Abstract
Most LuxR homologues function as activators of transcription during the process of quorum sensing, but a few, including EsaR and ExpREcc, negatively impact gene expression. The LuxR-activated luxI promoter and LuxR binding site, the lux box, were used in artificial contexts to assess the potential for transcriptional activation and DNA binding by EsaR and ExpREcc. Although the acyl-homoserine lactone responsiveness of both proteins is the opposite of that shown by most LuxR family members, EsaR and ExpREcc have preserved the ability to interact with RNA polymerase and activate transcription despite their low affinity for the lux box DNA.
The quorum sensing regulator LuxR of Vibrio fischeri and most homologous proteins found in various proteobacteria function as acyl-homoserine lactone (AHL)-dependent transcriptional activators (reviewed in references 10, 17, 26, and 28) (Fig. 1, top). However, a few LuxR-type transcription factors function as negative regulators of gene expression. The quorum sensing regulators EsaR and ExpREcc fall into this latter category.
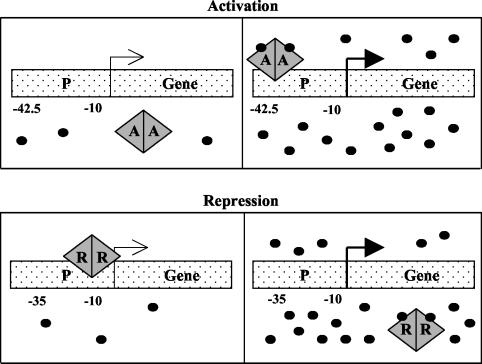
FIG. 1.
Cartoon model of quorum sensing regulation by an activator (A) or a repressor (R). (Top) An activator binds to the DNA at a position around −42.5 and initiates transcription only in the presence of a high concentration of AHL, represented by the small black circles. (Bottom) A repressor remains bound to the DNA near −10 until a high enough concentration of AHL is reached to neutralize the repressor activity.
EsaR of Pantoea stewartii subsp. stewartii normally governs the expression of specific target genes by repression and AHL-dependent derepression (3, 18). P. stewartii subsp. stewartii causes Stewart's wilt disease in maize, in part, through the synthesis of an exo/capsular polysaccharide (EPS) virulence factor that clogs the xylem of the plant host (4). EPS synthesis is tightly controlled by a multitiered regulatory cascade (2, 24). The dominant level of control is mediated by EsaR and the AHL produced by the cognate AHL synthase, EsaI (3, 18). The role of EsaR as a repressor of quorum sensing has been demonstrated genetically and biochemically (3, 18). The key to EsaR repressor activity is twofold. First, EsaR exists as a dimer and binds target promoters in the absence of AHL (18, 21). Second, the placement of the esaR box DNA binding site is positioned to block the transcriptional activity of RNA polymerase (2) (Fig. 1, bottom).
The ExpR proteins studied in isolates of Erwinia carotovora (ExpREcc) and Erwinia chrysanthemi (ExpREch) also have characteristics of a repressor-like activity, although their precise regulatory role related to exoenzyme production is unclear (1, 19, 27). However, it was shown that an expR mutant strain of E. carotovora strain SCC3193 exhibited a slight increase in pectinase production, suggesting a weak repressor role, possibly by sequestering AHL to limit the activation activity of an unidentified alternate AHL-responsive activator (1).
Overall, there exists only 18 to 25% amino acid sequence identity between the members of the LuxR protein family (26). EsaR and ExpREcc exhibit 24% and 23% amino acid identity with LuxR, respectively (1, 2), but are 47% identical to each other (1). Interestingly, all three proteins recognize the same AHL signaling molecule, the l isomer of 3-oxo-hexanoyl-homoserine (3-oxo-C6-HL). The binding site for AHL in the LuxR family is predicted to be within the N-terminal domain (15, 16, 29). Repressor activity during quorum sensing requires binding of the C-terminal domain of the protein to the target site (7), while activation requires, in addition to DNA binding, appropriate surfaces with which to establish a productive interaction with RNA polymerase (RNAP) (9, 23). The goal of this study was to measure the ability of EsaR and ExpREcc to bind to the lux box DNA and activate the lux operon and, thereby, further examine the level of structural and functional conservation among LuxR-type regulators.
Construction of derivatives of pBAD22 expressing luxR, esaR, and expR.
Escherichia coli DH5α (14) was used as the host organism for recombinant DNA manipulations. The luxR, esaR, and expR genes were amplified via PCR using the templates pJE202 (8), pSVB5-18 (2), and pSAO18 (1), respectively, with the appropriate primers listed in Table 1. In all three cases, the forward primer contained an EcoRI site and was designed to maintain optimal spacing between the Shine-Dalgarno sequence and start codon, while the reverse primer contained either a SmaI or XbaI site and two stop codons.
TABLE 1.
Strains, plasmids, phages, and primers
| Strain, plasmid, phage, or primer | Relevant information | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | F− φ80d lacZΔM15 Δ(lacZYA-argF)U169 deoR recA1 endA1 hsd17 phoA supE44 thi-1 gyrA96 relA1 | 14 |
| K-12 GS162 | MC4100 pheA905 thi Δ(argF-lac)U169 araD129 rpsL150 relA1 deoC1 fibB5301 ptsF25 rbsR | G. Stauffer |
| Top10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 deoR recA1 araD139 Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 nupG | 11 |
| Plasmids | ||
| pGEM-T Easy | Cloning vector, f1 ori, Apr, used as an intermediate cloning vector | Promega |
| pBAD22 | Arabinose inducible vector, Apr | 13 |
| pBAD-LuxR | luxR ligated into EcoRI and SmaI sites in pBAD22 | This study |
| pBAD-EsaR | esaR ligated into EcoRI sites in pBAD22, 15-bp carryover of pGEM vector | This study |
| pBAD-ExpR | expR ligated into EcoRI sites in pBAD22, 15-bp carryover of pGEM vector, single silent mutation creates a RsaI site | This study |
| pKE555 | luxICDABE, Cmr | 6 |
| pMC1403 | translational lacZ fusion vector, Apr | 5 |
| p35LB10 | Artificial LuxR-repressible lac promoter, Apr | 7 |
| pgcvB-lacZ+251 | Source of transcription terminators, Apr | 25 |
| pMU100 | Dual terminators in pMC1403, Apr | This study |
| pluxI-lacZ | luxI-lacZYA fusion, Apr | This study |
| Phages | ||
| λgt2 | λ cloning vector, cI857 repressor | 20 |
| λluxI-lacZ | luxI-lacZYA fusion in λgt2 | This study |
| λ35LB10 | 35LB10-lacZYA fusion in λgt2 | This study |
| Primers | ||
| luxR forward | 5′-CCGGAATTCACCATGAAAAACATAAATGCCGACGAC | This study |
| luxR reverse | 5′-TCCCCCGGGCTATTAATTTTTAAAGTATGGGCA | This study |
| esaR forward | 5′-GGAATTCACCATGTTTTCTTTTTTCCTTG | This study |
| esaR reverse | 5′-CTCTAGATCACTACCTGGCCGCTGAC | This study |
| expR forward | 5′-GGAATTCACCATGTCGCAGTTATTCTACAAC | This study |
| expR reverse | 5′-CTCTAGATCACTATGACTGAACCGGTCGG | This study |
| pBAD forward | 5′-TCGCAACTCTCTACTGTTTC | This study |
| pBAD reverse | 5′-CTTCTCTCATCCGCCAAAAC | This study |
| PluxI forward | 5′-AAGAATTCACAATGTACCATTTTAGTCATATCAG | This study |
| PluxI reverse | 5′-AAGGATCCTTATACTCCTCCGATGGAATTGCC | This study |
| lux box uppera | 5′-TCTTACCTGTAGGATCGTACAGGT | This study |
| lux box lowera | 5′-CTTAACCTGTACGATCCTACAGGT | This study |
| esaR box uppera | 5′-TCTTGCCTGTACTATAGTGCAGGT | This study |
| esaR box lowera | 5′-CTTAACCTGCACTATAGTACAGGC | This study |
The protein binding site is underlined.
The three PCR products were first cloned into pGEM-T Easy (Promega, Madison, Wis.) and were then subcloned into pBAD22 (13) using primer or vector-derived EcoRI-SmaI sites for luxR and EcoRI-EcoRI sites for esaR and expR. The pBAD22 constructs were named pBAD-LuxR, pBAD-EsaR, and pBAD-ExpR (Table 1). Sequencing reactions performed at the Core Sequencing Facility at the Virginia Bioinformatics Institute, Virginia Tech, with primers annealing to pBAD22 (Table 1) verified the fidelity of the DNA sequence for luxR and esaR; the expR construct does contain a single silent nucleotide change from the wild-type sequence. LuxR and EsaR protein expression from pBAD-LuxR and pBAD-EsaR was confirmed via Western immunoblotting, since polyclonal antisera against these two proteins were readily available (data not shown).
Development of a lambda-based reporter system.
The luxI-lacZ translational gene fusion was constructed by PCR generation of a luxI promoter fragment from the template pKE555 (6), carrying the luxICDABE genes from V. fischeri strain MJ1, using the appropriate primers (Table 1). After EcoRI-BamHI digestion, the resulting 396-bp fragment was cloned into the EcoRI and BamHI sites of the lacZYA reporter pMC1403 (5). In the resulting plasmid, pluxI-lacZ, the BamHI site fuses the 19th codon of luxI to the 8th codon of lacZ. Plasmid pluxI-lacZ was digested with EcoRI and MfeI, and the 5.8-kbp fragment carrying the luxI-lacZYA gene fusion was ligated into the EcoRI site of phage λgt2 (20). The ligated DNA was packaged into phage particles using the Gigapack λ packaging system (Stratagene, La Jolla, Calif.), and the packaging mixture was used to infect E. coli cells. A λluxI-lacZ fusion phage was isolated and used to lysogenize E. coli strain GS162. The lysogen GS162λluxI-lacZ (Table 1) was tested for a single copy of λ (22).
Phage λ35LB10 (Table 1) is derived from p35LB10 (7) and carries the LuxR-repressible artificial 35LB10-lacZ fusion. It was constructed using an intermediate plasmid, pMU100, in which the two E. coli gcvB transcription terminators t1 and t2 from pgcvB-lacZ+251 (25) were inserted upstream of the lacZ gene in pMC1403 (5). Plasmid p35LB10 was digested with HindIII and SacI, and a 2.2-kbp fragment carrying the 35LB10-lacZ fusion was used to replace the HindIII-SacI lacZ fragment in pMU100. The 5.7-kbp fragment carrying the t1 and t2 dual terminators followed by the 35LB10-lacZYA fusion was then excised from this intermediate plasmid with MfeI, cloned into the EcoRI site of phage λgt2 (20), packaged, and used to lysogenize E. coli GS162 as described above. The lysogen GS162λ35LB10 was also tested for a single copy of λ (22).
The λluxI-lacZ and λ35LB10 constructs were subsequently transduced into E. coli Top 10 (ΔaraBAD) (11) for use in the in vivo expression assays. The resulting Top 10λluxI-lacZ and λ35LB10 strains were chosen as single lysogens by comparison of their β-galactosidase levels to those of the confirmed single lysogens of E. coli GS162 described above.
Activation of the lux promoter in vivo by EsaR and ExpREcc.
The E. coli λluxI-lacZ strains separately containing pBAD22, pBAD-LuxR, pBAD-EsaR, and pBAD-ExpR were grown overnight at 30°C in RM minimal medium (2% Casamino Acids, 1× M9 salts [12.8 g of Na2HPO4 · 7H2O, 3 g of KH2PO4, 0.5 g of NaCl, and 1 g of NH4Cl per liter], 0.4% glucose, and 1 mM MgCl2) containing 100 μg of ampicillin ml−1 to an optical density at 600 nm (OD600) of 0.2 to 0.5. The strains were then subcultured by inoculating to an OD600 of 0.005 into six sets of RM minimal medium with ampicillin (100 μg ml−1) broth supplemented either with or without the PBAD inducer 0.2% l-(+)-arabinose, in the absence or presence of a 1 or 100 μM concentration of a d,l-racemic mixture of 3-oxo-C6-HL (Fig. 2). At an OD600 of 0.5 (mid-exponential log-phase growth), 5 μl of cells was diluted 1:200 in Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, 1 mM MgSO4) with 400 μM dithiothreitol and lysed with 50 μl of chloroform. Chemiluminescent β-galactosidase assays (Tropix, Bedford, Mass.) were performed on 10-μl aliquots of the cell lysate in a Lucy 1 microplate luminometer (Rosys Anthos, Wals, Austria) over a 20-s integration time as previously described (7). Each sample was tested in triplicate, and light output was measured in relative light units.
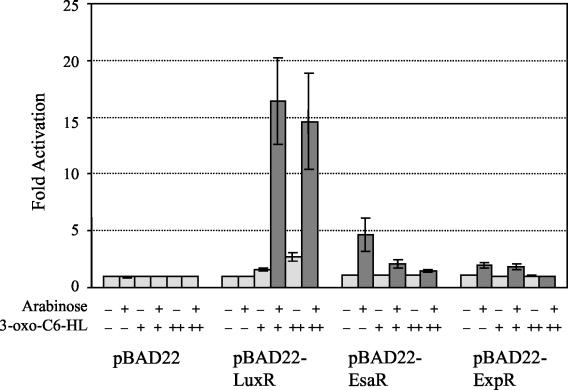
FIG. 2.
Activation assays: β-galactosidase assays using the E. coli Top10λluxI strain transformed with pBAD22, pBAD-LuxR, pBAD-EsaR, or pBAD-ExpR. The cells were grown in six sets of RM minimal medium with 100 μg of ampicillin ml−1 either without (−) or with (+) 0.2% l-(+)-arabinose and either without (−) or with a 1 μM (+) or 100 μM (++) concentration of a d,l-racemic mixture of 3-oxo-C6-HL. Samples from two independent trials were tested in triplicate. Error bars represent the range of each experiment from the mean. The negative control (pBAD22, no supplements) was set at 1 for each experiment, with the actual average value being equivalent to 3.04 ± 0.364 relative light units. Results discussed in the text are highlighted in dark grey.
In this system, E. coli Top10λluxI-lacZ transformed with pBAD22 constitutes the negative control and indicates the basal level of expression of the reporter in the absence of any regulators. Addition of arabinose, 3-oxo-C6-HL, or both had no impact on the level of β-galactosidase produced by this strain (Fig. 2). As expected, the positive control reporter strain E. coli Top10λluxI-lacZ transformed with pBAD-LuxR was stimulated to express lacZ at levels roughly 16- or 14-fold above the negative control in the presence of arabinose plus 1 or 100 μM 3-oxo-C6-HL, respectively (Fig. 2).
The E. coli Top10λluxI-lacZ strain expressing EsaR was able to activate transcription of the luxI-lacZ fusion at levels about fourfold above the background in the presence of arabinose only, suggesting that EsaR can enter into a productive interaction with RNAP at the luxI promoter. Addition of 1 μM 3-oxo-C6-HL inhibited EsaR-mediated luxI-lacZ expression about twofold, and a concentration of 100 μM 3-oxo-C6-HL was needed to more fully neutralize EsaR activation. This is consistent with previous in vivo and surface plasmon resonance analyses that indicated the ability of EsaR to bind to its DNA recognition site is antagonized by the presence of 3-oxo-C6-HL (18). Finally, ExpREcc could only activate the luxI-lacZ reporter at levels about twofold above the background and also required 100 μM 3-oxo-C6-HL for complete inhibition of β-galactosidase expression.
These findings suggest that EsaR and ExpREcc have retained the ability to function as activators of transcription when bound in the appropriate manner to promoter DNA, creating a functional transcription initiation complex with RNAP. The efficiency of activation of the luxI promoter by EsaR and ExpREcc is four- and eightfold lower, respectively, than that with LuxR. In order to determine if this difference is due to inefficient recognition of the heterologous target DNA, protein-protein interactions, or both, we measured the ability of EsaR and ExpREcc to bind to the lux box by using an in vivo transcriptional repression assay with an artificial promoter construct.
In vivo analysis of the binding of EsaR and ExpR to the lux box.
Strains of E. coli Top10λ35LB10 containing the pBAD series plasmids were grown and assayed for β-galactosidase production (Fig. 3) under the same conditions described above for the activation assays. In this assay, if LuxR, EsaR, or ExpREcc binds to the lux box, it should repress transcription from the artificial 35LB10-lacZ promoter fusion, resulting in a decrease in β-galactosidase levels (7). Thus, the degree to which transcription is repressed will reflect the relative affinity of the proteins for the lux box DNA target. The strain expressing LuxR demonstrated roughly eightfold repression in the presence of arabinose and 1 μM 3-oxo-C6-HL in comparison to the pBAD22 control. The ability of LuxR to repress β-galactosidase expression was improved to about 15-fold below the negative control with arabinose and 100 μM 3-oxo-C6-HL in the medium.
FIG. 3.
Repression assays: β-galactosidase assays using the E. coli Top10λ35LB10 strain transformed with pBAD22, pBAD-LuxR, pBAD-EsaR, or pBAD-ExpR. The cells were grown in six sets of RM minimal medium with 100 μg of ampicillin ml−1 either without (−) or with (+) 0.2% l-(+)-arabinose and either without (−) or with a 1 μM (+) or 100 μM (++) concentration of a d,l-racemic mixture of 3-oxo-C6-HL. Samples from two independent trials were tested in triplicate. Error bars represent the range of each experiment from the mean. The negative control (pBAD22, no supplements) was set at 1 for each experiment, with the actual average value being equivalent to 168 ± 14.0 relative light units. Results discussed in the text are highlighted in dark grey.
In this assay system, EsaR was able to repress lacZ expression roughly twofold in the presence of arabinose alone. The ability of EsaR to bind to the lux box and repress the 35LB10 promoter was decreased by the addition of 1 μM 3-oxo-C6-HL and abolished by the addition of 100 μM 3-oxo-C6-HL. No repression of the 35LB10 promoter was seen with ExpREcc. These data suggest that the lower levels of activation observed in the λluxI-lacZ reporter assay are due at least in part to a diminished affinity of EsaR and ExpREcc for the nonnative lux box DNA. Since EsaR appeared to retain some ability to bind to and recognize the lux box, a direct in vitro DNA binding assay was used to further examine this activity.
EMSAs measuring the ability of EsaR to recognize DNA targets.
EsaR protein was partially purified from E. coli Top10 pBAD-EsaR induced with 0.02% arabinose as previously described (18). Essentially, the cells were resuspended in buffer (50 mM Tris [pH 7.5], 10% glycerol) and lysed under pressure (20,000 lb/in2) using a French press. After centrifugation (30,000 × g for 30 min), the soluble lysate was fractionated by heparin column chromatography and NaCl (400 to 800 mM) gradient elution. Fractions containing EsaR were identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and pooled. This EsaR-enriched preparation, estimated by SDS-PAGE analysis to be 50% pure, was used in gel electromobility shift assays (EMSAs) along with 32P-end-labeled DNA probes generated by annealing oligonucleotides containing either the lux box or esaR box (Table 1). The lux box and esaR box are identical at 15 out of 20 positions.
EMSAs were performed essentially as described by Minogue et al. (18). Assays included partially purified EsaR protein as indicated in Fig. 4 and either 1.6 μM lux box or esaR box oligonucleotides in binding buffer [20 mM HEPES (pH 7.6), 1 mM EDTA, 10 mM (NH4)2SO4, 1 mM dithiothreitol, 0.2% Tween 20, 30 mM KCl, 50 mg of lambda DNA ml−1, and 150 mg of bovine serum albumin ml−1]. Reactions were incubated at 28°C for 30 min. Addition of excess (250 μM) unlabeled competitor DNA substrate was used to demonstrate the binding specificity of EsaR for the oligomeric substrates (Fig. 4, lanes 5 and 10). This unlabeled competitor DNA also annealed with radiolabeled single-stranded oligonucleotides, resulting in the enhanced radiolabeled double-stranded DNA band seen in the EMSA reactions containing competitor DNAs (Fig. 4, lanes 5 and 10). Reaction mixtures were resolved on a nondenaturing 6% polyacrylamide gel in Tris-borate-EDTA buffer (Fisher, Pittsburgh, Pa.) at 4°C, 200 V, for 40 min. The probes were imaged with a Molecular Imager FX system (Bio-Rad) and quantified with the Quantity One software (Bio-Rad).
FIG. 4.
EsaR EMSAs. Synthetic 28-bp radiolabeled oligonucleotides (1.6 μM) specifying either the esaR box (lanes 1 to 5) or the lux box (lanes 6 to 10) were incubated with semipure EsaR at concentrations of 0 (lanes 1 and 6), 2 nM (lanes 2 and 7), 20 nM (lanes 3 and 8), 200 nM (lanes 4 and 9), and 200 nM plus excess competitor DNA (250 μM) (lanes 5 and 10) and resolved by nondenaturing gel electrophoresis. ss DNA, single-stranded 24-base complementary oligonucleotide DNAs; ds DNA, annealed double-stranded 28-bp substrate DNA.
The relative binding efficiency of EsaR for the separate target DNAs was calculated as the percent total radiolabeled double-stranded DNA present in the protein-DNA complex. EsaR protein at concentrations of 2, 20, and 200 nM was found to bind the lux box with efficiencies of 0.6, 2, and 13%, respectively, while the same concentrations of EsaR bound the native esaR box with efficiencies of 2, 11, and 52% (Fig. 4). Thus, the esaR box DNA is recognized by EsaR about fivefold more efficiently than is lux box DNA. This difference in binding affinity corresponds roughly to the degree of difference measured in the in vivo activation assays and suggests that the primary reason for the decreased activation of the luxI-lacZ fusion exhibited by EsaR in vivo is its inability to bind the lux box as efficiently as LuxR.
Conclusions.
The LuxR homologue EsaR, previously shown to function as a repressor, retains an ability to function as an activator of transcription by RNAP. For most LuxR family members, AHL binding by the apo-activator is thought to facilitate formation of the active DNA binding conformation. In contrast, EsaR DNA binding is neutralized by 3-oxo-C6-HL, as shown indirectly in the in vivo β-galactosidase activation and repression assays and directly in the in vitro EMSAs, consistent with previous studies (3). Moreover, both the in vivo and in vitro assays showed that ApoEsaR can bind to the heterologous lux box, which differs from the esaR box at 5 of 20 positions. However, EsaR binds less efficiently to the lux box DNA than to the native esaR box, which may explain the weaker activation exhibited by EsaR in the in vivo assays.
ExpREcc fails to repress the 35LB10 promoter fusion in vivo, which may reflect an even lower binding affinity of ExpREcc for the lux box DNA. ExpREcc is able to activate expression of the luxI-lacZ fusion at rates just barely above background. RNAP may be required to help ExpREcc stabilize its contacts with the DNA in this artificial context. The fact that the low level of activation produced by ExpREcc is abolished by addition of 3-oxo-C6-HL suggests that it responds to the signal in a manner similar to EsaR.
It was demonstrated previously that the LuxR homologue, LasR, to a degree, can recognize the lux box and activate transcription in the presence of its cognate AHL and, conversely, LuxR can recognize the LasR binding site and activate transcription in the presence of its cognate AHL (12). This suggested that there was functional conservation in the DNA binding domain of the LuxR family of proteins and the targets that they recognize. The data reported are consistent with this study showing the ability of two other LuxR homologues to bind to the lux box and activate the luxI promoter.
However, what is clear from this and previous studies is that EsaR and ExpR have an ability to fold into a stable DNA binding protein in the absence of AHL. There is good evidence that AHL serves as a scaffold for folding and stabilizes the DNA binding conformation of the activator TraR and that the lack of AHL promotes the proteolytic degradation of the nascent TraR protein (30). Conversely, AHL binding to EsaR promotes structural changes that result in reduced DNA binding potential (18). Whether or not these conformational changes also render AHL-EsaR sensitive to proteolysis remains to be established. Ultimately, more detailed comparative structural analyses of representative members of the LuxR family that bind to the DNA in the presence of AHL versus those that bind to the DNA in the absence of AHL will be necessary to fully understand the functional differences between the two groups.
Future studies that make use of reporter constructs incorporating the native binding sites for EsaR and ExpREcc should allow for a thorough analysis of the protein-protein interactions that occur between these proteins and RNAP in the transcription initiation complex. These studies would yield additional insights into the degree of functional conservation that exists among the LuxR family of proteins involved in the quorum sensing response of proteobacteria and contribute to a broader understanding of mechanisms of transcription initiation and protein evolution in bacteria.
Acknowledgments
We thank John Varga for his preliminary work on the project and E. Peter Greenberg for his encouragement. We also thank Frank Bernhard and Markus Wehland for their contribution in the initial EMSA studies.
Jessica K. Ball, Marie A. Faini, Carmen M. Herrera, Timothy D. Minogue, and Mark L. Urbanowski contributed equally and are listed alphabetically in the byline.
This work was funded by National Science Foundation (NSF) Career Award MCB-9875479 (A.M.S.), NSF grant MCB-0211687 (S.v.B.), and the W. M. Keck Foundation Medical Science Grant Program (M.L.U. and E.P.G.).
REFERENCES
- 1.Andersson, R. A., A. R. B. Eriksson, R. Heikinheimo, A. Mäee, M. Pirhonen, V. Koiv, H. Hyytiaeinen, A. Tuikkala, and E. T. Palva. 2000. Quorum sensing in the plant pathogen Erwinia carotovora subsp. carotovora: the role of expREcc. Mol. Plant-Microbe Interact. 13:384-393. [DOI] [PubMed] [Google Scholar]
- 2.Beck von Bodman, S., and S. K. Farrand. 1995. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J. Bacteriol. 177:5000-5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck von Bodman, S., D. R. Majerczak, and D. L. Coplin. 1998. A negative regulator mediates quorum sensing control of exopolysaccharide production in Pantoea stewartii subsp. stewartii. Proc. Natl. Acad. Sci. USA 95:7687-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradshaw-Rouse, J. J., M. A. Whatley, D. L. Coplin, A. Woods, L. Sequeira, and A. Kelman. 1981. Agglutination of Erwinia stewartii strains with a corn agglutinin: correlation with extracellular polysaccharide production and pathogenicity. Appl. Environ. Microbiol. 42:344-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban, M. J., J. Chou, and S. N. Cohen. 1980. In vitro gene fusions that join an enzymatically active β-galactosidase segment to amino-terminal fragments of exogenous proteins: Escherichia coli plasmid vectors for the detection and cloning of translational initiation signals. J. Bacteriol. 143:971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egland, K. A., and E. P. Greenberg. 1999. Quorum sensing in Vibrio fischeri: elements of the luxI promoter. Mol. Microbiol. 31:1197-1204. [DOI] [PubMed] [Google Scholar]
- 7.Egland, K. A., and E. P. Greenberg. 2000. Conversion of the Vibrio fischeri transcriptional activator, LuxR, to a repressor. J. Bacteriol. 182:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engebrecht, J., K. Nealson, and M. Silverman. 1983. Bacterial bioluminescence: isolation and genetic analysis of functions from Vibrio fischeri. Cell 32:773-781. [DOI] [PubMed] [Google Scholar]
- 9.Finney, A. H., R. J. Blick, K. Murakami, A. Ishihama, and A. M. Stevens. 2002. Role of the C-terminal domain of the alpha subunit of RNA polymerase in LuxR-dependent transcriptional activation of the lux operon during quorum sensing. J. Bacteriol. 184:4520-4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuqua, C., M. R. Parsek, and E. P. Greenberg. 2001. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 35:439-468. [DOI] [PubMed] [Google Scholar]
- 11.Grant, S. G., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 87:4645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray, K. M., L. Passador, B. H. Iglewski, and E. P. Greenberg. 1994. Interchangeability and specificity of components from the quorum-sensing regulatory systems of Vibrio fischeri and Pseudomonas aeruginosa. J. Bacteriol. 176:3076-3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 15.Hanzelka, B. L., and E. P. Greenberg. 1995. Evidence that the N-terminal region of the Vibrio fischeri LuxR protein constitutes an autoinducer-binding domain. J. Bacteriol. 177:815-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo, Z. Q., A. J. Smyth, P. Gao, Y. Qin, and S. K. Farrand. 2003. Mutational analysis of TraR. Correlating function with molecular structure of a quorum-sensing transcriptional activator. J. Biol. Chem. 278:13173-13182. [DOI] [PubMed] [Google Scholar]
- 17.Miller, M. B., and B. L. Bassler. 2001. Quorum sensing in bacteria. Annu. Rev. Microbiol. 55:165-199. [DOI] [PubMed] [Google Scholar]
- 18.Minogue, T. D., M. Wehland-von Trebra, F. Bernhard, and S. B. von Bodman. 2002. The autoregulatory role of EsaR, a quorum sensing regulator in Pantoea stewartii subsp. stewartii: evidence for a repressor function. Mol. Microbiol. 44:1625-1635. [DOI] [PubMed] [Google Scholar]
- 19.Nasser, W., M. L. Bouillant, G. P. C. Salmond, and S. Reverchon. 1998. Characterization of the Erwinia chrysanthemi expI-expR locus directing the synthesis of two N-acyl-homoserine lactone signal molecules. Mol. Microbiol. 29:1391-1405. [DOI] [PubMed] [Google Scholar]
- 20.Panasenko, S. M., J. R. Cameron, R. W. Davis, and I. R. Lehman. 1977. Five hundredfold overproduction of DNA ligase after induction of a hybrid lambda lysogen constructed in vitro. Science 196:188-189. [DOI] [PubMed] [Google Scholar]
- 21.Qin, Y., Z. Q. Luo, A. J. Smyth, P. Gao, S. Beck von Bodman, and S. K. Farrand. 2000. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J. 19:5212-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimada, K., R. A. Weisberg, and M. E. Gottesman. 1972. Prophage lambda at unusual chromosomal locations. I. Location of the secondary attachment sites and the properties of the lysogens. J. Mol. Biol. 63:483-503. [DOI] [PubMed] [Google Scholar]
- 23.Stevens, A. M., N. Fujita, A. Ishihama, and E. P. Greenberg. 1999. Involvement of the RNA polymerase α-subunit C-terminal domain in LuxR-dependent activation of the vibrio fischeri luminescence genes. J. Bacteriol. 181:4704-4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres-Cabassa, A., S. Gottesman, R. D. Frederick, P. J. Dolph, and D. L. Coplin. 1987. Control of extracellular polysaccharide synthesis in Erwinia stewartii and Escherichia coli K-12: a common regulatory function. J. Bacteriol. 169:4525-4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urbanowski, M. L., L. T. Stauffer, and G. V. Stauffer. 2000. The gcvB gene encodes a small untranslated RNA involved in expression of the dipeptide and oligopeptide transport systems in Escherichia coli. Mol. Microbiol. 37:856-868. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead, N. A., A. M. L. Barnard, H. Slater, N. J. L. Simpson, and G. P. C. Salmond. 2001. Quorum-sensing in gram-negative bacteria. FEMS Microbiol. Rev. 25:365-404. [DOI] [PubMed] [Google Scholar]
- 27.Whitehead, N. A., J. T. Byers, P. Commander, M. J. Corbett, S. J. Coulthurst, L. Everson, A. K. P. Harris, C. L. Pemberton, N. J. L. Simpson, H. Slater, D. S. Smith, M. Welch, N. Williamson, and G. P. C. Salmond. 2002. The regulation of virulence in phytopathogenic Erwinia species: quorum sensing, antibiotics and ecological considerations. Antonie Leeuwenhoek 81:223-231. [DOI] [PubMed] [Google Scholar]
- 28.Withers, H., S. Swift, and P. Williams. 2001. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 4:186-193. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, R. G., T. Pappas, J. L. Brace, P. C. Miller, T. Oulmassov, J. M. Molyneaux, J. C. Anderson, J. K. Bashkin, S. C. Winans, and A. Joachimiak. 2002. Structure of a bacterial quorum-sensing transcription factor complexed with pheromone and DNA. Nature 417:971-974. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, J., and S. C. Winans. 2001. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc. Natl. Acad. Sci. USA 98:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]