Abstract
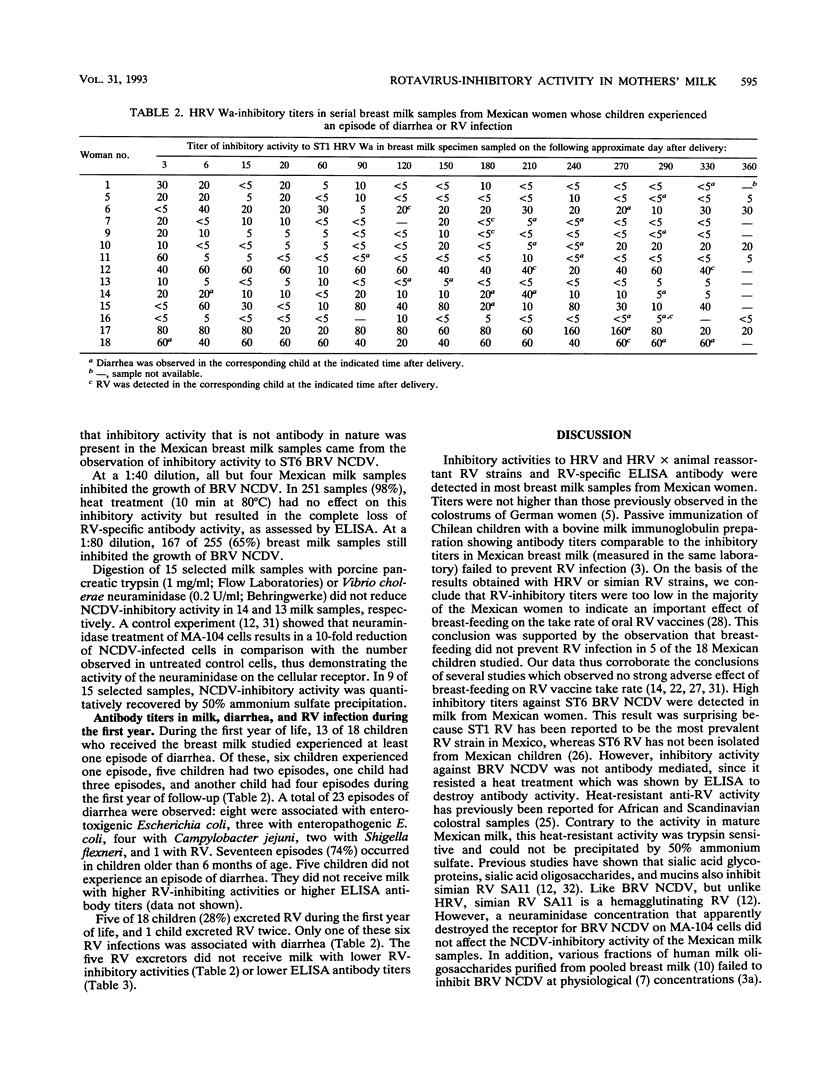
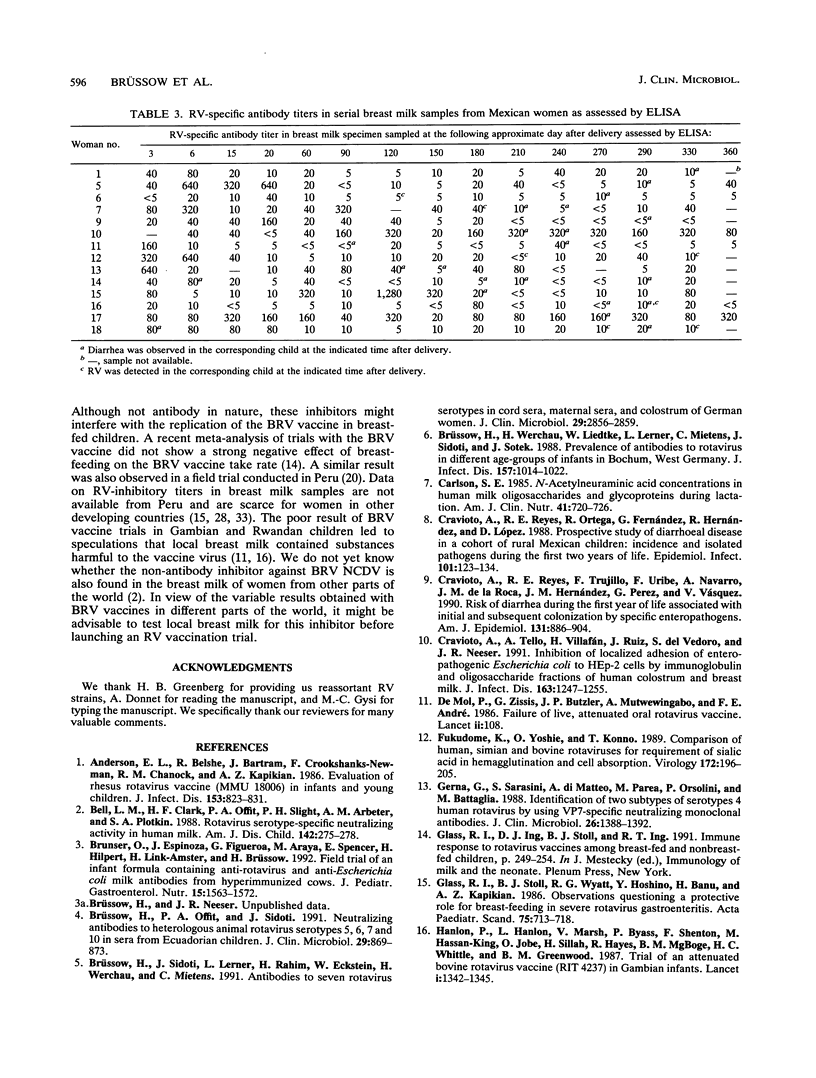
A total of 75 children born in rural Mexico were followed for diarrheal diseases and rotavirus (RV) excretion during the first year of life. For 18 children, an average of 14 serial breast milk samples were obtained between days 2 and 360 after delivery and were tested for RV-inhibitory activity. Of these samples, 70, 62, and 85% showed inhibitory activity against serotype (ST) 1 human RV, ST4 human RV, and ST3 simian RV, respectively; the median titers were 10, 10, and 20, respectively. Some 89% of the milk samples showed RV-specific antibodies in an enzyme-linked immunosorbent assay (median titer, 20). Surprisingly, 98% of the milk samples inhibited ST6 bovine RV. ST6, but not ST1, RV-inhibitory activity survived heat treatment (10 min at 80 degrees C). Of the 18 children tested, 13 children experienced 23 episodes of diarrhea (enterotoxigenic [n = 8] and enteropathogenic [n = 3] Escherichia coli, Campylobacter jejuni [n = 4], Shigella flexneri [n = 2], RV [n = 1]) and 5 children experienced 6 RV infections. Only one RV infection was associated with diarrhea. The five RV excretors did not differ from the nonexcretors with respect to the RV-inhibitory activity in the breast milk fed to them. The RV-inhibitory titers were too low in the majority of the studied Mexican milk samples to indicate an important effect of breast-feeding on the take rate of oral human, simian, or reassortant RV vaccines. Breast-feeding might, however, inhibit the take rate of a bovine RV vaccine.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. L., Belshe R. B., Bartram J., Crookshanks-Newman F., Chanock R. M., Kapikian A. Z. Evaluation of rhesus rotavirus vaccine (MMU 18006) in infants and young children. J Infect Dis. 1986 May;153(5):823–831. doi: 10.1093/infdis/153.5.823. [DOI] [PubMed] [Google Scholar]
- Bell L. M., Clark H. F., Offit P. A., Slight P. H., Arbeter A. M., Plotkin S. A. Rotavirus serotype-specific neutralizing activity in human milk. Am J Dis Child. 1988 Mar;142(3):275–278. [PubMed] [Google Scholar]
- Brüssow H., Offit P. A., Sidoti J. Neutralizing antibodies to heterologous animal rotavirus serotypes 5, 6, 7, and 10 in sera from Ecuadorian children. J Clin Microbiol. 1991 May;29(5):869–873. doi: 10.1128/jcm.29.5.869-873.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Sidoti J., Lerner L., Rahim H., Eckstein W., Werchau H., Mietens C. Antibodies to seven rotavirus serotypes in cord sera, maternal sera, and colostrum of German women. J Clin Microbiol. 1991 Dec;29(12):2856–2859. doi: 10.1128/jcm.29.12.2856-2859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Werchau H., Liedtke W., Lerner L., Mietens C., Sidoti J., Sotek J. Prevalence of antibodies to rotavirus in different age-groups of infants in Bochum, West Germany. J Infect Dis. 1988 May;157(5):1014–1022. doi: 10.1093/infdis/157.5.1014. [DOI] [PubMed] [Google Scholar]
- Carlson S. E. N-acetylneuraminic acid concentrations in human milk oligosaccharides and glycoproteins during lactation. Am J Clin Nutr. 1985 Apr;41(4):720–726. doi: 10.1093/ajcn/41.4.720. [DOI] [PubMed] [Google Scholar]
- Cravioto A., Reyes R. E., Ortega R., Fernández G., Hernández R., López D. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Infect. 1988 Aug;101(1):123–134. doi: 10.1017/s0950268800029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravioto A., Reyes R. E., Trujillo F., Uribe F., Navarro A., De La Roca J. M., Hernández J. M., Pérez G., Vázquez V. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am J Epidemiol. 1990 May;131(5):886–904. doi: 10.1093/oxfordjournals.aje.a115579. [DOI] [PubMed] [Google Scholar]
- Cravioto A., Tello A., Villafán H., Ruiz J., del Vedovo S., Neeser J. R. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis. 1991 Jun;163(6):1247–1255. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- De Mol P., Zissis G., Butzler J. P., Mutwewingabo A., André F. E. Failure of live, attenuated oral rotavirus vaccine. Lancet. 1986 Jul 12;2(8498):108–108. doi: 10.1016/s0140-6736(86)91643-0. [DOI] [PubMed] [Google Scholar]
- Fukudome K., Yoshie O., Konno T. Comparison of human, simian, and bovine rotaviruses for requirement of sialic acid in hemagglutination and cell adsorption. Virology. 1989 Sep;172(1):196–205. doi: 10.1016/0042-6822(89)90121-9. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., di Matteo A., Parea M., Orsolini P., Battaglia M. Identification of two subtypes of serotype 4 human rotavirus by using VP7-specific neutralizing monoclonal antibodies. J Clin Microbiol. 1988 Jul;26(7):1388–1392. doi: 10.1128/jcm.26.7.1388-1392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. I., Ing D. J., Stoll B. J., Ing R. T. Immune response to rotavirus vaccines among breast-fed and nonbreast-fed children. Adv Exp Med Biol. 1991;310:249–254. doi: 10.1007/978-1-4615-3838-7_33. [DOI] [PubMed] [Google Scholar]
- Glass R. I., Stoll B. J., Wyatt R. G., Hoshino Y., Banu H., Kapikian A. Z. Observations questioning a protective role for breast-feeding in severe rotavirus diarrhea. Acta Paediatr Scand. 1986 Sep;75(5):713–718. doi: 10.1111/j.1651-2227.1986.tb10279.x. [DOI] [PubMed] [Google Scholar]
- Hanlon P., Hanlon L., Marsh V., Byass P., Shenton F., Hassan-King M., Jobe O., Sillah H., Hayes R., M'Boge B. H. Trial of an attenuated bovine rotavirus vaccine (RIT 4237) in Gambian infants. Lancet. 1987 Jun 13;1(8546):1342–1345. doi: 10.1016/s0140-6736(87)90649-0. [DOI] [PubMed] [Google Scholar]
- Kalica A. R., Flores J., Greenberg H. B. Identification of the rotaviral gene that codes for hemagglutination and protease-enhanced plaque formation. Virology. 1983 Feb;125(1):194–205. doi: 10.1016/0042-6822(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Lanata C. F., Black R. E., del Aguila R., Gil A., Verastegui H., Gerna G., Flores J., Kapikian A. Z., Andre F. E. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989 Mar;159(3):452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- Larralde G., Li B. G., Kapikian A. Z., Gorziglia M. Serotype-specific epitope(s) present on the VP8 subunit of rotavirus VP4 protein. J Virol. 1991 Jun;65(6):3213–3218. doi: 10.1128/jvi.65.6.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madore H. P., Christy C., Pichichero M., Long C., Pincus P., Vosefsky D., Kapikian A. Z., Dolin R. Field trial of rhesus rotavirus or human-rhesus rotavirus reassortant vaccine of VP7 serotype 3 or 1 specificity in infants. The Elmwood, Panorama, and Westfall Pediatric Groups. J Infect Dis. 1992 Aug;166(2):235–243. doi: 10.1093/infdis/166.2.235. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Hoshino Y., Kapikian A. Z., Chanock R. M. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986 Nov;24(5):822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otnaess A. B., Orstavik I. Effect of fractions of Ethiopian And Norwegian colostrum on rotavirus and Escherichia coli heat-labile enterotoxin. Infect Immun. 1981 Aug;33(2):459–466. doi: 10.1128/iai.33.2.459-466.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Noriega L., Arias C. F., López S., Puerto F., Snodgrass D. R., Taniguchi K., Greenberg H. B. Diversity of rotavirus serotypes in Mexican infants with gastroenteritis. J Clin Microbiol. 1990 Jun;28(6):1114–1119. doi: 10.1128/jcm.28.6.1114-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichichero M. E. Effect of breast-feeding on oral rhesus rotavirus vaccine seroconversion: a metaanalysis. J Infect Dis. 1990 Sep;162(3):753–755. doi: 10.1093/infdis/162.3.753. [DOI] [PubMed] [Google Scholar]
- Rimer H. C., Wasserman S. S., Flores J., Pichichero M. E., Losonsky G. A. Rotavirus-specific breast milk antibody in two populations and possible correlates of interference with rhesus rotavirus vaccine seroconversion. J Infect Dis. 1992 May;165(5):826–830. doi: 10.1093/infdis/165.5.826. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., D'Hondt E., Delem A., André F. E., Zissis G. Protection of infants against rotavirus diarrhoea by RIT 4237 attenuated bovine rotavirus strain vaccine. Lancet. 1984 May 5;1(8384):977–981. doi: 10.1016/s0140-6736(84)92323-7. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., d'Hondt E., André F. E., Beards G. M., Flewett T. H. Clinical efficacy of the RIT 4237 live attenuated bovine rotavirus vaccine in infants vaccinated before a rotavirus epidemic. J Pediatr. 1985 Aug;107(2):189–194. doi: 10.1016/s0022-3476(85)80123-2. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Ruuska T., Bogaerts H., Delem A., André F. Dose-response study of RIT 4237 oral rotavirus vaccine in breast-fed and formula-fed infants. Pediatr Infect Dis. 1985 Nov-Dec;4(6):622–625. doi: 10.1097/00006454-198511000-00005. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Willoughby R., Wee S. B., Miskuff R., Vonderfecht S. Sialic acid glycoproteins inhibit in vitro and in vivo replication of rotaviruses. J Clin Invest. 1987 Jan;79(1):148–154. doi: 10.1172/JCI112775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolken R. H., Wyatt R. G., Mata L., Urrutia J. J., Garciá B., Chanock R. M., Kapikian A. Z. Secretory antibody directed against rotavirus in human milk--measurement by means of enzyme-linked immunosorbent assay. J Pediatr. 1978 Dec;93(6):916–921. doi: 10.1016/s0022-3476(78)81211-6. [DOI] [PubMed] [Google Scholar]


