Abstract
Unlike small molecule drugs, the conformational properties of protein biopharmaceuticals in solution are influenced by a variety of factors that are not solely defined by their covalent chemical structure. Since the conformation (or higher order structure) of a protein is a major modulator of its biological activity, the ability to detect changes in both the higher order structure and conformational dynamics of a protein, induced by an array of extrinsic factors, is of central importance in producing, purifying, and formulating a commercial biopharmaceutical with consistent therapeutic properties. In this study we demonstrate that two complementary mass spectrometry-based approaches (analysis of ionic charge state distribution and hydrogen/deuterium exchange) can be a potent tool in monitoring conformational changes in protein biopharmaceuticals. The utility of these approaches is demonstrated by detecting and characterizing conformational changes in the biopharmaceutical product interferon β-1a (IFN-β1a). The protein degradation process was modeled by inducing a single chemical modification of IFN-β1a (alkylation of its only free cysteine residue with N-ethylmaleimide), which causes significant reduction in its antiviral activity. Analysis of IFN-β1a ionic charge state distributions unequivocally reveals a significant decrease of conformational stability in the degraded protein, while hydrogen/deuterium exchange measurements provide a clear indication that the higher order structure is affected well beyond the covalent modification site. Importantly, neither technique required that the location or indeed the nature of the chemical modification be known prior to or elucidated in the process of the analysis. In contrast, application of the standard armamentarium of biophysical tools, which are commonly employed for quality control of protein pharmaceuticals, met with very limited success in detection and characterization of conformational changes in the modified IFN-β1a. This work highlights the role mass spectrometry can and should play in the biopharmaceutical industry beyond the presently assigned task of primary structure analysis.
Introduction
Biopharmaceuticals (the vast majority of which are recombinant proteins) constitute the fastest growing segment among the new pharmaceutical products. Their share of the market is expected to continue to expand and outpace the traditional small-molecule pharmaceuticals in the foreseeable future (1, 2). Since the biological activity of all proteins is intimately related to their higher order structure, the therapeutic properties of protein biopharmaceuticals are uniquely determined by their conformation. In the case of small-molecule drugs conformational issues can also be important, however they arise only in the solid state where different conformers of the same molecule can lead to different crystal states, or polymorphs (3). Polymorphs of the same drug may differ dramatically from one another in their physical properties leading in some cases to significant changes in pharmacokinetic profiles, sometimes with catastrophic consequences (4). Nevertheless, there is no evidence that these conformational differences persist in solution. This is in stark contrast to the solution behavior of protein biopharmaceuticals where partial or complete loss of a native folded structure is common and can dramatically influence their therapeutic properties (thereby adversely affecting the efficacy), their ability to elicit an immunogenic response (5), or even trigger aggregation (6). As a result, both the physical and chemical stability of a protein biopharmaceutical need to be carefully investigated and controlled during and after its production.
Protein conformations are largely determined by the primary structure (amino acid sequence), although they can also be significantly influenced by an array of post-translational modifications (PTM). While most enzymatically-controlled PTMs are central to the protein's ability to function in vivo, a large number of modifications are non-enzymatic and are associated with protein ageing, degradation, and ultimately, loss of function (7). Oxidation, deamidation, isomerization, and racemization of amino acids residues, as well as disulfide scrambling, are among the most common non-enzymatic PTMs inflicting damage on proteins both in vitro and in vivo (8), and have been traditionally an obvious focus of quality control efforts (9). However, in many cases detection and even localization of these PTMs is not sufficient to reveal their potential consequences to the integrity of the protein's higher order structure. Indeed, the conformational repercussions of various PTMs can vary significantly from one protein to another. In some cases non-enzymatic PTMs exert little or no effect on a protein conformation, while in other cases even subtle changes in the covalent structure of a protein can lead to profound changes in the higher order structure, many of which are far removed from the site of modification. This unpredictability in the impact of PTMs on the conformational properties of a protein biopharmaceutical (and ultimately, on its efficacy, immunogenicity, and stability) places a premium on the ability to detect and characterize changes in the higher order structure and dynamics of these proteins triggered by a host of different physical and chemical environments encountered during the manufacturing, storage, and administration of these complex drugs.
The availability of robust and reliable analytical tools capable of characterizing the higher order structure and dynamics of protein biopharmaceuticals is critical in accelerating the successful development of these novel drugs (10, 11). Such tools will also play an important role in setting specification levels to which these chemical changes can be tolerated, in stability and comparability studies (when changes or optimization (7) are made to the production process), and in allowing meaningful comparison to determine if follow-on biologies (biosimilars) are in fact appropriately similar to the original innovator product (12, 13).
Until very recently, however, there was a lack of simple, effective and reliable experimental tools to meet this analytical objective. X-ray crystallography is obviously biased towards the most stable crystal conformations (which may allow the non-native conformations in solution to escape detection), while high-resolution NMR spectroscopy still suffers from rather unforgiving molecular weight limitations and high material requirements. Although the higher order structure of protein drugs can also be probed using separation techniques, such as size exclusion chromatography (SEC), native gel electrophoresis (14), and analytical centrifugation (15), a variety of optical spectroscopic methods, such as circular dichroism and fluorescence (16, 17), as well as light scattering techniques (18) and calorimetry (19), the structural information deduced from these measurements is usually very limited and lacking in both sensitivity and specificity.
This technological gap is being filled by mass spectrometry (MS), which in recent years has matured into a technique capable of providing structural information on biopolymers at a variety of levels (20, 21). Although most current applications of MS in the analysis of biopharmaceuticals are limited to characterization of covalent (primary) structure, including both amino acid sequence and PTMs (22), rapid progress in developing MS-based approaches to probe protein conformation and dynamics suggests that its potential spans well beyond the routine sequencing tasks. Among several MS techniques used to assess protein higher order structure, hydrogen/deuterium exchange (HDX) (23) and analysis of ionic charge state distributions in electrospray ionization (ESI) mass spectra (24) appear to be particularly promising. A unique advantage of these techniques is the ability to detect very small amounts of non-native conformers within the background of natively folded protein species. This is in contrast to MS structural techniques based on chemical cross-linking (25) or chemical modification (26), which produce low yields of the target protein and thus would preclude the detection of conformers present in minute quantities. Furthermore, combination of HDX with protein fragmentation prior to MS analysis (proteolysis in solution under slow exchange conditions (27) or ion dissociation in the gas phase (28)) provides an opportunity to obtain polypeptide backbone flexibility maps and offers the capability to specifically identify segments of a protein that have unfolded. As an analytical technique, HDX MS continues to enjoy spectacular growth, as suggested by the rapid expansion of both the number of applications and the scope of biophysical information that is extracted from these measurements. It is therefore surprising that HDX MS has seen very few applications in the field of biopharmaceutical analysis, where a capability to detect and characterize conformational changes is urgently needed (29). In addition, the use of ESI MS to assess the integrity of higher order structure by analyzing a protein's ion charge state distributions (30) has never been utilized as a means to detect conformational changes in protein biopharmaceuticals resulting from chemical modifications and/or exposure to the array of physical and chemical environments during all phases of its manufacturing and storage.
In the present work we investigate structural changes that result from the chemical modification of interferon β1a (IFN-β1a), a cytokine defined by its anti-viral activities. Essential to its biological activity is the recruitment of a heterodimeric cell surface receptor composed of two transmembrane subunits, IFN receptor 1 (IFNAR1) and IFN receptor 2 (IFNAR2), to form the ternary IFNAR-1/IFN-β1a/IFNAR-2 complex (31). Specific covalent modification of the sole free cysteine of IFN-B1a leads to a protein with impaired biological activity as well as a suspected structural change. Utilizing this altered IFN-β1a we demonstrate that ion charge state distribution analysis in ESI MS in combination with HDX MS is a sensitive and reliable toolset for monitoring changes in the higher order structure of this commercial biopharmaceutical product. The high sensitivity, accuracy, and ability to provide detailed structural information make these MS-based methods very attractive additions or potential alternatives to the “classical” biophysical techniques commonly employed by the industry to assess and monitor comparability and stability of the higher order structure of protein biopharmaceuticals.
Materials and Methods
IFN-β1a sample preparation and treatment
The IFN-β1a used in this study was obtained from Biogen Idec, Inc. (Cambridge, MA). N-ethylmaleimide (NEM) was obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO). All other chemicals and solvents were of analytical grade or higher. IFN-β1a alkylation with NEM was carried out in buffer A (100 mM sodium phosphate, 200 mM sodium chloride, pH 7.2) at an approximate protein concentration of 0.25 mg/mL with an NEM final concentration of 0.5 mM (using a stock NEM solution of 100 mM). The reaction was then allowed to proceed for 1 hour at ambient temperature in the dark. After incubation, the NEM-IFN-β1a sample was buffer exchanged into buffer A using a 4 mL 10K MWCO centrifugal membrane filters (Amicon, Millipore). The buffer exchange was performed at 5 °C and 4,000 rpm and was repeated 3 times to ensure removal of excess NEM. The concentration of NEM-IFN-β1a was determined by UV absorption measurements at 280 nm using the extinction coefficient of 1.50 OD280 nm/mg/mL (samples were typical concentrated to a final concentration of about 0.25 mg/mL). NEM-IFN-β1a was then characterized by mass spectrometry to determine the extent of alkylation (in terms of both NEM : IFN-β1a stoichiometry and the fraction of modified IFN-β1a molecules). Intact mass analysis and peptide mapping confirmed that a single NEM molecule was covalently attached to IFN-β1a cysteine 17 and that the labeling efficiency at this site was > 95 %. SEC analysis was then conducted on NEM- IFN-β1a (vide infra) to ensure quantitative retention time shift of the IFN-β1a material and its homogeneity.
Size exclusion chromatography
SEC analyses were carried out using a G2000SWxl column (Tosoh Bioscience, San Francisco, CA) at ambient temperature in a mobile phase of buffer A (flow rate of 1.0 mL/min) with sample injection volumes ranging from 25 to 100 μL. Protein elution profiles were recorded by monitoring absorption at a wavelength of 280 nm.
Circular dichroism spectrophotometry
All CD measurements were carried out using a Jasco J-715 spectropolarimeter (Jasco, Inc., Easton, MD). Sample concentrations for the near-UV CD measurements were 20 μM in buffer A; the spectra were recorded in the 250-360 nm range in 0.1 nm steps using a 1 cm quartz cuvette. Sample concentrations for far-UV measurements were 4 μM in 100 mM ammonium acetate (pH 6.8). The data were collected in the 190-250 nm range in 0.5 nm steps (1 nm bandwidth, 20 nm min-1 scan rate) using a 0.1 cm quartz cuvette. Three scans were averaged for each spectrum to ensure adequate signal-to-noise ratio. All samples were kept at 25 °C during the data acquisition.
Fluorescence
The fluorescence emission spectra were recorded with Jasco FP-750 spectrofluorometer (Jasco, Inc., Eaton, MD) using 1 cm optical path length quartz cuvettes. The instrument settings were as follows: excitation bandwidth, 10 nm; emission band, 5 nm; response speed, slow; excitation wavelength, 280 nm; spectroscopic data spacing, 1 nm; scanning speed, 60 nm/min; and sensitivity settings, medium. Three consecutive scans (300-480 nm range) were recorded and averaged for each spectrum. No correction for the observed fluorescence photo bleaching effect was introduced. Protein concentration used for measurements was 0.023 mg/ml in 50 mM sodium phosphate, 100 mM NaCI buffer (pH 6.8).
Analytical ultracentrifugation
All AUC experiments were conducted in a Beckman/Coulter (Fullerton, CA) XL-I analytical ultracentrifuge at 20 °C, at a speed of 50K rpm. UV sedimentation profiles were recorded using UV absorption at a wavelength of 238 nm. Sample concentrations were in the range of 0.2 to 0.3 mg/mL in buffer A. Centrifugation cell consisted of a 12 mm charcoal filled epon centerpiece and sapphire windows. Data analysis was conducted with the computer program SEDFIT developed by Peter Schuck and co-workers (32).
HDX MS
HDX for intact proteins was initiated by diluting the protein stock solutions (50 μM in 20 mM ammonium acetate) 10-fold into exchange buffer (20 mM ammonium acetate in D2O) equilibrated to 25°C. At a designated time the sample was placed in an ice bath for 15 seconds and then an equal volume of pre-chilled quench solution (acetonitrile containing 0.2% formic acid) was added and rapidly mixed, yielding a pH of 2.4. Using a denaturing quench dramatically increased the resolution of the mass measurements, which were recorded immediately following the quench. Forward exchange was measured for each protein by preparing a sample in which the protein was added last to a prechilled, quenched solution. Plots of increase in mass over time were prepared by subtracting the average mass of the forward exchange control sample from the average mass calculated at various HDX time points.
To probe protein conformational stability locally, HDX was initiated by diluting the protein stock solutions (50 μM in 100 mM sodium phosphate pH 7.2,100 mM NaCI) 10-fold into exchange buffer (50 mM sodium phosphate D2O, pD 7.2, uncorrected for the isotope effect) equilibrated to 25 °C. At a designated time an aliquot of the solution was placed in an ice bath for 15 sec and then an equal volume of pre-chilled quench solution (3.6 M guanidine HCI, 0.5 % trifluoroacetic acid, 50 mM tris(2-carboxyethyl) phosphine HCI) was added and rapidly mixed, yielding pH 2.4. All subsequent steps were performed in an ice bath. The quenched sample was digested online using two immobilized protease columns in tandem, first pepsin and then type XIII fungal protease (33), at a flow rate of 0.16 ml min-1 using 0.1 % formic acid as the mobile phase. The resulting peptides were collected and desalted with an inline nanotrap cartridge then resolved by a C18 column (Armor Advance, 5 μm, 50 × 2.1 mm) using a rapid gradient from 5% to 65% acetonitrile containing 0.1 % formic acid and a flow rate of 0.3 ml min-1. The total time for the digest and desalting was 3 min, all peptides had eluted from the C18 column by 9 min. MS measurements were carried out with a hybrid quadrupole/time-of-flight mass spectrometer (QStar-XL, MDS Sciex/Applied Biosystems, Toronto, Canada) equipped with a standard Turbospray™ source.
Two data sets were used in our comparison of IFN-β1a with NEM-IFN-β1a by local HDX MS. One data set consisted of 3 exchange time points (10 s, 30 s, and 120 s) for each sample. The other consisted of a single 5 min exchange time point performed in triplicate for each sample. Proteolytic peptides were identified using a combination of exact mass and MS/MS data for unlabeled protein utilizing a shallower elution gradient (such proteolytic fragments cover over 75 % of the protein sequence). The extent of deuterium incorporation was calculated by monitoring the average mass increase for each identified peptide. The theoretical maximum deuterium incorporation value was calculated for each peptide based on the dilution factor and number of exchangeable amides, with the assumption that exchange of N-terminal amide is lost by back exchange during sample workup(34).
Direct ESI MS measurements for the analysis of IFN-β1a ion charge state distributions and IFN-β1a/IFNAR-2 binding studies
The extracellular portion of IFNAR-2, which was expressed and purified at Biogen Idec (35), and NEM-IFN-β1a were buffer exchanged into 100 mM ammonium acetate pH 6.8 through repeated concentration and dilution using Centricon (Millipore) centrifugal filters with a 10 kDa cut-off (fixed angle rotor operated at 4,000 g and 4 °C). A mixture of 4 μM IFNAR-2 and 1.5 μM NEM-IFN-β1a prepared in 100 mM ammonium acetate was analyzed on the QStar-XL (MDS Sciex/Applied Biosystems, Toronto, Canada) equipped with a nanospray source.
Biological activity assay
Antiviral activities of IFN-β1a and NEM-IFN-β1a were assessed using the method of Alam et al. (36). In summary, the assay measures the ability of IFN-β1a to protect human lung carcinoma (A549) cells from the cytopathic effect (CPE) of the encephalomyocarditis (EMC) virus. IFN-B1a samples are diluted in cell growth medium to the working range of the assay (10000 U/mL). On day 1 of the assay, samples are serially diluted in cell growth medium on assay plates along with an assay standard and control. A549 cells (37) are added to the plates and incubated 15-20 hours at which point the EMC virus is added to the plates and incubated an additional 30 hours. Each plate then is stained with crystal violet, washed and dried, and then read visually to determine the lowest concentration of IFN-B1a protecting the cells from the CPE of EMC virus.
Results and Discussion
IFN-β1a is a 23 kDa glycoprotein (38), several forms of which are used for treatment of relapsing forms of multiple sclerosis (39); it also exhibits anti-viral and anti-cancer activity (31). While recent attempts to introduce IFN-β1a directly into targeted tissue using gene transfer techniques showed much promise (40), administration of recombinant forms of interferons remains the only approved form of treatment, with several follow-on versions currently in development. As is the case with most proteins, interferons have a problematic propensity to misfold, which leads to activity loss, aggregation and increased immunogenic response (5). IFN-β1a misfolding can be accelerated by a variety of factors, including chemical modifications, interaction of proteins with surfaces, exposure to elevated temperatures, lyophilization, etc.
Earlier studies of interferon degradation identified several amino acid residues that are common targets of chemical modifications, including three (out of four) methoinine residues and a single free cysteine residue (41), a conclusion that was confirmed in our more recent study. While it is impossible to achieve a selective oxidation of any single methionine side chain (or their combination) in IFN-β1a, protein alkylation with N-ethylmaleimide (NEM) can produce an almost homogenous product, where >95% of protein molecules are modified at cysteine at position 17, without affecting the rest of the protein. Despite the limited range of alkylation targets in IFN-β1a (compared to oxidation), it was found to result in ca. 50% loss in activity as detected by standard anti-viral assays. Therefore, this alkylated form of IFN-β1a (NEM-IFN-β1a) renders itself as an attractive model of protein degradation, whereby any change in protein activity and conformation must necessarily originate from the single covalent modification.
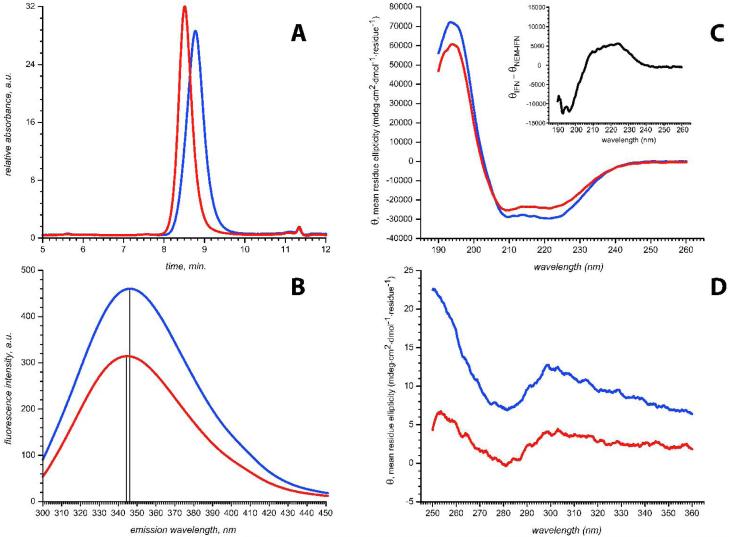
Biological activity loss resulting from IFN-β1a alkylation is likely mediated by changes in the protein higher order structure. However, classical biophysical tools provide little information on conformational consequences of IFN-β1a alkylation. Although alkylation of IFN-β1a with NEM does result in a reduction of SEC elution time (Figure 1A), a similar shift was also observed in H2O2-treated samples, which do not suffer any detectable activity loss (data not shown). Analytical ultracentrifugation analysis indicated only a very slight reduction (of about 0.6%) in the sedimentation coefficient of the NEM-IFN-β1a monomer in comparison to the native IFN-β1a monomer. Although this difference is very small, it has been reproducibly repeated and is consistent with the SEC data suggesting a possible increase in hydrodynamic volume on alkylation. Such an increase in hydrodynamic volume without a change in molecular weight (as suggested by light scattering measurements) would yield a reduced sedimentation coefficient; however, given the magnitude of the SEC shift a much larger difference in the sedimentation coefficients between IFN-β1a and NEM-IFN-β1a was anticipated. Fluorescence measurements (Figure 1B) yield a ca. 15% reduction in the NEM-IFN-β1a emission intensity and a very small blue shift (ca. 3 nm). Although these results are consistent with the notion of less stable tertiary structure of NEM-IFN-β1a (e.g., by indicating transfer of aromatic residues from a hydrophobic to aqueous environment), the paucity of the spectral changes makes them unsuited for gauging the integrity of the higher order structure.
Figure 1.
Comparison of IFN-β1a (blue traces) and NEM-IFN-β1a (red traces) using classical biophysical techniques: SEC profiles (A), fluorescence emission spectra (B), far-UV CD spectra (C) and near-UV CD spectra (D). Gray trace in panel A corresponds to incompletely alkylated protein. Inset in panel C s show the difference between the two spectra (normalized to protein concentration).
In the case of the far-UV CD spectra for NEM- IFN-β1αa small reduction in the total helical content is indicated (Figure 1C), with the difference between the far-UV spectra of intact and alkylated forms of IFN-β1a bearing signature features of a small increase in random coil on alkylation (see inset in Figure 1C). This result suggests a helix-to-coil conversion in some (yet unidentified) region(s) of the protein triggered by alkylation. However, the amplitude decrease of the negative bands at 208 nm and 222 nm, which are signatures of α-helical structure, is not very significant and is not well suited to serve as a marker of the structure loss, especially in a situation when stress-affected IFN-β1a molecules constitute only a fraction of the entire protein population. Ellipticity changes in the near-UV region indicate a partial loss of the protein's tertiary structure (or, more precisely, a decrease in the order in the spatial arrangement of its aromatic residues) upon alkylation, and the relative magnitude of this change is much more pronounced compared to the differences observed in the far-UV CD spectra (Figure 1D). However, the intrinsic weakness of the near-UV CD signal makes it again hardly suitable for reliable detection of unfolding, especially if structurally compromised species represent only a fraction the entire ensemble of protein molecules.
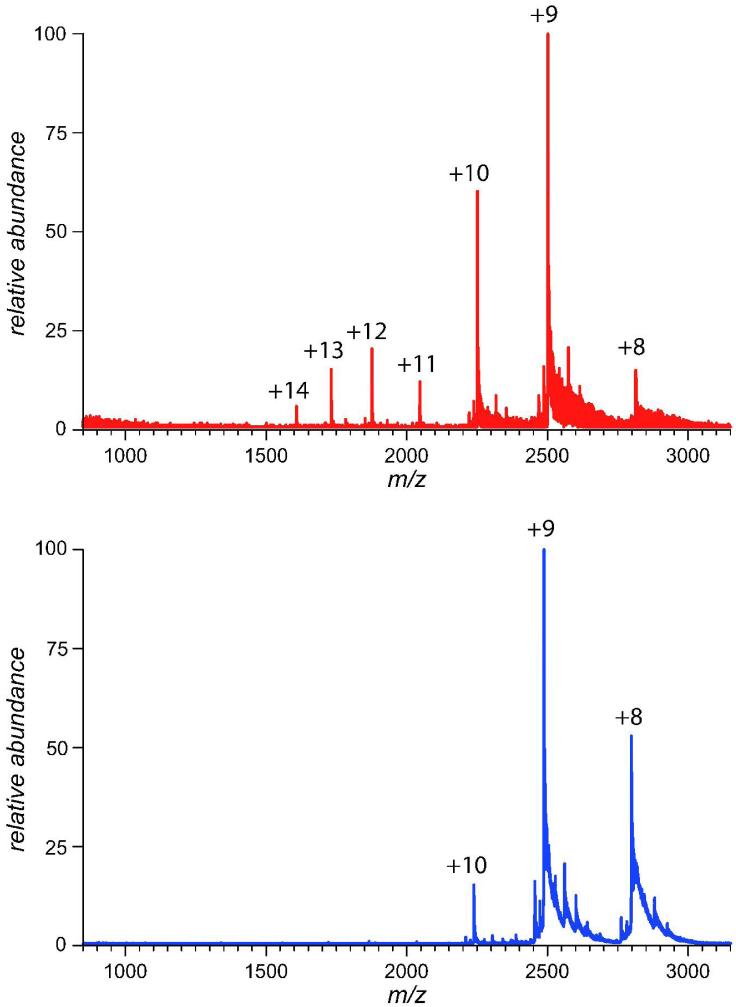
Overall, the classical biophysical measurements provided little insight as to where these changes were occurring on IFN-β1a and in some cases were questionable as to their significance. However, in the case of ESI-MS analysis evidence of partial unfolding of IFN-β1a triggered by its alkylation is readily provided. Protein ion charge state distributions in the mass spectra of NEM-IFN-β1a acquired under near-native conditions are bimodal (Figure 2), suggesting that at least two protein conformations are populated at equilibrium (42, 43). The compactness of one of these states is indistinguishable from that of intact IFN-β1a, whose ionic charge state distribution is characteristic of a natively folded protein. Higher charge density ionic population of NEM-IFN-β1a (charge states +11 through +14) represents a less compact state with a diminished tertiary structure (30). Although this population of high charge-density ions is also present in the spectrum of intact IFN-β1a, the relative abundance is significantly lower, making these ions barely detectable. Interestingly, the presence of this non-native state could not be detected following protein oxidation with H2O2, which in comparison to NEM treatment does not appear to compromise IFN-β1a activity as reported by Orr et. al. (29) and confirmed by our own work (data not shown).
Figure 2.
Charge state distributions of NEM-IFN-β1a (top) and intact IFN-β1a (bottom) ions in ESI MS acquired under near-native conditions following buffer exchange of protein solutions to 100 mM ammonium acetate.
While the appearance of the high charge-density protein ions in ESI mass spectra of NEM-IFN-β1a indicates partial unfolding, consistent with the partial loss of higher order structure observed in CD measurements, ESI MS measurements provide significantly more information on the alkylation-induced conformational change. The bimodal charge state distribution of NEM-IFN-β1a ions clearly indicate that the conformation of the chemically modified protein is not permanently altered. In other words, instead of locking the protein in a non-native state, alkylation makes the native conformation less stable and increases the frequency of sampling less ordered conformations, thereby elevating their Boltzmann weight and making them visible in ESI MS. At the same time, the ionic signal representing the compact, native-like state of IFN-β1a remains more abundant than the signal corresponding to the non-native species following protein alkylation. Fractional concentration of the partially unfolded NEM-IFN-β1a molecules in solution is difficult to estimate, but it is unlikely to be higher than the relative abundance of the corresponding ions in ESI MS (44).
The direct ESI MS measurements and the subsequent analyses of NEM-IFN-β1a ionic charge state distributions are very informative, as they clearly reveal the presence of two protein conformations at equilibrium and unequivocally link this behavior to the chemical modification event. However, the direct measurements can only be carried out in volatile buffer systems, thereby disallowing analysis of protein Pharmaceuticals in the context of their specific matrices. Structural integrity of therapeutic proteins may be compromised by a variety of factors, including composition of their formulations (45). Therefore, it may be argued that the transfer of a protein drug from its formulation-specific environment to the ESI-compatible solution may lead in some cases to a false-negative or false-positive detection of unfolding. Furthermore, no structural characteristics of the non-native protein state can be deduced from such measurements, information that can be very valuable for the optimization of the production and storage processes.
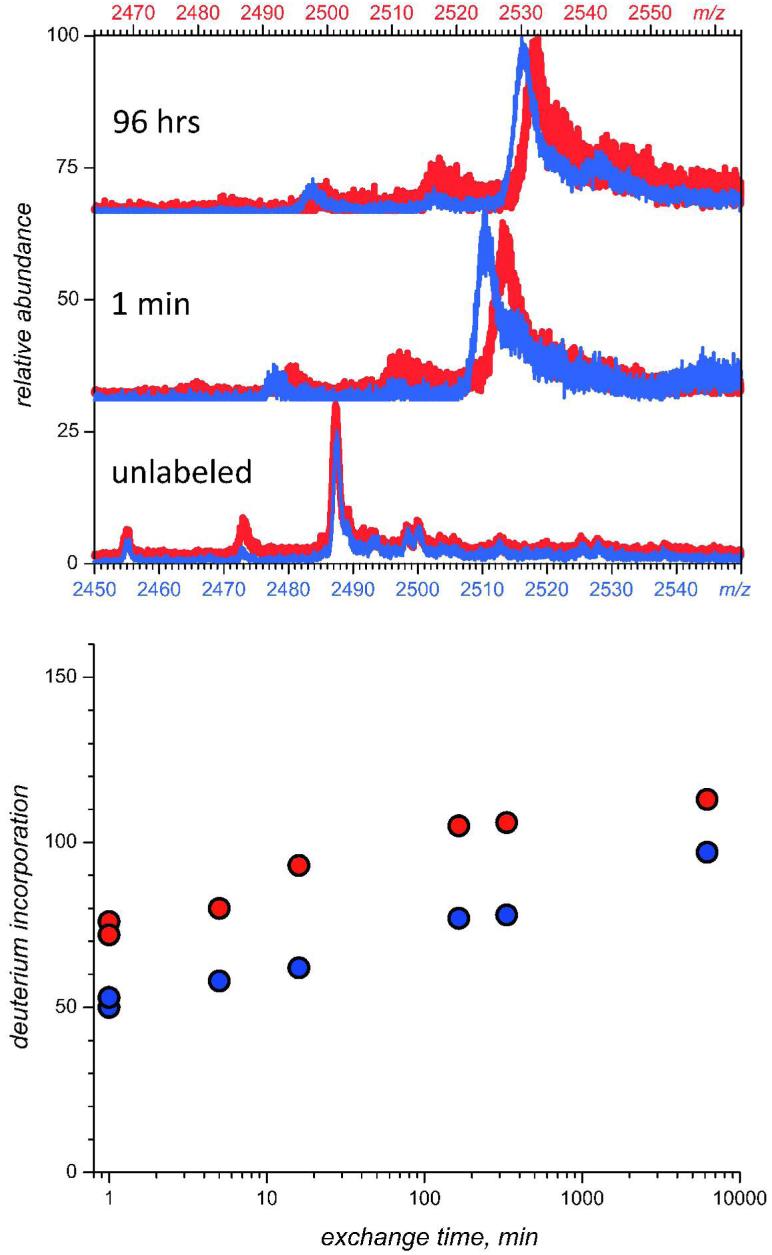
To enable detection and characterization of conformational changes triggered by alkylation of IFN-β1a in the relevant environment, the backbone dynamics of IFN-β1a and NEM-IFN-β1a were probed with HDX MS. Global HDX MS measurements provide clear evidence of increased flexibility of the modified protein, as suggested by the accelerated kinetics of deuterium incorporation (Figure 3). In order to understand how the local dynamics of different protein segments are affected by chemical modification at a single site, the spatial distribution of deuterium incorporation was evaluated for both unmodified and alkylated forms of IFN-β1a. Deuterium content measurements of various proteolytic fragments yield valuable stability information throughout the range of structural elements within native IFN-β1a, as well as the effect exerted by the alkylation on local dynamics in various parts of the protein. While most of the proteolytic fragments exhibited a low-to moderate increase in deuterium uptake kinetics (< 20%) as a result of protein alkylation, the effect is much more significant in several peptides. An example is presented in Figure 4, where the kinetics of deuterium uptake within the peptic fragment spanning fifteen amino acid residues (88-102) is very slow in the native unmodified IFN-β1a, but is accelerated dramatically in the alkylated form of the protein. A closer examination of the time evolution of the isotopic distribution of this peptide fragment derived from NEM-IFN-β1a reveals the presence of two distinct peptide ion populations. Deuterium content of one of them is indistinguishable from that of the same peptic fragment derived from the unmodified form of IFN-β1a; another population exhibits significantly lower protection levels. Such bimodal isotopic distributions usually signal the occurrence of the so-called correlated exchange, a signature of the EX1 exchange regime (46). In our case, ca. 5% of the ion population may also represent a contribution of unmodified IFN-β1a present in the NEM-IFN-β1a sample.
Figure 3.
Global HDX kinetics of intact IFN-β1a (blue) and NEM-IFN-β1a (red). The top panel shows raw HDX MS data (charge state +9), where the m/z scale for IFN-NEM ions was offset such that the observed difference in the ion peak position is due to the different rates of deuterium incorporation. Using the same color coding, the bottom panel shows the incorporation of deuterium by each protein versus time, calculated as described in the materials and methods.
Figure 4.
Evolution of isotopic distributions of a peptide fragment (88-102) derived from unmodified IFN-β1a (blue) and NEM-IFN-β1a (red) at different time points of the exchange reaction. The gray trace shows the isotopic distribution of the fully exchanged peptide (HDX end-point).
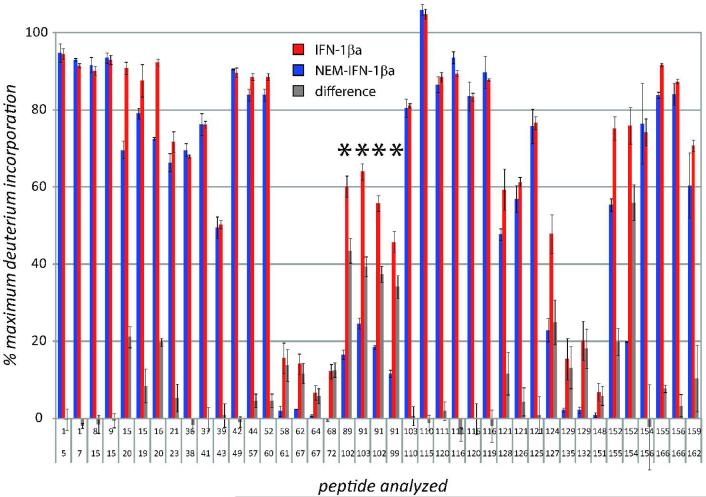
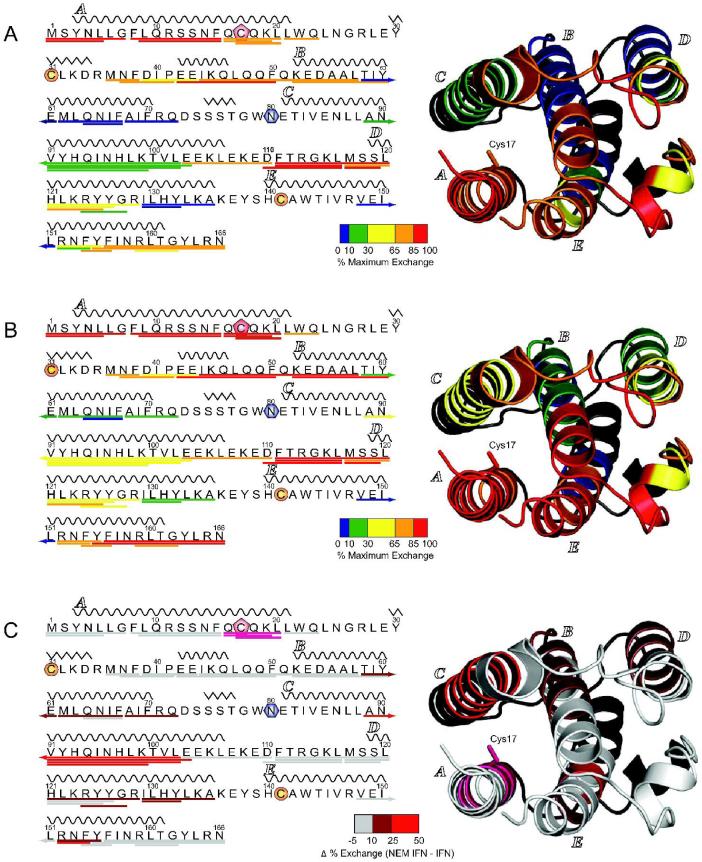
Bimodal isotopic distributions were also observed for several other peptides whose exchange kinetics are influenced by protein alkylation (marked with asterisks in Figure 5). Although the differences between the two forms of the protein can be seen as early as 10 sec of the exchange reaction (Figure 4), a clear distinction between the two populations of (88-102) peptide ions can be made following 5 min of exchange. Therefore the HDX MS data presented in Figure 5 shows the levels of deuterium incorporation in IFN-β1a and NEM-IFN-β1a at 5 min. The results of HDX MS measurements for IFN-β1a and NEM-IFN-β1a presented in Figure 5 were mapped onto the crystal structure of IFN-β1a (38) (Figure 6A and B) and provide a clear indication that the backbone flexibility is not distributed evenly throughout the polypeptide chain.
Figure 5.
Levels of deuterium incorporation following 5 min of exchange within segments representing all observed proteolytic fragments derived from unmodified IFN-β1a (blue) and NEM-IFN-β1a (red). Gray bars plot the difference between IFN-β1a and NEM-IFN-β1a. Peptides that exhibited bimodal exchange in the NEM-IFN-β1a sample are indicated with an asterisk.
Figure 6.
Segments representing all observed proteolytic fragments of unmodified NEM-IFN-β1a (A) and IFN-β1a (B) colored according to their deuteration level following 5 min of exchange mapped on the 1° and 3° structures of the protein. Color coding reflects flexibility of various segments ranging from high protection levels (blue) to very low protection (red). The bottom diagram (C) shows protein segments where flexibility is changed as a result of alkylation. These are colored by difference in % exchange as indicated by the scale, except for the residues in pink, which exhibited a marked difference in exchange though the fast exchange kinetics in this region prevented an accurate difference calculation. In all 3° structures residues not observed were colored in black. Helical segments are indicated by wavy lines above the amino acid sequence and labeled alphabetically by convention. Cysteine residues involved in a disulfide bond are colored in orange circles, the free (alkylated) cysteine residue is marked with a red pentagon, and the first residue of the CHO chain is marked with a blue hexagon.
Comparison of the flexibility maps of the unmodified and alkylated IFN-β1a is a very useful tool for visualizing the structural elements whose stability is affected by the chemical modification (Figure 6C). It is not surprising that the flexibility of the protein segments containing the Cys17 residue increase following IFN-β1a alkylation. However, the affected regions are not confined by primary structure to the polypeptide region containing the chemically modified residue (Cys17). One of the most affected segments (88-102) is very distant in sequence from the alkylated residue, but is proximally located in the structure on helix C facing the Cys17 side chain. Alkylation of Cys17 with NEM apparently introduces a significant steric constraint in the protein interior, which distorts helix packing, thereby adversely affecting the stability of the higher order structure. The flexibility maps also allow one to identify several other protein segments (e.g., helix D), whose stability is adversely affected by the chemical modification of Cys17 through some yet unknown allosteric mechanisms, despite being distant from the modification site both in the sequence and in the 3D structure.
The backbone flexibility maps also provide some clues regarding the molecular mechanism of IFN-β1a inactivation following its alkylation. We note that the two helices forming the interface with high-affinity IFN-β1a receptor IFNAR-2 (A and E (38)) are among the most dynamic (least protected) elements of IFN-β1a secondary structure. Elevated polypeptide chain flexibility has been suggested in the past to play an important role in protein interactions (47), and transient structural disorder was shown in the past to be an important facilitator of protein binding to small ligands (48) and other proteins (49). Should the IFN-β1a chain flexibility be a factor catalyzing the IFN-β1a/IFNAR-2 binding as well, a modest mobility increase within a short segment of helix A induced by IFN-β1a alkylation would be unlikely to disrupt the binding. Indeed, preliminary studies utilizing direct ESI MS as well as SEC indicate the presence of a NEM-IFN-β1a/IFNAR-2 complex, which is readily formed in a manner comparable to that of unmodified IFN-β1a (data not shown). Should this be the case, the increased backbone mobility observed for NEM-IFN-β1a may have a deleterious effect towards IFNAR-1 recognition, which in contrast to IFNAR2 could proceed through a classical docking mechanism (50). Among the structural elements of IFN-β1a proposed to form the IFNAR-1 binding interface, helix C exhibits the most significant gain in backbone mobility upon protein alkylation (Figure 6).
The involvement of helix C in IFN-β1a/receptor interaction and its prominent role in partial unfolding of the protein following its alkylation make it a convenient reporter of compromised integrity of native conformation in response to IFN-β1a degradation. Evolution of the isotopic distribution of peptic fragments located in this structural element of NEM-IFN-β1a (Figure 4) provides very readily conclusive evidence of diminished structural stability. Importantly, the bimodal character of the isotopic distributions observed at up to 5 min of HDX provides an easy and reliable way to make a distinction between the two forms of the protein, one whose structural integrity has not been compromised (low deuterium uptake level) and the conformer characterized by high backbone mobility in this region (high deuterium uptake level). This feature would allow the signature of partial unfolding to be seen even in the background of protein molecules whose structural integrity has not been compromised. In fact, the abundance ratio of the two ionic populations may be used to calculate the relative amount of the degraded protein in the sample.
Conclusions
In the present study we have demonstrated that HDX MS along with ion charge state distribution analysis can be confidently used to monitor the stability of protein biopharmaceutical products. In particular, HDX MS provided reliable and detailed characterization information on the native IFN-β1a conformation and dynamics. In addition, the direct comparison of native IFN-β1a to a form chemically modified at its free cysteine is particularly enlightening in assessing the impact of this simple modification. Although some evidence of the diminished conformational stability of NEM-IFN-β1a could be obtained from measurements employing classical biophysical tools, specifically by CD, HDX MS is unrivaled in its ability to detect and characterize the large scale dynamic events within the protein that affect its function. Such specific detailed information on the higher order structure of protein biopharmaceuticals cannot be obtained by the standard biophysical characterization commonly used in the biopharmaceutical industry. Since this experimental technique probes conformational stability directly without relying on covalent markers of protein degradation, it may be used in a variety of settings, including situations where protein structural integrity is compromised while its covalent structure remains intact (structural changes that arise solely from noncovalent changes). This capability opens up a host of new and exciting opportunities in the field of characterization of the higher order structure and stability of protein Pharmaceuticals that could have significant benefit to protein design, stability, and comparability studies.
Acknowledgements
This work was supported in part by a grant from the National Institutes of Health R01 GM061666. The authors would like to also thank Dr. Darren Baker (Biogen Idec, Molecular Discovery) for samples of IFNAR2 and Peter Reavey (Biogen Idec, Bioanalytical Quality Control) for conducting the antiviral assay on IFN-β1a and NEM-IFN-β1a samples.
Footnotes
In this paper PTM will be used to imply chemical modifications that occur to protein after the biosynthesis of its polypeptide that are concerned with either signal transduction or/and degradation.
References
- 1.Walsh G. Biopharmaceutical benchmarks 2006. Nat. Biotech. 2006;24:769–776. doi: 10.1038/nbt0706-769. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence S. Pipelines turn to biotech. Nat. Biotech. 2007;25:1342–1342. doi: 10.1038/nbt1207-1342. [DOI] [PubMed] [Google Scholar]
- 3.Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv. Drug Del. Rev. 2004;56:335–347. doi: 10.1016/j.addr.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Bauer J, Spanton S, Henry R, Quick J, Dziki W, Porter W, Morris J. Ritonavir: an extraordinary example of conformational polymorphism. Pharm. Res. 2001;18:859–866. doi: 10.1023/a:1011052932607. [DOI] [PubMed] [Google Scholar]
- 5.Maas C, Hermeling S, Bouma B, Jiskoot W, Gebbink MFBG. A role for protein misfolding in immunogenicity of biopharmaceuticals. J. Biol. Chem. 2007;282:2229–2236. doi: 10.1074/jbc.M605984200. [DOI] [PubMed] [Google Scholar]
- 6.Morozova-Roche L, Malisauskas M. A false paradise - mixed blessings in the protein universe: the amyloid as a new challenge in drug development. Curr. Med. Chem. 2007;14:1221–1230. doi: 10.2174/092986707780597989. [DOI] [PubMed] [Google Scholar]
- 7.Soskic V, Groebe K, Schrattenholz A. Nonenzymatic posttranslational protein modifications in ageing. Exp. Gerontol. 2008;43:247–257. doi: 10.1016/j.exger.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Hipkiss AR. Accumulation of altered proteins and ageing: Causes and effects. Exp. Gerontol. 2006;41:464–473. doi: 10.1016/j.exger.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Federici MM. The quality control of biotechnology products. Biologicals. 1994;22:151–159. doi: 10.1006/biol.1994.1021. [DOI] [PubMed] [Google Scholar]
- 10.Walsh G. Second-generation biopharmaceuticals. Eur. J. Pharm. Biopharm. 2004;58:185–196. doi: 10.1016/j.ejpb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Szymkowski DE. Creating the next generation of protein therapeutics through rational drug design. Curr. Opin. Drug Discov. Devel. 2005;8:590–600. [PubMed] [Google Scholar]
- 12.Roger SD, Mikhail A. Biosimilars: opportunity or cause for concern? J. Pharm. Pharm. Sci. 2007;10:405–410. [PubMed] [Google Scholar]
- 13.Kuhlmann M, Covic A. The protein science of biosimilars. Nephrol. Dial. Transplant. 2006;21:v4–8. doi: 10.1093/ndt/gfl474. [DOI] [PubMed] [Google Scholar]
- 14.Oliva A, Farina JB, Llabres M. New trends in analysis of biopharmaceutical products. Curr. Pharm. Anal. 2007;3:230–248. [Google Scholar]
- 15.Berkowitz SA. Role of analytical ultracentrifugation in assessing the aggregation of protein biopharmaceuticals. AAPS J. 2006;8:E590–605. doi: 10.1208/aapsj080368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luykx DMAM, Goerdayal SS, Dingemanse PJ, Jiskoot W, Jongen PMJM. HPLC and tandem detection to monitor conformational properties of biopharmaceuticals. J. Chromatogr. B. 2005;821:45–52. doi: 10.1016/j.jchromb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Raso SW, Abel J, Barnes JM, Maloney KM, Pipes G, Treuheit MJ, King J, Brems DN. Aggregation of granulocyte-colony stimulating factor in vitro involves a conformationally altered monomeric state. Protein Sci. 2005;14:2246–2257. doi: 10.1110/ps.051489405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye H. Simultaneous determination of protein aggregation, degradation, and absolute molecular weight by size exclusion chromatography-multiangle laser light scattering. Anal. Biochem. 2006;356:76–85. doi: 10.1016/j.ab.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 19.Brewer JM. The use of differential scanning calorimetry (DSC) to determine the correctness of folding of cloned proteins. Biotechnol. Appl. Biochem. 1999;30:173–175. [PubMed] [Google Scholar]
- 20.Kaltashov IA, Eyles SJ. Studies of biomolecular conformations and conformational dynamics by mass spectrometry. Mass Spectrom. Rev. 2002;21:37–71. doi: 10.1002/mas.10017. [DOI] [PubMed] [Google Scholar]
- 21.Kaltashov IA, Eyles SJ. Mass spectrometry in molecular biophysics: conformation and dynamics of biomolecules. John Wiley; Hoboken, N.J.: 2005. [Google Scholar]
- 22.Srebalus-Barnes CA, Lim A. Applications of mass spectrometry for the structural characterization of recombinant protein Pharmaceuticals. Mass Spectrom. Rev. 2007;26:370–388. doi: 10.1002/mas.20129. [DOI] [PubMed] [Google Scholar]
- 23.Wales TE, Engen JR. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom. Rev. 2006;25:158–170. doi: 10.1002/mas.20064. [DOI] [PubMed] [Google Scholar]
- 24.Kaltashov IA, Abzalimov RR. Do ionic charges in ESI MS provide useful information on macromolecular structure? J. Am. Soc. Mass Spectrom. 2008;19 doi: 10.1016/j.jasms.2008.05.018. in press. [DOI] [PubMed] [Google Scholar]
- 25.Andrea S. Chemical cross-linking and mass spectrometry to map three-dimensional protein structures and protein-protein interactions. Mass Spectrom. Rev. 2006;25:663–682. doi: 10.1002/mas.20082. [DOI] [PubMed] [Google Scholar]
- 26.Konermann L, Tong X, Pan Y. Protein structure and dynamics studied by mass spectrometry: H/D exchange, hydroxyl radical labeling, and related approaches. J. Mass Spectrom. 2008 doi: 10.1002/jms.1435. in press. [DOI] [PubMed] [Google Scholar]
- 27.Engen JR, Smith DL. Investigating protein structure and dynamics by hydrogen exchange MS. Anal. Chem. 2001;73:256A–265A. doi: 10.1021/ac012452f. [DOI] [PubMed] [Google Scholar]
- 28.Kaltashov IA, Eyles SJ. Crossing the phase boundary to study protein dynamics and function: combination of amide hydrogen exchange in solution and ion fragmentation in the gas phase. J. Mass Spectrom. 2002;37:557–565. doi: 10.1002/jms.338. [DOI] [PubMed] [Google Scholar]
- 29.Tobler SA, Holmes BW, Cromwell MEM, Fernandez EJ. Benzyl alcohol-induced destabilization of interferon-g: a study by hydrogen-deuterium isotope exchange. J. Pharm. Sci. 2004;93:1605–1617. doi: 10.1002/jps.10589. [DOI] [PubMed] [Google Scholar]
- 30.Konermann L, Douglas DJ. Acid-Induced unfolding of cytochrome c at different methanol concentrations: Electrospray ionization mass spectrometry specifically monitors changes in the tertiary structure. Biochemistry. 1997;36:12296–12302. doi: 10.1021/bi971266u. [DOI] [PubMed] [Google Scholar]
- 31.Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat. Rev. Drug Discov. 2007;6:975–990. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cravello L, Lascoux D, Forest E. Use of different proteases working in acidic conditions to improve sequence coverage and resolution in hydrogen/deuterium exchange of large proteins. Rapid Commun. Mass Spectrom. 2003;17:2387–2393. doi: 10.1002/rcm.1207. [DOI] [PubMed] [Google Scholar]
- 34.Bai Y, Milne JS, Mayne L, Englander SW. Primary structure effects on peptide group hydrogen exchange. Proteins. 1993;17:75–86. doi: 10.1002/prot.340170110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arduini RM, Strauch KL, Runkel LA, Carlson MM, Hronowski X, Foley SF, Young CN, Cheng W, Hochman PS, Baker DP. Characterization of a soluble ternary complex formed between human interferon-beta-1a and its receptor chains. Protein Sci. 1999;8:1867–1877. doi: 10.1110/ps.8.9.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alam J, Goelz S, Rioux P, Scaramucci J, Jones W, McAllister A, Campion M, Rogge M. Comparative pharmacokinetics and pharmacodynamics of two recombinant human interferon beta-1 a (IFN beta-1 a) products administered intramuscularly in healthy male and female volunteers. Pharm. Res. 1997;14:546–549. doi: 10.1023/a:1012128406432. [DOI] [PubMed] [Google Scholar]
- 37.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int. J. Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 38.Whitty A, Karpusas M. The structure of human interferon-b-1a (Avonex™) and its relation to activity: a case study of the use of structural data in the arena of protein Pharmaceuticals. In: Chasman DL, editor. Protein Structure: Determinantion, Analysis and Applications for Drug Discovery. Marcel Dekker; New York-Basel: 2003. pp. 483–519. [Google Scholar]
- 39.Bermel RA, Rudick RA. Interferon-b treatment for multiple sclerosis. Neurotherapeutics. 2007;4:633–646. doi: 10.1016/j.nurt.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sterman DH, Recio A, Carroll RG, Gillespie CT, Haas A, Vachani A, Kapoor V, Sun J, Hodinka R, Brown JL, Corbley MJ, Parr M, Ho M, Pastan I, Machuzak M, Benedict W, Zhang X.-q., Lord EM, Litzky LA, Heitjan DF, June CH, Kaiser LR, Vonderheide RH, Albelda SM. A phase I clinical trial of single-dose intrapleural IFN-b gene transfer for malignant pleural mesothelioma and metastatic pleural effusions: high rate of antitumor immune responses. Clin. Cancer Res. 2007;13:4456–4466. doi: 10.1158/1078-0432.CCR-07-0403. [DOI] [PubMed] [Google Scholar]
- 41.Orru S, Amoresano A, Siciliano R, Napoleoni R, Finocchiaro O, Datola A, De Luca E, Sirna A, Pucci P. Structural analysis of modified forms of recombinant IFN-b produced under stress-simulating conditions. Biol. Chem. 2000;381:7–17. doi: 10.1515/BC.2000.002. [DOI] [PubMed] [Google Scholar]
- 42.Dobo A, Kaltashov IA. Detection of multiple protein conformational ensembles in solution via deconvolution of charge state distributions in ESI MS. Anal. Chem. 2001;73:4763–4773. doi: 10.1021/ac010713f. [DOI] [PubMed] [Google Scholar]
- 43.Mohimen A, Dobo A, Hoerner JK, Kaltashov IA. A chemometric approach to detection and characterization of multiple protein conformers in solution using electrospray ionization mass spectrometry. Anal. Chem. 2003;75:4139–4147. doi: 10.1021/ac034095+. [DOI] [PubMed] [Google Scholar]
- 44.Kuprowski MC, Konermann L. Signal response of coexisting protein conformers in electrospray mass spectrometry. Anal. Chem. 2007;79:2499–2506. doi: 10.1021/ac0620056. [DOI] [PubMed] [Google Scholar]
- 45.Frokjaer S, Otzen DE. Protein drug stability: A formulation challenge. Nat. Rev. Drug Discov. 2005;4:298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 46.Xiao H, Hoerner JK, Eyles SJ, Dobo A, Voigtman E, Mel'cuk AI, Kaltashov IA. Mapping protein energy landscapes with amide hydrogen exchange and mass spectrometry: I. A generalized model for a two-state protein and comparison with experiment. Protein Sci. 2005;14:543–557. doi: 10.1110/ps.041001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shoemaker BA, Portman JJ, Wolynes PG. Speeding molecular recognition by using the folding funnel: the fly-casting mechanism. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8868–8873. doi: 10.1073/pnas.160259697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao H, Kaltashov IA. Transient structural disorder as a facilitator of protein-ligand binding: native H/D exchange-mass spectrometry study of cellular retinoic acid binding protein I. J. Am. Soc. Mass Spectrom. 2005;16:869–879. doi: 10.1016/j.jasms.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Griffith WP, Kaltashov IA. Protein conformational heterogeneity as a binding catalyst: ESI-MS study of hemoglobin H formation. Biochemistry. 2007;46:2020–2026. doi: 10.1021/bi062032q. [DOI] [PubMed] [Google Scholar]
- 50.Camacho CJ, Vajda S. Protein-protein association kinetics and protein docking. Curr. Opin. Struct. Biol. 2002;12:36–40. doi: 10.1016/s0959-440x(02)00286-5. [DOI] [PubMed] [Google Scholar]