Abstract
Members of the carcinoembryonic antigen family (CEACAMs) are widely expressed, and, depending on the tissue, capable of regulating diverse functions including tumor promotion, tumor suppression, angiogenesis, and neutrophil activation. Four members of this family, CEACAM1, CEACAM8, CEACAM6, and CEACAM3 (recognized by CD66a, CD66b, CD66c, and CD66d mAbs, respectively), are expressed on human neutrophils. CD66a, CD66b, CD66c, and CD66d antibodies each increase neutrophil adhesion to human umbilical vein endothelial cell monolayers. This increase in neutrophil adhesion caused by CD66 antibodies is blocked by CD18 mAbs and is associated with upregulation of CD11/CD18 on the neutrophil surface. To examine potential interactions of CEACAMs in neutrophil signaling, the effects on neutrophil adhesion to human umbilical vein endothelial cells of a set of CD66 mAbs was tested following desensitization to stimulation by various combinations of these mAbs. Addition of a CD66 mAb in the absence of calcium results in desensitization of neutrophils to stimulation by that CD66 mAb. The current data show that desensitization of neutrophils to any two CEACAMs results in selective desensitization to those two CEACAMs, while the cells remain responsive to the other two neutrophil CEACAMs. In addition, cells desensitized to CEACAM-3, -6, and -8 were still responsive to stimulation of CEACAM1 by CD66a mAbs. In contrast, desensitization of cells to CEACAM1 and any two of the other CEACAMs left the cells unresponsive to all CD66 mAbs. Cells desensitized to any combination of CEACAMs remained responsive to the unrelated control protein CD63. Thus, while there is significant independence of the four neutrophil CEACAMs in signaling, CEACAM1 appears to play a unique role among the neutrophil CEACAMs. A model in which CEACAMs dimerize to form signaling complexes could accommodate the observations. Similar interactions may occur in other cells expressing CEACAMs.
Background
The carcinoembryonic antigen (CEA)2 family consists of two subfamilies, the CEACAM subgroup and the pregnancy specific glycoprotein (PSG) subgroup. Members of this family have been redundantly named [for review see [1-4]], but subsequent consensus unified the nomenclature for the CEACAM family [5]. CEACAM family members are widely expressed in epithelial, endothelial, and hematopoietic cells, including neutrophils, T-cells, and NK cells. CEACAMs appear to be capable of transmitting signals that result in a variety of effects depending on the tissue, including tumor suppression, tumor promotion, angiogenesis, neutrophil activation, lymphocyte activation, regulation of the cell cycle, and regulation of adhesion [2,3,5-42]. In many tissues, more than one CEACAM family member are expressed concurrently. For example, CEACAMs 1, 5, and 6 are often expressed in ovarian, endometrial, cervical, breast, lung, and colon carcinomas, and may be useful as biomarkers in cancer [43-47]. A CEACAM5 expressing measles virus has entered phase I trials in ovarian cancer [48]. CD66mAbs that recognize CEACAMs are also in clinical trials as part of conditioning regimens in allogeneic stem cell transplantation for acute leukemia [49,50]
The CEACAM gene family contains more than seventeen expressible closely related genes that belong to the immunoglobulin (Ig) gene superfamily [for review see [1,2,4,5,22] and cea.klinikum.uni-muenchen.de]. Each of the human CEACAM family molecules contains one amino-terminal (N) domain of 108–110 amino acid residues homologous to Ig variable domains, followed by a differing number of Ig constant-like domains. CD66 mAbs react with members of the CEACAM family. Clearly characterized mAbs belonging to the CD66 cluster are described by their reactivity with each family member as indicated by a lower case letter after "CD66" as follows: CD66a mAb, CEACAM1, biliary glycoprotein; CD66b mAb, CEACAM8, CGM6; CD66c mAb, CEACAM6, NCA; CD66d mAb, CEACAM3, CGM1; and CD66e mAb, CEACAM5 or CEA [3]. CEACAM-1, -3, -6, and-8, but not CEACAM-5 (CEA), are expressed on human neutrophils. In humans, at least eight forms of CEACAM1, produced by differential splicing of the single CEACAM1 gene, have been identified [51-55]. In neutrophils, CEACAM1 and CEACAM3 exist as transmembrane proteins with cytoplasmic tails, while CEACAM8 and CEACAM6 are linked to the membrane via a glycosyl-phosphatidylinositol anchor.
CD66 mAbs have been reported to activate neutrophils [23,24,27,37,39-41]. By use of specific mAbs, each of the CEACAM family members expressed on neutrophils, CEACAM1, CEACAM8, CEACAM6, and CEACAM3 (recognized by CD66a, CD66b, CD66c, and CD66d mAbs, respectively) have been shown to be capable of activating neutrophils as determined by the physiologic response of adhesion to human umbilical vein endothelial cells (HUVECs) [37]. CD66 mAb binding to the neutrophil surface triggers a transient activation signal that requires extracellular calcium and regulates the adhesive activity of CD11/CD18 [37]. In the absence of extracellular calcium, this activation state decays and is no longer functional after 10 min.
The similarity in structure among the CEACAMs, and their ability to undergo homotypic and heterotypic interactions with other members of the family, led us to question the degree of interdependency of CEACAM signaling in neutrophils. To examine potential interactions among CEACAM members in transmitting signals in neutrophils, the effects of a set of well characterized CD66 mAbs on neutrophil adhesion to HUVECs was studied. The ability of combinations of CD66 mAbs, in the absence of calcium, to desensitize neutrophils to subsequent simulation by CD66 mAbs was examined. The data demonstrate significant functional independence of the four CEACAM molecules in signaling, but also suggest a unique role for CEACAM-1 in CEACAM signaling in neutrophils.
Methods
Cell preparation
Normal peripheral blood neutrophils were prepared by a modification of the method of Boyum as previously described [56], and were suspended at the indicated concentrations in Hanks' balanced salt solution (HBSS) with or without Ca2+ (Gibco, Grand Island, NY), as indicated. Differential cell counts on Wright-stained cells routinely revealed greater than 95% neutrophils. Viability as assessed by trypan blue dye exclusion was greater than 98%.
Antibodies and reagents
The CD45 mAb AHN-12 (IgG1) [57], the CD63 mAb AHN-16.1 (IgG1) [58], and the anti-HLA class I mAb W6/32 (IgG2a) [59] have been previously described. CD66 mAbs were obtained from the CD66 section of the Sixth International Workshop and Conference on Human Leukocyte Differentiation Antigens and included the following CD66 mAbs: B13.9 (IgG1) (CD66b), C11228.2C (IgG1) (CD66c), Bu-104 (IgG1) (CD66ae), and COL-1 (IgG2a) (CD66de) [3].
The PE-labeled CD11b mAb (Leu 15) was obtained from Becton Dickenson (Mountain View, CA). The source of mAbs was either hybridoma cell culture supernatants, purified antibody, or ascites fluid diluted in PBS containing 1 mg/ml BSA as indicated. All sera and ascites were heat inactivated at 56°C for 30 min and clarified by centrifugation at 13,000 × g at 4°C for 15 min before use. N-formyl-met-leu-phe (FMLP) and normal mouse serum (NMS) were purchased from Sigma Chemical Co. (St. Louis, MO).
Fluorescence labeling of cells
Neutrophils were labeled with calcein AM (Molecular Probes, Eugene, OR) [60] by incubating 5 × 106 cells/ml with 50 ug of calcein AM for 30 min at 37°C in 18 ml of calcein labeling buffer [HBSS without Ca2+ or Mg2+ containing 0.02% BSA]. Cells were then washed twice with calcein labeling buffer at 23°C and resuspended in the desired media.
Endothelial cell adhesion assay
Neutrophil adhesion to human umbilical vein endothelial cells (HUVECs) was performed as previously described [37]. Briefly, HUVECs (Clonetics Corp., San Diego, CA) were passaged 1:5 in T-25 flasks (Costar) no more than three times before plating in 96 well microtiter plates at 3000 cells/well. HUVECs were grown to confluence in 96 well microtiter plates in EGM media (Clonetics) and fed every 24 hours. Using the adhesion assay described below, no difference in resting and stimulated neutrophil adhesion was observed, and, as expected [37,61], no difference in surface expression of CD54 (ICAM-1) or CD62E (E selectin, ELAM-1) in resting or TNF stimulated cells was noted, using HUVECs passaged once compared with those passaged five times. In some experiments, the HUVECs were stimulated by culture for 4 hours at 37°C with 50 ng/ml TNFα (Cetus, Emeryville, CA). The wells were then washed four times with calcium free wash buffer (HBSS without Ca2+ plus 4% HIFBS) and 25 ul of calcium free wash buffer containing the indicated antibody (10 ug/ml final concentration) was added to each well. One hundred ul of calcium free wash buffer containing 105 calcein-labeled neutrophils was added. After the indicated time, 25 ul of calcium-free wash buffer containing the indicated mAb (10 ug/ml final concentration) and 10.8 mM Ca2+ was then added to yield a final physiologic calcium concentration (1.8 mM), and the plates were incubated at 37°C in 5% CO2 for 30 min. The wells were then aspirated and washed 4 times with endo wash buffer (HBSS plus 4% HIFBS), and the fluorescence was quantitated with a Millipore fluorescence plate reader using an excitation wavelength of 485 nm and an emission wavelength of 530 nm. For each condition, quadruplicate wells were tested and values are reported as the mean +/- SD. Each experiment was performed at least four times using different HUVEC subcultures. The data in Figures 1 and 2 are shown as the percent of added neutrophils remaining adherent to the monolayers, and represent the means +/- SD of 4 separate determinations. While the SD is shown in each figure, in some panels it is sufficiently small that it is not possible to see on the scale shown.
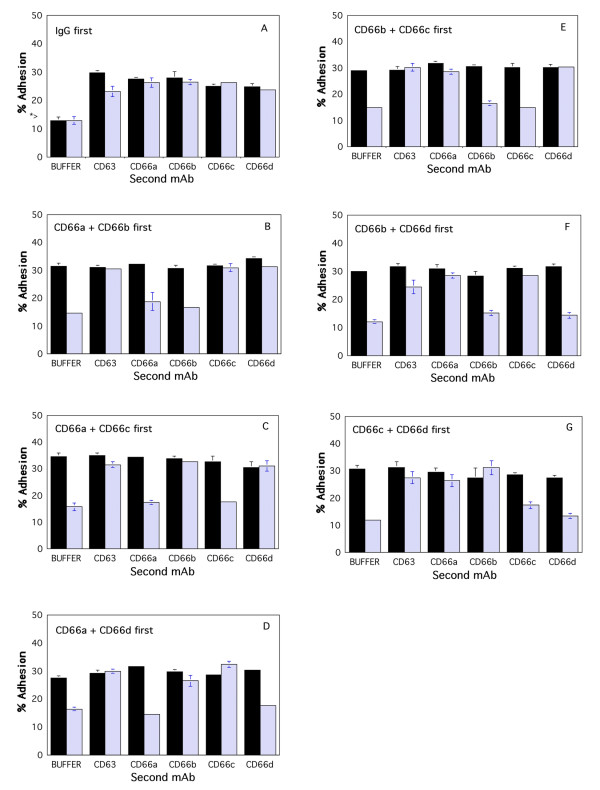
Figure 1.
Cross desensitization with two CD66 mAbs to further stimulation of neutrophil adhesion to HUVECs. TNF-stimulated HUVECs were washed, and Ca2+ free buffer containing IgG (panel A), the CD66ae mAb and CD66b mAb (panel B), the CD66ae mAb and CD66c mAb (panel C), the CD66ae mAb and CD66de mAb (panel D), the CD66b mAb and CD66c mAb (panel E), the CD66b mAb and CD66de mAb (panel F), or the CD66c mAb and CD66de mAb (panel G), were added (see Methods). Neutrophils in Ca2+ free buffer were then added. After 15 sec (solid bars) or 15 min (hatched bars), the indicated next mAb or buffer, and Ca2+ (1.8 mM final concentration) were added. After 30 min the wells were washed. The * > (Panel A) indicates the amount of adhesion observed when neutrophils were incubated in the wells for 30 min in the presence of buffer containing Ca2+ with or without 10 ug/ml IgG (final concentration). The percent of neutrophils adherent to the monolayers are shown. Selective desensitization at 15 min was statistically significant (p < 0.05).
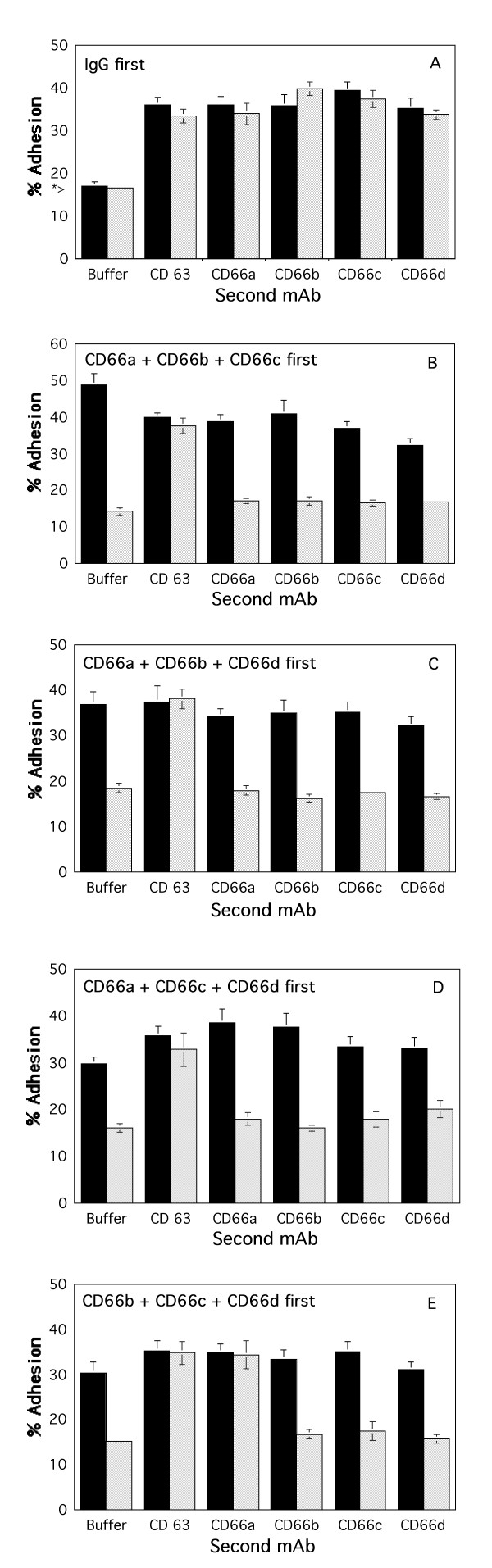
Figure 2.
Cross desensitization with three CD66 mAbs to further stimulation of neutrophil adhesion to HUVECs. TNF-stimulated HUVECs were washed and Ca2+ free buffer containing 10 ug/ml final concentration each of IgG (panel A), the CD66ae mAb, CD66b mAb, and CD66c mAb (panel B), the CD66ae mAb, CD66b mAb, and CD66de mAb (panel C), the CD66ae mAb, CD66c mAb, and CD66de mAb (panel D), or the CD66b mAb, CD66c mAb, and CD66de mAb (panel E), were added (see Methods). Neutrophils in Ca2+ free buffer were then added. After 15 sec (solid bars) or 15 min (hatched bars), buffer containing 10 ug/ml final concentration of the indicated next mAb or buffer, and Ca2+ (1.8 mM final concentration) were added. After 30 min the wells were washed. The * > (panel A) indicates the amount of adhesion observed when neutrophils were incubated for 30 min in the presence of buffer containing Ca2+ with or without 10 ug/ml IgG (final concentration). The percent of neutrophils remaining adherent are shown. Selective desensitization at 15 min was statistically significant (p < 0.05).
Statistical analyses
Effects of mAbs on neutrophil adhesion to HUVECs was analyzed by the Mann Whitney U test when appropriate.
Results
Effects of CD66 mAbs on neutrophil adhesion to endothelial cells
Because CEACAM-1, -3, -6, and -8 are highly homologous structurally, and can undergo a number of different homotypic and heterotypic adhesion reactions among themselves [2,26,62-72], it is possible that they might interact on the neutrophil surface. To better characterize possible interactions among the CEACAMs in signaling on human neutrophils, we utilized calcium-dependent desensitization by CD66 mAbs to examine individual CEACAM-mediated signaling. As expected, when neutrophils were incubated for 30 min with HUVECs and 10-7 M FMLP in the presence of normal mouse IgG (IgG) or mAb, and washed as described in the Methods, each of the CD66ae, CD66b, CD66c, CD66de, and the control CD63 mAbs augmented neutrophil adhesion approximately two-fold compared with IgG or media [not shown and [37,58]]. In contrast, neither the CD45 mAb nor the anti-HLA class I mAb altered neutrophil adhesion (not shown).
Cross desensitization to pairs of CD66a, CD66b, CD66c, and CD66d mAbs
Desensitization of neutrophils to further stimulation by mAbs directed to specific CEACAM family members by exposure of the neutrophils to the mAbs in the absence of calcium was used to examine the independence of signaling mechanisms triggered by each CD66 mAb. Although these CD66 mAbs stimulated neutrophil adhesion to resting HUVEC [37], for the experiments reported here, TNF-treated HUVECs were used because these conditions yielded a stronger signal in the assay. HUVECs were stimulated for 4 hours with 50 ng/ml TNFα, washed, and neutrophils were added with desensitizing mAbs, incubated in the absence of calcium, washed, and stimulated with other mAbs and cell adhesion quantitated as described in the Methods. First, IgG was added to the microtiter wells containing the TNF stimulated HUVECs in the absence of Ca2+ (Fig 1, panel A). As expected [37,58], when neutrophils were added to the wells in the absence of Ca2+ and allowed to incubate for 15 sec before Ca2+ was added (solid bars) stimulated neutrophil adhesion was observed when aliquots of CD66ae mAb, CD66b mAb, CD66c, CD66de, or CD63 mAbs were added, but not when buffer was added. Since the CD66e antigen is not expressed in neutrophils, the available CD66ae and CD66de mAbs can be used effectively as CD66a and CD66d mAbs, respectively, in this cell system. When neutrophils were added to the wells in the absence of Ca2+ and allowed to incubate for 15 min before Ca2+ was added (hatched bars), stimulated neutrophil adhesion to the HUVECs following the addition of aliquots of CD66ae, CD66b, CD66c, CD66de, and CD63 mAbs, but not buffer was also observed.
Next, the CD66ae and CD66b mAbs were added to the microtiter wells containing the TNF stimulated HUVECs in the absence of Ca2+ (Fig 1, panel B). As expected, when neutrophils were added to the wells in the absence of Ca2+ and allowed to incubate for 15 sec before Ca2+ was added (solid bars) stimulated neutrophil adhesion was observed when aliquots of buffer, CD66ae mAb, or CD66b mAb, were added. Adhesion was also observed when aliquots of CD66c mAb, CD66de mAb, or CD63 mAb were added. When neutrophils were added to the wells in the absence of Ca2+ and allowed to incubate for 15 min before Ca2+ was added (hatched bars), there was a marked decrease in neutrophil adhesion to the HUVECs following the addition of aliquots of buffer, CD66ae mAb, or CD66b mAb. In contrast, the cells were still responsive to stimulation by CD66c, CD66de, and CD63 mAbs as evidenced by an increase in adhesion.
Similarly, desensitization of neutrophils to stimulation by the CD66ae and CD66c mAbs selectively desensitized the cells to further stimulation by the CD66ae mAb and the CD66c mAb, but not by CD66b, CD66de, or CD63 mAbs (Fig 1, panel C). Finally, desensitization to the CD66ae and CD66de mAbs left the cells unresponsive to CD66ae and CD66de mAbs, but they remained responsive to CD66b, CD66c, and CD63 mAbs (Fig 1, panel D).
When cells were desensitized to CD66b and CD66c mAbs, the cells were unresponsive to CD66b and CD66c mAbs, but were still responsive to stimulation by CD66ae, CD66de, and CD63 mAbs as evidenced by an increase in adhesion (Fig 1, panel E). Similarly, desensitization of neutrophils to stimulation by the CD66b and CD66de mAbs selectively desensitized the cells to further stimulation by the CD66b and CD66de mAbs, but not by CD66ae, CD66c, or CD63 mAbs (Fig 1, panel F). Similar selectivity of this desensitization was observed when cells were desensitized with the CD66c mAb and the CD66de mAb, in that the cells were desensitized to CD66c and CD66de mAbs, but not to CD66ae, CD66b, or CD63 mAbs (Fig 1, panel G).
Cross desensitization to combinations of three CD66 mAbs
Desensitization with various combinations of three CD66 mAbs was next examined. First, IgG was added to the microtiter wells containing the TNF stimulated HUVECs in the absence of Ca2+ (Fig 2, panel A). As expected, when neutrophils were added to the wells in the absence of Ca2+ and allowed to incubate for 15 sec (solid bars) or 15 min (hatched bars) before Ca2+ was added, stimulated neutrophil adhesion was similar to that observed in Figure 1, panel A. Next, the CD66ae, CD66b, and CD66c mAbs were added to the microtiter wells containing the TNF stimulated HUVECs in the absence of Ca2+ (Fig 2, panel B). As expected, when neutrophils were added to the wells in the absence of Ca2+ and allowed to incubate for 15 sec before Ca2+ was added (solid bars), stimulated neutrophil adhesion was observed when aliquots of buffer, CD66ae mAb, CD66b mAb, CD66c mAb, CD66de mAb, or CD63 mAb were added. When neutrophils were added to the wells in the absence of Ca2+ and allowed to incubate for 15 min before Ca2+ was added (hatched bars), there was a marked decrease in neutrophil adhesion to the HUVECs following the addition of aliquots of buffer, CD66ae mAb, CD66b mAb, or CD66c mAb. In addition, the cells were no longer responsive to stimulation by the CD66de mAb. In contrast, the cells were still responsive to stimulation by CD63 mAbs as evidenced by an increase in adhesion. Thus, cells were desensitized to CD66de mAb stimulation with a combination of mAbs that does not bind the CD66d antigen. Similarly, desensitization of neutrophils to stimulation by the CD66ae, CD66b, and CD66de mAbs desensitized the cells to further stimulation by the CD66c mAb, as well as CD66ae, CD66b, and CD66de mAbs, but not by CD63 mAbs (Fig 2, panel C). Similar selectivity of this desensitization was observed when cells were desensitized with the CD66ae, CD66c, and CD66de mAbs, in that the cells were desensitized to CD66ae, CD66b, CD66c, and CD66de mAbs, but not to CD63 mAbs (Fig 2, panel D). In contrast, desensitization to the CD66b, CD66c, and CD66de mAbs left the cells unresponsive to CD66b, CD66c, and CD66de mAbs, but they remained responsive to both CD66ae and CD63 mAbs (Fig 2, panel E).
Discussion
While it has been shown that CEACAM-1, -8, -6, and -3 can each independently transduce signals in neutrophils resulting in activation of CD11/CD18, and an increase in neutrophil adhesion to endothelial cells [37], potential interactions among these molecules in neutrophil activation are not well defined. Experiments in which CD66 mAbs were allowed to bind to the neutrophils for various lengths of time in the absence of calcium before calcium repletion, suggested that the binding of CD66 mAbs to the neutrophil surface results in a transient activation state during which time a signal can be transmitted to CD11/CD18 if extracellular calcium is present. In the absence of extracellular calcium, this activation state decayed significantly within 1 min, and is no longer functional after 10 min, i.e. the cell is desensitized to stimulation by that mAb [37]. This observation allowed the current study to be performed.
This study demonstrates that desensitization of neutrophils to stimulation by any two neutrophil CEACAMs allows the cell to respond to stimulation by the other two neutrophil CEACAMs. However, neutrophils desensitized to CEACAM-1 and any other two neutrophil CEACAMs, are unresponsive to the remaining neutrophil CEACAM, while retaining responsiveness to the unrelated membrane protein CD63. In contrast, neutrophils desensitized to CEACAM-8, -6, and -3, were still responsive to both CEACAM-1 and CD63. Thus, CEACAM-1 appears to have a unique role in CEACAM signaling in neutrophils.
We feel the observed results are due to mAbs binding their specific antigens on the neutrophil surface. There are potential alternative explanations for the results observed in this study. CEACAM1 can be expressed on HUVECs. Therefore, in earlier studies, a series of experiments addressed the possibility that the observed results could be due to CD66 mAb binding the HUVECs [37]. Preincubation of HUVECs with mAb under various conditions, followed by washing, indicated that the effects of CD66 mAbs were due to mAbs binding to the neutrophils and not the HUVECs [37].
Furthermore, it was also possible that the Fc fragments of these mAbs could alter signaling. The CD66 mAbs used here could also induce a conformational change in a CEACAM, or possibly cluster surface CEACAMs. These possibilities were addressed in an earlier report in which F(ab')2 fragments of the CD66ae, CD66be, and CD66c mAbs were found to stimulate neutrophil adhesion to HUVECs in this assay, as did the intact IgGs [37]. In contrast, Fab fragments of the CD66ae mAb had little effect on neutrophil adhesion in this assay, suggesting that cross-linking or clustering of CEACAMs could play a role in the observed effects [37].
The molecular explanation for these observations is unclear. CD66b and CD66c mAbs triggered an activation signal, despite the fact that they bind GPI-linked surface proteins, as has been previously reported [37]. MAb binding to other GPI-liniked proteins can also transduce signals [27]. While the details of the "activation signal" transmitted by CEACAMs are not known, the finding of tyrosine kinase activity, including lyn and hck, associated with CEACAM-1, CEACAM-6, and CEACAM-8, and src with CEACAM-1, suggests that these kinase activities may be involved in signal transduction via CEACAM family members [73,74]. CEACAM1 is also associated with protein tyrosine phosphatase activity [75]. CEACAM1 in neutrophils also undergoes transient changes in phosphorylation following stimulation with chemotactic agents, suggesting that phosphorylation may be involved in regulating CEACAM-1 function as well [73,74]. CEACAM3 is tyrosine phosphorylated upon binding gonococci expressing CEACAM ligand Opa protein variants [76]. Tyrosine kinase activity in neutrophils has also been reported to be associated with CD63, the control signaling molecule used in this study [58], while serine kinase activity has been reported to associate with CD63 in melanoma cells [77].
Although mAbs to both CEACAM1 and CEACAM3 triggered neutrophil activation in this study, the cytoplasmic domain of CEACAM1 has an ITIM motif, while that of CEACAM3 contains an ITAM sequence. In a transfected HeLa epithelial cell model, uptake of gonococci mediated by CEACAM1 and CEACAM3 differed with regard to their sensitivity to tyrosine kinase inhibitors [78]. Other studies have also found differences in the mechanism of CEACAM3 and CEACAM6 mediated uptake; the former being dependent on tyrosine kinase activity and the latter requiring the integritiy of cholesterol-rich membrane microdomains [79,80].
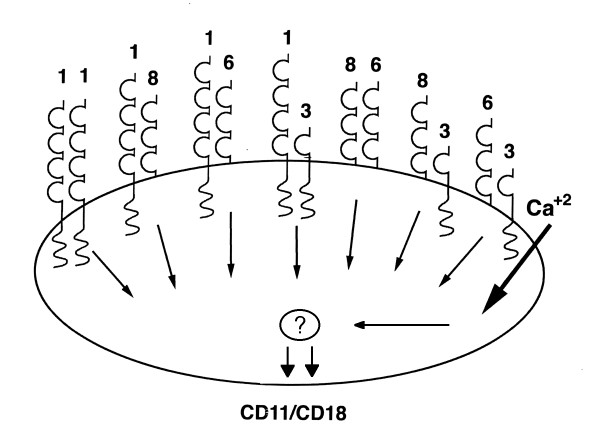
The data are consistent with the existence of signaling complexes containing more than one CEACAM on the neutrophil surface. CEACAMs have been shown to undergo homotypic and heterotypic adhesion [55,62,65-67,70-72,81-83]. CEACAM8 exhibits heterotypic adhesion with CEACAM6, while CEACAM-1, -6, and -5 exhibit both homotypic and heterotypic adhesion. For example, a model in which CEACAMs exist as heterodimers containing two different CEACAMs or CEACAM-1-CEACAM-1 homodimers in a signaling complex, in which an active CEACAM dimer is required for signal transmission, could explain the current observations (Fig 3). For example, in this model, desensitization of CEACAM-1 would allow signaling by CEACAM-8/6; 8/3; or 6/3 dimers, while desensitization of CEACAM-1 and any other two CEACAMs would leave no active dimers. In contrast, desensitization of CEACAMs-8, 6, and 3 would leave active CEACAM-1 homodimers. Association of CEACAMs into larger complexes containing more than just two CEACAMs is also possible. Data have been reported showing that CEACAM-1 can form dimers in solution and on an epithelial cell surface [84]. Dr. Singer and colleagues have provided evidence that complex formation among CEACAMs in neutrophils is possible [35,85]. Despite having tried a number of experimental approaches, including immunoprecipitation, immunoblotting, and surface labeling with 125I and biotin, we have not been able to detect the existence of such complexes in neutrophils (data not shown). Given the convergence of signaling by the different CEACAMs with different cytoplasmic domains, it is possible that another molecule may act as an intermediary in CEACAM signaling.
Figure 3.
Model of potential CEACAM dimers in signaling complexes on neutrophils. A possible model of CEACAM signaling complexes on neutrophils that is compatible with observed desensitization data is shown. In this model, CEACAMs can exist on the neutrophil surface as heterodimers or as CEACAM-1 homodimers. Signaling would require an active dimer. For example, desensitization of CEACAM-1 would allow signaling by CEACAM-8/6; 8/3; or 6/3 dimers, while desensitization of CEACAM-1 and any other two CEACAMs would leave no active dimers. In contrast, desensitization of CEACAMs-8, 6, and 3 would leave active CEACAM-1 homodimers. The existence of potential unidentified cooperative signaling molecules is denoted by the "?"
The role(s) of CEACAMs in neutrophil function are complex. However, ligation of CEACAM-1, -8, -6, and -3 by CD66a, CD66b, CD66c, and CD66d mAbs, respectively, transduce signals in neutrophils resulting in activation of CD11/CD18, and an increase in neutrophil adhesion to endothelial cells, one of the critical first steps of inflammation [37]. In addition, several other reports have also suggested that CEACAMs are capable of regulating the function of CD11/CD18 [24,39,40], and induce an increase in intracytoplasmic calcium and an oxidative burst in neutrophils [27]. CEACAM1 also regulates neutrophil apoptosis, thus possibly influencing the resolution of inflammation [34]. Finally, studies have shown that certain bacteria bind to some CEACAM family members on neutrophils, and this interaction may also result in signal transduction resulting in modification of neutrophil activity [6,8,22,86-96]. Thus, CEACAMs appear to be involved in neutrophil adhesion by transmitting some form of activation signal that regulates the activity of other adhesion molecules, as well as possibly by homotypic or heterotypic adhesion. CEACAMs-1, -8, and -6, are upregulated to the neutrophil surface from intracellular stores following stimulation [97-99].
The current observations may also be relevant to other cells expressing CEACAMs. CEACAM1 and CEACAM6 have been reported to present selectin ligands to CD62E (ELAM-1, E-selectin) on endothelial cells [23], and appear to be involved in angiogenesis [9,16,28,100]. A role for a soluble form of CEACAM1 in angiogenesis has also been demonstrated [100]. CEACAM1 also appears to play a critical role in tumor lymphangiogenesis [15], and can regulate cell migration via interaction with filamin A [17]. CEACAM1 associates with the beta 3-integrin, and this association is dependent on the phosphorylation of Tyr-488 in the cytoplasmic domain of CEACAM1; this complex may play a role in cell invasion [101]. During cell-matrix adhesion of endothelial cells, CEACAM1 associates with talin, a regulator of integrin function [28]. CEACAMs serve as a receptor for murine hepatitis virus [102-106], and as a human receptor for Neisseria meningiditis and Neisseria gonorrhea [8,22,86-91,94,95]. CEACAMs can also transmit signals regulating proliferation of epithelial cells and lymphocytes [2,6-8,13,14,22,35,36,107,108]. Thus, interactions among CEACAMs in signaling may occur in various cell systems.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
KMS participated in study design, data analysis, and helped draft the manuscript.
APNS participated in study design, data analysis, and helped draft the manuscript.
All authors read and approved the manuscript.
Acknowledgments
Acknowledgements
We thank Kenneth Campbell for technical assistance and Dr. Jane Little for a critical review of the manuscript.
Supported in part by the American Heart Association, Minnesota Affiliate, NIH grant CA60658, the Office of the Vice President for Research and Dean of the Graduate School of the University of Minnesota, the Minnesota Medical Foundation, and the Masonic Memorial Hospital Fund, Inc.
Presented in part at the 8th International CEA/PSG Workshop, Estes Park, Colorado, September 6–9, 1997.
Contributor Information
Keith M Skubitz, Email: skubi001@tc.umn.edu.
Amy PN Skubitz, Email: skubi002@umn.edu.
References
- Khan WN, Frangsmyr L, Teglund S, Israelsson A, Bremer K, Hammarstrom S. Identification of three new genes and estimation of the size of the carcinoembryonic antigen family. Genomics. 1992;14:384–390. doi: 10.1016/S0888-7543(05)80230-7. [DOI] [PubMed] [Google Scholar]
- Obrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skubitz KM, Grunert F, Jantscheff P, Kuroki M, Skubitz AP. Summary of the CD66 Cluster Workshop. In: Kishimoto T, Kikutani H, von dem Borne A, Goyert S, Mason D, Misasaka M, Moretta L, Okumura K, Shaw S, Springer T, et al, editor. Leukocyte Typing VI: White cell differentiation antigens. New York and London: Garland Publishing, Inc; 1997. pp. 922–1000. [Google Scholar]
- Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarstrom S, Holmes KV, Karlsson A, Kuroki M, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol. 2006;6:433–446. doi: 10.1038/nri1864. [DOI] [PubMed] [Google Scholar]
- Greicius G, Severinson E, Beauchemin N, Obrink B, Singer BB. CEACAM1 is a potent regulator of B cell receptor complex-induced activation. J Leukoc Biol. 2003;74:126–134. doi: 10.1189/jlb.1202594. [DOI] [PubMed] [Google Scholar]
- Hammarstrom S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- Horst AK, Ito WD, Dabelstein J, Schumacher U, Sander H, Turbide C, Brummer J, Meinertz T, Beauchemin N, Wagener C. Carcinoembryonic antigen-related cell adhesion molecule 1 modulates vascular remodeling in vitro and in vivo. J Clin Invest. 2006;116:1596–1605. doi: 10.1172/JCI24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh JT, Luo W, Song W, Wang Y, Kleinerman DI, Van NT, Lin SH. Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res. 1995;55:190–197. [PubMed] [Google Scholar]
- Ilantzis C, Jothy S, Alpert LC, Draber P, Stanners CP. Cell-surface levels of human carcinoembryonic antigen are inversely correlated with colonocyte differentiation in colon carcinogenesis. Lab Invest. 1997;76:703–716. [PubMed] [Google Scholar]
- Izzi L, Turbide C, Houde C, Kunath T, Beauchemin N. cis-Determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene. 1999;18:5563–5572. doi: 10.1038/sj.onc.1202935. [DOI] [PubMed] [Google Scholar]
- Kammerer R, Hahn S, Singer BB, Luo JS, von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol. 1998;28:3664–3674. doi: 10.1002/(SICI)1521-4141(199811)28:11<3664::AID-IMMU3664>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kammerer R, Stober D, Singer BB, Obrink B, Reimann J. Carcinoembryonic antigen-related cell adhesion molecule 1 on murine dendritic cells is a potent regulator of T cell stimulation. J Immunol. 2001;166:6537–6544. doi: 10.4049/jimmunol.166.11.6537. [DOI] [PubMed] [Google Scholar]
- Kilic N, Oliveira-Ferrer L, Neshat-Vahid S, Irmak S, Obst-Pernberg K, Wurmbach JH, Loges S, Kilic E, Weil J, Lauke H, et al. Lymphatic reprogramming of microvascular endothelial cells by CEA-related cell adhesion molecule-1 via interaction with VEGFR-3 and Prox1. Blood. 2007;110:4223–4233. doi: 10.1182/blood-2007-06-097592. [DOI] [PubMed] [Google Scholar]
- Kilic N, Oliveira-Ferrer L, Wurmbach JH, Loges S, Chalajour F, Neshat-Vahid S, Weil J, Fernando M, Ergun S. Pro-angiogenic signaling by the endothelial presence of CEACAM1. J Biol Chem. 2005;280:2361–2369. doi: 10.1074/jbc.M409407200. [DOI] [PubMed] [Google Scholar]
- Klaile E, Muller MM, Kannicht C, Singer BB, Lucka L. CEACAM1 functionally interacts with filamin A and exerts a dual role in the regulation of cell migration. J Cell Sci. 2005;118:5513–5524. doi: 10.1242/jcs.02660. [DOI] [PubMed] [Google Scholar]
- Kleinerman DI, Dinney CP, Zhang WW, Lin SH, Van NT, Hsieh JT. Suppression of human bladder cancer growth by increased expression of C-CAM1 gene in an orthotopic model. Cancer Res. 1996;56:3431–3435. [PubMed] [Google Scholar]
- Kleinerman DI, Troncoso P, Lin SH, Pisters LL, Sherwood ER, Brooks T, von Eschenbach AC, Hsieh JT. Consistent expression of an epithelial cell adhesion molecule (C-CAM) during human prostate development and loss of expression in prostate cancer: implication as a tumor suppressor. Cancer Res. 1995;55:1215–1220. [PubMed] [Google Scholar]
- Kleinerman DI, Zhang WW, Lin SH, Nguyen TV, von Eschenbach AC, Hsieh JT. Application of a tumor suppressor (C-CAM1)-expressing recombinant adenovirus in androgen-independent human prostate cancer therapy: a preclinical study. Cancer Res. 1995;55:2831–2836. [PubMed] [Google Scholar]
- Krop-Watorek A, Laskowska A, Salwa J, Klopocki AG, Grunert F, Ugorski M. CEA-related proteins on human urothelial cell lines of different transformation grades. Cancer Lett. 1999;139:15–22. doi: 10.1016/S0304-3835(98)00363-2. [DOI] [PubMed] [Google Scholar]
- Kuespert K, Pils S, Hauck CR. CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol. 2006;18:565–571. doi: 10.1016/j.ceb.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Hoogerwerf M, Laan LJ van der, Nagel G, Schoot CE van der, Grunert F, Roos D. CD66 nonspecific cross-reacting antigens are involved in neutrophil adherence to cytokine-activated endothelial cells. J Cell Biol. 1992;118:457–466. doi: 10.1083/jcb.118.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Schoot CE van der, Hoogerwerf M, Roos D. Cross-linking of the carcinoembryonic antigen-like glycoproteins CD66 and CD67 induces neutrophil aggregation. J Immunol. 1993;151:4934–4940. [PubMed] [Google Scholar]
- Kunath T, Ordonez-Garcia C, Turbide C, Beauchemin N. Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene. 1995;11:2375–2382. [PubMed] [Google Scholar]
- Lucka L, Budt M, Cichocka I, Danker K, Horstkorte R, Reutter W. C-CAM-mediated adhesion leads to an outside-in dephosphorylation signal. Eur J Biochem. 1999;262:541–546. doi: 10.1046/j.1432-1327.1999.00402.x. [DOI] [PubMed] [Google Scholar]
- Lund-Johansen F, Olweus J, Symington FW, Arli A, Thompson JS, Vilella R, Skubitz K, Horejsi V. Activation of human monocytes and granulocytes by monoclonal antibodies to glycosylphosphatidylinositol-anchored antigens. Eur J Immunol. 1993;23:2782–2791. doi: 10.1002/eji.1830231110. [DOI] [PubMed] [Google Scholar]
- Muller MM, Singer BB, Klaile E, Obrink B, Lucka L. Transmembrane CEACAM1 affects integrin-dependent signaling and regulates extracellular matrix protein-specific morphology and migration of endothelial cells. Blood. 2005;105:3925–3934. doi: 10.1182/blood-2004-09-3618. [DOI] [PubMed] [Google Scholar]
- Nair KS, Zingde SM. Adhesion of neutrophils to fibronectin: role of the cd66 antigens. Cell Immunol. 2001;208:96–106. doi: 10.1006/cimm.2001.1772. [DOI] [PubMed] [Google Scholar]
- Nollau P, Prall F, Helmchen U, Wagener C, Neumaier M. Dysregulation of carcinoembryonic antigen group members CGM2, CD66a (biliary glycoprotein), and nonspecific cross-reacting antigen in colorectal carcinomas. Comparative analysis by northern blot and in situ hybridization. Am J Pathol. 1997;151:521–530. [PMC free article] [PubMed] [Google Scholar]
- Nollau P, Scheller H, Kona-Horstmann M, Rohde S, Hagenmuller F, Wagener C, Neumaier M. Expression of CD66a (human C-CAM) and other members of the carcinoembryonic antigen gene family of adhesion molecules in human colorectal adenomas. Cancer Res. 1997;57:2354–2357. [PubMed] [Google Scholar]
- Scheffrahn I, Singer BB, Sigmundsson K, Lucka L, Obrink B. Control of density-dependent, cell state-specific signal transduction by the cell adhesion molecule CEACAM1, and its influence on cell cycle regulation. Exp Cell Res. 2005;307:427–435. doi: 10.1016/j.yexcr.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Screaton RA, Penn LZ, Stanners CP. Carcinoembryonic antigen, a human tumor marker, cooperates with Myc and Bcl-2 in cellular transformation. J Cell Biol. 1997;137:939–952. doi: 10.1083/jcb.137.4.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BB, Klaile E, Scheffrahn I, Muller MM, Kammerer R, Reutter W, Obrink B, Lucka L. CEACAM1 (CD66a) mediates delay of spontaneous and Fas ligand-induced apoptosis in granulocytes. Eur J Immunol. 2005;35:1949–1959. doi: 10.1002/eji.200425691. [DOI] [PubMed] [Google Scholar]
- Singer BB, Scheffrahn I, Heymann R, Sigmundsson K, Kammerer R, Obrink B. Carcinoembryonic antigen-related cell adhesion molecule 1 expression and signaling in human, mouse, and rat leukocytes: evidence for replacement of the short cytoplasmic domain isoform by glycosylphosphatidylinositol-linked proteins in human leukocytes. J Immunol. 2002;168:5139–5146. doi: 10.4049/jimmunol.168.10.5139. [DOI] [PubMed] [Google Scholar]
- Singer BB, Scheffrahn I, Obrink B. The tumor growth-inhibiting cell adhesion molecule CEACAM1 (C-CAM) is differently expressed in proliferating and quiescent epithelial cells and regulates cell proliferation. Cancer Res. 2000;60:1236–1244. [PubMed] [Google Scholar]
- Skubitz KM, Campbell KD, Skubitz AP. CD66a, CD66b, CD66c, and CD66d each independently stimulate neutrophils. J Leukoc Biol. 1996;60:106–117. doi: 10.1002/jlb.60.1.106. [DOI] [PubMed] [Google Scholar]
- Stanners CP, DeMarte L, Rojas M, Gold P, Fuks A. Opposite functions for two classes of genes of the human carcinoembryonic antigen family. Tumour Biol. 1995;16:23–31. doi: 10.1159/000217925. [DOI] [PubMed] [Google Scholar]
- Stocks SC, Kerr MA. Stimulation of neutrophil adhesion by antibodies recognizing CD15 (Le(X)) and CD15-expressing carcinoembryonic antigen-related glycoprotein NCA-160. Biochem J. 1992;288:23–27. doi: 10.1042/bj2880023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocks SC, Kerr MA, Haslett C, Dransfield I. CD66-dependent neutrophil activation: a possible mechanism for vascular selectin-mediated regulation of neutrophil adhesion. J Leukoc Biol. 1995;58:40–48. doi: 10.1002/jlb.58.1.40. [DOI] [PubMed] [Google Scholar]
- Stocks SC, Ruchaud-Sparagano MH, Kerr MA, Grunert F, Haslett C, Dransfield I. CD66: role in the regulation of neutrophil effector function. Eur J Immunol. 1996;26:2924–2932. doi: 10.1002/eji.1830261218. [DOI] [PubMed] [Google Scholar]
- Turbide C, Kunath T, Daniels E, Beauchemin N. Optimal ratios of biliary glycoprotein isoforms required for inhibition of colonic tumor cell growth. Cancer Res. 1997;57:2781–2788. [PubMed] [Google Scholar]
- Esteban JM, Felder B, Ahn C, Simpson JF, Battifora H, Shively JE. Prognostic relevance of carcinoembryonic antigen and estrogen receptor status in breast cancer patients. Cancer. 1994;74:1575–1583. doi: 10.1002/1097-0142(19940901)74:5<1575::AID-CNCR2820740513>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Jantscheff P, Terracciano L, Lowy A, Glatz-Krieger K, Grunert F, Micheel B, Brummer J, Laffer U, Metzger U, Herrmann R, Rochlitz C. Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J Clin Oncol. 2003;21:3638–3646. doi: 10.1200/JCO.2003.55.135. [DOI] [PubMed] [Google Scholar]
- Kim J, Kaye FJ, Henslee JG, Shively JE, Park JG, Lai SL, Linnoila RI, Mulshine JL, Gazdar AF. Expression of carcinoembryonic antigen and related genes in lung and gastrointestinal cancers. Int J Cancer. 1992;52:718–725. doi: 10.1002/ijc.2910520509. [DOI] [PubMed] [Google Scholar]
- Koops MD, Thompson J, Zimmermann W, Stanners CP. Transcriptional regulation of the non-specific cross-reacting antigen gene, a member of the carcinoembryonic antigen gene family up-regulated in colorectal carcinomas. Eur J Biochem. 1998;253:778–786. doi: 10.1046/j.1432-1327.1998.2530778.x. [DOI] [PubMed] [Google Scholar]
- Thompson J, Mossinger S, Reichardt V, Engels U, Beauchemin N, Kommoss F, von Kleist S, Zimmermann W. A polymerase-chain-reaction assay for the specific identification of transcripts encoded by individual carcinoembryonic antigen (CEA)-gene-family members. Int J Cancer. 1993;55:311–319. doi: 10.1002/ijc.2910550223. [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Pham L, O'Connor MK, Federspiel MJ, Russell SJ, Peng KW. Dual therapy of ovarian cancer using measles viruses expressing carcinoembryonic antigen and sodium iodide symporter. Clin Cancer Res. 2006;12:1868–1875. doi: 10.1158/1078-0432.CCR-05-1803. [DOI] [PubMed] [Google Scholar]
- Ringhoffer M, Blumstein N, Neumaier B, Glatting G, von Harsdorf S, Buchmann I, Wiesneth M, Kotzerke J, Zenz T, Buck AK, et al. 188Re or 90Y-labelled anti-CD66 antibody as part of a dose-reduced conditioning regimen for patients with acute leukaemia or myelodysplastic syndrome over the age of 55: results of a phase I-II study. Br J Haematol. 2005;130:604–613. doi: 10.1111/j.1365-2141.2005.05663.x. [DOI] [PubMed] [Google Scholar]
- Zenz T, Glatting G, Schlenk RF, Buchmann I, Dohner H, Reske SN, Bunjes D. Targeted marrow irradiation with radioactively labeled anti-CD66 monoclonal antibody prior to allogeneic stem cell transplantation for patients with leukemia: results of a phase I-II study. Haematologica. 2006;91:285–286. [PubMed] [Google Scholar]
- Barnett TR, Drake L, Pickle W., 2nd Human biliary glycoprotein gene: characterization of a family of novel alternatively spliced RNAs and their expressed proteins. Mol Cell Biol. 1993;13:1273–1282. doi: 10.1128/mcb.13.2.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett TR, Kretschmer A, Austen DA, Goebel SJ, Hart JT, Elting JJ, Kamarck ME. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989;108:267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M, Arakawa F, Matsuo Y, Oikawa S, Nakazato H, Matsuoka Y. Three novel molecular forms of biliary glycoprotein deduced from cDNA clones from a human leukocyte library. Biochem Biophys Res Commun. 1991;176:578–585. doi: 10.1016/S0006-291X(05)80223-2. [DOI] [PubMed] [Google Scholar]
- Singer BB, Lucka L. CEACAM1. UCSD-Nature Molecule Pages. 2005.
- Watt SM, Fawcett J, Murdoch SJ, Teixeira AM, Gschmeissner SE, Hajibagheri NM, Simmons DL. CD66 identifies the biliary glycoprotein (BGP) adhesion molecule: cloning, expression, and adhesion functions of the BGPc splice variant. Blood. 1994;84:200–210. [PubMed] [Google Scholar]
- Skubitz KM, Snook RW., 2nd Monoclonal antibodies that recognize lacto-N-fucopentaose III (CD15) react with the adhesion-promoting glycoprotein family (LFA-1/HMac-1/gp 150,95) and CR1 on human neutrophils. J Immunol. 1987;139:1631–1639. [PubMed] [Google Scholar]
- Harvath L, Balke JA, Christiansen NP, Russell AA, Skubitz KM. Selected antibodies to leukocyte common antigen (CD45) inhibit human neutrophil chemotaxis. J Immunol. 1991;146:949–957. [PubMed] [Google Scholar]
- Skubitz KM, Campbell KD, Iida J, Skubitz AP. CD63 associates with tyrosine kinase activity and CD11/CD18, and transmits an activation signal in neutrophils. J Immunol. 1996;157:3617–3626. [PubMed] [Google Scholar]
- Parham P. Purification of immunologically active HLA-A and -B antigens by a series of monoclonal antibody columns. J Biol Chem. 1979;254:8709–8712. [PubMed] [Google Scholar]
- Vaporciyan AA, Jones ML, Ward PA. Rapid analysis of leukocyte-endothelial adhesion. J Immunol Methods. 1993;159:93–100. doi: 10.1016/0022-1759(93)90145-W. [DOI] [PubMed] [Google Scholar]
- Wertheimer SJ, Myers CL, Wallace RW, Parks TP. Intercellular adhesion molecule-1 gene expression in human endothelial cells. Differential regulation by tumor necrosis factor-alpha and phorbol myristate acetate. J Biol Chem. 1992;267:12030–12035. [PubMed] [Google Scholar]
- Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- Eidelman FJ, Fuks A, DeMarte L, Taheri M, Stanners CP. Human carcinoembryonic antigen, an intercellular adhesion molecule, blocks fusion and differentiation of rat myoblasts. J Cell Biol. 1993;123:467–475. doi: 10.1083/jcb.123.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup JM, Kim JC, Thomas P, Ishii S, Ford R, Shively JE, Durbin H, Stanners CP, Fuks A, Zhou H, et al. Adhesion to carcinoembryonic antigen by human colorectal carcinoma cells involves at least two epitopes. Int J Cancer. 1993;55:262–268. doi: 10.1002/ijc.2910550216. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Inuzuka C, Kuroki M, Arakawa F, Matsuoka Y, Kosaki G, Nakazato H. A specific heterotypic cell adhesion activity between members of carcinoembryonic antigen family, W272 and NCA, is mediated by N-domains. J Biol Chem. 1991;266:7995–8001. [PubMed] [Google Scholar]
- Oikawa S, Inuzuka C, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. Cell adhesion activity of non-specific cross-reacting antigen (NCA) and carcinoembryonic antigen (CEA) expressed on CHO cell surface: homophilic and heterophilic adhesion. Biochem Biophys Res Commun. 1989;164:39–45. doi: 10.1016/0006-291X(89)91679-3. [DOI] [PubMed] [Google Scholar]
- Oikawa S, Kuroki M, Matsuoka Y, Kosaki G, Nakazato H. Homotypic and heterotypic Ca(++)-independent cell adhesion activities of biliary glycoprotein, a member of carcinoembryonic antigen family, expressed on CHO cell surface. Biochem Biophys Res Commun. 1992;186:881–887. doi: 10.1016/0006-291X(92)90828-9. [DOI] [PubMed] [Google Scholar]
- Olsson H, Wikstrom K, Kjellstrom G, Obrink B. Cell adhesion activity of the short cytoplasmic domain isoform of C-CAM (C-CAM2) in CHO cells. FEBS Lett. 1995;365:51–56. doi: 10.1016/0014-5793(95)00436-D. [DOI] [PubMed] [Google Scholar]
- Rojas M, DeMarte L, Screaton RA, Stanners CP. Radical differences in functions of closely related members of the human carcinoembryonic antigen gene family. Cell Growth Differ. 1996;7:655–662. [PubMed] [Google Scholar]
- Rojas M, Fuks A, Stanners CP. Biliary glycoprotein, a member of the immunoglobulin supergene family, functions in vitro as a Ca2(+)-dependent intercellular adhesion molecule. Cell Growth Differ. 1990;1:527–533. [PubMed] [Google Scholar]
- Teixeira AM, Fawcett J, Simmons DL, Watt SM. The N-domain of the biliary glycoprotein (BGP) adhesion molecule mediates homotypic binding: domain interactions and epitope analysis of BGPc. Blood. 1994;84:211–219. [PubMed] [Google Scholar]
- Zhou H, Fuks A, Alcaraz G, Bolling TJ, Stanners CP. Homophilic adhesion between Ig superfamily carcinoembryonic antigen molecules involves double reciprocal bonds. J Cell Biol. 1993;122:951–960. doi: 10.1083/jcb.122.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer J, Neumaier M, Gopfert C, Wagener C. Association of pp60c-src with biliary glycoprotein (CD66a), an adhesion molecule of the carcinoembryonic antigen family downregulated in colorectal carcinomas. Oncogene. 1995;11:1649–1655. [PubMed] [Google Scholar]
- Skubitz KM, Campbell KD, Ahmed K, Skubitz AP. CD66 family members are associated with tyrosine kinase activity in human neutrophils. J Immunol. 1995;155:5382–5390. [PubMed] [Google Scholar]
- Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–790. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- McCaw SE, Schneider J, Liao EH, Zimmermann W, Gray-Owen SD. Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol Microbiol. 2003;49:623–637. doi: 10.1046/j.1365-2958.2003.03591.x. [DOI] [PubMed] [Google Scholar]
- Iida J, Skubitz AP, McCarthy JB, Skubitz KM. Protein kinase activity is associated with CD63 in melanoma cells. J Transl Med. 2005;3:42. doi: 10.1186/1479-5876-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaw SE, Liao EH, Gray-Owen SD. Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect Immun. 2004;72:2742–2752. doi: 10.1128/IAI.72.5.2742-2752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitter T, Pils S, Sakk V, Frank R, Fischer KD, Hauck CR. The granulocyte receptor carcinoembryonic antigen-related cell adhesion molecule 3 (CEACAM3) directly associates with Vav to promote phagocytosis of human pathogens. J Immunol. 2007;178:3797–3805. doi: 10.4049/jimmunol.178.6.3797. [DOI] [PubMed] [Google Scholar]
- Schmitter T, Pils S, Weibel S, Agerer F, Peterson L, Buntru A, Kopp K, Hauck CR. Opa proteins of pathogenic neisseriae initiate Src kinase-dependent or lipid raft-mediated uptake via distinct human carcinoembryonic antigen-related cell adhesion molecule isoforms. Infect Immun. 2007;75:4116–4126. doi: 10.1128/IAI.01835-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucka L, Cichocka I, Baumler K, Bechler K, Reutter W. A short isoform of carcinoembryonic-antigen-related rat liver cell-cell adhesion molecule (C-CAM/gp110) mediates intercellular adhesion. Sequencing and recombinant functional analysis. Eur J Biochem. 1995;234:527–535. doi: 10.1111/j.1432-1033.1995.527_b.x. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Durbin H, Bodmer WF. Carcinoembryonic antigen functions as an accessory adhesion molecule mediating colon epithelial cell-collagen interactions. Proc Natl Acad Sci USA. 1990;87:1541–1545. doi: 10.1073/pnas.87.4.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Stanners CP, Fuks A. Specificity of anti-carcinoembryonic antigen monoclonal antibodies and their effects on CEA-mediated adhesion. Cancer Res. 1993;53:3817–3822. [PubMed] [Google Scholar]
- Hunter I, Sawa H, Edlund M, Obrink B. Evidence for regulated dimerization of cell-cell adhesion molecule (C-CAM) in epithelial cells. Biochem J. 1996;320:847–853. doi: 10.1042/bj3200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer BB. doctoral dissertation. Albert-Ludwigs-Universitat Freiburg; 1996. Molekulare organization von CD66-molekullen afu neutrophilen granulozyten. Doctoral dissertation. [Google Scholar]
- Bos MP, Grunert F, Belland RJ. Differential recognition of members of the carcinoembryonic antigen family by Opa variants of Neisseria gonorrhoeae. Infect Immun. 1997;65:2353–2361. doi: 10.1128/iai.65.6.2353-2361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Gotschlich EC. CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc Natl Acad Sci USA. 1996;93:14851–14856. doi: 10.1073/pnas.93.25.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T, Grunert F, Medina-Marino A, Gotschlich EC. Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J Exp Med. 1997;185:1557–1564. doi: 10.1084/jem.185.9.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Owen SD, Dehio C, Haude A, Grunert F, Meyer TF. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. Embo J. 1997;16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Owen SD, Lorenzen DR, Haude A, Meyer TF, Dehio C. Differential Opa specificities for CD66 receptors influence tissue interactions and cellular response to Neisseria gonorrhoeae. Mol Microbiol. 1997;26:971–980. doi: 10.1046/j.1365-2958.1997.6342006.x. [DOI] [PubMed] [Google Scholar]
- Hauck CR, Meyer TF, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. Embo J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusch HG, Drzeniek Z, Markos-Pusztai Z, Wagener C. Binding of Escherichia coli and Salmonella strains to members of the carcinoembryonic antigen family: differential binding inhibition by aromatic alpha-glycosides of mannose. Infect Immun. 1991;59:2051–2057. doi: 10.1128/iai.59.6.2051-2057.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter SL, Rutherfurd SM, Wagener C, Shively JE, Hefta SA. Binding of nonspecific cross-reacting antigen, a granulocyte membrane glycoprotein, to Escherichia coli expressing type 1 fimbriae. Infect Immun. 1991;59:2485–2493. doi: 10.1128/iai.59.7.2485-2493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M, Evans D, Hadfield A, Grunert F, Teixeira AM, Watt SM. Critical determinants of host receptor targeting by Neisseria meningitidis and Neisseria gonorrhoeae: identification of Opa adhesiotopes on the N-domain of CD66 molecules. Mol Microbiol. 1999;34:538–551. doi: 10.1046/j.1365-2958.1999.01620.x. [DOI] [PubMed] [Google Scholar]
- Virji M, Makepeace K, Ferguson DJ, Watt SM. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Gray-Owen SD, Knorre A, Meyer TF, Dehio C. Opa binding to cellular CD66 receptors mediates the transcellular traversal of Neisseria gonorrhoeae across polarized T84 epithelial cell monolayers. Mol Microbiol. 1998;30:657–671. doi: 10.1046/j.1365-2958.1998.01102.x. [DOI] [PubMed] [Google Scholar]
- Ducker TP, Skubitz KM. Subcellular localization of CD66, CD67, and NCA in human neutrophils. J Leukoc Biol. 1992;52:11–16. doi: 10.1002/jlb.52.1.11. [DOI] [PubMed] [Google Scholar]
- Kuroki M, Matsuo Y, Kinugasa T, Matsuoka Y. Augmented expression and release of nonspecific cross-reacting antigens (NCAs), members of the CEA family, by human neutrophils during cell activation. J Leukoc Biol. 1992;52:551–557. doi: 10.1002/jlb.52.5.551. [DOI] [PubMed] [Google Scholar]
- Tetteroo PA, Bos MJ, Visser FJ, von dem Borne AE. Neutrophil activation detected by monoclonal antibodies. J Immunol. 1986;136:3427–3432. [PubMed] [Google Scholar]
- Ergun S, Kilik N, Ziegeler G, Hansen A, Nollau P, Gotze J, Wurmbach JH, Horst A, Weil J, Fernando M, Wagener C. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000;5:311–320. doi: 10.1016/S1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- Brummer J, Ebrahimnejad A, Flayeh R, Schumacher U, Loning T, Bamberger AM, Wagener C. cis Interaction of the cell adhesion molecule CEACAM1 with integrin beta(3) Am J Pathol. 2001;159:537–546. doi: 10.1016/s0002-9440(10)61725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler G, Nedellec P, Lu JH, Keck U, Basile A, Cardellichio C, Zimmermann W, Beauchemin N, Holmes KV. Characterization of a new gene that encodes a functional MHV receptor and progress in the identification of the virus-binding site(s) Adv Exp Med Biol. 1995;380:345–350. doi: 10.1007/978-1-4615-1899-0_56. [DOI] [PubMed] [Google Scholar]
- Dveksler GS, Dieffenbach CW, Cardellichio CB, McCuaig K, Pensiero MN, Jiang GS, Beauchemin N, Holmes KV. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J Virol. 1993;67:1–8. doi: 10.1128/jvi.67.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dveksler GS, Pensiero MN, Cardellichio CB, Williams RK, Jiang GS, Holmes KV, Dieffenbach CW. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991;65:6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KV, Dveksler G, Gagneten S, Yeager C, Lin SH, Beauchemin N, Look AT, Ashmun R, Dieffenbach C. Coronavirus receptor specificity. Adv Exp Med Biol. 1993;342:261–266. doi: 10.1007/978-1-4615-2996-5_40. [DOI] [PubMed] [Google Scholar]
- Holmes KV, Tresnan DB, Zelus BD. Virus-receptor interactions in the enteric tract. Virus-receptor interactions. Adv Exp Med Biol. 1997;412:125–133. doi: 10.1007/978-1-4899-1828-4_20. [DOI] [PubMed] [Google Scholar]
- Kammerer R, von Kleist S. The carcinoembryonic antigen (CEA) modulates effector-target cell interaction by binding to activated lymphocytes. Int J Cancer. 1996;68:457–463. doi: 10.1002/(SICI)1097-0215(19961115)68:4<457::AID-IJC10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Morales VM, Christ A, Watt SM, Kim HS, Johnson KW, Utku N, Texieira AM, Mizoguchi A, Mizoguchi E, Russell GJ, et al. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a) J Immunol. 1999;163:1363–1370. [PubMed] [Google Scholar]