Introduction (Canine Genomics)
A dog genome sequence was made publicly available 2004, and is the basis of a new paradigm in the study, diagnosis, and treatment of inherited diseases in dogs. In reality, the way in which veterinary scientists think about and approach the study of genetic disease has not changed, but the tools we have available have and will continue to change, allowing us to study increasingly complex problems, and to make more rapid advances in context of simple problems. In order to put these advances in perspective, we will first give a historical perspective on the approaches to studying genetic diseases, particularly in humans, and then outline the advances that have become possible with the availability of the dog genome sequence. Then we will discuss two inherited defects that are associated with urolithiasis, in particular those responsible for cystine and purine (uric acid and its salts) stone formation. Together, these two conditions illustrate the contemporary use of a broad range of genetic approaches.
No matter what species are investigated, geneticists, base their approach to the study of a disease on the information available about that disease. The information falls into two broad categories. One category encompasses what is known at the protein, cellular, organ, and whole organism level. The second category encompasses what is known at the DNA level. Using these terms loosely, let’s think of the information as based either on the phenotype or on genome category. On one hand, phenotypic information can consist of specific enzyme activity or results of, any specific laboratory or clinical assay. On the other hand, the genomic category may include information about anatomical, developmental, behavioral, or abnormalities of a cell type, organ, or the entire living being. Genomic information can range from 1) understanding of the mode of inheritance through the study of families in which the disease is found, 2) the description of a chromosomal abnormality, to 3) any knowledge of the location of the DNA sequence variation that is responsible for a disease or other non-deleterious phenotypic characteristic. Information about a particular companion animal disease should also take into account what is known about similar diseases in other species, particularly in other mammals, such as humans and mice.
Most of the initial studies that identified DNA mutations underlying genetic disease were based on phenotypic knowledge of the defective, absent or inappropriate protein, abnormally expressed in a human disease. Through time-consuming and laborious methods, the cDNAs (complementary DNA; also called copy DNA. cDNA is DNA produced by reverse transcriptase acting on messenger RNA) and genes for these proteins were cloned and sequenced from affected and normal individuals, leading to discovery of disease-causing DNA mutations. This knowledge spilled over into veterinary medicine, leading to identification of underlying disease-causing mutations for diseases such as hemophilia, lysosomal storage diseases, and other simple inherited diseases due to enzyme deficiencies. For example, canine phosphofructokinase deficiency and MPS VII were understood at the molecular level in this fashion 43, 50. In these cases, the absence of a particular protein or enzyme activity indicated which genes might be defective. Prior cloning of these genes in humans or other mammals provided information and reagents that allowed their study in companion animals. In other words, the early approaches to understanding the DNA basis of genetic diseases, including the choice of genes that could be considered as candidate-genes to study, were based entirely on phenotypic information about the diseases and their pathology.
In the 1980’s, the approach to study of genetic disease based on genome position began to be used, and is generally referred to as “positional cloning”. This approach initially used a combination of cytogenetic data (l.e. observations of deletions of particular parts of chromosomes in affected individuals) and genetic mapping data. Genetic mapping refers to analyzing polymorphic sites (places in the genome where the DNA is known to vary in sequence between different individuals in the species) in families that contain one or more individuals affected with the disease of interest. The goal is to identify sites of DNA variation at variant alleles (alleles are different forms or variants of a gene found at the same place, or locus, on a chromosome) that travel from generation to generation with the disease. Various manifestations of the disease occur when the site of DNA variation is located on the same chromosome and close to the gene and mutation that causes disease. Consequently, by knowing the chromosomal location of the variable DNA sequence (also known as a “linked-marker”), one can detemine the approximate location of a disease-causing gene. The next step is to search the DNA surrounding the linked marker for the disease-causing gene and mutation. At one time, this was extremely time consuming. Fortunately, recent advances, including the development of polymerase chain reaction (PCR) technology, the availability of whole genome sequences, and some other “genomic” tools, have considerably simplified the last steps involved in positional cloning.
What is a genome sequence, and how is it used? A genome sequence is the complete DNA sequence of all of the chromosomes of a particular living being (except the Y chromosome in some cases). For mammalian species, a genome sequence is comprised of between 2.4 and 3 billion nucleotides. Genome sequences are currently generated by isolating genomic DNA from the organism of interest, and breaking or cutting the chromosome-length DNA molecules into predetermined sizes that can be readily cloned and sequenced. Once the sequencing of the ends of millions of clones has been completed, a super computer is used to 1) compare the sequences to one another to identify overlapping sequences 2) organize the individual sequences and, 3) determine the consensus sequence of each chromosome. If more DNA is sequenced, each individual base is sequenced more frequently, and the resulting genome sequence is likely to be more accurate. For the dog genome sequence, over 31 million sequences were generated (each data set consisting of between 500 and 600 nucleotides on average) and resulted in assemble-genome-sequences where each nucleotide was sequenced on the average of 7.6 times. This is referred to as a 7.6X genome sequence, and represents 99% of the dog genome.
Other sequence-based resources include databases of expressed-sequence-tags (ESTs). ESTs are generated from cloning all mRNA molecules from particular tissues or cell types, then sequencing the ends of these clones in effort to capture all of the mRNA molecules made by a particular being. If exhaustively cloned and analyzed, these mRNA molecules define all of the possible protein sequences encoded by the DNA of a particular species. When aligned to a whole genome sequence to show regions of identical DNA sequence, they pinpoint the location of the gene that encodes the mRNA and protein.
Other extremely useful DNA sequences are those that are variable within a species, (aka, polymorphisms). Databases exist that annotate all known single-nucleotide-polymorphisms (SNPs). This type of variation is particularly useful because high-throughput-assays have been developed (so-called SNP chips) that can determine the alleles present at hundreds of thousands of SNPs of an individual in a single experiment. The dog genome sequencing project identified more than 2.5 million SNPs. In addition, the technology now exists to simultaneously analyze more than 120,000 dog SNPs.
With so much DNA sequence information, the only practical method of interpreting and annotating the sequences is via a computer. Several genome browsers have been developed that are extremely helpful in integrating available DNA sequence data, with graphical interfaces that identify the location of gene, mRNAs, ESTs, SNPs, and evolutionarily conserved sequences that reside along the linear length of the chromosome. There are three main genome browsers available for the dog, NCBI, Santa Cruz Genome browser and Ensembl (http://www.ncbi.nlm.nih.gov/genome/guide/dog/, http://genome.ucsc.edu/, http://www.ensembl.org/index.html ).
How will availability of genome sequences, and other sequence-based information and technologies change the way genetic diseases can be studied in the future? Knowledge of as much about the phenotype of the disease as possible will still be of the utmost importance. As we will explain, technologically advanced genetic studies will remain of limited use if disease phenotypes are not well-defined, with appropriate information available for affected and unaffected animals. Family studies will still be relevant, since this information will continue to be useful in predicting the mode of inheritance of a particular disease or trait, and also to identify breeds that are at increased risk for a particular disease. However, genome sequences and related technologies are accelerating the rate at which discoveries are made and confirmed, and allow study of diseases that are more complex in their inheritance (e.g. susceptibility to cancer and autoimmunity), compared to those which are simple inherited diseases.
The current approach to a complex genetic disease is based on the assumption that, particularly within a breed, the disease is due to the same DNA mutation that was inherited from a common ancestor to all affected dogs. Therefore, all affected dogs share at least the part of the ancestral chromosome that contains the gene that suffered the disease causing mutation. They will also share some amount of DNA surrounding that gene, and that DNA will in essence be “marked” by the particular pattern of DNA variation that was present in the ancestor dog. For some diseases, the original disease-causing mutation may have occurred before the breed was formed, so that today the same mutation occurs in different breeds. Based on these assumptions, the current approach to a genetic disease will typically proceed in the following manner.
A disease is recognized as having a genetic basis because it has a particularly high incidence in one breed, a somewhat high incidence in several breeds, while multiple breeds also appear to be protected from the disease. In high incidence breed (s), there have been reports of litters with multiple affected offspring, but there have been enough investigations of the disease to demonstrate that parent-to-offspring transmission is a rare event. This information leads to the conclusion that the disease does not show simple autosomal dominant transmission, and that the disease occurs with equal frequency in males and females. DNA is collected from affected dogs in the high risk breed, as well as unaffected dogs of the same breed that have been thoroughly examined and when possible, are older than the age at which the disease is usually diagnosed). Appropriate evaluations have proved that they do not have the disease. The DNA sample from each dog of this group is analyzed to determine the location of alleles (genotype) at tens of thousands of SNPs across the dog genome, using available high-throughput technologies, such as “SNP chips”(currently both Affymetrix and Illumina offer canine SNP arrays). The massive amount of data that is obtained is statistically analyzed to identify SNPs at which the allele frequencies vary significantly between the affected dogs (cases) and the unaffected dogs (controls). An example would be the extreme case where, at a particular SNP, all affected dogs have an adenosine nucleotide (A) on both copies of the chromosome, while all the control dogs are either heterozygous for A and C (cytosine) or homozygous for C. The assumption in this example is that the association of the A allele with the disease phenotype is due to the original disease-causing mutation that occurred nearby on the chromosome that contained an A allele at this particular SNP. It has traveled with the disease-causing mutation through the generations that have passed since the mutation occurred. Since the location of the SNP is known, the next step is to look at the dog genome sequence in the region of the SNP, determining what genes are nearby, and finding out what is known about the function and expression of those genes. If it seems possible that the genes could be involved in the disease, they are then considered as candidate genes. Candidate genes are then sequenced in normal and affected dogs to determine if there are any DNA differences that might account for the disease. If so, additional experiments can be designed to prove that the DNA difference is actually the cause of the disease. Once candidate genes have been identified, much of what is known about a gene is likely to be information obtained from the study of humans and mice.
Once a disease-causing mutation has been identified, the potential benefits are both immediate and long-term. Of immediate benefit is the ability to use the knowledge of the specific disease-causing DNA change for genetic testing to provide information needed to reduce the incidence of the particular genetic disease. Of long-term benefit is the possibility that the knowledge of the molecular basis of the disease will lead to new therapies that could range from the development of a new pharmacological agent to the administration of a DNA-based (gene-) or cell-based (stem cell) therapy.
Genetic basis for urolithiasis in dogs
Certain types of uroliths are more likely to have a genetic basis than others. Primary changes in metabolism that lead to increased urine concentration of compounds that can form uroliths are the most obvious inherited causes of urolithiasis. In dogs, both cystine and uric acid can be excreted in urine in increased quantities due to metabolic changes resulting in cystinuria or hyperuricosuria. Some of the genes responsible for these disorders have been identified. In both of these cases, the metabolic change that leads to abnormally high concentrations of uric acid or cystine results in an increased risk for urolith formation.
In addition to these primary causes, predisposing factors exist that could have a genetic basis. For example, genetic factors that predispose dogs to urinary tract infections could increase the prevalence of infection-induced struvite uroliths. Another example is studies evaluating breed associations with specific types of uroliths. 33 These studies have provided some evidence that struvite urolithiasis occurs in some particular breeds more than in others. In particular, breeds with a male predisposition to struvite urolithiasis (Cocker spaniels, Springer spaniels and Labrador retrievers) have the potential to harbor a susceptibility locus since infection-induced struvite uroliths are generally less common in male dogs.
Urinary inhibitors and promoters of uroliths have been observed in the urine of humans and several other species including dogs 17,35. Genetic changes that lead to variation in the concentrations of these compounds in the urine could lead to increased risk for urolithaisis 12. General susceptibility to urolithiasis could also occur due to inherited anatomic differences of dogs. For example, the dependency of an individual dog’s bladder could lead to a predisposition to urethral obstruction. Anytime there is a breed predisposition to a particular urolith type, there is probably an underlying genetic factor that affects the prevalence in that breed.
Hyperuricosuria
Hyperuricosuria is defined as abnormal increase in the concentration of uric acid in the urine. Comparison of the excretion and/or concentration of urine uric acid in Dalmatian dogs to some (but by no means all) different types of dogs revealed that when specific and sensitive methodology for quantification of urine uric acid was used, all the Dalmatians had hyperuricosuria 6. Normal concentration of uric acid in dogs with normal renal function is considered to be low at ~ 9.5 mg/dl 37. Humans excrete about 3.5 times this quantity due to a defect in the enzyme uricase (aka, uric acid oxidase) that converts uric acid to allantoin in the liver 57. Allantoin is the end product of DNA and RNA metabolism in dogs, and is much more soluble in urine than uric acid. In addition to hyperuricosuria, humans have hyperuricemia (high serum concentrations of uric acid). Table 1 summarizes the relative amounts of serum and urine uric acid in dogs and humans. These values do not reflect the body weights of the different species which would make the canine numbers even higher. Humans suffer from consequences of elevated serum uric acid including gout, which is caused by precipitation of uric acid in the joints. Dalmatian dogs with mild increases of serum uric acid do not develop signs typical of gout (Table 1)
Table 1.
Relative amounts of uric acid in serum and urine in dogs and people
Values in mg/100ml, converted from 37.
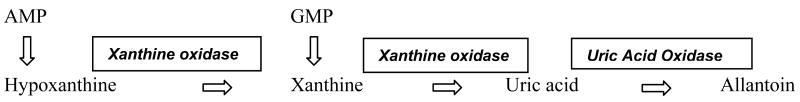
In dogs, excessive urinary excretion of uric acid can occur secondary to metabolic defects or due to particular disease states including generalized liver disease and portosystemic shunts. The enzyme urate oxidase appears to be exclusively produced in the liver. Consequently, severe liver disease can lead to a reduced quantity of uricase and varying degrees of hyperuricemia 26. In humans, tumor lysis syndrome leads to excess uric acid in serum and subsequently in urine. However this syndrome has not been documented in dogs. The biochemical pathway of purine degradation is shown in Figure 1. Decreased quantities of urate oxidase leads to hyperuricemia, in humans and great apes. The same phenotype can be seen in dogs with severe liver damage or port-systemic shunts.
Figure 1.
Biochemical steps in the degradation of purines (enzymes that catalyze the reactions are boxed). AMP=Adenine monophosphate GMP=Guanine monophosphate
Metabolic hyperuricosuria has been extensively studied in the Dalmatian breed of dog. There is a consensus of opinion that all Dalmatians are affected by this disorder 6,27. In these dogs, the defect is not in the enzyme urate oxidase as it is in humans, although on the surface the phenotypes appear similar 46. In contrast to humans, Dalmatians excrete more uric acid into the urine, resulting in lower serum uric acid concentrations than occurs in humans.
Normally, uric acid is filtered by glomerului and then reabsorbed by the proximal tubules where it re-enters circulation. There are species-specific differences in relative amounts of reabsorption and secretion of uric acid by the proximal tubules 44. The proteins involved in the reabsorption of uric acid from the ultrafiltrate are just beginning to be discovered. Uric acid must be transported across the apical membrane of the proximal tubules and then across the basolateral membrane in order to re-enter the systemic circulation. Micropuncture experiments were used to demonstrate that there is a reduction of proximal tubular reabsorption of uric acid in Dalmatian kidneys 45.
Based on studies which localized the Dalmatian defect to the liver 29, Giesecke & Tiemeyer studied the biochemical function of the enzyme uric acid oxidase18. These researchers demonstrated that while uric acid oxidase functions in non-Dalmatian liver homogenates and liver slices, Dalmatian uric acid oxidase was only able to convert uric acid to allantoin in liver homogenates and not in liver slices. On the basis of these observations, it was suggested that the Dalmatian phenotype could be explained by the lack of function of a urate transporter in the liver 18,28,53.
Since the Dalmatian phenotype involves liver metabolism and urinary excretion of uric acid, researchers studied liver and kidney tissues to determine the organ responsible for the problem. The hyperuricosuria defect was localized to the liver of affected dogs by transplantation studies. It is possible to correct the defect in Dalmatians and induce hyperuricosuria in wild-type dogs by transplanting the liver or hepatocytes between Dalmatian and non-Dalmatian dogs 29. When similar studies were done with kidney transplants, the affected phenotype could be improved but not corrected completely 3. Based on these observations, it is currently the general consensus of opinion that hyperuricosuria is due to a kidney phenotype and a liver phenotype in the Dalmatian dog and is associated with a defect in a uric acid transporter in both tissues.
Based on the outcomes of experimental crosses between a few different breeds and Dalmatians, Dalmatian hyperuricosuria is thought to be inherited as a simple autosomal recessive trait. 27,48. Clinical manifestations of this change in urinary metabolism are estimated to occur in about 25% of male Dalmatians 4. The clinical outcome of hyperuricosuria in the Dalmatian is apparently limited to urate uroliths. Ammonium urate uroliths and uroliths composed of other salts of uric acid are much less frequently observed in female Dalmatians as compared to male Dalmatians 1.
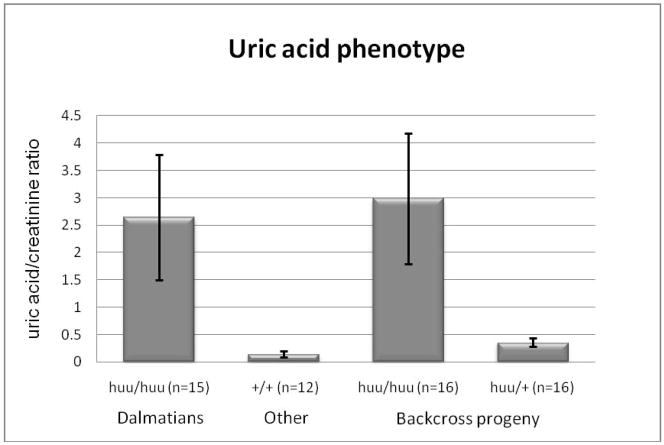
Since all Dalmatians are apparently affected with hyperuricosuria, cross breeding is necessary to identify the gene and the mutation that causes hyperuricosuria. To maintain the breed characteristics of the Dalmatian, Dr. Robert Schaible performed a (Dalmatian X Pointer) X (Dalmatian) backcross to segregate offspring into two groups: a low urine uric acid group and a high urine uric acid group. To determine if offspring excreted high or low concentrations of uric acid, puppies from this backcross were tested for urine uric acid/creatinine values when they were 4 to 7 weeks of age. Figure 2 shows a comparison of urine uric acid/creatinine values for pure Dalmatians, non-Dalmatians and two classes of backcross dogs (low urine uric acid and high urine uric acid).
Figure 2.
Uric Acid/creatinine ratios were measured on 4 to 6 week old puppies from the breeds shown in the figure. The animal’s genotype at the hyperuricosuria locus (huu) and the number of animals sampled in each column shown is shown below each bar. Statistically significant differences were obtained by ANOVA between the +/+, huu/+ and huu/huu categories (p = 8.33 × 10−13, alpha 0.05). In addition there was no difference between the two huu/huu categories (Dalmatian vs. backcross).
In order to map the hyperuricosuria locus, a set of markers were used to scan the canine chromosomes and identify a region that was inherited along with uric acid levels. A total of 148 markers were genotyped on 25 members of the backcross family. A single marker on chromosome CFA03, REN153P03, revealed linkage to the phenotype with a significant LOD (Logarithm of the Odds score) score of 3.99. Additional markers from the region were tested on 36 family members and a maximum LOD score of 6.55 was obtained for REN153P03 47. Additional markers tested in this family localized the critical interval containing the gene to a small region of the genome containing 19 candidate genes.
Since all Dalmatians are homozygous for the hyperuricosuria locus, the next approach was to identify the region of the chromosome where the DNA is identical in all Dalmatians. This further narrowed the list of genes to four candidates. Sequence analysis revealed a mutation in one gene that completely segregated with high uric acid status in the backcross dogs and was homozygous in 200 unrelated Dalmatians. The mutation was not detected in 300 non-Dalmatians (Bannasch unpublished data).
Other breeds have been reported to have a higher than expected incidence of urate stones, including the Bulldog and the Black Russian Terrier 5,33. When we tested dogs from these two breeds that had formed urate uroliths, they were also homozygous for the mutation. Since there is no known direct relationship between the Dalmatian, Bulldog and Black Russian Terrier, we believe that this mutation is very old and likely to occur in other breeds as well. In the Bulldog and Black Russian Terrier, the mutation segregates within the breeds, allowing breeders to use genetic testing of the mutation to select against hyperuricosuria. In the Dalmatian breed, the only way to eliminate hyperuricosuria, and therefore the predisposition to form urate uroliths, is by using the low uric acid backcross dogs (99.6% Dalmatian) in breeding programs. Currently, the United Kennel Club, the second largest purebred dog registry in the USA, allows these animals to be registered and therefore alter the prevalence of hyperuricoruia in the breed. At the time of writing this article, these dogs were not registered with the American Kennel Club, the largest registry in the USA due to a decision made by the parent club for the breed, the Dalmatian Club of America. Although breed purity is important in purebred dogs, the small amount of Pointer DNA remaining in these dogs (<0.05%) seems a small price to pay for disease elimination.
Cystinuria
Cystinuria is a disorder of transmembrane transport of the sulfur-containing non-essential amino acid cystine and dibasic amino acids lysine, ornithine, and arginine, which normally occurs in renal tubule cells after glomerular filtration, and also in the intestine. Clinical manifestations result from impaired reabsorption of cystine from glomerular filtrate, leading to excess cystine in the urine. Cystine, but not the dibasic amino acids, has low solubility in acidic urine, with only small elevations above the normal concentration exceeding the acid urine saturation point 51. As a result of cystinuria, affected humans and animals are at increased risk for formation of cystine uroliths. The impaired intestinal absorption of these four amino acids does not appear to lead to any nutritional deficiency states in dogs or humans, presumably because they are non-essential amino acids 41.
Development of a cystine urolith in a dog was first recognized in 1823, and the first documented case of cystinuria in a dog with evidence of a metabolic defect (elevated cystine levels in the urine accompanied by urolith formation) appeared in 1935 30,36. Recognition of the heritable nature of cystine stone formation in dogs soon followed when Brand and colleagues’ reported the disease in Irish terriers 19. Increased risk for cystine urolith formation has subsequently been documented for many breeds (summarized below).
Cystinuria has been recognized in humans for even longer than it has been documented in dogs, with discoveries in humans influencing studies in dogs. Cystinuria is recognized in two forms in humans; Type I and Non-Type I. Type I refers to cases that show recessive inheritance, and in which carriers have normal urinary amino acid concentrations. In Non-Type I cystinuria, the parents of patients show moderately elevated urine concentratons of cystine and the dibasic amino acids, lysine, arginine, and ornithine, and have a low incidence of cystine urolith formation. This genetic pattern is described as autosomal dominant inheritance with incomplete penetrance based on the rare incidence of cystine urolith formation in obligate carrier individuals 41.
The molecular basis for cystinuria in humans is well-documented, beginning with the first discovery of mutations in human patients 11. It is now known that mutations in two different genes account for the disease state in greater than 95% of human patients 16. These two genes, SLC3A1 and SLC7A9, encode the polypeptide subunits of the b0,+ amino acid transporter (for reviews, see 40–42). The system b0,+ amino acid transport is one of several known heteromeric amino acid transporters, which are composed of a heavy chain of the SLC3 (solute carrier family 3) family, and a light chain of the SLC7 family (Author; Please define SLC3, etc.). The SLC7 subunits, composed of 12 transmembrane domains, provide the specific amino acid transport activity, and are linked to the heavy subunit by a single disulfide bond. The SLC3 subunits have a single transmembrane domain with a cytoplasmic amino terminus. The majority of this heavy chain is extracellular and heavily glycosylated. The SLC3 heavy chain appears to be essential for localization of the heteromeric transporter to the plasma membrane 42.
The defective genes in human cystinuria are SLC3A1 encoding a protein called rBAT, and SLC7A9 encoding the protein referred to as b0,+AT. Over 170 different mutations in these two genes have been identified in cystinuric human patients 16. With only one exception 49, all of the SLC3A1 mutations are associated with Type I cystinuria. In SLC7A9, nearly 85% of the SLC7A9 mutations are associated with the Non-Type I urinary amino acid pattern and the remaining 15% with Type-I cystinuria 15,42. The disease causing mutations remain unidentified in approximately 15% of cystinuric patients 16. These unexplained cystinuria cases may be the result of 1) mutations in the promoter or intronic regions, 2) the combined results of SLC3A1 or SLC7A9 polymorphisms in combination with specific cystinuria-causing mutations, or 3) mutations in as yet unidentified genes 16.
In dogs, cystinuria has primarily been diagnosed by analysis of the mineral composition of uroliths that have been removed from dogs suffering from some degree of urinary tract obstruction. Due to the availability of free urolith analysis at the Universities of Minnesota and California, a large amount of data has been accumulated. As for most types or uroliths, cystine uroliths, are found predominantly in male dogs 10,14,34,39,56. Approximately 1% of all uroliths in dogs in the United States are cystine stones 34,39, with stone analysis laboratories in Europe reporting as high as 20% cystine stones (see for example 54; 23. The mean age of dogs when cystine calculi are retrieved is between 4.8 years and 5.6 years 10,23,34.
Urinary cystine crystals have a characteristic hexagonal shape, providing a useful diagnostic sign 38. Cystinuric dogs can also be identified by analysis of urine for cystine. The cyanide nitroprusside reaction is a simple qualitative screening test for cystinuria, giving a positive result when 75–125 mg cystine/g creatinine are present in a urine sample. Quantification of all urinary amino acids by high pressure liquid chromatography can also be performed, but is expensive and not a routine veterinary test. In dogs, single urine specimens are collected without regard to time since most recent feeding. In contrast, in human patients a 24-hour urine sample is analyzed, making correlations of the canine and human disease more difficult. However, when amino acid concentrations are adjusted to urinary creatinine concentrations 51, there is a strong correlation between single sample and 24-hour cystine values 24. Tsan recognized the difficulty in diagnosing aminoaciduria from a single urine sample, when he stated “A low cystine value from a single urinary sample does not prove that the dog is not cystinuric” 52. There may be multiple factors contributing to this phenomenon, such as diet and diurnal variation in cystine secretion 31.
Cystine uroliths have been recovered from dogs of approximately 70 breeds 32,39. The following findings have been taken as evidence of a genetic basis: 1) increased incidence or risk of cystine urolith formation (based on data from urolith analysis laboratories), 2) multiple related cystinuric dogs or test matings that produce affected dogs, 3) detection of excess urinary cystine and dibasic amino acids or abnormal cystine renal clearance in urolith-forming dogs, and 4) urolith formation documented in female dogs. One or both of the first two criteria are found in several breeds (Australian cattle dog, Australian shepherd, Basenji, Basset hound, Bullmastiff, Chihuahua, Dachshund, English bulldog, Irish terrier, Mastiff, Newfoundland, Scottish deerhound, Scottish terrier, Staffordshire terrier, and Welsh corgi, while one of the four criteria has been observed in at least 23 dog breeds (reviewed in20).
Mating studies have been conducted for several breeds, beginning with the early studies of Irish terriers, where a cystinuric nephew of the propositus was identified 8 and additional matings produced 12 cystinuric dogs 9. In a mating of a cystinuric male to two of his sisters, cystine urinary excretion was reported as slightly higher than normal in several dogs of one mating, while the other mating produced two cystinuric males and one cystinuric female. It was concluded that the inheritance of cystinuria was recessive, and while most affected dogs were males, an autosomal trait could not be excluded. The analysis of a Scottish terrier pedigree is suggestive of X-linked recessive inheritance 52. In a similar study, only male Basset hounds were cystinuric 2. However, all unaffected dams were siblings or daughters of cystinuric dogs, hence, autosomal recessive inheritance could not be excluded. Breeding studies in Newfoundland dogs excluded the X-linked recessive mode of inheritance and showed that cystinuria in this breed is inherited in an autosomal recessive fashion. Aminoaciduria is present in male and female Newfoundlands at equal frequencies, with clinical signs predominating in males due to anatomical differences 13.
Combined with breeding studies, urine amino acid analysis and metabolic studies, cystinuria in Newfoundland dogs closely resembles the type-I disease in humans 13. Affected male dogs formed cystine uroliths as early as 4 to 6 months of age, while females formed uroliths less commonly and later in life. Cloning and sequencing the canine SLC3A1 cDNA and genes from normal and affected animals revealed that affected dogs were homozygous for a nonsense mutation in exon 2 of the gene. This mutation results in a severely truncated protein of 197 amino acids compared to the normal polypeptide length of 700 amino acids 21. Testing for this mutation has been commercially available for several years, allowing breeders to detect which dogs are clinically normal carriers of the mutation and, and to choose mating pairs that cannot produce affected offspring. A similar mutation in SLC3A1 has been detected in cystinuric Labrador retrievers, which have a disease phenotype that resembles the Newfoundland Type I cystinuria (P. Henthorn et al., unpublished data).
While Newfoundlands and Labrador retrievers clearly have a severe (early age of urolith formation) Type I form of cystinuria, the clinical and biochemical expression of cystinuria in other breeds seems to be quite variable. While some differences in various studies may be related to degree of clinical and laboratory investigation as well as methodological differences, multiple reports of variant aspects of phenotypes have emerged from independent sources over the years. These include variable cystine excretion in urolith forming dogs, ranging from normal to approximately 100 times normal 23, both normal and elevated cystine excretion at different times in stone-forming dogs 25. Variation in the underlying renal transport defect has been documented in renal clearance studies demonstrating variation from 50% of the normal reabsorption to active secretion within a breed and between breeds 7. Multiple reports document urolith formation in dogs in which excess urine cystine concentrations were not detected 22,24. These cases suggest that elevated urinary cystine concentration is not the only factor to consider as a cause of cystine urolith formation, as well as the possibility of a non-genetic form of cystinuria, where cystine urolith formation occurs in the absence of an amino acid transport defect 23.
The estimated age of the dog at the time of urolith formation appears to be an important factor in classifying canine cystinuria. While the mean age at which cystine urolithiasis occurs in dogs is about 5 years, 11% of dogs form stones at less than 2 years of age 55, with variation both between canine breeds and within individual cystinuric dogs of a breed 23. Urolith-forming dogs with documented high urine cystine values that decreased with age to within the normal range 24, and apparent spontaneous resolution of cystinuria have also been observed in a Dachshund 39.
Taking all the phenotypic and genetic data together, including breed-specific information, it is clear the canine cystinuria is genetically heterogeneous. Cystinuria in Newfoundlands (and also Labrador retrievers) clearly differs from the “average” phenotype of cystinuria in other dog breeds 13, including higher incidence of urolith formation in female dogs, and the juvenile age of urolith formation in male dogs 39. In non-Newfoundland dogs, cystine and the dibasic amino acids appear to be excreted in the urine at lower concentrations than in Newfoundlands (Table 2). While the urine cystine and urine dibasic amino acid ranges in Newfoundland dogs 13 and those seen in 24 cystinuric dogs of other breeds 25 overlap, mean values for Newfoundland dogs are at least three times as high as for the group of the other 24 cystinuric dogs. There is also molecular evidence for genetic heterogeneity in canine cystinuria. Sequencing of the SLC3A1 and SLC7A9 protein coding regions in a various breedsof cystine urolith forming dogs of various breeds have not revealed disease-causing mutations 21.
Table 2.
Urine Cystine and Dibasic Amino Acid Levels in Normal and Cystinuric Dogs
| Cystine | Ornithine | Lysine | Arginine | |
|---|---|---|---|---|
| Dogs of 9 breeds (not Newfoundland) 25 mean (SD; range) | ||||
| Cystinuric | 368 (291; 17–1115) | 152 (215; 18–1062) | 1283 (1558; 169–6859) | 177 (157; 12–655) |
| Normal | 39 (26; 8–92) | 39 (16; 9–73) | 190 (58; 56–286) | 84 (96; 14–335) |
| Newfoundland dogs 13 mean ± SD | ||||
| Cystinuric | 1081 ± 446 | 1930 ± 2414 | 3494 ± 3667 | 4552 ± 5173 |
| Normal Relatives | 54 ± 38 | 71± 36 | 143±102 | 83 ±86 |
| Normal Unrelated | <179 | <202 | <464 | <452 |
While these values were not obtained from the same laboratory, examination of the normal values for each group indicates that they are roughly comparable.
Based upon breed predilections, varied clinical features, and urinary amino acid patterns, there are at least two types of cystinuria in the dog. Type I cystinuria, documented in Newfoundlands and Labrador retrievers, is homologous to type I cystinuria in humans with mutations in the SLC3A1 gene. It is characterized by early age of cystine urolith formation in males, occasional urolith occurrence in females, and markedly high urinary excretion of cystine and the dibasic amino acids. Cystinuria in many other breeds is characterized by cystine urolith formation later in life (not before adulthood), moderately elevated and variable urinary excretion of cystine and dibasic amino acids, with an as yet unknown mode of inheritance. Therefore, while mutations in one of cystine transporter genes have been found to cause cystinuria in some dogs, the genetic basis of canine cystinuria appears to be more complex than in humans 13,21. Discovery of the genetic basis of the more complex forms will undoubtedly rely on the new approaches made possible by the dog genome sequence.
Acknowledgments
The authors thank Drs. Urs Giger, Margret L. Casal, Peter F. Jezyk, Kenneth Bovee, Stanton Segal as well as Junlong Liu, Angela Huff, Adam Sang, Tanya Gidalevitz, and Ping Wang for their contributions to the various canine cystinuria studies performed at the University of Pennsylvania. The authors also acknowledge the work of Drs. Noa Safra, R. Schaible, and G. V. Ling on hyperuricosuria.
This work was supported by grants from the National Institute of Health (NIDDK DK074954 and RR02512), and grants from the Morris Animal Foundation and the Canine Health Foundation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albasan H, Lulich JP, Osborne CA, et al. Evaluation of the association between sex and risk of forming urate uroliths in Dalmatians. J Am Vet Med Assoc. 2005;227:565. doi: 10.2460/javma.2005.227.565. [DOI] [PubMed] [Google Scholar]
- 2.Albrecht F. Symptoms, diagnosis, and treatment of cystinuria in the dog. [German] Kleintier-Praxis. 1974;19:202. [Google Scholar]
- 3.Appleman R, Hallenbeck G, RG S. Effect of reciprocal allogeneic renal transplantation between Dalmatian and non-dalmatian dogs on urinary excretion of uric acid. Proceedings of the Society of Experimental Biological Medicine. 1966;121:1094. doi: 10.3181/00379727-121-30975. [DOI] [PubMed] [Google Scholar]
- 4.Bannasch DL, Ling GV, Bea J, et al. Inheritance of urinary calculi in the Dalmatian. J Vet Intern Med. 2004;18:483. doi: 10.1892/0891-6640(2004)18<483:ioucit>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 5.Bende B, Nemeth T. High prevalence of urate urolithiosis in the Russian black terrier. Vet Rec. 2004;155:239. doi: 10.1136/vr.155.8.239. [DOI] [PubMed] [Google Scholar]
- 6.Benedict SR. The Harvey Lectures page 346. Journal of Laboratory and Clinical Medicine. 1916;1 [Google Scholar]
- 7.Bovee KC, Thier SO, Rea C, et al. Renal clearance of amino acids in canine cystinuria. Metabolism Clinical and Experimental. 1974;23:51. doi: 10.1016/0026-0495(74)90103-6. [DOI] [PubMed] [Google Scholar]
- 8.Brand E, Cahill GF. Canine cystinuria. III. Journal of Biological Chemistry. 1936;114:xv. [Google Scholar]
- 9.Brand E, Cahill GF, Kassell B. Canine cystinuria V. Family history of two cystinuric Irsih terriers and cystine determinations in dog urine. J Biol Chem. 1940;133:431. [Google Scholar]
- 10.Brown NO, Parks JL, Greene RW. Canine urolithiasis: retrospective analysis of 438 cases. J Am Vet Med Assoc. 1977;170:414. [PubMed] [Google Scholar]
- 11.Calonge MJ, Gasparini P, Chillaron J, et al. Cystinuria caused by mutations in rBAT, a gene involved in the transport of cystine [see comments] Nature Genetics. 1994;6:420. doi: 10.1038/ng0494-420. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho MLJ, Osborne CA, Nakagawa Y. Defective urinary crystallization inhibition and urinary stone formation. Int Braz J Urol. 2006;32:342. doi: 10.1590/s1677-55382006000300016. [DOI] [PubMed] [Google Scholar]
- 13.Casal ML, Giger U, Bovee KC, et al. Inheritance of cystinuria and renal defect in Newfoundlands. Journal of the American Veterinary Medical Association. 1995;207:1585. [PubMed] [Google Scholar]
- 14.Case LC, Ling GV, Franti CE, et al. Cystine-containing urinary calculi in dogs: 102 cases (1981–1989) Journal of the American Veterinary Medical Association. 1992;201:129. [PubMed] [Google Scholar]
- 15.Dello Strologo L, Pras E, Pontesilli C, et al. Comparison between SLC3A1 and SLC7A9 cystinuria patients and carriers: a need for a new classification. J Am Soc Nephrol. 2002;13:2547. doi: 10.1097/01.asn.0000029586.17680.e5. [DOI] [PubMed] [Google Scholar]
- 16.Font-Llitjós M, Jimenez-Vidal M, Bisceglia L, et al. New insights into cystinuria: 40 new mutations, genotype-phenotype correlation, and digenic inheritance causing partial phenotype. J Med Genet. 2005;42:58. doi: 10.1136/jmg.2004.022244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forterre SRJ, Kohn B, Brunnberg L, Schweigert FJ. Protein profiling of organic stone matrix and urine from dogs with urolithiasis. J Anim Physiol Anim Nutr (Berl) 2006;90:192. doi: 10.1111/j.1439-0396.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- 18.Giesecke D, Tiemeyer W. Defect of uric acid uptake in Dalmatian dog liver. Experientia. 1984;40:1415. doi: 10.1007/BF01951919. [DOI] [PubMed] [Google Scholar]
- 19.Green DF, Morris ML, Cahill GF, et al. Canine cystinuria II. Analysis of cystine calculi and sulfur distribution in the urine. J Biol Chem. 1936;114:91. [Google Scholar]
- 20.Henthorn PS, Giger U. Cystinuria. In: Ostrander G, Lindblad-Toh, editors. The Dog and Its Genome. Cold Spring Harbor Laboratory Press; 2006. p. 349. [Google Scholar]
- 21.Henthorn PS, Liu J, Gidalevich T, et al. Canine cystinuria: polymorphism in the canine SLC3A1 gene and identification of a nonsense mutation in cystinuric Newfoundland dogs. Human Genetics. 2000;107:295. doi: 10.1007/s004390000392. [DOI] [PubMed] [Google Scholar]
- 22.Holtzapple PG, Rea C, Bovee K, et al. Characteristics of cystine and lysine transport in renal jejunal tissue from cystinuric dogs. Metabolism: Clinical & Experimental. 1971;20:1016. doi: 10.1016/0026-0495(71)90024-2. [DOI] [PubMed] [Google Scholar]
- 23.Hoppe A, Denneberg T. Cystinuria in the dog: clinical studies during 14 years of medical treatment. [erratum appears in J Vet Intern Med 2001 Nov-Dec;15(6):594] Journal of Veterinary Internal Medicine. 2001;15:361. [PubMed] [Google Scholar]
- 24.Hoppe A, Denneberg T, Jeppsson JO, et al. Canine cystinuria: an extended study on the effects of 2-mercaptopropionylglycine on cystine urolithiasis and urinary cystine excretion. [see comment] British Veterinary Journal. 1993;149:235. doi: 10.1016/S0007-1935(05)80170-8. [DOI] [PubMed] [Google Scholar]
- 25.Hoppe A, Denneberg T, Jeppsson JO, et al. Urinary excretion of amino acids in normal and cystinuric dogs. [see comment] British Veterinary Journal. 1993;149:253. doi: 10.1016/S0007-1935(05)80171-X. [DOI] [PubMed] [Google Scholar]
- 26.Alvares KRJW, Abu-Jawdeh GM, Schmidt JV, Yeldandi AV, Rao MS, Reddy JK. Rat urate oxidase produced by recombinant baculovirus expression: formation of peroxisome crystalloid core-like structures. PNAS. 1992;89:4908. doi: 10.1073/pnas.89.11.4908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keeler CE. The Inheritance of Predisposition to Renal Calculi in the Dalmatian. Journal of American Veterinary Medical Association. 1940;96:507. [Google Scholar]
- 28.Kocken JM, Borel Rinkes IH, Bijma AM, et al. Correction of an inborn error of metabolism by intraportal hepatocyte transplantation in a dog model. Transplantation. 1996;62:358. doi: 10.1097/00007890-199608150-00010. [DOI] [PubMed] [Google Scholar]
- 29.Kuster G, Shorter R, Dawson B, et al. Uric acid metabolism in dalmatians and other dogs. Role of the liver Archives of Internal Medicine. 1972;129:492. [PubMed] [Google Scholar]
- 30.Lassaigne JL. Observation sur l’existence de l’oxide cystique dans un calcul vesical du chien, et essai analytique sur la composition elementaire de cette substance particuliere. Ann Chim Phys. 1823;23:328. [Google Scholar]
- 31.Lindell A, Denneberg T, Jeppsson JO, et al. Measurement of diurnal variations in urinary cystine saturation. Urol Res. 1995;23:215. doi: 10.1007/BF00393301. [DOI] [PubMed] [Google Scholar]
- 32.Ling GV, Franti CE, Ruby AL, et al. Urolithiasis in dogs II: breed prevalence, and interrelations of breed, sex, age, and mineral composition. American Journal of Veterinary Research. 1998;59:630. [PubMed] [Google Scholar]
- 33.Ling GV, Franti CE, Ruby AL, et al. Urolithiasis in dogs. II: Breed prevalence, and interrelations of breed, sex, age, and mineral composition. Am J Vet Res. 1998;59:630. [PubMed] [Google Scholar]
- 34.Ling GV, Franti CE, Ruby AL, et al. Urolithiasis in dogs I: mineral prevalence and interrelations of mineral composition, age, and sex. American Journal of Veterinary Research. 1998;59:624. [PubMed] [Google Scholar]
- 35.Marangella M, Bagnis C, Bruno M, et al. Crystallization inhibitors in the pathophysiology and treatment of nephrolithiasis. Urol Int. 2004;72 (Suppl 1):6. doi: 10.1159/000076583. [DOI] [PubMed] [Google Scholar]
- 36.Morris ML, Green DF, Dinkel JH, et al. Canine Cystinuria. An unusual case of urinary calculi in the dog. N Amer Vet. 1935;16:16. [Google Scholar]
- 37.Moulin B, Vinay P, Duong N, et al. Net urate reabsorption in the Dalmatian coach hound with a note on automated measurement of urate in species with low plasma urate. Can J Physiol Pharmacol. 1982;60:1499. doi: 10.1139/y82-221. [DOI] [PubMed] [Google Scholar]
- 38.Osborne CA, O’Brien TD, Ghobrial HK, et al. Crystalluria. Observations, interpretations, and misinterpretations Veterinary Clinics of North America. Small Animal Practice. 1986;16:45. doi: 10.1016/s0195-5616(86)50004-8. [DOI] [PubMed] [Google Scholar]
- 39.Osborne CA, Sanderson SL, Lulich JP, et al. Canine cystine urolithiasis. Cause, detection, treatment, and prevention Veterinary Clinics of North America. Small Animal Practice. 1999;29:193. doi: 10.1016/s0195-5616(99)50011-9. [DOI] [PubMed] [Google Scholar]
- 40.Palacin M, Estevez R, Bertran J, et al. Molecular biology of mammalian plasma membrane amino acid transporters. Physiological Reviews. 1998;78:969. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 41.Palacin M, Goodyer P, Nunes V, et al. Cystinuria. In: Scriver CR, Beaudet AL, Sly WS, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. III. New York: McGraw-Hill; 2001. p. 4909. [Google Scholar]
- 42.Palacin M, Nunes V, Font-Llitjos M, et al. The genetics of heteromeric amino acid transporters. Physiology (Bethesda) 2005;20:112. doi: 10.1152/physiol.00051.2004. [DOI] [PubMed] [Google Scholar]
- 43.Ray JBA, DeSanto C, Fyfe JC, Xu D, Wolfe JH, Aguirre GD, Patterson DF, Haskins MEHP. Cloning of the canine beta-glucuronidase cDNA, mutation identification in canine MPS VII, and retroviral vector-mediated correction of MPS VII cells. Genomics. 1998;48:248. doi: 10.1006/geno.1997.5189. [DOI] [PubMed] [Google Scholar]
- 44.Roch-Ramel F, Peters G. Urinary excretion of uric acid in nonhuman mammalian species. In: Weiner KWIM, editor. Uric Acid. Berlin: Springer-Verlag; 1978. p. 211. [Google Scholar]
- 45.Roch-Ramel F, Wong NL, Dirks JH. Renal excretion of urate in mongrel and Dalmatian dogs: a micropuncture study. Am J Physiol. 1976;231:326. doi: 10.1152/ajplegacy.1976.231.2.326. [DOI] [PubMed] [Google Scholar]
- 46.Safra N, Ling GV, Schaible RH, et al. Exclusion of urate oxidase as a candidate gene for hyperuricosuria in the Dalmatian dog using an interbreed backcross. J Hered. 2005;96:750. doi: 10.1093/jhered/esi078. [DOI] [PubMed] [Google Scholar]
- 47.Safra N, Schaible RS, Bannasch DL. Linkage analysis with an interbreed backcross maps Dalmatian hyperuricosuria to CFA03. Mammalian Genome. 2006;17:340. doi: 10.1007/s00335-005-0137-5. [DOI] [PubMed] [Google Scholar]
- 48.Schaible RH. Genetic predisposition to purine uroliths in Dalmatian dogs. Vet Clin North Am Small Anim Pract. 1986;16:127. doi: 10.1016/s0195-5616(86)50007-3. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt C, Vester U, Wagner CA, et al. Significant contribution of genomic rearrangements in SLC3A1 and SLC7A9 to the etiology of cystinuria. Kidney Int. 2003;64:1564. doi: 10.1046/j.1523-1755.2003.00250.x. [DOI] [PubMed] [Google Scholar]
- 50.Smith BFSH, Rajpurohit Y, Henthorn PS, Wolfe JH, Patterson DF, Giger U. Molecular basis of canine muscle type phosphofructokinase deficiency. J Biol Chem. 1996;271:20070. doi: 10.1074/jbc.271.33.20070. [DOI] [PubMed] [Google Scholar]
- 51.Treacher RJ. Urolithiasis in the dog - II Biochemical aspects. J Small Anim Pract. 1966;7:537. doi: 10.1111/j.1748-5827.1966.tb04483.x. [DOI] [PubMed] [Google Scholar]
- 52.Tsan MF, Jones TC, Thornton GW, et al. Canine cystinuria: its urinary amino acid pattern and genetic analysis. American Journal of Veterinary Research. 1972;33:2455. [PubMed] [Google Scholar]
- 53.Vinay P, Gattereau A, Moulin B, et al. Normal urate transport into erythrocytes in familial renal hypouricemia and in the Dalmatian dog. Can Med Assoc J. 1983;128:545. [PMC free article] [PubMed] [Google Scholar]
- 54.Wallerstrom BI, Wagberg TI. Canine Urolithiasis in Sweden and Norway - Retrospective Survey of Prevalence and Epidemiology. Journal of Small Animal Practice. 1992;33:534. [Google Scholar]
- 55.Wallerstrom BI, Wagberg TI, Lagergren CH. Cystine Calculi in the Dog - an Epidemiologic Retrospective Study. Journal of Small Animal Practice. 1992;33:78. [Google Scholar]
- 56.Weaver AD. Canine urolithiasis: incidence, chemical composition and outcome of 100 cases. J Small Anim Pract. 1970;11:93. doi: 10.1111/j.1748-5827.1970.tb06134.x. [DOI] [PubMed] [Google Scholar]
- 57.Wu XW, Muzny DM, Lee CC, et al. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. 1992;34:78. doi: 10.1007/BF00163854. [DOI] [PubMed] [Google Scholar]