold) on the basal transcription activity of both pKT267 and pKT260 in the absence of HPV16 or BPV1 E2. This could reflect an influence of methylation on transcriptional regulation mediated by other papillomavirus DNA sequences within the LCR-derived sequences or the minimal BamC promoter itself. Therefore, we monitored the influence of global methylation in the context of a third luciferase reporter plasmid, pBS1073, which contains four copies of a consensus E2BS positioned upstream of a minimal herpes simplex virus tk promoter driving transcription of the firefly luciferase gene. Methylated or unmethylated pBS1073 was transfected with or without the HPV16 E2 expression plasmid into the human kidney epithelial 293 cell line, and luciferase expression at 48 h posttransfection was measured (Fig. 4). HPV16 E2 produced a 20-fold induction of luciferase expression from the unmethylated pBS1073. In contrast there was no measurable induction by E2 of luciferase expression from the methylated pBS1073 template. The ability of E2 to induce luciferase expression was largely dependent on the presence of the E2BS in cis as there was less than a twofold induction of luciferase expression from the unmethylated or methylated parental control template, pBS1013, which does not contain the four E2BSs (data not shown). This set of experiments indicated that the only papillomavirus sequences required to see an influence of methylation on E2 function were the E2BSs.
Effect of methylation of only E2BS within a transcriptional template on the activation of transcription by E2.
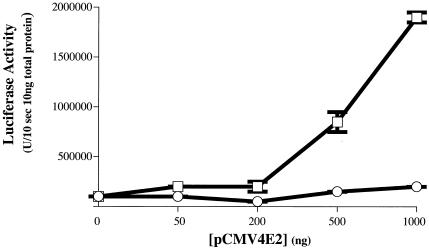
The effects of the DNA methylation monitored in the experiments described above (Fig. 2 to 4) were based on the global methylation of transcriptional templates through the in vitro treatment of DNA with a CpG methylase. In these experiments, methylation at cytosines within CpG dinucleotides elsewhere in the transcriptional template than at E2BSs could influence the transcriptional competence of the template DNA. To address whether the methylation status of E2BS alone influences E2's transcription activity, we generated transcriptional templates selectively methylated at E2BSs. This was achieved by ligating a double-stranded DNA oligonucleotide that contained either a methylated or unmethylated E2BS into the luciferase reporter plasmid pGL2-promoter containing the minimal (enhancerless) simian virus 40 (SV40) early promoter driving expression of the firefly luciferase gene. A fraction of each ligation was transformed into E. coli, and 20 independent clones from each ligation were sequenced. From this analysis we determined that, for both ligation reactions, the ligation products had on average 1.5 copies of the E2BS. Two micrograms of the ligation products was directly transfected into human kidney epithelial 293 cells with or without various amounts of the HPV16 E2 expression plasmid pCMV4E2 (Fig. 5). We observed a dose-dependent induction of luciferase activity by E2 from the pGL2-promoter-based transcription template generated with the unmethylated E2BS double-stranded oligonucleotide. However we saw little to no induction of luciferase by E2 in the cells that received the pGL2-promoter-based transcription template generated with the methylated E2BS double-stranded oligonucleotide. This result demonstrates that E2's ability to activate transcription is influenced specifically by the methylation status of its cognate E2BS.
FIG. 5.
Effect of E2BS-specific methylation on E2-dependent transcription. Shown are the luciferase activities measured in lysates of cells transfected with a luciferase reporter plasmid containing methylated or unmethylated E2BS. In vitro-methylated and unmethylated E2BS double-stranded oligonucleotides were ligated to the linearized form of the luciferase reporter plasmid pGL2-promoter (Promega), which contains the enhancerless SV40 early promoter directing transcription of the firefly luciferase gene. Two micrograms of the ligated plasmid containing either methylated (circles) or unmethylated (squares) E2BSs (ligated plasmids contained on average 1.5 copies of the methylated or unmethylated oligonucleotide based on sequence analysis of multiple bacterial transformants of each ligation reaction [see Materials and Methods and Results]) was transfected with Fugene into 293 cells with the indicated amounts of the HPV16 E2 expression plasmid pCMV4E2. Luciferase activities were measured in 48 h.
Papillomavirus DNA methylation in cervical epithelial cells harboring extrachromosomal HPV16 genomes.
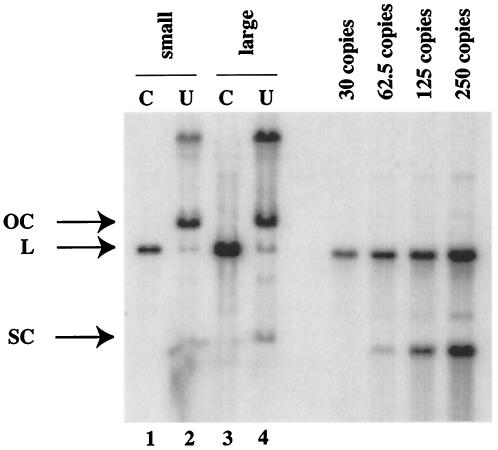
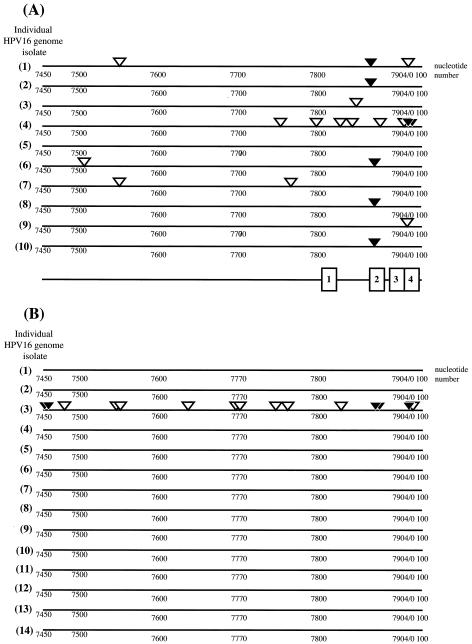
The HPV life cycle is tied to the differentiation of the host epithelial cell with the productive stage of the viral life cycle, which is characterized by the amplification of the viral genome and its encapsidation into viral capsids, arising in cells undergoing terminal differentiation (7). The activities of DNA methylases can be influenced by the differentiation state of the cell (1, 12, 44, 55, 64). To investigate the methylation status of the HPV16 genome during the viral life cycle, we analyzed a clone of cervical epithelial cells, W12E, isolated from a low-grade, intraepithelial lesion surgically removed from the cervix of a women infected with HPV16. This cell line stably harbors the HPV16 genome as a nuclear plasmid (27). W12E cells were grown on fibroblast feeder cells, which allow for the maintenance of epithelial cells in a poorly differentiated, basal cell-like state. In these cultures, however, a small percentage of the epithelial cells within confluent portions of the epithelial cell colonies spontaneously undergo differentiation. Chris Fisher and colleagues subjected these W12E cell populations to centrifugal elutriation to separate cells by the size. The majority of the epithelial cells, being poorly differentiated and basal cell-like in character, were small. Less abundant, larger epithelial cells were also isolated; these cells displayed a differentiated character, as evidenced by increased abundance of keratin 10 and involucrin proteins (22), markers for keratinocyte differentiation, and harbored amplified copies of viral DNA based on real-time PCR (see Fig. 2 of reference 22) as well as Southern analysis (Fig. 6). We analyzed the low-molecular-weight DNAs isolated from these different pooled elutriated fractions of W12E cells containing small, undifferentiated or large, differentiated cells for the methylation status of the LCR of HPV16 using bisulfite treatment (20). We focused on the 3′ region of the LCR that contains all four E2BSs. The bisulfite analysis involves bisulfite-induced modification of target DNA under chemically reactive conditions in which cytosine is converted to uracil but methylcytosine is nonreactive. Following amplification of target DNA by PCR, all modified uracil residues are amplified as thymidine and only methylcytosine residues are amplified as cytosine. The PCR products are then cloned into a bacterial vector, and plasmid DNAs are isolated from multiple independent bacterial transformants sequenced to identify the location of methylated cytosine on the sequence of interest. To assess completion of the bisulfite reaction, we first carried out the bisulfite reaction on a bacterially synthesized recombinant clone of the HPV16 genome isolated from W12 cells by analyzing a region of the HPV16 genome (nt 6833 to 6921) that contains two dcm E. coli methylation target sites (data not shown). In five of the six independent amplimer clones analyzed, the cytosine residues within both dcm recognition sites were determined to be methylated, as judged by their resistance to bisulfite-mediated conversion to uracils. This was expected given that the E. coli strain used to produce the recombinant HPV16 plasmid was dcm positive. For the sixth amplimer analyzed one of the two dcm sites was found to be methylated but the second was unmethylated, likely reflecting the incomplete efficiency of E. coli dcm methylation (48). Importantly, all other cytosines (n = 44) in the region of the bacterially synthesized HPV16 plasmids that was analyzed were found to be unmethylated (and therefore converted to thymidine in the amplimer) in all six amplimers analyzed. These results demonstrated that the conditions for the bisulfite reaction were suitably complete to discriminate between methylated and unmethylated cytosines. We next analyzed the methylation status of the LCR of HPV16 DNAs isolated from the elutriated fractions of W12E cells that were enriched for small, poorly differentiated cells. Of the 10 amplimer clones analyzed, all contained methylated cytosines within the LCR (Fig. 7A). Six of the 10 clones contained methylated cytosines within one or more E2BSs. Thus, in poorly differentiated cells, cytosine methylation within the LCR is common, with E2BS2 being the most frequently methylated E2BS. A strikingly different result was observed with HPV16 DNAs isolated from the more highly differentiated, large-size cells. In 13 of the 14 amplimer clones generated from HPV16 DNA isolated from the elutriated fraction enriched for differentiated cells, no cytosines within the LCR, including all E2BSs, were methylated (Fig. 7B). The one amplimer clone from this large-cell fraction that displayed evidence for methylation was heavily methylated, with five cytosine nucleotides in three of the E2BSs methylated along with 10 additional sites of methylation at residues outside of the E2BSs in the LCR. We also found an absence of any methylation within a region of the L1 ORF (nt 6833 to 6921) upstream of the LCR in 12 independent amplimer clones of HPV16 DNA isolated from the large-cell fraction (data not shown). These data indicate that the methylation state of the viral genome varies depending on the differentiation state of the cell.
FIG. 6.
Amplification of HPV16 DNA in large-cell fractions of elutriated W12E cells. Shown is a phosphorimager-derived image from an HPV16-specific Southern analysis of the DNA samples from pools of small-cell or large-cell fractions of elutriated W12E cells that were used in the bisulfite analysis (Fig. 7). Cell equivalents (106) of DNAs from the pooled small-cell (lanes 1 and 2) and large-cell (lanes 3 and 4) fractions that were (lanes 1 and 3) or were not (lanes 2 and 4) digested with BamHI to linearize the viral genome were electrophoresed in 0.8% agarose, transferred to nitrocellulose, and subjected to HPV16-specific hybridization. Shown at the right are copy number standards generated with an HPV16 recombinant plasmid cleaved with BamHI to release the full-length HPV16 genome from the bacterial vector. This plasmid was used as the probe for this Southern analysis. SC, supercoiled; L, linear; OC, relaxed open circular. Based on quantitative analysis of the phosphorimage, the pooled large-cell fraction harbored an eightfold higher level of viral DNA per cell than the small-cell fraction.
FIG. 7.
Differential cytosine methylation patterns in small-cell versus large-cell fractions of W12E cells. Shown are locations of methylated cytosines within (solid arrowheads) and outside (open arrowheads) E2BSs in individually cloned and sequenced amplimers of bisulfite-treated HPV16 DNAs isolated from small-cell (A) and large-cell (B) fractions of elutriated W12E cells. The line drawing at the bottom of panel A shows approximate locations of the four E2BSs: E2BS1 (1), E2BS2 (2), E2BS3 (3), and E2BS4 (4). For exact nucleotide positions of E2BSs, see the Fig. 1 legend. Nucleotide positions of methylated cytosines in individual amplimers are provided in Table 1.
DISCUSSION
In this study we present evidence that the global cytosine methylation at CpG dinucleotides in E2-responsive transcriptional templates or the specific cytosine methyation of CpG dinucleotides in E2BSs inhibits transcriptional activation by either BPV1 or HPV16 E2 proteins. Our results prove correct the hypothesis put forth by Thain and colleagues (59) that methylation of E2BSs influences E2's ability to carry out those biological functions dependent on its binding to E2BSs. Given our results, it is reasonable to predict that other biological functions of E2 that rely on binding to E2BSs, such as E1-dependent replication and BPV-1 plasmid DNA maintenance, will be likewise influenced by the methylation status of relevant E2BSs. It also raises the possibility that the methylation status at E2BSs might modulate the biological activities of the other E2 ORF gene products, including E2TR (E2C) and E8/E2TR (E8/E2), which inhibit E2 (30-32), arguably through competitive DNA binding to the E2BS or by formation of heterodimers (4, 37), and which can also inhibit basal (i.e., E2-independent) transcription from viral promoters (53, 60).
Our data demonstrating the influence of CpG methylation on E2 function led us to address the question of whether DNA methylation plays a role in regulating E2 functions in the viral life cycle. We found that the methylation state of the viral genome varies depending on the differentiation state of the host epithelial cell, with a comparatively high degree of methylation at E2BSs and elsewhere in the HPV16 LCR seen in the less well differentiated cervical epithelial cells. In contrast, we primarily observed a hypomethylated state for the LCRs of viral genomes isolated from more highly differentiated cells that supported viral DNA amplification. Thus the methylation status of the HPV16 genome varies with the differentiation state of the host cell, raising the possibility that there exists a cause-and-effect relationship between viral DNA methylation and one or more functional processes during the viral life cycle.
Different E2BSs have been implicated in mediating different biological activities of E2, including transcriptional activation, transcriptional repression, and E1-dependent replication. Three E2BSs, E2BS2 to E2BS4, mediate E2's ability to repress the promoter (P107 in HPV18 and P97 in HPV16) positioned just upstream of the E6 ORF in mucosotropic HPVs (14, 16, 46, 49, 60). This repression is thought to result from E2's ability, upon binding to E2BS4, to occlude TATA box binding protein from binding to the adjoining TATA box within the HPV18 P107 and the HPV16 P97 promoters (17). E2BS3 is thought to mediate E2's repression of the HPV18 P107 and HPV16 P97 promoter by competing with the cellular transcription factor SP1, which binds to a site overlapping E2BS3 (14, 56). It is not understood mechanistically how E2's binding to E2BS2 leads to repression. These same three E2BSs, E2BS2 to E2BS4, also can contribute to E1-dependent replication (15), with E2BS3 being sufficient to support E2's ability to recruit the viral E1 DNA helicase to the viral DNA genome (50, 67). E2BS1 can mediate E2's activation of transcription from the promoter positioned just upstream of E6, especially under conditions where the other E2BSs are disrupted (14). The highly conserved nature of all four E2BSs within the mucosotropic HPVs leads to the prediction that each site plays a critical role in the life cycle.
In our study we found that E2BS2 was the site most likely to be methylated in less well differentiated W12E cells, with methylation at both E2BS3 and E2BS4 seen in one case in which E2BS2 was not methylated. Interestingly, all three of these E2BSs have been implicated in E2-mediated repression. The reduced affinity of E2 for these sites when methylated would therefore be predicted to lead to a more highly active transcriptional state that is not susceptible to E2-mediated repression. However, were these same methylation sites at the E2BSs as well as methylation sites elsewhere in the LCR also to lead to the recruitment of HDACs and induction of a more tightly packed chromatin state, the overall consequence on transcription may be the opposite: a transcriptional silencing. This is consistent with our observations that, in these W12E cells, levels of early viral gene expression are highly attenuated (27) and is supported by the recent observation by Bechtold and colleagues, based on the use of trichostatin A, that the HPV16 genome is normally in a highly silent transcriptional state in W12E cells (6). The frequent methylation of E2BS2 and less frequent methylation of E2BS3 and E2BS4, the sites that mediate E2 repression, may also account in part for why Bechtold and colleagues failed to see repression by E2 in W12E cells (6) and why disruption, in particular of E2BS2 in HPV31, leads to no observable phenotype (52).
Levels of viral transcription increase as the host cells undergo differentiation, and this increase is thought to contribute to the productive stage of the viral life cycle. We found that, in the less well differentiated cells, the overall methylation status of the LCR was greater than that seen in the more highly differentiated cells. Cytosine methylation can inhibit gene expression either by direct interference of transcription factors with their recognition sequences, e.g., E2, or, via interactions with methyl-CpG-binding proteins and HDACs, by leading to the formation of condensed chromatin, which is less accessible to the cellular transcriptional apparatus (3, 28, 39, 40). Therefore, methylcytosine-enriched regions in the viral genome could lead to transcriptional silencing. DNA methylation may also influence mammalian DNA replication. Two replication origins, one in hamster cells (ori-RPS14) and one in human cells (ori-dnmt1C1), have been identified; these sites are associated with dense clusters of methylated CpG dinucleotides. A 500-bp region near the Chinese hamster dihydrofolate reductase ori locus is densely methylated, but only when cells are actively replicating their DNAs (47, 58). The hypomethylated state of the viral genome found in the more well differentiated cells, alternatively, could lead to formation of a more acetylated, more open chromatin state, which is conducive to transcription and perhaps also to DNA replication. Thus the hypomethylated state may contribute both to transcription from the late-specific viral promoter and vegetative amplification of viral DNA. We also infer from our findings that viral genomes encapsidated in progeny virus in the highly differentiated cells are hypomethylated and therefore in a chromatin state that would be more permissive for viral transcription and DNA amplification upon initial infection of the host cell. This prediction may explain why, early in infection, HPV31 E1^E4 spliced mRNAs, which can arise from either the early or late promoter, are expressed transiently to high levels (42).
De novo methylation of DNA is thought to occur primarily in stem cells early in development and to contribute to global epigenetic reprogramming of gene expression (34, 45). De novo methylation is mediated by the DNA methylases DNMT3a and DNMT3b. Unlike DNMT1, which methylates newly replicated hemimethylated DNA, the DNMT3a and -3b enzymes methylate DNA de novo not only at CpG dinucleotides but also at CpA and to a lesser extent at CpT dinucleotides. Our methylation analysis of the HPV16 genomes in W12E cells reveals a pattern of methylation at cytosine residues that is indicative of de novo methylation. We found frequent methylation of cytosines at CpA as well as CpG dinucleotides and infrequent methylation at CpT dinucleotides (Table 1). The relative frequencies of methylation at cytosines in non-CpG dinucleotides in the HPV16 LCR in W12E cells (CpA > CpT > CpC) are consistent with the general preference for non-CpG methylation observed in stem cells, where de novo methylation on cellular DNA is found. We infer from the W12E-derived HPV16 methylation pattern that HPVs are subjected to de novo cytosine methylation. This has interesting implications both for the host and the virus. De novo cytosine methylation has been invoked as a host cellular-defense mechanism that is designed to suppress transcription from genomes of invading viral pathogens and parasitic DNAs. Patterns of methylation indicative of de novo methylation have been seen in endogenous retroviral and line elements as well as SV40 and adenovirus DNAs in virally transformed cell lines (25). Furthermore, increases were found in the methylation of EBV genomes immortalized B cells as those cells were passaged, and this immortalization was found to correlate with the extinction of viral transcription (33, 43). The de novo methylation of HPV16 in W12E cells may provide evidence that cervical epithelial cells invoke a host defense mechanism to suppress transcription of papillomavirus genomes. This may also explain in part why we previously found viral gene expression in W12E cells to be extremely low, despite the fact that this clonal population of cells (when not fractionated by elutriation) harbors 500 to 1,000 copies of the HPV16 genome (26). In contrast, in clones of W12 cells in which the viral DNA is integrated as a concatemer at low copy numbers ranging from several to approximately 50 copies/cell (W12I), viral gene expression is greatly enhanced, with mRNAs arising preferentially from the junctional copy of the viral genome at the site of integration (27). We previously described evidence indicating that this increased abundance of viral transcripts in W12I cells is at least partly due to differences in mRNA half lives (27). However, we also noted that the severalfold difference in mRNA half lives between W12E and W12I clones likely cannot account completely for the increased abundance of viral mRNAs in the W12I clones, which were calculated to be in the range of 2 orders of magnitude greater per viral genome copy than that in the W12E clones. It is intriguing to speculate that changes in DNA methylation patterns at junctional copies of the HPV genomes following viral DNA integration could contribute to the increased expression of viral mRNAs from these viral templates. Consistent with this possibility, Badal and associates have recently assessed the methylation patterns in cervical lesions from patients infected with HPVs and found there to be a progressive lessening in the degree of CpG methylation as lesions progressed to frank cervical cancer (2).
TABLE 1.
Nucleotide positions of methylated cytosines in individual amplimer clones of the HPV16 LCR from W12E cells
| Sample no.a | Nucleotide position(s) of methylated cytosine (dinucleotide type)b |
|---|---|
| Small-cell DNA | |
| A 1 | 7550 (CpA), 7860 (CpG), 7861 (CpG), 31 (CpG), 32 (CpG) |
| A 2 | 7860 (CpG), 7861 (CpG) |
| A 3 | 7846 (CpA) |
| A 4 | 7751 (CpT), 7802 (CpA), 7824 (CpA), 7832 (CpA), 7883 (CpT), 43 (CpG), 44 (CpG), 52 (CpG), 53 (CpG), 58 (CpG), 59 (CpG) |
| A 6 | 7507 (CpA), 7866 (CpA) |
| A 7 | 7551-2 (CpG), 7778 (CpA) |
| A 8 | 7866 (CpA) |
| A 9 | 31 (CpG), 32 (CpG) |
| A 10 | 7866 (CpA) |
| Large-cell DNA | |
| B 3 | 7453 (CpG), 7454 (CpG), 7459 (CpG), 7460 (CpG), 7475 (CpA), 7545 (CpA), 7554 (CpA), 7632 (CpA), 7696 (CpA), 7718 (CpA), 7744 (CpA), 7766 (CpT), 7822 (CpA), 7860 (CpG), 7861 (CpG) 7866 (CpA), 43 (CpG), 44 (CpG), 48 (CpA) |
Indicated are the sample numbers from Fig. 7A and B of the sequenced amplimer clones obtained from bisulfite-treated DNAs isolated from small- (A) or large-cell (B) fractions of elutriated W12E cells.
Acknowledgments
We thank Toni Gahn for providing us pBS1073 and Chen-Yu Wang for contributing to the experiments described in Fig. 4. We thank Bill Sugden for critical reading of this manuscript and Uli Bernard for sharing his manuscript prior to publication.
This study was supported by grants from the National Institutes of Health (CA22443 and CA14520).
REFERENCES
- 1.Aguirre-Arteta, A. M., I. Grunewald, M. C. Cardoso, and H. Leonhardt. 2000. Expression of an alternative Dnmt1 isoform during muscle differentiation. Cell Growth Differ. 11:551-559. [PubMed] [Google Scholar]
- 2.Badal, V., L. S. H. Chuang, E. H.-H. Tan, S. Badal, L. L. Villa, C. M. Wheeler, B. F. L. Li, and H.-U. Bernard. 2003. CpG methylation of human papillomavirus type 16 DNA in cervical cancer cell lines and in clinical specimens: genomic hypomethylation correlates with carcinogenic progression. J. Virol. 77:6227-6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballestar, E., and A. P. Wolffe. 2001. Methyl-CpG-binding proteins. Targeting specific gene repression. Eur. J. Biochem. 268:1-6. [DOI] [PubMed] [Google Scholar]
- 4.Barsoum, J., S. S. Prakash, P. Han, and E. J. Androphy. 1992. Mechanism of action of the papillomavirus E2 repressor: repression in the absence of DNA binding. J. Virol. 66:3941-3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastien, N., and A. A. McBride. 2000. Interaction of the papillomavirus E2 protein with mitotic chromosomes. Virology 270:124-134. [DOI] [PubMed] [Google Scholar]
- 6.Bechtold, V., P. Beard, and K. Raj. 2003. Human papillomavirus type 16 E2 has no effect on transcription from episomal viral DNA. J. Virol. 77:2021-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bedell, M. A., J. B. Hudson, T. R. Golub, M. E. Turyk, M. Hosken, G. D. Wilbanks, and L. A. Laimins. 1991. Amplification of human papillomavirus genomes in vitro is dependent on epithelial differentiation. J. Virol. 65:2254-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouvard, V., A. Storey, D. Pim, and L. Banks. 1994. Characterization of the human papillomavirus E2 protein: evidence of trans-activation and trans-repression in cervical keratinocytes. EMBO J. 13:5451-5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang, C. M., M. Ustav, A. Stenlund, T. F. Ho, T. R. Broker, and L. T. Chow. 1992. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc. Natl. Acad. Sci. USA 89:5799-5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chuang, L. S., H. I. Ian, T. W. Koh, H. H. Ng, G. Xu, and B. F. Li. 1997. Human DNA-(cytosine-5) methyltransferase-PCNA complex as a target for p21WAF1. Science 277:1996-2000. [DOI] [PubMed] [Google Scholar]
- 11.Clark, S. J., J. Harrison, C. L. Paul, and M. Frommer. 1994. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 22:2990-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Creusot, F., G. Acs, and J. K. Christman. 1982. Inhibition of DNA methyltransferase and induction of Friend erythroleukemia cell differentiation by 5-azacytidine and 5-aza-2′-deoxycytidine. J. Biol. Chem. 257:2041-2048. [PubMed] [Google Scholar]
- 13.Del Vecchio, A. M., H. Romanczuk, P. M. Howley, and C. C. Baker. 1992. Transient replication of human papillomavirus DNAs. J. Virol. 66:5949-5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demeret, C., C. Desaintes, M. Yaniv, and F. Thierry. 1997. Different mechanisms contribute to the E2-mediated transcriptional repression of human papillomavirus type 18 viral oncogenes. J. Virol. 71:9343-9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demeret, C., M. M. Le, M. Yaniv, and F. Thierry. 1995. Control of HPV 18 DNA replication by cellular and viral transcription factors. Nucleic Acids Res. 23:4777-4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dong, G., T. R. Broker, and L. T. Chow. 1994. Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J. Virol. 68:1115-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dostatni, N., P. F. Lambert, R. Sousa, J. Ham, P. M. Howley, and M. Yaniv. 1991. The functional BPV-1 E2 trans-activating protein can act as a repressor by preventing formation of the initiation complex. Genes Dev. 5:1657-1671. [DOI] [PubMed] [Google Scholar]
- 18.Flores, E. R., B. L. Allen-Hoffmann, D. Lee, C. A. Sattler, and P. F. Lambert. 1999. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte cell line. Virology 262:344-354. [DOI] [PubMed] [Google Scholar]
- 19.Fontaine, V., E. van der Meijden, J. de Graaf, J. ter Schegget, and L. Struyk. 2000. A functional NF-κB binding site in the human papillomavirus type 16 long control region. Virology 272:40-49. [DOI] [PubMed] [Google Scholar]
- 20.Frommer, M., L. E. McDonald, D. S. Millar, C. M. Collis, F. Watt, G. W. Grigg, P. L. Molloy, and C. L. Paul. 1992. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA 89:1827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fuks, F., W. A. Burgers, A. Brehm, L. Hughes-Davies, and T. Kouzarides. 2000. DNA methyltransferase Dnmt1 associates with histone deacetylase activity. Nat. Genet. 24:88-91. [DOI] [PubMed] [Google Scholar]
- 22.Garner-Hamrick, P. A., and C. Fisher. 2002. HPV episomal copy number closely correlates with cell size in keratinocyte monolayer cultures. Virology 301:334-341. [DOI] [PubMed] [Google Scholar]
- 23.Graham, F. L., and A. J. van der Eb. 1973. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 52:456-467. [DOI] [PubMed] [Google Scholar]
- 24.Hegde, R. S. 2002. The papillomavirus E2 proteins: structure, function, and biology. Annu. Rev. Biophys. Biomol. Struct. 31:343-360. [DOI] [PubMed] [Google Scholar]
- 25.Heller, H., C. Kammer, P. Wilgenbus, and W. Doerfler. 1995. Chromosomal insertion of foreign (adenovirus type 12, plasmid, or bacteriophage lambda) DNA is associated with enhanced methylation of cellular DNA segments. Proc. Natl. Acad. Sci. USA 92:5515-5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon, S., B. L. Allen-Hoffmann, and P. F. Lambert. 1995. Integration of human papillomavirus type 16 into the human genome correlates with a selective growth advantage of cells. J. Virol. 69:2989-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon, S., and P. F. Lambert. 1995. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc. Natl. Acad. Sci. USA 92:1654-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, P. L., G. J. Veenstra, P. A. Wade, D. Vermaak, S. U. Kass, N. Landsberger, J. Strouboulis, and A. P. Wolffe. 1998. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat. Genet. 19:187-191. [DOI] [PubMed] [Google Scholar]
- 29.Kirchmaier, A. L., and B. Sugden. 1997. Dominant-negative inhibitors of EBNA-1 of Epstein-Barr virus. J. Virol. 71:1766-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert, P. F., N. L. Hubbert, P. M. Howley, and J. T. Schiller. 1989. Genetic assignment of multiple E2 gene products in bovine papillomavirus-transformed cells. J. Virol. 63:3151-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert, P. F., B. C. Monk, and P. M. Howley. 1990. Phenotypic analysis of bovine papillomavirus type 1 E2 repressor mutants. J. Virol. 64:950-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert, P. F., B. A. Spalholz, and P. M. Howley. 1987. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell 50:69-78. [DOI] [PubMed] [Google Scholar]
- 33.Lewin, N., J. Minarovits, G. Weber, B. Ehlin-Henriksson, T. Wen, H. Mellstedt, G. Klein, and E. Klein. 1991. Clonality and methylation status of the Epstein-Barr virus (EBV) genomes in in vivo-infected EBV-carrying chronic lymphocytic leukemia (CLL) cell lines. Int. J. Cancer 48:62-66. [DOI] [PubMed] [Google Scholar]
- 34.Li, E., C. Beard, and R. Jaenisch. 1993. Role for DNA methylation in genomic imprinting. Nature 366:362-365. [DOI] [PubMed] [Google Scholar]
- 35.Li, R., J. Knight, G. Bream, A. Stenlund, and M. Botchan. 1989. Specific recognition nucleotides and their DNA context determine the affinity of E2 protein for 17 binding sites in the BPV-1 genome. Genes Dev. 3:510-526. [DOI] [PubMed] [Google Scholar]
- 36.Mansky, K. C., A. Batiza, and P. F. Lambert. 1997. Bovine papillomavirus type 1 E1 and simian virus 40 large T antigen share regions of sequence similarity required for multiple functions. J. Virol. 71:7600-7608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McBride, A. A., J. C. Byrne, and P. M. Howley. 1989. E2 polypeptides encoded by bovine papillomavirus type 1 form dimers through the common carboxyl-terminal domain: transactivation is mediated by the conserved amino-terminal domain. Proc. Natl. Acad. Sci. USA 86:510-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Middleton, T., and B. Sugden. 1992. A chimera of EBNA1 and the estrogen receptor activates transcription but not replication. J. Virol. 66:1795-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nan, X., H. H. Ng, C. A. Johnson, C. D. Laherty, B. M. Turner, R. N. Eisenman, and A. Bird. 1998. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393:386-389. [DOI] [PubMed] [Google Scholar]
- 40.Nan, X., P. Tate, E. Li, and A. Bird. 1996. DNA methylation specifies chromosomal localization of MeCP2. Mol. Cell. Biol. 16:414-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okano, M., D. W. Bell, D. A. Haber, and E. Li. 1999. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell 99:247-257. [DOI] [PubMed] [Google Scholar]
- 42.Ozbun, M. A. 2002. Human papillomavirus type 31b infection of human keratinocytes and the onset of early transcription. J. Virol. 76:11291-11300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulson, E. J., J. D. Fingeroth, J. L. Yates, and S. H. Speck. 2002. Methylation of the EBV genome and establishment of restricted latency in low-passage EBV-infected 293 epithelial cells. Virology 299:109-121. [DOI] [PubMed] [Google Scholar]
- 44.Persengiev, S. P., and D. L. Kilpatrick. 1996. Nerve growth factor induced differentiation of neuronal cells requires gene methylation. Neuroreport 8:227-231. [DOI] [PubMed] [Google Scholar]
- 45.Ramsahoye, B. H., D. Biniszkiewicz, F. Lyko, V. Clark, A. P. Bird, and R. Jaenisch. 2000. Non-CpG methylation is prevalent in embryonic stem cells and may be mediated by DNA methyltransferase 3a. Proc. Natl. Acad. Sci. USA 97:5237-5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rapp, B., A. Pawellek, F. Kraetzer, M. Schaefer, C. May, K. Purdie, K. Grassmann, and T. Iftner. 1997. Cell-type-specific separate regulation of the E6 and E7 promoters of human papillomavirus type 6a by the viral transcription factor E2. J. Virol. 71:6956-6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rein, T., H. Zorbas, and M. L. DePamphilis. 1997. Active mammalian replication origins are associated with a high-density cluster of mCpG dinucleotides. Mol. Cell. Biol. 17:416-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ringquist, S., and C. L. Smith. 1992. The Escherichia coli chromosome contains specific, unmethylated dam and dcm sites. Proc. Natl. Acad. Sci. USA 89:4539-4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a.Robertson, K. D., and R. F. Ambinder. 1997. Mapping promoter regions that are hypersensitive to methylation-mediated inhibition of transcription: application of the methylation cassette assay to the Epstein-Barr virus major latency promoter. J. Virol. 71:6445-6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Romanczuk, H., F. Thierry, and P. M. Howley. 1990. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J. Virol. 64:2849-2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarafi, T. R., and A. A. McBride. 1995. Domains of the BPV-1 E1 replication protein required for origin-specific DNA binding and interaction with the E2 transactivator. Virology 211:385-396. [DOI] [PubMed] [Google Scholar]
- 51.Simon, D., H. Stuhlmann, D. Jahner, H. Wagner, E. Werner, and R. Jaenisch. 1983. Retrovirus genomes methylated by mammalian but not bacterial methylase are non-infectious. Nature 304:275-277. [DOI] [PubMed] [Google Scholar]
- 52.Stubenrauch, F., H. B. Lim, and L. A. Laimins. 1998. Differential requirements for conserved E2 binding sites in the life cycle of oncogenic human papillomavirus type 31. J. Virol. 72:1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stubenrauch, F., T. Zobel, and T. Iftner. 2001. The E8 domain confers a novel long-distance transcriptional repression activity on the E8E2C protein of high-risk human papillomavirus type 31. J. Virol. 75:4139-4149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stunkel, W., Z. Huang, S. H. Tan, M. J. O'Connor, and H. U. Bernard. 2000. Nuclear matrix attachment regions of human papillomavirus type 16 repress or activate the E6 promoter, depending on the physical state of the viral DNA. J. Virol. 74:2489-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szyf, M., J. Rouleau, J. Theberge, and V. Bozovic. 1992. Induction of myogenic differentiation by an expression vector encoding the DNA methyltransferase cDNA sequence in the antisense orientation. J. Biol. Chem. 267:12831-12836. [PubMed] [Google Scholar]
- 56.Tan, S. H., L. E. Leong, P. A. Walker, and H. U. Bernard. 1994. The human papillomavirus type 16 E2 transcription factor binds with low cooperativity to two flanking sites and represses the E6 promoter through displacement of Sp1 and TFIID. J. Virol. 68:6411-6420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taniguchi, A., K. Kikuchi, K. Nagata, and S. Yasumoto. 1993. A cell-type-specific transcription enhancer of type 16 human papillomavirus (HPV 16)-P97 promoter is defined with HPV-associated cellular events in human epithelial cell lines. Virology 195:500-510. [DOI] [PubMed] [Google Scholar]
- 58.Tasheva, E. S., and D. J. Roufa. 1994. Densely methylated DNA islands in mammalian chromosomal replication origins. Mol. Cell. Biol. 14:5636-5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thain, A., O. Jenkins, A. R. Clarke, and K. Gaston. 1996. CpG methylation directly inhibits binding of the human papillomavirus type 16 E2 protein to specific DNA sequences. J. Virol. 70:7233-7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thierry, F., and P. M. Howley. 1991. Functional analysis of E2-mediated repression of the HPV18 P105 promoter. New Biol. 3:90-100. [PubMed] [Google Scholar]
- 61.Ustav, M., and A. Stenlund. 1991. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 10:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaillancourt, P., T. Nottoli, J. Choe, and M. R. Botchan. 1990. The E2 transactivator of bovine papillomavirus type 1 is expressed from multiple promoters. J. Virol. 64:3927-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Velazquez Torres, A., and P. Gariglio Vidal. 2002. Possible role of transcription factor AP1 in the tissue-specific regulation of human papillomavirus. Rev. Investig. Clin. 54:231-242. [PubMed] [Google Scholar]
- 64.Walsh, C. P., and T. H. Bestor. 1999. Cytosine methylation and mammalian development. Genes Dev. 13:26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wolffe, A. P., and M. A. Matzke. 1999. Epigenetics: regulation through repression. Science 286:481-486. [DOI] [PubMed] [Google Scholar]
- 66.Yang, L., R. Li, I. J. Mohr, R. Clark, and M. R. Botchan. 1991. Activation of BPV-1 replication in vitro by the transcription factor E2. Nature 353:628-632. [DOI] [PubMed] [Google Scholar]
- 67.Yasugi, T., J. D. Benson, H. Sakai, M. Vidal, and P. M. Howley. 1997. Mapping and characterization of the interaction domains of human papillomavirus type 16 E1 and E2 proteins. J. Virol. 71:891-899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.zur Hausen, H. 1991. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology 184:9-13. [DOI] [PubMed] [Google Scholar]