Abstract
Treponema denticola harbours a genetic locus with significant homology to most of the previously characterized Treponema pallidum tro operon. Within this locus are five genes (troABCDR) encoding for the components of an ATP-binding cassette cation-transport system (troABCD) and a DtxR-like transcriptional regulator (troR). In addition, a σ70-like promoter and an 18 bp region of dyad symmetry were identified upstream of the troA start codon. This putative operator sequence demonstrated similarity to the T. pallidum TroR (TroRTp) binding sequence; however, the position of this motif with respect to the predicted tro promoters differed. Interestingly, unlike the T. pallidum orthologue, T. denticola TroR (TroRTd) possesses a C-terminal Src homology 3-like domain commonly associated with DtxR family members. In the present study, we show that TroRTd is a manganese- and iron-dependent transcriptional repressor using Escherichia coli reporter constructs and in T. denticola. In addition, we demonstrate that although TroRTd possessing various C-terminal deletions maintain metal-sensing capacities, these truncated proteins exhibit reduced repressor activities in comparison with full-length TroRTd. Based upon these findings, we propose that TroRTd represents a novel member of the DtxR family of transcriptional regulators and is likely to play an important role in regulating both manganese and iron homeostases in this spirochaete.
Introduction
Treponema denticola, a member of the ‘red complex’, is a motile, asaccharolytic, anaerobic spirochaete that is common to the normal oral flora of humans (Socransky et al., 1998; Sela, 2001). Studies indicate that it represents one of approximately 60 treponemal species associated with subgingival plaque (Paster et al., 2001). The organism is thought to contribute significantly to the development of a number of acute and chronic periodontal diseases, including severe periodontitis, a polymicrobial infection of gingival tissues that if left untreated can lead to bone resorbtion and tooth loss (Loesche and Grossman, 2001). Although not generally considered to be life-threatening, the economic impact of periodontal disease in adult populations is substantial (Satcher, 2000; Loesche and Grossman, 2001). T. denticola has been shown to express a variety of adhesins, proteases, cytolysins and immunomodulatory factors (Sela, 2001). Many of these are predicted to play important role(s) in the pathogenesis of diseases caused by this organism.
In order to successfully colonize human hosts, bacteria must be able to acquire nutrients and overcome the constant pressure of host defences. Because metal ions are important for many biological processes, the ability of bacteria such as T. denticola to obtain essential metals from the environmental niches in which they reside is critical for their survival (Outten and O'Halloran, 2001; Blencowe and Morby, 2003; Cornelissen, 2003; Mulrooney and Hausinger, 2003; Rensing and Grass, 2003). Although human tissues, fluids and secretions are seemingly rich in nutrients, many elements, including Fe2+, Zn2+ and Mn2+, are often not freely available for use by bacteria (Ratledge and Dover, 2000; Kaplan, 2002; Braun, 2003). In order to cope with this problem, microorganisms have evolved both passive and active mechanisms for acquiring metals from these environments (Ferguson and Deisenhofer, 2004). In general, passive mechanisms are associated with high rates of transport and low metal-binding affinities, while active mechanisms show slower transport kinetics with higher binding affinities (Nikaido and Saier, 1992; Brown and Holden, 2002; Cornelissen, 2003; Faraldo-Gomez and Sansom, 2003). With regard to iron acquisition, T. denticola has been shown to bind both lactoferrin and haemin but does not produce siderophores (Scott et al., 1993; Chu et al., 1994; Xu et al., 2001; Xu and Kolodrubetz, 2002). At present, the mechanisms by which spirochaetes such as T. denticola obtain other essential metals from host environments remains poorly understood.
Consistent with the predicted metabolic diversity of T. denticola, genome analyses indicate the presence of a variety of putative metal transport-related loci (Seshadri et al., 2004; Gherardini et al., 2006). In addition to possessing homologues of Treponema pallidum ZnuABC and TP0972, T. denticola appears to possess eight ATP-binding cassette (ABC)-type transporters specifically devoted to the transport of Fe2+, implicating the importance of this metal to the organism (Seshadri et al., 2004; Gherardini et al., 2006; Desrosiers et al., 2007). Particularly interesting, however, is the presence of a Tro ABC-transport system similar to that identified in T. pallidum (Hardham et al., 1997; Gherardini et al., 2006). Comparative analyses of the T. pallidum and T. denticola tro operons have demonstrated that the genetic organization of the troABCDR locus in both species is identical and that a high degree of sequence similarity exists between the various gene products at the amino acid level (Gherardini et al., 2006). In the present study we utilize a combination of molecular genetic and biochemical approaches to demonstrate that T. denticola TroR (TroRTd) is a cation-dependent transcriptional repressor possessing structural and biochemical properties distinct from its T. pallidum orthologue.
Results
Identification of the tro operon in T. denticola
Analysis of the T. denticola ATCC 35405 genome sequence revealed the presence of a genetic locus demonstrating significant similarity to the T. pallidum tro operon. In T. denticola, the tro operon consists of five open reading frames: troA, troB, troC, troD and troR. This operon is predicted to encode the components of an ABC cation-transport system composed of TroA, a solute-binding protein; TroB, an ATPase; TroC and TroD, cytoplasmic membrane permeases; and TroR, a metal-dependent transcriptional regulator (Hardham et al., 1997). In contrast to T. pallidum, however, gpm encoding the glycolytic pathway enzyme, phosphoglycerate mutase is absent and the genes flanking the operons are not conserved (Fig. 1). Results of comparative analyses of the tro operons from these two organisms are shown in Table 1. The troABCD orthologues are highly similar in both species; however, unlike T. pallidum TroR (TroRTp), TroRTd harbours a 70-amino-acid C-terminal extension.
Fig. 1.
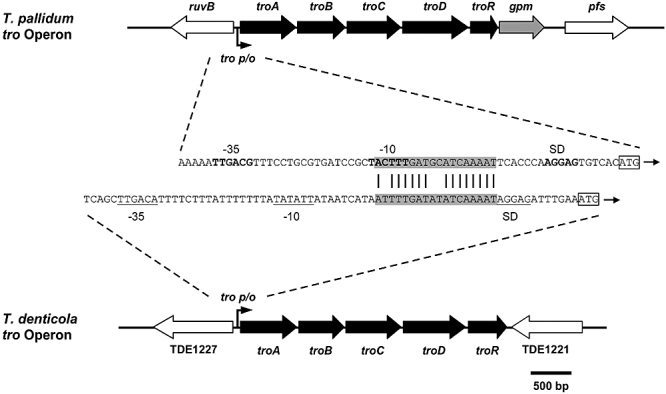
Genetic organization of the T. pallidum and the T. denticola tro operons and sequence alignment of the T. pallidum and T. denticola tro P/O regions located immediately upstream of the troA ORFs. troA is predicted to encode a periplasmic binding protein; troB is predicted to encode the ATP-binding component, while troC and troD are predicted to encode the membrane components of ABC transporters; troR is homologous to a number of genes encoding Gram-positive iron-activated repressor proteins including DtxR and SirR; gpm encodes the glycolytic pathway enzyme, phosphoglycerate mutase. The σ70-like promoters (−10 and −35 sequences) are indicated in bold (T. pallidum) or underlined (T. denitcola). The 18 bp operator motifs are highlighted in grey. Sequence identity between the operators is indicated by vertical lines. Putative ribosome binding sites are in bold (T. pallidum) or underlined (T. denitcola) and indicated by SD. The troA start codons are indicated by boxes.
Table 1.
Pairwise comparisons of T. denticola and T. pallidum open reading frames.
| T. denticola/T. pallidum | |||||
|---|---|---|---|---|---|
| Open reading frame | Amino acids | Predicted mass | Predicted pI | % Identity | % Similarity |
| troA | 312/308 | 34583/33570 | 5.58/6.21 | 54 | 73 |
| troB | 255/266 | 28572/29360 | 7.69/6.46 | 74 | 88 |
| troC | 304/298 | 32359/31540 | 10.14/10.26 | 61 | 79 |
| troD | 366/367 | 39760/38776 | 8.60/8.60 | 60 | 77 |
| troR | 222/153 | 25412/17122 | 9.15/8.47 | 55 | 74 |
Inspection of the region immediately upsteam of the T. denticola troA start codon revealed the presence of a σ70-like promoter sequence and an 18 bp region of dyad symmetry (Fig. 1). DNA sequence alignment of this region with that of T. pallidum revealed that 15/18 bp wasidentical to the previously described TroRTp-binding sequence. Interestingly, the relative locations of the putative T. denticola tro promoter and operator (tro-P/O) sequences differ relative to the T. pallidum tro-P/O sequences (Fig. 1). While the T. pallidum tro-P/O sequences have been determined to be overlapping (Posey et al., 1999), the predicted T. denticola tro-P/O sequences are separated by 10 bp (Fig. 1).
Bioinformatic analysis of TroRTd and purification of TroRTd-6×His
TroRTd is predicted to be a metal-dependent transcriptional regulator based on homology to the DtxR family of metalloregulators. This group of transcriptional regulators contain several Fe-activated repressor proteins, including Corynebacterium diptheriae DtxR, Mycobacterium tuberculosis IdeR and Streptomyces spp.; DesR, the Mn2+-activated regulators TroRTp and Bacillus subtilis MntR; and the Fe- and Mn2+-activated repressors Staphylococcus epidermidis SirR and Group A Streptococcus MtsR (Hill et al., 1998; Bates et al., 2005; Pennella and Giedroc, 2005). An alignment of the primary amino acid sequences of TroRTd with TroRTp, DtxR (Cd DtxR) and MntR (Bs MntR) is shown in Fig. 2. In general, Fe-responsive repressors have N-terminal domains (∼126 amino acids) containing helix–turn–helix DNA binding motifs (α2α3) and two metal ion binding sites (D'Aquino and Ringe, 2003). A short proline-containing region (∼26 amino acids) links this domain to the C-terminal region (∼76 amino acids) (Brennan and Matthews, 1989; Qiu et al., 1995; Schiering et al., 1995; D'Aquino and Ringe, 2003). TroRTd is predicted to have secondary structure similar to DtxR and to contain a comparable N-terminal DNA-binding domain followed by a helical dimerization domain. Additionally, similar to DtxR, TroRTd appears to possess an extended C-terminal Src homology 3-like (SH3-like) domain that is absent from TroRTp and MntR.
Fig. 2.
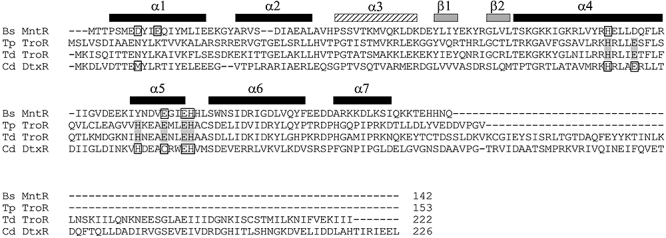
Primary sequence alignment of B. subtilis MntR (Bs MntR), C. diptheriae DtxR (Cd DtxR) and the T. pallidum (Tp) and T. denticola (Td) TroR proteins. The secondary structure of DtxR family metalloregulators is indicated above the sequence alignments: α-helices (α1, 2, 4, 5, 6) are shown in black, the DNA recognition helix (α3) is shown as a hatched line, β-strands (β1–2) are shown in grey and the proline-containing tether region (α7) is shown in black. The black boxes outline conserved metal co-ordination site residues in Bs MntR or Cd DtxR sequences. Putative TroR metal co-ordination site residues are highlighted in grey.
Two distinct metal co-ordination sites have been identified in DtxR: the ancillary cation binding site (site 1:H79, E83, H98) is thought to stabilize dimer formation and allow metal ion binding at the primary site (site 2: M10 and C102, E105, H106), which is the putative metalloregulatory site that is required for metal ion activation (Qiu et al., 1995; Ding et al., 1996; Love et al., 2003; Pennella and Giedroc, 2005). In comparison with DtxR, MntR and TroRTp, TroRTd demonstrates a high degree of conservation at both sites 1 and 2 with 5/7, 4/7 and 7/7 identical residues respectively (Fig. 2, shaded amino acids). Notably, an important difference at site 2 in both TroRTd and TroRTp is the presence of an asparagine (N10) corresponding to DtxR M10 and MntR D8. Additionally, the C102 residue of DtxR is substituted by E102 in MntR, TroRTd and TroRTp. All four proteins are predicted to have tether regions linking the N- and C-terminal regions of the proteins; this region is designated α7 in MntR and is proline-containing in DtxR and TroRTd (Fig. 2). The biological function of the DtxR C-terminal SH3-like domain is unclear; however, it has been proposed to modulate repressor activation through interactions with site 1 residues, and to contribute two ligands (E170 and Q173) involved in metal ion binding (D'Aquino and Ringe, 2003; Love et al., 2003; Chou et al., 2004). These two residues do not appear to be conserved in TroRTd (Fig. 2).
In order to obtain purified protein, T. denticola troR was cloned into the pBAD/HisA expression vector resulting in pPJB114. The His-tagged protein (TroRTd-6xHis) was overexpressed in Escherichia coli, producing a 26 kDa protein that localized to the insoluble fraction of the cell lysate. Following removal of soluble proteins, TroRTd was extracted from the insoluble pellet with 0.2% Sarkosyl and purified with a Nickel-agarose affinity column as described in the Experimental procedures section. This procedure yielded TroRTd-6xHis that was purified to apparent homogeneity as determined by SDS-PAGE (data not shown) and was used for antibody production. Following numerous attempts using a variety of conditions, including those previously used to purify TroRTp (Posey et al., 1999), we were unable to obtain TroRTd from the soluble fraction, and a minimum of 0.2% sarkosyl was required to obtain TroRTd from the insoluble fraction. Because of the insolubility of recombinant TroRTd, we were unable to conduct in vitro DNA binding experiments.
TroRTd expression negatively regulates both T. denticola and T. pallidum tro promoter activity
Studies by Posey et al. (1999) have previously demonstrated that purified TroRTp can bind to the tro-P/O region in a Mn2+-dependent manner. To assess the ability of TroRTd to bind to and affect transcription from the tro-P/O region, we employed lacZ-transcriptional fusion analyses in E. coli. The tro-P/O regions from both T. denticola and T. pallidum were cloned into pPBMB101, generating lacZ reporter constructs, pTDE100 and pTPA100 respectively. β-Galactosidase activity was measured in E. coli TOP10 cells harbouring pTDE100 or pTPA100 in the presence of either pPJB113 (expresses TroRTd) or pBAD/HisA (vector control). Immunoblot analysis of E. coli lysates showed that TroRTd was expressed from pPJB113, but not from the empty vector control (data not shown). Results demonstrated that β-galactosidase activity from strains DEN100 (harbouring pTDE100 and pBAD/HisA) and PAL100 (harbouring pPAL100 and pBAD/HisA) was high in the absence of TroRTd (Fig. 3A and B). In contrast, when TroR was expressed, β-galactosidase activity decreased significantly in strains DEN113 (harbouring pTDE100 and pPJB113; 96% repression) and PAL113 (harbouring pPAL100 and pPJB113; 88% repression), indicating that TroRTd exerted a negative regulatory effect on both of the tro promoters (Fig. 3A and B). These results are consistent with previous transcriptional fusion studies involving TroRTp (Posey et al., 1999) and suggest that TroRTd acts as a repressor of the tro operons.
Fig. 3.
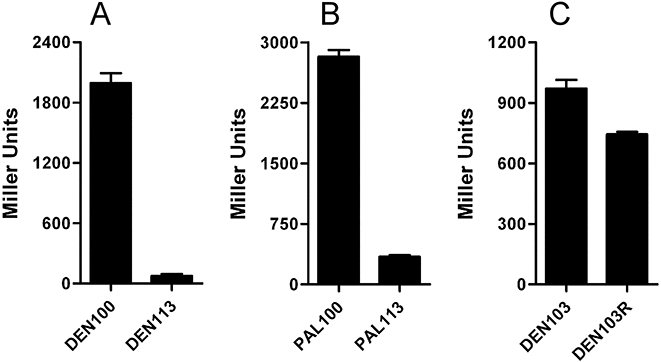
TroRTd expression negatively regulates both T. denticola and T. pallidum tro promoter activity. β-Galactosidase activity was measured from: (A) strains DEN100 and DEN113 harbouring pTDE100 (T. denticola troP/O-lacZ reporter) and either pBAD/HisA (control) or pPJB113 (expresses TroRTd); (B) strains PAL100 and PAL113 harbouring pPAL100 (T. pallidum troP/O-lacZ reporter) and either pBAD/HisA (control) or pPJB113 (expresses TroRTd); or (C) strains DEN103 and DEN103R harbouring pTDE103 (T. denticola troP/O-lacZ reporter with a disrupted operator region) and either pBAD/HisA (control) or pPJB113 (expresses TroRTd). Cultures were grown aerobically for 12 h in LBL media and β-galactosidase activity was determined as described by Miller (1972). Results represent the means and standard deviations of three independent experiments.
To confirm the importance of the putative T. denticola tro operator sequence for TroRTd binding, this sequence was mutated and assessed using a lacZ reporter fusion. Plasmid pTDE103 harbouring the T. denticola tro-P/O was constructed with four base changes in the putative operator sequence (AGCTTCATATTTCAAAAT, changes in bold, Table 2). Results of β-galactosidase assays using strains DEN103 and DEN103R demonstrated that promoter activity from pTDE103 decreased by only 23% in the presence of TroR (Fig. 3C). Additionally, the β-galactosidase activity from pTDE103 was approximately twofold lower than pTDE100 (Fig. 3A and C), suggesting that changes in the operator may affect other factors involved in transcription from the tro-P/O region. These findings further support that TroRTd is a negative regulator of the tro operon.
Table 2.
PCR primers, complementary oligonucleotides and qRT-PCR primers and probes used in this study.
| PCR primers | |
| OriCm-F1 | 5′-GATGCTAGATCTTCGAATTTCTGCCATTCATCCGC-3′ |
| OriCm-R1 | 5′-GATGCTCTGCAGACTAGTCGACCCGGGATCCTCTAGAAATATTTTATCTGATTAATAAGATG-3′ |
| LacZ-F2 | 5′-GATGCTCTGCAGCTCGAGTTCACACAGGAAACAGCTATGATAGATCCCGTCGTTTTACAACG-3′ |
| LacZ-R2 | 5′-GATGCTAGATCTTACTCAGGAGAGCGTTCACCG-3′ |
| Km-FNh | 5′-CCCAACGGTCTCACTAGAGCGAACCGGAATTGCCAGCTG-3′ |
| Km-RNc | 5′-CCCAACGGTCTCACATGCTCAGAAGAACTCGTCAAGAAG-3′ |
| TdtroR-F2 | 5′-CCCAACCGTCTCACATGAAAATCTCGCAAATTACAACCG-3′ |
| TdtroR-R3 | 5′-CCCAACCGTCTCAAGCTTATTATATTCCGCATTTTACTTTATCTAAACTTG-3′ |
| TdtroR-RA | 5′-CCCAACCGTCTCAAGCTTACTAAATAATAATTTTTTCGACAAATATATTTTTTAAAATCATCG-3′ |
| TdtroR-RB | 5′-CCCAACCGTCTCAAGCTTATTAATCTGTACATGTATATTCCTTTTG-3′ |
| TdtroR-RC | 5′-CCCAACCGTCTCAAGCTTATTATCTGGGTATCATTGCTCCATGGG-3′ |
| TdtroR-RD | 5′-CCCAACCGTCTCAAGCTTATTATTTAGGATGTCCTAAATATTTGTC-3′ |
| TdtroR-RH2 | 5′-CCCAACCGTCTCAAGCTTATTAATGATGATGATGATGATGAATAATAATTTTTTCGACAAATATATTTTTTAAAATCATCG |
| Complementary oligonucleotides | |
| Tdtrop-A | 5′-GATCGGGATTCAGCTTGACATTTTCTTTATTTTTTTATATATTATAATCATAATTTTGATATATCAAAATAGGAGATTTGAA-3′ |
| Tdtrop-B | 5′-TCGATTCAAATCTCCTATTTTGATATATCAAAATTATGATTATAATATATAAAAAAATAAAGAAAATGTCAAGCTGAATCCC-3′ |
| Tptrop-A | 5′-GATCTACTGAAAAATTGACGTTTCCTGCGTGATCCGCTACTTTGATGCATCAAAATTCACCCAAGGAGTGTCAC-3′ |
| Tptrop-B | 5′-TCGAGTGACACTCCTTGGGTGAATTTTGATGCATCAAAGTAGCGGATCACGCAGGAAACGTCAATTTTTCAGTA-3′ |
| Tdtrop-mutF3 | 5′-GATCGGGATTCAGCTTGACATTTTCTTTATTTTTTTATATATTATAATCATAAGCTTCATATTTCAAAATAGGAGATTTGAA-3′ |
| Tdtrop-mutR3 | 5′-TCGATTCAAATCTCCTATTTTGAAATATGAAGCTTATGATTATAATATATAAAAAAATAAAGAAAATGTCAAGCTGAATCCC-3′ |
| qRT-PCR primers and probes | |
| troA-F1 | 5′-GCATGGTTGCCGACATAGC-3′ |
| troA-R1 | 5′-CCCGCACCCATAAGAGCTT-3′ |
| troA-P1 | FAM-CACATTAACTTCATCTCCGCCGACAACTTT-TAMRA |
| flaA-F1 | 5′-TGACGGCTGGAGAGAATTGG-3′ |
| flaA-R1 | 5′-GGATAAAGCCTCAATTCCCTAGACT-3′ |
| flaA-P1 | FAM-ATGGAACAATCCTTCATACATTGCGAACGT-TAMRA |
FAM, 6-carboxyflourescein; TAMRA, 5-carboxytetramethylrhodamin.
TroRTd is a Mn2+- and Fe2+-dependent repressor
TroRTp has previously been shown to be a Mn2+-dependent repressor (Posey et al., 1999). In order to determine the metal responsiveness of TroRTd expressed in E. coli, we measured β-galactosidase activity in strains DEN113 and PAL113 in response to different metal ions. Both strains were grown in M9CG individually supplemented with 5 μM Cd2+, Co2+, Cu2+, Fe2+, Mn2+, Ni2+ and Zn2+. Addition of either Mn2+ or Fe2+ resulted in substantially decreased β-galactosidase activity from both DEN113 and PAL113, indicating that TroRTd repressor function was primarily responsive to these two metals (Fig. 4A and B). Similarly, the addition of Co2+ partially repressed promoter activity; however, no effect was observed upon addition of Cd2+, Ni2+, Zn2+, or in the absence of metals (Fig. 4A and B). These results are in agreement with previous reports demonstrating that TroRTp responds to Mn2+ in E. coli (Posey et al., 1999; Hazlett et al., 2003). However, our results demonstrated that TroRTd also appears to respond to Fe2+. This observation is consistent with other members of the DtxR family that are primarily known to be Fe-responsive. In contrast to TroRTp that has been reported to be Zn2+-responsive (Hazlett et al., 2003), TroRTd did not repress promoter activity in response to Zn2+ supplementation. Taken together, these findings suggest that TroRTd is unique in its metal-sensing capacities in comparison with TroRTp, and shares properties with both MntR- and DtxR-type metalloregulators.
Fig. 4.
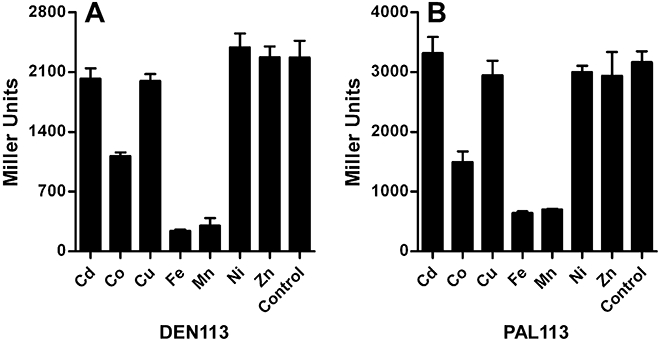
The TroRTd repressor responds to Mn2+ and Fe2+ in E. coli. (A) Strain DEN113 expressing pTDE100 (T. denticola troP/O-lacZ reporter) and pPJB113 (expresses TroRTd) or (B) strain PAL113 expressing pPAL100 (T. pallidum troP/O-lacZ reporter) and pPJB113 (expresses TroRTd) were grown aerobically overnight in M9CG media supplemented with various divalent cations (5 μM) or in unsupplemented M9CG media (control). β-Galactosidase activity of the overnight cultures was determined as described by Miller (1972). Results represent the means and standard deviations of three independent experiments.
T. denticola tro operon is negatively regulated by Mn2+ and Fe2+
To determine the response of the T. denticola tro operon to specific cations, spirochaetes were grown in NOS-EC media (chelated NOS media with EX-CYTE) supplemented with 5 μM Fe2+, Mn2+ or Zn2+. At 24 and 48 h post inoculation, RNA was harvested and troA transcript levels were quantified by qRT-PCR. Relative amounts of the troA transcripts from spirochaetes grown in the presence of cations were then compared with transcript levels from unsupplemented controls. All reactions were normalized to flaA as an internal control (Frederick et al., 2008). At 24 h, a fourfold decrease in troA transcript levels was observed in the presence of Mn2+ while troA expression from cultures supplemented with Fe2+ or Zn2+ were similar to control levels (Fig. 5A). Interestingly, at 48 h, a three- to fourfold decrease in troA transcript levels was observed in the presence of Fe2+ and Mn2+ respectively, while expression from cultures supplemented with Zn2+ remained unchanged (Fig. 5A). Based upon these results and consistent with the E. coli lacZ-transcriptional fusion assays, it appears that the T. denticola tro operon is negatively regulated by Mn2+ and Fe2+, but not Zn2+.
Fig. 5.
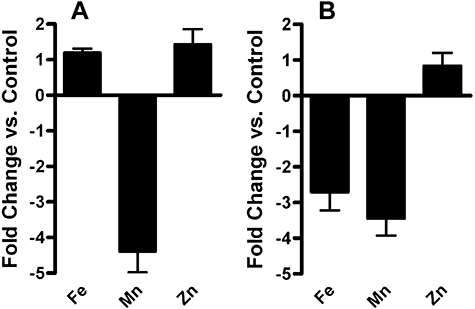
T. denticola tro operon is negatively regulated by Mn2+ and Fe2+. Expression of troA was analysed by qRT-PCR. RNA extracted from T. denticola grown in NOS-EC media supplemented with 5 μM Fe2+, Mn2+ or Zn2+ was quantified at (A) 24 h or (B) 48 h post inoculation using specific primers and probes with the Taqman system. Values have been normalized to the internal control, flaA. Fold changes are relative to spirochaetes grown in NOS-EC media lacking metal supplmentation (control). Results represent the means and standard deviations of three independent experiments performed in quadruplicate.
TroRTd proteins lacking the SH3-like domain demonstrate decreased repression of T. denticola P/Otro-lacZ, but not T. pallidum P/Otro-lacZ
Both TroRTp and B. subtilis MntR lack the C-terminal regions present in many members of the DtxR family of metalloregulators; however, both demonstrate repressor activity in the absence of this domain. In order to assess the importance of the TroRTd C-terminal SH3-like domain with respect to repressor activity, full-length and truncated troR alleles were cloned into pBAD/HisA and expressed in E. coli. A schematic representing the C-terminal TroRTd truncations is shown in Fig. 6A. Immunoblot analysis demonstrated that all of the truncated proteins were expressed in E. coli at the expected sizes and at similar levels (Fig. 6B). Removal of the C-terminal region of TroRTd did not improve solubility of the recombinant proteins (data not shown). β-Galactosidase assays were conducted in E. coli strains harbouring the tro-P/O lacZ-transcriptional fusion constructs, pTDE100 or pTPA100, along with plasmids expressing full-length TroRTd (strain DEN113) or various truncated derivatives as follows: TroRTdΔ157−222 (strain DEN113A), TroRTdΔ147−222 (strain DEN113B), TroRTdΔ137−222 (strain DEN113C) and TroRTdΔ127−222 (strain DEN113D). Results for the T. denticola tro-P/O region demonstrated significantly decreased β-galactosidase activity in strain DEN113 (96% repression), compared with the control strain DEN100, as expected. In contrast, expression of the truncated TroRTd proteins in strains DEN113A, DEN113B and DEN113C demonstrated increased β-galactosidase activity, corresponding to 64%, 58% and 43% repression respectively, indicating decreased repressor function. Expression of TroRTdΔ127−222, a deletion extending into the proline-containing region of the protein, in strain DEN113D resulted in maximal β-galactosidase activity (similar to DEN100), indicating virtually complete loss of repressor function (Fig. 6C). These results suggest that the TroRTd SH3-like domain is important for repressor activity and presumably binding to the T. denticola tro-P/O region.
Fig. 6.
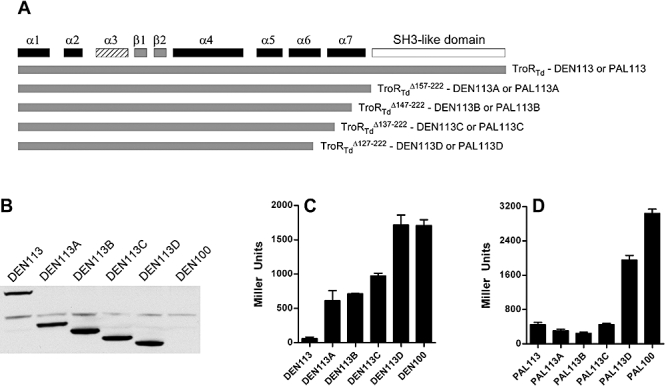
TroRTd deletion mutants exhibit variable repressor activity in E. coli.
A. Physical maps of wild type and C-terminally truncated TroRTd proteins. TroRTd proteins are represented as grey lines and amino acid deletions are indicated. Names of the strains expressing the various TroRTd deletion constructs in the presence of pTDE100 (T. denticola troP/O-lacZ reporter) or pPAL100 (T. pallidum troP/O-lacZ reporter) are also indicated. DtxR secondary structure is indicated: α-helices (α1, 2, 4, 5, 6) are shown in black, the DNA recognition helix (α3) is shown as a hatched line, β-strands (β1–2) are shown in grey, the proline-containing tether region (α7) is shown in black and SH3-like domain is shown in white. Full-length and mutant troR alleles were PCR-amplified from T. denticola ATCC 35405 chromosomal DNA and cloned into the NcoI and HindIII restriction sites of pBAD/HisA to facilitate the expression of TroRTd proteins.
B. Immunoblot analysis of full-length and truncated TroRTd proteins expressed by: DEN113, DEN113A, DEN113B, DEN113C, DEN113D and DEN100. Effect of C-terminal deletion mutations on the expression of (C) pTDE100 or (D) pTPA100 reporter constructs. E. coli TOP10 cells harbouring both a TroRTd expression construct and a tro-P/O-lacZ reporter plasmid were grown aerobically overnight in LBL media. β-Galactosidase activity of the 12 h cultures was determined as described by Miller (1972). Results represent the means and standard deviations of three independent experiments.
Transcriptional fusion analyses with the T. pallidum tro-P/O region in E. coli revealed that expression of full-length TroRTd, TroRTdΔ157−222, TroRTdΔ147−222 or TroRTdΔ137−222 resulted in maximal repressor activity. As shown in Fig. 6D, expression of full-length TroRTd in strain, PAL113 resulted in decreased β-galactosidase activity corresponding to 85% repression compared with control strain PAL100. Similarly, β-galactosidase activity decreased in strains PAL113A, PAL113B and PAL113C (90%, 92% and 85% repression respectively), indicating intact repressor function. In contrast, TroRTdΔ127−222 expression in strain PAL113D resulted in only slightly decreased β-galactosidase activity corresponding to partial repressor activity (36%) (Fig. 6D). Taken together, these results suggest that the SH3-like domain of TroRTd is required for maximal repression of the T. denticola tro-P/O; however, this C-terminal extension does not appear to be critical for repression of the T. pallidum tro-P/O. In addition, it is possible that the differences observed in the positions of the −10 and −35 sequences within the T. denticola and T. pallidum tro-P/O regions may affect repressor binding.
T. denticola TroRTdΔ157−222 maintains metal specificity
Unlike other members of the DtxR family of metalloregulators, both TroRTp and B. subtilis MntR are manganese-responsive and lack C-terminal SH3-like domains. In order to assess the importance of the TroRTd C-terminal domain with respect to metal specificity, β-galactosidase activity from strains DEN113A and PAL113A was measured in response to metal ion supplementation. In the presence of TroRTdΔ157−222 expression, β-galactosidase activity from both DEN113A and PAL113A decreased in response to Mn2+ and Fe2+ and also decreased slightly in response to Co2+ (Fig. 7A and B). β-Galactosidase activity remained unchanged in the presence of Cd2+, Cu2+, Ni2+ and Zn2+ (Fig. 7A and B). These results indicated that similar to full-length TroRTd, TroRTdΔ157−222 maintained repressor activity in the presence of Mn2+, Fe2+ and Co2+. Based on these results, it appears that the C-terminal domain of TroRTd is not important for metal specificity and the role of this region of the protein in metal binding, if any, remains unclear.
Fig. 7.
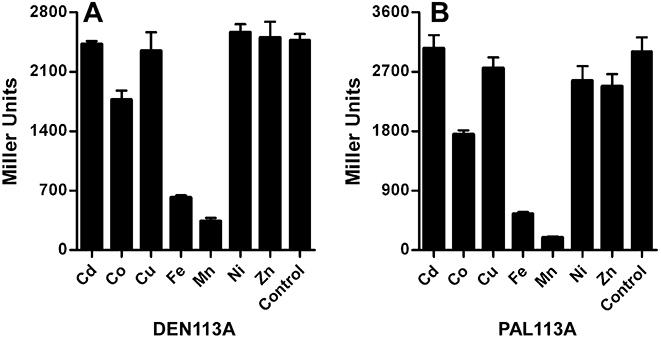
TroRTdΔ157−222 repressor responds to Mn2+ and Fe2+ in E. coli. (A) Strain DEN113A harbouring pTDE100 (T. denticola troP/O-lacZ reporter) and pPJB113A (expresses TroRTdΔ157−222) or (B) strain PAL113A harbouring pPAL100 (T. pallidum troP/O-lacZ reporter) and pPJB113A (expresses TroRTdΔ157−222) were grown aerobically overnight in M9CG assay media supplemented with various divalent cations (5 μM) or in unsupplemented M9CG media (control). β-Galactosidase activity of the overnight cultures was determined as described by Miller (1972). Results represent the means and standard deviations of three independent experiments.
Discussion
As intracellular concentrations of both essential and nonessential metals can be toxic to bacterial cells, their uptake is tightly regulated by metal-dependent, transcriptional repressor proteins (Outten and O'Halloran, 2001; Patzer and Hantke, 2001; Glasfeld et al., 2003). These regulatory proteins (e.g. DtxR and Fur families) serve as direct links between intracellular metal levels and the expression of appropriate uptake, efflux and storage systems. In general, metal cations bind reversibly to a metal binding site altering the conformation of the repressor to effect changes in gene expression. The affinity of metal ions for this site serves to maintain the intracellular concentration of metals within strict, biologically acceptable limits in a cell. The selectivity of the site is essential to ensure that other metals do not interfere with these homeostasis mechanisms (Guedon and Helmann, 2003). In addition to mediating physiological responses to environmental levels of metals, it is well established that metal-dependent repressors, such as DtxR and Fur, modulate the virulence phenotypes of a number of important human pathogens (Jakubovics and Jenkinson, 2001; Guedon and Helmann, 2003).
DxtR and its homologues represent a distinct family of metal-dependent repressors that primarily regulate the expression of genes involved in both metal uptake and virulence. In Corynebacterium diphtheria, genes encoding iron transport proteins are negatively regulated by DtxR (Tao et al., 1994; Schmitt, 1999). As intracellular concentrations of ferrous iron increase, DtxR becomes iron-loaded, dimerize, and binds to a palindromic target sequence within DtxR-regulated promoters, repressing the transcription of downstream genes (Qiu et al., 1995; Tao et al., 1995). The X-ray crystal structure of DtxR has been solved and has led to a better understanding of the interaction of DtxR with its metal ligands (Qiu et al., 1995; D'Aquino et al., 2007). Apo-DtxR contains two metal binding sites: binding site 1, which co-ordinates a metal between H79, E83 and H98 (C-terminal SH3 domain residues E170 and Q173 may also contribute), is considered an auxiliary binding site that may act cooperatively to enhance binding of a second metal ion to the primary binding site, binding site 2 (Qiu et al., 1995; Pennella and Giedroc, 2005; D'Aquino et al., 2007). This binding site is required for metal-dependent DtxR repressor activity and co-ordinates the metal between M10, C102, E105 and H106. While Ni2+, Co2+ and Mn2+ have been shown to activate DtxR in vitro, Fe2+ is the specific activator in vivo (Ikeda et al., 2005; Pennella and Giedroc, 2005).
A much more distant member of the family is MntR, a DtxR homologue from B. subtilis that has been shown to be selective for Mn2+ and regulates the expression of two manganese transporters under low Mn2+ conditions (Glasfeld et al., 2003; Kehres and Maguire, 2003; Pennella and Giedroc, 2005). Unlike DtxR, MntR binds Mn2+ in a binuclear manganese cluster, in which two metal ions are co-ordinated between D8, E11, H77, E99, E102 and H103 (see Fig. 2) This unique configuration confers selectivity for Mn2+ over Fe2+. Recent studies have demonstrated that the D8 residue confers some of the Mn2+ specificity in MntR, as changing that amino acid to M8 (DtxR-like) relaxes the specificity for Mn2+ (Guedon and Helmann, 2003; Golynskiy et al., 2005). While DtxR and MntR have some key active site amino acid residues in common (e.g. H79, E105 and H106), MntR lacks the SH3-like domain, has different amino acids in key metal binding site residues and functions in a different cytosolic environment so that the two proteins function/respond quite differently in vivo (Guedon and Helmann, 2003).
Other DtxR family members include the Fe- and Mn2+-responsive repressors group A Streptococcus MtsR and S. epidermidis SirR. Both MtsR and SirR have been shown to possess the majority of the conserved metal co-ordination sites identified in DtxR and are predicted to have similar structural domains (Hill et al., 1998; Bates et al., 2005). SirR, however, is most similar (38%) at the amino acid level to T. pallidum TroR (Hill et al., 1998), a distinct member of this group that has been identified and partially characterized. TroRTp has structural features that suggest that it may represent a functional intermediate of the Fe2+-dependent DtxR and the Mn2+-dependent MntR (Hardham et al., 1997; Posey et al., 1999; Hazlett et al., 2003). For example, TroRTp is similar to MntR in overall protein structure as both proteins lack the characteristic DtxR SH3-like domain and have some amino acid residues that correspond to key residues involved in MntR's binuclear Mn2+ metal pocket (see Fig. 2, shaded amino acids). Interestingly, TroRTp also has residues that correspond to key residues in DtxR sites 1 and 2 (see Fig. 2, shaded amino acids). Experimentally, TroRTp has been shown to be Mn2+-specific when assayed using purified protein in gel mobility shift assays using the tro operon-P/O as a target sequence (Posey et al., 1999). In contrast, Hazlett et al. (2003) showed that recombinant TroRTp responds more efficiently to Zn2+ than Mn2+, using troP/O-lacZ reporter constructs in E. coli. Fe2+ did not affect TroRTp binding to the Tro P/O in either assay system. It is interesting to note that while TroRTp effectively regulates the Mn/Zn/Fe TroABCD transport system (Posey et al., 1999; Hazlett et al., 2003), it does not appear to regulate the specific, high-affinity Zn2+ uptake system (Znu operon) in T. pallidum (Desrosiers et al., 2007). As these studies on TroRTp were done in different strains and genetic backgrounds, it is difficult to determine the exact metal specificity of this protein. Therefore, the exact metal specificity of TroRTp in T. pallidum remains in question.
In the present study, we identified the troABCDR operon in the T. denticola ATCC 35405 genome and characterized the activity of TroRTd as a metal-dependent repressor that responded primarily to Mn2+ and Fe2+. Based on previous studies with TroRTp, we predicted that TroRTd would bind to the putative TroR binding sequence upstream of the T. denticola tro operon to repress expression of these genes. Our results demonstrated that TroRTd repressed transcription of both T. denticola and T. pallidum tro-P/O lacZ reporter gene fusions in E. coli. In addition, mutation of the putative TroRTd binding sequence upstream of troA resulted in a considerable decrease in repressor activity. These findings provide evidence that TroRTd is a negative regulator of the tro operon. Interestingly, significant repression was demonstrated in response to Mn2+ or Fe2+, and partial repression was observed in response to Co2+ while Zn2+ had no effect on gene expression. Consistent with these findings, qRT-PCR analyses conducted with T. denticola grown under metal-deplete or -replete conditions demonstrated that troA expression decreased in the presence of Mn2+ and Fe2+, but not Zn2+. Taken together these results suggest that TroRTd has different metal specificity than TroRTp (Mn2+ or Zn2+) or MntR (Mn2+) and may be more similar to DtxR, SirR and MtsR.
Previous studies by Guedon and Helmann (2003) have demonstrated that, as important as the active site residues are in metal specificity, the cytosolic metal environment affects the metal-dependent response of a particular repressor in different host backgrounds. When they ‘converted’ DtxR to a Mn-dependent repressor by introducing M10D and C102E (MntR-like) and expressed the mutant protein in B. subtilis, DtxR (M10D/C102E) as predicted responded to Mn2+. However, when intracellular concentrations of Fe2+ were increased by deregulating iron uptake in a B. subtilis fur mutant, DtxR (M10D/C102E) also responded to Fe2+ (Guedon and Helmann, 2003). Thus, they showed that while key residues within the binding site affect metal specificity, the concentration of specific metals within a particular cell also influence the regulatory capacity of a given metal-dependent repressor protein. Interestingly, Posey and Gherardini (2000) have measured and compared the intracellular levels of Mn2+ (0.79 and 0.24 nmol mg−1 of protein respectively) and Fe2+ (4.2 and 3.5 nmol mg−1 of protein respectively) in E. coli and T. denticola. While there are some differences in the intracellular levels of these metals (particularly Mn) under the conditions tested, it is interesting to note that the data presented in this report, as measured in E. coli (lacZ reporter assays) and T. denticola (qRT-PCR), indicate that TroRTd responded similarly to Mn2+ and Fe2+ in both cytosolic backgrounds.
Secondary structure predictions and amino acid comparisons of TroRTd with DxtR, MntR and TroRTp revealed the presence of conserved N-terminal helical domains as well as conserved metal binding site residues. Additionally, TroRTd had a C-terminal SH3-like domain. The importance of the C-terminal extension present on TroRTd was investigated using a number of C-terminally truncated TroR proteins. Our results demonstrated that loss of the C-terminal region of TroRTd affected repressor function at the T. denticola tro-P/O, but not at the T. pallidum tro-P/O. These results are consistent with the fact that TroRTp naturally lacks this C-terminal extension. It is possible that the differences observed in the tro-P/O regions of these two treponemes may reflect their different-sized TroRs; i.e. each species has optimized its P/O region for TroR binding. Conversely, the different-length TroRs may be produced to compensate for the altered tro-P/O regions. In any case, it appears that the TroRTd requires an extended C-terminal region for maximal repression of the T. denticola tro-P/O. Further results indicated that the proline-containing region may be necessary for TroR to function at both T. denticola and T. pallidum tro-P/O regions as a deletion affecting this region-abrogated repression. This domain may function in protein–protein interactions that may be important for subsequent DNA binding; however, this remains to be experimentally determined. The metal specificity of C-terminally truncated TroR remained unchanged, suggesting that this domain was not involved in metal binding. Similar to DtxR, the specific function of TroRTd C-terminal domain remains unclear.
In summary, we have demonstrated that TroRTd is a Mn2+- and Fe2+-dependent transcriptional regulator that negatively regulates expression of the T. denticola tro operon. Structural predictions show that TroRTd possesses conserved metal binding site residues as well as an SH3-like domain. Overall, these findings suggest that TroRTd is a novel member of the DtxR family of transcriptional regulators and would be predicted to play a critical role in regulating Mn2+ and Fe2+ levels in T. denticola. Further studies will be required to determine the role of the T. denticola tro operon in metal ion homeostasis in natural infections. Studies are ongoing to obtain soluble TroR for further biochemical and crystallography analyses to gain further insight into the structure and function of TroRTd.
Experimental procedures
Bacterial strains, plasmids, chemical reagents and growth conditions
The bacterial strains and plasmids used in this study are described in Table 3. Puratronic metals were obtained from Alfa Aesar: Cd as Cadmium sulphate, 99.999%; Co as Cobalt (II) sulphate 99.999%; Cu as Copper (II) sulphate, 99.999%; Fe as Iron (II) sulphate, 99.999%; Mn as Manganese (II) sulphate, 99.999%; Ni as Nickel (II) sulphate, 99.9985%; Zn as Zinc sulphate, 99.999%; Magnesium sulphate, 99.997%; Calcium chloride, 99.9965%. Tris(2-carboxyethyl)-phosphine hydrochloride (TCEP) was obtained from Pierce. All other chemicals were obtained from Sigma. E. coli strains were grown at 37°C on Difco LB-Lennox (LBL) agar or in LBL broth unless otherwise stated. For metal specificity assays, E. coli strains were grown in Difco M9 Minimal Salts supplemented with 0.5% w/v Bacto Casamino Acids and 0.2% v/v glycerol (M9CG). When appropriate, antibiotics were used at the following concentrations: 100 μg ml−1 ampicillin, 25 μg ml−1 chloramphenicol and 25 μg ml−1 kanamycin (Km).
Table 3.
Bacterial strains and plasmids used in this study.
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strain | ||
| T. denticola | ||
| ATCC 35405 | Type strain; human oral isolate | Chan et al. (1993) |
| E. coli | ||
| TOP10 | High-efficiency transformation: Aps, Cms, Kms | Invitrogen |
| LYS114 | TOP10 (pLysE + pPJB114): Apr, Cmr | This study |
| DEN100 | TOP10 (pTDE100 + pBAD/HisA): Apr, Kmr | This study |
| DEN113 | TOP10 (pTDE100 + pPJB113): Apr, Kmr | This study |
| DEN103 | TOP10 (pTDE103 + pBAD/HisA): Apr, Kmr | This study |
| DEN103R | TOP10 (pTDE103 + pPJB113): Apr, Kmr | This study |
| DEN113A | TOP10 (pTDE100 + pPJB113A): Apr, Kmr | This study |
| DEN113B | TOP10 (pTDE100 + pPJB113B): Apr, Kmr | This study |
| DEN113C | TOP10 (pTDE100 + pPJB113C): Apr, Kmr | This study |
| DEN113D | TOP10 (pTDE100 + pPJB113D): Apr, Kmr | This study |
| PAL100 | TOP10 (pTPA100 + pBAD/HisA): Apr, Kmr | This study |
| PAL113 | TOP10 (pTPA100 + pPJB113): Apr, Kmr | This study |
| PAL113A | TOP10 (pTPA100 + pPJB113A): Apr, Kmr | This study |
| PAL113B | TOP10 (pTPA100 + pPJB113B): Apr, Kmr | This study |
| PAL13C | TOP10 (pTPA100 + pPJB113C): Apr, Kmr | This study |
| PAL113D | TOP10 (pTPA100 + pPJB113D): Apr, Kmr | This study |
| Plasmids | ||
| pACYC184 | Cloning vector; p15A ori: Cmr, Tcr | New England Biolabs |
| pBAD/His/lacZ | Arabinose inducible LacZ expression vector; pMB1 ori: Apr | Invitrogen |
| pUni/V5-His A | Echo Cloning vector; R6K ori: Kmr | Invitrogen |
| pBAD/His A | Arabinose inducible expression vector; pMB ori: Apr | Invitrogen |
| pLysE | Constitutively expresses T7 lysozyme; p15A ori: Cmr | Invitrogen |
| pPBMB100 | Promoterless lacZ reporter vector; p15A ori: Cmr | This study |
| pPBMB101 | Promoterless lacZ reporter vector; p15A ori: Kmr | This study |
| pTPA100 | T. pallidum troP/O-lacZ reporter construct; pPBMB101 derivative: Kmr | This study |
| pTDE100 | T. denticola troP/O-lacZ reporter construct; pPBMB101 derivative: Kmr | This study |
| pTDE103 | T. denticola troP/O-lacZ reporter construct; mutated operator sequence; pPBMB101 derivative: Kmr | This study |
| pPJB113 | TroRTd expression construct; pBAD/His A derivative: Apr | This study |
| pPJB113A | TroRTdΔ157−222 expression construct; pBAD/His A derivative: Apr | This study |
| pPJB113B | TroRTdΔ147−222 expression construct; pBAD/His A derivative: Apr | This study |
| pPJB113C | TroRTdΔ137−222 expression construct; pBAD/His A derivative: Apr | This study |
| pPJB113D | TroRTdΔ127−222 expression construct; pBAD/His A derivative: Apr | This study |
| pPJB114 | TroRTd-6×His expression construct; pBAD/His A derivative: Apr | This study |
Treponema denticola ATCC 35405 was grown at 37°C under anaerobic conditions (4% H2, 5% CO2, 91% N2) in NOS media (ATCC medium 1494) supplemented with 1% EX-CYTE (Millipore; NOS-E media) instead of rabbit serum. Growth rates in NOS-E media were found to be similar to those in NOS media. Metal-deplete NOS-E media (NOS-EC media) was prepared by chelating NOS-E media two times with 2.5% (w/v) Chelex 100 under anaerobic conditions. NOS-EC media was then supplemented with 1 mM MgSO4 and 0.1 mM CaCl2 and filter-sterilized.
Recombinant DNA techniques
DNA manipulations were performed using standard methods. PCR was performed using the Expand High Fidelity PCR System (Roche Applied Science). PCR and restriction enzyme-digested products were purified using a QIAquick Gel Extraction Kit (Qiagen). Plasmids were purified using a QIAprep Spin Miniprep Kit (Qiagen). Ligation reactions were performed using a Fast-Link Quick Ligase Kit (Epicentre Technologies). Chemically competent E. coli TOP 10 cells were transformed as per manufacturer's instructions (Invitrogen). DNA sequencing was performed by ACGT.
Construction of promoterless lacZ reporter vectors
A 1.96 kb fragment harbouring the p15A origin of replication and chloramphenicol-resistance marker from pACYC184 in addition to a 3.38 kb fragment containing the lacZ locus from pBAD/His/lacZ were PCR-amplified using the OriCm-F1/OriCm-R1 and LacZ-F2/LacZ-R2 primer pairs respectively (the PstI and BglII sites in the linker regions are underlined; Table 2). The amplified products were then restriction-digested and ligated to one another to produce pPBMB100. A 0.80 kb fragment containing the kanamycin-resistance marker from pUni/V5-His A was subsequently amplified via PCR using the Km-FNh/Km-RNc primer pair (the BsaI/NheI and BsaI/NcoI sites in the linker regions are underlined; Table 2) and cloned into pPBMB100 digested with NheI and NcoI to yield pPBMB101.
Construction of troP/O-lacZ reporter vectors
Complementary oligonucleotides (Tdtrop-A/Tdtrop-B and Tptrop-A/Tptrop-B) encoding the T. denticola and T. pallidum tro-P/O regions were mixed at concentrations of 5 μM each, heated to 95°C for 5 min and then allowed to cool to room temperature (RT) to facilitate annealing (the 5′-BamHI and 3′-XhoI 4 bp overhangs incorporated into the ends of oligonucleotides are underlined; Table 2). The resulting double-stranded DNA fragments were then cloned into pPBMB101 digested with BamHI and XhoI yielding pTDE100 and pTPA100 respectively (Table 3). Complementary oligonucleotides (Tdtrop-mutF3/Tdtrop-mutR3) encoding the T. denticola tro-P/O with four changes in the predicted operator sequence (see bolded bases in Table 2) were annealed and cloned as described above resulting in pTDE103. Constructs were confirmed by sequencing.
Construction of TroRTd expression vectors
Full-length, truncated and 6×His-tagged troR alleles were amplified from frozen stocks of T. denticola ATCC 35405 using the following primer pairs: TdtroR-F2/TdtroR-R3, TdtroR-F2/TdtroR-RA, TdtroR-F2/TdtroR-RB, TdtroR-F2/TdtroR-RC, TdtroR-F2/TdtroR-RD and TdtroR-F2/TdtroR-RH2 (the BsmBI/NcoI and BsmBI/HindIII sites in the linker regions are underlined; Table 2). The amplified products were then cloned into pBAD/HisA digested with NcoI and HindIII yielding pPJB113, pPJB113A, pPJB113B, pPJB113C, pPJB113D and pPJB114 respectively (Table 3). Constructs were confirmed by sequencing.
β-Galactosidase assays
Combinations of the TroRTd expression and lacZ reporter constructs were cotransformed into E. coli Top10 cells (Table 3). In order to assay promoter activities under metal-rich conditions, cotransformants were inoculated (1/3000) into LBL broth plus antibiotics from overnight cultures and grown for 12 h at 37°C with shaking. In order to assay promoter activities under metal-deplete conditions, cotransformants were inoculated (1/3000) into M9CG plus antibiotics supplemented with 5 μM Cd2+, Co2+, Cu2+, Fe2+, Mn2+, Ni2+ or Zn2+ from overnight cultures and grown for 12 h at 37°C with shaking. TroRTd expression was found to be sufficiently leaky from the araBAD promoters of pPJB113, pPJB113A, pPJB113B, pPJB113C, pPJB113D during these assays and did not warrant the use of l-arabinose as an inducer. β-Galactosidase assays were performed as previously described by Miller (1972).
T. denticola RNA purification, cDNA synthesis and qRT-PCR
For metal regulation assays, T. denticola was grown to late log phase in NOS-E media, pelleted by centrifugation (2800 g, 10 min) and then re-suspended in NOS-EC media. NOS-EC media supplemented with 5 μM Fe2+, Mn2+ or Zn2+ was inoculated with ∼107 spirochaetes ml−1 and RNA was harvested at 24 and 48 h. qRT-PCR experiments were performed as previously described for Borrelia burgdorferi (Burtnick et al., 2007). Briefly, T. denticola RNA was extracted using TRI-Reagent (Sigma) as described by the manufacturer. RNA was treated with RNase-free DNase (Qiagen) and purified using the RNeasy miniprep kit (Qiagen). SuperScript III (Invitrogen) was used to synthesize first-strand cDNA following manufacturer's instructions. qRT-PCR primers and probes specific for troA and flaA were designed using Primer Express 1.0 and are shown in Table 2. Reactions were performed in a total volume of 20 μl using TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA), 1–2 ng of first-strand cDNA, 300 nm forward and reverse primers and 250 nm probe. All reactions were carried out on the ABI PRISM 7900HT Sequence Detection System (Applied BioSystems) using a PCR cycle of 2 min at 50°C, 10 min at 95°C, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min. Each transcript was normalized by comparison with the constitutively expressed, internal control flaA (Frederick et al., 2008). Three individual assays were performed in quadruplicate. Fold changes were calculated using the ΔΔCT method.
TroRTd purification and antibody production
For purification of recombinant protein, TroRTd-6×His was overexpressed from E. coli LYS114 (Table 3). Bacteria were grown in 500 ml of LBL broth plus antibiotics at 37°C with aeration. When the culture reached an OD600 of 0.8, protein expression was induced using 0.02% l-arabinose for 2 h. Cells were harvested by centrifugation, re-suspended in Lysis Buffer A (B-PER plus 2 μg ml−1 DNase I; Pierce) and incubated for 10 min at RT. The insoluble material was pelleted by centrifugation (27 000 g, 15 min, 4°C), re-suspended in Lysis Buffer B (B-PER) and incubated for 10 min at RT. The insoluble material was pelleted, re-suspended in Solubilization Buffer [50 mM Tris (pH 8.0), 50 mM NaCl, 10 mM Imidazole, 0.25 mM TCEP and 0.2% Sarkosyl] and gently agitated for 60 min at 4°C. The remaining insoluble material was removed by centrifugation, the supernatant was filter-sterilized and allowed to batch-bind to HIS-Select Nickel Affinity Gel (Sigma) prior to loading onto a gravity fed column. The flow through was collected and applied to the column a second time, followed by washing of the column with Wash Buffer [50 mM Tris (pH 8.0), 300 mM NaCl, 10% glycerol, 40 mM Imidazole and 0.25 mM TCEP]. Protein was eluted with Elution Buffer [50 mM Tris (pH 8.0), 50 mM NaCl, 10% glycerol, 300 mM Imidazole and 0.25 mM TCEP]. Fractions were analysed by SDS-PAGE and those containing TroRTd were pooled, concentrated and stored in Storage Buffer [20 mM Tris (pH 8.0), 50 mM NaCl, 10% glycerol and 0.25 mM TCEP]. Protein concentrations were determined using a BCA protein assay kit (Pierce). Purified protein was used to raise anti-TroRTd polyclonal antiserum in female New Zealand White rabbits at Cocalico Biologicals using a standard protocol.
SDS-PAGE and immunoblot analysis
Whole-cell lysates were prepared by pelleting 1 ml each of the E. coli cultures and re-suspending the cells in 200 μl of 1× SDS-PAGE sample buffer prior to heating at 100°C for 10 min. Once cooled, 1 μl of DNase (10 000 U ml−1; Pierce) was added to each sample to decrease viscosity. Treated lysates were then electrophoresed on 4–20% Express Gels (ISC BioExpress). Proteins were electrophoretically transferred to nitrocellulose membranes (0.45 μm pore size; Invitrogen). Immunoblot analysis was performed as follows at RT: membranes were blocked with TBS-TS [20 mM Tris-HCl (pH 7.5), 500 mM NaCl, 0.1% Tween 20 and 3% skim milk] for 60 min, followed by incubation for 1 h with a 1/2000 dilution of the primary antibody (anti-TroR polyclonal antiserum) in TBS-T [20 mM Tris-HCl (pH 7.5), 500 mM NaCl and 0.1% Tween 20]. Membranes were washed three times with TBS-T, followed by incubation for 1 h with a 1/5000 dilution of the secondary antibody (anti-rabbit IgG-HRP conjugate; Sigma) in TBS-T. Membranes were washed three times with TBS-T and blots were visualized with ECL Plus Western Blotting Detection Reagents (GE Healthcare) as per manufacturer's instructions.
Acknowledgments
We would like to thank Patricia Rosa and Mollie Jewett for critical review of this manuscript. We would like to thank Dan Sturdevant for Taqman primer and probe design and Brandon Kramer for help with NOS-E media. We are grateful to Gary Hettrick and Anita Mora for graphics support. This research was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases.
References
- Bates CS, Toukoki C, Neely MN, Eichenbaum Z. Characterization of MtsR, a new metal regulator in group A streptococcus, involved in iron acquisition and virulence. Infect Immun. 2005;73:5743–5753. doi: 10.1128/IAI.73.9.5743-5753.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe DK, Morby AP. Zn(II) metabolism in prokaryotes. FEMS Microbiol Rev. 2003;27:291–311. doi: 10.1016/S0168-6445(03)00041-X. [DOI] [PubMed] [Google Scholar]
- Braun V. Iron uptake by Escherichia coli. Front Biosci. 2003;8:s1409–s1421. doi: 10.2741/1232. [DOI] [PubMed] [Google Scholar]
- Brennan RG, Matthews BW. The helix-turn-helix DNA binding motif. J Biol Chem. 1989;264:1903–1906. [PubMed] [Google Scholar]
- Brown JS, Holden DW. Iron acquisition by Gram-positive bacterial pathogens. Microbes Infect. 2002;4:1149–1156. doi: 10.1016/s1286-4579(02)01640-4. [DOI] [PubMed] [Google Scholar]
- Burtnick MN, Downey JS, Brett PJ, Boylan JA, Frye JG, Hoover TR, Gherardini FC. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol Microbiol. 2007;65:277–293. doi: 10.1111/j.1365-2958.2007.05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EC, Siboo R, Keng T, Psarra N, Hurley R, Cheng SL, Iugovaz I. Treponema denticola (ex Brumpt 1925) sp. nov., nom. rev., and identification of new spirochete isolates from periodontal pockets. Int J Syst Bacteriol. 1993;43:196–203. doi: 10.1099/00207713-43-2-196. [DOI] [PubMed] [Google Scholar]
- Chou CJ, Wisedchaisri G, Monfeli RR, Oram DM, Holmes RK, Hol WG, Beeson C. Functional studies of the Mycobacterium tuberculosis iron-dependent regulator. J Biol Chem. 2004;279:53554–53561. doi: 10.1074/jbc.M407385200. [DOI] [PubMed] [Google Scholar]
- Chu L, Kennell W, Holt SC. Characterization of hemolysis and hemoxidation activities by Treponema denticola. Microb Pathog. 1994;16:183–195. doi: 10.1006/mpat.1994.1019. [DOI] [PubMed] [Google Scholar]
- Cornelissen CN. Transferrin-iron uptake by Gram-negative bacteria. Front Biosci. 2003;8:d836–847. doi: 10.2741/1076. [DOI] [PubMed] [Google Scholar]
- D'Aquino JA, Ringe D. Determinants of the SRC homology domain 3-like fold. J Bacteriol. 2003;185:4081–4086. doi: 10.1128/JB.185.14.4081-4086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aquino JA, Lattimer JR, Denninger A, D'Aquino KE, Ringe D. Role of the N-terminal helix in the metal ion-induced activation of the diphtheria toxin repressor DtxR. Biochemistry. 2007;46:11761–11770. doi: 10.1021/bi7007883. [DOI] [PubMed] [Google Scholar]
- Desrosiers DC, Sun YC, Zaidi AA, Eggers CH, Cox DL, Radolf JD. The general transition metal (Tro) and Zn2+ (Znu) transporters in Treponema pallidum: analysis of metal specificities and expression profiles. Mol Microbiol. 2007;65:137–152. doi: 10.1111/j.1365-2958.2007.05771.x. [DOI] [PubMed] [Google Scholar]
- Ding X, Zeng H, Schiering N, Ringe D, Murphy JR. Identification of the primary metal ion-activation sites of the diphtheria tox repressor by X-ray crystallography and site-directed mutational analysis. Nat Struct Biol. 1996;3:382–387. doi: 10.1038/nsb0496-382. [DOI] [PubMed] [Google Scholar]
- Faraldo-Gomez JD, Sansom MS. Acquisition of siderophores in gram-negative bacteria. Nat Rev Mol Cell Biol. 2003;4:105–116. doi: 10.1038/nrm1015. [DOI] [PubMed] [Google Scholar]
- Ferguson AD, Deisenhofer J. Metal import through microbial membranes. Cell. 2004;116:15–24. doi: 10.1016/s0092-8674(03)01030-4. [DOI] [PubMed] [Google Scholar]
- Frederick JF, Rogers EA, Marconi RT. Analysis of a growth phase regulated-two component regulatory system in the periodontal pathogen, Treponema denticola. J Bacteriol. doi: 10.1128/JB.00046-08. doi: 10.1128/JB.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gherardini FC, Boylan JA, Brett PJ. Metal utilization and oxidative stress. In: Radolf JD, Lukehart SA, editors. Pathogenic Treponema: Molecular and Cellular Biology. Norwich: Caister Academic Press; 2006. pp. 101–126. [Google Scholar]
- Glasfeld A, Guedon E, Helmann JD, Brennan RG. Structure of the manganese-bound manganese transport regulator of Bacillus subtilis. Nat Struct Biol. 2003;10:652–657. doi: 10.1038/nsb951. [DOI] [PubMed] [Google Scholar]
- Golynskiy MV, Davis TC, Helmann JD, Cohen SM. Metal-induced structural organization and stabilization of the metalloregulatory protein MntR. Biochemistry. 2005;44:3380–3389. doi: 10.1021/bi0480741. [DOI] [PubMed] [Google Scholar]
- Guedon E, Helmann JD. Origins of metal ion selectivity in the DtxR/MntR family of metalloregulators. Mol Microbiol. 2003;48:495–506. doi: 10.1046/j.1365-2958.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- Hardham JM, Stamm LV, Porcella SF, Frye JG, Barnes NY, Howell JK, et al. Identification and transcriptional analysis of a Treponema pallidum operon encoding a putative ABC transport system, an iron-activated repressor protein homolog, and a glycolytic pathway enzyme homolog. Gene. 1997;197:47–64. doi: 10.1016/s0378-1119(97)00234-5. [DOI] [PubMed] [Google Scholar]
- Hazlett KR, Rusnak F, Kehres DG, Bearden SW, La Vake CJ, La Vake ME, et al. The Treponema pallidum tro operon encodes a multiple metal transporter, a zinc-dependent transcriptional repressor, and a semi-autonomously expressed phosphoglycerate mutase. J Biol Chem. 2003;278:20687–20694. doi: 10.1074/jbc.M300781200. [DOI] [PubMed] [Google Scholar]
- Hill PJ, Cockayne A, Landers P, Morrissey JA, Sims CM, Williams P. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect Immun. 1998;66:4123–4129. doi: 10.1128/iai.66.9.4123-4129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda JS, Janakiraman A, Kehres DG, Maguire ME, Slauch JM. Transcriptional regulation of sitABCD of Salmonella enterica serovar Typhimurium by MntR and Fur. J Bacteriol. 2005;187:912–922. doi: 10.1128/JB.187.3.912-922.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubovics NS, Jenkinson HF. Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology. 2001;147:1709–1718. doi: 10.1099/00221287-147-7-1709. [DOI] [PubMed] [Google Scholar]
- Kaplan J. Mechanisms of cellular iron acquisition: another iron in the fire. Cell. 2002;111:603–606. doi: 10.1016/s0092-8674(02)01164-9. [DOI] [PubMed] [Google Scholar]
- Kehres DG, Maguire ME. Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev. 2003;27:263–290. doi: 10.1016/S0168-6445(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev. 2001;14:727–752. doi: 10.1128/CMR.14.4.727-752.2001. Table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love JF, VanderSpek JC, Murphy JR. The src homology 3-like domain of the diphtheria toxin repressor (DtxR) modulates repressor activation through interaction with the ancillary metal ion-binding site. J Bacteriol. 2003;185:2251–2258. doi: 10.1128/JB.185.7.2251-2258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- Mulrooney SB, Hausinger RP. Nickel uptake and utilization by microorganisms. FEMS Microbiol Rev. 2003;27:239–261. doi: 10.1016/S0168-6445(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Nikaido H, Saier MH., Jr Transport proteins in bacteria: common themes in their design. Science. 1992;258:936–942. doi: 10.1126/science.1279804. [DOI] [PubMed] [Google Scholar]
- Outten CE, O'Halloran TV. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science. 2001;292:2488–2492. doi: 10.1126/science.1060331. [DOI] [PubMed] [Google Scholar]
- Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, Levanos VA, et al. Bacterial diversity in human subgingival plaque. J Bacteriol. 2001;183:3770–3783. doi: 10.1128/JB.183.12.3770-3783.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer SI, Hantke K. Dual repression by Fe(2+)-Fur and Mn(2+)-MntR of the mntH gene, encoding an NRAMP-like Mn(2+) transporter in Escherichia coli. J Bacteriol. 2001;183:4806–4813. doi: 10.1128/JB.183.16.4806-4813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennella MA, Giedroc DP. Structural determinants of metal selectivity in prokaryotic metal-responsive transcriptional regulators. Biometals. 2005;18:413–428. doi: 10.1007/s10534-005-3716-8. [DOI] [PubMed] [Google Scholar]
- Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- Posey JE, Hardham JM, Norris SJ, Gherardini FC. Characterization of a manganese-dependent regulatory protein, TroR, from Treponema pallidum. Proc Natl Acad Sci USA. 1999;96:10887–10892. doi: 10.1073/pnas.96.19.10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Verlinde CL, Zhang S, Schmitt MP, Holmes RK, Hol WG. Three-dimensional structure of the diphtheria toxin repressor in complex with divalent cation co-repressors. Structure. 1995;3:87–100. doi: 10.1016/s0969-2126(01)00137-x. [DOI] [PubMed] [Google Scholar]
- Ratledge C, Dover LG. Iron metabolism in pathogenic bacteria. Annu Rev Microbiol. 2000;54:881–941. doi: 10.1146/annurev.micro.54.1.881. [DOI] [PubMed] [Google Scholar]
- Rensing C, Grass G. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol Rev. 2003;27:197–213. doi: 10.1016/S0168-6445(03)00049-4. [DOI] [PubMed] [Google Scholar]
- Satcher DS. Surgeon General's report on oral health. Public Health Rep. 2000;115:489–490. doi: 10.1093/phr/115.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering N, Tao X, Zeng H, Murphy JR, Petsko GA, Ringe D. Structures of the apo- and the metal ion-activated forms of the diphtheria tox repressor from Corynebacterium diphtheriae. Proc Natl Acad Sci USA. 1995;92:9843–9850. doi: 10.1073/pnas.92.21.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt MP. Identification of a two-component signal transduction system from Corynebacterium diphtheriae that activates gene expression in response to the presence of heme and hemoglobin. J Bacteriol. 1999;181:5330–5340. doi: 10.1128/jb.181.17.5330-5340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D, Siboo IR, Chan EC, Klitorinos A, Siboo R. Binding of hemin and congo red by oral hemolytic spirochetes. Oral Microbiol Immunol. 1993;8:245–250. doi: 10.1111/j.1399-302x.1993.tb00568.x. [DOI] [PubMed] [Google Scholar]
- Sela MN. Role of Treponema denticola in periodontal diseases. Crit Rev Oral Biol Med. 2001;12:399–413. doi: 10.1177/10454411010120050301. [DOI] [PubMed] [Google Scholar]
- Seshadri R, Myers GS, Tettelin H, Eisen JA, Heidelberg JF, Dodson RJ, et al. Comparison of the genome of the oral pathogen Treponema denticola with other spirochete genomes. Proc Natl Acad Sci USA. 2004;101:5646–5651. doi: 10.1073/pnas.0307639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- Tao X, Schiering N, Zeng HY, Ringe D, Murphy JR. Iron, DtxR, and the regulation of diphtheria toxin expression. Mol Microbiol. 1994;14:191–197. doi: 10.1111/j.1365-2958.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Tao X, Zeng HY, Murphy JR. Transition metal ion activation of DNA binding by the diphtheria tox repressor requires the formation of stable homodimers. Proc Natl Acad Sci USA. 1995;92:6803–6807. doi: 10.1073/pnas.92.15.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Kolodrubetz D. Construction and analysis of hemin binding protein mutants in the oral pathogen Treponema denticola. Res Microbiol. 2002;153:569–577. doi: 10.1016/s0923-2508(02)01370-0. [DOI] [PubMed] [Google Scholar]
- Xu X, Holt SC, Kolodrubetz D. Cloning and expression of two novel hemin binding protein genes from Treponema denticola. Infect Immun. 2001;69:4465–4472. doi: 10.1128/IAI.69.7.4465-4472.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


