Abstract
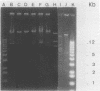
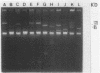
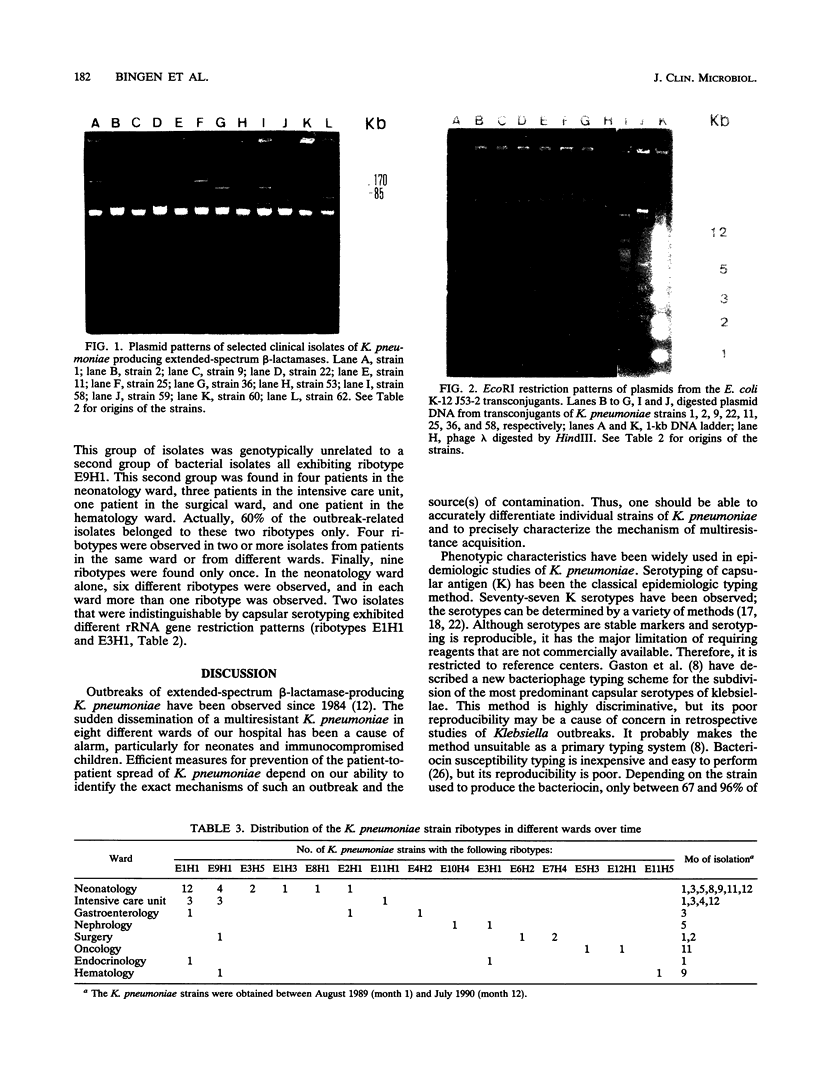
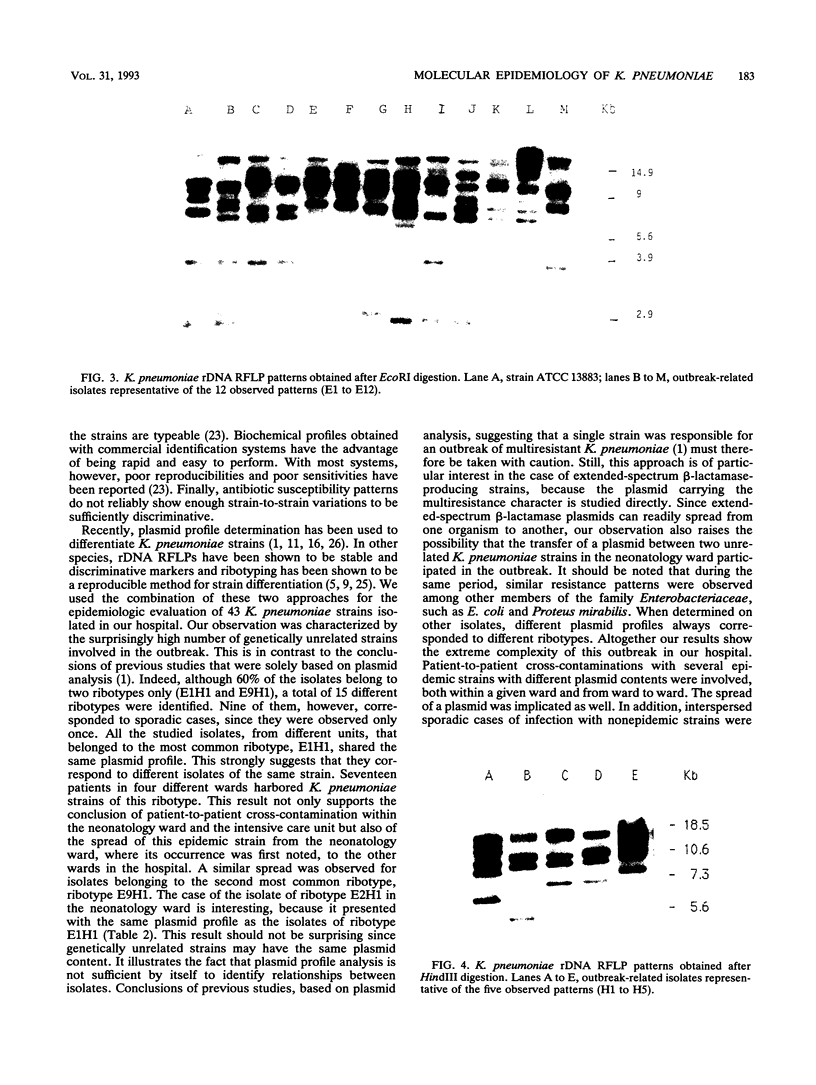
Over a 12-month period, 43 children in eight different wards of our hospital (Hôpital Robert Debré) were infected or colonized with Klebsiella pneumoniae strains producing extended broad-spectrum beta-lactamases. The epidemiology of the outbreak was studied by a molecular approach including the determination of the beta-lactamase physicochemical parameters and plasmid profiles, as well as analysis of the restriction fragment length polymorphisms of the rDNA regions (ribotyping). The last approach produced 12 and 5 different patterns with EcoRI and HindIII, respectively, thus identifying 15 different ribotypes among the 43 clinical K. pneumoniae strains. However, 60% of the strains in six wards belonged to only two ribotypes, whereas nine ribotypes were observed only once. Twelve isolates from different wards that were representative of the eight most common ribotypes showed four different beta-lactamase isoelectric focusing patterns and seven different plasmid profiles by direct analysis or after EcoRI digestion. Thus, at least two genetically unrelated strains in the same ward were found to have the same plasmid content. Our results show the complexity of the outbreak, which was associated with patient-to-patient cross-contamination with several epidemic strains with different plasmid contents, interspersed sporadic cases with nonepidemic strains, and the possible spread of a plasmid. The combination of plasmid profile analysis and ribotyping therefore seems to be powerful at deciphering the details of such outbreaks.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlet G., Sanson-le Pors M. J., Rouveau M., Fournier G., Marie O., Schlemmer B., Philippon A. Outbreak of nosocomial infections due to Klebsiella pneumoniae producing SHV-4 beta-lactamase. Eur J Clin Microbiol Infect Dis. 1990 Nov;9(11):797–803. doi: 10.1007/BF01967377. [DOI] [PubMed] [Google Scholar]
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Bourdois A., Mariani-Kurkdjian P., Cezard J. P., Navarro J., Elion J. DNA restriction fragment length polymorphism differentiates crossed from independent infections in nosocomial Xanthomonas maltophilia bacteremia. J Clin Microbiol. 1991 Jul;29(7):1348–1350. doi: 10.1128/jcm.29.7.1348-1350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Mariani-Kurkdjian P., Lambert-Zechovsky N. Y., Desjardins P., Denamur E., Aujard Y., Vilmer E., Elion J. Ribotyping provides efficient differentiation of nosocomial Serratia marcescens isolates in a pediatric hospital. J Clin Microbiol. 1992 Aug;30(8):2088–2091. doi: 10.1128/jcm.30.8.2088-2091.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E., Denamur E., Lambert-Zechovsky N., Aujard Y., Brahimi N., Geslin P., Elion J. Analysis of DNA restriction fragment length polymorphism extends the evidence for breast milk transmission in Streptococcus agalactiae late-onset neonatal infection. J Infect Dis. 1992 Mar;165(3):569–573. doi: 10.1093/infdis/165.3.569. [DOI] [PubMed] [Google Scholar]
- Bingen E., Lambert-Zechovsky N. Technical aspects of the quantitative and differential analysis of the microbial intestinal ecosystem. Dev Pharmacol Ther. 1984;7 (Suppl 1):134–137. doi: 10.1159/000457243. [DOI] [PubMed] [Google Scholar]
- Brun-Buisson C., Legrand P., Philippon A., Montravers F., Ansquer M., Duval J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet. 1987 Aug 8;2(8554):302–306. doi: 10.1016/s0140-6736(87)90891-9. [DOI] [PubMed] [Google Scholar]
- Gaston M. A., Ayling-Smith B. A., Pitt T. L. New bacteriophage typing scheme for subdivision of the frequent capsular serotypes of Klebsiella spp. J Clin Microbiol. 1987 Jul;25(7):1228–1232. doi: 10.1128/jcm.25.7.1228-1232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Jarlier V., Nicolas M. H., Fournier G., Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988 Jul-Aug;10(4):867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- John J. F., Jr, McKee K. T., Jr, Twitty J. A., Schaffner W. Molecular epidemiology of sequential nursery epidemics caused by multiresistant Klebsiella pneumoniae. J Pediatr. 1983 Jun;102(6):825–830. doi: 10.1016/s0022-3476(83)80006-7. [DOI] [PubMed] [Google Scholar]
- Kliebe C., Nies B. A., Meyer J. F., Tolxdorff-Neutzling R. M., Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert-Zechovsky N., Bingen E., Denamur E., Brahimi N., Brun P., Mathieu H., Elion J. Molecular analysis provides evidence for the endogenous origin of bacteremia and meningitis due to Enterobacter cloacae in an infant. Clin Infect Dis. 1992 Jul;15(1):30–32. doi: 10.1093/clinids/15.1.30. [DOI] [PubMed] [Google Scholar]
- Legrand P., Fournier G., Buré A., Jarlier V., Nicolas M. H., Decré D., Duval J., Philippon A. Detection of extended broad-spectrum beta-lactamases in Enterobacteriaceae in four French hospitals. Eur J Clin Microbiol Infect Dis. 1989 Jun;8(6):527–529. doi: 10.1007/BF01967473. [DOI] [PubMed] [Google Scholar]
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Mayer L. W. Use of plasmid profiles in epidemiologic surveillance of disease outbreaks and in tracing the transmission of antibiotic resistance. Clin Microbiol Rev. 1988 Apr;1(2):228–243. doi: 10.1128/cmr.1.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfreyman J. M. Klebsiella serotyping by counter-current immunoelectrophoresis. J Hyg (Lond) 1978 Oct;81(2):219–225. doi: 10.1017/s0022172400025043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A., Labia R., Jacoby G. Extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1989 Aug;33(8):1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L. B., Willey S. H., Papanicolaou G. A., Medeiros A. A., Eliopoulos G. M., Moellering R. C., Jr, Jacoby G. A. Outbreak of ceftazidime resistance caused by extended-spectrum beta-lactamases at a Massachusetts chronic-care facility. Antimicrob Agents Chemother. 1990 Nov;34(11):2193–2199. doi: 10.1128/aac.34.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riser E., Noone P., Bonnet M. L. A new serotyping method for Klebsiella species: evaluation of the technique. J Clin Pathol. 1976 Apr;29(4):305–308. doi: 10.1136/jcp.29.4.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin S. J. Klebsiella marker systems. Infect Control. 1985 Feb;6(2):59–63. doi: 10.1017/s0195941700062615. [DOI] [PubMed] [Google Scholar]
- Sirot D., Sirot J., Labia R., Morand A., Courvalin P., Darfeuille-Michaud A., Perroux R., Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 1987 Sep;20(3):323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- Stull T. L., LiPuma J. J., Edlind T. D. A broad-spectrum probe for molecular epidemiology of bacteria: ribosomal RNA. J Infect Dis. 1988 Feb;157(2):280–286. doi: 10.1093/infdis/157.2.280. [DOI] [PubMed] [Google Scholar]
- Walia S., Madhavan T., Reuman P., Tewari R., Duckworth D. Plasmid profiles and klebocin types in epidemiologic studies of infections by Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis. 1988 Apr;7(2):279–284. doi: 10.1007/BF01963102. [DOI] [PubMed] [Google Scholar]
- Wheatcroft R., Williams P. A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981 Jun;124(2):433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]
- de Champs C., Sirot D., Chanal C., Poupart M. C., Dumas M. P., Sirot J. Concomitant dissemination of three extended-spectrum beta-lactamases among different Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991 Apr;27(4):441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]