Abstract
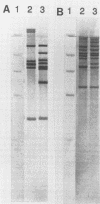
DNA probes specific for genes encoding rRNA and the glycopeptide resistance gene vanA were used to investigate a cluster of vancomycin-resistant (MICs, > 512 mg/liter) Enterococcus faecalis and Enterococcus faecium isolated from separate patients in a renal unit in a London hospital. When digested with BamHI, 12 of 13 vancomycin-resistant E. faecalis isolates exhibited a common restriction fragment length polymorphism pattern of rRNA genes (ribotype). A vanA probe hybridized with chromosomal DNA in these 12 isolates. The other isolate of vancomycin-resistant E. faecalis had a different ribotype and the vanA gene was located on plasmid DNA. These data suggest that cross-infection with a single strain of vancomycin-resistant E. faecalis occurred in most instances. In contrast, 23 vancomycin-resistant E. faecium isolates showed greater heterogeneity, comprising 8 ribotypes, suggesting that multiple strains were present in the unit. Twenty-one of these 23 isolates harbored a 24-MDa plasmid which hybridized with the vanA probe, implying that interstrain dissemination of a vancomycin resistance plasmid may have occurred among E. faecium isolates in the renal unit.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Elion J. Evidence for the genetic unrelatedness of nosocomial vancomycin-resistant Enterococcus faecium strains in a pediatric hospital. J Clin Microbiol. 1991 Sep;29(9):1888–1892. doi: 10.1128/jcm.29.9.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandis H., Plećeas P., Andries L. Die Typisierung von Streptococcus faecalis- und Streptococcus faecium-Stämmen mit Bakteriophagen. Zentralbl Bakteriol Mikrobiol Hyg A. 1985 Oct;260(2):206–215. [PubMed] [Google Scholar]
- Caprioli T., Zaccour F., Kasatiya S. S. Phage typing scheme for group D streptococci isolated from human urogenital tract. J Clin Microbiol. 1975 Oct;2(4):311–317. doi: 10.1128/jcm.2.4.311-317.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coudron P. E., Mayhall C. G., Facklam R. R., Spadora A. C., Lamb V. A., Lybrand M. R., Dalton H. P. Streptococcus faecium outbreak in a neonatal intensive care unit. J Clin Microbiol. 1984 Dec;20(6):1044–1048. doi: 10.1128/jcm.20.6.1044-1048.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Leclercq R., Coutant V., Duval J., Courvalin P. Phenotypic and genotypic heterogeneity of glycopeptide resistance determinants in gram-positive bacteria. Antimicrob Agents Chemother. 1990 Oct;34(10):1875–1879. doi: 10.1128/aac.34.10.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka-Malen S., Molinas C., Arthur M., Courvalin P. The VANA glycopeptide resistance protein is related to D-alanyl-D-alanine ligase cell wall biosynthesis enzymes. Mol Gen Genet. 1990 Dec;224(3):364–372. doi: 10.1007/BF00262430. [DOI] [PubMed] [Google Scholar]
- Emanuel J. R. Simple and efficient system for synthesis of non-radioactive nucleic acid hybridization probes using PCR. Nucleic Acids Res. 1991 May 25;19(10):2790–2790. doi: 10.1093/nar/19.10.2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French G., Abdulla Y., Heathcock R., Poston S., Cameron J. Vancomycin resistance in south London. Lancet. 1992 Mar 28;339(8796):818–819. doi: 10.1016/0140-6736(92)91954-7. [DOI] [PubMed] [Google Scholar]
- Garaizar J., Kaufmann M. E., Pitt T. L. Comparison of ribotyping with conventional methods for the type identification of Enterobacter cloacae. J Clin Microbiol. 1991 Jul;29(7):1303–1307. doi: 10.1128/jcm.29.7.1303-1307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross P. A., Harkavy L. M., Barden G. E., Flower M. F. The epidemiology of nosocomial enterococcal urinary tract infection. Am J Med Sci. 1976 Jul-Aug;272(1):75–81. doi: 10.1097/00000441-197607000-00009. [DOI] [PubMed] [Google Scholar]
- Hall L. M., Duke B., Guiney M., Williams R. Typing of Enterococcus species by DNA restriction fragment analysis. J Clin Microbiol. 1992 Apr;30(4):915–919. doi: 10.1128/jcm.30.4.915-919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Z., Kuhn M., Lannigan R., Austin T. W. Microbiological investigation of an outbreak of bacteraemia due to Streptococcus faecalis in an intensive care unit. J Hosp Infect. 1988 Nov;12(4):263–271. doi: 10.1016/0195-6701(88)90068-0. [DOI] [PubMed] [Google Scholar]
- Jarvis W. R., Martone W. J. Predominant pathogens in hospital infections. J Antimicrob Chemother. 1992 Apr;29 (Suppl A):19–24. doi: 10.1093/jac/29.suppl_a.19. [DOI] [PubMed] [Google Scholar]
- Kühnen E., Richter F., Richter K., Andries L. Establishment of a typing system for group D streptococci. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Jan;267(3):322–330. doi: 10.1016/s0176-6724(88)80048-8. [DOI] [PubMed] [Google Scholar]
- Kühnen E., Rommelsheim K., Andries L. Combined use of phage typing, enterococcinotyping and species differentiation of group D streptococci as an effective epidemiological tool. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Oct;266(3-4):586–595. doi: 10.1016/s0176-6724(87)80242-0. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Markowitz S. M., Lopardo H. A., Patterson J. E., Zervos M. J., Rubeglio E., Eliopoulos G. M., Rice L. B., Goldstein F. W. Evidence for clonal spread of a single strain of beta-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J Infect Dis. 1991 Apr;163(4):780–785. doi: 10.1093/infdis/163.4.780. [DOI] [PubMed] [Google Scholar]
- Murray B. E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990 Jan;3(1):46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Masecar B. L., Kauffman C. A., Schaberg D. R., Hierholzer W. J., Jr, Zervos M. J. Gentamicin resistance plasmids of enterococci from diverse geographic areas are heterogeneous. J Infect Dis. 1988 Jul;158(1):212–216. doi: 10.1093/infdis/158.1.212. [DOI] [PubMed] [Google Scholar]
- Pitcher D., Johnson A., Allerberger F., Woodford N., George R. An investigation of nosocomial infection with Corynebacterium jeikeium in surgical patients using a ribosomal RNA gene probe. Eur J Clin Microbiol Infect Dis. 1990 Sep;9(9):643–648. doi: 10.1007/BF01964264. [DOI] [PubMed] [Google Scholar]
- Pleceas P., Bogdan C., Vereanu A. Enterocine-typing of group D streptococci. Zentralbl Bakteriol Orig A. 1972 Jul;221(2):173–181. [PubMed] [Google Scholar]
- Rahman M., Noble W. C., Cookson B. Transmissible mupirocin resistance in Staphylococcus aureus. Epidemiol Infect. 1989 Apr;102(2):261–270. doi: 10.1017/s0950268800029939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinehart E., Smith N. E., Wennersten C., Gorss E., Freeman J., Eliopoulos G. M., Moellering R. C., Jr, Goldmann D. A. Rapid dissemination of beta-lactamase-producing, aminoglycoside-resistant Enterococcus faecalis among patients and staff on an infant-toddler surgical ward. N Engl J Med. 1990 Dec 27;323(26):1814–1818. doi: 10.1056/NEJM199012273232606. [DOI] [PubMed] [Google Scholar]
- SHARPE M. E. SEROLOGICAL TYPES OF STREPTOCOCCUS FAECALIS AND ITS VARIETIES AND THEIR CELL WALL TYPE ANTIGEN. J Gen Microbiol. 1964 Jul;36:151–160. doi: 10.1099/00221287-36-1-151. [DOI] [PubMed] [Google Scholar]
- SHARPE M. E., SHATTOCK P. M. F. The serological typing of group D streptococci associated with outbreaks of neonatal diarrhoea. J Gen Microbiol. 1952 Feb;6(1-2):150–165. doi: 10.1099/00221287-6-1-2-150. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Smyth C. J., Matthews H., Halpenny M. K., Brandis H., Colman G. Biotyping, serotyping and phage typing of Streptococcus faecalis isolated from dental plaque in the human mouth. J Med Microbiol. 1987 Feb;23(1):45–54. doi: 10.1099/00222615-23-1-45. [DOI] [PubMed] [Google Scholar]
- Uttley A. H., George R. C., Naidoo J., Woodford N., Johnson A. P., Collins C. H., Morrison D., Gilfillan A. J., Fitch L. E., Heptonstall J. High-level vancomycin-resistant enterococci causing hospital infections. Epidemiol Infect. 1989 Aug;103(1):173–181. doi: 10.1017/s0950268800030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford N., McNamara E., Smyth E., George R. C. High-level resistance to gentamicin in Enterococcus faecium. J Antimicrob Chemother. 1992 Apr;29(4):395–403. doi: 10.1093/jac/29.4.395. [DOI] [PubMed] [Google Scholar]