The α-cell of the pancreatic islet modulates glucose homeostasis by secreting glucagon that acts primarily by driving hepatic glucose production. Glucose sensing of the α-cell becomes defective in both type 1 and type 2 diabetes, resulting in hyperglucagonemia that likely contributes to hyperglycemia (1). Thus, it is important to elucidate the signals that trigger glucagon secretion and the transduction of these signals within the α-cell. Glucagon secretion has been linked to several triggers: the α-cell detecting a fall in circulating glucose levels directly, a paracrine response to signal(s) from the islet β-cell (e.g., insulin, γ-aminobutyric acid [GABA], or Zn2+ ions) or the islet δ-cell (somatostatin), or a response to neural signals (2–8). In all likelihood, an interaction of several signals regulates glucagon secretion in vivo.
There is good reason to believe that glucagon release, like insulin release, is influenced by physiological α-cell electrical activity and Ca2+ influx and fundamentally resembles the excitation-secretion coupling seen in many secretory cell types (9). Stimulus-induced α-cell electrical activity results from depolarization-induced opening of voltage-gated Ca2+ and K+ channels (2,9–11). The depolarization first activates the low voltage–activated T-type Ca2+ channels, which have been implicated in action potential initiation (10). Activation of K+ channels then shapes the α-cell action potential upstroke. The resulting depolarization activates high voltage–activated Ca2+ channels including the N-type and L-type Ca2+ channels, which coordinate Ca2+-induced glucagon release (11). Action potential repolarization then follows with the activation of voltage-gated potassium (N) channels (12).
Hypoglycemic conditions can promote glucagon secretion by stimulating α-cell electrical activity and Ca2+ entry. In islet β-cells, elevations in glucose increase the ATP-to-ADP ratio, resulting in the closure of ATP-sensitive K+ (KATP) channels and causing action potential firing. α-Cell electrical activity is also sensitive to modulators of the KATP channel; thus, glucose responsiveness has been linked to the activity of KATP, which sets the resting membrane potential of pancreatic β-cells. In contrast to β-cells, KATP channel closure in α-cells has been linked to the termination of action potential firing (10). KATP channels can affect human glucagon secretion as evidenced in carriers of the E23K variant of KATP, which is linked to an increased incidence in adult-onset diabetes and perturbations in glucose regulation of glucagon secretion (13). Similarly, a mouse model with a defective KATP channel subunit sulfonurea receptor 1 (SUR1) also shows perturbations in glucagon secretion (14). Thus, one might suspect that KATP channels are involved in the α-cell response to hypoglycemia. In addition, islet paracrine factors such as insulin, somatostatin, Zn2+, and GABA have the ability to cause islet α-cells to fire action potentials (2–8), in some cases by regulating ion channels such as KATP (15).
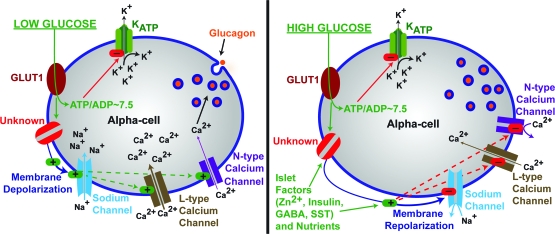
In this issue of Diabetes, Quoix et al. (16) investigated the relationship of KATP channels and paracrine factors with glucose sensing. Electrical activity and Ca2+ flux were examined in dispersed islets isolated from mice expressing a fluorescent protein specifically in islet α-cells. A subset of these α-cells responded to low glucose (0.5 mmol/l), adrenaline, and KATP channel inhibition with Ca2+ elevations in a manner similar to the responses in control α-cells. On the other hand, when KATP channels were opened with diazoxide or inhibitors of metabolism, Ca2+ fluctuations induced by low glucose or KATP channel inhibition returned to basal levels. Interestingly, this predicts that KATP channels may be mainly closed under low glucose conditions in α-cells due to metabolism. The authors confirm that KATP channel currents are mostly closed in low glucose (0.5 mmol/l) and can be activated by diazoxide or metabolic poisons. To address whether this may be due to increased metabolism in α-cells under low glucose conditions, the authors determined changes in the metabolic product NAD(P)H. Levels of NAD(P)H in β-cells were found to respond to glucose, as expected; however, α-cell NAD(P)H levels did not vary substantially, thereby corroborating previous α-cell studies in which equivalent ATP-to-ADP ratios and NAD(P)H or FAD fluorescence under low- and high-glucose conditions were observed (16,17). Thus, this work leads to several important conclusions concerning α-cell glucose-sensing mechanisms. First, glucose sensing in α-cells is independent of glucose metabolism, whereas the responses to glucose are independent of KATP channels, as illustrated in Fig. 1. However, these data do confirm that KATP activity can regulate α-cell Ca2+ entry.
FIG. 1.
KATP-independent glucose regulation of α-cell glucagon secretion. Hypoglycemic stimulation of glucagon secretion occurs through membrane depolarization, which is independent of KATP activity (left panel). The stimulation of α-cells with glucose leads to reduction of glucagon secretion in part by reducing calcium influx presumably through membrane repolarization through an unresolved pathway, which is illustrated as “Unknown” and labeled in red. The unknown is sensitive to glucose levels, whereas it is insensitive to glucose metabolites; it may be an ion channel or receptor and may also be an intracellular signaling mechanism. SST, somatostatin.
So, if islet α-cells do not signal via the glucose-ATP-KATP channel system to control glucagon secretion in response to glucose levels, how do they detect and respond to hypoglycemia? Paracrine factors released from β-cells have been linked to KATP channel modulation in α-cells and, thus, may regulate the total glucose-dependent glucagon secretion independently of ATP (15). α-Cells may require interaction with an intact islet or islet paracrine factors for normal glucose sensing. Quoix et al. (16) found that on single α-cells the islet paracrine factors Zn2+, insulin, or GABA have modest effects on α-cell Ca2+ levels under low glucose conditions (2–6). However, the α-cell Ca2+ levels are reduced by only 28% when switching from low to high glucose, thus implicating another unknown modulator that may work with glucose to further reduce α-cell electrical signaling and Ca2+ influx. All three paracrine factors are secreted under elevated glucose conditions, and it could be that these factors may further reduce the Ca2+ level while glucose conditions are elevated (2–6). The authors find that glucose can reduce glucagon secretion by 70% while incubated with insulin, indicating that glucose is significantly more important to glucagon secretion than insulin. There are contradictory reports on the role of paracrine factors in the regulation of glucagon secretion, and it could be that β-cell paracrine regulation of α-cell Ca2+ fluctuation is minimal (2–6,18–19). However, it may also be that the α-cell population is heterogeneous and that some of the α-cells in this report that do not respond to low glucose with Ca2+ fluctuations are the cells that can respond to paracrine factors in intact islets. The regulation of paracrine factors may also require intact β-cell–to–α-cell contacts to exert influence on α-cell Ca2+ dynamics. These might include gap junctions.
Finally, it should not be forgotten that autonomic influences play an important role in α-cell electrical activity and may modulate glucose sensing through innervation and circulating neurotransmitter levels (8). It is likely that such neurotransmitters play major roles in regulating glucagon secretion in ways both dependent and independent of the KATP channel.
In summary, the work of Quoix et al. provides evidence that the KATP channel is not the primary α-cell target responsible for hypoglycemia-induced glucagon secretion (16). Interestingly, the work also shows that, at least in isolated cells, Zn2+, insulin, or GABA minimally regulates α-cell glucose-induced Ca2+ flux. These are intriguing observations that should stimulate future studies designed to understand the glucose-sensing mechanisms of the α-cell.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
See accompanying original article, p. 412.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Unger RH, Aguilar-Parada E, Müller WA, Eisentraut AM: Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest 49: 837–848, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gromada J, Franklin I, Wollheim CB: Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev 28: 84–116, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Maruyama H, Hisatomi A, Orci L, Grodsky GM, Unger RH: Insulin within islets is a physiologic glucagon release inhibitor. J Clin Invest 74: 2296–2299, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenbaum CJ, Havel PJ, Taborsky GJ Jr, Klaff LJ: Intra-islet insulin permits glucose to directly suppress pancreatic A cell function. J Clin Invest 88: 767–773, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ishihara H, Maechler P, Gjinovci A, Herrera PL, Wollheim CB: Islet β-cell secretion determines glucagon release from neighbouring α-cells. Nat Cell Biol 5: 330–335, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Rorsman P, Berggren PO, Bokvist K, Ericson H, Mohler H, Ostenson CG, Smith PA: Glucose-inhibition of glucagon secretion involves activation of GABAA-receptor chloride channels. Nature 341: 233–236, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Iversen J: Inhibition of pancreatic glucagon release by somatostatin: in vitro. Scand J Clin Lab Invest 33: 125–129, 1974 [PubMed] [Google Scholar]
- 8.Ahren B: Autonomic regulation of islet hormone secretion: implications for health and disease. Diabetologia 43: 393–410, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Olsen HL, Theander S, Bokvist K, Buschard K, Wollheim CB, Gromada J: Glucose stimulates glucagon release in single rat α-cells by mechanisms that mirror the stimulus-secretion coupling in β-cells. Endocrinology 146: 4861–4870, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Gopel SO, Kanno T, Barg S, Weng XG, Gromada J, Rorsman P: Regulation of glucagon release in mouse α-cells by KATP channels and inactivation of TTX sensitive Na+ channels. J Physiol 528: 509–520, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gromada J, Bokvist K, Ding WG, Barg S, Buschard K, Renstrom E, Rorsman P: Adrenaline stimulates glucagon secretion in pancreatic α-cells by increasing the Ca2+ current and the number of granules close to the L-type Ca2+ channels. J Gen Physiol 110: 217–228, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barg S, Galvanovskis J, Gopel SO, Rorsman P, Eliasson L: Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting α-cells. Diabetes 49: 1500–1510, 2000 [DOI] [PubMed] [Google Scholar]
- 13.Tschritter O, Stumvoll M, Machicao F, Holzwarth M, Weisser M, Maerker E, Teigeler A, Häring H, Fritsche A: The prevalent Glu23Lys polymorphism in the potassium inward rectifier 6.2 (KIR6.2) gene is associated with impaired glucagon suppression in response to hyperglycemia. Diabetes 51: 2854–2860, 2002 [DOI] [PubMed] [Google Scholar]
- 14.Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren PO, Rorsman P: ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes 53 (Suppl. 3): S181–S189, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Leung YM, Ahmed I, Sheu L, Gao X, Hara M, Tsushima RG, Diamant NE, Gaisano HY: Insulin regulates islet alpha-cell function by reducing KATP channel sensitivity to adenosine 5′-triphosphate inhibition. Endocrinology 147: 2155–2162, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Quoix N, Cheng-Xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois M, Henquin JC, Gilon P: Glucose and pharmacological modulators of ATP-sensitive K+ channels control [Ca2+]c by different mechanisms in isolated mouse α-cells. Diabetes 58: 412–421, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Detimary P, Dejonghe S, Ling Z, Pipeleers D, Schuit F, Henquin JC: The changes in adenine nucleotides measured in glucose-stimulated rodent islets occur inS cells but not in α cells and are also observed in human islets. J Biol Chem 273: 33905–33908, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Vieira E, Salehi A, Gylfe E: Glucose inhibits glucagon secretion by a direct effect on mouse pancreatic alpha cells. Diabetologia 50: 370–379, 2007 [DOI] [PubMed] [Google Scholar]
- 19.MacDonald PE, De Marinis YZ, Ramracheya R, Salehi A, Ma X, Johnson PR, Cox R, Eliasson L, Rorsman P: A K ATP channel-dependent pathway within alpha cells regulates glucagon release from both rodent and human islets of Langerhans. PLoS Biol 5: e143, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]