Abstract
Bacillus anthracis is a soil pathogen capable of causing anthrax. To establish a method for specifically detecting B. anthracis for practical applications, such as for the inspection of slaughterhouses, the cap region, which is essential for encapsulation in B. anthracis, was used in a DNA hybridization study by polymerase chain reaction (PCR). Oligonucleotide primers were designed to amplify a 288-bp DNA fragment within the capA gene by PCR. The amplified DNA sequence specifically hybridized to the DNA of B. anthracis but not to that of other bacterial strains tested. Since this PCR-based method efficiently and specifically detected the capA sequence of bacteria in blood and spleen samples of mice within 8 h after the administration of live B. anthracis, this PCR system could be used for practical applications. By using lysis methods in preparing the samples for PCR, it was possible to amplify the 288-bp DNA segment from samples containing very few bacteria, as few as only 1 sporeforming unit, indicating that the PCR detection method developed in this study will permit the monitoring of B. anthracis contamination in the environment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEALL F. A., TAYLOR M. J., THORNE C. B. Rapid lethal effect in rats of a third component found upon fractionating the toxin of Bacillus anthracis. J Bacteriol. 1962 Jun;83:1274–1280. doi: 10.1128/jb.83.6.1274-1280.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN E. R., MOODY M. D., TREECE E. L., SMITH C. W. Differential diagnosis of Bacillus cereus, Bacillus anthracis, and Bacillus cereus var. mycoides. J Bacteriol. 1958 May;75(5):499–509. doi: 10.1128/jb.75.5.499-509.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada K., Oka S., Kimura S., Shimada K., Serikawa T., Yamada J., Tsunoo H., Egawa K., Nakamura Y. Detection of Pneumocystis carinii sequences by polymerase chain reaction: animal models and clinical application to noninvasive specimens. J Clin Microbiol. 1991 Sep;29(9):1985–1990. doi: 10.1128/jcm.29.9.1985-1990.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Uchida I., Terakado N., Yoshikawa M. Cloning and CO2-dependent expression of the genetic region for encapsulation from Bacillus anthracis. Mol Microbiol. 1988 May;2(3):371–376. doi: 10.1111/j.1365-2958.1988.tb00041.x. [DOI] [PubMed] [Google Scholar]
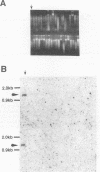
- Makino S., Uchida I., Terakado N., Sasakawa C., Yoshikawa M. Molecular characterization and protein analysis of the cap region, which is essential for encapsulation in Bacillus anthracis. J Bacteriol. 1989 Feb;171(2):722–730. doi: 10.1128/jb.171.2.722-730.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANLEY J. L., SARGEANT K., SMITH H. Purification of factors I and II of the anthrax toxin produced in vivo. J Gen Microbiol. 1960 Feb;22:206–218. doi: 10.1099/00221287-22-1-206. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Steffan R. J., Atlas R. M. Polymerase chain reaction: applications in environmental microbiology. Annu Rev Microbiol. 1991;45:137–161. doi: 10.1146/annurev.mi.45.100191.001033. [DOI] [PubMed] [Google Scholar]
- THORNE C. B., GOMEZ C. G., NOYES H. E., HOUSEWRIGHT R. D. Production of glutamyl polypeptide by Bacillus subtilis. J Bacteriol. 1954 Sep;68(3):307–315. doi: 10.1128/jb.68.3.307-315.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE C. B., MOLNAR D. M., STRANGE R. E. Production of toxin in vitro by Bacillus anthracis and its spearation into two components. J Bacteriol. 1960 Mar;79:450–455. doi: 10.1128/jb.79.3.450-455.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull P. C., Hutson R. A., Ward M. J., Jones M. N., Quinn C. P., Finnie N. J., Duggleby C. J., Kramer J. M., Melling J. Bacillus anthracis but not always anthrax. J Appl Bacteriol. 1992 Jan;72(1):21–28. doi: 10.1111/j.1365-2672.1992.tb04876.x. [DOI] [PubMed] [Google Scholar]
- Uchida I., Sekizaki T., Hashimoto K., Terakado N. Association of the encapsulation of Bacillus anthracis with a 60 megadalton plasmid. J Gen Microbiol. 1985 Feb;131(2):363–367. doi: 10.1099/00221287-131-2-363. [DOI] [PubMed] [Google Scholar]