Abstract
Prolonged light exposure is a determinant factor in inducing neurodegeneration of photoreceptors by apoptosis. Yet, the molecular bases of the pathways and components triggering this cell death event are elusive. Here, we reveal a prominent age-dependent increase in the susceptibility of photoreceptor neurons to undergo apoptosis under light in a mouse model. This is accompanied by light-induced subcellular changes of photoreceptors, such as dilation of the disks at the tip of the outer segments, prominent vesiculation of nascent disks, and autophagy of mitochondria into large multilamellar bodies. Notably, haploinsufficiency of Ran-binding protein-2 (RanBP2) suppresses apoptosis and most facets of membrane dysgenesis observed with age upon light-elicited stress. RanBP2 haploinsufficiency promotes decreased levels of free fatty acids in the retina independent of light exposure and turns the mice refractory to weight gain on a high-fat diet, whereas light promotes an increase in hydrogen peroxide regardless of the genotype. These studies demonstrate the presence of age-dependent and RanBP2-mediated pathways modulating membrane biogenesis of the outer segments and light-elicited neurodegeneration of photoreceptors. Furthermore, the findings support a mechanism whereby the RanBP2-dependent production of free fatty acids, metabolites thereof or the modulation of a cofactor dependent on any of these, promote apoptosis of photoreceptors in concert with the light-stimulated production of reactive oxygen species.
Keywords: Ran-binding protein 2 (RanBP2), neuroprotection, light, free fatty acids, photoreceptor neurons, apoptosis
Introduction
The retina is comprised of a well-defined neurocircuitry mediating the capture, processing and transmission of photon stimuli to high-order processing centers in the brain. The primary neurons of the retina, rod and cone photoreceptors, mediate the physicochemical transduction of light. While several components of the light-transduction cascade promote the degeneration of photoreceptors upon inherited mutations in the cognate genes 1, light acts also as a powerful inducer of degeneration of these neurons in wild-type mouse strains.2 Neurodegeneration elicited by light and age appears to vary in multiple genetic backgrounds, thus supporting the presence of various genetic modifiers of cell death upon selective stressors.3–5
To this date, few loci conferring resistance to light damage have been identified in genetically altered mice. These include mice lacking the expression of Rpe65, Rho, and mice harboring the Rpe65 Leu450Met mutation.5–9 Although some of these loci appear to have no impact on age-related retinal degeneration, quantitative trait loci have been implicated in age-related retinal degeneration, but the identities of the genes implicated in this process remain elusive.3, 4 Regardless, cumulative damage from oxidative stress appears to play a determinant role in the development of age-related phenotypes of photoreceptors in part as the result of marked and uneven oxygen tension, and metabolic demands, across the retina, that make photoreceptors particularly vulnerable to oxidative damage.10–13 To this effect, overexpression of erythropoietin in transgenic mice is neuroprotective against light-induced but not inherited retinal degeneration.14 Hence, the data hint of a link between light- and age-dependent death of photoreceptor neurons. On the other hand, the phenotypic analyses of genetically engineered mouse models support that light-induced degeneration is independent of the activation of phototransduction, but dependent on the light-receptor, rhodopsin, and that independent and poorly defined mechanisms triggering apoptosis may operate for light-induced, age-dependent, and inherited forms of retinal degeneration.5, 8 In addition, light-elicited degeneration of photoreceptors may act synergistically with certain forms of inherited degeneration selectively affecting these neurons, because neurodegeneration is exacerbated by light in certain mouse models with inherited degeneration of photoreceptors.15–19 Hence, the identification of novel components modulating the death of photoreceptors upon light and aging are likely to provide critical insights to novel pathways underlying the molecular bases of neurodegeneration upon various stress stimuli.
The Ran-binding protein-2 (RanBP2) is at the nexus of multiple subcellular and molecular processes underlying nuclear-cytoplasmic trafficking 20–22, transport and function of mitochondria 23, modulation of proteasome function and protein homeostasis 23–26, and modulation of protein-protein interaction by sumoylation in culture cells.27–30 Notably, haploinsufficiency of RanBP2 in combination with diet and genetic background triggers defined age-related phenotypes manifested by perturbation of growth and glucose catabolism.31 In addition, further decrease of the levels of RanBP2 in a mouse model harboring a hypomorphic allele of RanBP2 promotes missegregation of chromosomes (aneuploidy) in mitotic cells and carcinogen-elicited and age-dependent tumorigenesis without overall impairment of nuclear-cytoplasmic trafficking, mitotic spindle formation, and protein SUMO-modification.32 Here, we reveal that light-induced susceptibility to damage and apoptosis of photoreceptor neurons increases prominently between 12- and 24-week old inbred 129P2/OlaHsd mice, and that haploinsufficiency of RanBP2 in these mice suppresses strongly the age- and light-dependent increase of damage and cell-death of photoreceptors. Moreover, the neuroprotective effects caused by a deficit in RanBP2 is reflected by a significant decrease of free fatty acids, which upon light-induced oxidative stress, may suppress apoptosis and preceding phenotypes such as membrane dysgenesis.
Results
Light-induced morphological changes of photoreceptor neurons by RanBP2 haploinsufficiency and age
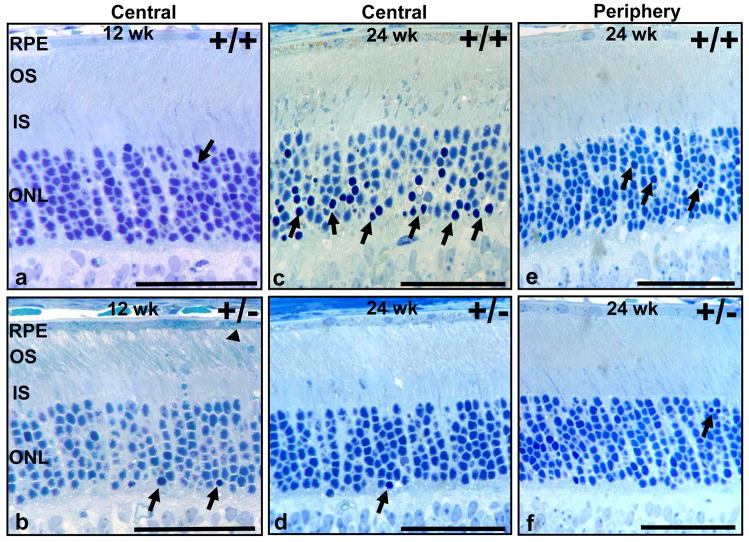
We previously identified phenotypes in RanBP2 haploinsufficient mice that are manifested in an age-dependent fashion.31 In light of the role of RanBP2 in retinal function 31 and multiple processes that may contribute to the development of manifestations linked to cell death events, we assessed the role of RanBP2 in the development of light- and age-dependent phenotypes linked to the damage and death of photoreceptor neurons. Because age-dependent manifestations were observed between 12-and 24-week old RanBP2+/+ and RanBP2+/− mice on an inbred 129P2/OlaHsd background 31, we examined the effect of constant white illumination for a period of 48 hours on inducing gross morphological abnormalities of retinal neurons, in particular photoreceptors, between 12- and 24-week old RanBP2+/+ and RanBP2+/− mice on the same genetic background. We found that 12-week old RanBP2+/− mice presented vacuolization of the outer segment compartment of photoreceptors that was limited to the distal (upper) portion of this compartment. This damage was less apparent in RanBP2+/+ mice (Figures 1a and b). In addition, few scattered and pyknotic nuclei were visible in both RanBP2+/+ and RanBP2+/− mice, but no significant changes were observed in the organization of the outer nuclear layer and inner segment compartment of photoreceptors. In contrast, the central regions of the retina of 24-week old RanBP2+/+ mice presented drastic morphological changes of the outer and inner segment compartments of photoreceptors, including condensed inner segments, overall disorganization of outer segments and outer nuclear layer, the presence of cystic spaces and cellular-like debris in the inner segments, and a strong increase of widespread condensed nuclei of photoreceptors that led to a decrease of the thickness of the outer nuclear layer (rows of nuclei) (Figure 1c, supplementary Figure 1). These phenotypic abnormalities in the central regions of the retina were largely alleviated in RanBP2+/− mice (Figure 1d) and peripheral regions of the retina of both genotypes (Figs. 1e and f).
Figure 1.
Age-dependent light damage of photoreceptors is strongly reduced in RanBP2 haploinsufficient mice. Light photomicrographs of methylene blue-stained sections of central (a-d) and peripheral (e, f) regions of the retina of 12-(a, b) and 24-week old (c-f) RanBP2+/+ (a, c, e) and RanBP2+/− mice (b, d, f). There is a strong increase in pyknotic nuclei in 24-week old wild-type mice compared with 12-week old wild-type mice (arrows pointing to intense nuclei staining) in the central retina (a, c) that is accompanied by the disorganization of the outer nuclear layer. The periphery of the retina is spared largely from pyknosis (e). In contrast, no apparent differences in pyknosis are observed between 12- and 24-week old RanBP2+/− mice (b, d, f), but some vacuolization of the tip of the outer segments can be noted (arrowhead). Legend: RPE, retina pigment epithelium; OS, outer segment of rod photoreceptors; IS, inner segment of rod photoreceptors; ONL, outer nuclear layer (nuclei of photoreceptors). Scale bar, 40 μm.
Haploinsufficiency of RanBP2 alleviates membrane dysgenesis in photoreceptors triggered by prolonged light exposure
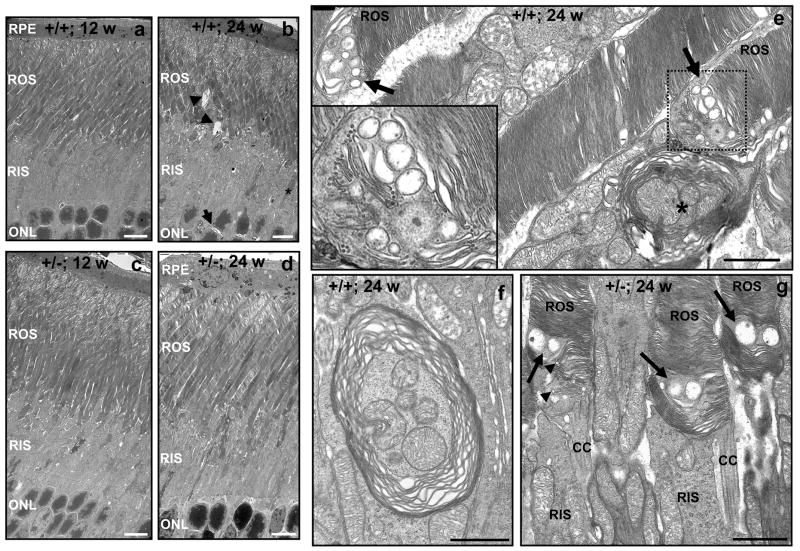
We assessed further the abnormalities of photoreceptors upon light-induced degeneration at ultrastructural level. There was a strong increase in the overall disorganization of the rod and inner segment subcellular compartments of photoreceptors in 24-week old RanBP2+/+ mice compared with 12-week RanBP2+/+ mice (Figures 2a, b). This increase in disorganization was largely suppressed in RanBP2+/− mice (Figures 2c, d). The subcellular changes in photoreceptors induced by light comprised a substantial increase of dilated disks at the tip of the outer segments, the presence of condensed (electrodense) inner segments (star, Figure 2b), large cystic spaces between outer segments and between nuclei (arrowheads, Figure 2b), vacuoles in the inner segments, and migration of mitochondria to the outer nuclear layer. These age-dependent pathological phenotypes were lessened in RanBP2+/− mice (Figures 2c, d). In addition, there are at least two unique features at ultrastructural level that were observed in photoreceptors of 24-week old RanBP2+/+, but not RanBP2+/− mice. First, RanBP2+/+ mice presented a prominent accumulation of multivesicular bodies at the base of the outer segments (Figures 2b, e) that disrupted the stacking of the nascent disks of the outer segments. This was accompanied often by fragmentation of the nascent disks, formation of large lateral disk lamellas and numerous vesicular bodies of various sizes (Figure 2e, inset picture). Conversely, RanBP2+/− mice appear largely to present normal formation of nascent disks, but two large vesicular bodies were often visible between nascent disks causing the focal disruption of their stacking (Figure 2f, arrows), and small crescent vesicular bodies appear to bulge also from the rims of some nascent disks (Figure 2f, arrowheads). In both genotypes such vesicular bodies seem comprised of a single membrane leaflet or no membrane could be discerned. Second, the inner segment compartment often presented large multilamellar bodies (MLBs) with loose lamellae of variable interlamellar space surrounding multiple mitochondria, indicating possibly the degeneration of mitochondria into MLBs (Figures 2e, g). This phenotype was not observed in RanBP2+/− mice. Mitochondria presented also dilation and fragmentation of the cristae. Thus, the data support the existence of a RanBP2-dependent switch rendering photoreceptors susceptible to damage by light with age and of a neuroprotective mechanism by RanBP2 that halts the age-dependent effect of light degeneration. Interestingly, all mice exhibited the dilation and disruption of the disks at the tip of the outer segments of photoreceptors independently of their age and genotype (Figures 2a-d). This phenotype was not observed in RanBP2+/+ mice reared under low (<70 lux) illumination (supplementary Figure 2). Hence, there is a non-selective RanBP2-independent effect of light in the development of dilated and disorganized disks at the tip of photoreceptors that is common at early stages of light-induced damage of photoreceptors.
Figure 2.
Electron micrographs depicting the age-dependent ultrastructural changes of inner and outer segments of photoreceptors of 12- and 24-week old RanBP2+/+ and RanBP2+/− mice upon prolonged light exposure. There is an overall increase in the subcellular disorganization of morphological features of the inner and outer segments of photoreceptors in 24-week old RanBP2+/+ mice (b) compared to 12-week old RanBP2+/− mice (a). This is reflected by an increase of cystoid spaces between nuclei (arrow) and outer segments (arrowheads), shrinking and condensed inner segments (star), formation of vacuoles in the inner segment, and dilation of the disks at the tip of the outer segments. The development of these subcellular pathologies, except for the dilation of the disks, was strongly decreased in 24-week old RanBP2+/− mice (d) compared to 12-week old RanBP2+/− mice (c). e, f, and g, depict high magnification images of single membrane multivesicular bodies at the base of the outer segments (e), large vesicular body duets (arrows) and crescent vesicles in nascent disk rims (arrowheads) at the base of the outer segments of RanBP2+/− mice (f), and multilamellar bodies engulfing mitochondria in RanBP2+/+ mice (e, star; g). Legend: RPE, retina pigment epithelium; ROS, rod outer segment of photoreceptors; RIS, rod inner segment of photoreceptors; ONL, outer nuclear layer (nuclei of photoreceptors); CC, connecting cilium. Scale bar in a-d, 6 μm; scale bar in e-g, 1 μm.
Differences in light-induced apoptosis of photoreceptors across the retina caused by aging and RanBP2 haploinsufficiency
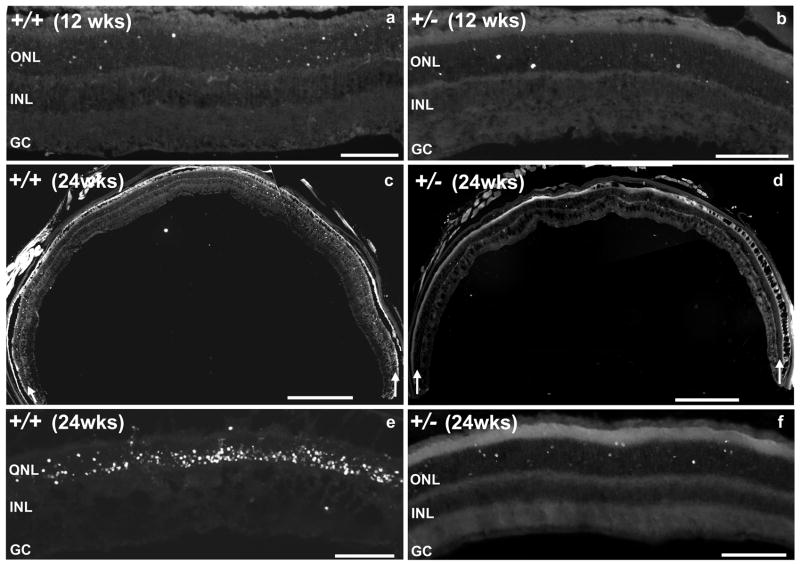
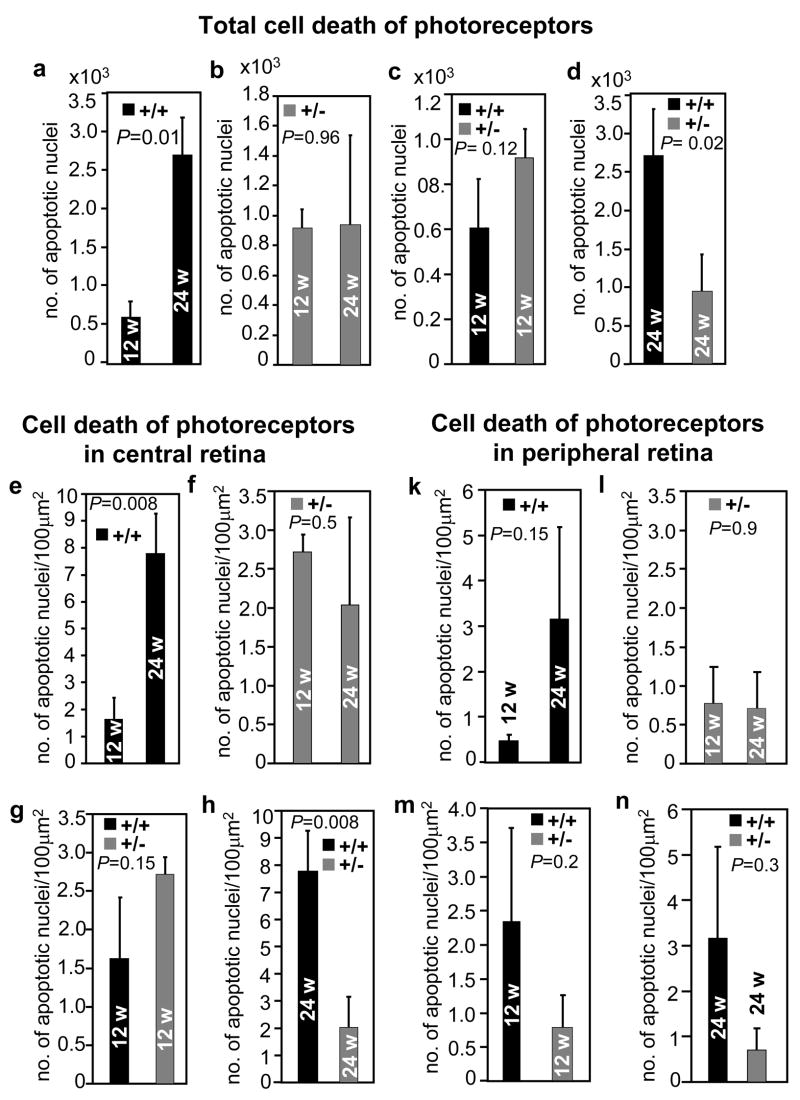
We performed morphometric analyses on the impact of light on eliciting apoptosis of photoreceptor neurons in 12- and 24-week old RanBP2+/+ and RanBP2+/− mice. We surveyed retina sections for the presence of DNA fragmentation in nuclei by TdT-mediated dUTP nick end-labeling (TUNEL) assay. Among retinal neurons, light-elicited apoptosis was restricted to nuclei of photoreceptor neurons regardless of the age and genotype of the mice (Figure 3). There was no apparent difference in cell death between 12-week old RanBP2+/+ and RanBP2+/−mice (Figures 3a and b). Conversely, 24-week old RanBP2+/+ and RanBP2+/−mice presented a substantial difference in the number of apoptotic nuclei in photoreceptors (Figures 3c - f). To this effect, wild-type mice exhibited a strong increase in apoptosis of photoreceptors (Figures 3c, e) in comparison to age-matched RanBP2+/− mice (Figures 3d, f). The same was also observed between 12- and 24-week old wild-type mice (Figures 3a, c and e), but not between 12-and 24-week old RanBP2+/− mice (Figures 3b, d and f). These observations were confirmed by quantitative analyses of TUNEL-positive nuclei of photoreceptors derived from several sections of the eyecup. A 5-fold significant increase of the total number of apoptotic photoreceptors was observed in 24-week old RanBP2+/+ mice compared with 12-week RanBP2+/+ mice (Figure 4a), while there was not a significant difference between 12- and 24-week old RanBP2+/− mice (Figure 4b). At the age of 12 weeks, both wild-type and RanBP2+/− mice had similar total number of apoptotic cells (Figure 4c). However, RanBP2+/− mice exhibited significantly less apoptotic photoreceptors compared with RanBP2+/+ mice at the age of 24 weeks (Figure 4d). Hence, there is an age-dependent increase in the susceptibility of photoreceptors to undergo cell death upon light-elicited stress that is suppressed by haploinsufficiency of RanBP2.
Figure 3.
Light-elicited and age-dependent accumulation of apoptotic nuclei selectively in photoreceptors is substantially reduced in RanBP2 haploinsufficient mice. TUNEL staining reflecting the nucleosomal DNA fragmentation of nuclei of photoreceptors show comparable cell-death between 12-week old RanBP2+/+ and RanBP2+/− mice (a, b), while there is a substantial increase in apoptosis in 24-week old RanBP2+/+, but not RanBP2+/− mice (c-f). a, b, e and f are high power micrographs of the central region of the retina. c and d are low power micrographs of the whole retina attached to the eyecup (white arrows point to the outer nuclear layer of photoreceptors). Legend: ONL, outer nuclear layer (nuclei of photoreceptors); INL, inner outer nuclear layer (nuclei of second-order neurons); GC, ganglion cell layer. Scale bar in a, b, e and f, 100 μm; scale bar in c and d, 500 μm.
Figure 4.
Quantitative morphometric analyses of apoptosis of photoreceptor neurons in 12- and 24-week old RanBP2+/+ and RanBP2+/− mice upon prolonged light exposure. There is a ~5-fold significant increase in the absolute number of apoptotic nuclei in 24-week old RanBP2+/+ mice compared with 12-week old ones (a) that is not observed in RanBP2+/− mice (b). At the age of 12 weeks, there was no difference in apoptosis between RanBP2+/+ and RanBP2+/− mice (c), but RanBP2+/− mice had significantly less apoptotic cells than RanBP2+/+ mice at the age of 24 weeks (d). There were also significant regional differences in apoptosis between the central (e-h) and peripheral (k-n) sections of retinas. There is a ~5-fold significant increase in the number of apoptotic nuclei from 12- to 24-week old RanBP2+/+ mice (e), but not RanBP2+/− mice (f). No difference in cell death is noticeable between 12-week old RanBP2+/+ and RanBP2+/− mice (g). At the age of 24 weeks, RanBP2+/+ mice present a significant increase in cell death, which is suppressed in RanBP2+/− mice (h). Although not significant, similar trends were observed in the peripheral regions of the retina (k-n). No difference in apoptosis was observed between 12- and 24-week old RanBP2+/− mice in either the central (f) or the peripheral (l) regions of the retina. Results shown represent the mean ± S.D. (n=4).
In addition, quantitative morphometric analysis was done on multiple central and peripheral sections of retinas to probe for potential regional differences of susceptibility to apoptosis of photoreceptors. The amount of cell death was normalized against the area of outer nuclear region analyzed. A ~5-fold increase of the total number of apoptotic photoreceptors was observed in 24-week old RanBP2+/+ mice compared with 12-week RanBP2+/+ mice (Figure 4e). In contrast, comparison of 12- and 24-week old RanBP2+/− mice showed no significant difference in cell death (Figure 4f). Both wild-type and RanBP2+/− mice had similar total number of apoptotic cells at the age of 12 weeks (Figure 4g), while there was a significant increase of apoptosis in wild-type, but not RanBP2+/−mice, at the age of 24 weeks (Figure 4h). On the other hand, similar analyses of the peripheral regions of the retinas showed that there was not a significant age-dependent increase to light-elicited degeneration in these regions of the retinas, although such trend was observed (Figures 4k-n). Finally, analysis of cell death in central and peripheral regions of age- and genotype-matched retinas shows that apoptosis is prominent in the central region of the retina (supplementary Figures 3a-d). Apoptosis increases with age in RanBP2+/+, but not RanBP2+/−mice (supplementary Figures 3a-d). Collectively, the data show that the central retina presents the highest susceptibility to cell death upon light-elicited stress and haploinsufficiency of RanBP2 blocks this effect.
The expression and SUMOylation of Topo IIα and levels of opsin apoprotein are not affected by RanBP2 in retinal neurons
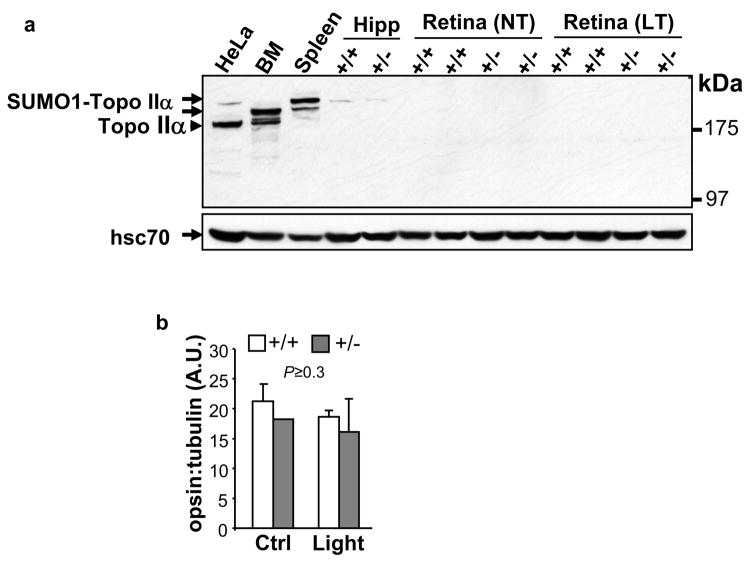
RanBP2 was shown physiologically to modulate the SUMOylation of topoisomerase-IIα (Topo IIα) and its localization 32, whereas reduced levels of opsin apoprotein of rod photoreceptors protects these neurons from light-elicited degeneration.9 Hence, to gain further insight into the molecular basis of the neuroprotective effects of a deficit of RanBP2 in photoreceptor neurons, we examined whether haploinsufficiency of RanBP2 affected the levels of SUMOylation of Topo IIα and opsin apoprotein in the absence and presence of light stress. Conversely to other tissues and HeLa cells with high mitotic activity, we found that Topo IIα or its sumoylated isoform is not detectable in retinal neurons (Figure 5a) and haploinsufficiency of RanBP2 did not cause a change in the levels of opsin apoprotein under either light condition (Figure 5b). Hence, these data do not support Topo IIα- or opsin-dependent effects promoted by RanBP2 in neuroprotection.
Figure 5.
Haploinsufficiency of RanBP2 has no effect on the levels of topoisomerase-IIα (Topo IIα), SUMOylated Topo IIα α (SUMO1-Topo IIα), and apoprotein opsin, in the retina. (a) The expression of Topo IIα and sumoylated Topo IIα in retinas of non (NT)- and light-treated (LT) 24-week old RanBP2+/+ and RanBP2+/− mice was assessed by immunoblot analyses of retinal extracts (100 μg) and compared to extracts (100 μg) of HeLa, bone marrow (BM) from the femoral bone, spleen, and hippocampus (Hipp). Regardless of the light-treatment and genotype, Topo IIα and sumoylated Topo IIα were not detected in the retinas, but they were expressed in HeLa, bone marrow and spleen, whereas traces of sumoylated Topo IIα were detected in the hippocampus. Blot was reprobed for the cytosolic heat shock 70 protein (hsc70) as loading control. (b) The expression of the apoprotein opsin in retinas of 24-week old RanBP2+/+ and RanBP2+/− mice under normal cyclic (Ctrl) and prolonged light exposure (light) was quantified from immunoblot analyses of retinal extracts (2 μg) by densitometry and normalized for tubulin expression. No changes in apoprotein opsin expression were observed regardless the exposure to light and genotype. Results shown represent the mean ± S.D. (n=4). A.U. arbitrary units.
RanBP2+/− mice present a deficit in fat mass
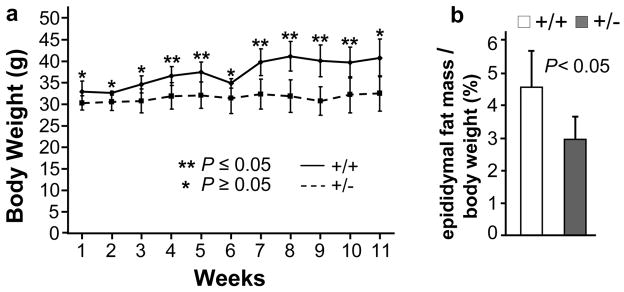
Several lines of evidence from this and previous work suggest that lipid metabolism may be deregulated in RanBP2+/− mice. First, several facets of membrane dysgenesis are significantly alleviated in RanBP2+/− compared to RanBP2+/+ mice. In particular, the formation large multilamellar bodies and vesicular deposits with single or no discernable membrane leaflet reminiscent of lipid droplets structures are observed often in disorders affecting various facets of lipid metabolism or trafficking.33–38 Second, RanBP2 haploinsufficient mice present age-dependent decreased weight gain on a high-energy diet.31 Finally, gene expression profiling between RanBP2+/+ and RanBP2+/− mice suggest that lipid metabolism may be deregulated systemically in RanBP2+/− mice (unpublished observations). To further probe whether haploinsufficiency of RanBP2 modulated lipid metabolism, RanBP2+/+ and RanBP2+/− mice were placed on a high-fat diet (40%) for 12 weeks. In contrast to RanBP2+/+ mice, RanBP2+/− are remarkably refractory to body weight gain (Figure 6a) and present significantly less accumulation of epididymal fat mass (Figure 6b). These results support that lipogenesis is down-regulated in RanBP2+/− mice.
Figure 6.
Haploinsufficiency of RanBP2 renders 24-week old mice refractory to gain of body weight and epididymal fat mass when placed on a high-fat (40%) diet. RanBP2+/+ but not RanBP2+/− mice gain significant body weight (a) and epididymal fat mass (b) when placed on a high-fat diet for 12 weeks. In (a) double asterisks represent significant differences between the groups (Student’s t test, P=0.05). Statistical significance was also found across the time-course of the experiment (repeated measures 2-way ANOVA, P=0.03). Results shown represent the mean ± S.D. (n=4).
Haploinsufficiency of RanBP2, age and light promote differential changes in lipid metabolites
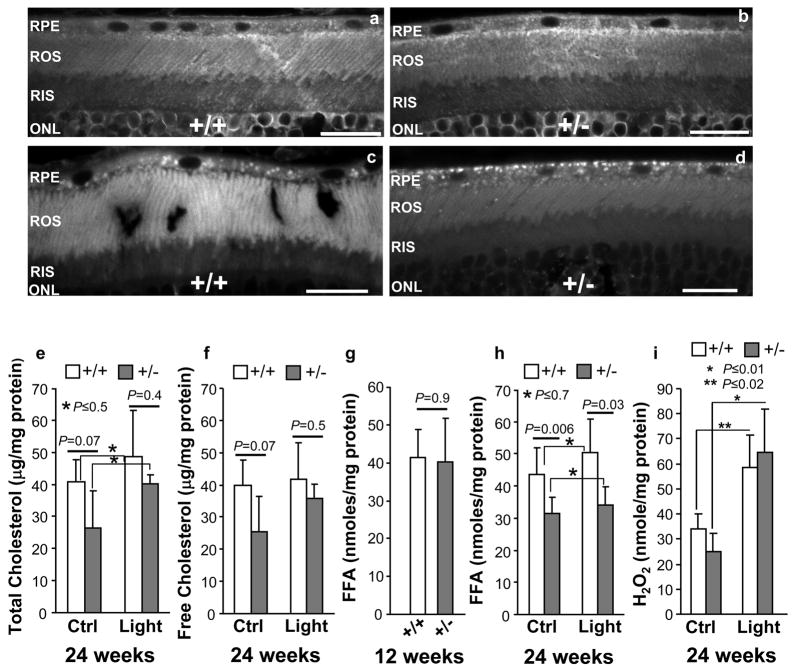
Cholesterol and free fatty acids, such as docosahexaenoic acid (DHA), are abundant and critical components of membranes of the outer segments of photoreceptors and other neurons 39–43 and abnormalities in the level of these have been linked to several syndromic and non-syndromic retinal dystrophies.34, 44–55 Moreover, light-elicited lipid peroxidation and deregulation of the production of free fatty acids (or metabolites thereof) are thought to promote the degeneration of photoreceptors, endothelial cells and pancreatic β-cells, by mechanisms that remain largely elusive.10, 13, 56–60 Hence, we examined whether the levels and distribution of cholesterol and free fatty acids are deregulated in the retina of haploinsufficient RanBP2 mice. As shown in Figure 7, we did not discern changes in cholesterol lipid droplets (LD) in photoreceptors between 24-week old RanBP2+/+ and RanBP2+/− mice in the absence or presence of chronic light-stress. However, we noted that the distribution of LD was disturbed differently in retina pigment epithelium (RPE) cells located adjacent to the tip of the outer segments of RanBP2+/+ and RanBP2+/− mice, but only when these were exposed to light-elicited stress (Figure 7a-d). To this effect, RanBP2+/+ and RanBP2+/− mice on a 12 hour light-dark cyclic illumination presented LD finely distributed throughout the RPE cells (Figures 7a-b). In contrast, upon light-elicited stress the LD deposits (aggregates) were seen uniformly distributed in the RPE of RanBP2+/+ mice (Figure 7c), whereas such deposits were localized prominently to the basal end of RPE cells of RanBP2+/−mice (Figure 7d). Examination of cholesterol and free fatty acids (FFA) content of retinas shows that neither the level of total nor free cholesterol were changed between 24-week old RanBP2+/+ and RanBP2+/− mice regardless of the light exposure, although there was a trend for RanBP2+/− mice to present lower levels of total and free (non-esterified) cholesterol on a normal 12 hour light-dark cyclic illumination (Figures 7e, f). In contrast, we found that the content of FFA in the retina varied significantly with age and genotype of RanBP2 mice in a light-independent fashion. There was no difference in FFA content between 12-week old RanBP2+/+ and RanBP2+/− mice (Figure 7g), whereas 24-week old RanBP2+/−mice presented reduced levels (~35–40%) of FFA compared to RanBP2+/+ (Figure 7h). Finally, we probed further whether prolonged exposure to light promotes oxidative stress in the retina in a light-and genotype-dependent manner, since such stress insult is thought to play a role in degeneration of photoreceptors.10, 13 As shown in Figure 7i, there was a significant increase of hydrogen peroxide (H2O2) production in retinal neurons upon light-elicited stress and the levels of H2O2 production did not vary with the genotype of RanBP2 mice.
Figure 7.
Lipid metabolic deficits and reactive oxygen species production in 24-week old RanBP2+/+ and RanBP2+/− mice upon light-elicited stress. Filipin staining of cholesterol/lipid droplets (LD) shows that RanBP2+/+ (a) and RanBP2+/− (b) under normal cyclic light present no visible difference in LD, whereas under light-elicited stress there is an apparent coalescence of LD throughout the perikarya of the retinal pigment epithelium (RPE) in RanBP2+/+ mice (c) and coalescence and polarized localization of LD to the basal end of the RPE of RanBP2+/− mice (d). No significant and discernable changes in LD distribution were observed in the outer (ROS) and inner segments (RIS) of rod photoreceptor neurons (a-d). Total (e) and free (f) cholesterol were not significantly altered in retinas of RanBP2+/+ and RanBP2+/− mice regardless of the light treatment, although RanBP2+/− mice exhibited a trend to present lower levels of total (e) and free (f) cholesterol than RanBP2+/+ mice when reared under cyclic light, but not prolonged light exposure (n=4). The content of free fatty acids (FFA) in the retina remained unaltered between RanBP2+/+ and RanBP2+/− mice at the age of 12-weeks (n=4) (g), whereas 24-week old RanBP2+/− mice presented ~ 35% decreased levels of FFA than RanBP2+/+ mice and this remained unchanged under prolonged light exposure (n=7 for RanBP2+/+ mice under normal cyclic light, n=6 for RanBP2+/− mice under normal cyclic light, n=4 for mice under prolonged light exposure) (h). (i) The level of hydrogen peroxide (H2O2) increases in retinas upon light-elicited stress regardless of the genotype of RanBP2 mice (n=4). Results shown represent the mean ± S.D.. Legend: ROS, rod outer segment (compartment) of photoreceptors; RIS, rod inner segment (compartment) of photoreceptors; ONL, outer nuclear layer (nuclei of photoreceptors); RPE, retinal pigment epithelium.
Discussion
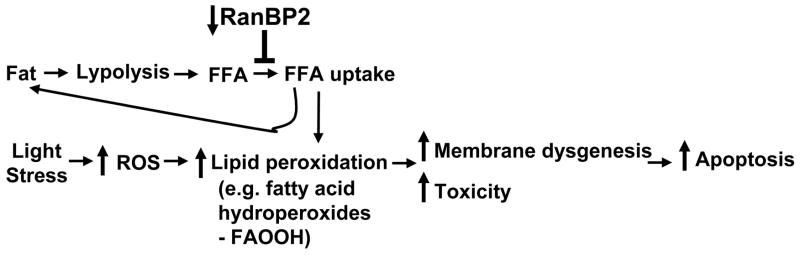
Our studies reveal an age-dependent switch between 12- and 24-week old mice on the 129P2/OlaHsd background that significantly increases the susceptibility of photoreceptors to light-induced degeneration and membrane dysgenesis of photoreceptor neurons. RanBP2 haploinsufficiency significantly blocks this switch thus rendering older photoreceptors much less susceptible to light-elicited degeneration and suppressing several facets of membrane dysgenesis, such as the formation of multilamellar bodies and disruption of nascent disk formation. Strikingly, a genotype-dependent, but light-independent, decrease in FFA of the retina in 24-week old but not 12-week old RanBP2+/− mice correlates well with the morphological phenotypes observed upon light-elicited stress. Hence, the data support a model whereby a RanBP2-dependent reduction of FFA content renders neuroprotection to photoreceptor neurons to light-induced damage (Figure 8). The decrease of free fatty acids in the retina of RanBP2+/− mice, and the resistance to weight-gain and formation of adipose tissue of RanBP2+/− mice when place on a high fat diet, suggest that FFA uptake is compromised in RanBP2+/− mice. The deregulation of FFA uptake may also affect the transport (and distribution) of cholesterol in lipid droplets61 as observed in retina pigment epithelial cells of RanBP2+/− mice, a phenotype likely secondary to deficits in FFA. Lower levels of FFA in FFA-enriched photoreceptor neurons likely decreases lipid peroxidation, such as formation of fatty acid hydroperoxides, which are implicated in the propagation of lipid peroxidation.62 The major outcomes of lipid peroxidation in photoreceptors are twofold. First, it promotes the generation of toxic FFA metabolites, which by themselves or in concert with other ligands, promote apoptosis.60, 63–65 Second, changes in the orientation of the acyl chain of peroxidized fatty acids toward the hydrophilic exterior of the lipid bilayer (e.g. lipid whisker model 66) may disrupt critical protein-protein and protein-lipid contacts required for the membrane biogenesis of nascent disks of photoreceptors or even generate novel pathological ligand(s) contributing to the death of photoreceptors.
Figure 8.
Model of the effect of haploinsufficiency of RanBP2 and light-elicited stress, in the production of free fatty acids (FFA), lipid peroxidation by reactive oxygen species (ROS), and downstream effect of these, in membrane dysgenesis, toxicity, and stimulation of apoptosis (see text for details). Legend. Upward arrows indicate an increased level/effect; downward arrow indicates a decreased level/effect.
The light-elicited pathways and mechanisms leading to the apoptosis of photoreceptors remain largely elusive. In particular, the identity of the FFA or metabolites thereof underlying neuroprotection and exacerbating apoptosis in the central region of the retina upon light-induced damage are unknown. In light of the large content of DHA in the membrane of the outer segment of photoreceptors 43, DHA is a strong candidate to mediate neuroprotection when in deficit or to stimulate apoptosis when at high levels. Indeed, a diet deficit in the linolenic acid precursor of DHA of albino rats confers neuroprotection to photoreceptor neurons upon light-induced damage.67 Collectively, these observations support that photoreceptors contain an excessive pool of DHA with deleterious implications upon light-elicited damage and that the bioavailability of FFA to photoreceptors differ between the central and peripheral regions of the retina. On the other hand, light-elicited degeneration of photoreceptors of Drosophila due to deficits in phosphatidylinositol-phospholipase Cβ4 (PI-PLCβ4)-mediated production of diacylglycerol (DAG) 68 and generation of FFA and metabolites thereof from DAG by a DAG lipase encoded by inaE 59, suggest another vital role of FFA signaling in the survival of photoreceptor neurons (and Drosophila). Although it remains unclear whether such PI-PLC-dependent signaling pathway is conserved in vertebrate photoreceptor neurons, the mammalian homolog of the PI-PLCβ4 of Drosophila is present in photoreceptor neurons.69, 70 Hence, this PI-PLC signaling pathway may be another source for the generation of FFA from phosphatidylinositol 4,5-biphosphate (PIP2).71–79
Finally, analysis of haploinsufficiency of RanBP2 greatly facilitates the identification and dissection of primary phenotypes from confounding secondary phenotypes, which are often difficult to parse in severe phenotypes.31, 32 However, genetic tools to manipulate and analyze a diverse but limited set of partners, each associating specifically to selective domains of RanBP2, will provide novel insights into what specific biological activities directly linked to each of the functions of RanBP2 20–23, 31 confers light-elicited neuroprotection and modulates lipid metabolism. The data here presented do not suggest a direct role of sumoylation of Topo IIα32, and opsin apoprotein9, in such processes, because of the absence of the former from retinal neurons and the lack of change in the levels of the latter in RanBP2+/− mice, respectively. It is of interest to note that an autosomal dominant acute necrotizing encephalopathy triggered by the onset of a febrile illness in the human maps to 2q12.1-2q13, where RanBP2 is localized 80–82. Identification of such potential genetic lesion(s) in RanBP2 may provide new clues of the role of RanBP2 and its partners in neurological function and cell death upon various stressors. Regardless, the RanBP2 mouse model defines a new genetic tool and framework to partition, isolate, and define, molecularly and genetically, activities and subcellular processes linked to RanBP2 and aging that are important in modulating the survival and death of photoreceptors and other neurons, and manifestations of aging-related diseases affecting the retina (e.g. macular dystrophies) and elsewhere, upon exposure to deleterious stressors.
Material and methods
Mice
RanBP2+/− mice were described elsewhere.31 RanBP2+/+ and RanBP2+/−mice were in an inbred 129P2/OlaHsd background and reared in 70 lux of diffused white fluorescent light and 12:12 light-dark (LD) cycle. Mice were fed with a standard chow diet (~10% fat; Test Diet 5LJ5, Purina) or placed whenever applicable on a high fat (~40%) diet (Test Diet DIO 58Y1, Purina) for 12 weeks. Animal protocols were approved by the Institutional Animal Care and Use Committee at Duke University and the procedures adhered to the ARVO guidelines for the Use of Animals in Vision Research.
Light exposure
Mice were placed in a white reflective cage with food and water and fluorescent light bulbs mounted at the top of the cage. Illuminance was measured at the bottom of the cage with a traceable dual-range light meter (Fisher Scientific). Mice were exposed to 1,200 lux of continuous, cooled, and diffused fluorescent white light for 48 hrs. Mice were sacrificed and the eyeballs immediately collected and processed for histology and morphometric analyses.
Histology
Eyes were prepared for light and electron microscopy by fixing eyecups overnight in 2% glutaraldehyde and 2% paraformaldehyde in 0.1% cacodylate buffer, pH 7.2 at 4oC. Semi-thin sections (0.5 μm) along the vertical meridian were mounted on slides and stained with 1% methylene blue. Light images of the retina were acquired with a Nikon C1-Plus light microscope equipped with Nomarski optics and coupled to a SPOT RT-SE digital camera (Diagnostic Instruments).
TUNEL Staining and quantitative morphometric analysis of apoptosis
Apoptosis was detected in situ with the DeadEnd Fluorometric TUNEL System (Promega) on retinas fixed with 4% paraformaldehyde. Images of TUNEL-positive nuclei of sections of eyecups were captured with a laser confocal Nikon C1-Plus light microscope equipped with epifluorescence and coupled to a Cascade 1K digital camera (Roper Scientific). Images were acquired with 4x and 10x objectives. Digital stitching of composite images acquired with the 4x objective was performed with Photoshop CS (Adobe). Quantitative morphometric analyses of images analyzed with Metamorph v6.2 (Molecular Devices) were performed on eight sections per eye along the vertical meridian with a 10x-objective. Typically, images of the whole section of the retina comprised 7–8 image fields. Peripheral images of the retina containing the marginal areas of the retina comprised the first and last image fields. Central regions of the retina comprised typically the third and fourth image fields of the retina. Statistical analysis of apoptosis was performed with two-tailed equal variance t-test.
Transmission electron microscopy
Posterior eyes were processed as described in the Histology section followed by post-fixation in 2% osmium tetraoxide in 0.1% cacodylate buffer and embedded in Spurr resin. Sixty-five nanometer-thick sections were cut with a ultramicrotome and stained with uranyl acetate and lead citrate. Specimens were visualized with JEOL 1200 EX and Philips CM 12 transmission electron microscopes. Low magnification images were captured with the Philips TEM coupled with an AMT camera and processed with the Image Capturing Engine software (version 5.42355). High magnification images were captured with the JEOL TEM and negatives were scanned with Photoshop CS.
Immunoblot analyses
Retina samples were homogenized with a Kontes Microtube Pellet Pestle Rods with Motor in NP-40 buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1% Nonidet P-40 (NP-40), with Complete protein inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Samples were centrifuged at 10,000g for 15 min and supernatants collected. Protein concentration was measured by Bradford method using BSA as standard. Protein extracts were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting was carried out as described elsewhere 83. Primary antibodies used: Mouse anti-acetylated tubulin (Sigma, 1:40,000), rabbit anti-rhodopsin (Affinity Bioreagents, 1:20,000), rabbit anti-Hsc70 (Stressgen, 1:3,000), rabbit anti-Topo IIα (Topogen, 1:3,000).
Filipin staining
Radial retinal cryosections (~10 μm thick) mounted on slides were rinsed 3 times with phosphate-saline pH 7.4 (PBS) and incubated with 0.05 mg/ml of filipin (Sigma) for 2hr at room temperature in the dark. Specimens were then rinsed 3 times with PBS and mounted with ProLong Gold antifade (Invitrogen).
Hydrogen Peroxide Measurement
The hydrogen peroxide levels were assayed using the Amplex Red Hydrogen Peroxide/Peroxidase Assay Kit (Invitrogen) as per manufacturer’s instructions. Ten μl of NP40 extract was used for each measurement. Results were normalized against soluble protein contents in the extract. Two-tailed equal variance t-test statistical analysis was performed.
Free cholesterol and total cholesterol quantitation
Free cholesterol and total cholesterol were determined with the Cholesterol and Cholesterol Ester Quantitation kit (Biovision, Mountain View, CA) as per manufacture’s instructions. Briefly, 3 μl retinal NP40 extracts was diluted with assay buffer for measurement by fluorescence (Excitation/emission/cutoff=560/590/590 nm; SpectraMax M5, Molecular Devices). Total cholesterol was measured with cholesterol esterase added, free cholesterol was measured without cholesterol esterase. Results were normalized to soluble protein content in the extracts. Two-tailed equal variance t-test statistical analysis was performed.
Free Fatty Acid Quantification
Free fatty acids (FFA) were determined with the Free Fatty Acid Quantification kit (Biovision, Mountain View, CA) to detect C-8 (octanoate) and longer fatty acids as per manufacture’s instructions. Briefly, retinal NP40 extracts were used for each reaction. FFA content was measured by a colorimetric assay (Ab570) with palmitic acid employed as a standard. FFA results were normalized against soluble protein contents in the extract. Two-tailed equal and unequal variance t-test statistical analysis was performed.
Supplementary Material
Acknowledgments
This work was supported by NIH grant EY11993 and Pearle Vision Foundation to P.A.F and NIH 2P30-EY005722-21. A.Y. was supported by undergraduate research scholarships from Howard Hughes Medical Institute and American Federation Aging Research. P.A.F. is the Jules & Doris Stein Research to Prevent Blindness Professor.
Abbreviations list
- RanBP2
Ran-binding protein 2
- TUNEL
TdT-mediated dUTP nick end-labeling
- MLBs
multilamellar bodies
- SUMO-1
Small Ubiquitin-like Modifier-1
- RPE65
retinal pigment epithelium-specific protein 65kDa
- Topo IIα
topoisomerase-IIα
- LD
lipid droplets
- FFA
free fatty acids
- DHA
docosahexaenoic acid
- DAG
diacylglycerol
- H2O2
hydrogen peroxide
- PIP2
phosphatidylinositol 4,5-biphosphate
- TEM
transmission electron microscope
- PI-PLCβ4
phosphatidylinositol-phospholipase Cβ4
- DAG
diacylglycerol
- ROS
rod outer segment (compartment) of photoreceptors
- RIS
rod inner segment (compartment) of photoreceptors
- ONL
outer nuclear layer (nuclei of photoreceptors)
- RPE
retinal pigment epithelium
References
- 1.Pacione LR, Szego MJ, Ikeda S, Nishina PM, McInnes RR. Progress toward understanding the genetic and biochemical mechanisms of inherited photoreceptor degenerations. Annu Rev Neurosci. 2003;26:657–700. doi: 10.1146/annurev.neuro.26.041002.131416. [DOI] [PubMed] [Google Scholar]
- 2.Reme CE. The dark side of light: rhodopsin and the silent death of vision the proctor lecture. Invest Ophthalmol Vis Sci. 2005;46:2671–2682. doi: 10.1167/iovs.04-1095. [DOI] [PubMed] [Google Scholar]
- 3.Danciger M, Lyon J, Worrill D, LaVail MM, Yang H. A strong and highly significant QTL on chromosome 6 that protects the mouse from age-related retinal degeneration. Invest Ophthalmol Vis Sci. 2003;44:2442–2449. doi: 10.1167/iovs.02-1252. [DOI] [PubMed] [Google Scholar]
- 4.Danciger M, Yang H, Ralston R, Liu Y, Matthes MT, Peirce J, et al. Quantitative genetics of age-related retinal degeneration: a second F1 intercross between the A/J and C57BL/6 strains. Mol Vis. 2007;13:79–85. [PMC free article] [PubMed] [Google Scholar]
- 5.Hao W, Wenzel A, Obin MS, Chen CK, Brill E, Krasnoperova NV, et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002;32:254–260. doi: 10.1038/ng984. [DOI] [PubMed] [Google Scholar]
- 6.Grimm C, Wenzel A, Williams T, Rol P, Hafezi F, Reme C. Rhodopsin-mediated blue-light damage to the rat retina: effect of photoreversal of bleaching. Invest Ophthalmol Vis Sci. 2001;42:497–505. [PubMed] [Google Scholar]
- 7.Wenzel A, Reme CE, Williams TP, Hafezi F, Grimm C. The Rpe65 Leu450Met variation increases retinal resistance against light-induced degeneration by slowing rhodopsin regeneration. J Neurosci. 2001;21:53–58. doi: 10.1523/JNEUROSCI.21-01-00053.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodruff ML, Wang Z, Chung HY, Redmond TM, Fain GL, Lem J. Spontaneous activity of opsin apoprotein is a cause of Leber congenital amaurosis. Nat Genet. 2003;35:158–164. doi: 10.1038/ng1246. [DOI] [PubMed] [Google Scholar]
- 9.Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Reme CE. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat Genet. 2000;25:63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- 10.Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, et al. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linsenmeier RA. Effects of light and darkness on oxygen distribution and consumption in the cat retina. J Gen Physiol. 1986;88:521–542. doi: 10.1085/jgp.88.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright AF, Jacobson SG, Cideciyan AV, Roman AJ, Shu X, Vlachantoni D, et al. Lifespan and mitochondrial control of neurodegeneration. Nat Genet. 2004;36:1153–1158. doi: 10.1038/ng1448. [DOI] [PubMed] [Google Scholar]
- 13.Imamura Y, Noda S, Hashizume K, Shinoda K, Yamaguchi M, Uchiyama S, et al. Drusen, choroidal neovascularization, and retinal pigment epithelium dysfunction in SOD1-deficient mice: a model of age-related macular degeneration. Proc Natl Acad Sci U S A. 2006;103:11282–11287. doi: 10.1073/pnas.0602131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm C, Wenzel A, Stanescu D, Samardzija M, Hotop S, Groszer M, et al. Constitutive overexpression of human erythropoietin protects the mouse retina against induced but not inherited retinal degeneration. J Neurosci. 2004;24:5651–5658. doi: 10.1523/JNEUROSCI.1288-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cideciyan AV, Hood DC, Huang Y, Banin E, Li ZY, Stone EM, et al. Disease sequence from mutant rhodopsin allele to rod and cone photoreceptor degeneration in man. Proc Natl Acad Sci U S A. 1998;95:7103–7108. doi: 10.1073/pnas.95.12.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galy A, Roux MJ, Sahel JA, Leveillard T, Giangrande A. Rhodopsin maturation defects induce photoreceptor death by apoptosis: a fly model for RhodopsinPro23His human retinitis pigmentosa. Hum Mol Genet. 2005;14:2547–2557. doi: 10.1093/hmg/ddi258. [DOI] [PubMed] [Google Scholar]
- 17.Naash ML, Peachey NS, Li ZY, Gryczan CC, Goto Y, Blanks J, et al. Light-induced acceleration of photoreceptor degeneration in transgenic mice expressing mutant rhodopsin. Invest Ophthalmol Vis Sci. 1996;37:775–782. [PubMed] [Google Scholar]
- 18.Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Susceptibility to retinal light damage in transgenic rats with rhodopsin mutations. Invest Ophthalmol Vis Sci. 2003;44:486–492. doi: 10.1167/iovs.02-0708. [DOI] [PubMed] [Google Scholar]
- 19.Vaughan DK, Coulibaly SF, Darrow RM, Organisciak DT. A morphometric study of light-induced damage in transgenic rat models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2003;44:848–855. doi: 10.1167/iovs.02-0709. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama N, Hayashi N, Seki T, Pante N, Ohba T, Nishii K, et al. A giant nucleopore protein that binds Ran/TC4. Nature. 1995;376:184–188. doi: 10.1038/376184a0. [DOI] [PubMed] [Google Scholar]
- 21.Bernad R, van der Velde H, Fornerod M, Pickersgill H. Nup358/RanBP2 attaches to the nuclear pore complex via association with Nup88 and Nup214/CAN and plays a supporting role in CRM1-mediated nuclear protein export. Mol Cell Biol. 2004;24:2373–2384. doi: 10.1128/MCB.24.6.2373-2384.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutten S, Kehlenbach RH. Nup214 is required for CRM1-dependent nuclear protein export in vivo. Mol Cell Biol. 2006;26:6772–6785. doi: 10.1128/MCB.00342-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho KI, Cai Y, Yi H, Yeh A, Aslanukov A, Ferreira PA. Association of the Kinesin-Binding Domain of RanBP2 to KIF5B and KIF5C Determines Mitochondria Localization and Function. Traffic. 2007;8:1722–1735. doi: 10.1111/j.1600-0854.2007.00647.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferreira PA, Nakayama TA, Travis GH. Interconversion of red opsin isoforms by the cyclophilin-related chaperone protein Ran-binding protein 2. Proc Natl Acad Sci U S A. 1997;94:1556–1561. doi: 10.1073/pnas.94.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferreira PA, Hom JT, Pak WL. Retina-specifically expressed novel subtypes of bovine cyclophilin. J Biol Chem. 1995;270:23179–23188. doi: 10.1074/jbc.270.39.23179. [DOI] [PubMed] [Google Scholar]
- 26.Ferreira PA, Nakayama TA, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383:637–640. doi: 10.1038/383637a0. [DOI] [PubMed] [Google Scholar]
- 27.Lee GW, Melchior F, Matunis MJ, Mahajan R, Tian Q, Anderson P. Modification of Ran GTPase-activating protein by the small ubiquitin-related modifier SUMO-1 requires Ubc9, an E2-type ubiquitin-conjugating enzyme homologue. J Biol Chem. 1998;273:6503–6507. doi: 10.1074/jbc.273.11.6503. [DOI] [PubMed] [Google Scholar]
- 28.Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex protein RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- 29.Mahajan R, Gerace L, Melchior F. Molecular characterization of the SUMO-1 modification of RanGAP1 and its role in nuclear envelope association. J Cell Biol. 1998;140:259–270. doi: 10.1083/jcb.140.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matunis MJ, Coutavas E, Blobel G. A novel ubiquitin-like modification modulates the partitioning of the Ran-GTPase-activating protein RanGAP1 between the cytosol and the nuclear pore complex. J Cell Biol. 1996;135:1457–1470. doi: 10.1083/jcb.135.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aslanukov A, Bhowmick R, Guruju M, Oswald J, Raz D, Bush RA, et al. RanBP2 Modulates Cox11 and Hexokinase I Activities and Haploinsufficiency of RanBP2 Causes Deficits in Glucose Metabolism. PLoS Genet. 2006;2:e177. doi: 10.1371/journal.pgen.0020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawlaty MM, Malureanu L, Jeganathan KB, Kao E, Sustmann C, Tahk S, et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIalpha. Cell. 2008;133:103–115. doi: 10.1016/j.cell.2008.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.German DC, Liang CL, Song T, Yazdani U, Xie C, Dietschy JM. Neurodegeneration in the Niemann-Pick C mouse: glial involvement. Neuroscience. 2002;109:437–450. doi: 10.1016/s0306-4522(01)00517-6. [DOI] [PubMed] [Google Scholar]
- 34.Karan G, Lillo C, Yang Z, Cameron DJ, Locke KG, Zhao Y, et al. Lipofuscin accumulation, abnormal electrophysiology, and photoreceptor degeneration in mutant ELOVL4 transgenic mice: a model for macular degeneration. Proc Natl Acad Sci U S A. 2005;102:4164–4169. doi: 10.1073/pnas.0407698102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci U S A. 2007;104:11026–11031. doi: 10.1073/pnas.0702504104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel SC, Suresh S, Kumar U, Hu CY, Cooney A, Blanchette-Mackie EJ, et al. Localization of Niemann-Pick C1 protein in astrocytes: implications for neuronal degeneration in Niemann- Pick type C disease. Proc Natl Acad Sci U S A. 1999;96:1657–1662. doi: 10.1073/pnas.96.4.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips SE, Woodruff EA, 3rd, Liang P, Patten M, Broadie K. Neuronal loss of Drosophila NPC1a causes cholesterol aggregation and age-progressive neurodegeneration. J Neurosci. 2008;28:6569–6582. doi: 10.1523/JNEUROSCI.5529-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitz G, Muller G. Structure and function of lamellar bodies, lipid-protein complexes involved in storage and secretion of cellular lipids. J Lipid Res. 1991;32:1539–1570. [PubMed] [Google Scholar]
- 39.Anderson RE. Lipids of ocular tissues. IV A comparison of the phospholipids from the retina of six mammalian species. Exp Eye Res. 1970;10:339–344. doi: 10.1016/s0014-4835(70)80046-x. [DOI] [PubMed] [Google Scholar]
- 40.Andrews LD, Cohen AI. Freeze-fracture evidence for the presence of cholesterol in particle-free patches of basal disks and the plasma membrane of retinal rod outer segments of mice and frogs. J Cell Biol. 1979;81:215–228. doi: 10.1083/jcb.81.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Brien JS, Sampson EL. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. J Lipid Res. 1965;6:545–551. [PubMed] [Google Scholar]
- 42.Albert AD, Boesze-Battaglia K. The role of cholesterol in rod outer segment membranes. Prog Lipid Res. 2005;44:99–124. doi: 10.1016/j.plipres.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 44.Anderson RE, Maude MB, Alvarez RA, Acland GM, Aguirre GD. Plasma lipid abnormalities in the miniature poodle with progressive rod-cone degeneration. Exp Eye Res. 1991;52:349–355. doi: 10.1016/0014-4835(91)90100-s. [DOI] [PubMed] [Google Scholar]
- 45.Anderson RE, Maude MB, Nilsson SE, Narfstrom K. Plasma lipid abnormalities in the abyssinian cat with a hereditary rod-cone degeneration. Exp Eye Res. 1991;53:415–417. doi: 10.1016/0014-4835(91)90249-e. [DOI] [PubMed] [Google Scholar]
- 46.Connor WE, Weleber RG, DeFrancesco C, Lin DS, Wolf DP. Sperm abnormalities in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1997;38:2619–2628. [PubMed] [Google Scholar]
- 47.Correa-Cerro LS, Wassif CA, Kratz L, Miller GF, Munasinghe JP, Grinberg A, et al. Development and characterization of a hypomorphic Smith-Lemli-Opitz syndrome mouse model and efficacy of simvastatin therapy. Hum Mol Genet. 2006;15:839–851. doi: 10.1093/hmg/ddl003. [DOI] [PubMed] [Google Scholar]
- 48.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 49.Vaughan DK, Peachey NS, Richards MJ, Buchan B, Fliesler SJ. Light-induced exacerbation of retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82:496–504. doi: 10.1016/j.exer.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bazan NG, Scott BL, Reddy TS, Pelias MZ. Decreased content of docosahexaenoate and arachidonate in plasma phospholipids in Usher’s syndrome. Biochem Biophys Res Commun. 1986;141:600–604. doi: 10.1016/s0006-291x(86)80215-7. [DOI] [PubMed] [Google Scholar]
- 51.Delton-Vandenbroucke I, Maude MB, Chen H, Aguirre GD, Acland GM, Anderson RE. Effect of diet on the fatty acid and molecular species composition of dog retina phospholipids. Lipids. 1998;33:1187–1193. doi: 10.1007/s11745-998-0322-7. [DOI] [PubMed] [Google Scholar]
- 52.Gong J, Rosner B, Rees DG, Berson EL, Weigel-DiFranco CA, Schaefer EJ. Plasma docosahexaenoic acid levels in various genetic forms of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1992;33:2596–2602. [PubMed] [Google Scholar]
- 53.Hoffman DR, DeMar JC, Heird WC, Birch DG, Anderson RE. Impaired synthesis of DHA in patients with X-linked retinitis pigmentosa. J Lipid Res. 2001;42:1395–1401. [PubMed] [Google Scholar]
- 54.Maude MB, Anderson EO, Anderson RE. Polyunsaturated fatty acids are lower in blood lipids of Usher’s type I but not Usher’s type II. Invest Ophthalmol Vis Sci. 1998;39:2164–2166. [PubMed] [Google Scholar]
- 55.Simonelli F, Manna C, Romano N, Nunziata G, Voto O, Rinaldi E. Evaluation of fatty acids in membrane phospholipids of erythrocytes in retinitis pigmentosa patients. Ophthalmic Res. 1996;28:93–98. doi: 10.1159/000267880. [DOI] [PubMed] [Google Scholar]
- 56.Richards MJ, Nagel BA, Fliesler SJ. Lipid hydroperoxide formation in the retina: correlation with retinal degeneration and light damage in a rat model of Smith-Lemli-Opitz syndrome. Exp Eye Res. 2006;82:538–541. doi: 10.1016/j.exer.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Artwohl M, Roden M, Waldhausl W, Freudenthaler A, Baumgartner-Parzer SM. Free fatty acids trigger apoptosis and inhibit cell cycle progression in human vascular endothelial cells. Faseb J. 2004;18:146–148. doi: 10.1096/fj.03-0301fje. [DOI] [PubMed] [Google Scholar]
- 58.Shimabukuro M, Zhou YT, Levi M, Unger RH. Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc Natl Acad Sci U S A. 1998;95:2498–2502. doi: 10.1073/pnas.95.5.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, Zhou Y, et al. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron. 2008;58:884–896. doi: 10.1016/j.neuron.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Das UN. Essential fatty acids, lipid peroxidation and apoptosis. Prostaglandins Leukot Essent Fatty Acids. 1999;61:157–163. doi: 10.1054/plef.1999.0085. [DOI] [PubMed] [Google Scholar]
- 61.Johnson RA, Hamilton JA, Worgall TS, Deckelbaum RJ. Free fatty acids modulate intermembrane trafficking of cholesterol by increasing lipid mobilities: novel 13C NMR analyses of free cholesterol partitioning. Biochemistry. 2003;42:1637–1645. doi: 10.1021/bi0264465. [DOI] [PubMed] [Google Scholar]
- 62.Aikens J, Dix TA. Perhydroxyl radical (HOO.) initiated lipid peroxidation. The role of fatty acid hydroperoxides. J Biol Chem. 1991;266:15091–15098. [PubMed] [Google Scholar]
- 63.Spiteller G. Are changes of the cell membrane structure causally involved in the aging process? Ann N Y Acad Sci. 2002;959:30–44. doi: 10.1111/j.1749-6632.2002.tb02080.x. [DOI] [PubMed] [Google Scholar]
- 64.Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000;29:323–333. doi: 10.1016/s0891-5849(00)00302-6. [DOI] [PubMed] [Google Scholar]
- 65.Kim JS, He L, Lemasters JJ. Mitochondrial permeability transition: a common pathway to necrosis and apoptosis. Biochem Biophys Res Commun. 2003;304:463–470. doi: 10.1016/s0006-291x(03)00618-1. [DOI] [PubMed] [Google Scholar]
- 66.Greenberg ME, Li XM, Gugiu BG, Gu X, Qin J, Salomon RG, et al. The lipid whisker model of the structure of oxidized cell membranes. J Biol Chem. 2008;283:2385–2396. doi: 10.1074/jbc.M707348200. [DOI] [PubMed] [Google Scholar]
- 67.Organisciak DT, Darrow RM, Jiang YL, Blanks JC. Retinal light damage in rats with altered levels of rod outer segment docosahexaenoate. Invest Ophthalmol Vis Sci. 1996;37:2243–2257. [PubMed] [Google Scholar]
- 68.Bloomquist BT, Shortridge RD, Schneuwly S, Perdew M, Montell C, Steller H, et al. Isolation of a putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 69.Ferreira PA, Pak WL. Bovine phospholipase C highly homologous to the norpA protein of Drosophila is expressed specifically in cones. J Biol Chem. 1994;269:3129–3131. [PubMed] [Google Scholar]
- 70.Ferreira PA, Shortridge RD, Pak WL. Distinctive subtypes of bovine phospholipase C that have preferential expression in the retina and high homology to the norpA gene product of Drosophila. Proc Natl Acad Sci U S A. 1993;90:6042–6046. doi: 10.1073/pnas.90.13.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghalayini A, Anderson RE. Phosphatidylinositol 4,5-bisphosphate: light-mediated breakdown in the vertebrate retina. Biochem Biophys Res Commun. 1984;124:503–506. doi: 10.1016/0006-291x(84)91582-1. [DOI] [PubMed] [Google Scholar]
- 72.Ghalayini AJ, Tarver AP, Mackin WM, Koutz CA, Anderson RE. Identification and immunolocalization of phospholipase C in bovine rod outer segments. J Neurochem. 1991;57:1405–1412. doi: 10.1111/j.1471-4159.1991.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 73.Choe HG, Ghalayini AJ, Anderson RE. Phosphoinositide metabolism in frog rod outer segments. Exp Eye Res. 1990;51:167–176. doi: 10.1016/0014-4835(90)90069-7. [DOI] [PubMed] [Google Scholar]
- 74.Hayashi F, Amakawa T. Light-mediated breakdown of phosphatidylinositol-4,5-bisphosphate in isolated rod outer segments of frog photoreceptor. Biochem Biophys Res Commun. 1985;128:954–959. doi: 10.1016/0006-291x(85)90139-1. [DOI] [PubMed] [Google Scholar]
- 75.Hayashi F, Sumi M, Amakawa T. Phosphatidylinositol stimulates phosphorylation of protein components I and II in rod outer segments of frog photoreceptors. Biochem Biophys Res Commun. 1987;148:54–60. doi: 10.1016/0006-291x(87)91075-8. [DOI] [PubMed] [Google Scholar]
- 76.Millar FA, Fisher SC, Muir CA, Edwards E, Hawthorne JN. Polyphosphoinositide hydrolysis in response to light stimulation of rat and chick retina and retinal rod outer segments. Biochim Biophys Acta. 1988;970:205–211. doi: 10.1016/0167-4889(88)90180-2. [DOI] [PubMed] [Google Scholar]
- 77.Gehm BD, Mc Connell DG. Phosphatidylinositol-4,5-bisphosphate phospholipase C in bovine rod outer segments. Biochemistry. 1990;29:5447–5452. doi: 10.1021/bi00475a006. [DOI] [PubMed] [Google Scholar]
- 78.Gehm BD, Pinke RM, Laquerre S, Chafouleas JG, Schultz DA, Pepperl DJ, et al. Activation of bovine rod outer segment phosphatidylinositol-4,5-bisphosphate phospholipase C by calmodulin antagonists does not depend on calmodulin. Biochemistry. 1991;30:11302–11306. doi: 10.1021/bi00111a016. [DOI] [PubMed] [Google Scholar]
- 79.Peng YW, Rhee SG, Yu WP, Ho YK, Schoen T, Chader GJ, et al. Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments. Proc Natl Acad Sci U S A. 1997;94:1995–2000. doi: 10.1073/pnas.94.5.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neilson DE, Eiben RM, Waniewski S, Hoppel CL, Varnes ME, Bangert BA, et al. Autosomal dominant acute necrotizing encephalopathy. Neurology. 2003;61:226–230. doi: 10.1212/01.wnl.0000073544.28775.1a. [DOI] [PubMed] [Google Scholar]
- 81.Neilson DE, Feiler HS, Wilhelmsen KC, Lynn A, Eiben RM, Kerr DS, et al. Autosomal dominant acute necrotizing encephalopathy maps to 2q12.1-2q13. Ann Neurol. 2004;55:291–294. doi: 10.1002/ana.10849. [DOI] [PubMed] [Google Scholar]
- 82.Krebber H, Bastians H, Hoheisel J, Lichter P, Ponstingl H, Joos S. Localization of the gene encoding the Ran-binding protein RanBP2 to human chromosome 2q11–q13 by fluorescence in situ hybridization. Genomics. 1997;43:247–248. doi: 10.1006/geno.1997.4777. [DOI] [PubMed] [Google Scholar]
- 83.Ferreira PA. Characterization of RanBP2-associated molecular components in neuroretina. Methods in enzymology. 2000;315:455–468. doi: 10.1016/s0076-6879(00)15861-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.