Abstract
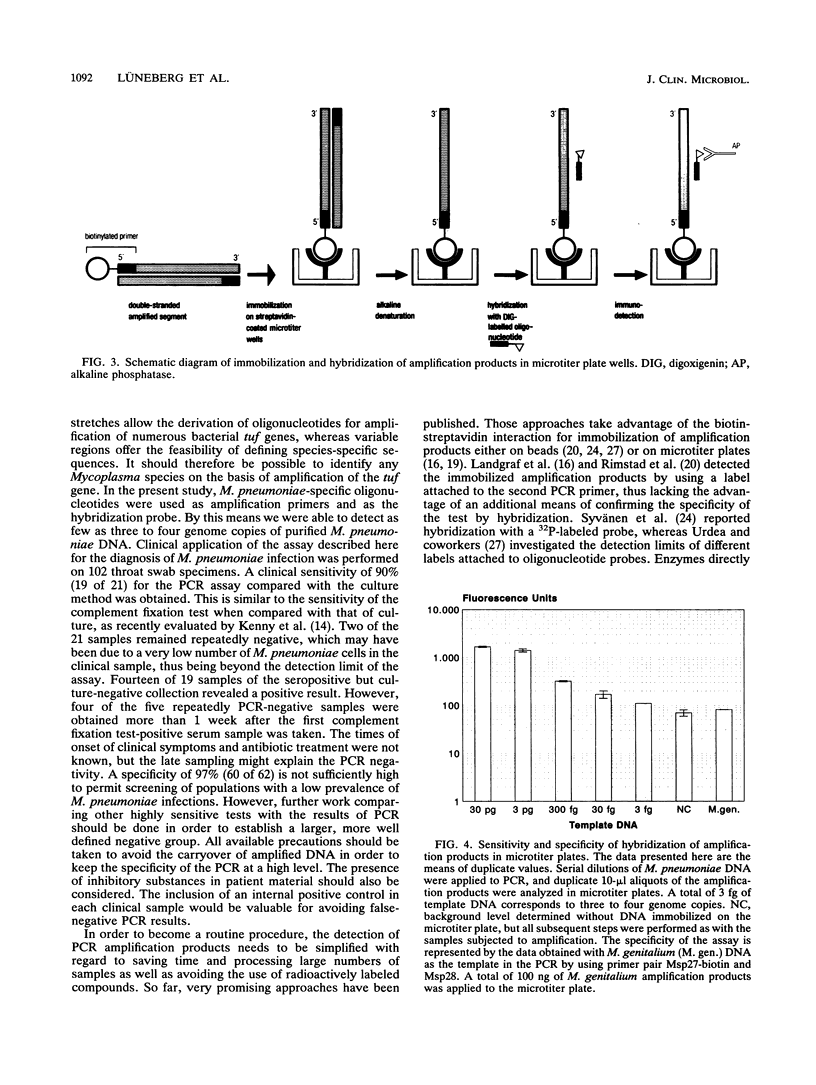
In order to improve the diagnosis of a Mycoplasma pneumoniae infection, we developed a polymerase chain reaction (PCR)-based assay. The gene encoding elongation factor Tu (tuf) was selected as the target sequence. Oligonucleotides derived from variable stretches of the tuf gene were able to prime the amplification of a 950-bp fragment exclusively when M. pneumoniae DNA was used as the template. The sensitivity of the assay was increased 10-fold when the amplification products were hybridized with an internal M. pneumoniae-specific oligonucleotide. The use of three to four genome copies for PCR was sufficient for obtaining a hybridization signal. In addition, we substituted radioactive filter hybridization with a microtiter plate assay. Via a biotin moiety of one PCR primer, the amplification products were immobilized on streptavidin-coated microtiter plates. Subsequent hybridization with a digoxigenin-labeled oligonucleotide resulted in the same sensitivity and specificity as those obtained by filter hybridization. Clinical application of the assay was performed on 102 throat swab specimens from patients with respiratory tract infections. Of 21 culture-positive samples, 19 were confirmed to be positive in the PCR-based assay (sensitivity, 90%). Furthermore, 14 of 19 seropositive but culture-negative samples gave a positive hybridization signal. Of 62 culture-negative and seronegative specimens, 60 gave a negative result in our assay (specificity, 97%). Of the 33 samples that were positive in our PCR-based assay, 5 samples initially gave false-negative results because of the presence of inhibitory substances in those specimens. Inhibition of Taq polymerase in these five cases was prevented by an additional step of phenol extraction and subsequent ethanol precipitation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baseman J. B., Dallo S. F., Tully J. G., Rose D. L. Isolation and characterization of Mycoplasma genitalium strains from the human respiratory tract. J Clin Microbiol. 1988 Nov;26(11):2266–2269. doi: 10.1128/jcm.26.11.2266-2269.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernet C., Garret M., de Barbeyrac B., Bebear C., Bonnet J. Detection of Mycoplasma pneumoniae by using the polymerase chain reaction. J Clin Microbiol. 1989 Nov;27(11):2492–2496. doi: 10.1128/jcm.27.11.2492-2496.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLYDE W. A., Jr MYCOPLASMA SPECIES IDENTIFICATION BASED UPON GROWTH INHIBITION BY SPECIFIC ANTISERA. J Immunol. 1964 Jun;92:958–965. [PubMed] [Google Scholar]
- Dallo S. F., Chavoya A., Su C. J., Baseman J. B. DNA and protein sequence homologies between the adhesins of Mycoplasma genitalium and Mycoplasma pneumoniae. Infect Immun. 1989 Apr;57(4):1059–1065. doi: 10.1128/iai.57.4.1059-1065.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer D., Furano A. V. Duplication of the tuf gene, which encodes peptide chain elongation factor Tu, is widespread in Gram-negative bacteria. J Bacteriol. 1981 Dec;148(3):1006–1011. doi: 10.1128/jb.148.3.1006-1011.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer D., Furano A. V. Portions of the gene encoding elongation factor Tu are highly conserved in prokaryotes. J Biol Chem. 1980 Jan 25;255(2):728–734. [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hyman H. C., Yogev D., Razin S. DNA probes for detection and identification of Mycoplasma pneumoniae and Mycoplasma genitalium. J Clin Microbiol. 1987 Apr;25(4):726–728. doi: 10.1128/jcm.25.4.726-728.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamine J. M., Loechel S., Collier A. M., Barile M. F., Hu P. C. Nucleotide sequence of the MgPa (mgp) operon of Mycoplasma genitalium and comparison to the P1 (mpp) operon of Mycoplasma pneumoniae. Gene. 1989 Oct 30;82(2):259–267. doi: 10.1016/0378-1119(89)90051-6. [DOI] [PubMed] [Google Scholar]
- Inamine J. M., Loechel S., Hu P. C. Nucleotide sequence of the tuf gene from Mycoplasma gallisepticum. Nucleic Acids Res. 1989 Dec 11;17(23):10126–10126. doi: 10.1093/nar/17.23.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J. S., Søndergård-Andersen J., Uldum S. A., Lind K. Detection of Mycoplasma pneumoniae in simulated clinical samples by polymerase chain reaction. Brief report. APMIS. 1989 Nov;97(11):1046–1048. doi: 10.1111/j.1699-0463.1989.tb00516.x. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Kaiser G. G., Cooney M. K., Foy H. M. Diagnosis of Mycoplasma pneumoniae pneumonia: sensitivities and specificities of serology with lipid antigen and isolation of the organism on soy peptone medium for identification of infections. J Clin Microbiol. 1990 Sep;28(9):2087–2093. doi: 10.1128/jcm.28.9.2087-2093.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause D. C., Mawn C. B. Physical analysis and mapping of the Mycoplasma pneumoniae chromosome. J Bacteriol. 1990 Sep;172(9):4790–4797. doi: 10.1128/jb.172.9.4790-4797.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf A., Reckmann B., Pingoud A. Direct analysis of polymerase chain reaction products using enzyme-linked immunosorbent assay techniques. Anal Biochem. 1991 Oct;198(1):86–91. doi: 10.1016/0003-2697(91)90510-z. [DOI] [PubMed] [Google Scholar]
- Loechel S., Inamine J. M., Hu P. C. Nucleotide sequence of the tuf gene from Mycoplasma genitalium. Nucleic Acids Res. 1989 Dec 11;17(23):10127–10127. doi: 10.1093/nar/17.23.10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüneberg E., Kamla V., Hadding U., Frosch M. Sequence and expression in Escherichia coli of a Mycoplasma hominis gene encoding elongation factor Tu. Gene. 1991 Jun 15;102(1):123–127. doi: 10.1016/0378-1119(91)90550-u. [DOI] [PubMed] [Google Scholar]
- Mantero G., Zonaro A., Albertini A., Bertolo P., Primi D. DNA enzyme immunoassay: general method for detecting products of polymerase chain reaction. Clin Chem. 1991 Mar;37(3):422–429. [PubMed] [Google Scholar]
- Rimstad E., Hornes E., Olsvik O., Hyllseth B. Identification of a double-stranded RNA virus by using polymerase chain reaction and magnetic separation of the synthesized DNA segments. J Clin Microbiol. 1990 Oct;28(10):2275–2278. doi: 10.1128/jcm.28.10.2275-2278.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt K., Däubener W., Bitter-Suermann D., Hadding U. A safe and efficient method for elimination of cell culture mycoplasmas using ciprofloxacin. J Immunol Methods. 1988 Apr 22;109(1):17–25. doi: 10.1016/0022-1759(88)90437-1. [DOI] [PubMed] [Google Scholar]
- Syvänen A. C., Bengtström M., Tenhunen J., Söderlund H. Quantification of polymerase chain reaction products by affinity-based hybrid collection. Nucleic Acids Res. 1988 Dec 9;16(23):11327–11338. doi: 10.1093/nar/16.23.11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Whitcomb R. F., Clark H. F., Williamson D. L. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new spiroplasma. Science. 1977 Mar 4;195(4281):892–894. doi: 10.1126/science.841314. [DOI] [PubMed] [Google Scholar]
- Uldum S. A., Jensen J. S., Søndergård-Andersen J., Lind K. Enzyme immunoassay for detection of immunoglobulin M (IgM) and IgG antibodies to Mycoplasma pneumoniae. J Clin Microbiol. 1992 May;30(5):1198–1204. doi: 10.1128/jcm.30.5.1198-1204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdea M. S., Warner B. D., Running J. A., Stempien M., Clyne J., Horn T. A comparison of non-radioisotopic hybridization assay methods using fluorescent, chemiluminescent and enzyme labeled synthetic oligodeoxyribonucleotide probes. Nucleic Acids Res. 1988 Jun 10;16(11):4937–4956. doi: 10.1093/nar/16.11.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel R., Herrmann R. Physical mapping of the Mycoplasma pneumoniae genome. Nucleic Acids Res. 1988 Sep 12;16(17):8323–8336. doi: 10.1093/nar/16.17.8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev D., Sela S., Bercovier H., Razin S. Nucleotide sequence and codon usage of the elongation factor Tu(EF-Tu) gene from Mycoplasma pneumoniae. Mol Microbiol. 1990 Aug;4(8):1303–1310. doi: 10.1111/j.1365-2958.1990.tb00709.x. [DOI] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]