Abstract
Parthenolide has been shown to have anti-inflammatory and antitumor properties. However, whether and how parthenolide enhances tumor sensitivity to radiation therapy are unknown. In this study, we show that inhibition of the nuclear factor-κB (NF-κB) pathway is a common mechanism for the radiosensitization effect of parthenolide in prostate cancer cells LNCaP, DU145, and PC3. Parthenolide inhibits radiation-induced NF-κB DNA-binding activity and the expression of its downstream target sod2, the gene coding for an important antiapoptotic and antioxidant enzyme (manganese superoxide dismutase) in the three prostate cancer cells. Different susceptibilities to parthenolide’s effect are observed in two radioresistant cancer cells, DU145 and PC3, with DU145 cells showing higher sensitivity. This differential susceptibility to parthenolide is due, in part, to the fact that in addition to NF-κB inhibition, parthenolide activates the phosphatidylinositol-3-kinase/Akt prosurvival pathway in both cell lines. However, the activated Akt in DU145 cells is kept at a relatively low level compared with that in PC3 cells due to the presence of functional PTEN. Transfection of wild-type PTEN into PTEN-null cells, PC3, confers the enhanced radiosensitization effect of parthenolide in PTEN-expressing cells. When PTEN expression is knocked down in DU145 cells, the cells become more resistant to parthenolide’s effect. Taken together, these results suggest that parthenolide inhibits the NF-κB pathway and activates the phosphatidylinositol-3-kinase/Akt pathway in prostate cancer cells. The radiosensitization effect of parthenolide is due, in part, to the inhibition of the NF-κB pathway. The presence of PTEN enhances the radiosensitization effect of parthenolide, in part, by suppressing the absolute amount of activated p-Akt.
Introduction
Prostate cancer is the most common cancer type and was the third leading cause of cancer death among U.S. men in 2006 (1). Radiation therapy is frequently used to treat early stage and inoperable locally advanced prostate cancer. The outcome of radiation therapy can be greatly improved if higher doses of radiation are applied, especially for patients with unfavorable tumors (i.e., stage T3 lesions, pretreatment prostate-specific antigen levels >10 ng/mL, or biopsy Gleason scores ≥7). For these patients, the 5-year cancer-free survival rate increased from 41% to 75% when radiation dose was increased to >7,200 cGy (2). However, the side effects and late complications resulting from high-dose radiation will increase to an unacceptable level, which limits the use of high doses of radiation. Another problem in radiation therapy is that tumor cells develop radio-resistance. Therefore, it is important to find agents that sensitize malignant tumor cells to radiation therapy, thus minimizing radiation toxicity to surrounding organs by lowering effective therapeutic doses.
Many mechanisms are involved in the development of radioresistance in tumor cells. One of the possible mechanisms is activation of the nuclear factor-κB (NF-κB) signaling pathway. It has been shown that approximately two-thirds of the biological damage by radiation is due to free radical–mediated indirect action (3). NF-κB is a redox-sensitive transcription factor family regulating cell survival and death. In response to radiation-induced reactive oxygen species, the NF-κB pathway is activated (4). There are five members in the NF-κB family: RelA (p65), RelB, c-Rel, NF-κB1 (p105/p50), and NF-κB2 (p100/p52). NF-κB is normally sequestered in the cytoplasm by its inhibitor IκB family members in an inactive complex. Two NF-κB activation pathways exist. The classical pathway activates the IκB kinase complex, which consists of IKKα, IKKβ, and IKKγ, leading to IκBα phosphorylation, ubiquitination, and further degradation by the 26S proteasome. As a result, the p50:RelA dimer is released and translocated into the nucleus. The alternative pathway activates the IKKα homodimer, leading to the partial degradation of p100 and activation of the p52:RelB dimer. Russell and Tofilon reported that radiation activates the classical NF-κB pathway by selective degradation of plasma membrane–associated IκBα (5). Our previous study shows that RelB is also activated by radiation (6). Activation of NF-κB induces the transcription of its target genes, which are involved in antiapoptosis and tumor metastasis (7). Among numerous NF-κB downstream targets, manganese superoxide dismutase (MnSOD) has been identified as a constitutively and immediately accessible NF-κB target (8). MnSOD is a nuclear-encoded primary antioxidant enzyme localized in the mitochondria. The known function of MnSOD is to remove superoxide radicals in mitochondria (9). Overexpression of MnSOD is protective against radiation-induced cell death (6, 10, 11).
We and others have shown that NF-κB is constitutively activated in aggressive prostate cancer (6, 12), which may be responsible for the intrinsic radioresistance of some prostate cancer cells. Thus, inhibition of the NF-κB pathway represents a target to enhance the sensitivity of prostate cancer to radiation therapy.
Parthenolide is a major active component of the herbal medicine feverfew (Tanacetum parthenium), which is conventionally used in Europe to treat inflammatory diseases such as fever, migraine, and arthritis (13). Parthenolide has been shown to inhibit growth or induce apoptosis in a number of tumor cell lines (14–17). More remarkably, it has been shown that parthenolide is cytotoxic to hepatoma cells and leukemia cells whereas sparing normal liver cells and hematopoietic cells (14, 16), suggesting that the cytotoxic effect of parthenolide may be selective for tumor cells. Many mechanisms are postulated to be involved in the antitumor effect of parthenolide, including inhibition of nucleic acid synthesis (18), depletion of thiols, induction of oxidative stress (14, 15), induction of mitochondria dysfunction (14), disruption of intracellular calcium equilibrium (15), induction of cell cycle G2-M phase arrest (14, 19), sustained activation of c-Jun-NH2-kinase (20, 21), and inhibition of NF-κB (21). It has also been shown that parthenolide sensitizes cancer cells to various apoptosis-inducing agents mainly through inhibition of NF-κB (22). However, whether parthenolide sensitizes cancer cells to radiation-induced cell death and whether inhibition of NF-κB is sufficient for the radiosensitization effect of parthenolide remain unknown.
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN) is a tumor suppressor protein with dual-specificity protein phosphatase activity (23). It dephosphorylates focal adhesion kinase, a major regulator of the integrin signaling pathway (24), as well as the Src-homology collagen protein (Shc), and thus inhibits the growth factor–induced mitogen-activated protein kinase signaling pathway (25). PTEN also functions as a lipid phosphatase and dephosphorylates the second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3) to antagonize the phosphatidylinositol-3-kinase (PI3K) signaling pathway (26). Loss of chromosome 10q, which harbors the PTEN gene, is found in ~60% of advanced prostate cancers (27). Functional loss of PTEN and subsequent activation of the PI3K/Akt (v-akt murine thymoma viral oncogene homologue 1, also called protein kinase B) pathway have been widely implicated in the progression to metastasis of prostate cancer. In this study, we used three prostate cancer cell lines, including the radiosensitive LNCaP cells and two radioresistant cell lines, DU145 (PTEN wild-type) and PC3 (PTEN null), to investigate whether PTEN cooperates with NF-κB in the radiosensitization effect of parthenolide.
Materials and Methods
Cell Culture and Treatment
Human prostate cancer cell lines LNCaP, DU145, and PC3 were obtained from American Type Culture Collection. LNCaP and PC3 cells were cultured in RPMI 1640 (Invitrogen) supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin mixture, 1 mmol/L of sodium pyruvate, 10 mmol/L of HEPES, 1% NEAA mixture (Cambrex), 1% MEM vitamin mixture (Cellgro), and 2 mmol/L of L-glutamine. DU145 cells were cultured in MEM (Sigma) supplemented with 10% fetal bovine serum, 1% penicillin and streptomycin mixture, 1 mmol/L of sodium pyruvate, and 0.1 mmol/L of nonessential amino acids. Cells were grown in a 5% CO2 atmosphere at 37°C. The parthenolide stock solution (5 mmol/L) was prepared in DMSO and diluted in culture medium to the indicated final concentration for cell treatment. DMSO (0.1%) diluted in medium was used as vehicle control. A 130 kv X-ray machine (Faxitron X-ray Corporation) was used to radiate cells, with a dose rate of 89.7 cGy/min.
Clonogenic Survival Assay
Cells were trypsinized and plated in triplicate into six-well plates at different densities based on cell types and doses of radiation. LNCaP, DU145, and PC3 cells were plated at a density of 2,000 to 10,000 cells/well, 100 to 600 cells/well, and 100 to 500 cells/well, respectively. Cells were treated with the indicated concentrations of parthenolide or vehicle control for 24 h prior to exposure to the indicated doses of radiation. Twenty-four hours after radiation treatment, the medium containing parthenolide was removed and cells were maintained in normal culturing medium. Twelve days after the cells were plated, they were washed and stained with crystal violet, and the colonies containing >50 cells were counted. Plating efficiency was calculated by dividing the average number of cell colonies per well by the amount of cells plated. Survival fractions were calculated by normalization to the plating efficiency of appropriate control groups. The dose-modifying factor is calculated from the ratio of the doses of radiation in the absence or presence of the drug to achieve the same cell survival.
3-(4,5-Methylthiazol-2-yl)-2,5-Diphenyl-Tetrazolium Bromide Assay
Cells were plated at a density of 5,000 cells/well into 96-well plates and were grown overnight. Then cells were pretreated with the indicated concentrations of parthenolide for 3 h, and exposed to 6 Gy of radiation or sham-irradiated. Twenty-four hours after the radiation, parthenolide-containing medium was replaced with normal culturing medium. Five days after radiation, 3-(4,5-methylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (50 μg/well) was added and incubated at 37°C for 1 h. After removal of medium, 200 μL of DMSO was added to each well to dissolve the purple formazan crystal. The absorbance was measured at 540 nm. The cell survival was referenced to the control group.
Nuclear Extracts Preparation and Electrophoretic Mobility Shift Assay
Cells were collected and suspended in 500 μL of buffer A (10 mmol/L HEPES-KOH with 1.5 mmol/L MgCl2, 10 mmol/L KCl, 0.2 mmol/L phenylmethylsulfonyl fluoride, 500 μmol/L of DTT, and protease inhibitors). The samples were kept on ice for 15 min and vortexed vigorously for 15 s. The lysate was then centrifuged at 14,000 rpm for 30 s. The pellet was dissolved in 100 μL of buffer B [20 mmol/L HEPES/KOH with 1.5 mmol/L MgCl2, 420 mmol/L NaCl, 25% glycerol, 0.2 mmol/L phenylmethylsulfonyl fluoride, 500 μmol/L DTT, 0.2 mmol/L EDTA (pH 8.0), and protease inhibitors] and kept on ice for 20 min followed by centrifugation at 12,000 rpm for 2 min. The supernatant, identified as the nuclear extract, was frozen at −80°C. Protein concentration was determined by Bradford method. Double-stranded oligonucleotides corresponding to the consensus sequence of the NF-κB–binding site (5′-GAGACTGGGGAATACCCCAGT-3′) was labeled with [32P]ATP. Reaction solution (20 μL) containing 5 μg of the nuclear extract, 4 μL of 5× binding buffer [50 mmol/L Tris-HCl (pH 7.5), with 20% glycerol, 5 mmol/L MgCl2, 2.5 mmol/L EDTA, 5 mmol/L DTT, and 0.25 mg/mL poly dI-dC] and 1 μL of [32P]-labeled probe, was incubated at room temperature for 20 min. Samples were separated on a 4% polyacrylamide gel and visualized by phosphorimaging.
Western Blot Analysis
For each treatment group, certain amounts of the whole cell lysate were separated on 10% SDS-PAGE gel and transferred onto a nitrocellulose membrane. After blocking in 5% milk for 1 h, the membrane was incubated with the primary antibody, followed by the corresponding secondary antibody. The signals were detected by the enhanced chemiluminescence system and quantified by Quantity One (Bio-Rad). Anti-MnSOD antibody was purchased from Upstate, antiactin antibody from Sigma, Akt and phosphor-Akt (Ser473) antibody from Cell Signaling Technology, PTEN antibody from Santa Cruz Biotechnology.
SOD Activity Gel Electrophoresis
Cellular SOD activities were measured based on the inhibition of the reduction of nitroblue tetrazolium by SOD as described by Beauchamp and Fridovich (28). Briefly, 50 μg protein samples in 0.05 mol/L of phosphate buffer were loaded on a 12.5% native polyacrylamide gel. Electrophoresis was carried out at 4°C overnight. Following electrophoresis, the gel was stained in the dark for SOD activity with 2.43 mmol/L of nitroblue tetrazolium for 20 min, riboflavin-TEMED solution for 15 min, and then exposed to light. The achromatic bands showed the presence of SOD activity.
Transfection of Cells with Plasmid DNA and Short Interfering RNA and Determination of Cell Survival by Trypan Blue Exclusion Assay
The green fluorescent protein (GFP)-PTEN expression construct was kindly provided by Dr. Vivek M. Rangnekar (University of Kentucky). PTEN short interfering RNA (siRNA) and scrambled control siRNA were purchased from Santa Cruz Biotechnology. Cells were plated into 12-well plates, and transiently transfected with GFP-PTEN expression plasmid and GFP control plasmid, PTEN siRNA, and control siRNA by using Lipofect AMINE 2000 (Invitrogen) according to the manufacturer’s instructions. Briefly, 1 μg of plasmid DNA or 36 pmol of siRNA was mixed with 50 μL of incomplete medium without fetal bovine serum and antibiotics, and then complexed with a mixture of 3 μL of Lipofect AMINE and 50 μL of incomplete medium for 20 min at room temperature. The mixture was diluted with 500 μL of incomplete medium and added to the cells. After 5 h, the medium was replaced with 1 mL of complete medium containing 10% fetal bovine serum and incubated overnight. Parthenolide was added to the medium, and 24 h later, cells were treated with 6 Gy of radiation or sham-irradiated. After another 24 h, the cells were processed for trypan blue exclusion assay and collected for Western blot analysis. Cell suspension (20 μL) was mixed with 20 μL of 0.04% trypan blue solution, loaded on to a hemocytometer. Cells were counted under light microscope. Dead cells retained the dye whereas the viable cells excluded trypan blue and appeared bright. The cell survival was calculated against the relative control group.
Statistical Analysis
Statistical analysis was done by using either Student’s t test (for two-group comparison) or one-way ANOVA (for multiple-group comparison). Data are reported as mean ± SD.
Results
Parthenolide’s Efficiency in Sensitizing LNCaP, DU145, and PC3 Cells to Radiation Treatment Differs
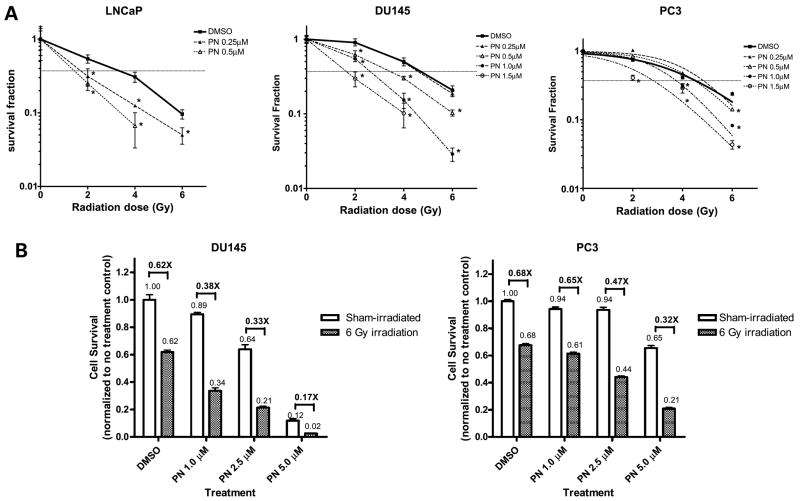
To determine whether parthenolide can enhance the sensitivity of prostate cancer cells to radiation treatment, three human prostate cancer cell lines, LNCaP, DU145, and PC3, were plated for clonogenic cell survival assay. In the absence of parthenolide, LNCaP cells were much more sensitive to radiation treatment than DU145 and PC3 cells were (Fig. 1A), which is consistent with our previous result (6). Exposure to 6 Gy of radiation killed 90% of LNCaP cells but only ~80% of DU145 and PC3 cells. Parthenolide sensitized all three prostate cancer cell lines to radiation treatment in a dose-dependent manner. LNCaP cells were most sensitive to parthenolide treatment. The lowest concentration of parthenolide to show the radiosensitization effect at 2 Gy radiation was 0.25 μmol/L in LNCaP cells, 0.5 μmol/L in DU145 cells, and 1.5 μmol/L in PC3 cells. Because LNCaP cells showed high sensitivity to radiation alone, we focused our study of the radiosensitization effect of parthenolide on the radioresistant aggressive prostate cancer cell lines, DU145 and PC3. In these two cell lines, DU145 showed significantly greater sensitivity to parthenolide treatment. The dose-modifying factor of 1.0 μmol/L of parthenolide at 0.37 survival fraction (D1) were 1.8 and 1.3 in DU145 and PC3 cells, respectively. For 1.5 μmol/L of parthenolide, the dose-modifying factors were 2.6 and 1.6 in DU145 and PC3 cells, respectively. The different radiosensitization efficiency of parthenolide in DU145 and PC3 cells was further shown by 3-(4,5-methylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay (Fig. 1B). Due to the higher cell density used in 3-(4,5-methylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay compared with clonogenic assay, higher concentrations of parthenolide were used. Consistent with the results of clonogenic survival assay, the radiosensitization effect of parthenolide was dose-dependent in both DU145 and PC3 cells (Fig. 1B), with 5 μmol/L of parthenolide showing the most remarkable effect. There was no significant difference between the radiosensitivity of DU145 cells and PC3 cells in the absence of parthenolide. The relative cell survival after 6 Gy of radiation was 0.62 ± 0.03 in DU145 cells, and 0.68 ± 0.03 in PC3 cells (P > 0.05) in the absence of parthenolide. Parthenolide showed higher toxicity and a higher radiosensitization effect in DU145 cells than in PC3 cells. At the 5th day after 5 μmol/L of parthenolide treatment, the relative cell survival fraction was only 0.12 in DU145 cells, whereas it was 0.65 in PC3 cells. After 6 Gy of radiation, the relative cell survival decreased from 0.12 to 0.02 (decrease of 83.3%) in the presence of 5 μmol/L of parthenolide in DU145 cells, whereas in PC3 cells, it decreased from 0.65 to 0.21 (decrease of 67.8%). Due to the high cell density for determining biochemical and molecular biology end points, we used 5 μmol/L of parthenolide in all subsequent studies.
Figure 1.
Parthenolide sensitizes prostate cancer cells to radiation treatment. A, clonogenic survival assay. Cells were treated with the indicated concentrations of parthenolide (PN) for 24 h, and then exposed to the indicated doses of radiation. Twenty-four hours after radiation, the medium containing parthenolide was removed. Cells were then maintained for another 9 d. The cultures were stained and the colonies containing >50 cells were counted. Survival fraction was determined by dividing the plating efficiency of radiated cultures by the plating efficiency of nonradiated cultures. Points, means of triplicates; bars, SD. *, P < 0.05 compared with DMSO-treated counterpart. B, 3-(4,5-methylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide assay. Cells were pretreated with the indicated concentrations of parthenolide for 3 h, and then exposed to 6 Gy of radiation. Twenty-four hours after radiation, the medium containing parthenolide was removed. Cells were then maintained for another 4 d.
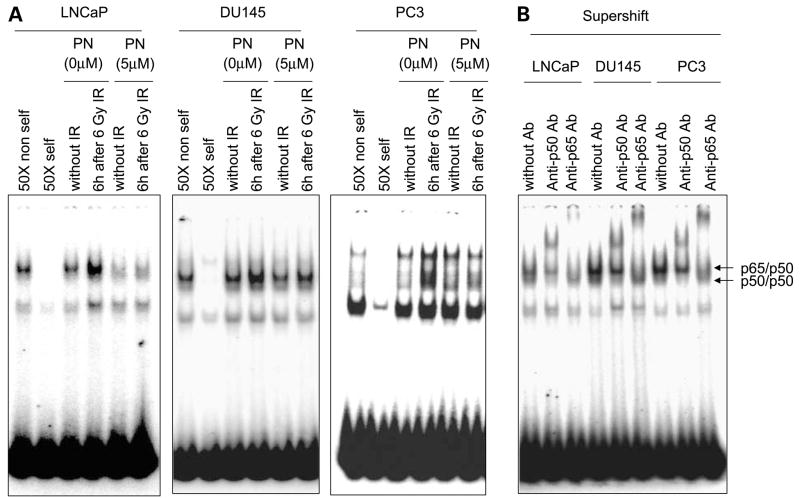
Parthenolide Inhibits Radiation-Induced NF-κB Activation in Prostate Cancer Cells
Nuclear extracts from cells treated with radiation in the absence or presence of parthenolide were analyzed for NF-κB DNA-binding activity by electrophoretic mobility shift assay. NF-κB DNA-binding activity was clearly increased by 6 h after radiation in all three cell lines (Fig. 2A). DU145 and PC3 showed higher NF-κB DNA-binding activity compared with LNCaP cells (Fig. 2B), which is consistent with the aggressive and radioresistant characteristics of these two cell lines. Parthenolide inhibited radiation-induced NF-κB DNA-binding activity in all three cancer cell lines, especially the p65/p50 heterodimer-binding activity, which is shown as the top band on the gel because it can be supershifted by both p65 and p50 antibodies (Fig. 2B). This result is consistent with previous reports that parthenolide could inhibit NF-κB activity by directly targeting the IκB kinase complex (29, 30), thus inhibiting NF-κB nuclear translocation, especially the p65 complex (31). Because NF-κB activation has been well-established as a mediator of radioresistance (32), these results suggest that parthenolide may exert its radiosensitization effect in prostate cancer cells by inhibiting radiation-induced NF-κB activation, thereby suppressing the transcription of NF-κB target genes involved in regulating cell survival and death.
Figure 2.
Parthenolide inhibits radiation-induced NF-κB DNA-binding activity in prostate cancer cells. A, cells were treated with DMSO or 5 μmol/L of parthenolide for 3 h before ionizing radiation (IR). Nuclear extracts were prepared at 6 h after 6 Gy of ionizing radiation for electrophoretic mobility shift assay with radiolabeled NF-κB probes. B, anti-p50 and anti-p65 antibodies were used for supershift.
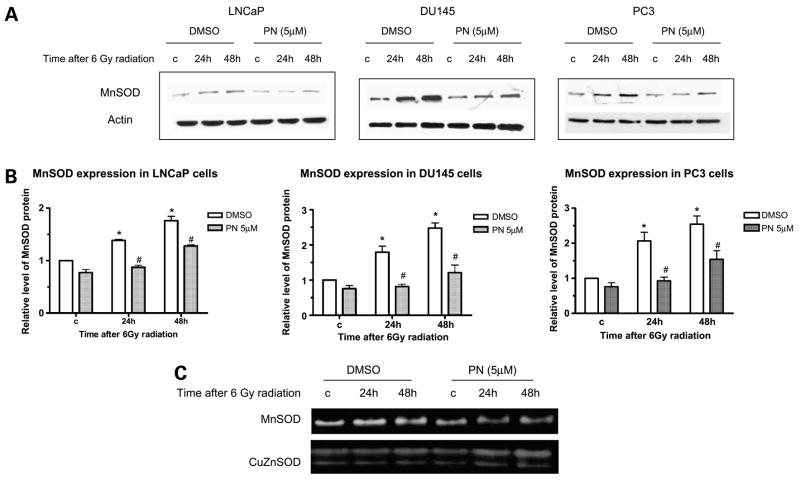
Parthenolide Suppresses MnSOD Induction by Radiation in Prostate Cancer Cells
Radiation exerts its effect largely by inducing oxidative stress. MnSOD is an important antioxidant enzyme induced by radiation. Therefore, we determined the effect of parthenolide on the expression of MnSOD, a well-established radiation-induced NF-κB target gene (6, 33). Radiation induced MnSOD protein levels in all three prostate cancer cell lines 24 and 48 h after radiation (Fig. 3A). In DU145 and PC3 cells, the induction of MnSOD was higher than in LNCaP cells (Fig. 3B), which is consistent with the relative radioresistance of these two cell lines. Parthenolide suppressed radiation-induced MnSOD in all three cancer cell lines used. This further showed that the transcription activity of NF-κB was inhibited in all three cancer cell lines. Consistent with the changes in MnSOD protein levels, the increase in MnSOD activity by radiation, determined by SOD activity gel electrophoresis, was also suppressed in the presence of parthenolide in PC3 cells (Fig. 3C). CuZnSOD activity was shown as a loading control which did not change significantly with treatment. Suppression of MnSOD will expose cells to radiation-induced oxidative stress; thus, this result suggests that the radiosensitization effect of parthenolide may be partially mediated through the inhibition of radiation-induced MnSOD expression.
Figure 3.
Parthenolide suppresses MnSOD induction by radiation in prostate cancer cells. Cells were treated with DMSO or 5 μmol/L of parthenolide for 3 h before radiation. Whole cell lysates were prepared at the indicated times after 6 Gy of radiation for detection of MnSOD protein levels and activities. A, representative Western blots. B, quantitation of MnSOD protein levels from three independent experiments. *, P < 0.05, compared with controls not treated with radiation; #, P < 0.05, compared with DMSO-treated counterparts. C, representative SOD activity gels of PC3 cells from four independent experiments.
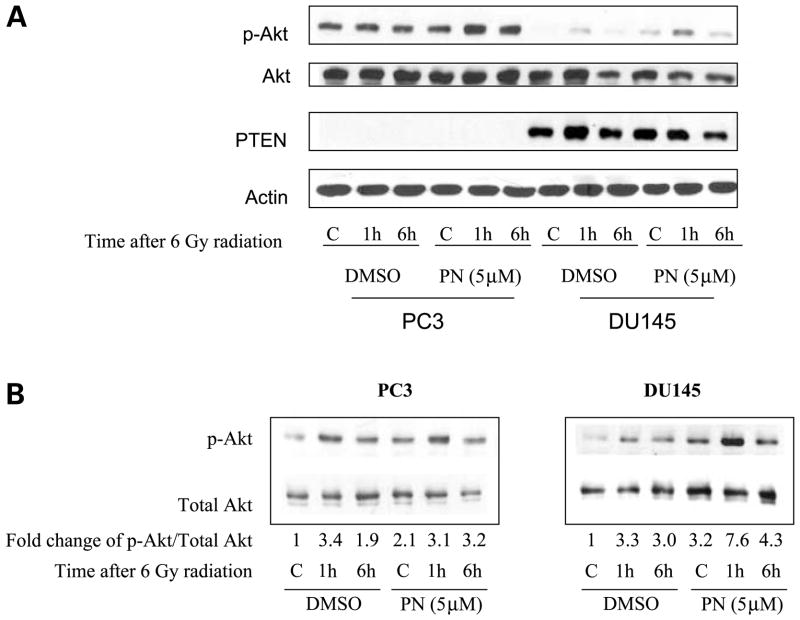
Parthenolide Activates Akt
Greater radiosensitization effect of parthenolide was observed in DU145 cells than in PC3 cells (Fig. 1). Both DU145 and PC3 cells are androgen-independent radioresistant aggressive prostate cancer. Neither has any functional p53 and they are known to have comparable levels of NF-κB (34). One of the major differences of these two cell lines is their PTEN status. DU145 cell is known to have functional PTEN, whereas PC3 is PTEN-null. The major function of PTEN is to dephosphorylate the second messenger PIP3 and to antagonize the PI3K/Akt, a prosurvival pathway. To elucidate the effects of PTEN on the differential radiosensitization efficiency of parthenolide in these two prostate cancer cell lines, we determined the effect of parthenolide on Akt activation. When we loaded 30 μg of proteins from both PC3 and DU145 whole cell lysates, as expected, PTEN-competent DU145 cells expressed a much lower level of activated p-Akt compared with PC3 cells at baseline (Fig. 4A). However, in order to ensure the detection of p-Akt in DU145 cells, protein was overloaded in PC3 cells, and therefore, the changes of p-Akt level caused by radiation and parthenolide were not in the linear range for densitometry. To show a better trend of treatment-induced changes in the p-Akt levels in these two cell lines, we loaded different amounts of protein for these two cell lines so that the levels of p-Akt were in the linear range. Radiation induced the phosphorylation of Akt in both cell lines, with the induction peak at 1 h after radiation (Fig. 4B). A previous study showed that activation of Akt after radiation was very rapid (10–15 min; ref. 35), and our data confirmed that this was an early event. The differences in peak times seem to be cell type–specific. Parthenolide alone increased the phosphorylation of Akt. When radiation was combined with parthenolide, greater induction of Akt phosphorylation was observed in both cell lines. In DU145 cells, which have wild-type PTEN, the phosphorylation of Akt was kept at a relatively low level compared with that in PC3 cells. Even with combined radiation and parthenolide treatments, the level of p-Akt in DU145 cells remained lower than the basal level in PC3 cells (Fig. 4A). Consistent with the prosurvival role of the p-Akt pathway, the radiosensitization effect of parthenolide was lower in PC3 cells, which have much higher levels of p-Akt after parthenolide treatment compared with DU145 cells similarly treated.
Figure 4.
Parthenolide activates Akt in PC3 and DU145 cells. Cells were pretreated with DMSO or 5 μmol/L of parthenolide for 3 h before radiation. Whole cell lysates were prepared at the indicated times after 6 Gy of radiation. The fold changes of p-Akt/total Akt were normalized to controls with no treatment. A, protein samples (30 μg) from both cell lines were loaded onto the same gel to compare the relative protein levels of these two cell lines. B, protein samples from PC3 cells (15 μg) and from DU145 cells (50 μg) were loaded separately to show changes in protein levels after treatments.
PTEN Enhances the Radiosensitization Effect of Parthenolide
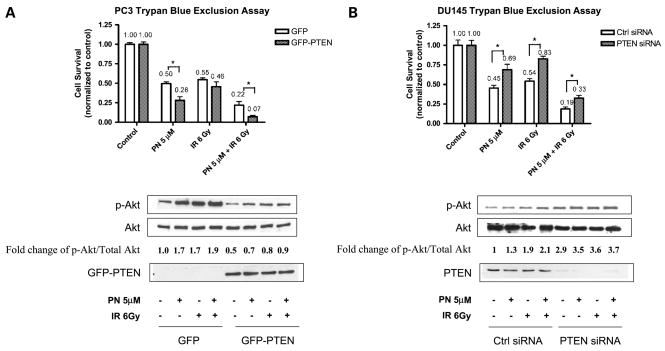
To directly test the role of PTEN in the radiosensitization effect of parthenolide in the two prostate cancer cell lines, we overexpressed PTEN in PTEN-null cell line PC3 and knocked down PTEN expression in PTEN wild-type cell line DU145 and determined cell survival after radiation and parthenolide treatments. We transfected GFP control plasmid and GFP-PTEN expression plasmids into PC3 cells. The transfection efficiency was ~85% based on the percentage of GFP-positive cells under fluorescent microscope (data not shown). Compared with the cells transfected with GFP alone, in the presence of GFP-PTEN expression, the normalized cell survival was decreased by 44% when treated with 5 μmol/L of parthenolide alone, 16% when treated with 6 Gy of radiation alone, and 68% when treated with parthenolide combined with radiation (Fig. 5A). Overexpression of GFP-PTEN in PC3 cells conferred the cells more susceptible to parthenolide and radiation toxicity, and also enhanced the radiosensitization effect of parthenolide. Consistent with the role of PTEN, the phosphorylation of Akt induced by parthenolide, radiation, and combined treatment was inhibited in the presence of GFP-PTEN (Fig. 5A). In a complementary experiment, PTEN was knocked down in DU145 cells by using PTEN siRNA. Compared with the cells transfected with control siRNA, in the cells in which PTEN expression was knocked down, the normalized cell survival was increased by 53% when treated with 5 μmol/L of parthenolide alone, 54% when treated with 6 Gy of radiation alone, and 74% when treated with parthenolide combined with radiation (Fig. 5B). Abrogation of PTEN expression in DU145 cells rendered the cells more resistant to the combined effect of parthenolide and radiation. Consistently, the phosphorylation of Akt induced by parthenolide, radiation, and combined treatment was further increased when PTEN expression was knocked down (Fig. 5B). Together, these results suggest that the presence of PTEN enhanced the radiosensitization effect of parthenolide.
Figure 5.
The presence of PTEN enhances the radiosensitization effect of parthenolide. A, PC3 cells were transiently transfected with GFP control plasmid and GFP-PTEN expression plasmid. Parthenolide was added to the medium, and 24 h later, cells were treated with 6 Gy of radiation or sham-irradiated. After an additional 24 h, the cells were processed for trypan blue exclusion assay and collected for Western blotting. Cytotoxicity was normalized to the corresponding control group. *, P <0.05, compared with the cells transfected with GFP control plasmid. The fold changes of p-Akt/total Akt were normalized to controls with no treatment. B, DU145 cells were transiently transfected with control siRNA and PTEN siRNA. Cells were treated in the same way as in A for cytotoxicity assay and Western blot. *, P < 0.05, compared with the cells transfected with control siRNA.
Discussion
Our results show that parthenolide sensitized prostate cancer cells to radiation treatment. Among the three prostate cancer cell lines used in our study, LNCaP cells express androgen receptor and have wild-type p53. Both DU145 and PC3 cells are androgen receptor–negative and have no functional p53 (36). The androgen-responsive LNCaP cells showed higher sensitivity to radiation treatment, which is consistent with previous studies (6, 37). Several factors can contribute to the fact that LNCaP cells are sensitive to radiation: (a) LNCaP cells have functional p53, (b) low activity of NF-κB in LNCaP cells, and especially, a low level of RelB (6), and (c) the presence of androgen receptor in LNCaP cells is antagonistic to NF-κB function (38, 39). Because LNCaP cells are sensitive to radiation treatment, our studies of the radiosensitization effect of parthenolide have been focused on the radioresistant cell lines, DU145 and PC3.
It has been shown that parthenolide inhibits the NF-κB pathway by targeting the IκB kinase complex (29, 30) or directly modifying p65 (31). The α-methylene-γ-lactone functional group in parthenolide can react with nucleophiles, such as cysteine sulfhydryl groups, in a Michael addition reaction (40). Inhibition of the NF-κB pathway by parthenolide is considered to be a consequence of alkylation of cysteine 179 of IKKβ or cysteine 38 of p65. Previous studies have shown that parthenolide sensitizes cancer cells to various apoptosis-inducing agents through inhibition of NF-κB (22). In our study, inhibition of NF-κB and its downstream target, MnSOD, seems to be a common mechanism for the radiosensitization effect of parthenolide in the prostate cancer cell lines studied. NF-κB activation induces many genes with antiapoptotic activities, including MnSOD, a mitochondria antioxidant enzyme that scavenges reactive oxygen species. Inhibition of the NF-κB pathway has been shown to sensitize prostate cancer cells to radiation treatment. Kim et al. (41) showed that using proteosome inhibitor-1 to inhibit NF-κB activation could increase the radiosensitivity of Ki-Ras–transformed human prostate epithelial 267B1/K-ras cells. Our previous finding showed that selective inhibition of RelB by dominant negative p100 significantly sensitized prostate cancer cells to ionizing radiation (6). The present study extends those findings to show that parthenolide inhibited radiation-induced NF-κB activation in all prostate cancer cell lines tested (Fig. 2A) and sensitized them to radiation treatment.
MnSOD is a key antioxidant enzyme that regulates cell transformation, tumor growth, and cell response to stress-inducing therapeutic regimens (42). Previous studies have shown that radiation induces cellular reactive oxygen species levels and MnSOD expression (6, 43). Inhibition of MnSOD expression by antisense MnSOD or selective inhibition of RelB can enhance the radiosensitivity of tumor cells (6, 43). In the present study, we show that parthenolide inhibited radiation-induced MnSOD expression in three prostate cancer cell lines (Fig. 3). Because MnSOD is capable of removing superoxides generated by radiation, the radiosensitization effect of parthenolide is likely to be mediated, in part, by the inhibition of MnSOD expression and activity.
Although our results showed that parthenolide sensitized both radioresistant prostate cancer cells, DU145 and PC3, to radiation in a dose-dependent manner (Fig. 1A), the efficiencies of parthenolide’s effect were different in the two cell lines. Higher toxicity and a higher radiosensitization effect of parthenolide were observed in DU145 cells. Because similar activities (Fig. 2B) and levels (34) of NF-κB, and a similar level of inhibition of the NF-κB target gene, MnSOD, by parthenolide (Fig. 3B) were detected in the two radioresistant prostate cancer cell lines, the different susceptibilities of the two cell lines to parthenolide suggests that besides inhibition of NF-κB, other mechanisms might be involved in the effect of parthenolide. We tested this hypothesis by examining the effect of parthenolide on the PI3K/Akt pathway in the two cell lines with different PTEN status. Our results suggest that the PI3K/Akt pathway is activated by parthenolide and the different cellular status of PTEN makes a difference in the cell susceptibility to parthenolide’s effect. The PI3K/Akt pathway is activated in response to growth factors and adhesion to the matrix or other cells. It promotes normal cell growth and proliferation. Activated PI3K converts PI(4,5)P2 to PI(3,4,5)P3 (PIP3), which is a lipid second messenger that activates many downstream molecules by binding to their pleckstrin-homology domains. PIP3 recruits Akt to the cell membrane and allows phosphati-dylinositol-dependent kinase 1 (PDK1) and a second kinase (termed PDK2, although not yet conclusively identified) to phosphorylate and activate Akt at Thr308 and Ser473, respectively. Activated Akt promotes both cell growth and cell survival by phosphorylation and inactivation of its downstream substrates including glycogen synthase kinase 3 (GSK3), the proapoptotic protein BCL2-antagonist of cell death (BAD), and the forkhead (FOXO) family of transcription factors, which promotes expression of p27-Kip1, a cell cycle inhibitor. It also activates the mammalian target of rapamycin by phosphorylating and inactivating tuberous sclerosis complex 2, thus promoting protein synthesis. The prosurvival role of Akt accounts for its transforming potential and for the resistance of cancer cells to some chemotherapeutic agents and ionizing radiation (44, 45). Radiation-induced activation of the PI3K/Akt prosurvival pathway is considered to be an important contributor to radioresistance in cancer cells. Gottschalk et al. (46) showed that PI3K inhibitor LY294002 sensitized prostate cancer cells to radiation through inactivation of Akt. Cao et al. (47) showed that the mammalian target of rapamycin inhibitor, RAD001, sensitized prostate cancer cells to radiation treatment. Our results show (Fig. 4B) that the PI3K/Akt pathway was activated by radiation in two radioresistant prostate cancer cell lines. This may contribute to their radioresistance. When parthenolide activates the PI3K/Akt pathway, its radiosensitization effect, resulting from inhibition of the NF-κB pathway, may be counteracted. Thus, the activation of the PI3K/Akt pathway by parthenolide participates in determining cell susceptibility to its radiosensitization effect. The fact that a significant radiosensitization effect was observed in the two radioresistant prostate cancer cell lines in spite of the activation of the PI3K/Akt pathway suggests that parthenolide’s inhibition of the NF-κB pathway overwhelms its effect of activating the PI3K/Akt pathway in determining cell response to radiation.
PTEN is a tumor suppressor which antagonizes PI3K by degrading PI(3,4,5)P3 back to PI(4,5)P2. PTEN mutations have been identified in 10% to 15% of all prostate cancers (48) and in up to 60% of advanced prostate cancers with multiple metastases or in prostate cancer cell lines (27). Haploinsufficiency of the PTEN gene has been shown to promote prostate cancer progression in a transgenic mouse model (49). The PI3K/Akt pathway can be activated by the deletion of PTEN (46). Loss of functional PTEN and the subsequent activation of the PI3K/Akt pathway may render cells more resistant to radiation treatment in advanced prostate cancer. Rosser et al. (50) showed that adenoviral-mediated PTEN transgene expression sensitizes prostate cancer cells to radiation. Our study confirms the results from the earlier study and extend to show that the presence of PTEN enhances the radiosensitization effect of parthenolide, in part, by suppressing the absolute amount of activated p-Akt. Our data suggest that the radiosensitization effect of parthenolide will be less effective in aggressive prostate cancer cells lacking wild-type PTEN. Thus, in order to get a better radiosensitization effect from parthenolide, an analogue of parthenolide which inhibits NF-κB but does not activate the PI3K/Akt pathway should be used. Alternatively, inhibitors of the PI3K/Akt pathway should be combined with parthenolide for cells deficient in PTEN functions.
In summary, the present study shows that parthenolide exerted its radiosensitization effect in prostate cancer cells, in part, by inhibiting the NF-κB pathway and its downstream target MnSOD. However, parthenolide also activated the PI3K/Akt prosurvival pathway, which might affect cell susceptibility to parthenolide’s effect. The presence of PTEN enhanced the radiosensitization effect of parthenolide by antagonizing the PI3K/Akt pathway. Understanding the mechanisms that are involved in the radiosensitization effect of parthenolide will enhance our ability to further improve the use of parthenolide as an effective adjuvant in radiation therapy of prostate cancer.
Acknowledgments
We thank Drs. Yong Xu and Yumin Chen for valuable comments, scientific and critical review of the manuscript.
Grant support: NIH grant CA49797.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Pirtskhalaishvili G, Hrebinko RL, Nelson JB. The treatment of prostate cancer: an overview of current options. Cancer Pract. 2001;9:295–306. doi: 10.1046/j.1523-5394.2001.96009.x. [DOI] [PubMed] [Google Scholar]
- 3.Hall EJ. Radiobiology for the radiologist. Philadelphia: J.B. Lippincott Company; 1994. p. 12. [Google Scholar]
- 4.Criswell T, Leskov K, Miyamoto S, Luo G, Boothman DA. Transcription factors activated in mammalian cells after clinically relevant doses of ionizing radiation. Oncogene. 2003;22:5813–27. doi: 10.1038/sj.onc.1206680. [DOI] [PubMed] [Google Scholar]
- 5.Russell JS, Tofilon PJ. Radiation-induced activation of nuclear factor-κB involves selective degradation of plasma membrane-associated I(κ)B(α) Mol Biol Cell. 2002;13:3431–40. doi: 10.1091/mbc.E02-05-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josson S, Xu Y, Fang F, Dhar SK, St Clair DK, St Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene. 2005;25:1554–9. doi: 10.1038/sj.onc.1209186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo JL, Kamata H, Karin M. IKK/NF-κB signaling: balancing life and death—a new approach to cancer therapy. J Clin Invest. 2005;115:2625–32. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saccani S, Pantano S, Natoli G. Two waves of nuclear factor κB recruitment to target promoters. J Exp Med. 2001;193:1351–9. doi: 10.1084/jem.193.12.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weisiger RA, Fridovich I. Superoxide dismutase. Organelle specificity. J Biol Chem. 1973;248:3582–92. [PubMed] [Google Scholar]
- 10.Hirose K, Longo DL, Oppenheim JJ, Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J. 1993;7:361–8. doi: 10.1096/fasebj.7.2.8440412. [DOI] [PubMed] [Google Scholar]
- 11.Eastgate J, Moreb J, Nick HS, Suzuki K, Taniguchi N, Zucali JR. A role for manganese superoxide dismutase in radioprotection of hematopoietic stem cells by interleukin-1. Blood. 1993;81:639–46. [PubMed] [Google Scholar]
- 12.Sweeney C, Li L, Shanmugam R, et al. Nuclear factor-κB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–7. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 13.Knight DW. Feverfew: chemistry and biological activity. Nat Prod Rep. 1995;12:271–6. doi: 10.1039/np9951200271. [DOI] [PubMed] [Google Scholar]
- 14.Wen J, You KR, Lee SY, Song CH, Kim DG. Oxidative stress-mediated apoptosis. The anticancer effect of the sesquiterpene lactone parthenolide. J Biol Chem. 2002;277:38954–64. doi: 10.1074/jbc.M203842200. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S, Ong CN, Shen HM. Critical roles of intracellular thiols and calcium in parthenolide-induced apoptosis in human colorectal cancer cells. Cancer Lett. 2004;208:143–53. doi: 10.1016/j.canlet.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 16.Guzman ML, Rossi RM, Karnischky L, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–9. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Liu L, Lee SO, Kim YT, You KR, Kim DG. Susceptibility of cholangiocarcinoma cells to parthenolide-induced apoptosis. Cancer Res. 2005;65:6312–20. doi: 10.1158/0008-5472.CAN-04-4193. [DOI] [PubMed] [Google Scholar]
- 18.Woynarowski JM, Konopa J. Inhibition of DNA biosynthesis in HeLa cells by cytotoxic and antitumor sesquiterpene lactones. Mol Pharmacol. 1981;19:97–102. [PubMed] [Google Scholar]
- 19.Pozarowski P, Halicka DH, Darzynkiewicz Z. Cell cycle effects and caspase-dependent and independent death of HL-60 and Jurkat cells treated with the inhibitor of NF-κB parthenolide. Cell Cycle. 2003;2:377–83. [PubMed] [Google Scholar]
- 20.Nakshatri H, Rice SE, Bhat-Nakshatri P. Antitumor agent parthenolide reverses resistance of breast cancer cells to tumor necrosis factor-related apoptosis-inducing ligand through sustained activation of c-Jun N-terminal kinase. Oncogene. 2004;23:7330–44. doi: 10.1038/sj.onc.1207995. [DOI] [PubMed] [Google Scholar]
- 21.Zhang S, Lin ZN, Yang CF, Shi X, Ong CN, Shen HM. Suppressed NF-κB and sustained JNK activation contribute to the sensitization effect of parthenolide to TNF-alpha-induced apoptosis in human cancer cells. Carcinogenesis. 2004;25:2191–9. doi: 10.1093/carcin/bgh234. [DOI] [PubMed] [Google Scholar]
- 22.Patel NM, Nozaki S, Shortle NH, et al. Paclitaxel sensitivity of breast cancer cells with constitutively active NF-κB is enhanced by IκBα super-repressor and parthenolide. Oncogene. 2000;19:4159–69. doi: 10.1038/sj.onc.1203768. [DOI] [PubMed] [Google Scholar]
- 23.Myers MP, Stolarov JP, Eng C, et al. P-TEN, the tumor suppressor from human chromosome 10q23, is a dual-specificity phosphatase. Proc Natl Acad Sci USA. 1997;94:9052–7. doi: 10.1073/pnas.94.17.9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura M, Gu J, Danen EH, Takino T, Miyamoto S, Yamada KM. PTEN interactions with focal adhesion kinase and suppression of the extracellular matrix-dependent phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1999;274:20693–703. doi: 10.1074/jbc.274.29.20693. [DOI] [PubMed] [Google Scholar]
- 25.Gu J, Tamura M, Yamada KM. Tumor suppressor PTEN inhibits integrin- and growth factor-mediated mitogen-activated protein (MAP) kinase signaling pathways. J Cell Biol. 1998;143:1375–83. doi: 10.1083/jcb.143.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–5. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray IC, Phillips SM, Lee SJ, Neoptolemos JP, Weissenbach J, Spurr NK. Loss of the chromosomal region 10q23-25 in prostate cancer. Cancer Res. 1995;55:4800–3. [PubMed] [Google Scholar]
- 28.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 29.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb feverfew directly binds to and inhibits IκB kinase. Chem Biol. 2001;8:759–66. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 30.Hehner SP, Hofmann TG, Droge W, Schmitz ML. The antiinflammatory sesquiterpene lactone parthenolide inhibits NF-κB by targeting the IκB kinase complex. J Immunol. 1999;163:5617–23. [PubMed] [Google Scholar]
- 31.Garcia-Pineres AJ, Castro V, Mora G, et al. Cysteine 38 in p65/NF-κB plays a crucial role in DNA binding inhibition by sesquiterpene lactones. J Biol Chem. 2001;276:39713–20. doi: 10.1074/jbc.M101985200. [DOI] [PubMed] [Google Scholar]
- 32.Magne N, Toillon RA, Bottero V, et al. NF-κB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–68. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Amundson SA, Bittner M, Chen Y, Trent J, Meltzer P, Fornace AJ., Jr Fluorescent cDNA microarray hybridization reveals complexity and heterogeneity of cellular genotoxic stress responses. Oncogene. 1999;18:3666–72. doi: 10.1038/sj.onc.1202676. [DOI] [PubMed] [Google Scholar]
- 34.Gasparian AV, Yao YJ, Kowalczyk D, et al. The role of IKK in constitutive activation of NF-κB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;115:141–51. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 35.Contessa JN, Hampton J, Lammering G, et al. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cells. Oncogene. 2002;21:4032–41. doi: 10.1038/sj.onc.1205500. [DOI] [PubMed] [Google Scholar]
- 36.van Bokhoven A, Varella-Garcia M, Korch C, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–25. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 37.Scott SL, Gumerlock PH, Beckett L, Li Y, Goldberg Z. Survival and cell cycle kinetics of human prostate cancer cell lines after single- and multifraction exposures to ionizing radiation. Int J Radiat Oncol Biol Phys. 2004;59:219–27. doi: 10.1016/j.ijrobp.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Palvimo JJ, Reinikainen P, Ikonen T, Kallio PJ, Moilanen A, Janne OA. Mutual transcriptional interference between RelA and androgen receptor. J Biol Chem. 1996;271:24151–6. doi: 10.1074/jbc.271.39.24151. [DOI] [PubMed] [Google Scholar]
- 39.McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-κB and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–59. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt TJ. Helenanolide-type sesquiterpene lactones. III Rates and stereochemistry in the reaction of helenalin and related helenanolides with sulfhydryl containing biomolecules. Bioorg Med Chem. 1997;5:645–53. doi: 10.1016/s0968-0896(97)00003-5. [DOI] [PubMed] [Google Scholar]
- 41.Kim BY, Kim KA, Kwon O, et al. NF-κB inhibition radiosensitizes Ki-Ras-transformed cells to ionizing radiation. Carcinogenesis. 2005;26:1395–403. doi: 10.1093/carcin/bgi081. [DOI] [PubMed] [Google Scholar]
- 42.Wang T, Zhang X, Li JJ. The role of NF-κB in the regulation of cell stress responses. Int Immunopharmacol. 2002;2:1509–20. doi: 10.1016/s1567-5769(02)00058-9. [DOI] [PubMed] [Google Scholar]
- 43.Guo G, Yan-Sanders Y, Lyn-Cook BD, et al. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol. 2003;23:2362–78. doi: 10.1128/MCB.23.7.2362-2378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 45.Kim D, Cheng GZ, Lindsley CW, Yang H, Cheng JQ. Targeting the phosphatidylinositol-3 kinase/Akt pathway for the treatment of cancer. Curr Opin Investig Drugs. 2005;6:1250–8. [PubMed] [Google Scholar]
- 46.Gottschalk AR, Doan A, Nakamura JL, Stokoe D, Haas-Kogan DA. Inhibition of phosphatidylinositol-3-kinase causes increased sensitivity to radiation through a PKB-dependent mechanism. Int J Radiat Oncol Biol Phys. 2005;63:1221–7. doi: 10.1016/j.ijrobp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 47.Cao C, Subhawong T, Albert JM, et al. Inhibition of mammalian target of rapamycin or apoptotic pathway induces autophagy and radiosensitizes PTEN null prostate cancer cells. Cancer Res. 2006;66:10040–7. doi: 10.1158/0008-5472.CAN-06-0802. [DOI] [PubMed] [Google Scholar]
- 48.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 49.Kwabi-Addo B, Giri D, Schmidt K, et al. Haploinsufficiency of the Pten tumor suppressor gene promotes prostate cancer progression. Proc Natl Acad Sci U S A. 2001;98:11563–8. doi: 10.1073/pnas.201167798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosser CJ, Tanaka M, Pisters LL, et al. Adenoviral-mediated PTEN transgene expression sensitizes Bcl-2-expressing prostate cancer cells to radiation. Cancer Gene Ther. 2004;11:273–9. doi: 10.1038/sj.cgt.7700673. [DOI] [PubMed] [Google Scholar]