Abstract
Diarrhea is widespread in intestinal diseases involving ischemia and/or hypoxia. Since hypoxia alters stimulated Cl− and water flux, we investigated the influence of such a physiologically and pathophysiologically important signal on expression of the cystic fibrosis transmembrane conductance regulator (CFTR). Located on the apical membrane, this cAMP-activated Cl− channel determines salt and fluid transport across mucosal surfaces. Our studies revealed depression of CFTR mRNA, protein, and function in hypoxic epithelia. Chromatin immunoprecipitation identified a previously unappreciated binding site for the hypoxia inducible factor-1 (HIF-1), and promoter studies established its relevance by loss of repression following point mutation. Consequently, HIF-1 overexpressing cells exhibited significantly reduced transport capacity in colorimetric Cl− efflux studies, altered short circuit measurements, and changes in transepithelial fluid movement. Whole-body hypoxia in wild-type mice resulted in significantly reduced small intestinal fluid and HCO3− secretory responses to forskolin. Experiments performed in Cftr−/− and Nkcc1−/− mice underlined the role of altered CFTR expression for these functional changes, and work in conditional Hif1a mutant mice verified HIF-1-dependent CFTR regulation in vivo. In summary, our study clarifies CFTR regulation and introduces the concept of a HIF-1-orchestrated response designed to regulate ion and fluid movement across hypoxic intestinal epithelia.—Zheng, W., Kuhlicke, J., Jäckel, K., Eltzschig, H. K., Singh, A., Sjöblom, M., Riederer, B., Weinhold, C., Seidler, U., Colgan, S. P., Karhausen, J. Hypoxia inducible factor-1 (HIF-1) -mediated repression of cystic fibrosis transmembrane conductance regulator (CFTR) in the intestinal epithelium.
Keywords: anion transporter, ion movement, fluid secretion, mucosa
Control of vectorial salt and liquid movement is a key function of epithelia. It is a prerequisite for body fluid and electrolyte homeostasis and for regulation of the surface liquid volume of the mucus membrane itself. In epithelia Cl− secretion is dependent on entry of Cl− (1, 2) as well as on Cl− efflux, which depends primarily on the cystic fibrosis transmembrane conductance regulator (CFTR) (3). Abnormalities of ion transporter function have been implicated in diarrheal diseases, while conditions of decreased ion transport capacity such as in the genetic disease cystic fibrosis have highlighted the importance of mucosal hydration and mucus clearance as a primary innate defense against pathogens (4).
For reasons of its unique anatomical setting, in the intestinal mucosa even small perturbations of blood flow can lead to rapid metabolic changes characteristic of ischemia and resultant hypoxia. In a number of pathological conditions, reduced availability of oxygen is a common feature causing barrier dysfunction and contributing as proinflammatory stimulus (5). However, previous studies have also revealed adaptive responses, most notably mediated by the hypoxia inducible factor-1 (HIF-1) (5,6,7).
Studies on the influence of hypoxia on epithelial electrogenic Cl− secretion established a direct, noncytotoxic attenuation of stimulated Cl− transport (8,9,10). This is consistent with the recent observation of a HIF-1-mediated alteration of Cl− influx via down-regulation of the Na-K-2Cl cotransporter NKCC1 (11). Our work identifying a parallel repression of CFTR not only proposes novel insights into mucosal physiology but also suggests the presence of an HIF-1-orchestrated mechanism controlling epithelial ion and water transport.
MATERIALS AND METHODS
Epithelial cell culture and in vitro exposure to hypoxia
T84 and Caco-2 epithelial cells were cultured as described (12) and seeded at 106 cells/ml. Standard hypoxic conditions (based on previous work; ref 6) were pO2 20 torr, pCO2 35 torr. At the start of each experiment, medium was exchanged with preequilibrated normoxic or hypoxic medium maintaining consistent medium layer depth throughout the project. Normoxic activation of HIF-1 was achieved with dimethyloxallyl glycine (DMOG, Cayman Chemical, Ann Arbor, MI, USA) at 1 mM for 24 h or following transduction with a constitutive active form of HIF-1 via lentiviral gene transfer as described (13).
Fluid transport assay
T84 cells were seeded onto semipermeable filter supports (Costar, Inc., Cambridge, MA, USA) and grown to confluence. After 5–7 d of culture, resistance was 1095 ± 98 Ω · cm2 for T84 and 1265 ± 89 Ω · cm2 for HIFΔODD-T84. Transmonolayer fluid movement was measured in absence or presence of 50 μM of the cAMP agonist forskolin (Biaffin, Kassel, Germany) as described (10). After 24 h of experimental conditions apical medium was quantified by weight. Barrier integrity was assayed by measurement of lactate dehydrogenase (LDH) in cell medium (CytoTox96 nonradioactive assay, Promega, Madison, WI, USA) or by measuring transepithelial resistance (TER) using a voltmeter (Evohm; World Precision Instruments, New Haven, CT, USA).
Transcriptional analysis
Quantitative genechip expression array from total RNA was performed on T84 cells (Affymetrix, Santa Clara, CA, USA) (14). Samples for polymerase chain reaction (PCR) were obtained using the Nucleospin RNA isolation kit (Macherey-Nagel, Düren, Germany) and the i-script cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA). mRNA expression was quantified by real-time PCR (iCycler, Bio-Rad) and analyzed following the principles established by Pfaffl (15). β-Actin served as a housekeeping gene. Primer sets are specified in Supplemental Table 1. Murine Cftr was quantified using FAM-labeled D-lux primer sets targeted against the exon/exon junction 17/18 and JOE-labeled certified D-lux β-actin primer set in a multiplex reaction (Invitrogen, Carlsbad, CA, USA).
Immunoblotting experiments
Total whole-cell proteins from colonic mucosal scrapings, T84 cells, or Caco-2 cells were analyzed by Western blot as described (16). Where indicated, mouse monoclonal anti-CFTR (Abcam, Cambridge, UK) or rabbit polyclonal anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was applied. HIF-1α was detected in nuclear lysates obtained with the NE-PER nuclear extraction reagents (Pierce, Rockford, IL, USA) using the HIF-1α antibody from Transduction Laboratories (Lexington, KY, USA). The obtained bands were quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA), and results are represented as fold change in ratio of CFTR to respective β-actin optical density.
Functional evaluation of CFTR
Functional in vitro analysis of CFTR function was performed with the colorimetric technique of Tang and Wildey (17). Briefly, microplates containing T84 cells were incubated with iodine-loading buffer. After washing, CFTR was activated through addition of forskolin at indicated concentrations for 5 min. Supernatant was then removed, and cells were washed and lysed. Iodine concentration of lysates was measured with a modified Sandell-Kolthoff reaction. CFTR channel function was calculated and plotted as a coefficient of iodine secretion J, expressed as μeq/h/cm2. Based on conditions established previously (10), a subset of hypoxic cells was reoxygenated for 2 h in fresh medium containing 1 mM 8-bromo-cAMP before functional analysis. Specificity of the assay was evaluated in cells treated with 50 μM forskolin and increasing concentrations of CFTR inhibitor CFTRinh172 (18).
Electrophysiological studies
To measure agonist-stimulated short-circuit current, transepithelial potential, and resistance, we used a voltage clamp (Iowa Dual Voltage Clamps, Bioengineering, University of Iowa, Iowa City, IA, USA) interfaced with an equilibrated pair of calomel electrodes and a pair of Ag-AgCl electrodes. Cl− secretory responses are expressed as peak short-circuit current Isc across the monolayer. Using the same principle, Isc measurements were performed in isolated jejunal, ileal, proximal colonic, and distal colonic mucosa, as detailed elsewhere (19).
Chromatin immunoprecipitation (ChIP) assay
ChIP was performed on Caco-2 cells as described elsewhere (13). Following isolation, sheared chromatin was incubated with anti-HIF-1α antibody, and immune complexes were precipitated with protein A-sepharose beads. HIF-1 binding to the CFTR 5′ untranslated region was quantified by standard PCR (primers: sense, 5′-ACACTCGCGCGCTCGCTC-3′; antisense, 5′-GGCCGCGGCTCCATAGCTGC-3′), which amplified a 217 bp region spanning the putative HIF-1 binding site. Chromatin incubated with immunoglobulin G (IgG) plus sepharose or with beads alone was used to control for nonspecific binding of DNA.
Luciferase reporter assay
The CFTR-pTL-LUC promoter construct (Panomics, Fremont, CA, USA) contains the 5′-flanking sequence upstream of exon 1 of the human CFTR gene including the transcriptional start site. Reporter assays were performed on Caco-2 cells applying standard methods of overnight transfection and the PolyFect transfection reagent (Qiagen, Hilden, Germany), followed by hypoxic or normoxic exposure as indicated. Luciferase activity was assessed on a TD20/20 luminometer (Turner Designs, Fresno, CA, USA) and the dual luciferase assay system (Promega, Madison, WI, USA). All activity was normalized to the activity of cotransfected Renilla control reporter.
Site-directed mutagenesis
The core consensus sequence of the HIF responsive element (HRE) was altered from 5′-ACGTG-3′ (WT-CFTRpluc) into 5′-ACATA-3′ (ΔHRE-CFTRpluc) using the QuickChange site-directed mutagenesis kit (Stratagene, LaJolla, CA, USA). These changes did not modify other transcription factor binding sites as predicted by computational simulation (Matinspector; http://www.genomatix.de). Successful mutation was confirmed by sequencing.
Animal studies
All studies were approved by the University of Tübingen and Hannover Medical School committee on investigations involving animals and the respective governmental agencies. Animals were matched for age and sex, the former being 6–8 wk (hypoxia experiments) and 2–3 months (Cftr−/− and Nkcc1−/− mice due to delay in body weight of Nkcc1-deficient mice). The Nkcc1-deficient mice had been generated in the laboratory of Gary Shull (University of Cincinnati, Cincinnati, OH, USA) (20). Both Cftrtm1Cam and Nkcc1 animals were bred for many generations in the NMRI backround. The whole-body hypoxia model was performed on wild-type (WT) C57BL/6/129 svj mice or conditional Hif1a-mutant mice and their littermate controls. Mice were exposed to either normobaric hypoxia (8% O2, 92% N2) or room air for 4 or 24 h (n=4–6/condition), as described previously (21). For normoxic induction of HIF-1, a subset of WT mice was treated with 8 mg DMOG by intraperitoneal injection for 48 h prior to initiation of hypoxia. Such treatment had induced activation of HIF-1 in a previous study (22). Control mice were injected with PBS. Mucosal scrapings were used for both mRNA and protein assays.
Surgical procedure for measurement of duodenal HCO3− secretion
Earlier work had established anesthesia and standard surgical procedures (23). Through a left carotid artery catheter, a continuous infusion of isotonic sodium carbonate solution (200 mM Na+ and 100 mM CO3; ref. 2) at 0.3 ml/h was commenced 1 h before start of measurements. Blood pH and [HCO3−] were 7.39 ± 0.04 and 28 ± 6 mM, respectively. The duodenum was cannulated as described and gently perfused at 0.25 ml/min with prewarmed 154 mmol/L NaCl. Following a recovery period, basal bicarbonate secretion was measured before luminal addition of 10−4 M forskolin. HCO3− outputs were determined in 10-min periods by back-titration (PHM82 Standard pH meter; Radiometer, Copenhagen, Denmark) as reported (24). HCO3− measured in the effluents from the duodenal segment ranged from 0.1 to 1.0 mmol/L, and standards showed excellent correlation between amounts of HCO3− added and recovered. Rates of luminal alkalinization were expressed as micromoles of base secreted per centimeter of intestine per hour (μmol/cm/h).
Measurement of jejunal fluid absorption and secretion
The method for measurement of jejunal fluid movements was adapted from Clayburgh et al. (25). A 5-cm segment of midjejunum was cannulated as described for the duodenum. To prevent fluid accumulation, the distal end of the jejunum preceding this isolated loop was drained. The jejunal lumen was perfused with 5 ml of prewarmed Hepes-buffered Ringer solution containing 2 mM ferrocyanide in a recirculating system at 0.5 ml/min. Forskolin-stimulated secretion was determined by measuring the concentration of nonabsorbable ferrocyanide in recirculating fluid before the start of the experiment, at the end of the basal period, and after application of 10−5 M luminal forskolin for 30 min.
Data analysis
Bivariate analysis was performed using Student’s t test. In vivo HCO3− secretion was compared with ANOVA. Values are expressed as means ± se. Values of P < 0.05 were considered significant.
RESULTS
Transcriptional repression of CFTR in hypoxic epithelia
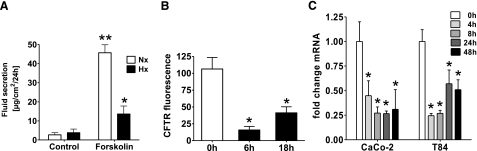
Epithelia (T84) displayed a markedly blunted response to forskolin-stimulated fluid movement after 24 h hypoxic exposure [Fig. 1A; 45.7±1.5 vs. 13.6±4.2 μg/cm2/24 h for normoxic (Nx) and hypoxic (Hx) treatment, respectively; P≤0.05], indicating a decreased sensitivity of the cAMP-responsive ion and water flux pathway. Such differences were not seen across unstimulated monolayers (2.7±1.1 vs. 3.8±1.9 μg/cm2/24 h, Nx vs. Hx) and were not attributable to changes in resistance, a reliable marker of barrier integrity (99.2±8.1 and 97.9±13.2% of initial TER at 24 and 48 h). Furthermore, LDH was not elevated after hypoxic exposure (data not shown). To characterize such secretory changes, transcriptional profiling of T84 cells was performed (Fig. 1B) and identified a selective hypoxia-dependent down-regulation of CFTR (0.15±0.05- and 0.39±0.08-fold at 6 and 18 h; P≤0.05). In confirmation, real-time PCR (Fig. 1C) revealed decreased transcript levels with maximal inhibition after 8 h in Caco-2 (0.27±0.06-fold) and 4 h in T84 (0.24±0.01-fold; P≤0.02). During ongoing hypoxia, however, repression abated but remained significantly depressed after 48 h (0.51±0.1- and 0.31±0.02-fold for Caco-2 and T84; P≤0.05).
Figure 1.
Altered epithelial fluid transport in hypoxia and transcriptional repression of CFTR. A) Basolateral to apical fluid movement across T84 monolayers was measured in hypoxic (Hx; black bars) vs. normoxic epithelia (Nx; open bars) in absence or presence of 50 μM forskolin. B) Confluent T84 cells were exposed to normoxia or 6 and 18 h of hypoxia, and relative expression of CFTR was quantified by microarray analysis. C) Confirmation of CFTR hypoxic repression by real-time PCR in Caco-2 and T84 cells as indicated. Results are pooled from 3–5 individual monolayers in each condition from 3 separate experiments and expressed as means ± se; *P ≤ 0.05 vs. respective normoxic condition, **P ≤ 0.05 vs. respective unstimulated control.
Functionally relevant repression of CFTR protein in hypoxia
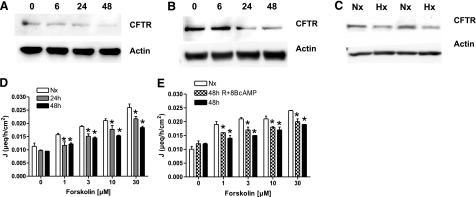
SDS-gel electrophoresis from epithelial cell lysates demonstrated a hypoxia time-dependent decrease of CFTR both in Caco-2- (Fig. 2A) and T84 cells (Fig. 2B), resulting in a 0.22 ± 0.05-fold (Caco-2) and 0.05 ± 0.008-fold (T84) change after 48 h of hypoxia (Supplemental Fig. 1A, B). In vivo relevance was obtained in a murine whole-body hypoxia model (4 h, 8% O2, Fig. 2C), revealing reduced CFTR protein levels after hypoxic exposure in small intestinal mucosal scrapings (0.33±0.12-fold, Supplemental Fig. 1C). Functional consequences of altered CFTR expression were determined using stimulated efflux of iodine as a colorimetrically measurable surrogate (26). Hypoxic T84 cells (Fig. 2D) displayed reduced sensitivity to forskolin-stimulated iodine efflux (10 μM forskolin; 0.021±0.0007, 0.0177±0.00015, and 0.0153±0.0002 μeq/h/cm2 for Nx, 24 h, and 48 h treatment; P≤0.05). Specificity for CFTR was established by preliminary experiments showing a dose-dependent antagonism to forskolin-stimulated iodine efflux by the CFTR inhibitor CTRInh172 (data not shown). States of metabolic stress are known to decrease cellular cAMP levels and therefore CFTR activity. To discriminate the influence of transcriptional regulation, T84 cells were exposed to hypoxia in presence of 8-bromo-cAMP to add back cellular cAMP and were reoxygenated before analysis (48+R+cAMP treatment). Following this treatment, only small differences in iodine efflux capacity were observed (10 μM forskolin; 0.021±0.001, 0.017±0.001, and 0.018±0.0002 μeq/h/cm2, for Nx, 48 h Hx, and 48+R+cAMP treatment; P≤0.05). Taken together, these data indicate that transcriptional regulation of CFTR abundance is associated with a consistent reduction of stimulated chloride efflux capacity in hypoxic epithelia.
Figure 2.
Epithelial hypoxia attenuates CFTR protein and function. A, B) Caco-2 (A) and T84 cells (B) were exposed to normoxia or indicated times of hypoxia, and cell lysates were examined by Western blot using specified antibodies. Blots are representative of 3 independent experiments. C) In a whole-body murine hypoxia model (Hx; 4 h), Western blot analysis for CFTR was performed on small intestinal mucosal scrapings. β-Actin served as control. Western blots are representative for 3 separate experiments; n = 2/condition. Iodine efflux in hypoxic epithelia was measured colorimetrically. D) T84 cells were exposed to indicated times of hypoxia, and efflux of loaded iodine was measured. E) T84 cells were either exposed to normoxia (empty bars) and hypoxia (48 h; filled bars) alone or exposed to hypoxia and reoxygenated in presence of 8-bromo-cAMP before measuring iodine efflux (48h+R+cAMP; checkered bars). Data are expressed as coefficient of iodine secretion J ± sem; *P ≤ 0.05 vs. normoxia.
Hypoxic repression of CFTR is HIF-1 dependent
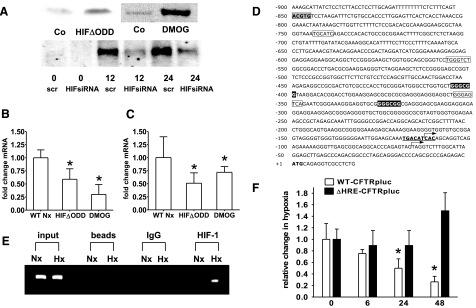
As HIF-1 is known to influence components of electrogenic Cl− transport (11), we investigated its influence on CFTR expression. For purposes of HIF-1 overexpression, we used cell lines that expressed oxygen-stable HIF-1 (HIFΔODD) or were treated with DMOG (1 mM for 24 h). Figure 3A demonstrates successful alteration of the HIF-1 activation in Caco-2 cells but is representative also for T84 cells. Such normoxic HIF-1 activation caused down-regulation of CFTR transcript (Caco-2: 0.61±0.05- and 0.62±0.1-fold for HIFΔODD and DMOG, Fig. 3B; T84: 0.59±0.06- and 0.3±0.09-fold for HIFΔODD and DMOG, Fig. 3C; P≤0.05). Analysis of the CFTR promoter sequence (Fig. 3D) yielded a previously unappreciated HRE at −850 bp from the initiation codon identified by White et al. (27). Physical interaction of HIF-1 to this site was verified by ChIP (Fig. 3E), which showed enhanced HIF-1 binding under hypoxic conditions. Next, we analyzed a luciferase construct bearing the CFTR promoter (Fig. 3F). Hypoxic exposure decreased luciferase activity in this construct by 0.49 ± 0.02-fold after 24 h and 0.27 ± 0.03-fold after 48 h (P≤0.05). Inactivation of the HRE by point mutation, however, abolished repression, while having no effect on CFTR basal activity. This identifies HIF-1 as regulator of the CFTR hypoxic response.
Figure 3.
CFTR repression is mediated by HIF-1. A) Western blot analysis of nuclear Caco-2 protein samples. Constitutive HIF-1 activation was achieved by using cells expressing HIFΔODD or following hydroxylase inhibition (DMOG; 1 mM for 24 h). B, C) CFTR mRNA levels in Caco-2 (B) and T84 cells (C) under conditions of normoxic HIF-1 activation. Results are means ± se from 3 separate experiments; *P ≤ 0.05 vs. control, which integrates results from respective controls for each HIF-1 intervention. D) Sequence of the CFTR promoter; numbering is relative to the initiation codon (boldface). Putative hypoxia responsive element (gray box), AP-1 sites (boxed), GC-box (inverted type), and a binding site for CREB (bold, underlined) are highlighted. Arrows indicate the tissue-specific transcription start sites identified for the small intestine and intestinal epithelial cell lines (27, 43). E) ChIP demonstrates HIF-1 binding to genomic DNA corresponding to the CFTR 5′-UTR in normoxic (Nx) and hypoxic (Hx) Caco-2 cells (24 h). Reaction controls included PCR performed on genomic DNA (input), on samples precipitated by protein A sepharose beads (beads) or by beads with IgG. F) CFTR promoter activity was examined with luciferase constructs driven by the CFTR promoter. WT (WT-CFTRpLuc) was compared to a promoter construct bearing a mutation in the HIF-1 binding site (ΔHRE-CFTRpLuc). Promoter activity was evaluated following indicated periods of hypoxia in Caco-2 cells. Results are calculated from the ratio of luciferase-activity to activity of cotransfected control plasmid (renilla), presented as means ± se from 3 experiments; n = 3/condition; *P ≤ 0.05 vs. normoxia.
In vitro relevance of CFTR regulation by HIF-1
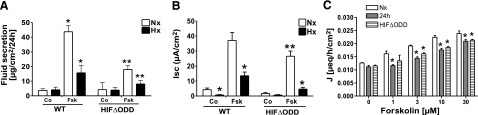
As proof of principle, we investigated the water movement in T84 cells constitutively overexpressing HIF-1 (HIFΔODD). These cells exhibited an abrogated fluid response to forskolin stimulation (4.28±0.3- vs. 11.8-fold change on forskolin stimulation in HIFΔODD-Nx and WT-Nx, respectively) indicating a relevant inhibition of the responsible mechanism by isolated HIF-1 activation (Fig. 4A). As previously demonstrated, hypoxia resulted in attenuated 1 μM forskolin-stimulated electrogenic Cl− secretion (Fig. 4B), measured in T84 cells as a function of short-circuit current Isc (37.05±5.21 vs. 13.53±2.47 μA/cm2 for Nx and Hx; P≤0.05). Overexpression of HIF-1 (HIFΔODD) blunted such Isc responses in normoxia and hypoxia (26.52±3.45 vs. 4.61±1.2 μA/cm2 for HIFΔODD-Nx and -Hx; P≤0.05 vs. respective WT value), thereby implicating HIF-1 pathways in this response. These changes were not mirrored by alterations in resistance, which indicated increased values for HIF overexpressing cells (200.7±10.4 and 169.1±5.3% at 24 and 48 h vs. 103.7±11.4 and 98.9±9.1% of initial TER in WT-T84). Normoxic HIF-1 activation also decreased stimulated iodine efflux (Fig. 4C), which was less sensitive to forskolin in HIFΔODD-T84 cells. Such changes paralleled largely the characteristics of hypoxic WT T84 cells and indicated a similar decline in Cl− transport capacity under both conditions (10 μM forskolin; 0.0224±0.0006, 0.01757±0.00058, and 0.0186±0.00027 μeq/h/cm2 for Nx, 24 h Hx, and HIFΔODD; P≤0.05).
Figure 4.
HIF-1-mediated alterations of water and ion movement in vitro. A) Fluid movement was determined in T84 cells constitutively overexpressing HIF-1 (HIFΔODD). A subset of cells was stimulated with forskolin (Fsk; 50 μM), and monolayers were exposed to either normoxia (Nx; open bars) or hypoxia (Hx; solid bars). Total amount of fluid on the apical surface was determined by weight. B) Measurement of 1 μM forskolin-stimulated electrogenic Cl− secretion, as a function of Isc. Cl− secretory responses were compared in WT vs. HIF-1-overexpressing (HIFΔODD) T84 cells subjected to normoxia or 24 h hypoxia. Data are means ± se; *P ≤ 0.05 vs. WT normoxia; **P ≤ 0.05 vs. respective WT condition. C) Calorimetric assay of iodine efflux in normoxic T84 cells constitutively overexpressing HIF-1 (HIFΔODD; striped bars) compared to WT T84 cells subjected to normoxia (open bars) or 24 h hypoxia (solid bars). Data are expressed as coefficient of iodine secretion J ± sem (μeq/h/cm2); *P ≤ 0.05 vs. normoxia. All represented data are calculated from 3 separate experiments; n ≥ 3 monolayers/condition.
Functionally relevant repression of CFTR by HIF-1 in vivo
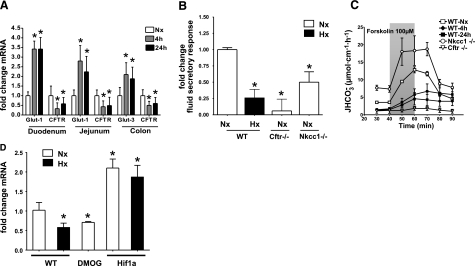
To establish conditions for ensuing experiments, we determined CFTR mRNA levels in small and large intestinal samples of mice exposed to 4 and 24 h of hypoxia (8% O2). As shown in Fig. 5A, CFTR transcript levels were diminished compared to normoxic controls in duodenum (0.33±0.31 vs. 0.58±0.33 at 4 and 24 h; P≤0.05), jejunum (0.43±0.31 vs. 0.49±0.43 at 4 and 24 h; P≤0.05) and colon (0.28±0.21 vs. 0.6±0.31 at 4 and 24 h; P≤0.05). The known HIF-1 target genes glucose transporter 1 and 3 served as positive controls for known HIF-1-dependent gene regulation. In contrast, Slc26a3 mRNA levels did not differ significantly in hypoxia (Supplemental Fig. 2). This suggests that hypoxia may differentially affect the expression of anion transporters with a predominantly secretory (CFTR) vs. absorptive (Slc26a3) function.
Figure 5.
Functionally relevant repression of CFTR by HIF-1 in vivo. A) Quantitative PCR for glucose transporter 1 and 3 or CFTR after normoxic (Nx; open bars) or hypoxic (Hx; 4 h, gray bars; 24 h, solid bars) exposure in WT mice. RNA was isolated from mucosal scrapings of duodenum or jejunum as indicated. B) In vivo measurement of jejunal fluid absorption. Results are calculated as fold change of forskolin stimulated fluid secretory response vs. response obtained from WT normoxic mice. Integrated into control data are results from the respective WT littermates of the following experimental groups: WT hypoxic animals (24 h), Cftr−/− animals, and Nkcc1−/− animals. Results are means ± se; n = 6–10/condition, n = 18 for integrated control group; *P ≤ 0.05 vs. control. C) In vivo duodenal HCO3− measurement after whole-body hypoxia compared to Cftr−/− and Nkcc1−/− mice. Control group data are merged from respective normoxic WT littermates for each experimental population. Results are means ± se; n = 6–10/condition, n = 22 for control group. D) Quantitative RT-PCR for CFTR from mucosal scrapings of conditional HIF1a mutant mice (HIF1a), WT mice treated with DMOG, and WT control mice in a whole-body hypoxia model [Nx, open bars; Hx (4 h), solid bars]. Results are mean ± se fold change vs. WT normoxia from 3 separate experiments; *P ≤ 0.05.
No significant differences in the percentage of bumetanide-sensitive Isc response vs. total Isc response to forskolin in small or large intestinal mucosa were observed (Supplemental Fig. 3). In Nkcc1−/− animals, forskolin-stimulated Isc was 48 ± 9% of that in control tissues in the jejunum (n=7), and 46 ± 6% (n=6) of that in control tissue in the proximal colon; the bumetanide-sensitive Isc was 0. In contrast, fluid secretory response (Fig. 5B) was significantly reduced in Cftr-deficient mice (0.06±0.18-fold change; P≤0.05) and after 24 h of hypoxia (0.26±0.13-fold change; P≤0.05) to stimulation with 10−4 M forskolin applied to an isolated jejunal loop, while the response in normoxic WT animals was robust (0.12±0.019 ml/cm/h for all control mice and across all genotypes; n=16). These differences were seen despite the fact that hypoxic animals were reoxygenated for 1 h and given intravascular fluid and base supplementation to restore normal blood pH. The response in Nkcc1-mutant mice was decreased (0.5±0.16-fold change; P≤0.05) to a lesser extent, indicating that effects of chronic hypoxia on NKCC1 expression could not be exclusively responsible for secretory changes in hypoxia.
In forskolin-stimulated duodenal HCO3− secretion (Fig. 5C), Nkcc1-deficient animals displayed a distinct increase in basal HCO3− secretion rate and an enhanced stimulated response (P≤0.05; ANOVA). However, while normoxic controls showed a robust HCO3− secretory response to forskolin, this response was strongly diminished in mice exposed to 4 and 24 h of hypoxia and was absent in Cftr-deficient animals (each P≤0.05; ANOVA).
To demonstrate HIF-1 dependency of CFTR repression also in vivo, we analyzed conditional Hif1a-mutant animals, which lack detectable Hif1a expression in >70% of intestinal epithelial cells (21). In real-time PCR analysis, these animals exhibited a 2.1 ± 0.26-fold (P≤0.05) increase of CFTR mRNA in colonic epithelia (Fig. 5D), and CFTR expression changed little after hypoxic exposure vs. a 0.46 ± 0.08-fold change in WT hypoxic mice (P≤0.05). Normoxic HIF-1 overexpression induced by systemic treatment with the HIF-1 activator DMOG caused CFTR repression to a similar degree seen after hypoxic exposure (0.51±0.02; P≤0.05).
DISCUSSION
CFTR is the major mechanism for epithelial Cl− secretion and therefore a central modulator of Na+ and water flux. Recent work established additional roles of CFTR as regulator of a number of epithelial transport proteins and further underlined CFTR’s importance for transmucosal electrolyte transport (28). However, our understanding of CFTR transcriptional regulation itself is still incomplete. First, indications that CFTR may be hypoxia responsive stem from the work of Bebök et al. (29), who underscored the influence of specific metabolic profiles for the cellular hypoxia response. While we have normalized such factors as cell density (30) and liquid layer depth (29) known to affect in vitro cellular oxygenation, the conditions of physiological normoxia remain undefined. In the context of intestinal epithelial cells 147 torr is probably unphysiologically high, even when taking into account differences between gas phase and epithelial surface. To be consistent with previous work, we defined 20 torr as hypoxia, but in vivo measurements suggest a pO2 of ∼36 torr for the untreated colonic epithelial surface and as low as 5 torr during inflammation (31).
States of epithelial hypoxia are clinically associated with diarrhea (32), attributed to changes in permeability (33), reduced absorptive processes (34), and mediator-induced stimulation of Cl− secretion (35). Thus, the consistent observation (10, 11) of reduced electrogenic Cl− secretion in hypoxic epithelia would not have been easily predicted. However, besides being an adaptive response to compensate for fluid losses, down-regulation of this highly energy-consuming secretory machinery serves to maintain energy stores and is observed in various epithelial cells in dependence to oxygen availability (9,10,11, 36). In addition, other metabolic sensors are known to influence CFTR activity, such as AMPkinase (37) and cAMP-PKA-dependent phosphorylation (38). We have previously shown that cAMP levels decrease following hypoxic exposure and that this is responsible in part for diminished stimulated Cl− conductance in hypoxic epithelia (10, 16). However, in agreement with this work (10), reoxygenation and cAMP addback did not lead to recovery of ion transport characteristics in our hands, and secretory responses in small intestine of mice subjected to hypoxia remained severely dampened after more than 2 h of reoxygenation. This suggested that transcriptional regulation of CFTR abundance substantially influences ion transport under hypoxic conditions.
HIF-1 is a ubiquitous hypoxia responsive factor involved in a number of mechanisms that help maintain barrier integrity and is known to affect epithelial ion movement (11). Using 2 in vitro systems of HIF-1 overexpression, we consequently demonstrated HIF-1 involvement in CFTR regulation. CFTR transcriptional control, however, is complex and other pathways of hypoxic CFTR modulation cannot be excluded. A number of transcription factors relevant for CFTR regulation, such as NFκB (39) and the cAMP responsive element binding protein (CREB) (40) are also hypoxia responsive (41). CREB degradation seen under hypoxic conditions (42) could thus contribute to transcriptional repression of CFTR documented by our data. This is hard to fully rule out, because of the importance of CREB binding for CFTR basal expression and the consequent difficulty to appreciate HIF repression in a promoter bearing a mutated CREB binding site. Still, experiments under conditions of normoxic HIF-1 activation support HIF-1 to be the major factor in CFTR hypoxic repression. Furthermore, our finding of a previously unappreciated HIF-1 responsive element at −850 bp is consistent with previous work on promoter truncations, which had suggested a repressive element between −1036 and −718 (43). Loss of repression on mutation of this HRE consequently establishes HIF-1 regulatory involvement. The effect of HRE disruption on the hypoxic response cannot be easily foreseen, however, and the finding of increased ΔHRE-CFTRpluc activity in late hypoxia might indicate that such an alteration allows for a CFTR stimulatory mechanism to gain significance. Similarly, PCR data had indicated a blunted response of CFTR mRNA repression in chronic hypoxia. Conflicting results also exist for oxygen-dependent CFTR regulation in different tissues (44,45,46). Currently, too little is known about the molecular switches of CFTR to fully explain such differences. However, because CFTR is differentially expressed in various tissues and bears tissue-specific transcription start sites (27), it is conceivable that CFTR activity is not required at the same level in all tissues and at all times.
The forskolin-induced secretory response displays CFTR dependency in a variety of CFTR-expressing cell lines, based on its absence after siRNA-mediated CFTR knockdown (47, 48). The significant decrease specifically in forskolin-stimulated fluid secretion in hypoxic cells and the abrogation of this response in both normoxic and hypoxic HIF-overexpressing cells is therefore a strong indication for an HIF-1 modulated, CFTR-dependent secretory response. Equally, forskolin-induced Isc was reduced in HIF-1-overexpressing T84 cells under control conditions and further decreased in hypoxia. However, HIF-1 being regulator of a comprehensive hypoxia adaptive response implies alterations of barrier phenotype distinct from CFTR-expression changes and constituting a considerable experimental challenge. The exact role of HIF-1 in barrier maintenance is unclear at this point, as some barrier protective mechanisms are induced by HIF-1 (6, 7, 12), while conversely a number of tight junctional proteins are down-regulated (49, 50). To control such factors in vitro, care was taken that monolayers had a comparable TER at begin of the functional assays. Still, we cannot rule out that differences in barrier maturity were basis to some of the changes seen, for example, when measuring water flux. Although this assay implicates immediate physiological relevance, it measures net effects influenced by a number of proteins, some of which, such as Aquaporin 5 (51) or EnaC (52), have been associated with altered function in hypoxic states or direct HIF-1 responsiveness.
Equally, experiments in the native intestine bear a number of experimental challenges. As such, the hypoxic response is known to alter during chronic hypoxia due to counteradaptive mechanisms (53, 54). Therefore, in vivo experiments were performed after prolonged hypoxia to characterize the stability of this response. However, since many of our earlier data stem from shorter hypoxic exposures (6, 12), key experiments were performed following shorter hypoxic exposure times. Both our in vitro and in vivo expression studies suggest, as indicated above, that CFTR suppression is most pronounced during early hypoxia, whereas functional data establish the prolonged consequences of such down-regulation.
Experiments to fully characterize the CFTR-dependent secretory responses in hypoxic animals were impeded by technical reasons in the colon, probably because the manipulation necessary to empty the stool elicits long-lasting secretory reflexes, which prevent stable baseline measurements. Measurement of Isc responses to forskolin in native intestine did not demonstrate a distinct hypoxic epithelial phenotype. This measurement is dependent on functional CFTR expression (55, 56), but it has been observed that approximately 20% of CFTR protein expression is able to generate the same cAMP-dependent Isc as observed in WT tissue (57). Conversely, the study of isolated intestinal mucosa in Ussing chambers is well suited to pick up dysregulation of CFTR due to trafficking defects, lack of CFTR-binding proteins (58), AMPK-activation (59), or NKCC1 function (2). The lack of difference in bumetanide-sensitive Isc in any of the intestinal segments thus argues against these mechanisms being the primary reason for secretory dysfunction after hypoxic exposure.
In contrast to Isc measurements, we and others had observed a significant difference in small intestinal fluid secretory response to forskolin in Cftr+/− mice in vivo (ref. 60 and unpublished observations), suggesting that reductions in CFTR expression to the degree observed during hypoxia (below 50% but above 20%) may cause a measurable decrease in fluid secretory response. Likewise, we had observed that the HCO3− secretory response in vivo in Cftr+/− duodenum is decreased compared to controls. In addition, both assays distinguished clearly between NKCC1 deficiency on the one hand and CFTR deficiency and the hypoxic phenotype on the other hand. During stimulation of intestinal anion secretion, NKCC1 is responsible for ∼50% of the basolateral Cl− uptake destined for secretion and alternative anion uptake pathways exist (2). CFTR, in contrast, is the major pathway for stimulated anion secretion in the brush border membrane of enterocytes. However, despite the precautions we took to reoxygenate and correct the blood pH, it is clear that additional, presently unknown factors may influence the anion secretory response in hypoxia, and other electrolyte transport abnormality were observed (e.g., a reduction in basal fluid absorption and a decrease in blood to lumen clearance for BSA as well as 51Cr-EDTA).
In summary, evidence exists that the necessity of the intestinal epithelium to function within an adverse microenvironment might have lead to a distinct hypoxia adaptive response (12). Because of the role of CFTR-dependent Cl− secretion in enterotoxin-mediated intestinal fluid secretion in infectious diarrheas (18), CFTR becomes an attractive target for development of inhibitors with antidiarrheal efficacy. Given that HIF-1 activation is observed under various conditions of inflammation (21, 61) and during infection (62), HIF-1-mediated CFTR depression might constitute a novel endogenous compensatory mechanism. Here our data document that altered HIF expression is sufficient to cause significant down-regulation of CFTR and measurably change the behavior of the Cl− secretory machinery. Such an innate, adaptive response is of immediate importance for conditions involving intestinal epithelial hypoxia or inflammation and enhances our understanding of intestinal fluid and electrolyte movements.
Acknowledgments
Support came from grants KA2330/1-2 (J. Karhausen) and Se460/13-4 (U.S.) of the Deutsche Forschungsgemeinschaft, SFB621 grant C9 (U.S.), a training grant of EuroCareCF (to J. Karhausen; EuroCareCF is supported by the European Community’s Sixth Framework Programme for Research, contract LSHM-CT-2005-018932), and U.S. National Institutes of Health grants HL60569 and DK50189 (S.P.C.). The Interdisziplinäres Zentrum für Klinische Forschung, University of Tübingen, gave support through a Verbundprojekt (J. Karhausen and H.K.E.) and within the Promotionskolleg (K.J.). M.S. was supported by an exchange scientists grant from the Lower Saxony Ministry of Science, and A.S. by a Ph.D. grant from the Hannover Biomedical Research School. The authors thank Dr. B. Scholte and Prof. K. Unertl for exceedingly helpful discussions and support and Michaela Hoch-Gutbrot and Irene Vollmer for technical assistance.
References
- Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- Walker N M, Flagella M, Gawenis L R, Schull G E, Clarke L L. An alternate pathway of cAMP-stimulated cl secretion across the NKCC1-null murine duodenum. Gastroenterology. 2002;123:531–541. doi: 10.1053/gast.2002.34757. [DOI] [PubMed] [Google Scholar]
- Field M. Intestinal ion transport and the pathophysiology of diarrhea. J Clin Invest. 2003;111:931–943. doi: 10.1172/JCI18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M R, Boucher R C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta G T, Dzus A L, Taylor C T, Colgan S P. Parallel induction of epithelial surface-associated chemokine and proteoglycan by cellular hypoxia: implications for neutrophil activation. J Leukoc Biol. 2000;68:251–259. [PubMed] [Google Scholar]
- Synnestvedt K, Furuta G T, Comerford K M, Louis N, Karhausen J, Eltzschig H K, Hansen K R, Thompson L F, Colgan S P. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 (HIF-1) mediated permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford K M, Wallace T J, Karhausen J, Louis N A, Montalto M C, Colgan S P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62:3387–3394. [PubMed] [Google Scholar]
- Papen M, Wodopia R, Bartsch P, Mairbaurl H. Hypoxia-effects on Ca(i)-signaling and ion transport activity of lung alveolar epithelial cells. Cell Physiol Biochem. 2001;11:187–196. doi: 10.1159/000047805. [DOI] [PubMed] [Google Scholar]
- Mairbaurl H, Wodopia R, Eckes S, Schulz S, Bartsch P. Impairment of cation transport in A549 cells and rat alveolar epithelial cells by hypoxia. Am J Physiol. 1997;273:L797–L806. doi: 10.1152/ajplung.1997.273.4.L797. [DOI] [PubMed] [Google Scholar]
- Taylor C T, Lisco S J, Awtrey C S, Colgan S P. Hypoxia inhibits cyclic nucleotide-stimulated epithelial ion transport: role for nucleotide cyclases as oxygen sensors. J Pharmacol Exp Ther. 1998;284:568–575. [PubMed] [Google Scholar]
- Ibla J C, Khoury J, Kong T, Robinson A, Colgan S P. Transcriptional repression of Na-K-2Cl cotransporter NKCC1 by hypoxia-inducible factor-1. Am J Physiol Cell Physiol. 2006;291:C282–289. doi: 10.1152/ajpcell.00564.2005. [DOI] [PubMed] [Google Scholar]
- Furuta G T, Turner J R, Taylor C T, Hershberg R M, Comerford K, Narravula S, Podolsky D K, Colgan S P. Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med. 2001;193:1027–1034. doi: 10.1084/jem.193.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H K, Abdulla P, Hoffman E, Hamilton K E, Daniels D, Schönfeld C, Löffler M, Reyes G, Duszenko M, Karhausen J, Robinson A, Westerman K A, Coe I R, Colgan S P. HIF-1-dependent repression of equilibrative nucleoside transporter (ENT) in hypoxia. J Exp Med. 2005;202:1493–1505. doi: 10.1084/jem.20050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart D J, Dong H, Byrne M C, Follettie M T, Gallo M V, Chee M S, Mittmann M, Wang C, Kobayashi M, Horton H, Brown E L. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nat Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- Pfaffl M W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C T, Fueki N, Agah A, Hershberg R M, Colgan S P. Critical role of cAMP response element binding protein expression in hypoxia-elicited induction of epithelial tumor necrosis factor-α. J Biol Chem. 1999;274:19447–19454. doi: 10.1074/jbc.274.27.19447. [DOI] [PubMed] [Google Scholar]
- Tang W, Wildey M J. Development of a colorimetric method for functional chloride channel assay. J Biomol Screen. 2004;9:607–613. doi: 10.1177/1087057104266740. [DOI] [PubMed] [Google Scholar]
- Ma T, Thiagarajah J R, Yang H, Sonowane N D, Folli C, Galietta L J V, Verkman A S. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler U, Rottinghaus I, Hillesheim J, Chen M, Riederer B, Krabbenhöft A, Engelhardt R, Wiemann M, Wang Z, Barone S, Manns M P, Soleimani M. Sodium and chloride absorptive defects in the small intestine in Slc26a6 null mice. Eur J Physiol. 2007;455:757–766. doi: 10.1007/s00424-007-0318-z. [DOI] [PubMed] [Google Scholar]
- Flagella M, Clarke L L, Miller M L, Erway L C, Giannella R A, Andringa A, Gawenis L R, Kramer J, Duffy J J, Doetschman T, Lorenz J N, Yamoah E N, Cardell E L, Schull G E. Mice lacking the basolateral Na-K-2Cl cotransporter have impaired epithelial chloride secretion and are profoundly deaf. J Biol Chem. 1999;274:26946–26955. doi: 10.1074/jbc.274.38.26946. [DOI] [PubMed] [Google Scholar]
- Karhausen J, Furuta G T, Tomaszewski J E, Johnson R S, Colgan C T, Haase V H. Epithelial hypoxia-inducible factor 1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins E P, Seeballuck F, Keely S J, Mangan N E, Callanan J J, Fallon P G, Taylor C T. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Sjöblom M, Nylander O. Isoflurane-induced acidosis depresses basal and PGE2 stimulated duodenal bicarbonate secretion in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G899–G904. doi: 10.1152/ajpgi.00398.2006. [DOI] [PubMed] [Google Scholar]
- Hogan D L, Crombie D L, Isenberg J I, Svendsen P, Schaffalitzki de Muckadell O B, Ainsworth M A. Acid-stimulated duodenal bicarbonate secretion involves a CFTR-mediated transport pathway in mice. Gastroenterology. 1997;113:533–541. doi: 10.1053/gast.1997.v113.pm9247473. [DOI] [PubMed] [Google Scholar]
- Clayburgh D R, Barrett T A, Tang Y, Meddings J B, van Edlik L J, Watterson M, Clarke L L, Mrsny R J, Turner J R. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduke M, Miller C, Mindell J. A decade of CLC chloride channels: structure, mechanism and many unsettled questions. Annu Rev Biophys Biomol Struct. 2000;29:411–438. doi: 10.1146/annurev.biophys.29.1.411. [DOI] [PubMed] [Google Scholar]
- White N L, Higgins C F, Trezise A E O. Tissue-specific in vivo transcription start sites of the human and murine cystic fibrosis genes. Human Mol Gen. 1998;7:363–369. doi: 10.1093/hmg/7.3.363. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K. CFTR: Interacting with everything? News Physiol Sci. 2001;16:167–170. doi: 10.1152/physiologyonline.2001.16.4.167. [DOI] [PubMed] [Google Scholar]
- Beböck Z, Tousson A, Schwiebert L M, Venglarik C J. Improved oxygenation promotes CFTR maturation and trafficking in MDCK monolayers. Am J Physiol Cell Physiol. 1999;280:C135–C145. doi: 10.1152/ajpcell.2001.280.1.C135. [DOI] [PubMed] [Google Scholar]
- Sheta E A, Trout H, Gildea J J, Harding M A, Theodorescu D. Cell density mediated pericellular hypoxia leads to induction of HIF-1alpha via nitric oxide and Ras/MAP kinase mediated signaling pathways. Oncogene. 2001;20:7624–7634. doi: 10.1038/sj.onc.1204972. [DOI] [PubMed] [Google Scholar]
- Hauser C J, Locke R R, Kao H W, Patterson J, Zipser R D. Visceral surface oxygen tension in experimental colitis in the rabbit. J Lab Clin Med. 1988;112:68–71. [PubMed] [Google Scholar]
- Sreenarasimhaiah J. Diagnosis and management of ischemic colitis. Curr Gastroenterol Rep. 2005;7:421–426. doi: 10.1007/s11894-005-0013-1. [DOI] [PubMed] [Google Scholar]
- Robinson J W, Mirkovitch V, Winistorfer B, Saegesser F. Response of intestinal mucosa to ischaemia. Gut. 1981;22:512–527. doi: 10.1136/gut.22.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kles K A, Tappenden K A. Hypoxia differentially regulates nutrient transport in rat jejunum regardless of luminal nutrient present. Am J Physiol Gastrointest Liver Physiol. 2002;283:1336–1342. doi: 10.1152/ajpgi.00055.2002. [DOI] [PubMed] [Google Scholar]
- Matthews J B, Tally K J, Smith J A, Zeind A J, Hrnjez B J. Activation of Cl secretion during chemical hypoxia by endogenous release of adenosine in intestinal epithelial monolayers. J Clin Invest. 1995;96:117–125. doi: 10.1172/JCI118010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dada L A, Chandel N S, Ridge K M, Pedemonte C, Bertorello A M, Sznajder J I. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallows K R, Kobinger G P, Wilson J M, Witters L A, Foskett J K. Physiological modulation of CFTR activity by AMP-activated protein kinase in polarized T84 cells. Am J Physiol Cell Physiol. 2003;284:C1297–C1308. doi: 10.1152/ajpcell.00227.2002. [DOI] [PubMed] [Google Scholar]
- Chappe V, Irvine T, Liao J, Evagelidis A, Hanrahan J W. Phosphorylation of CFTR by PKA promotes binding of the regulatory domain. EMBO J. 2005;24:2730–2740. doi: 10.1038/sj.emboj.7600747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferata E G A, González Guerrico A M, Pivetta O H, Santa-Coloma T A. NFkB activation is involved in regulation of cystic fibrosis transmembrane conductance regulator (CFTR) by Interleukin-1b. J Biol Chem. 2001;276:15441–15444. doi: 10.1074/jbc.M010061200. [DOI] [PubMed] [Google Scholar]
- Matthews R P, McKnight G S. Characterization of the cAMP response element of the cystic fibrosis transmembrane conductance regulator gene promoter. J Biol Chem. 1996;271:31869–31877. doi: 10.1074/jbc.271.50.31869. [DOI] [PubMed] [Google Scholar]
- Cummins E P, Taylor C T. Hypoxia-responsive transcription factors. Eur J Physiol. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- Taylor C T, Furuta G T, Synnestvedt K, Colgan S P. Phosphorylation-dependent targeting of cAMP response element binding protein to the ubiquitin/proteasome pathway in hypoxia. Proc Natl Acad Sci U S A. 2000;97:12091–12096. doi: 10.1073/pnas.220211797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Nakamura H, Trapnell B C, Dalemans W, Pavirani A, Lecocq J P, Crystal R G. The cystic firbrosis gene has a “housekeeping”-type promoter and is expressed at low levels in cells of epithelial origin. J Biol Chem. 1991;14:9140–9144. [PubMed] [Google Scholar]
- Fouassier L, Beaussier M, Schiffer E, Rey C, Barbu V, Mergey M, Wendum D, Callard P, Scoazec J Y, Lasnier E, Stieger B, Lienhart A, Housset C. Hypoxia-induced changes in the expression of rat hepatobiliary transporter genes. Am J Physiol Gastrointest Liver Physiol. 2007;293:G25–G35. doi: 10.1152/ajpgi.00175.2006. [DOI] [PubMed] [Google Scholar]
- Zaidi T, Mowrey-McKee M, Pier G B. Hypoxia increases corneal cell expression of CFTR leading to increased Pseudomonas aeruginosa binding, internalization and initiation of inflammation. Invest Ophthalmol Vis Sci. 2004;45:4066–4074. doi: 10.1167/iovs.04-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uramoto H, Takahashi N, Dutta A, Sabirov R, Ando-Akatsuka Y, Morishima S, Okada Y. Ishemia-induced enhancement of CFTR expression on the plasma membrane in neonatal rat ventricular myocytes. Japan J Physiol. 2003;53:357–365. doi: 10.2170/jjphysiol.53.357. [DOI] [PubMed] [Google Scholar]
- Li J, Allen K T, Sun X C, Cui M, Bonanno J A. Dependence of cAMP meditated increases in Cl- and HCO(3)- permeability on CFTR in bovine corneal endothelial cells. Exp Eye Res. 2008;86:684–690. doi: 10.1016/j.exer.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVinish L J, Cope G, Ropenga A, Curthbert A W. Chloride transporting capability of Calu-3 epithelia following persistent knockdown of the cystic fibrosis transmembrane conductance regulator, CFTR. Br J Pharmacol. 2007;150:1055–1065. doi: 10.1038/sj.bjp.0707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger P, Khoury J, Kong T, Weissmüller T, Robinson A, Colgan C T. Identification of vasodilator-stimulated phosphoprotein (VASP) as an HIF-regulated tissue permeability factor during hypoxia. FASEB J. 2007;21:2613–2621. doi: 10.1096/fj.06-8004com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamachary B, Zagzag D, Nagasawa H, Rainey K, Okuzama H, Baek J H, Semenza G L. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731. doi: 10.1158/0008-5472.CAN-05-3719. [DOI] [PubMed] [Google Scholar]
- Zhou B, Ann D, Li X, Kim K, Lin H, Minoo P, Crandall E, Borok Z. Hypertonic induction of aquaporin-5: novel role of hypoxia-inducible factor-1a. Am J Physiol Cell Physiol. 2007;292:C1280–C1290. doi: 10.1152/ajpcell.00070.2006. [DOI] [PubMed] [Google Scholar]
- Wodopia R, Ko H, Billian J, Wiesner R, Bartsch P, Mairbaurl H. Hypoxia decreases proteins involved in epithelial electrolyte transport in A549 cells and rat lung. Am J Physiol. 2000;279:L1110–L1119. doi: 10.1152/ajplung.2000.279.6.L1110. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Duplan E, Boyer N, Vigne P, Frelin C. Hypoxia up-regulates prolyl hydroxylase activity: a feedback mechanism that limits HIF-1 responses during reoxygenation. J Biol Chem. 2003;278:38183–38187. doi: 10.1074/jbc.M302244200. [DOI] [PubMed] [Google Scholar]
- Karhausen J, Kong T, Narravula S, Colgan C T. Induction of the von Hippel-Lindau tumor suppressor gene by late hypoxia limits HIF-1 expression. J Cell Biolbiochem. 2005;95:1264–1275. doi: 10.1002/jcb.20489. [DOI] [PubMed] [Google Scholar]
- Cuthbert A W, Halstead J, Ratcliff R, Colledge W H, Evans M J. The genetic advantage hypothesis in cystic fibrosis heterozygotes: a murine study. J Physiol (Lond) 1995;482:449–454. doi: 10.1113/jphysiol.1995.sp020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler U, Blumenstein I, Kretz A, Viellard-Baron D, Rossmann H, Colledge W H, Evans M, Ratcliff R, Gregor M. A functional CFTR protein is required for mouse intestinal cAMP-, cGMP- and Ca2+-dependent HCO3- secretion. J Physiol. 1997;505:411–423. doi: 10.1111/j.1469-7793.1997.411bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jonge H R, Bijvelds M, Wilke M, Bot A, Jorna H, Colledge W H, Verkade H J, Sinaasappel M. Intestinal and pancreatic biomarkers for CF. Ped Pulmonol Suppl. 2006;29:148–149. [Google Scholar]
- Broere N, Hillesheim J, Tuo B, Jorna H, Houtsmuller A B, Shenolikar S, Weinman E J, Donowitz M, Seidler U, de Jonge H R, Hogema B M. Cystic fibrosis transmembrane conductance regulator activation is reduced in the small intestine of Na+/H+ exchanger 3 regulatory factor 1 (NHERF-1)- but not NHERF-2-deficient mice. J Biol Chem. 2007;282:37575–37584. doi: 10.1074/jbc.M704878200. [DOI] [PubMed] [Google Scholar]
- Walker J, Jijon H B, Churchill T, Kulka M, Madsen K L. Activation of AMP-activated protein kinase reduces cAMP-mediated epithelial chloride secretion. Am J Physiol. 2003;285:G850–G860. doi: 10.1152/ajpgi.00077.2003. [DOI] [PubMed] [Google Scholar]
- Gabriel S E, Brigmann K N, Koller B H, Boucher R C, Stutts M J. Cystic fibrosis heterozygote resistance to cholera toxin in the cystic fibrosis mouse model. Science. 1994;266:107–109. doi: 10.1126/science.7524148. [DOI] [PubMed] [Google Scholar]
- Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol. 2007;435:405–419. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]
- Hartmann H, Eltzschig H K, Wurz H, Hantke K, Rakin A, Yazdi A S, Matteoli G, Bohn E, Authenrieth I B, Karhausen J, Neumann D, Colgan C T, Kempf V A J. Hypoxia-independent activation of HIF-1 by Enterobacteriaceae and their siderophores. Gastroenterology. 2008;134:756–767. doi: 10.1053/j.gastro.2007.12.008. [DOI] [PubMed] [Google Scholar]