Abstract
OBJECTIVE— A restricted region of proinsulin located in the B chain and adjacent region of C-peptide has been shown to contain numerous candidate epitopes recognized by CD8+ T-cells. Our objective is to characterize HLA class I–restricted epitopes located within the preproinsulin leader sequence.
RESEARCH DESIGN AND METHODS— Seven 8- to 11-mer preproinsulin peptides carrying anchoring residues for HLA-A1, -A2, -A24, and -B8 were selected from databases. HLA-A2–restricted peptides were tested for immunogenicity in transgenic mice expressing a chimeric HLA-A*0201/β2-microglobulin molecule. The peptides were studied for binding to purified HLA class I molecules, selected for carrying COOH-terminal residues generated by proteasome digestion in vitro and tested for recognition by human lymphocytes using an ex vivo interferon-γ (IFN-γ) ELISpot assay.
RESULTS— Five HLA-A2–restricted peptides were immunogenic in transgenic mice. Murine T-cell clones specific for these peptides were cytotoxic against cells transfected with the preproinsulin gene. They were recognized by peripheral blood mononuclear cells (PBMCs) from 17 of 21 HLA-A2 type 1 diabetic patients. PBMCs from 25 of 38 HLA-A1, -A2, -A24, or -B8 patients produced IFN-γ in response to six preproinsulin peptides covering residues 2–25 within the preproinsulin region. In most patients, the response was against several class I–restricted peptides. T-cells recognizing preproinsulin peptide were characterized as CD8+ T-cells by staining with peptide/HLA-A2 tetramers.
CONCLUSIONS— We defined class I–restricted epitopes located within the leader sequence of human preproinsulin through in vivo (transgenic mice) and ex vivo (diabetic patients) assays, illustrating the possible role of preproinsulin-specific CD8+ T-cells in human type 1 diabetes.
Type 1 diabetes involves the activation of lymphocytes against β-cell autoantigens. In animals, the predominant role of T-cells is supported by experiments in which diabetes is transferred by diabetogenic T-cells, is prevented by antibodies that interfere with T-cell activation, or fails to develop in diabetes-prone mice in which key genes in T-cell differentiation or activation are deficient. In humans, T-cells are predominant within insulitis at early stages of diabetes. Moreover, type 1 diabetes has been reported in an immunodeficient patient deprived of B-cells (1).
Major histocompatibility complex (MHC) class II–restricted CD4+ T-cells are central in the diabetes process, but CD8+ T-cells play a pivotal role in its initiation in NOD mice (2). In human, CD8+ T-cells are predominant, and a high percentage of interferon-γ (IFN-γ)-positive cells is detected within insulitis in recent-onset diabetes in most observations (3,4,5,6). Recurrent diabetes in recipients of isografts from a discordant twin is accompanied by predominant CD8+ T-cell infiltration (7).
Among β-cell autoantigens, proinsulin has been ascribed a key role in diabetes. In humans, insulin and proinsulin are common targets of autoantibodies (8,9) and T-cells (10,11,12,13,14,15,16,17) in diabetic and pre-diabetic individuals. Anti-insulin antibodies are the first to be detected in children at risk for diabetes and carry a high positive predictive value for diabetes (9). In NOD mice, injection of insulin-specific T-cell clones accelerate diabetes (18). Protection from diabetes is obtained by injecting insulin in pre-diabetic mice (19). In addition, proinsulin 1−/− or 2−/− NOD mice show delayed or accelerated diabetes, respectively (20,21).
Several new β-cell HLA class I–restricted epitopes have been reported recently (22,23,24,25,26,27). We and others have shown that a restricted region of human proinsulin located in the B chain and adjacent C-peptide clusters proteasome cleavage sites generating correct COOH-termini of putative MHC class I peptides and many epitopes that are recognized by diabetic CD8+ T-cells (22,23). Recognition of epitopes that are located within the C-peptide and C-peptide–B chain junction, including residues that are excised during the secretion process, makes a strong case for proinsulin as an autoantigen in diabetes. Despite strong evidence that leader sequence peptides are presented by class I HLA molecules, especially HLA-A2.1 (28), only two HLA A2.1 preproinsulin leader sequence peptides have been identified (26,27).
To characterize class I–restricted epitopes within the preproinsulin leader sequence, we selected 8- to 11-mer peptides carrying anchoring residues for class I molecules. These peptides were studied for immunogenicity in HLA-A*0201 transgenic mice (29). Mouse CD8+ T-cell clones specific to HLA-A*0201–restricted peptides were tested for cytotoxicity against HLA-A2 target cells transfected with the preproinsulin gene. In human, peptides were studied for binding to common class I molecules, for carrying COOH-terminal residues generated by proteasome digestion, and for recognition by peripheral blood mononuclear cells (PBMCs) from diabetic patients.
RESEARCH DESIGN AND METHODS
Mice.
HHD mice express the chimeric HLA-A*0201 HHD monochain containing the HLA-A*0201 α1/α2 domains and the H-2Db α3 domain linked at its NH2- terminus to the human β2m COOH-terminus by a 15-residue linker peptide (29).
Cytotoxicity assays.
Cytotoxicity assays were performed using RMA-S cells transfected with the HHD monochain or P815-HTR (high-efficiency transfection recipient, DBA/2, H-2d) mouse mastocytoma cells expressing HLA-A2.1 and human preproinsulin as targets. P815-HTR cells were electroporated with respectively 20 μg HLA-A2.1 plasmid (blue script) and 2 μg construct plasmid preproinsulin human linearized DNA (PcDNA3.1+; Invitrogen) at 250 V using an Easyject gene pulser. After 24 h, cells were transferred in selective medium containing 500 μg/ml G418 (Gibco-BRL, Paisley, U.K.) and cloned by limiting dilution. Neomycine-resistant clones expressing the HLA-A2.1 monochain were analyzed by flow cytometry using anti-Hβ2m fluorescein isothiocyanate (FITC)-conjugated antibody. Transfected cells expressing high preproinsulin RNA levels were selected by RT-PCR using β-actin RNA as internal standard.
Peptide immunizations were performed by injecting HHD mice with 100 μg peptide and 140 μg I-Ab–restricted helper peptide from the hepatitis B core protein (TPPAYRPPNAPIL) in incomplete Freund's adjuvant subcutaneously at the base of the tail. At day 11, spleen cells were restimulated in vitro for 6 days in the presence of irradiated (3,500 rad) HHD mouse spleen cells preactivated for 3 days by 10 μg/ml lipopolysaccharide 055:B5 (Sigma) and then pulsed with 5 μg/ml peptides. Spleen cells were recovered and tested for cytotoxicity against HHD-transfected RMA-S cells pulsed with tested or control peptide using chromium release assay.
CTL lines and clones were obtained by culturing cells showing cytolytic activity in RPMI supplemented with 10% FCS and 0.1 mmol/l 2-mercaptoethanol and restimulated weekly with peptide (1 μg/ml down to 0.05 μg/ml)-pulsed HHD cells.
Patients.
Type 1 diabetic patients had anti-GAD, -insulin, –islet antigen 2, or –islet cell antibodies at diagnosis.
Recent-onset patients (n = 26, 12 women and 14 men) were studied within 3 months of diagnosis. Long-standing patients (n = 12, 4 women and 8 men) had been insulin treated for 5–40 years at study. Control subjects included 12 healthy blood donors (4 women and 8 men) and 6 type 2 diabetic patients (2 women and 4 men), 3 of whom were insulin treated (Table 1). Informed consent was obtained from all patients. PBMCs were isolated by Ficoll Paque density gradient centrifugation (Pharmacia Biotech, Uppsala, Sweden) and analyzed immediately. HLA class I and class II alleles were determined by serological typing and genotyping, respectively, and class I alleles were confirmed by genotyping.
TABLE 1.
Patients and control individuals
| P | -DRB1* | -A, -B* | P | -DRB1* | -A, -B* | C | -DRB1* | -A, -B* |
|---|---|---|---|---|---|---|---|---|
| R1 | 03/04 | A2–3, B18–38 | R20 | 03/04 | A1–24, B8- | C1 | nd | A2–29, B7–44 |
| R2 | 04/13 | A2–24, B27- | R21 | 03/04 | A24–25, B18–56 | C2 | nd | A2–3, B38–51 |
| R3 | 03/11 | A2–30, B16–18 | R22 | 03/03 | A1–24, B16–39 | C3 | nd | A24–28, B7–8 |
| R4 | 03/16 | A2-, B45–18 | R23 | 04/08 | A3–24, B39–49 | C4 | nd | A1–30, B8–13 |
| R5 | 01/03 | A2-, B8- | R24 | 04/08 | A24–80, B44–51 | C5 | nd | A1–32, B8–13 |
| R6 | 03/16 | A2–11, B7–55 | R25 | 03/03 | A9–10, B8–14 | C6 | nd | A2–31, B35–60 |
| R7 | 03/04 | A2-, B62–52 | R26 | 03/04 | A1–2, B8–15 | C7 | nd | A2-, B8- |
| R8 | 03/04 | A2-, B8–44 | L1 | 03/04 | A2-, B35–62 | C8 | nd | A2–3, B7–12 |
| R9 | 03/11 | A2-, B8–51 | L2 | 03/04 | A2–29, B7–35 | C9 | nd | A2-, B27–44 |
| R10 | 04/04 | A2–3, B14–44 | L3 | 01/01 | A2–24, B7–35 | C10 | 03/04 | A1–2, B8–50 |
| R11 | 04/13 | A2–3, B15–50 | L4 | 04/04 | A2-, B39–62 | C11 | nd | A2–3, B7–50 |
| R12 | 01/04 | A2–3, B15–35 | L5 | 03/03 | A1–24, B8–45 | C12 | nd | A1–3, B8–27 |
| R13 | 03/04 | A2–24, B49- | L6 | 03/04 | A1–24, B8–49 | D1 | nd | A2–10, B12–44 |
| R14 | 07/13 | A1–2, B12–44 | L7 | 04/13 | A11–24, B50–62 | D2 | 03/15 | A2-, B35–44 |
| R15 | 03/13 | A2-, B8- | L8 | 03/04 | A1–3, B8–49 | D3 | 03/14 | A1–11, B8–55 |
| R16 | 03/04 | A2–3, B8–18 | L9 | 03/03 | A3–31, B8–51 | D4† | 03/13 | A2-, B38–45 |
| R17 | 04/16 | A1–24, B8–44 | L10 | 03/04 | A1–9, B8–21 | D5† | 01/11 | A1–2, B7- |
| R18 | 03/08 | A3–24, B8–58 | L11 | 03/04 | A1-, B8–38 | D6† | 03/07 | A1–19, B8–44 |
| R19 | 01/04 | A3–24, B35–47 | L12 | 03/04 | A1-, B8–44 |
P, patients; R, recent-onset diabetes; L, long-standing diabetes; C, healthy control subjects; D, type 2 diabetic control subjects; nd, not done.
HLA alleles.
Insulin treated.
Peptides.
Seven 8- to 11-mer preproinsulin peptides carrying putative binding motifs for HLA-A1, -A2, -A24, and -B8 alleles were selected as predicted from known viral peptide sequences and databases (http://www-bimass.cit.nih.gov; http://www.syfpeithi.de). Selection was based on bimass and syfpeithi scores ≥40 and 14, respectively, or on known viral peptide sequences (J.C., personal communication). Six HLA-A2–restricted peptides (one of them carrying binding motifs for HLA-A24 and -B8) and one peptide containing binding motifs for HLA-A1 and -A24 were selected (Table 2). Peptides were synthesized and purified (23). Peptide nomenclature refers to NH2- and COOH-terminal positions along the human preproinsulin sequence.
TABLE 2.
Binding of preproinsulin peptides to selected HLA class I alleles
| Peptide* | Sequence | HLA I | HLA motif† | Anchoring fit‡ | Binding§
|
Type of binder¶ | |
|---|---|---|---|---|---|---|---|
| 10−4 | 10−6 | ||||||
| 1–8 | MALWMRLL | A2 | MALWMRLL | + | 46 | 0 | L |
| 2–11 | ALWMRLLPLL | A2 | ALWMRLLPLL | ++ | 55 | 53 | H |
| A24 | ALWMRLLPLL | + | 80 | 100 | H | ||
| B8 | ALWMRLLPLL | + | 24 | 20 | I | ||
| 6–14 | RLLPLLALL | A2 | RLLPLLALL | ++ | 36 | 65 | H |
| 6–16 | RLLPLLALLAL | A2 | RLLPLLALLAL | ++ | 96 | 71 | H |
| 14–23 | LALWGPDPAA | A2 | LALWGPDPAA | + | 44 | 36 | I |
| 15–24 | ALWGPDPAAA | A2 | ALWGPDPAAA | + | 96 | 94 | H |
| 15–25 | ALWGPDPAAAF | A1 | ALWGPDPAAAF | + | 18 | 41 | I |
| A24 | ALWGPDPAAAF | ++ | 82 | 100 | H | ||
Data are percent.
NH2- and COOH-terminal positions in preproinsulin.
Anchoring positions are in bold characters.
HLA anchoring motifs: ++, strict; +, tolerated.
Binding (%) = binding of tested peptide/binding of viral reference peptide.
L, low binder (binding <20% at 10−6 mol/l and binding >20% at 10−4 mol/l); I, intermediate binder (binding between 20 and 50% at 10−6 mol/l); H, high binder (binding >50% at 10−6 mol/l).
Peptide-binding assay.
HLA heavy (H) chains prepared as reported previously (30) were incubated with 2 μg/ml exogenous β2m (Sigma, Steinheim, Germany) and with 10−4, 10−6, and 10−8 mol/l exogenous peptides in Eppendorf microtubes (Eppendorf-Netheler-Hinz, Hamburg, Germany) for 1 h at room temperature and then for 24 h at 4°C. Reassembled HLA molecules were further incubated for 90 min at 37°C in 96-well microtiter plates coated with anti-HLA monoclonal antibodies (BB7.2 for HLA-A2, B1.23.2 for HLA-B molecules, and PA2.6 for HLA-A and -B molecules). Correctly folded HLA complexes were revealed with anti-β2m antibodies coupled to alkaline phosphatase and 4-methyl-umbelliferyl phosphate (M-8883; Sigma) as substrate. Fluorescence generated was measured at 360/460 nm in a Microfluor reader (Victor 1420; Wallac, Turku, Finland). Percentage of binding was defined as the binding of tested peptide over the binding of the reference viral peptide × 100. Reference peptides were influenza virus matrix M.58–66 (GILGFVFTL) for HLA-A2, influenza virus PB1 peptide 591–599 (VSDGGPNLY) for HLA-A1, EBV LMP2 419–427 (TYGPFMSL) for A-24, and HIV Nef 90–97 (FLKEKGGL) for HLA-B8.
Proteasome digestion.
Preproinsulin peptide 1–28, which covers leader sequence and adjacent B chain residues (MALWMRLLPLLALLALWGPDPAAAFVNQ) was digested by a proteasome-enriched extract obtained from T1 lymphoblastoid cells (23). Cleavage products were separated by reversed- phase high-performance liquid chromatography (RP-HPLC; Perkin-Elmer, Norwalk, CT) on a C18 Nucleosil column (10 μm, 250 × 4.0 mm; Macherey-Nagel, Hoerdt, France). Mass analyses were performed on a MALDI-Tof spectrometer in a reflectron-delayed extraction ion source over a mass range of 500–3,200 Da and recorded with a Voyager-DE-Pro mass spectrometer (PerSeptive Biosystems, Framingham, MA). Monoisotopic masses were calculated, and peptides corresponding to computed masses were identified (23).
Enzyme-linked immunospot assay.
IFN-γ enzyme-linked immunospot (ELISpot) was performed as previously described (23). Background IFN-γ response was evaluated in three to six wells containing 3 × 105 cells/well incubated without peptide. Responses were considered positive when the number of spots in the presence of peptide was above background +3 SD. Positive controls consisted of three wells containing 3 × 104 cells/well stimulated with 1 μg/ml phytohemagglutinin and three wells containing 3 × 105 cells/well stimulated with 10 μg/ml viral peptides (peptide M.58–66 from influenza virus matrix for HLA-A2, nucleoprotein NP44–52 for HLA-A1, and NP380–388 for HLA-B8). Negative controls were HIV Nef 83–91 peptide for HLA-A2 and Nef 182–189 peptide for HLA-B8. A stimulation score was calculated to take into account interassay variability (stimulation score = mean number of spots in response to peptide − mean number of spots in absence of peptide).
Expansion of human proinsulin-specific T-cells.
PBMCs (3 × 106 cells/ml) were incubated in presence of 10 μg/ml peptide for 2 h at 37°C, washed, seeded in a six-well plate, maintained for 14 days, and fed on days 4 and 7 by replacing one-half supernatant with fresh medium containing 10 units/ml interleukin-2 and 5 and 2 μg/ml proinsulin peptide, respectively.
MHC class I tetramers and flow cytometry.
HLA-A2 tetramers were produced as previously described (31). Tetramers were titered individually by staining a relevant peptide-specific CD8+ T-cell line and used at 5–10 μg/ml. For staining, 106 PBMCs were incubated at 37°C for 30 min with 5–10 μg/ml PE-labeled tetramer and then with anti–CD8-APC and anti–CD3-FITC–labeled antibodies (BD/Pharmingen, San Jose, CA) for 15 min at 4°C. Small lymphocytes were gated according to forward/side scatter profiles. CD8+ cells were selected among CD3+ cells. Staining with 7-AAD (Pharmingen) was used to exclude dead cells. Data were collected on a FACSCalibur flow cytometer and analyzed using Cell Quest software (Becton Dickinson).
Statistics.
Comparison of stimulation score values of patients and control subjects used nonparametric Mann-Whitney test. Student's t test was used for 6–14 peptide/HLA-A2 tetramer assessment.
RESULTS
Immunogenicity of HLA-A2–binding peptides.
Six preproinsulin peptides (peptides 1–8, 2–11, 6–14, 6–16, 14–23, and 15–24) selected for predicted binding to HLA-A2 were tested for immunogenicity in HLA-A2 transgenic mice. As shown in Fig. 1, spleen cells from mice immunized against five of six preproinsulin peptides (2–11, 6–14, 6–16, 14–23, and 15–24) showed significant cytotoxic response to HHD-transfected RMA-S cells pulsed with the immunizing peptide. Immunogenic peptides induced cytotoxic T-cells, which displayed increasing lytic activity after each of four in vitro restimulations with 1, 0.33, 0.1, and 0.05 μg/ml peptide, respectively (data not shown).
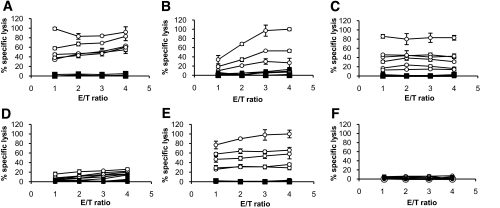
FIG. 1.
Cytotoxic responses of peptide-specific HLA-A2.1–restricted murine T-cells against preproinsulin peptides. Five to six HHD mice were immunized in vivo for each preproinsulin peptide (peptides 6–14, [A]; 6–16, [B]; 2–11, [C]; 14–23, [D]; 15–24, [E]; and 1–8, [F]). Spleen cells were restimulated in vitro 11 days later using irradiated peptide-pulsed HHD lymphoblasts. Spleen cells were then tested for cytolytic activity against HHD-transfected RMA-S targets loaded with relevant preproinsulin peptide (○) or irrelevant negative control peptide (▪) using chromium release assay. The figure represents the percentage of specific lysis (vertical axis) obtained for 1:1, 2:1, 3:1, and 4:1 effector:target (E/T) ratio (horizontal axis).
Murine preproinsulin leader sequence-specific T-cell clones.
As four peptides covered overlapping residues, murine T-cell clones were generated against preproinsulin peptides 6–14, 6–16, 14–23, and 15–24. All 6–14–and 6–16–specific clones were cytotoxic against 6–14–and 6–16–pulsed HHD-transfected RMA-S cells, indicating that cytotoxic T-cells recognize a unique epitope. As a rule, clones generated against peptides 6–14 or 6–16 showed higher cytotoxicity to 6–14–pulsed than to 6–16–pulsed HHD-transfected RMA-S cells. In contrast, 14–23 and 15–24 peptide-specific T-cells showed low cross-reactivity with peptides 15–24 and 14–23, respectively (not shown).
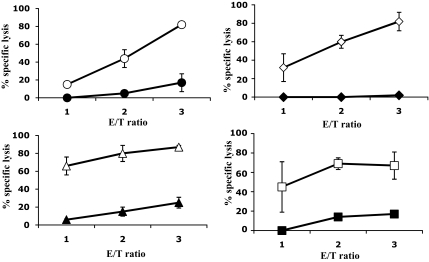
To evaluate processing of peptides 2–11, 6–14, 14–23, and 15–24 from the source protein, we tested whether peptide-specific CD8+ T-cell clones were cytotoxic against P815 cells cotransfected with the HHD-encoding and human proinsulin genes (Fig. 2). T-cell clones specific for all four preproinsulin peptides were cytotoxic, indicating that corresponding epitopes were generated from full-length preproinsulin through the endogenous processing pathway.
FIG. 2.
Cytotoxicity of anti–2–11, anti–6–14, anti–14–23, and anti–15–24 CD8+ T-cell clones against P815 cells transfected with the HHD-encoding and the human preproinsulin genes. Individual CD8+ T-cell clones were tested for cytolytic activity against HHD- and preproinsulin-transfected P815 targets loaded with relevant preproinsulin peptide (circles for 2–11, rectangles for 6–14, triangles for 14–23, squares for 15–24; open symbols) or without peptide (closed symbols, respectively), using chromium release assay. The figure represents the percentage of specific lysis (vertical axis) obtained for 1:1, 2:1, and 3:1 effector:target (E/T) ratio (horizontal axis).
Binding of selected peptides to HLA-A1, -A2, -A24, and -B8.
Among the six peptides selected for possible binding to HLA-A2, one peptide (2–11) contained binding motifs for HLA-A24 and -B8 in addition to HLA-A2. Another peptide (15–25) contained binding motifs for HLA-A1 and -A24. These peptides were studied for binding to purified HLA molecules. Peptides that yielded >50, 20–50, and <20% binding at 10−6 mol/l were considered high, intermediate, and low binders, respectively. Four of six peptides were high binders to HLA-A2 (2–11, 6–14, 6–16, and 15–24), whereas peptide 14–23 showed intermediate binding, and peptide 1–8 showed no binding at 10−6 mol/l and low binding at 10−4 mol/l. Peptide 2–11 was a high binder to HLA-A24 and an intermediate binder to HLA-B8. Peptide 15–25 was an intermediate binder to HLA-A1 and a high binder to HLA-A24. Overall, six peptides showed binding to HLA class I alleles. Two peptides (2–11 and 15–25) showed high or intermediate binding to different HLA class I molecules (Table 2). Noticeably, binding of preproinsulin peptides to class I molecules was weaker than that of the control viral peptides with the exception of peptides 2–11 and 15–25, which showed a binding equal to the reference viral peptide (100% binding) to HLA-A24 at 10−6 mol/l (not shown). Peptide 14–23, which elicited low cytotoxicity against 14–23–pulsed HHD-transfected RMA-S cells but significant binding to HLA-A2, was included in further experiments. Peptide 1–8, showing no specific cytotoxicity and no significant binding, was excluded.
Processing of preproinsulin 1–28 by proteasome.
We determined whether COOH-terminal residues of preselected peptides were generated by proteasome digestion. Database (http://www.paproc2.de.paproc2/cgi-bin) predicts proteasome cleavage in preproinsulin leader sequence in positions 10–14, 16, and 22–24, pointing to peptides 2–11, 6–14, 6–16, 14–23, and 15–24 as possible candidates. We analyzed peptides resulting from digestion of preproinsulin peptide 1–28 by a proteasome-enriched T1 cell extract (23). Several digestions were performed that yielded identical HPLC profiles. A 20-h incubation was retained for fraction collection. Multiple peaks appeared between 18 and 74 min of HPLC column retention time, and a late peak corresponding to the entire preproinsulin 1–28 peptide was evidenced. Mass spectrometry analysis of total proteasome digests defined cleavage sites that clustered in positions 17 and 22–25. HPLC fractions yielded the same cleavage sites as total digests and additional cleavage sites in 9–16, 18–21, 26, and 27. Although numerous cleavage sites predicted by current databases were confirmed in positions 10–24, cleavage sites that were not predicted were characterized (in positions 9, 15, 18–21, 26, and 27). The COOH-terminal residues of the six preselected peptides that showed a significant binding to HLA class I alleles (peptides 2–11, 6–14, 6–16, 14–23, 15–24, and 15–25) were thus generated by proteasome.
Recognition of leader sequence preproinsulin peptides by human T-cells.
In our study, the most frequent class I alleles were HLA-A2 (21 patients and 12 control subjects), HLA-B8 (17 patients and 8 control subjects), HLA-A24 (14 patients and 1 control), and HLA-A1 (11 patients and 7 control subjects) (Table 1). Peptide-binding studies and, in the case of HLA-A2–restricted peptides, immunogenicity in HHD transgenic mice defined six possible HLA-A1–, -A2–, -A24–, or -B8–restricted epitopes that carry COOH-terminal residues generated by proteasome cleavage in vitro. Preproinsulin and control viral peptides were tested for recognition by T-cells from control and type 1 diabetic individuals using an IFN-γ ELISpot assay.
Viral peptides.
To evaluate IFN-γ responses to known CD8+ T-cell epitopes, responses of control and patient PBMCs to viral peptides were studied. Eighteen of 20 HLA-A2 patients and 8 of 11 HLA-A2 control subjects showed a positive response to influenza M.58–66 that ranged between 3 and 20 SD above background. Six of nine HLA-A1 individuals (3 of 6 patients and 3 of 3 control subjects) tested against influenza NP44–52 and 9 of 20 HLA-B8 individuals (7 of 13 patients and 2 of 7 control subjects) tested against influenza NP380–388 showed a positive response that ranged within 3 and 6 SD above background. No HLA-A2 or -B8 individuals showed a response to HIV peptides. No difference in the response to viral peptides used as positive and negative control subjects was observed between diabetic and control individuals (Table 3).
TABLE 3.
Recognition of viral peptides by PBMCs of patients and of control individuals
| V* | Epitope | Sequence | HLA† | Frequencies of recognition‡
|
Responses§
|
|||
|---|---|---|---|---|---|---|---|---|
| Patients | Control subjects | Patients | Control subjects | Comparison (P) | ||||
| IV | M0. 58–66 | GILGFVFTL | A2 | 18/20 | 8/11 | 89 (0–676) | 49 (0–246) | 0.36 |
| IV | NP 44–52 | CTELKLSDY | A1 | 3/6 | 3/3 | 39 (7–40) | 14 (10–20) | 0.43 |
| IV | NP 380–388 | ELRSRYWAI | B8 | 7/13 | 2/7 | 8 (0–119) | 5 (2–131) | 0.30 |
| HIV | Nef 83–91 | AAVDLSHFL | A2 | 0/12 | 0/4 | 3 (0–20) | 0 (0–5) | 0.06 |
| HIV | Nef 182–189 | EWRFDSRLA | B8 | 0/8 | 0/3 | 1 (0–35) | 0 (0–0) | 0.07 |
Data are n or median (mean).
V, virus; IV, influenza virus.
HLA, presenting HLA molecule.
ELISpot responses, number of responders/number of individuals tested.
Median (range) of stimulation score; stimulation score = mean number of spots in response to peptide − mean number of spots in absence of peptide; P, P values comparing the median responses in patients versus control subjects; nonparametric Mann-Whitney test.
Preproinsulin peptides.
All preproinsulin peptides tested were recognized by PBMCs of diabetic patients (Tables 4 and 5). When pooling control and patient responses to preproinsulin peptides, 0 and 65%, respectively, showed numbers of spots in the presence of peptide that were 3 SD above background. Setting the threshold for positive responses at background +3 SD (23), a positive IFN-γ response was observed against at least one preproinsulin leader sequence peptide with the same frequency in recent-onset (17 of 26) and long-standing (8 of 12) type 1 diabetic patients. No IFN-γ response to preproinsulin peptides was detected in 18 control individuals, including 6 type 2 diabetic patients. Some peptides were recognized with a comparable frequency in recent-onset and long-standing diabetic patients, i.e., peptides 6–14 and 6–16 for HLA-A2. Others peptides were selectively recognized in recent-onset patients (2–11, 14–23, and 15–24 for HLA-A2; and 2–11 and 15–25 for HLA-A24). When considering peptides tested with PBMCs of more than six patients carrying a defined class I allele, four peptides (2–11 for HLA-B8, 14–23 and 15–24 for HLA-A2, and 15–25 for HLA-A24) were recognized in >50% of recent-onset patients. Two peptides (6–16 for HLA-A2 and 2–11 for HLA-A2 and -A24) were recognized in at least 30% of recent-onset patients (Table 5).
TABLE 4.
Relative recognition frequencies of preproinsulin peptides in diabetic versus control subjects
| P* | HLA | Frequencies of recognition†
|
Comparison
|
|||||
|---|---|---|---|---|---|---|---|---|
| L | R | C | D | Patients | Control subjects | P‡ | ||
| 2–11 | A2 | 0/4 | 5/17 | 0/7 | 0/4 | 5/21 | 0/11 | 0.01 |
| A24 | 0/4 | 3/10 | 0/1 | — | 3/14 | 0/1 | — | |
| B8 | 4/10 | 4/7 | 0/6 | 0/2 | 8/17 | 0/8 | 0.0008 | |
| 6–16 | A2 | 2/4 | 7/16 | 0/8 | 0/4 | 9/20 | 0/12 | 0.0004 |
| 6–14 | A2 | 1/4 | 4/14 | 0/4 | 0/2 | 5/18 | 0/6 | 0.01 |
| 14–23 | A2 | 0/4 | 9/14 | 0/4 | 0/2 | 9/18 | 0/6 | 0.0003 |
| 15–24 | A2 | 1/4 | 7/13 | 0/4 | 0/2 | 8/17 | 0/6 | 0.0008 |
| 15–25 | A1 | 2/3 | 1/3 | 0/1 | — | 3/6 | 0/1 | — |
| A24 | 0/3 | 10/10 | 0/1 | — | 10/13 | 0/1 | — | |
Data are n. P, peptide; R, recent-onset diabetes; L, long-standing diabetes; C, healthy control subjects; D, type 2 diabetic control subjects.
NH2- and COOH-terminal amino acids in preproinsulin.
ELISpot responses, number of responders/number of individuals tested.
P values comparing the relative epitope recognition frequencies in patients (L + R) versus control subjects (C + D); nonparametric Mann-Whitney test.
TABLE 5.
Recognition of preproinsulin peptides by PBMCs of diabetic patients: median values of stimulation score
| P* | HLA | Responses†
|
Comparison‡
|
|||||
|---|---|---|---|---|---|---|---|---|
| L | R | C | D | Patients | Control subjects | P value | ||
| 2–11 | A2 | 3.3 (0–12.2) | 10.0 (0–41.8) | 0.56 (0–8.3) | 0 (0–0) | 7.8 (0–41.8) | 0 (0–8.3) | 0.01 |
| A24 | 5.2 (2.2–12.2) | 0 (0–41.8) | — | — | 2.8 (0–41.8) | — | — | |
| B8 | 25 (0–105) | 8.2 (0–37.2) | 0 (0–1.1) | 0 (0–5.6) | 15.6 (0–105) | 0 (0–5.6) | 0.01 | |
| 6–16 | A2 | 12.2 (10–54.5) | 5 (0–111.7) | 0 (0–9.5) | 0 (0–0) | 7.3 (0–111.7) | 0 (0–9.5) | 0.004 |
| 6–14 | A2 | 28.9 (21.1–51.1) | 30.6 (7.8–144) | 4.7 (3.9–7.2) | — | 30.6 (7.8–144) | 4.7 (3.9–7.2) | 0.01 |
| 14–23 | A2 | 11.1 (7.8–12.2) | 7.7 (54–0) | 1.4 (0–5.6) | — | 8.9 (54–0) | 1.4 (0–5.6) | 0.04 |
| 15–24 | A2 | 12.8 (2.4–32.2) | 8.7 (0–52.8) | 0 (0–0) | — | 12.2 (0–52.8) | 0 (0–0) | 0.002 |
| 15–25 | A1 | 13.6 (6.1–25.6) | 1.7 (0–14.4) | — | — | 9.2 (0–25.6) | — | — |
| A24 | 11.1 (5.8–25.6) | 0 (0–14.4) | — | — | 0 (0–25.6) | — | — | |
Data are median (range). P, peptide; R, recent-onset diabetes; L, long-standing diabetes; C, healthy control subjects; D, type 2 diabetic control subjects.
NH2- and COOH-terminal amino acids in preproinsulin.
ELISpot responses; median (range) of stimulation score; stimulation score = mean number of spots in response to peptide − mean number of spots in absence of peptide.
P values comparing the median responses in patients versus control subjects; nonparametric Mann-Whitney test.
Characterization of 6–14–specific T-cells as CD8+ T-cells.
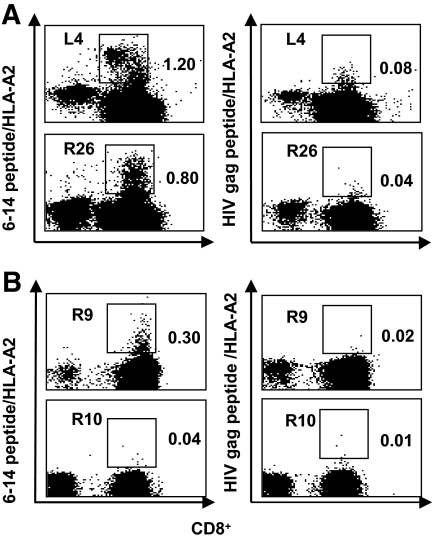
To set up the staining of CD8+ T-cells with 6–14 peptide/HLA-A2 tetramers, cells from patients L4 and R26 were expanded for 14 days with peptide 6–14 and studied for recognition of preproinsulin 6–14/HLA-A2 and HIV gag/HLA-A2 control tetramers. Staining with 6–14/HLA-A2 tetramer was at 1.2 and 0.8% CD8+ T-cells from patients L4 and R26, respectively, whereas the staining with HIVgag/HLA-A2 tetramer was at 0.08 and 0.04%, respectively (Fig. 3A). In subsequent experiments, some patients who showed a positive ELISpot response to peptide 6–14 were studied for 6–14 peptide/HLA-A2 tetramer binding. In four ELISpot-positive patients, 0.13, 0.14, 0.17, and 0.30% 6–14 peptide/HLA-A2–positive cells were detected in the CD8+ T-cell fraction, whereas in ELISpot-negative patients, the average of 6–14 peptide/HLA-A2 tetramer binding was 0.05 ± 0.01% (range 0.04–0.08%, n = 7, P < 0.02), bringing evidence that 6–14 peptide-reactive T-cells were clustered in the CD8+ T-cell subset as shown in an illustrative experiment (Fig. 3B). For comparison, mean HIV gag/HLA-A2 tetramer detection in the 11 patients was 0.04 ± 0.05.
FIG. 3.
Peptide/HLA-A2 tetramers staining of CD8+ T-cells from diabetic patients. A: Staining of expanded CD8+ T-cells from two diabetic patients. PBMCs from one long-standing (L4) diabetic patient and one recent-onset (R26) diabetic patient were cultured in presence of peptides (peptide 6–14 or HIV gag peptide) and then stained by 6–14 peptide/HLA-A2 and HIV gag peptide/HLA-A2 tetramers. Cultures were harvested for tetramer staining on day 14. HIV gag peptide/HLA-A2 tetramer was used as a negative control. Detection of tetramer-stained cells was performed by gating out 7-AAD+ dead cells and selecting CD8+ T-cells. The figure shows the staining with 6–14 peptide/HLA-A2 tetramer (left) and with HIV gag peptide/HLA-A2 tetramer (right) at day 14 of cell expansion. Dot plots show tetramer (vertical axis) versus CD8+ cells (horizontal axis) staining. The numbers displayed in each dot plot indicate the percentages of cells stained by tetramers. B: Ex vivo tetramer staining of PBMCs from 6–14 peptide ELISpot-positive and ELISpot-negative diabetic patients. PBMCs from 6–14 peptide ELISpot-positive diabetic patients were stained ex vivo with 6–14 peptide/HLA-A2 and HIV gag peptide/HLA-A2 tetramers. The figure shows the ex vivo staining with 6–14 peptide/HLA-A2 tetramer (left) and with HIV gag peptide/HLA-A2 tetramer (right) for one 6–14 peptide ELISpot-positive patient (R9). As a control, a 6–14 peptide ELISpot-negative patient (R10).
DISCUSSION
Defining epitopes derived from β-cell autoantigens is expected to have wide-range implications in development of T-cell assays and peptide-based immunotherapy in type 1 diabetes. Although numerous HLA class II–restricted CD4+ T-cell have been defined in human type 1 diabetes (14) and transgenic mice expressing human class II alleles (32,33,34), epitopes presented by class I alleles to CD8+ T-cells remain largely unknown. An HLA-A2–restricted GAD epitope (GAD 114–123) has been defined as the target of cytotoxic T-cells in two subjects with preclinical and recent-onset type 1 diabetes (35). An HLA-A2 epitope derived from islet amyloid polypeptide (IAPP) has been characterized using an IFN-γ ELISpot assay (36). More recently, new β-cell HLA class I–restricted epitopes have been reported. The proinsulin A chain, B chain, C-peptide, and leader sequence contain epitopes recognized by CD8+ T-cells (17,22,23,37–41). Recognition of epitopes encompassing residues excised during insulin processing makes a strong case for proinsulin as an autoantigen in diabetes. However, there has been no systematic study of leader sequence epitopes.
Leader sequence peptides are commonly presented to CD8+, especially HLA-A2–restricted, T-cells (42). Using a systematic approach to define class I epitopes derived from the preproinsulin leader sequence, we bring evidence that PBMCs from diabetic patients secrete IFN-γ in response to several epitopes. Five preproinsulin leader sequence peptides were recognized in 80% HLA-A2 patients. We did not restrict our study to epitopes showing the highest binding affinity to purified class I molecules because rules governing the spectrum of epitopes presented in autoimmunity remain far from clear. All five peptides that showed significant binding to purified HLA-A2 molecules in vitro were highly immunogenic in HHD transgenic mice. One peptide (proinsulin 2–11) was recognized in 57% HLA-B8 and 30% HLA-A24 recent-onset diabetic patients. Two peptides covering the 6–16 preproinsulin region correspond to the same epitope because 6–14–and 6–16–specific T-cell clones recognize indistinctly both peptides. In contrast, the 14–25 region contains two epitopes that are distinctly recognized by 14–23–and 15–24–specific peptides. Using 6–14/HLA-A2 tetramers, we bring direct evidence that preproinsulin peptide 6–14 is recognized by human CD8+ T-cells. In contrast, the small number of patients studied using tetramers preclude firm conclusions as to the concordance observed between IFN-γ production and tetramer staining.
In most patients, the response to preproinsulin leader sequence was multiepitopic. The long preclinical phase characterizing diabetes development does not preclude, however, that a more restricted set of peptides is dominantly recognized at initiation of the autoimmune process, as reported in case of a CD4+ T-cell–restricted epitope in the NOD mouse (43). A2-restricted GAD, IAPP, and preproinsulin peptide 2–10 were predominantly recognized in recent-onset diabetic patients (35,36,27). This was the case in our study for peptides 2–11 and 14–23, although not for HLA A2–restricted peptide 6–14. It should be acknowledged that our data do not directly correlate recognition of preproinsulin peptides by CD8+ T-cells and the absence of residual C-peptide in long-standing diabetic patients studied. However, a comparable observation was reported for other proinsulin epitopes (23) and may reconcile with previous observations in human type 1 diabetes (7). This may indicate that long-term memory class I–restricted T-cells persist in patients who are likely to have been deprived of remnant β-cells for years while on exogenous insulin therapy. Finally, the evidence that murine T-cell clones that were specific for leader sequence preproinsulin peptides were cytotoxic to P815 target cells expressing the human preproinsulin gene indicate that corresponding CD8+ T-cells may point to useful disease markers.
Using a strategy based on peptide library-mediated in vitro assembly of class I molecules, preproinsulin peptides have been defined on the basis of their association with HLA-B8, -A2, and -B15. Several epitopes were shown to harbor anchor residues that were weakly predicted by commonly used algorithms or did not contain canonical allele-specific binding motifs (44). In our study, some of these peptides were recognized in >40% of patients, such as preproinsulin 2–11 in HLA-B8; 6–16, 14–23, and 15–24 in HLA-A2; or 15–25 in both HLA-A1 and -A24 patients.
Proteasome-mediated proteolysis is a major, although not exclusive, system generating COOH termini of class I epitopes. Intracellular mechanisms that define the sequence of peptides presented to CD8+ T-cells include, in addition, peptide translocation in the endoplasmic reticulum by transporter associated with antigen processing (TAP) and binding of peptides into the MHC class I groove. As reported in case of viral epitopes (45), we used 20S proteasome–enriched preparations extracted from the T1 lymphoblastoid cell, which contains IFN-γ–inducible LMP-2 and LMP-7 proteasome subunits. Among candidate peptides, six carried COOH-terminal flanking residues that were identified after proteasome processing in vitro. One peptide (1–8) that carried a leucine in position 8 was not identified as a proteasome cleavage site. This is not unexpected because many peptides from signal sequences have been characterized as presented by class I alleles in a proteasome-independent and TAP-independent manner (46,47). However, this peptide showed no detectable binding to HLA-A2 nor immunogenicity in HHD transgenic mice. In contrast, peptide 2–11 showed significant binding to HLA-A2, significant immunogenicity in HHD transgenic mice and recognition by PBMCs from 29% HLA-A2 and 57% HLA-B8 diabetic patients.
Considering previous reports (23,26) and the present data, at least nine HLA-A2–restricted epitopes have been identified as recognized by CD8+ T-cells from type 1 diabetic patients within preproinsulin. Similar evidence that points to the high diversity of epitopes recognized by CD8+ T-cells within restricted regions of myelin autoantigens has been obtained in multiple sclerosis, in contrast with the limited number of immunodominant epitopes usually reported on viral antigens (48). Furthermore, epitope diversity was not restricted to HLA-A2 in multiple sclerosis (49). In the same line, there was no clear correlation between the prevalence of positive responses to peptides within the restricted preproinsulin region studied and affinity levels of peptide binding to HLA class I molecules. In vivo priming in HHD mice and study of peptide binding in vitro possibly biased our peptide selection procedure toward peptides with significant affinity, possibly underestimating the number of peptides recognized along diabetes development. However, frequent responses to intermediate affinity peptides were observed, such as proinsulin 2–11 in HLA-B8 and 14–23 in HLA-A2 patients. Because autoimmune reactions develop against self proteins, the autoantigen-specific T-cell repertoire is expected to be purged from high-avidity T-cells specific for dominant T-cell epitopes, opening the likelihood of autoreactive T-cells that are specific for subdominant or cryptic epitopes.
Our data bring evidence that type 1 diabetic patients shows class I–restricted responses to 8- to 11-mer peptides within the preproinsulin leader sequence. The absence of recognition of preproinsulin peptides by PBMCs from control individuals may point to leader sequence preproinsulin-specific CD8+ T-cells as useful disease markers.
Acknowledgments
A.T. is a recipient of a research fellowship from the Ministère de la Recherche et de la Technologie. This work was supported by Programme Hospitalier de Recherche Clinique P051078, by Agence Nationale de la Recherche Grant MELTD1, and by the Ministère de la Recherche et de la Technologie.
No other potential conflicts of interest relevant to this article were reported.
Published ahead of print at http://diabetes.diabetesjournals.org on 14 November 2008.
A.T. and T.L. contributed equally to this study.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Martin S, Wolf-Eichbaum D, Duinkerken G, Scherbaum WA, Kolb H, Noordzij JG, Roep BO: Development of type 1 diabetes despite severe hereditary B-lymphocyte deficiency. N Engl J Med 345: 1036–1040, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Verdaguer J, Schmidt D, Amrani A, Anderson B, Averill N, Santamaria P: Spontaneous autoimmune diabetes in monoclonal T cell nonobese diabetic mice. J Exp Med 186: 1663–1676, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh N, Hanafusa T, Miyazaki A, Miyagawa J, Yamagata K, Yamamoto K, Waguri M, Imagawa A, Tamura S, Inada M, Kawata S, Tarui S, Kono N, Matsuzawa Y: Mononuclear cell infiltration and its relation to the expression of major histocompatibility complex antigens and adhesion molecules in pancreas biopsy specimens from newly diagnosed insulin-dependent diabetes mellitus patients. J Clin Invest 92: 2313–2322, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Somoza N, Vargas F, Roura-Mir C, Vives-Pi M, Fernandez-Figueras MT, Ariza A, Gomis R, Bragado R, Marti M, Jaraquemada D: Pancreas in recent onset insulin-dependent diabetes mellitus: changes in HLA, adhesion molecules and autoantigens, restricted T cell receptor V. J Immunol 153: 1360–1377, 1994 [PubMed] [Google Scholar]
- 5.Conrad B, Weidmann E, Trucco G, Rudert WA, Behboo R, Ricordi C, Rodriquez-Rilo H, Finegold D, Trucco M: Evidence for superantigen involvement in insulin-dependent diabetes mellitus aetiology. Nature 371: 351–355, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Foulis AK, McGill M, Farquharson MA: Insulitis in type 1 (insulin-dependent) diabetes mellitus in man: macrophages, lymphocytes, and interferon-gamma containing cells. J Pathol 165: 97–103, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Sibley RK, Sutherland DE, Goetz F, Michael AF: Recurrent diabetes mellitus in the pancreas iso- and allograft: a light and electron microscopic and immunohistochemical analysis of four cases. Lab Invest 53: 132–144, 1985 [PubMed] [Google Scholar]
- 8.Yu L, Robles DT, Abiru N, Kaur P, Rewers M, Kelemen K, Eisenbarth GS: Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 97: 1701–1706, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler AG, Hummel M, Schenker M, Bonifacio E: Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 48: 460–468, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Keller RJ: Cellular immunity to human insulin in individuals at high risk for the development of type I diabetes mellitus. J Autoimmun 3: 321–327, 1990 [DOI] [PubMed] [Google Scholar]
- 11.Schloot NC, Roep BO, Wegmann D, Yu L, Chase HP, Wang T, Eisenbarth GS: Altered immune response to insulin in newly diagnosed compared to insulin-treated diabetic patients and healthy control subjects. Diabetologia 40: 564–572, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Rudy G, Stone N, Harrison LC, Colman PG, McNair P, Brusic V, French MB, Honeyman MC, Tait B, Lew AM: Similar peptides from two beta cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Mol Med 1: 625–633, 1995 [PMC free article] [PubMed] [Google Scholar]
- 13.Dubois-LaForgue D, Carel JC, Bougneres PF, Guillet JG, Boitard C: T-cell response to proinsulin and insulin in type 1 and pretype 1 diabetes. J Clin Immunol 19: 127–134, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Alleva DG, Crowe PD, Jin L, Kwok WW, Ling N, Gottschalk M, Conlon PJ, Gottlieb PA, Putnam AL, Gaur A: A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J Clin Invest 107: 173–180, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petersen LD, Duinkerken G, Bruining GJ, van Lier RA, de Vries RR, Roep BO: Increased numbers of in vivo activated T cells in patients with recent onset insulin-dependent diabetes mellitus. J Autoimmun 9: 731–737, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Durinovic-Bello I, Schlosser M, Riedl M, Maisel N, Rosinger S, Kalbacher H, Deeg M, Ziegler M, Elliott J, Roep BO, Karges W, Boehm BO: Pro- and anti-inflammatory cytokine production by autoimmune T cells against preproinsulin in HLA-DRB1*04, DQ8 type 1 diabetes. Diabetologia 47: 439–450, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Naquet P, Ellis J, Tibensky D, Kenshole A, Singh B, Hodges R, Delovitch TL: T cell autoreactivity to insulin in diabetic and related non-diabetic individuals. J Immunol 140: 2569–2578, 1988 [PubMed] [Google Scholar]
- 18.Daniel D, Gill RG, Schloot N, Wegmann D: Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur J Immunol 25: 1056–1062, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Daniel D, Wegmann DR: Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9–23). Proc Natl Acad Sci U S A 93: 956–960, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thebault-Baumont K, Dubois-Laforgue D, Krief P, Briand JP, Halbout P, Vallon-Geoffroy K, Morin J, Laloux V, Lehuen A, Carel JC, Jami J, Muller S, Boitard C: Acceleration of type 1 diabetes mellitus in proinsulin 2-deficient NOD mice. J Clin Invest 111: 851–857, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moriyama H, Abiru N, Paronen J, Sikora K, Liu E, Miao D, Devendra D, Beilke J, Gianani R, Gill RG, Eisenbarth GS: Evidence for a primary islet autoantigen (preproinsulin 1) for insulitis and diabetes in the nonobese diabetic mouse. Proc Natl Acad Sci U S A 100: 10376–10381, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hassainya Y, Garcia-Pons F, Kratzer R, Lindo V, Greer F, Lemonnier FA, Niedermann G, van Endert PM: Identification of naturally processed HLA-A2–restricted proinsulin epitopes by reverse immunology. Diabetes 54: 2053–2059, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Toma A, Haddouk S, Briand JP, Camoin L, Gahery H, Connan F, Dubois-Laforgue D, Caillat-Zucman S, Guillet JG, Carel JC, Muller S, Choppin J, Boitard C: Recognition of a subregion of human proinsulin by class I-restricted T cells in type 1 diabetic patients. Proc Natl Acad Sci U S A 102: 10581–10586, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarchum I, Nichol L, Trucco M, Santamaria P, DiLorenzo TP: Identification of novel IGRP epitopes targeted in type 1 diabetes patients. Clin Immunol 127: 359–365, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker C, Petrich de Marquesini LG, Bishop AJ, Hedges AJ, Dayan CM, Wong FS: Human CD8 responses to a complete epitope set from preproinsulin: implications for approaches to epitope discovery. J Clin Immunol 28: 350–360, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Mallone R, Martinuzzi E, Blancou P, Novelli G, Afonso G, Dolz M, Bruno G, Chaillous L, Chatenoud L, Bach JM, van Endert P: CD8+ T-cell responses identify β-cell autoimmunity in human type 1 diabetes. Diabetes 56: 613–621, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Martinuzzi E, Novelli G, Scotto M, Blancou P, Bach JM, Chaillous L, Bruno G, Chatenoud L, van Endert P, Mallone R: The frequency and immunodominance of islet-specific CD8+ T-cell responses change after type 1 diabetes diagnosis and treatment. Diabetes 57: 1312–1320, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Henderson RA, Michel H, Sakaguchi K, Shabanowitz J, Appella E, Hunt DF, Engelhard VH: HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science 255: 1264–1266, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Pascolo S, Bervas N, Ure JM, Smith AG, Lemonnier FA, Perarnau B: HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med 185: 2043–2051, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choppin J, Cohen W, Bianco A, Briand JP, Connan F, Dalod M, Guillet JG: Characteristics of HIV-1 Nef regions containing multiple CD8+ T cell epitopes: wealth of HLA-binding motifs and sensitivity to proteasome degradation. J Immunol 166: 6164–6169, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM: Phenotypic analysis of antigen-specific T lymphocytes. Science 274: 94–96, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Raju R, Munn SR, David CS: T cell recognition of human pre-proinsulin peptides depends on the polymorphism at HLA DQ locus: a study using HLA DQ8 and DQ6 transgenic mice. Hum Immunol 58: 21–29, 1997 [DOI] [PubMed] [Google Scholar]
- 33.Endl J, Otto H, Jung G, Dreisbusch B, Donie F, Stahl P, Elbracht R, Schmitz G, Meinl E, Hummel M, Ziegler AG, Wank R, Schendel DJ: Identification of naturally processed T cell epitopes from glutamic acid decarboxylase presented in the context of HLA-DR alleles by T lymphocytes of recent onset IDDM patients. J Clin Invest 99: 2405–2415, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herman AE, Tisch RM, Patel SD, Parry SL, Olson J, Noble JA, Cope AP, Cox B, Congia M, McDevitt HO: Determination of glutamic acid decarboxylase 65 peptides presented by the type I diabetes-associated HLA-DQ8 class II molecule identifies an immunogenic peptide motif. J Immunol 163: 6275–6282, 1999 [PubMed] [Google Scholar]
- 35.Panina-Bordignon P, Lang R, van Endert PM, Benazzi E, Felix AM, Pastore RM, Spinas GA, Sinigaglia F: Cytotoxic T cells specific for glutamic acid decarboxylase in autoimmune diabetes. J Exp Med 181: 1923–1927, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panagiotopoulos C, Qin H, Tan R, Verchere CB: Identification of a β-cell–specific HLA class I restricted epitope in type 1 diabetes. Diabetes 52: 2647–2651, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Ouyang Q, Standifer NE, Qin H, Gottlieb P, Verchere CB, Nepom GT, Tan R, Panagiotopoulos C: Recognition of HLA class I-restricted β-cell epitopes in type 1 diabetes. Diabetes 55: 3068–3074, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Standifer NE, Ouyang Q, Panagiotopoulos C, Verchere CB, Tan R, Greenbaum CJ, Pihoker C, Nepom GT: Identification of novel HLA-A*0201-restricted epitopes in recent-onset type 1 diabetic subjects and antibody-positive relatives. Diabetes 55: 3061–3067, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Pinkse GG, Boitard C, Tree TI, Peakman M, Roep BO: HLA class I epitope discovery in type 1 diabetes: independent and reproducible identification of proinsulin epitopes of CD8 T cells: report of the IDS T Cell Workshop Committee. Ann N Y Acad Sci 1079: 19–23, 2006 [DOI] [PubMed] [Google Scholar]
- 40.van Endert P, Hassainya Y, Lindo V, Bach JM, Blancou P, Lemonnier F, Mallone R: HLA class I epitope discovery in type 1 diabetes. Ann N Y Acad Sci 1079: 190–197, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Pinkse GG, Tysma OH, Bergen CA, Kester MG, Ossendorp F, van Veelen PA, Keymeulen B, Pipeleers D, Drijfhout JW, Roep BO: Autoreactive CD8 T cells associated with beta cell destruction in type 1 diabetes. Proc Natl Acad Sci U S A 102: 18425–18430, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gueguen M, Biddison WE, Long EO: T cell recognition of an HLA-A2-restricted epitope derived from a cleaved signal sequence. J Exp Med 180: 1989–1994, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen W, Bergerot I, Elliott JF, Harrison LC, Abiru N, Eisenbarth GS, Delovitch TL: Evidence that a peptide spanning the B-C junction of proinsulin is an early Autoantigen epitope in the pathogenesis of type 1 diabetes. J Immunol 167: 4926–4935, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Chang L, Kjer-Nielsen L, Flynn S, Brooks AG, Mannering SI, Honeyman MC, Harrison LC, McCluskey J, Purcell AW: Novel strategy for identification of candidate cytotoxic T-cell epitopes from human preproinsulin. Tissue Antigens 62: 408–417, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Kessler JH, Beekman NJ, Bres-Vloemans SA, Verdijk P, van Veelen PA, Kloosterman-Joosten AM, Vissers DC, ten Bosch GJ, Kester MG, Sijts A, Drijfhout JW, Ossendorp F, Offringa R, Melief CJ: Efficient identification of novel HLA-A(*)0201-presented cytotoxic T lymphocyte epitopes in the widely expressed tumor antigen PRAME by proteasome-mediated digestion analysis. J Exp Med 193: 73–88, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kloetzel PM: Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII. Nat Immunol 5: 661–669, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Engelhard VH, Brickner AG, Zarling AL: Insights into antigen processing gined by direct analysis of the naturally processed class I MHC associated peptide repertoire. Mol Immunol 39: 127–137, 2002 [DOI] [PubMed] [Google Scholar]
- 48.Yewdell JW: Confronting complexity: real-world immunodominance in antiviral CD8+ T cell responses. Immunity 25: 533–543, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Berthelot L, Laplaud DA, Pettré S, Ballet C, Michel L, Hillion S, Braudeau C, Connan F, Lefrère F, Wiertlewski S, Guillet JG, Brouard S, Choppin J, Soulillou JP: Blood CD8(+) T cell responses against myelin determinants in multiple sclerosis and healthy individuals. Eur J Immunol 38: 1889–1899, 2008 [DOI] [PubMed] [Google Scholar]