Summary
Hypoxia modifies GABAA receptor (GABAAR) function, and can cause seizures, encephalopathy or myoclonus. To characterize the effects of hypoxia on neuronal GABAARs, we subjected rat cortical neurons to 1% O2 for 2, 4 or 8 h, followed by recovery times of 0 to 96 h, and used whole-cell and perforated-patch patch clamp recording to assess GABAAR currents and pharmacology. Hypoxic exposure for 4 h caused downregulation of maximal GABA current immediately following hypoxia and after 48 h recovery without changing the EC50 for GABA. Two and eight hour hypoxic exposures had inconsistent effects on GABAAR currents. Maximal diazepam potentiation was increased immediately following 4 h hypoxia, while potentiation by zolpidem was increased after 48 h recovery. Pentobarbital enhancement and zinc inhibition of GABA currents were unchanged. Hypoxia also caused a depolarizing shift in the reversal potential of GABA-induced Cl− currents after 24 h recovery. The L-type voltage-gated calcium channel (L-VGCC) blocker, nitrendipine, during hypoxia or control treatment prevented the reduction in GABAAR currents, and increased control currents over baseline. Nitrendipine also prevented the increase in zolpidem potentiation 48 h after hypoxia, and blocked the depolarizing shift in Cl− reversal potential 24 h after hypoxia. The effects of hypoxia on maximal GABAAR currents, zolpidem pharmacology and Cl− reversal potential thus require depolarization-induced calcium entry via L-VGCCs, and constitutive L-VGCC activity appears to reduce maximal GABAAR currents in control neurons via a calcium-dependent process. Calcium-dependent modulation of GABAAR currents via L-VGCCs may be a fundamental regulatory mechanism for GABA receptor function.
Keywords: Patch clamp, reversal potential, gramicidin, nitrendipine, benzodiazepine, zolpidem
1. Introduction
GABAA receptors (GABAARs) mediate fast inhibitory synaptic neurotransmission in the central nervous system. They are members of the ligand-gated ion channel family, composed of five subunits from multiple subunit subtypes (α1-α6, β1-β3, γ1-γ3, δ, ε, π, θ and ρ1-ρ3) (Macdonald and Olsen, 1994). The binding of γ-aminobutyric acid (GABA) regulates gating of the chloride ion channel, and a number of positive and negative allosteric regulators, including the benzodiazepines (BZ), barbiturates, neurosteroids, Zn2+ and other divalent cations, interact with GABAARs at binding sites formed by the presence of specific subunit subtypes in the holoreceptor (Korpi et al., 2002). The BZ full agonist diazepam binds to GABAARs containing α1, α2, α3 or α5 subunits, combined with a β and γ2 subunit (Korpi et al., 2002). High affinity zolpidem binding is conferred by the presence of an α1 subunit, combined with β and γ subunits. Presence of α2 or α3 subtype results in receptors with moderate zolpidem affinity (MacDonald and Kapur, 1999). Zn2+ inhibits GABA-evoked currents with higher potency at receptors containing an α4, α5 or α6 and lacking an α1 and/or a γ subunit (Macdonald et al., 1996). The pharmacological and biophysical properties of GABAARs thus depend on subunit composition (Sieghart et al., 1999).
Both excitatory and inhibitory neurotransmission are affected during and after hypoxia, leading to excitotoxicity (Yue et al., 1997). Modification of GABAA receptor function has been implicated in a range of hypoxia-related pathologies, including encephalopathy (Low et al., 1985), seizures (Bergamasco et al., 1984) and myoclonus (Hallett, 2000). GABA-mediated currents were reduced in CA1 pyramidal neurons in hippocampal slices exposed to hypoxia both in vivo and in vitro (Xu and Pulsinelli, 1994; Congar et al., 1995). Decreased GABAAR current in cultured hippocampal neurons subjected to experimental ischemia was attributed to depletion of ATP and increased intracellular Ca2+ (Harata et al., 1997). Within 1 h after cerebral ischemia, GABA-gated 36Cl− flux dramatically decreased in gerbil neurons (Verheul et al., 1993). Intracellular Cl− in rat hippocampal slices was increased early after ischemia, resulting in reduced Cl− driving force and GABAA responses (Inglefield and Schwartz-Bloom, 1998).
Our previous studies demonstrated that maximal GABA currents in NTera2 (NT2-N) neuronal cells changed in a biphasic manner after 8 h exposure to 1% O2. Maximal currents were significantly increased immediately following 8 h hypoxia, but were dramatically reduced after 48 h recovery, and then returned to baseline after 96 h recovery. GABAA receptor α1, α5, β2 and γ2 subunit mRNAs were downregulated after hypoxia (Gao et al., 2004). Since NT2-N cells are derived from a human tumor cell line, they may not completely reflect the behavior of brain-derived neurons. Here we investigated the effects of different durations of hypoxia and normoxic recovery on GABAAR currents and pharmacology in rat cortical neurons in primary culture. Hypoxic exposure reduced GABA currents immediately following hypoxia and after 48 h recovery, altered benzodiazepine pharmacology and shifted the chloride equilibrium potential. These post-hypoxic changes could be prevented by the voltage-gated calcium channel (L-VGCC) blocker, nitrendipine, during hypoxic treatment.
2. Methods
2.1 Cortical Neuron Culture
Cortices were dissected from E18 fetal Sprague Dawley rats after euthanasia of the dam by a protocol approved by University of Toledo College of Medicine IACUC. Primary culture of cortical neurons was performed according to a procedure modified from standard techniques (Banker and Cowan, 1997; Ransom et al., 1977; Porter et al. 1997). Dissected cortices were transferred to 0.25% Trypsin-ethylene diaminotetraacetic acid (EDTA) for 10 min at 37 °C, washed three times with Spiner’s modification of Eagle’s minimum essential medium (SMEM), and then dispersed by repeated trituration. The cell suspension was diluted with SMEM plus fetal bovine serum (5%) and horse serum (5%) to a final concentration of 3–5×105 cells/ml and plated onto a poly-D-lysine coated 35 mm plastic culture dishes. To inhibit proliferation of non-neuronal cells at 3 days in vitro (DIV), half of the medium was replaced with SMEM with horse serum (SMEM/HS) containing 5-fluoro-2′-deoxyuridine (FUDR) and uridine. Half of the medium was exchanged for fresh SMEM/HS at least twice a week (Porter et al., 1997). Cells were cultured for 13–15 DIV before use in the experiments below.
2.2 Hypoxia and reoxygenation
Neurons were examined microscopically to confirm viability before starting the experiment. Deoxygenated culture medium (SMEM/HS) was prepared by bubbling for 10 min with 95%N2/5%CO2 at 37°C. SMEM/HS was replaced with deoxygenated SMEM/HS and then placed in an O2- and CO2-controlled incubator (Innova CO-48, New Brunswick Scientific Co. Inc.) pre-equilibrated to 1% O2, 5% CO2 at 37 °C for 2, 4 or 8 h. After hypoxic exposure, the medium was replaced with fresh aerated SMEM/HS and neurons were either studied immediately or returned to the normoxic incubator (95%air/5%CO2, 37°C) for 24 h or 48 h prior to recording. For the “0 h” recovery time point, neurons were recorded within 1–2 h of termination of hypoxia; for 24 and 48 h recovery times, recordings occurred within ± 2 h of the stated time. For L-VGCC antagonist experiments, SMEM/HS was replaced with deoxygenated SMEM/HS containing 3 μM nitrendipine and kept in the hypoxic incubator (1% O2, 5% CO2 at 37 °C) for 4 h. After hypoxic exposure, the medium was replaced with fresh aerated SMEM/HS without nitrendipine and cells were returned to the normoxic incubator. Control experiments were similarly handled but maintained in a normoxic environment and solutions at all times.
2.3 Electrophysiology
2.3.1. Whole-cell patch-clamp recording
Whole-cell recordings were obtained using standard patch-clamp technique (Hamill et al., 1981) with an Axopatch 200B amplifier (Axon Instruments, Union City, CA). Cortical neurons were taken out from the incubator and the medium was replaced with external recording solution containing (in mM): 142 NaCl, 10 CaCl2, 6 MgCl2, 8.1 KCl, 10 glucose, 10 HEPES, 315–325 mOsm, pH 7.4. Patch-clamp electrodes of 2.5–5.0 MΩ were filled with internal solution containing (in mM): 153.3 KCl, 1 MgCl2, 10 HEPES, 5 EGTA, 4 MgATP, 305–312 mOsm, PH 7.3. Signals were digitized on line at 1000Hz using a Digidata 1200A Data Acquisition System (Axon instruments, Foster City, CA) running Clampex 9.2 software and subsequently analyzed off-line using Clampfit 9.2 (pClamp 9.2, Axon Instruments). Patch-clamp electrodes were pulled from Fisher Micro-hematocrit capillary tubes (Fisher Scientific) using a P-97 Flaming-Brown micropipette puller (Sutter Instrument Co.). For whole cell recordings, membrane potential was held at −70 mV. GABA and other drugs were sequentially applied in increasing concentrations using a pressurized drug application system to facilitate flow through 100 μM applicator tips. Peak GABAAR currents elicited by increasing GABA concentrations were fitted to a sigmoidal function using a four parameter logistic equation (sigmoidal C-R) with a variable slope: I = (Imax)/(1–10(log(EC50−[drug])×Hill slope)), where I is the peak current at a given GABA concentration, and Imax is the maximal GABAAR current. Curve fitting was performed using Prism 4.0 software (Graph Pad Software Inc., San Diego, CA). Maximal current and C-R curve fits were obtained from individual neurons, then averaged and compared by ANOVA with post hoc t-tests using Bonferroni’s correction for multiple comparisions. Cell capacitance was measured shortly after obtaining whole-cell configuration using the “Membrane Test” function of Clampex 9.2. The percent enhancement or inhibition produced by co-application of a modulator was determined by dividing the peak amplitude in the presence of modulator by the average of control currents elicited by GABA alone before and after co-application and multiplying by 100. Neurons with significant rundown (>20% over 10 min) were excluded from analysis.
2.3.2. Gramicidin perforated-patch recording
Gramicidin perforated-patch recordings were performed as previously reported (Gao et al., 2005) using similar methods as whole-cell recordings, except the plasma membrane was not mechanically ruptured after gigaseal formation. Gramicidin was dissolved in DMSO (10 mg/ml) and then diluted in the internal solution to a final concentration of 10 μg/ml. The electrode tip was filled with a small amount of gramicidin-free internal solution to avoid problems with seal formation. After seal formation, the progress of gramicidin Cl− impermeable pore formation was evaluated by monitoring the gradual reduction in membrane resistance. GABA (10 μM) was applied to the cell after the membrane resistance had stabilized to 300–400 MΩ which usually took about 12–20 min. The reversal potential for GABA currents (ECl) was determined by varying holding potential of neurons from −90 mV to +50 mV in 20 mV increments and measuring the resulting peak GABA-evoked currents. Linear regression was used to calculate a best-fit line for the voltage dependence of GABA currents, and the interpolated intercept of this line with the abscissa was taken as the ECl value. The slope of this line was used as the corresponding slope conductance. Perforated-patch recordings were terminated by increased suction on the micropipette or when the patch spontaneously ruptured into whole-cell configuration as evidenced by large depolarizing currents due to ECl of 0 mV.
3. Results
3.1. Effects of duration of hypoxia on GABAAR currents
After 13–15 DIV, healthy cortical neurons developed long axons and dendrites. No overt changes in cortical neuron morphology were observed immediately after exposure to 1% O2 for 2h, 4h or 8h of hypoxia or after 24h or 48h recovery under normoxic conditions (95% air/5% CO2, 37°C). This was consistent with our previous studies demonstrating that 8 h hypoxic exposure on NT2-N neuronal cells did not result in significant death or injury by trypan blue staining and lactate dehydrogenase (LDH) assay. There was no difference in initial membrane potential or membrane capacitance (Cm) between control cells and cells recorded after hypoxic exposure with recovery durations up to 48 h (Table 1). Since membrane capacitance is proportional to cell membrane surface area, hypoxia did not appear to cause somatic swelling or dendrite retraction, features commonly associated with neuronal injury.
Table 1. Rat Cortical Neuron Membrane Capacitance after Hypoxia.
Membrane capacitance (pF) at control and post-hypoxic recovery times after stated durations at 1% O2.
| Hypoxia | Control | 0 h Recovery | 24 h Recovery | 48 h Recovery |
|---|---|---|---|---|
| Duration | Mean ± SEM.(n) | Mean ± SEM.(n) | Mean ± SEM.(n) | Mean ± SEM.(n) |
| 2 h | 19.5 ± 0.46 (9) | 20.0 ± 0.93 (8) | 19.3 ± 0.77 (9) | 18.2 ± 1.3 (9) |
| 4 h | 20.3 ± 0.71 (10) | 20.1 ± 0.75 (9) | 19.1 ± 1.07 (7) | 19.3 ± 0.71 (10) |
| 8 h | 19.9 ± 0.88 (10) | 20.2 ± 1.06 (10) | 20.2 ± 0.58 (8) | 17.9. ± 084 (9) |
| 4 h + NT | 18.8 ± 0.67 (11) | 19.1 ± 0.89 (8) | 17.3 ± 0.75 (8) | 18.5 ± 0.67 (9) |
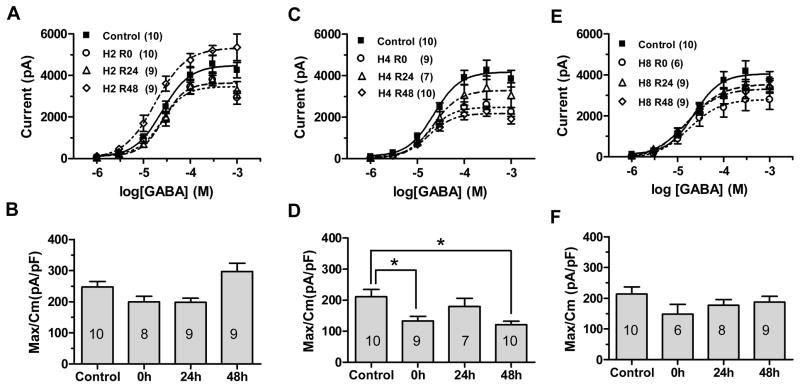
GABA-activated currents in cortical neurons were obtained by whole-cell patch-clamp recordings voltage clamped at −70mV. Brief (5 s) applications of increasing GABA concentrations (1 μM–1 mM) evoked concentration dependent inward currents from both control and hypoxia-treated cortical neurons (Fig. 1). Peak currents values were then fitted to a sigmoidal logistic function (Fig 2A, 2C, 2E) which showed no significant change in EC50. Maximal GABA-evoked currents after 2 h hypoxia were decreased after 0 h and 24 h recovery (Table 2, p<0.05 at 24 h, t test with Bonferroni correction after one way ANOVA) but trended toward an increase after 48 h recovery (Table 2, p>0.05). After 4 h hypoxia, maximal GABA current decreased after hypoxia; these reductions were significant the 0 h and 48 h recovery times at (Table 2, p<0.05, t test with Bonferroni correction after one way ANOVA). Hypoxic exposure for 8 h caused non-significant trends toward reduction in GABA currents at each recovery time (Table 2, p>0.05). These changes in maximal current were not due to altered cell size, as there was no significant difference in membrane capacitance between time points (Table 1). Current density, defined as maximal GABAAR current normalized to membrane capacitance, was significantly reduced immediately after 4 h hypoxia and after 48 h recovery (Table 3, Fig 2C). Current density did not change at any recovery time after 2 h (Fig. 2B, Table 3, p>0.05, t test with Bonferroni correction after ANOVA) or 8 h hypoxic exposures (Fig 2F, Table 3, p>0.05). Since both maximal currents and current density were significantly affected after 4 h hypoxia, with no change in the EC50 for GABA activation, this suggests either a reduction in the density of functional GABAAR channels per unit membrane, or a decrease in the conductance or kinetics of individual channels. A change in the driving force for chloride is also possible (see below), but not relevant to these recordings, as equal chloride concentrations were used in the extracellular and intracellular pipette solutions, creating a reversal potential of 0 mV and minimizing problems related to large ion gradients. Since the most consistent changes in GABAAR current occurred after 4 h of exposure to 1% O2, we investigated whether this hypoxic exposure also affected and GABAAR modulator pharmacology, which might suggest altered GABAAR subunit composition. In the following experiments, 4 h exposure to 1% O2 was used as the standard hypoxia treatment.
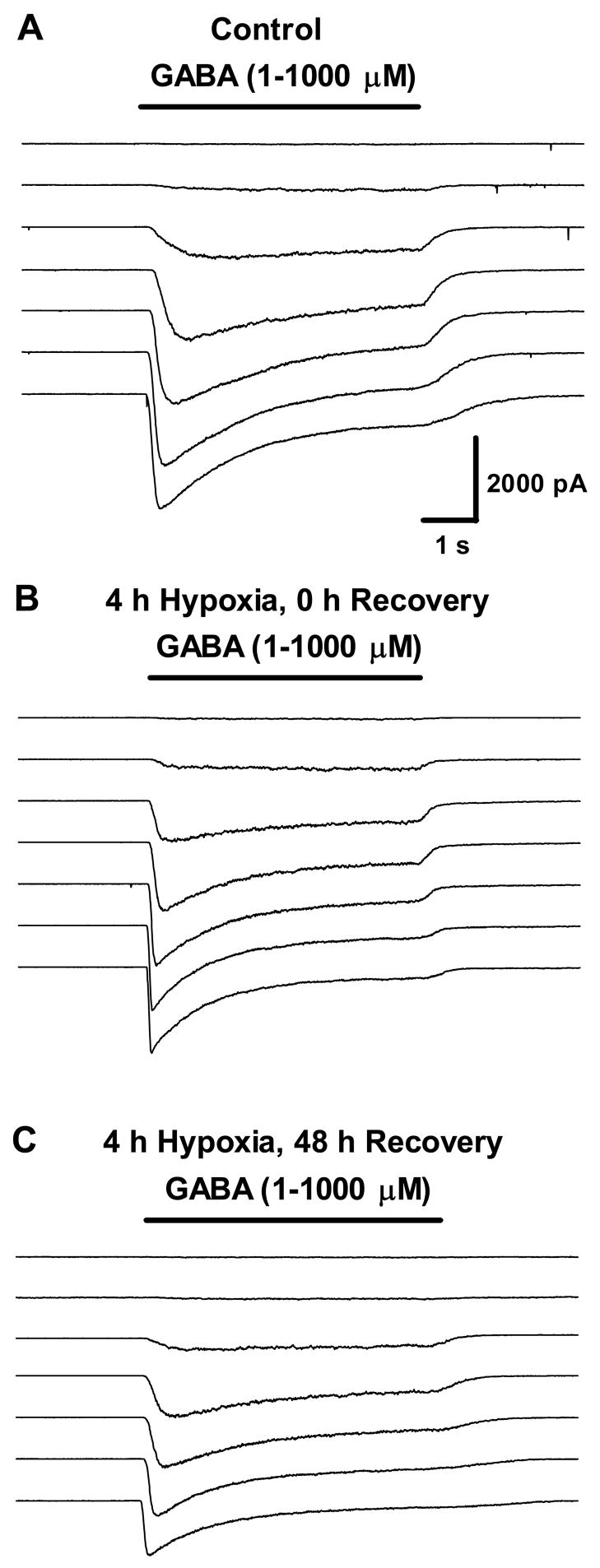
Figure 1.
GABAAR current traces after 4 h hypoxia. Whole-cell GABAAR currents evoked by increasing concentrations of GABA (1μM–1mM) in a control neuron (A); immediately following 4h hypoxia (B); 48 h recovery after 4 h hypoxia (C). Current amplitude and time scale as shown are the same for all traces.
Figure 2.
GABAAR current after 2 h, 4 h or 8h hypoxia. Peak currents elicited by increasing GABA concentrations in control cells (filled squares) and immediately (0 h, open circles), 24 h (open triangles) and 48 h (open diamonds) after 2 h hypoxia (A), 4 h hypoxia (C), or 8 h hypoxia (E). Each symbol represents means of peak currents evoked at that GABA concentration. Maximal GABA currents normalized to cell capacitance for control cells and at each recovery time points after 2 h hypoxia (B), 4 h hypoxia (D) or 8 h hypoxia (F). Bars represent mean ± SEM; the n for each condition is shown in the appropriate bar. There was no significant difference in current density between control and 2h or 8h hypoxia-treated cells at any recovery time after hypoxia (p>0.05, one way ANOVA). Current density was significantly reduced at 0 h and 48 h recovery after 4 h hypoxia (D). Asterisks show columns significantly different from each other as marked (*p < 0.05, **p<0.01, ***p<0.001 compared to control by one way ANOVA).
Table 2. Maximal GABAAR Currents after Hypoxia and Recovery.
Maximal GABA-evoked peak currents at specified recovery times after 3 different hypoxic durations. NT= nitrendipine (3 μM).
| Hypoxia | Control (pA) | 0 h Recovery (pA) | 24 h Recovery (pA) | 48 h Recovery (pA) |
|---|---|---|---|---|
| Duration | Mean ± SEM (n) | Mean ± SEM (n) | Mean ± SEM (n) | Mean ± SEM (n) |
| 2 h | 4848 ± 376 (9) | 3924 ± 281 (8) | 3791 ± 208 (9)* | 5303 ± 497 (9) |
| 4 h | 4289 ± 459 (10) | 2813 ± 246 (9)* | 3445 ± 547 (7) | 2362 ± 239 (10)** |
| 8 h | 4290 ± 505 (10) | 2872 ± 502 (6) | 3551 ± 310 (8) | 3334 ± 338 (9) |
| 4 h + NT | 6341± 484 (11)* | 6748 ± 820 (8)*** | 5409 ± 945 (8) | 6212± 524 (9)*** |
p<0.05,
p<0.01,
p<0.001 compared to control, t test with Bonferroni correction after one-way ANOVA. For 4 h + NT, comparisons are made to 4 h control and post-hypoxic time points; post-hypoxic values did not differ from control in the 4h + NT group.
Table 3. Maximal GABAAR Currents Normalized to Membrane Capacitance after Hypoxia and Recovery.
Maximal GABA-evoked currents normalized to membrane capacitance at specified recovery times after 3 different hypoxic durations.
| Hypoxia | Control (pA/pF) | 0 h Recovery (pA/pF)) | 24 h Recovery (pA/pF)) | 48 h Recovery (pA/pF) |
|---|---|---|---|---|
| Duration | Mean ± SEM (n) | Mean ± SEM (n) | Mean ± SEM (n) | Mean ± SEM (n) |
| 2 h | 247.2 ± 17.3 (9) | 199.5 ± 17.6 (8) | 198.4 ± 12.7 (9) | 296.9 ± 26.2 (9) |
| 4 h | 211.5 ± 22.5 (10) | 132.7 ± 15.6 (9)* | 180.0 ± 26.1 (7) | 121.2 ± 10.7 (10)** |
| 8 h | 214.0 ± 22.7 (10) | 148.4 ± 31.6 (6) | 177.3 ± 18.0 (8) | 187.4 ± 18.8 (9) |
| 4 h + NT | 341.1 ± 27.4 (11)** | 357.5 ± 36.0 (8)*** | 309.5 ± 48.7 (8)* | 337.8 ± 26.7 (9)*** |
p<0.05,
p<0.01 relative to control, t test with Bonferroni correction. after one way ANOVA.
3.2. Effects of 4 h hypoxia on GABAAR pharmacology
To investigate whether there was a change in GABAAR pharmacology after 4h hypoxia, individual cortical neurons were recorded at multiple recovery times after sham or 4 h hypoxic exposure, and the effects of subunit-specific modulators to enhance or inhibit GABA elicited currents was assessed by co-application of each modulator with GABA compared to currents elicited by GABA alone. The positive modulators diazepam, zolpidem and pentobarbital and the negative allosteric modulator, Zn2+ were chosen on the basis of their association with specific subunits for their binding and efficacy.
3.2.1 Diazepam
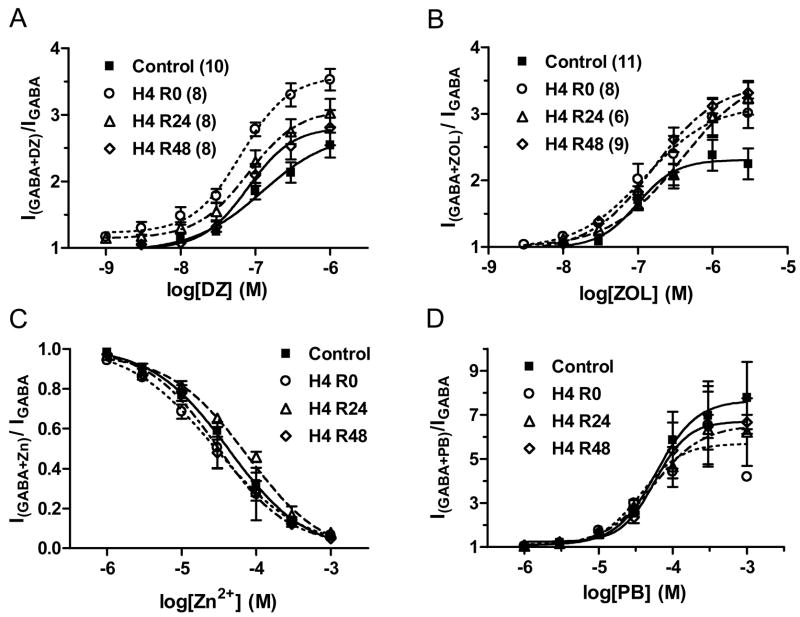
The benzodiazepine full agonist, diazepam, potentiated GABA currents in a concentration-dependent manner (Fig. 5A). Diazepam concentration-response curves were derived from coapplication of increasing concentrations of diazepam with 3 μM GABA. After 4 h hypoxia, there was an initial significant increase in maximal diazepam potentiation compared to control cells (p< 0.01, see Table 4), with no significant difference in EC50 (ρ>0.05 by one way ANOVA, see Table 5). This increase returned to control values after 24 h and 48 h recovery (Table 4).
Figure 5.
Nitrendipine prevented the decrease in GABA currents after 4h hypoxia. (A) Peak currents elicited by increasing GABA concentrations in control cells (filled squares) and immediately (0 h, open circles), 24 h (open triangles) and 48 h (open diamonds) after 4 h hypoxia in the presence of nitrendipine (NT, 3 μM). Each symbol represents means of peak currents evoked at that GABA concentration. (B) Maximal GABA currents normalized to cell capacitance for control cells and at each recovery time point after 4 h hypoxia, with or without NT. There was no significant difference in current density between control and 4 h hypoxia-treated neurons in the presence of NT during hypoxia (ρ>0.05, one way ANOVA).
Table 4. Maximal Enhancement or Inhibition of GABA Currents after 4 h Hypoxia Relative to Control.
| Control (% of control) | 0 h Recovery (% of control) | 24 h Recovery (% of control) | 48 h Recovery (% of control) | |
|---|---|---|---|---|
| Mean ± SEM (n) | Mean ± SEM (n) | Mean ± SEM (n) | Mean ± SEM (n) | |
| Diazepam | 256 ± 16.6 (10) | 358 ±13.9 (8)** | 303 ± 21.2 (8) | 274 ± 20.1 (8) |
| Zolpidem | 245 ± 21.7 (10) | 317 ± 24.5 (8) | 310 ± 25.3 (6) | 334 ± 14.4 (8)* |
| Pentobarbital | 777 ± 163 (6) | 654 ± 177 (2) | 635 ± 170 (5) | 668 ± 33.1 (4) |
| Zinc | 5.72±1.39 (6) | 6.58 ± 2.06 (5) | 7.70 ± 1.77 (6) | 4.90 ± 0.88 (6) |
p<0.05,
p<0.01,
p<0.001 by Bonferroni correction after one way ANOVA
Table 5. EC50 (or IC50) of Allosteric Agent Effects on GABA Currents After 4 h Hypoxia.
| Control | 0 h Recovery EC50 | 24 h Recovery EC50 | 48 h Recovery EC50 | |
|---|---|---|---|---|
| EC50 (μM) (95% CI, n) | EC50 (μM) (95% CI, n) | EC50 (μM) (95% CI, n) | EC50 (μM) (95% CI, n) | |
| Diazepam | 0.12 (0.05–0.30, 10) | 0.06 (0.04–0.09, 8) | 0.08 (0.04–0.14, 8) | 0.08 (0.05–0.13, 8) |
| Zolpidem | 0.10 (0.06–0.17, 10) | 0.12 (0.05–0.30, 8) | 0.38 (0.13–1.14, 6) | 0.16 (0.11–0.24, 8) |
| Pentobarbital | 59.7 (27.5–130, 8) | 37.3 (10.3–134, 2) | 54.7 (18.3–163, 6) | 57.1 (35.9–90.9, 7) |
| Zinc | 42.6 (29.5–61.6, 6) | 27.3 (13.9–53.7, 5) | 75.3 (48.3–117, 6) | 30.0 (20.7–43.5, 6) |
3.2.2 Zolpidem
Zolpidem, a benzodiazepine site agonist with high potency at α1-containing GABAARs and minimal efficacy at α5-containing receptors, produced concentration-dependent enhancement of GABAAR current in cortical neurons (Fig 5B, Tables 4 and 5). Zolpidem concentration-response curves were derived from coapplication of increasing concentrations of zolpidem with 3 μM GABA. After 4 h hypoxia, maximal enhancement by zolpidem tended to be increased (Table 4) with no significant difference in EC50 (Table 5, ρ>0.05 by one way ANOVA). The increase in maximal zolpidem potentiation was significant after 48 h normoxic recovery (ρ<0.05 by Bonferonni correction after one way ANOVA).
3.2.3 Pentobarbital
Pentobarbital potentiates GABA currents with no significant GABAAR subtype selectivity. Pentobarbital enhanced 3 μM GABA currents in a concentration-dependent manner (Fig 5C, Tables 4 and 5). There was no significant difference in maximal enhancement or EC50 for enhancement between control neurons and neurons at different recovery times after hypoxic treatment.
3.2.4 Zinc
The divalent cation, Zn2+, negatively modulates GABAAR currents with higher potency at receptors containing an α4, α5 or α6 subtype and lacking the α1 subunit or γ subunits (Smart et al., 1991). Zn2+ reduced whole-cell 10 μM GABA currents in a concentration-dependent manner (Fig 5D, Tables 4 and 5). Maximal inhibition and IC50 for Zn2+ inhibition of GABA currents were not significantly changed in post-hypoxic neurons at any of the recovery time points (Tables 4 and 5, ρ>0.05 by Bonferroni correction after one way ANOVA).
3.3 Effects of 4 h hypoxia on GABA current reversal potential
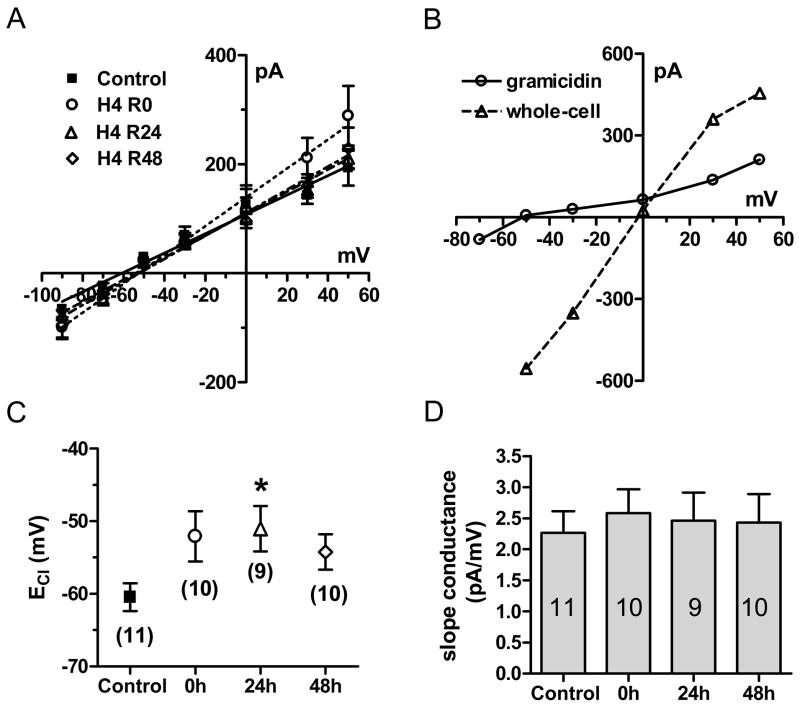
To examine whether hypoxia affected the Cl− reversal potential, we performed whole-cell gramicidin perforated-patch recording, which does not disturb intracellular Cl− concentration ([Cl−]i). The antibiotic gramicidin forms membrane pores impermeable to Cl−, but permeable to monovalent cations and small uncharged molecules and maintains [Cl−]i stable for times exceeding 60 min (Kyrozis and Reichling, 1995). I–V curves were plotted by peak currents obtained during application of 10 μM GABA at multiple holding potentials (Fig. 4A). A sample experiment is shown in Fig. 4B, with an individual neuron studied 24 h after 4 h hypoxia, initially recorded in perforated-patch mode and again after rupture of the patch to go into whole-cell recording mode. With rupture of the perforated patch, the slope conductance increased due to reduction in access resistance and the Cl− reversal potential shifted to ~0 mV, due to the equivalent chloride concentrations between external and internal pipette solutions. With cells recorded after hypoxia in perforated-patch mode to preserve intracellular chloride gradients, there was a depolarizing (positive) shift in the Cl− reversal potential after 4 h hypoxia relative to controls (Fig 4C) that was significant at the 24 h recovery point (Control: −59.5 ± 3.2 mV, n = 11; H4,R24: −51.0 ± 3.1 mV, n = 9, ρ<0.05 by Bonferonni correction after one way ANOVA). The chloride reversal potential returned toward control values after 48 h recovery (H4,R48: −54.3 ± 2.5 mV, n=10, ρ>0.05). Slope conductance at each recovery time point was not significantly different from control (Control : 2.27 ± 0.35 pA/mV, n = 11; 0 h: 2.58 ± 0.38 pA/mV, n = 10; 24 h: 2.46 ± 0.45 pA/mV, n = 9; 48 h: 2.43 ± 0.46 pA/mV, n = 10, ρ>0.05 by t test with Bonferonni correction after one way ANOVA, Fig. 6D). Hence, there was no evidence for a reduction in GABAAR conductance or rectification. A depolarizing shift in Cl− reversal potential could result in smaller outward GABA currents or even inward currents; however, it does not account for the change in GABA current amplitudes observed here under whole-cell recording conditions in which equivalent transmembrane Cl− concentrations were used.
Figure 4.
Hypoxia (4 h) shifted the reversal potential of GABA-induced Cl− currents (ECl). (A) GABA current-voltage relationship in control conditions (filled squares), immediately following 4 h hypoxia (0 h, open circles), 24 h (open triangles) and 48 h (open diamonds) recovery after 4 h hypoxia. (B) Sample I–V relationship of GABA currents in gramicidin perforated-patch and whole-cell patch mode in a single cell recorded 24 h after 4 h hypoxia. (C) The changes in ECl were significantly different between control and 24 h recovery time point. Data represents mean ± SEM; the n for each condition is shown in the appropriate symbol; Asterisks show columns significantly different from each other as marked (*p < 0.05 compared to control by one way ANOVA with post-hoc t test). (D) There was no significant difference in slope conductance after 4 h hypoxic exposure compared with control (ρ>0.05, one way ANOVA).
Figure 6.
The effect of nitrendipine on zolpidem pharmacology and GABA reversal potential. (A) Enhancement of 3 μM GABA currents by zolpidem (ZOL) significantly increased at 48 h recovery after 4 h hypoxia (ρ<0.01, one way ANOVA). NT (3 μM) during hypoxia not only prevented the increase in enhancement of GABA currents by ZOL, but significantly reduced ZOL enhancement at 48h recovery (p<0.05, student t test). (B) GABA I–V relationship in control conditions (filled squares), and at 24 h (open triangles) and 48 h (open diamonds) recovery after 4 h hypoxia in the presence of NT. (C) Summary of changes in ECl in control conditions or hypoxia conditions with or without NT. In the presence of NT during hypoxia, there was no difference in ECl between control and hypoxia-treated neurons. Data represents mean ± SEM; the n for each condition is shown at the appropriate symbol.
3.4 Prevention of hypoxic effects on GABA currents by nitrendipine
Hypoxia can regulate cell function through a variety of signaling pathways, including the elevation of intracellular calcium via several possible mechanisms. To determine whether the effects of hypoxia on cortical neuron GABA currents was due to calcium entry through L-type voltage-gated calcium channels (L-VGCCs), we co-incubated cortical neuron cultures in the L-VGCC blocker, nitrendipine (NT, 3 μM), during 4 h control or hypoxic exposure, with subsequent washout and replacement with aerated SMEM/HS culture medium. Since several L-VGCC antagonists can inhibit GABAAR currents, NT was chosen due to its lack of interaction with GABAAR at the concentration used (Das et al., 2004). Whole-cell and perforated-patch recordings were performed at the same recovery time points.
3.4.1 Maximal GABA current reduction blocked by nitrendipine
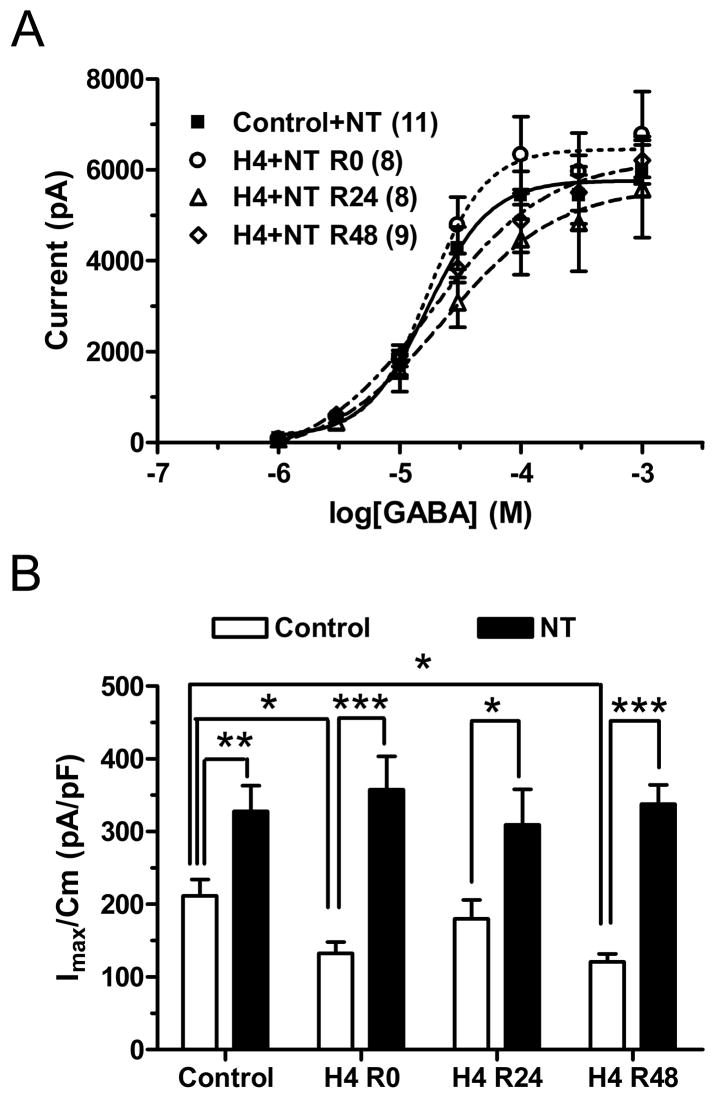
Whole-cell currents elicited by GABA after incubation in NT (3 μM, 4 h) were significantly larger than control currents from neurons not exposed to NT (Fig. 5A, B, p<0.05 by t test with Bonferonni correction after one way ANOVA). The EC50 value and Hill slope were unchanged from control. In cells recorded 0 h, 24 h or 48 h after 4 h hypoxia in the presence of NT (10 μM), there was no significant change in maximal current, EC50 or Hill slope from control cells (exposed to NT), and maximal current values at each post-hypoxic time point were significantly larger than those recorded at these time points after 4 h hypoxia in the absence of NT (Table 2, Fig 5A, B). When normalized to cell capacitance, control + NT maximal current density was significantly increased from control without NT (Table 3, Fig. 5A, B) and at each post-hypoxic time point, suggesting an increased number of functional channels on the surface membrane. NT thus prevented the hypoxia-induced reduction in cell surface GABAARs at the 0 h and 48 h recovery time points. There was no significant difference in membrane capacitance between neurons exposed or not exposed to NT (Table 1), hence exposure did not appear to alter cell size. These results suggest that L-VGCC activation may be required for hypoxia-induced reduction of GABAAR currents.
3.4.2 Nitrendipine prevented the hypoxia-induced change in BZ pharmacology
Since nitrendipine prevented the hypoxia-induced reduction in maximal GABA current, it was important to determine whether it could also affect the change in BZ pharmacology after hypoxia. Nitrendipine (3 μM) during hypoxic exposure prevented the increase in zolpidem enhancement of GABA currents after 48h recovery (Fig. 6A). Interestingly, the potentiation of GABA current by zolpidem was significantly reduced at 48 h recovery after 4h hypoxia in the presence of NT (control+NT: 269.4 ± 18.7 %; H4+NT,R48: 194.0 ± 16.3 %; P<0.05, student T-test; Fig 6B), in contrast to the increase observed at this time point after hypoxia in the absence of NT, suggesting that L-VGCC activation is involved in determining post-hypoxic BZ sensitivity.
3.4.3 Nitrendipine prevented the hypoxia-induced change in chloride reversal potential
We next explored whether NT exposure during hypoxia would affect the hypoxia-induced depolarizing shift in the chloride reversal potential. Gramicidin perforated-patch recordings were performed as before on cortical neurons under control conditions and at the 24 and 48 h recovery time points after 4 h hypoxia in the presence of NT (3 μM). The chloride reversal potential in control neurons exposed to NT (−50.41 ± 3.59 mV, n = 7) was less negative than control neurons not exposed to NT (−59.5 ± 3.1 mV, n = 11) but this difference was not significant (p>0.05, students t-test). The chloride reversal potential at 24 h and 48 h recovery after 4 h hypoxia in the presence of NT did not change compared with that at normoxia (H4+NT,R24: −52.93 ± 4.36 mV; H4+NT,R48: −51.77 ± 4.17 mV, p>0.05, t test with Bonferroni correction after one way ANOVA). Thus, NT also prevented the depolarizing shift in chloride reversal potential after hypoxia, suggesting that L-VGCC-dependent processes are involved in the post-hypoxic regulation of chloride homeostasis in cortical neurons.
4. Discussion
As in our previous studies using NT2-N neuronal cells (Gao et al., 2004), GABAAR currents in cortical neurons were significantly affected by transient exposure to hypoxia. However, GABAAR currents in rat primary cortical neurons in culture had variable responses to hypoxia. After 2 h exposure to 1% O2, maximal GABA-evoked currents tended to be smaller immediately after hypoxia, were significantly smaller 24 h afterwards, and tended to be larger than control after 48 h recovery. After 4 h hypoxic exposure, currents were significantly smaller at 0 and 48 h after hypoxia and non-significantly reduced 24 h after hypoxia. After 8 h hypoxia, currents were non-significantly reduced at all 3 post-hypoxic time points. These results differed from our prior studies in NT2-N neuronal cells, in which cells had a biphasic response after 8 h exposure to 1% O2. NT2-N cell GABA currents were increased immediately after hypoxia, returned toward control after 24 h recovery, were decreased (60% of control) after 48 h recovery, and returned to baseline at 96 h recovery (Gao et al., 2004). The key differences are that cortical neuron GABA currents did not increase at any post-hypoxic time-point after any of the 3 hypoxic durations. Moreover, the most significant reductions in maximal GABA current in cortical neurons were observed after 4 h rather than 8 h hypoxia.
Such contrasting effects of hypoxia on GABA-evoked currents might be due to differences in GABAAR subunit composition between NT2-N cells and cortical neurons, and to the variability of neuronal phenotypes in cortical cultures. NT2-N neuronal cells are derived from a human teratocarcinoma, and consistently express a subset of GABAAR subunit mRNAs at different stages of in vitro development (Neelands et al., 1999). These cells can be used as a model for GABAAR regulation due to the relative homogeneity of their responses to GABA and GABAAR modulators (Neelands et al., 1998). Although NT2-N cells are quite ‘neuronal’ in many repects, the present results suggest that the details of GABAAR regulation, function and subunit expression may differ in actual CNS neurons. Indeed, the present findings in cortical neurons in culture might also differ from neurons within the intact brain and in different brain regions.
Since the most consistent changes in maximal GABA-evoked current occurred after 4 h hypoxic exposure, this treatment was selected for further examination of post-hypoxic changes in GABAAR function, focusing on subunit-dependent pharmacology. Hypoxia (4 h at 1 % O2) increased diazepam efficacy for enhancing GABA-evoked currents immediately after hypoxic exposure and non-significantly elevated enhancement at later post-hypoxic time points. In contrast, GABAR current enhancement by the α1 subunit-selective imidazopyridine, zolpidem, was non-significantly increased at the 0 and 24 h time points but significantly increased after 48 h recovery. No changes in barbiturate or Zn2+ sensitivity were detected at any post-hypoxic time point.
In NT2-N neuronal cells, similar changes in BZ pharmacology were noted, associated with altered subunit mRNA expression (Gao et al., 2004). Maximal potentiation of GABA currents by diazepam was increased 48 h after (8 h) hypoxia, while zolpidem enhancement was decreased, and responses to pentobarbital and zinc were unchanged. Semiquantitative reverse transcriptase (RT)-PCR showed decreased α1, α5, β2 and γ2 subunit mRNA after hypoxia, correlating with decreased maximal currents. Such changes may be clinically relevant, as mRNA expression of the α1 and β2 subunits decreased within 30 minutes after reperfusion in hippocampal areas CA1, CA3 and dentate gyrus in an in vivo stroke model (Li et al., 1993). Moreover, in a rat stroke model, cortical reductions in the α1, α2, α5 and γ2 subunits were observed 1, 7 and 30 days after infarct (Redecker et al., 2002).
In cortical neurons, the early change in diazepam sensitivity and the late change in zolpidem sensitivity, both specific to BZ binding site, might have resulted from incorporation of different α subunits into the holoreceptor. The increase in diazepam efficacy could be related to incorporation of BZ-sensitive subunits (α1, α2, α3 or α5) in place of BZ-insensitive subunits (α4 or α6), loss of “γ-less” receptors, or the preferential loss of BZ-insensitive α subunits during downregulation of GABAAR current. The isolated increase in diazepam enhancement immediately after hypoxia, with no change in zolpidem enhancement, could be produced by an increased proportion of α5-containing receptors that respond to diazepam but not zolpidem. The later increase in zolpidem efficacy could have been produced by an increased proportion of GABAARs containing α1, α2 or α3, since zolpidem only binds to receptors containing these subunits. Further, the lack of increase in zolpidem potency argues against an increase in α1-containing receptors, since zolpidem has higher affinity at those sites. Hence, if subunit changes are involved, the early post-hypoxic period would require an increased proportion of α5 containing GABARs, while the late changes would need an increase in α2 or α3. Such changes are likely to be quite different from those associated with BZ tolerance after chronic exposure, which results in decreased BZ sensitivity associated with a variety of subunit changes in different model systems (e.g. (Bateson, 2002; Chen et al., 1999; Primus et al., 1996)). Future examination of specific α subunit expression patterns on the neuronal surface by immunocytochemistry could help determine whether such subunit changes occur.
The mechanism underlying the observed changes in GABAAR current and pharmacology might also involve regulation by protein kinases and/or phosphatases, particularly the Ca2+/calmodulin-dependent protein phosphatase 2B, calcineurin (CaN), as previously demonstrated in a perinatal model of hypoxia-induced seizures (Sanchez et al., 2005). In hypoxia-treated neonatal rats, a decrease in GABAergic inhibition in CA1 pyramidal neurons resulted from AMPA-receptor mediated activation of CaN, which dephosphorylated the GABAAR β2 or β3 subunit (Sanchez et al., 2005). CaN regulation of GABAAR function is important in mediating long-term depression (LTD), a decrease in GABA-mediated IPSP amplitude induced by an NMDA receptor-mediated mechanism similar to that underlying long term potentiation of AMPA receptor synapses (Lu et al., 2000) and involves a direct interaction between CaN and the γ2 subunit (Wang et al., 2003). In our cortical culture model, calcium entry was unlikely to be mediated by AMPA or NMDA receptor mechanisms as synaptic connectivity in culture is typically sparse and ambient glutamate and glycine levels are low. Rather, hypoxia might induce membrane depolarization (possibly due to lowered ATP levels and decreased Na+/K+ ATPase activity), which could lead to Ca2+ influx through VGCCs, particularly L-VGCCs which are slow to desensitize. An increase in intracellular Ca2+ could activate CaN, possibly resulting in the decrease in GABA current. We therefore sought to determine whether antagonism of L-VGCCs would block some or all of the effects of hypoxia on GABAAR function.
L-VGCC are highly expressed in the rat cerebral cortex and hippocampus with high density on neuronal cell bodies (Ricci et al., 2002). Blockade of L-VGCC during hypoxia prevented the reduction of GABA current at all time points after 4h hypoxia (Fig. 5), suggesting that L-VGCC are activated during hypoxia and that the reductions in GABAAR current may be Ca2+-mediated. In addition, the maximal GABA current in control cells significantly increased after 4 h exposure to nitrendipine, indicating that Ca2+-dependent downregulation of GABA currents likely occurs even under non-hypoxic conditions and may participate in setting the “basal tone” of GABAAR inhibition by determining the number of functional cell surface receptors. Indeed, blockade of L-type voltage-gated Ca2+ channels by nifedipine has been shown to inhibit down-regulation of GABAR number induced by 48 h exposure to 1 mM GABA (Lyons et al., 2001). VGCC regulation of GABAAR surface expression may be a mechanism common to a variety of stimuli, as the decrease in IPSC GABAAR conductance after chronic BZ treatment, likely induced by GABAAR-mediated depolarization and resulting VGCC activation, was reversed by systemic nimodipine injection (Xiang et al., 2007b).
In addition to preventing the hypoxia-induced decrease in GABAAR current, nitrendipine not only prevented the upregulation of zolpidem enhancement of GABA current after 48 h recovery, but actually reduced zolpidem potentiation at that recovery time point. Whether this was due to prevention of a change in GABAAR subunit composition (e.g. a hypoxia/Ca2+-induced decrease in α5-containing receptors) or a change in calcium-dependent phosphorylation is unclear. In earlier studies in NT2-N neuronal cells, we found that activation of protein kinase C (PKC) caused a rightward shift in the diazepam concentration-response curve without affecting maximal enhancement (Gao et al., 2005), hence PKC is unlikely to be directly involved. Other phosphorylation or dephosphorylation mechanisms are possible (Kittler et al., 2003), but few studies have investigated the effects of such post-translational regulation on BZ allosteric properties.
Finally, we used gramicidin perforated-patch recording to determine whether hypoxia altered the reversal potential for chloride in cortical neurons. We found a modest depolarizing shift of the chloride reversal potential which was significant 48 h after hypoxic exposure. Co-incubation with nitrendipine (3 μM) during hypoxia caused a non-significant depolarizing trend in the chloride reversal potential in control neurons and prevented the depolarizing shift after hypoxia. Hypoxia/ischemia has been shown to increase intracellular chloride concentration in adult rat CA1 hippocampal neurons (Comerford et al., 2003; Inglefield and Schwartz-Bloom, 1998; Katchman et al., 1994; Mittmann et al., 1998) though the mechanism remains controversial. Changes in Cl− reversal potential in CA1 neurons were also seen after chronic BZ treatment, accompanied by GABAAR-mediated depolarization (Zeng et al., 1997). In our previous experiments, the resting membrane potential in cortical neurons at 13–15 days in vitro was around −65 mV (data not shown), hence the shift in chloride reversal potential from −59.5 ± 3.2 mV in control cells to −51.0 ± 3.1 mV recorded 24 h after hypoxia implies that GABAAR currents become significantly depolarizing after hypoxia and could increase neuronal excitability. Indeed, GABA currents could facilitate L-VGCC-mediated calcium currents as suggested by the findings of Tiez et al. after chronic BZ treatment (Xiang et al., 2007a). Downregulation of GABAAR function by a calcium-dependent process might thus be protective against excessive depolarization associated with seizures and myoclonus, though the mechanisms underlying these changes require further investigation.
Figure 3.
Modulation of GABA currents after 4 h hypoxia. (A) Enhancement of 3 μM GABA currents by increasing concentrations of diazepam (DZ) in control cells (filled squares, n=10), 0 h (open circles, n=8), 24 h (open triangles, n=8) and 48 h (open diamonds, n=8) after 4 h hypoxia, normalized to control current amplitude. A significant increase in maximal enhancement was observed at 0 h after hypoxia (ρ<0.01) compared to control, with no change in EC50. (B) Enhancement of 3 μM GABA currents by zolpidem (ZOL) in control cells (filled squares, n=10), 0 h (open circles, n=8), 24 h (open triangles, n=6) and 48 h (open diamonds, n=8) after 4 h hypoxia. The EC50 for enhancement was unchanged, but maximal enhancement was significantly increased at the 48 h recovery time point after hypoxia (ρ<0.05). (C) Enhancement of 3 μM GABA currents by pentobarbital (PB) in control cells (filled squares, n=6), 0 h (open circles, n=2), 24 h (open triangles, n=6) and 48 h (open diamonds, n=6) after 4 h hypoxia. Maximal enhancement and EC50 were not significantly different (ρ>0.05). (D) Inhibition of 10 μM GABA currents by increasing concentrations of Zn2+ in control cells (filled squares, n=6), 0 h (open circles, n=5), 24 h (open triangles, n=6) and 48 h (open diamonds, n=6) after 4 h hypoxia. No difference in Zn2+ inhibition were observed (ρ>0.05). (See Table 4, 5)
Acknowledgments
We gratefully acknowledge the expert technical assistance of Brian Behrle, Julie Warner, Matt McClellan and Alison Fountain. We also thank Dr. Elizabeth I. Tietz for helpful comments on the manuscript.
This work was supported in part by R01-NS049389 and a research grant from the Myoclonus Research Foundation, Ft. Lee, N.J. to L.J.G., and an institutional predoctoral training award from the University of Toledo College of Medicine to L.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–425. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
- Bateson AN. Basic pharmacologic mechanisms involved in benzodiazepine tolerance and withdrawal. Curr Pharm Des. 2002;8(1):5–21. doi: 10.2174/1381612023396681. [DOI] [PubMed] [Google Scholar]
- Bergamasco B, Benna P, Ferrero P, Gavinelli R. Neonatal hypoxia and epileptic risk: a clinical prospective study. Epilepsia. 1984;25:131–136. doi: 10.1111/j.1528-1157.1984.tb04168.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Huang XH, Zeng XJ, Sieghart W, Tietz EI. Benzodiazepine-mediated regulation of α1–2, β1–3 and γ-aminobutyric acid type A receptor subtype proteins in the rat brain hippocampus and cortex. Neuroscience. 1999;93:33–44. doi: 10.1016/s0306-4522(99)00118-9. [DOI] [PubMed] [Google Scholar]
- Comerford KM, Leonard MO, Karhausen J, Carey R, Colgan SP, Taylor CT. Small ubiquitin-related modifier-1 modification mediates resolution of CREB-dependent responses to hypoxia. Proc Natl Acad Sci U S A. 2003;100:986–991. doi: 10.1073/pnas.0337412100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Congar P, Khazipov R, Ben-Ari Y. Direct demonstration of functional disconnection by anoxia of inhibitory interneurons from excitatory inputs in rat hippocampus. J Neurophysiol. 1995;73:421–426. doi: 10.1152/jn.1995.73.1.421. [DOI] [PubMed] [Google Scholar]
- Das P, Bell-Horner CL, Huang RQ, Raut A, Gonzales EB, Chen ZL, Covey DF, Dillon GH. Inhibition of type A GABA receptors by L-type calcium channel blockers. Neuroscience. 2004;124(1):195–206. doi: 10.1016/j.neuroscience.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Dallwig R, Deitmer JW, Backus KH. On the mechanism of GABA-induced currents in cultured rat cortical neurons. Pflugers Arch. 1999;437:289–297. doi: 10.1007/s004240050782. [DOI] [PubMed] [Google Scholar]
- Gao L, Greenfield LJ. Activation of protein kinase C reduces benzodiazepine potency at GABA(A) receptors in NT2-N neurons. Neuropharmacology. 2005;48(3):333–342. doi: 10.1016/j.neuropharm.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Gao L, Lyons AR, Greenfield LJ., Jr Hypoxia alters GABAA receptor function and subunit expression in NT2-N neurons. Neuropharmacology. 2004;46:318–330. doi: 10.1016/j.neuropharm.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Hallett M. Physiology of human posthypoxic myoclonus. Mov Disord 15 Suppl. 2000;1:8–13. doi: 10.1002/mds.870150703. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Archives. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harata N, Wu J, Ishibashi H, Ono K, Akaike N. Run-down of the GABAA response under experimental ischaemia in acutely dissociated CA1 pyramidal neurones of the rat. J Physiol. 1997;500(Pt 3):673–688. doi: 10.1113/jphysiol.1997.sp022052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XJ, Ticku MK. Development pattern of the GABAA-benzodiazepine receptor ionophore complex in primary cultures of cortical neurons. Brain Res Dev Brain Res. 1994;80:137–140. doi: 10.1016/0165-3806(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Inglefield JR, Schwartz-Bloom RD. Optical imaging of hippocampal neurons with a chloride-sensitive dye: early effects of in vitro ischemia. J Neurochem. 1998;70:2500–2509. doi: 10.1046/j.1471-4159.1998.70062500.x. [DOI] [PubMed] [Google Scholar]
- Katchman AN, Vicini S, Hershkowitz N. Mechanism of early anoxia-induced suppression of the GABAA-mediated inhibitory postsynaptic current. J Neurophysiol. 1994;71:1128–1138. doi: 10.1152/jn.1994.71.3.1128. [DOI] [PubMed] [Google Scholar]
- Kato-Negishi M, Muramoto K, Kawahara M, Kuroda Y, Ichikawa M. Developmental changes of GABAergic synapses formed between primary cultured cortical neurons. Brain Res Dev Brain Res. 2004;152:99–108. doi: 10.1016/j.devbrainres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol. 2003;13(3):341–347. doi: 10.1016/s0959-4388(03)00064-3. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABA(A) receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Methods. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Li H, Siegel RE, Schwartz RD. Rapid decline of GABAA receptor subunit mRNA expression in hippocampus following transient cerebral ischemia in the gerbil. Hippocampus. 1993;3:527–537. doi: 10.1002/hipo.450030412. [DOI] [PubMed] [Google Scholar]
- Low JA, Galbraith RS, Muir DW, Killen HL, Pater EA, Karchmar EJ. The relationship between perinatal hypoxia and newborn encephalopathy. Am J Obstet Gynecol. 1985;152:256–260. doi: 10.1016/s0002-9378(85)80205-2. [DOI] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26(1):197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Lyons HR, Land MB, Gibbs TT, Farb DH. Distinct signal transduction pathways for GABA-induced GABA(A) receptor down-regulation and uncoupling in neuronal culture: a role for voltage-gated calcium channels. J Neurochem. 2001;78(5):1114–1126. doi: 10.1046/j.1471-4159.2001.00501.x. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- MacDonald RL, Kapur J. Pharmacological properties of recombinant and hippocampal dentate granule cell GABAA receptors. Adv Neurol. 1999;79:979–990. [PubMed] [Google Scholar]
- Macdonald RL, Saxena NC, Angelotti TP. Functional expression of recombinant GABAA receptor channels in L929 fibroblasts. Epilepsy Res Suppl. 1996;12:177–185. [PubMed] [Google Scholar]
- Mittmann T, Qu M, Zilles K, Luhmann HJ. Long-term cellular dysfunction after focal cerebral ischemia: in vitro analyses. Neuroscience. 1998;85:15–27. doi: 10.1016/s0306-4522(97)00638-6. [DOI] [PubMed] [Google Scholar]
- Neelands TR, Zhang J, Macdonald RL. GABA(A) receptors expressed in undifferentiated human teratocarcinoma NT2 cells differ from those expressed by differentiated NT2-N cells. J Neurosci. 1999;19:7057–7065. doi: 10.1523/JNEUROSCI.19-16-07057.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelands TR, Greenfield LJ, Jr, Zhang J, Turner RS, Macdonald RL. GABAA receptor pharmacology and subtype mRNA expression in human neuronal NT2-N cells. J Neurosci. 1998;18:4993–5007. doi: 10.1523/JNEUROSCI.18-13-04993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter NM, Thibault O, Thibault V, Chen KC, Landfield PW. Calcium channel density and hippocampal cell death with age in long-term culture. J Neurosci. 1997;17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primus RJ, Yu J, Xu J, Hartnett C, Meyyappan M, Kostas C, Ramabhadran TV, Gallager DW. Allosteric uncoupling after chronic benzodiazepine exposure of recombinant γ-aminobutyric acidA receptors expressed in Sf9 cells: Ligand efficiency and subtype selectivity. J Pharmacol Exper Therap. 1996;276:882–890. [PubMed] [Google Scholar]
- Ransom BR, Neale E, Henkat M, Bullock PN, Nelson PG. Mouse spinal cord in cell culture. I. Morphology and intrinsic neuronal electrophysiologic properties. J Neurophysiol. 1977;40:1132–1150. doi: 10.1152/jn.1977.40.5.1132. [DOI] [PubMed] [Google Scholar]
- Redecker C, Wang W, Fritschy JM, Witte OW. Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: mediation by NMDA-dependent processes. J Cereb Blood Flow Metab. 2002;22:1463–1475. doi: 10.1097/01.WCB.0000034149.72481.BD. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Sanchez RM, Dai W, Levada RE, Lippman JJ, Jensen FE. AMPA/kainate receptor-mediated downregulation of GABAergic synaptic transmission by calcineurin after seizures in the developing rat brain. J Neurosci. 2005;25:3442–3451. doi: 10.1523/JNEUROSCI.0204-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Smart TG, Moss SJ, Xie X, Huganir RL. GABAA receptors are differentially sensitive to zinc: dependence on subunit composition. Br J Pharmacol. 1991;103:1837–1839. doi: 10.1111/j.1476-5381.1991.tb12337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verheul HB, de Leeuw FE, Scholten G, Tulleken CA, Lopes da Silva FH, Ghijsen WE. GABAA receptor function in the early period after transient forebrain ischaemia in the rat. Eur J Neurosci. 1993;5:955–960. doi: 10.1111/j.1460-9568.1993.tb00946.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Liu S, Haditsch U, Tu W, Cochrane K, Ahmadian G, Tran L, Paw J, Wang Y, Mansuy I, Salter MM, Lu YM. Interaction of calcineurin and type-A GABA receptor gamma 2 subunits produces long-term depression at CA1 inhibitory synapses. J Neurosci. 2003;23(3):826–836. doi: 10.1523/JNEUROSCI.23-03-00826.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang K, Tietz EI. Benzodiazepine-induced hippocampal CA1 neuron alpha-amino-3-hydroxy-5-methylisoxasole-4-propionic acid (AMPA) receptor plasticity linked to severity of withdrawal anxiety: differential role of voltage-gated calcium channels and N-methyl-D-aspartic acid receptors. Behav Pharmacol. 2007a;18(5–6):447–460. doi: 10.1097/FBP.0b013e3282d28f2b. [DOI] [PubMed] [Google Scholar]
- Xiang K, Tietz EI. Nimodipine prevents the reduction of hippocampal CA1 neuron mIPSC amplitude induced by chronic benzodiazepine administration. 2007 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience. 2007b. 2007 Online. [Google Scholar]
- Xu ZC, Pulsinelli WA. Responses of CA1 pyramidal neurons in rat hippocampus to transient forebrain ischemia: an in vivo intracellular recording study. Neurosci Lett. 1994;171:187–191. doi: 10.1016/0304-3940(94)90636-x. [DOI] [PubMed] [Google Scholar]
- Yue X, Mehmet H, Penrice J, Cooper C, Cady E, Wyatt JS, Reynolds EO, Edwards AD, Squier MV. Apoptosis and necrosis in the newborn piglet brain following transient cerebral hypoxia-ischaemia. Neuropathol Appl Neurobiol. 1997;23:16–25. [PubMed] [Google Scholar]
- Zeng X, Tietz EI. Depression of early and late monosynaptic inhibitory postsynaptic potentials in hippocampal CA1 neurons following prolonged benzodiazepine administration: role of a reduction in Cl− driving force. Synapse. 1997;25(2):125–136. doi: 10.1002/(SICI)1098-2396(199702)25:2<125::AID-SYN3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]