Abstract
Hypothalamic growth hormone-releasing hormone (GHRH) controls the release of growth hormone and acts as a growth factor in various tumors. Potent antagonistic analogues of GHRH have been synthesized that strongly suppress the growth of diverse cancers through several mechanisms. However, the influence of GHRH antagonists on the redox (reduction/oxidation) status of cancers has not been investigated. Cellular generation of reactive oxygen species (ROS) is central to redox signaling and is implicated in the initiation, development, and progression of cancer. In this study, we evaluated by Western blot the effects in vitro of GHRH and its antagonist JMR-132 on proliferating cell nuclear antigen, tumor suppressor protein p53, transcription factor NF-κB p50 and its phosphorylated form, caspase 3, and cleaved caspase 3 in the LNCaP human prostate cancer cell line. GHRH stimulated and GHRH antagonist inhibited the expression of the major antioxidant enzymes, as well as the expression of COX 2 and cytochrome c oxidase IV, which are enzymes involved in the generation of ROS. GHRH augmented and GHRH antagonist suppressed lipid and protein oxidative stress markers, as well as the intracellular generation of ROS. In all these tests, GHRH antagonists exerted strong antioxidant activity. Because the metabolism of ROS and oxidative stress have been associated with initiation and progression of not only prostate tumors but also other malignancies, our findings reinforce previous experimental evidence that GHRH antagonists could be useful for cancer therapy.
Keywords: GHRH, oxidative stress, ROS, antioxidative activity
A new therapy for cancer may emerge from the development of antagonistic analogues of growth hormone-releasing hormone (GHRH), which started more than a decade ago. GHRH neuropeptide, secreted by the hypothalamus, regulates the release of growth hormone from the anterior pituitary gland. GHRH was first isolated from human pancreatic tumors and was only subsequently identified in human hypothalamus (1–3).
The fact that GHRH is implicated as a growth factor in carcinogenesis was established only recently (4), although its initial identification from tumor tissue should have provided a hint about this likelihood (1). Thus, the expression of mRNA for GHRH and the presence of biologically active GHRH were demonstrated in several established cancer cell lines and human tumors. The suppression of proliferation of breast, prostate, and lung cancer cell lines after the knocking down of GHRH gene expression supports the concept that GHRH functions as a growth factor at least in these human cancers. Peptide receptors that mediate the effects of GHRH and its antagonists on tumors were also identified recently, with the demonstration that cancers can express splice variants (SVs) of the pituitary GHRH receptor as well as the pituitary type itself (5–9).
Several series of antagonistic analogues of GHRH were synthesized in our laboratory and were shown to inhibit the growth of a variety of experimental human cancers (1, 3, 7, 10–12) The inhibitory effect of antagonistic analogues of GHRH is exerted in part by endocrine mechanisms through the suppression of GHRH-evoked GH release from the pituitary, which in turn results in the reduction of hepatic insulin-like growth factor 1 (IGF-1) levels in serum. The antitumor effects of GHRH antagonists can also be exerted directly on tumors and are based on the blockade of action of autocrine GHRH in tumors, as well as the inhibition of the secretion of autocrine/paracrine IGF-1 or IGF-2 from the tumors (3).
The influence of the GHRH analogues in the redox (reduction/oxidation) status of cancers has not been investigated. The central role in redox signaling is played by reactive oxygen species (ROS), which are oxygen radicals and nonradical derivatives of O2, thus highly reactive molecules. When organic radicals are generated within an organism they can react rapidly with DNA, proteins, and lipids, causing chemical modification, collectively known as oxidative stress (13). ROS are produced continuously by the mitochondria, macrophages, and peroxisomes (14).
Reactions between ROS and redox active amino acid residues in transcription factors and enzymes can modulate the activities of these proteins. Cells possess effective mechanisms to control ROS. Among these is the synthesis of detoxifying enzymes, such as thioredoxins (Trxs), superoxide dismutases (SODs), glutathione peroxidases (GPxs), and quinone oxidoreductase 1 (NQO1), which convert ROS into less-active species (15, 16).
ROS and cellular oxidative stress are associated with cancer in a complex fashion (17–20). Cancer cells produce more ROS than normal cells, and ROS are thought to play a role in tumor initiation and progression (21, 22) and are also required for aggressive phenotype (23). Abnormal increases in ROS can be exploited to selectively kill cancer cells (24). Exogenous ROS-stressing agents can increase intracellular ROS to a toxic level or the threshold that triggers cell death (24, 25).
In the present study we showed by Western blot the expression of the GHRH receptor (GHRH-R) and its SV1 in the LNCaP prostate cancer cell line. We evaluated the effect of GHRH(1-29)NH2 and GHRH antagonist JMR-132 on the proliferation rate of LNCaP cells and on the expression of proliferating cell nuclear antigen (PCNA). We examined expression of wild-type tumor suppressor protein p53 (26), transcription factor NF-κB p50 and its phosphorylated form, as well as caspase 3 and cleaved caspase 3, which act on apoptosis (27).
We then evaluated whether GHRH(1-29)NH2 and the GHRH antagonist influence expression of the antioxidant enzymes SOD1, which is also a target for the inhibition of angiogenesis and tumor growth (28); NQO1, a cytosolic protein that reduces and detoxifies quinones protecting the cells against redox cycling and oxidative stress (29); GPx1, which is a main glutathione peroxidase (30); and Trx1, a major cytoplasmic antioxidant enzyme (31). In addition, we examined whether expression of COX 2 and cytochrome c oxidase IV (COX IV), enzymes that are involved in the generation of ROS, could be influenced by GHRH and GHRH antagonist.
A possible upregulation or downregulation of the major antioxidant enzymes by the antagonistic analogues of GHRH does not provide definite conclusions about the oxidative status of the cancer cells. Decreased expression of antioxidant enzymes can reflect either less oxidative stress (theory of redox homeostasis) (32) in the cells or more oxidative stress, which might result from the downregulation of their genes by the GHRH antagonists. Consequently, to elucidate the oxidative status of the prostate cancer cell line before and after treatment with the GHRH antagonist, we evaluated the expression of 3-nitrotyrosine (33–35) and the protein carbonyl groups, which are considered markers of protein oxidative modifications (36, 37), as well as malondialdehyde (MDA), which reflects the status of lipid peroxidation (37). In addition, we examined the influence of GHRH and JMR-132 on intracellular generation of ROS.
Results
Expression of GHRH Receptor and Its SV1 in the LNCaP Prostate Cancer Cell Line.
A band of 45 kDa, which reflects the production of GHRH-R (38), and a band of 39.5 kDa, which is consistent with the size of the SV1 receptor (39) (regularity index [RI]: 2.37 and 2.90, respectively) were detected in the LNCaP prostate cancer cell line. MCF7 breast cancer cells, which do not express GHRH-R or SV1 receptor, were used as negative control (9) (RI: 0.06 and 0.08, respectively). The results are shown in Fig. S1.
Effect of GHRH(1-29)NH2 and GHRH Antagonist JMR-132 on Proliferation Rate and Expression of PCNA in LNCaP Cancer Cells In Vitro.
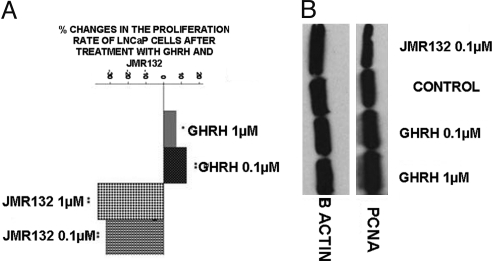
LNCaP prostate cancer cells were exposed to two concentrations of GHRH(1-29)NH2 and JMR-132. At the dose of 1 μM, GHRH(1-29)NH2 did not appreciably influence the proliferation rate of the cells, producing an increase of only 7%. However, GHRH(1-29)NH2 at a 0.1 μM concentration stimulated the proliferation rate of LNCaP cells by 13%. The GHRH antagonist JMR-132 at doses of 0.1 μM and 1 μM decreased proliferation of the LNCaP prostate cancer cell line by 32% and 37%, respectively. The results are shown in Fig. 1A. In addition, the expression levels of PCNA (molecular mass: 36 kDa) were evaluated by Western blot. PCNA protein expression increased in cells exposed to 0.1 μM and 1 μM of GHRH(1-29)NH2 (RI: 0.77 and 0.925) and decreased in cells incubated with 0.1 μM of GHRH antagonist JMR-132. (RI: 0.495) as compared with control (RI: 0.656). The results are shown in Fig. 1B.
Fig. 1.
Effect of GHRH and JMR-132 on the proliferation rate of LNCaP cancer cells, measured after an incubation of 72 h. (A) Changes in proliferation rate of LNCaP prostate cancer cells after exposure to GHRH antagonist JMR-132 and GHRH (1-29)NH2. Percentage increase or decrease are expressed vs. LNCaP cells cultured in the absence of JMR-132 or GHRH (1-29)NH2. *P < 0.05; **P < 0.005. (B) Western blot analysis of expression of PCNA in LNCaP prostate cancer cells after exposure to GHRH antagonist JMR-132 and GHRH(1-29)NH2; n = 2
Effect of GHRH(1-29)NH2 and JMR-132 on Expression of Wild-Type p53 Tumor Suppressor Protein in LNCaP Cancer Cells In Vitro.
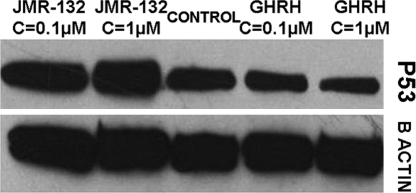
LNCaP prostate cancer cells cultured in vitro were exposed to two concentrations of JMR-132 and GHRH(1-29)NH2, and the expression level of the p53 tumor suppressor protein (molecular mass: 53 kDa) was measured by Western blot. The results are shown in Fig. 2. p53 protein expression was higher in cells exposed to 0.1 μM and 1 μM GHRH antagonist JMR-132 (RI: 0.583 and 0.658) and lower in cells incubated with 0.1 μM and 1 μM GHRH (1-29)NH2 (RI: 0.376 and 0.264) as compared with control (RI: 0.436).
Fig. 2.
Western blot analysis of expression of wild-type p53 tumor suppressor protein in LNCaP prostate cancer cells after 72-h exposure to GHRH antagonist JMR-132 and GHRH(1-29)NH2; n = 2
Effect of GHRH Antagonist JMR-132 and GHRH(1-29)NH2 on Expression of NF-κB p50 and Its Phosphorylated Form, Caspase 3, and Cleaved Caspase 3 Protein in LNCaP Prostate Cancer Cells In Vitro.
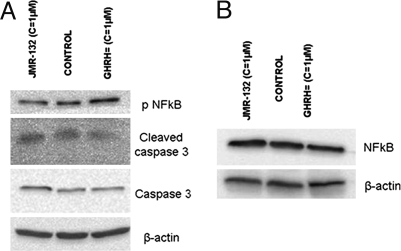
LNCaP cells cultured in vitro were exposed to 1 μM GHRH antagonist JMR-132 and 1 μM GHRH(1-29)NH2. The expression levels of NF-κB p50, phosphorylated NF-κB p50, caspase 3 (molecular mass: 35 kDa), and cleaved caspase 3 were detected by Western blot. The results are shown in Fig. 3A. The expression of phosphorylated NF-κB, caspase 3 protein, and its cleaved form was higher in cells exposed to GHRH antagonist JMR-132 (RI: 0.451, 0.120, and 0.391) and lower in cells cultured with GHRH (1-29)NH2 (RI: 0.623, 0.083, and 0.182) as compared with control (RI: 0.521, 0.108, and 0.320). The expression of NF-κB was not influenced by GHRH (1-29)NH2 or JMR-132 (RI: 0.766, 0.786, and 0.737). The results are shown in Fig. 3B.
Fig. 3.
Effect of GHRH and JMR-132 on the activation of caspase 3 and NF-κB p50 measured after an incubation of 72 h. (A) Western blot analysis of expression of phosphorylated NF-κB p50, caspase 3 protein, and its cleaved form in LNCaP prostate cancer cells after exposure to GHRH antagonist JMR-132 and GHRH (1-29)NH2; n = 2. (B) Western blot analysis of expression of NF-κB p50; n = 2
Effect of JMR-132 and GHRH(1-29)NH2 on Expression of Antioxidant Enzymes GPx1, SOD1, NQO1, and Trx1 in LNCaP Prostate Cancer Cells In Vitro.
LNCaP prostate cancer cells cultured in vitro were exposed to 1 μM JMR-132 and GHRH(1-29)NH2. The expression levels of the detoxifying enzymes were measured by Western blot. GPx1 (molecular mass: 22 kDa) protein expression was higher in cells exposed to GHRH (1-29)NH2 (RI: 0.126) and lower in cells incubated with GHRH antagonist JMR-132 (RI: 0.035) as compared with control (RI: 0.107). SOD1 protein expression (molecular mass: 18 kDa) was only detectable in cells that were incubated with GHRH (1-29)NH2 (RI: 0.111). NQO1 (molecular mass: 29 kDa) protein expression was higher in cells exposed to GHRH (1-29)NH2 (RI: 0.196) and much lower in cells incubated with GHRH antagonist JMR-132 (RI: 0.025) as compared with control (RI: 0.175). The levels of Trx1 (molecular mass: 12 kDa) were elevated in cells treated with GHRH (1-29)NH2 (RI: 0.277) and decreased in cells exposed to JMR-132 (RI: 0.196) as compared with control (RI: 0.210). The results are shown in Fig. S2.
Effect of GHRH Antagonist JMR-132 and GHRH(1-29)NH2 on Expression of COX 2 and COX IV Enzymes in LNCaP Prostate Cancer Cells In Vitro.
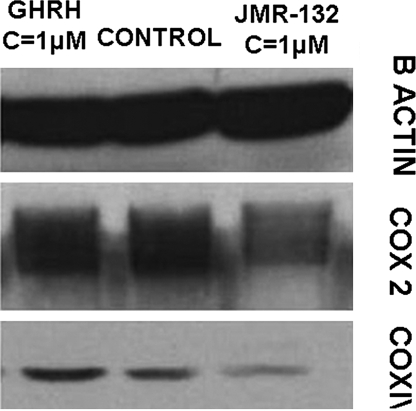
After LNCaP prostate cancer cells cultured in vitro were exposed to 1 μM GHRH antagonist JMR-132 and GHRH(1-29)NH2, the expression levels of the enzymes COX 2 and COX IV were measured by Western blot. COX 2 (molecular mass: 74 kDa) and COX IV (molecular mass: 17 kDa) protein expression was higher in cells exposed to GHRH (1-29)NH2 (RI: 0.928, 0.237) and lower in cells incubated with GHRH antagonist JMR-132 (RI: 0.532, 0.077) as compared with control (RI: 0.822, 0.139). The results are shown in Fig. 4.
Fig. 4.
Western blot analysis of expression of COX 2 and COX IV in LNCaP prostate cancer cells after 72-h incubation with GHRH antagonist JMR-132 and GHRH(1-29)NH2; n = 2
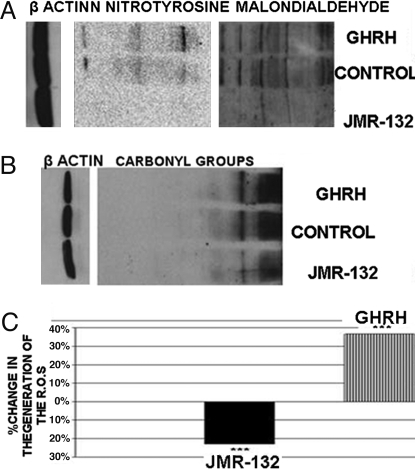
Effect of GHRH Antagonist JMR-132 and GHRH(1-29)NH2 on Protein and Lipid Oxidation and on Intracellular Generation of ROS in LNCaP Prostate Cancer Cells In Vitro.
LNCaP prostate cancer cells cultured in vitro were exposed to 1 μM JMR-132 or 1 μM GHRH(1-29)NH2. The levels of oxidation of proteins were determined by the detection of N-nitrotyrosine and the protein carbonyl groups. Both were elevated in cells exposed to GHRH(1-29)NH2 (RI: 3.282, 7.415) and decreased in cells incubated with GHRH antagonist JMR-132 (RI: 1.251, 4.275) as compared with control (RI: 2.903, 5.846). The results are shown in Fig. 5A. The levels of lipid peroxidation, determined by the detection of MDA, were increased in cells exposed to GHRH(1-29)NH2 (RI: 4.89) and decreased in cells incubated with GHRH antagonist JMR-132 (RI: 2.973) as compared with control (RI: 4.433). The results are shown in Fig. 5B. In addition, the generation of ROS was higher by 36% in cells incubated with GHRH (1-29)NH2 and lower by 23% in cells exposed to JMR-132 as compared with control. The results are shown in the Fig. 5C.
Fig. 5.
Effects of GHRH and JMR-132 on protein and lipid oxidation markers and on generation of ROS in LNCaP prostate cancer cells. (A) Detection of expression of oxidation markers (nitrotyrosine and MDA) in LNCaP prostate cancer cells after incubation for 72 h with GHRH antagonist JMR-132 and GHRH(1-29)NH2; n = 2. (B) Detection of expression of the carbonyl groups in LNCaP prostate cancer cells after 72-h treatment with GHRH antagonist JMR-132 and GHRH(1-29)NH2; n = 2. (C) Changes in generation of ROS after 30-min incubation. Percentage increase or decrease are expressed vs. LNCaP cells cultured in the absence of JMR-132 or GHRH (1-29)NH2. ***, P < 0.005.
Discussion
GHRH and its receptor(s) are expressed in a variety of human cancers and cancer cell lines, and antagonists of GHRH exert strong antiproliferative activity (2, 3, 7). However, the influence of GHRH and its antagonists on the redox status of cancer cell lines has not been investigated.
In the present study we detected expression of the pituitary type of GHRH-R and its SV1 in the LNCaP prostate cancer cell line, and we investigated the effects of GHRH and GHRH antagonist on its proliferation. The MCF-7 breast cancer cell line was used as the negative control (Fig. S1) because it does not respond to GHRH or JMR-132 (4, 9). We showed that 0.1 μM but not 1 μM of GHRH increased the proliferation rate, whereas 0.1 μM and 1 μM of GHRH antagonist JMR-132 decreased it. At the dose of 1 μM, GHRH(1-29)NH2 did not appreciably influence the proliferation rate of LNCaP cells, a phenomenon which was also reported in other cancer cell lines (4, 6, 9). This could be because LNCaP cells already produce significant amounts of endogenous GHRH, and the corresponding signaling pathways might be saturated after exposure to 1 μM GHRH. In a recent study we reported that after knocking down GHRH gene expression in LNCaP cells, their proliferation rate increased dramatically after supplementation with exogenous 1 μM GHRH(1-29)NH2 (4).
The wild-type tumor-suppressor protein p53, which is expressed in LNCaP cells (40, 41), acts as a major defense against cancer and can elicit apoptotic death, cell cycle arrest, or senescence through differential activation of target genes to maintain genomic integrity (26, 42). Given that both ROS and p53 participate in multiple cellular processes, interactions between them and their signaling pathways should exist (42). The induction of expression of wild-type p53 is related to antioxidant activities, which also contribute to tumor suppression (43–45). We examined whether expression of wild-type p53 is influenced by treatment with GHRH antagonist and GHRH(1-29)NH2. The results indicate that GHRH antagonist enhanced the expression of p53, whereas GHRH(1-29)NH2 suppressed it.
We then examined the expression of PCNA, which is considered a major proliferation marker. The levels of PCNA expression were elevated in cells exposed to GHRH(1-29)NH2 and decreased in cells cultured with GHRH antagonist. These results provide a confirmation of the experiment in vitro on the proliferation rate of the LNCaP prostate cancer cell line, shown in Fig. 1. Activation of the MAPK signaling pathway, which is stimulated by GHRH (46–49), is implicated in the progression of tumorigenesis (50). Wild-type p53 suppresses the promoter of PCNA to mediate DNA synthesis and repair processes (51). In addition, PCNA can play a critical role in regulating the stability of p53. The inactivation of PCNA can induce stabilization of p53 (52)
An upregulation of the tumor suppressor gene p53 can trigger apoptosis (53). To examine whether p53 leads to apoptotic death, we detected by Western blot the proapoptotic protein caspase 3. The expression of caspase 3 and cleaved caspase 3 was elevated in cells incubated with GHRH antagonist and downregulated after exposure to GHRH (1-29)NH2. These results are consistent with previous studies that supported the antiapoptotic role of GHRH in cancer cells (54, 55) and the induction of apoptosis by GHRH antagonists in tumors (55). In addition, inhibition of the MAPK pathway enhances apoptotic death (56, 57). The activation of the NF-κB p50, which promotes carcinogenesis (58–60), is enhanced by oxidative stress (59, 61, 62) and is inhibited by the overexpression of wild-type p53 (58, 63). The prostate cancer cells incubated with GHRH expressed increased levels of pNF-κB p50, in contrast to cells exposed to JMR-132, which expressed lower concentrations of this transcription factor.
We then examined the expression of the major antioxidant enzymes SOD1, GPx1, NQO1, and Trx1. SOD1 belongs to the SOD family of enzymes, which catalyze the dismutation of superoxide into H2O2 and oxygen. Malignant cells are highly dependent on SOD for survival and sensitive to its suppression (64). In addition, SOD 1 is essential for angiogenesis and tumor growth (65), and increased levels of SOD1 in human cells have been shown to augment the levels of ROS and stabilize hypoxia-inducible factor 1 (66). The expression levels of SOD1 were elevated in cells treated with GHRH, which indicates that GHRH and its analogues influence the expression of SOD1. The expression levels of SOD1 in cells cultured in free media or JMR-132 were not detectable, owing to low SOD activity in cancer cells (64). The expression of the cytosolic/mitochondrial selenium-dependent GPx1 was also decreased by GHRH antagonist and increased by GHRH. The overexpression of GPx1 is related to the protection of cancer cells against apoptosis, and its expression is regulated by a signaling pathway that is activated in oxidative stress response (67). Furthermore, the increase in MnSOD is associated with cancer progression (68).
NQO1 is a cytosolic protein that reduces and detoxifies quinones and their derivatives, thus protecting cells against redox cycling and oxidative stress (29). The expression levels of NQO1 were elevated in cells treated with GHRH and decreased in cells cultured with JMR-132. Overexpression of p53 suppresses the Nrf2-mediated activation of antioxidant response elements-containing promoters, including NQO1 gene (69). Trx1, a cell cytosolic detoxifying protein, was upregulated by GHRH and downregulated by JMR-132. Trx is implicated in the stimulation of hypoxia-inducible factor, which in turn stimulates VEGF and angiogenesis, thus promoting carcinogenesis (31).
COX 2, an enzyme that is induced in pathologic conditions leading to neuronal tissue death, is an important source of intracellular ROS. Enhanced COX 2 expression participates in inflammation and neuronal death in brains with ischemia and neurodegenerative diseases (70). Activation of the MAPK pathway induces the expression of the COX 2 enzyme (71), which also plays a significant role in the induction of cell migration (72). GHRH antagonist decreased the expression of COX 2, blocking the MAPK pathway. The expression of COX IV, which is also involved in the generation of ROS, was also downregulated by the GHRH antagonist, reflecting the decreased oxidative status of the cancer cell line.
We examined the protein and lipid oxidative status of the LNCaP prostate cancer cell line after treatment with GHRH and GHRH antagonist. The results indicate that GHRH antagonist JMR-132 possesses a strong antioxidant activity, whereas GHRH increases protein and lipid oxidation of the cells. The LNCaP prostate cancer cells exposed to JMR-132 generated fewer ROS than control cells, in marked contrast to cells exposed to GHRH, which produced increased amounts of ROS. Previous studies suggest that the activation of the MAPK pathway plays a key role in ROS generation (73) and indicate that the activation of MAPK is related to ROS-induced cellular events (22, 74–83). GHRH was shown to act through the phosphorylation of MAPK (46–49). A suppression of this pathway by GHRH antagonists results in reduction of oxidative stress in LNCaP.
This study reports antioxidant activity of GHRH antagonists. ROS, which participate in the initiation and progression of cancer (17, 66), are also strongly implicated in a variety of other diseases. Oxidative stress resulting from increased production of ROS plays a major role in aging (15, 84, 85), atherosclerosis, rheumatoid arthritis, obstructive sleep apnea, and in the pathogenesis of cardiovascular complications and ischemia (32), as well as in the multiple forms of insulin resistance (86) and the pathogenesis of diabetes and its major complications, including nephropathy, retinopathy, neuropathy, and macro- and microvascular damage (87). Increased oxidative stress seems to be involved in the development of neurodegenerative diseases like Alzheimer's and Parkinson's disease (88), amyotrophic lateral sclerosis, and Huntington's disease (89). Free radicals can also regulate angiogenesis and radiotherapy response (66) and VEGF production (87). The antioxidant activity of GHRH antagonists suggests that these compounds could be used not only as anticancer drugs but also for the treatment of diseases related to increased oxidative stress.
Materials and Methods
Cell Culture and Western Blotting.
LNCaP prostate cancer cells and MCF-7 breast cancer cells were obtained from American Type Culture Collection and were cultured as described previously (4). The antibodies that detect P53, PCNA, GPx1, SOD1, NQO1, Trx1, COX 2 and COX IV were purchased from Cell Signaling. The antibodies that detect β-actin, NF-κB50, pNF-κB50, caspase 3, and its cleaved form were purchased from Santa Cruz Biotechnology. The antibodies against GHRH-R (batch number: SV95) and SV1 (batch number: JH 2317/5) were raised in our laboratory. The signals for the immunoreactive proteins were visualized in a Chemi Doc XRS system (Bio-Rad). The Western blot assay and the quantification analysis of the blots were performed as described previously (4).
Detection of Protein and Lipid Oxidation.
The detection of the carbonyl groups, nitrotyrosine, and lipid peroxidation was performed with the Oxiselect Protein Carbonyl Immunoblot, the Oxiselect Nitrotyrosine Immunoblot Kit, and the Oxiselect Malondialdehyde Immunoblot Kit, respectively (Cell Biolabs) according the manufacturer's instructions. Lipid peroxidation was detected using a primary rabbit anti-MDA antibody (Cell Biolabs) according the manufacturer's instructions. The β-actin signal was used as control.
Measurement of Intracellular Generation of ROS.
The detection of ROS was carried out using aminophenyl fluorescein, an indicator for highly reactive oxygen species (Invitrogen). This fluorescein derivative is nonfluorescent until it reacts with the hydroxyl radical peroxynitrite anion or hypochlorite anion. Upon oxidation it exhibits green fluorescence, which can be detected with a fluorescence plate reader. LNCaP prostate cancer cells were seeded in 200-μl of RPMI 1640 containing 10 μM aminophenyl fluorescein at a density of 103 cells per well onto a 48-well plate and were incubated for 30 min at 37 °C with GHRH(1-29)NH2 or JMR-132 at a concentration of 10−6 M. Fluorescence was measured using a fluorescence plate reader (VICTOR3 Multilabel Plate Reader; Perkin-Elmer) with an excitation wavelength of 490 nm and an emission wavelength of 515 nm.
Cell Proliferation Rate Assay and Statistical Analysis.
The rate of cell proliferation was calculated by seeding 10,000 cells in six-well plates and after incubation for 4 days counting them under light microscope using the trypan blue assay or a Z series Coulter Counter (Beckman Coulter). The data are expressed as mean ± SEM. Statistical evaluation of the results was performed by the Student's t test (two-tailed). P values shown are against the control group.
Supplementary Material
Acknowledgments.
This study was supported by the Medical Research Service of the Veterans Affairs Department, the South Florida Veterans Affairs Foundation for Research and Education, and the University of Miami Miller School of Medicine Departments of Pathology and Medicine, Division of Hematology/Oncology (all to A.V.S.). N.B. is a recipient of a fellowship from the Alexander Onassis Public Benefit Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811209106/DCSupplemental.
References
- 1.Schally, Varga JL. Antagonistic analogs of growth hormone-releasing hormone: New potential antitumor agents. Trends Endocrinol Metab. 1999;10:383–391. doi: 10.1016/s1043-2760(99)00209-x. [DOI] [PubMed] [Google Scholar]
- 2.Schally AV. New approaches to the therapy of various tumors based on peptide analogues. Horm Metab Res. 2008;40:315–322. doi: 10.1055/s-2008-1073142. [DOI] [PubMed] [Google Scholar]
- 3.Schally AV, Varga JL, Engel JB. Antagonists of growth-hormone-releasing hormone: An emerging new therapy for cancer. Nat Clin Pract Endocrinol Metab. 2008;4:33–43. doi: 10.1038/ncpendmet0677. [DOI] [PubMed] [Google Scholar]
- 4.Barabutis N, Schally AV. Knocking down gene expression for growth hormone-releasing hormone inhibits proliferation of human cancer cell lines. Br J Cancer. 2008;98:1790–1796. doi: 10.1038/sj.bjc.6604386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havt A, et al. The expression of the pituitary growth hormone-releasing hormone receptor and its splice variants in normal and neoplastic human tissues. Proc Natl Acad Sci USA. 2005;102:17424–17429. doi: 10.1073/pnas.0506844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiaris H, Schally AV, Varga JL, Groot K, Armatis P. Growth hormone-releasing hormone: An autocrine growth factor for small cell lung carcinoma. Proc Natl Acad Sci USA. 1999;96:14894–14898. doi: 10.1073/pnas.96.26.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiaris H, Schally AV, Kalofoutis A. Extrapituitary effects of the growth hormone-releasing hormone. Vitam Horm. 2005;70:1–24. doi: 10.1016/S0083-6729(05)70001-7. [DOI] [PubMed] [Google Scholar]
- 8.Rekasi Z, Czompoly T, Schally AV, Halmos G. Isolation and sequencing of cDNAs for splice variants of growth hormone-releasing hormone receptors from human cancers. Proc Natl Acad Sci USA. 2000;97:10561–10566. doi: 10.1073/pnas.180313297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barabutis N, et al. Stimulation of proliferation of MCF-7 breast cancer cells by a transfected splice variant of growth hormone-releasing hormone receptor. Proc Natl Acad Sci USA. 2007;104:5575–5579. doi: 10.1073/pnas.0700407104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiaris H, Koutsilieris M, Kalofoutis A, Schally AV. Growth hormone-releasing hormone and extra-pituitary tumorigenesis: Therapeutic and diagnostic applications of growth hormone-releasing hormone antagonists. Expert Opin Investig Drugs. 2003;12:1385–1394. doi: 10.1517/13543784.12.8.1385. [DOI] [PubMed] [Google Scholar]
- 11.Kiaris H. GHRH analogs and cancer. Comb Chem High Throughput Screen. 2006;9:161. doi: 10.2174/138620706776055476. [DOI] [PubMed] [Google Scholar]
- 12.Siejka A, et al. GH-RH antagonist (MZ-4–71) inhibits VEGF secretion and proliferation of murine endothelial cells. Life Sci. 2003;72:2473–2479. doi: 10.1016/s0024-3205(03)00164-4. [DOI] [PubMed] [Google Scholar]
- 13.Jarrett JT. Biochemistry: Radicals by reduction. Nature. 2008;452:163–164. doi: 10.1038/452163a. [DOI] [PubMed] [Google Scholar]
- 14.Rigas B, Sun Y. Induction of oxidative stress as a mechanism of action of chemopreventive agents against cancer. Br J Cancer. 2008;98:1157–1160. doi: 10.1038/sj.bjc.6604225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Pham CG, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004;119:529–542. doi: 10.1016/j.cell.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 17.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–425. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schumacker PT. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Panayiotidis M. Reactive oxygen species (ROS) in multistage carcinogenesis. Cancer Lett. 2008;266:3–5. doi: 10.1016/j.canlet.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa K, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai T, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 24.Trachootham D, et al. Selective killing of oncogenically transformed cells through a ROS-mediated mechanism by beta-phenylethyl isothiocyanate. Cancer Cell. 2006;10:241–252. doi: 10.1016/j.ccr.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 26.Kastan MB. Wild-type p53: Tumors can't stand it. Cell. 2007;128:837–840. doi: 10.1016/j.cell.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 28.Juarez JC, et al. Superoxide dismutase 1 (SOD1) is essential for H2O2-mediated oxidation and inactivation of phosphatases in growth factor signaling. Proc Natl Acad Sci USA. 2008;105:7147–7152. doi: 10.1073/pnas.0709451105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long DJ, 2nd, et al. Disruption of the NAD(P)H:quinone oxidoreductase 1 (NQO1) gene in mice causes myelogenous hyperplasia. Cancer Res. 2002;62:3030–3036. [PubMed] [Google Scholar]
- 30.Sutton A, et al. Genetic polymorphisms in antioxidant enzymes modulate hepatic iron accumulation and hepatocellular carcinoma development in patients with alcohol-induced cirrhosis. Cancer Res. 2006;66:2844–2852. doi: 10.1158/0008-5472.CAN-05-2566. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Eleni C, Spyrou G, Brune B. The mitochondrial thioredoxin system regulates nitric oxide-induced HIF-1alpha protein. Free Radic Biol Med. 2008;44:91–98. doi: 10.1016/j.freeradbiomed.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 33.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 34.Souza JM, Peluffo G, Radi R. Protein tyrosine nitration—Functional alteration or just a biomarker? Free Radic Biol Med. 2008;45:357–366. doi: 10.1016/j.freeradbiomed.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Heijnen HF, et al. Subcellular localization of tyrosine-nitrated proteins is dictated by reactive oxygen species generating enzymes and by proximity to nitric oxide synthase. Free Radic Biol Med. 2006;40:1903–1913. doi: 10.1016/j.freeradbiomed.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 37.Danielson SR, Andersen JK. Oxidative and nitrative protein modifications in Parkinson's disease. Free Radic Biol Med. 2008;44:1787–1794. doi: 10.1016/j.freeradbiomed.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaylinn BD, et al. Molecular cloning and expression of a human anterior pituitary receptor for growth hormone-releasing hormone. Mol Endocrinol. 1993;7:77–84. doi: 10.1210/mend.7.1.7680413. [DOI] [PubMed] [Google Scholar]
- 39.Groot K, et al. Development of a radioimmunoassay for some agonists of growth hormone-releasing hormone. Int J Pept Protein Res. 1993;41:162–168. doi: 10.1111/j.1399-3011.1993.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 40.Gurova KV, et al. Expression of prostate specific antigen (PSA) is negatively regulated by p53. Oncogene. 2002;21:153–157. doi: 10.1038/sj.onc.1205001. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Li M, Wang H, Agrawal S, Zhang R. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc Natl Acad Sci USA. 2003;100:11636–11641. doi: 10.1073/pnas.1934692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu B, Chen Y, St Clair DK. ROS and p53: A versatile partnership. Free Radic Biol Med. 2008;44:1529–1535. doi: 10.1016/j.freeradbiomed.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bensaad K, Vousden KH. p53: New roles in metabolism. Trends Cell Biol. 2007;17:286–291. doi: 10.1016/j.tcb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Sablina AA, et al. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bensaad K, Vousden KH. Savior and slayer: The two faces of p53. Nat Med. 2005;11:1278–1279. doi: 10.1038/nm1205-1278. [DOI] [PubMed] [Google Scholar]
- 46.Siriwardana G, Bradford A, Coy D, Zeitler P. Autocrine/paracrine regulation of breast cancer cell proliferation by growth hormone releasing hormone via Ras, Raf, and mitogen-activated protein kinase. Mol Endocrinol. 2006;20:2010–2019. doi: 10.1210/me.2005-0001. [DOI] [PubMed] [Google Scholar]
- 47.Pombo CM, Zalvide J, Gaylinn BD, Dieguez C. Growth hormone-releasing hormone stimulates mitogen-activated protein kinase. Endocrinology. 2000;141:2113–2119. doi: 10.1210/endo.141.6.7513. [DOI] [PubMed] [Google Scholar]
- 48.Steinmetz R, Zeng P, King DW, Walvoord E, Pescovitz OH. Peptides derived from pro-growth hormone-releasing hormone activate p38 mitogen-activated protein kinase in GH3 pituitary cells. Endocrine. 2001;15:119–127. doi: 10.1385/ENDO:15:1:119. [DOI] [PubMed] [Google Scholar]
- 49.Zeitler P, Siriwardana G. Stimulation of mitogen-activated protein kinase pathway in rat somatotrophs by growth hormone-releasing hormone. Endocrine. 2000;12:257–264. doi: 10.1385/ENDO:12:3:257. [DOI] [PubMed] [Google Scholar]
- 50.Dolado I, et al. p38alpha MAP kinase as a sensor of reactive oxygen species in tumorigenesis. Cancer Cell. 2007;11:191–205. doi: 10.1016/j.ccr.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 51.Morris GF, Bischoff JR, Mathews MB. Transcriptional activation of the human proliferating-cell nuclear antigen promoter by p53. Proc Natl Acad Sci USA. 1996;93:895–899. doi: 10.1073/pnas.93.2.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banks, et al. L2DTL/CDT2 and PCNA interact with p53 and regulate p53 polyubiquitination and protein stability through MDM2 and CUL4A/DDB1 complexes. Cell Cycle. 2006;5:1719–1729. doi: 10.4161/cc.5.15.3150. [DOI] [PubMed] [Google Scholar]
- 53.D'Autreaux B, Toledano MB. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 54.Zeitler P, Siriwardana G. Antagonism of endogenous growth hormone-releasing hormone (GHRH) leads to reduced proliferation and apoptosis in MDA231 breast cancer cells. Endocrine. 2002;18:85–90. doi: 10.1385/ENDO:18:1:85. [DOI] [PubMed] [Google Scholar]
- 55.Rekasi Z, et al. Antagonist of growth hormone-releasing hormone induces apoptosis in LNCaP human prostate cancer cells through a Ca2+-dependent pathway. Proc Natl Acad Sci USA. 2005;102:3435–3440. doi: 10.1073/pnas.0410006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kurada P, White K. Ras promotes cell survival in Drosophila by downregulating hid expression. Cell. 1998;95:319–329. doi: 10.1016/s0092-8674(00)81764-x. [DOI] [PubMed] [Google Scholar]
- 57.Le Gall M, et al. The p42/p44 MAP kinase pathway prevents apoptosis induced by anchorage and serum removal. Mol Biol Cell. 2000;11:1103–1112. doi: 10.1091/mbc.11.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 59.Aggarwal BB. Nuclear factor-kappaB: The enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed KM, Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med. 2008;44:1–13. doi: 10.1016/j.freeradbiomed.2007.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan C, Li Q, Ross D, Engelhardt JF. Tyrosine phosphorylation of I kappa B alpha activates NF kappa B through a redox-regulated and c-Src-dependent mechanism following hypoxia/reoxygenation. J Biol Chem. 2003;278:2072–2080. doi: 10.1074/jbc.M206718200. [DOI] [PubMed] [Google Scholar]
- 62.Bar-Shai M, Carmeli E, Ljubuncic P, Reznick AZ. Exercise and immobilization in aging animals: The involvement of oxidative stress and NF-kappaB activation. Free Radic Biol Med. 2008;44:202–214. doi: 10.1016/j.freeradbiomed.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Shao J, et al. Overexpression of the wild-type p53 gene inhibits NF-kappaB activity and synergizes with aspirin to induce apoptosis in human colon cancer cells. Oncogene. 2000;19:726–736. doi: 10.1038/sj.onc.1203383. [DOI] [PubMed] [Google Scholar]
- 64.Huang, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 65.Blander G, de Oliveira RM, Conboy CM, Haigis M, Guarente L. Superoxide dismutase 1 knock-down induces senescence in human fibroblasts. J Biol Chem. 2003;278:38966–38969. doi: 10.1074/jbc.M307146200. [DOI] [PubMed] [Google Scholar]
- 66.Dewhirst MW, Cao Y, Moeller B. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat Rev Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gouaze V, et al. Glutathione peroxidase-1 protects from CD95-induced apoptosis. J Biol Chem. 2002;277:42867–42874. doi: 10.1074/jbc.M203067200. [DOI] [PubMed] [Google Scholar]
- 68.Cao C, Leng Y, Huang W, Liu X, Kufe D. Glutathione peroxidase 1 is regulated by the c-Abl and Arg tyrosine kinases. J Biol Chem. 2003;278:39609–39614. doi: 10.1074/jbc.M305770200. [DOI] [PubMed] [Google Scholar]
- 69.Faraonio R, et al. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J Biol Chem. 2006;281:39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- 70.Im JY, Kim D, Paik SG, Han PL. Cyclooxygenase-2-dependent neuronal death proceeds via superoxide anion generation. Free Radic Biol Med. 2006;41:960–972. doi: 10.1016/j.freeradbiomed.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Pratt PF, Bokemeyer D, Foschi M, Sorokin A, Dunn MJ. Alterations in subcellular localization of p38 MAPK potentiates endothelin-stimulated COX-2 expression in glomerular mesangial cells. J Biol Chem. 2003;278:51928–51936. doi: 10.1074/jbc.M309256200. [DOI] [PubMed] [Google Scholar]
- 72.Rodriguez C, et al. COX2 expression and Erk1/Erk2 activity mediate Cot-induced cell migration. Cell Signal. 2008;20:1625–1631. doi: 10.1016/j.cellsig.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 73.Griendling KK, Sorescu D, Lassegue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 74.Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17:287–296. doi: 10.1002/biof.5520170128. [DOI] [PubMed] [Google Scholar]
- 75.Touyz RM, Yao G, Viel E, Amiri F, Schiffrin EL. Angiotensin II and endothelin-1 regulate MAP kinases through different redox-dependent mechanisms in human vascular smooth muscle cells. J Hypertens. 2004;22:1141–1149. doi: 10.1097/00004872-200406000-00015. [DOI] [PubMed] [Google Scholar]
- 76.Suzaki Y, et al. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: Potential role in cell survival following oxidative insults. J Biol Chem. 2002;277:9614–9621. doi: 10.1074/jbc.M111790200. [DOI] [PubMed] [Google Scholar]
- 77.Mehdi MZ, Pandey NR, Pandey SK, Srivastava AK. H2O2-induced phosphorylation of ERK1/2 and PKB requires tyrosine kinase activity of insulin receptor and c-Src. Antioxid Redox Signal. 2005;7:1014–1020. doi: 10.1089/ars.2005.7.1014. [DOI] [PubMed] [Google Scholar]
- 78.Marques CA, et al. Neurotoxic mechanisms caused by the Alzheimer's disease-linked Swedish amyloid precursor protein mutation: Oxidative stress, caspases, and the JNK pathway. J Biol Chem. 2003;278:28294–28302. doi: 10.1074/jbc.M212265200. [DOI] [PubMed] [Google Scholar]
- 79.Furst R, et al. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96:43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- 80.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 81.Avogaro A, de Kreutzenberg SV, Fadini GP. Oxidative stress and vascular disease in diabetes: Is the dichotomization of insulin signaling still valid? Free Radic Biol Med. 2008;44:1209–1215. doi: 10.1016/j.freeradbiomed.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 82.Asaumi H, Watanabe S, Taguchi M, Tashiro M, Otsuki M. Externally applied pressure activates pancreatic stellate cells through the generation of intracellular reactive oxygen species. Am J Physiol Gastrointest Liver Physiol. 2007;293:G972–G978. doi: 10.1152/ajpgi.00018.2007. [DOI] [PubMed] [Google Scholar]
- 83.Bubici C, Papa S, Dean K, Franzoso G. Mutual cross-talk between reactive oxygen species and nuclear factor-kappa B: Molecular basis and biological significance. Oncogene. 2006;25:6731–6748. doi: 10.1038/sj.onc.1209936. [DOI] [PubMed] [Google Scholar]
- 84.Finkel T. Radical medicine: Treating ageing to cure disease. Nat Rev Mol Cell Biol. 2005;6:971–976. doi: 10.1038/nrm1763. [DOI] [PubMed] [Google Scholar]
- 85.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 86.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 87.Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 88.Przedborski S, Ischiropoulos H. Reactive oxygen and nitrogen species: Weapons of neuronal destruction in models of Parkinson's disease. Antioxid Redox Signal. 2005;7:685–693. doi: 10.1089/ars.2005.7.685. [DOI] [PubMed] [Google Scholar]
- 89.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.