Abstract
We dissect the transcriptional regulatory relationships coordinating the dynamic expression patterns of two signaling genes, wnt8 and delta, which are central to specification of the sea urchin embryo endomesoderm. cis-Regulatory analysis shows that transcription of the gene encoding the Notch ligand Delta is activated by the widely expressed Runx transcription factor, but spatially restricted by HesC-mediated repression through a site in the delta 5′UTR. Spatial transcription of the hesC gene, however, is controlled by Blimp1 repression. Blimp1 thus represses the repressor of delta, thereby permitting its transcription. The blimp1 gene is itself linked into a feedback circuit that includes the wnt8 signaling ligand gene, and we showed earlier that this circuit generates an expanding torus of blimp1 and wnt8 expression. The finding that delta expression is also controlled at the cis-regulatory level by the blimp1-wnt8 torus-generating subcircuit now explains the progression of Notch signaling from the mesoderm to the endoderm of the developing embryo. Thus the specific cis-regulatory linkages of the gene regulatory network encode the coordinated spatial expression of Wnt and Notch signaling as they sweep outward across the vegetal plate of the embryo.
Keywords: Blimp1, Delta, HesC, Notch, Wnt
The gene regulatory network (GRN) for sea urchin development represents the genomic code that specifies the regulatory interactions underlying specification of the embryonic territories, their subsequent subdivision, and then differentiation (1). Sequencing of the Strongylocentrotus purpuratus genome permitted the systematic identification by homology of all transcription factor genes encoded in this genome, and their developmental characterization (2–7). By incorporating all (or nearly all) of the relevant regulatory players, some portions of the GRN have achieved sufficient maturity to allow an overall causal understanding of development. This was shown recently for the network underlying specification and differentiation of the skeletogenic micromere lineages or skeletogenic mesoderm (SM) of this embryo (8). In this article we consider another major aspect of endomesodermal specification, a concentrically expanding progression of Notch and Wnt signaling that is initiated in the SM but then sweeps outward across the vegetal domains of the embryo (see fate map in Fig. 1). This signaling is required first for specification of the nonskeletogenic mesoderm (NSM), and it then participates in endoderm specification. We ask whether the static genomic regulatory code can also provide an explanation for this dynamic progression of intercellular signal transmission.
Fig. 1.
Dynamic expression of wnt8 and delta signaling genes. Schematic representations of delta (red shading) and wnt8 (blue) expression patterns are shown (also shown are similar patterns seen for several transcription factors). As illustrated in the bottom panel, the innermost cells (excluding small micromeres, white) are fated to become skeletogenic mesoderm (SM, brown); prospective nonskeletogenic mesoderm cells (NSM, green; endoderm, brown). At SM ingression, expression of delta moves from the SM to the NSM as wnt8 transitions out of the NSM to the endoderm.
The Notch receptor is initially expressed maternally and is globally distributed, although by early blastula stage it is regionally localized (9, 10). Before this, the location of intracellular Notch signaling is determined entirely by spatial control of transcriptional expression of the delta gene, encoding a Notch ligand. The delta gene is initially activated as an output of the SM GRN (8). The early SM Delta signal is critical for specification of the adjacent ring of cells as NSM beginning at the early blastula stage. In the NSM, reception of the Delta signal causes the essential transcriptional regulatory gene gcm to receive a direct Notch-mediated Su(H) input (9, 11–13), initiating NSM specification. When the SM cells ingress into the blastocoel at late (mesenchyme) blastula stage, the delta gene is turned off in these cells, but at this time it is activated in the NSM. In consequence, the surrounding ring of immediately adjacent cells, which become gut endoderm, now receive Notch signaling, and this is required for endoderm specification (14). Expression of wnt8 is also essential for specification of both NSM and endoderm. Thus, blocking Wnt8 translation with morpholino antisense oligonucleotides (MASO), or blocking nuclearization of its downstream effector, β-catenin, by overexpression of an intracellular fragment of Cadherin (15, 6), prevents both NSM and endoderm specification. These treatments interfere with activation of a wide number of endomesdoderm genes dependent on a β-catenin/Tcf input (1, 7).
Recent studies have revealed a dynamic sequence of regulatory gene expressions in the vegetal domains of the embryo that entrains a set of genes in an expanding-torus pattern of expression (Fig. 1). We showed that the regulatory circuitry underlying this phenomenon is a double-feedback loop linking the wnt8 and the blimp1 regulatory genes in a causal embrace (18). cis-Regulatory studies show that the wnt8 gene requires inputs from both β-catenin/Tcf (i.e., from the same signal transduction system that it activates) and from Blimp1 factor for expression, and conversely, the blimp1 gene requires inputs from the same Wnt8/Tcf signaling system as well as from another then ubiquitous factor, Otx (16, 18). However, the blimp1 gene also contains autorepression sites which, after some hours when the Blimp1 factor attains a high concentration, shut down its own expression, and therefore that of wnt8 as well (18, 19). Meanwhile, however, Wnt8 has diffused to the next ring of cells and activated the feedback circuit there. The consequence is that following their initial expression in the centrally located SM territory, expression of these genes disappears from the SM and is activated in the NSM; but again after some hours, it is shut off in the NSM and activated in the next outer rings of prospective endoderm cells, producing the expanding-torus pattern of gene expression seen in Fig. 1 (18). Additional cis-regulatory experiments showed that the hox11/13b and evenskipped genes are also directly controlled by this subcircuit (20). We noticed in the course of these studies that the dynamically changing locations of wnt8 expression and of delta expression remain perfectly complementary as the patterns expand outward: at the eighth cleavage, delta is expressed in the SM lineage, and wnt8 is expressed in the neighboring NSM; later, during skeletogenic cell ingression, wnt8 turns off in the NSM cells and is activated in the adjacent endoderm, while delta becomes expressed in the NSM. As a result, Wnt8 and Delta signaling domains remain aligned side by side. Here we show by direct cis-regulatory manipulation that the GRN subcircuit controlling the expanding-torus pattern of gene expression also explains the dynamic alignment of Delta and Wnt8 expression and, more generally, the progression of Delta-Notch signaling first in mesoderm and then in endoderm specification.
Results
Exclusive Expression of Delta and Its Repressor, HesC.
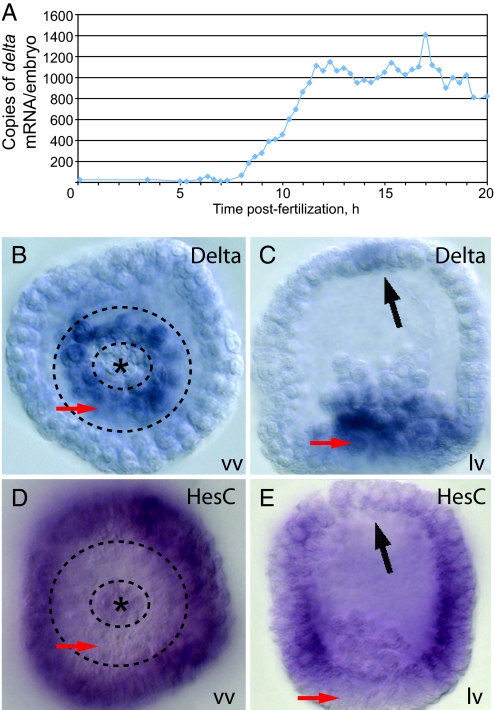
Expression of the delta gene begins between 8 h and 8 h 40 min postfertilization (i.e., late fifth cleavage). A high-resolution quantitative PCR (QPCR) time course is shown in Fig. 2A. Whole-mount in situ hybridization (WMISH) reveals that delta is expressed exclusively in the SM lineage until swimming blastula stage (19–20 hpf) (21). The delta gene then begins to be expressed in the adjacent NSM territory (Figs. 2B and 2C). Although not the focus of this study, delta is activated as well in the apical plate at early mesenchyme blastula stage (Fig. 2C). Early delta expression thus occurs in three territories: the SM from fifth cleavage to ingression, the NSM from just before skeletogenic cell ingression, and at this time also the apical plate.
Fig. 2.
Expression of delta and hesC. (A) High-resolution time course of delta mRNA accumulation determined by QPCR. Embryos were harvested at 20 min intervals. Expression of the delta gene in the SM begins between 8 h and 8 h 40 min PF (i.e., late fifth cleavage). (B and C) Detection of delta transcripts in 21 h mesenchyme blastulae by WMISH; vv, vegetal view; lv, lateral view. delta transcripts are present in NSM (red arrows) and apical plate (black arrow) but not in small micromeres (asterisk) or ingressed SM cells. (D and E) WMISH against hesC in embryos of same early mesenchyme blastula stage. Transcripts of this gene are present everywhere except in NSM, apical plate, and SM; symbols as in (B) and (C).
In its initial phase of expression in the skeletogenic lineage, delta is activated by ubiquitous factors and repressed by HesC (21). S. purpuratus HesC (5) belongs to a well-known class of transcriptional repressors (22–24) encoding group E bHLH and Orange domains and a C-terminal WRPW motif thought to recruit the Groucho/TLE corepressor (22, 25). The hesC gene is expressed zygotically, initially throughout the embryo, except for the SM lineage (21). There, its transcription is repressed by the product of the pmar1 gene (21). pmar1 is transcribed exclusively in the SM beginning at late fourth cleavage, until around the time of hatching (26). The initial phase of delta expression in the SM therefore follows that of pmar1, and it is controlled by a double-negative gate in which HesC repression of delta is relieved by Pmar1 repression of hesC (8, 21). Importantly for what follows, expression of the hesC gene turns off in the NSM and apical plate before delta expression in both of these territories (Figs. 2D and 2E), just as earlier it is turned off in the SM (i.e., by Pmar1) before expression of delta there. The expression domains of hesC and delta are thus specifically exclusive in all three territories.
Delta cis-Regulation.
Previous work identified a delta cis-regulatory module (CRM), the R11 CRM, located ∼16 kb downstream of the last delta exon, which when coupled to a heterologous basal promoter is sufficient to drive reporter expression in the SM (21). In response to pmar1 overexpression, an R11 reporter expresses throughout the embryo, as explicitly required by the double-negative gate. Three putative Ets1 binding sites were identified in the R11 CRM within a 75 bp stretch of sequence; when these Ets1 target sites were disrupted, sharply decreased reporter activity was observed (27). The R11 CRM was additionally found to respond to treatment with hesC MASO by producing global ectopic expression (21). However, the mode of HesC repression remained undetermined, as functional HesC binding sites were not identified in the R11 CRM.
We constructed a recombinant delta BAC clone that contains the GFP gene knocked into the delta coding sequence, the remainder of the gene, plus 66 kb of upstream and 60 kb of downstream noncoding sequence. This reporter construct faithfully expresses in the same domains in which endogenous delta transcripts are found (e.g., Fig. 3B). Using a series of deletion constructs, we identified a second functional delta CRM. This consisted of noncoding sequence from −90 to +484 into the 5′UTR. Its sequence is shown in supporting information (SI) Fig. S1A. This proximal CRM, in contrast to the R11 CRM, drives expression in the SM, the NSM, and the apical plate territories, thereby reproducing the temporal and spatial pattern of pregastrular delta expression in its entirety (Table 1 and Figs. 3A and 3C). In contrast to R11, the activity of the proximal CRM is not decreased by ets1 MASO perturbation (data not shown). However, like the R11 CRM, it does respond to hesC MASO, displaying ectopic expression throughout the embryo (Fig. 3D).
Fig. 3.
cis-Regulatory analysis of the delta gene. (A) Expression of reporter construct consisting of delta proximal module driving GFP in early mesenchyme blastula stage NSM. (B) Same pattern of expression generated by Delta:GFP knockin BAC. (C) Expression of proximal module reporter construct in apical plate of 24 h mesenchyme blastula embryo. (D) Same construct, expressing ectopically in presence of 400 μM morpholino antisense oligonucleotide against hesC. (E) Ectopic expression of same construct but with HesC target site mutated, in normal embryos. Quantitative data for experiments illustrated in (A–C) and (E) are in Table 1. (F) Summary network subcircuit showing delta and hesC genes: delta receives widespread activation input from the Runx transcription factor and dominant repression by HesC; hesC is repressed in the NSM (NSM R of HesC; see text).
Table 1.
Activity of Delta reporter constructs
| Reporter | No. injected | Correct GFP+/total GFP+* |
|---|---|---|
| Delta BAC | 361 | 104/113 |
| 5 kb reporter | 70 | 25/28 |
| Proximal module only | 131 | 37/50 |
| w/Hes site disruption | 94 | 2/78 |
| w/Runx site disruption | 50 | 4/7 |
*Correct reporter activity scored as fluorescence in one or more of the domains of endogenous delta expression and in no ectopic territory at 18 h and 27 h postfertilization.
A putative Runx binding site is present at +365 in the delta proximal CRM—namely, 5′-TGTGGGA-3′ (28). Mutation of the 5′-TGGGA-3′ of this Runx site caused a decrease in normalized reporter output to 12% ± 9% of control values, demonstrating potent activation mediated by Runx (Table 1). Sea urchin Runx is broadly expressed before gastrulation (29), including in the NSM, and these results implicate Runx as the major activator of delta in the NSM. However, this in turn requires repression outside of the NSM to explain why delta is expressed only there at mesenchyme blastula stage, rather than everywhere Runx is present. As we see in Figs. 2D and 2E, hesC is a prime candidate to be the repressor executing this function, because of its perfectly complementary expression. A possible HesC binding site, 5′-CACGCGTG-3′, is located at +359 within the delta proximal CRM (Fig. S1A). This site is an 8 bp palindrome, including an inverted 6 bp repeat of the class C, E-box binding site 5′-CACGCG-3′ (30, 31). Disruption of the proximal 5′-CACGCG-3′ resulted in ubiquitous reporter expression (Fig. 3E and Table 1), whereas mutation of a class B E-box, or of N-box sites within the module (Fig. S1A), had no effects.
These results indicate that the expression of delta in the NSM results from activation by Runx and repression by HesC through sites in the proximal CRM. Fig. 3F summarizes these relationships: Runx, broadly expressed at stages considered, activates delta transcription, while repression by HesC confines delta expression to the domain where HesC is not present, here, the NSM at mesenchyme blastula stage. Earlier, Pmar1 represses hesC in the SM, but the question now arises: What is the repressor of hesC in the NSM?
HesC cis-Regulation and the Role of Blimp1.
In the expanding-torus gene expression subcircuit reviewed briefly herein, the autorepression of blimp1 occurs in the NSM at about the same time as hesC expression is extinguished there. Thus Blimp1 could play the role of repressor of hesC at the same time as it represses itself. A candidate Blimp1 binding site is indeed present in the first intron of the hesC gene (Fig. S1B), the sequence of which, 5′-TACTTTCAACT-3′, conforms to the consensus target site for Blimp1, 5′(A/C/T)(A/G)(G/T)NGAAAG(G/T)(A/G/T)-3′ (18, 32, 33). This sequence is expected less than once per 8 kb of random sequence, and includes the invariant core CTTTC. Mutation of this site in a hesC reporter construct caused expression to remain strong in the NSM territory, in contrast to controls where, as we have seen, HesC expression is extinguished (Table 2 and Fig. 4 A and B and Fig. S4). Parallel experiments showed that hesC reporter activity also continues in the NSM of embryos treated with blimp1 MASO (Figs. S2A, S2C, and S2D); these same embryos contained ≈3-fold lower levels of endogenous delta mRNA at 21 h by QPCR measurement, evidently reflecting the continued, abnormal presence of the HesC repressor in the NSM. Overexpression of other candidate genes (foxA, gataE, hox11/13b, gcm, soxC, z13) produced no effect on hesC expression (Table S2), whereas blimp1 mRNA overexpression nearly abolished expression of the hesC BAC-GFP reporter (Figs. S2B and S2E–J). These experiments also revealed that Blimp1 is responsible for keeping hesC off in the skeletogenic mesoderm once pmar1 transcription ceases, because disruption of the Blimp1 site, or introduction of blimp1 MASO, resulted in return of hesC reporter activity to the skeletogenic mesenchyme after ingression, a result never observed with control constructs (Figs. 4 A and B, Table 2, and Fig. S2 C and D and Fig. S4). Blimp1-mediated repression in other organisms entails heterochromatin induction and long-term gene silencing, suggesting a mechanism for the persistent repression we observe (34–36). Note also that members of the Blimp transcription factor family are well known to act as activators, as we have shown that Blimp1 does in the wnt8 cis-regulatory system, and also as repressors, as in its own cis-regulatory system (18). Blimp1, in addition to shutting down its own transcription, is responsible for doing the same to hesC. Because HesC is the dominant repressor of delta, this demonstrates a causal chain linking Blimp1 with delta: Blimp1 represses hesC and HesC represses delta.
Table 2.
Activity of HesC reporter constructs
| Reporter | No. injected | WT pattern*/total GFP+ | Zone 4 | Strong zone 3 |
|---|---|---|---|---|
| HesC BAC | 223 | 70/89 | 0/89 | 10/89 |
| 10 kb reporter | 160 | 45/56 | 0/56 | 8/56 |
| w/Δ Blimp site | 127 | 0/36 | 31/36 | 24/36 |
| w/Δ Su(H) site | 119 | 0/32 | N/D | N/D |
*Wild-type reporter activity scored as pattern of fluorescence seen in BAC and 10 kb constructs (Fig. 5A) at 27 h postfertilization—namely, weak activity in zone 1, strong activity zone 2, intermediate activity zone 3 (NSM), and no activity zone 4 (ingressed skeletogenic mesoderm).
Fig. 4.
cis-Regulatory analysis of the hesC gene. (A) Expression pattern of the hesC GFP reporter reveals four domains at mesenchyme blastula stage: (1) low reporter activity in ectoderm outside of the apical plate and part of presumptive endoderm; (2) high activity in the preendoderm cells proximal to the NSM; (3) medium-low activity in NSM; and (4) no expression in the already ingressed SM cells (exposure time: 375 msec). The embryos were oriented identically and superimposed on them was the stenciled pattern shown in red. Quantitative data for embryos displaying each variant of the expression patterns in these experiments are given in Table 2. (B) Effect of disruption of Blimp1 binding site in intron 1 of the hesC locus. Strong expression of hesC reporter now occurs both in NSM (zone 3) and, unexpectedly, in SM (zone 4), demonstrating that Blimp1 is responsible for the persistent extinction of hesC transcription in both mesodermal territories (exposure time: 268 msec). (C) Effect of mutating Su(H) binding site in the hesC reporter. Activity is now lost in the zone 3 NSM, and greatly diminished activity in the endoderm of zone 2 (exposure time: 920 msec). (D) Subcircuit diagram showing Blimp1 repression input into both itself (18) and hesC (this work). (E) Subcircuit showing Delta-Notch signaling input (chevron) from neighboring cell into hesC gene and HesC repressive input back into delta.
Delta-Notch Signaling Input into the hesC Gene.
A wild-type reporter assay for hesC at mesenchyme blastula stage revealed a complex pattern of expression. There are four distinct domains of activity (Fig. 4A): (1) a low level of reporter activity in the ectoderm outside of the apical plate and extending into the presumptive Veg1 endoderm; (2) an extraordinarily high level of activity in the Veg2 tier of endoderm cells (also noted in analyses of hesC expression by WMISH); (3) a low level in the NSM; and (4) no wild-type expression in the (ingressed) SM. The cells receiving Notch signaling at this stage are those in the Veg2 endoderm adjacent to the NSM; the previous phase of Notch signaling was received by the NSM. Because high expression of the HesC reporter first in NSM and then in Veg2 endoderm is coincident with Notch signaling first within the NSM and then the Veg2 endoderm, the hesC gene itself could be a target of activation by Notch signaling. In fact, a consensus Su(H) target site exists at +2 from the start of transcription in the hesC gene (Fig. S1B). Mutation of this site led to sharp decreases in reporter activity in the Veg2 endoderm cells to the basal levels seen in the ectoderm, where no Delta-Notch signaling at this stage occurs (Figs. 4A and 4C). The mutation furthermore abolished activity entirely in NSM cells. The Delta-Notch signal therefore accounts for the unusually high level of fluorescence in the Veg2 endoderm cells receiving the signal at this stage of development. These data further suggest that the wild-type reporter activity seen in NSM cells, which previously received the Delta-Notch signal, reflects residual reporter protein and not continued expression. Thus Fig. 4 shows that the hesC cis-regulatory system responds negatively to Blimp1 repression and positively to Delta-Notch signaling, and that these together account for the dynamic pattern of spatial expression of the potent HesC repressor.
To close the circle, so to speak, we also found that the gene encoding the Notch receptor is itself under cis-regulatory control of the Blimp1 repressor, just as is the hesC gene. Following the gradual disappearance of maternal Notch protein (9), the notch gene is zygotically expressed like hesC in the Veg2 endoderm ring (10), where it is responsible for transducing the NSM Delta signal. Mutation of a cis-regulatory Blimp1 site in the Notch gene (Fig. S1C) causes massive vegetal expansion of expression of a Notch:GFP reporter construct into the NSM domain (Fig. S3). The activator of the notch gene remains unidentified.
In brief summary, the new results presented here show the following cis-regulatory linkages: the delta gene is repressed directly by HesC and activated directly by Runx; the HesC gene is repressed by Blimp1 and activated by Notch signaling; and the notch gene is also subject to direct Blimp1 repression. As we now discuss, these regulatory linkages, combined with those earlier discovered in the expanding-torus GRN subcircuit, suffice to explain the dynamic pregastrular progression of Notch signaling states across the endomesodermal domains of the embryo.
Discussion
The cis-regulatory target sites functionally analyzed in this work are structural features of the genomic sequence, but the regulatory linkages they specify produce a dynamic sequence of spatial signaling interactions. The motivating engine of this dynamic system is the wnt8-blimp1 expanding-torus subcircuit (18). As reviewed previously, this is a feedback subcircuit composed of the wnt8 and blimp1 genes, expression of which progresses concentrically outward from the SM to the NSM and then to the surrounding endoderm. At each stage, after some hours of transcription, the blimp1 gene represses itself, causing loss of wnt8 expression as well (16, 18), and also loss of expression of downstream regulatory genes (20) in what now becomes the silenced center of a torus of gene expression. Meanwhile, Wnt8 diffusion causes expansion of the subcircuit expression torus to the adjacent cells of the next domain. But we now see that the functional significance of this system extends even beyond the sweep of regulatory states across the vegetal plate: it specifically controls the activities of the Notch signaling system as well as maintaining an exact Boolean complementarity between Wnt and Notch signaling as the domains of each shift outward.
The sequence of regulatory transactions is summarized in Fig. 5. The overall subcircuit is shown in Fig. 5A, from results presented here and elsewhere (18, 20). The subcircuit thus portrayed indicates the relevant cis-regulatory linkages encoded in the genomic DNA (the “view from the genome”; ref. 1). The lynchpin of this subcircuit is the blimp1 gene and its autorepressive cis-regulatory module. The present study shows that blimp1 executes two crucial transitive repressions in addition to autorepression, the targets of which are the notch and hesC genes. Thus when blimp1 shuts itself down in the center of the torus it does the same to these genes. But HesC in turn controls transcription of the Notch ligand Delta; and Delta presentation, plus the zygotic expression of the Notch receptor, determines the changing locus of Notch signaling. The state of the subcircuit in the eighth cleavage NSM is shown in the “view from the nucleus” (1) of Fig. 5B. Here the torus subcircuit genes, including wnt8, are active in the NSM, as is hesC, driven by Notch signal input in response to the Delta ligand, then being expressed in the inner SM domain. Because HesC is present, delta is inactive. By early mesenchyme blastula stage in the NSM (VFN in Fig. 5C), the state of the subcircuit has changed. Blimp1 autorepression has eliminated blimp1 transcription in the NSM, and therefore wnt8 transcription, and Blimp1-mediated repression has extinguished hesC expression on the same schedule. Thus expression of delta, driven by the widespread activator Runx, is now allowed to occur for the first time in the NSM, but because maternal Notch is no longer available, the NSM cannot itself respond to Delta because the zygotic notch gene is also clamped silent by Blimp1 repression. At the same stage the state of the subcircuit is again different in the adjacent Veg2 endoderm (VFN of Fig. 5D). Here the cells receiving the Delta ligand are enabled to use it for Notch signaling, becaise zygotic notch is actively expressed, as is hesC, which silences delta. An important Notch target in the endoderm is the gatae gene (38). Meanwhile, the blimp1/wnt8 subcircuit is active in the endoderm, where their various targets are important for specification of the preendodermal state.
Fig. 5.
The gene regulatory network linkages that coordinate Delta-Notch signaling with the wnt8-blimp1 expanding-torus subcircuit. (A) Fate map and summary network subcircuit showing all linkages. Expression of delta is dominantly repressed by HesC and activated by the factor Runx, whereas hesC is activated broadly, amplified by receiving a cis-regulatory Delta-Notch signal input and repressed by Blimp1. For clarity the Wnt signaling pathway is simplified to show only the end result, nuclearized β-catenin interacting with the transcription factor Tcf (nb-Tcf); NICD, Notch intracellular domain; chevron, intercellular input. Fate map at left shows the future skeletal mesoderm (SM), purple; NSM, green; and endoderm, brown. (B–D) Depiction of linkages that are active in given stages and domains (i.e., views from the nuclei, VFNs). Note that NSM cells migrate toward vegetal pole as a consequence of skeletogenic cell ingression, and thus cells marked green in (B) and (C) are of the same lineage. Components shown in gray are inactive, and in color are active. (B) VFN for eighth cleavage NSM precursors. The blimp1/wnt8 subcircuit is active, whereas delta is not transcribed due to the presence of the dominant-acting HesC repressor. (C) VFN for NSM at early mesenchyme blastula. Expression of delta expression begins as hesC transcription ceases due to repression by Blimp1. Also due to Blimp1 repression, the blimp1/wnt8 subcircuit has been extinguished. The onset of delta expression in the NSMs is thereby synchronized with the disappearance of wnt8 from those same cells in the center of the moving torus. (D) VFN for endoderm at early mesenchyme blastula. The blimp1/wnt8 subcircuit is now active as a result of previous Wnt8 diffusion from the NSM (18). Delta-Notch signaling occurs in response to the NSM Delta ligand, further enhanced by transcription of the Notch receptor, which in this domain is not subject to Blimp1 repression; however, delta expression is reciprocally forbidden by Notch-driven HesC expression.
The structure of the subcircuit in Fig. 5A, particularly the causal link between blimp1 and hesC genes, thus ordains the two signaling regimes: as Wnt8 leaves the NSM so does HesC, thereby permitting delta expression in the wake of the expanding torus. The essential developmental result is that Delta and Wnt8 domains remain exclusive and adjacent in the NSM and endoderm (Figs. 5C and 5D), respectively, just as they were during the previous stage when Delta was expressed in the SM and Wnt8 in the NSM (Fig. 5B). Although biochemical constants of protein/mRNA synthesis and turnover, and protein-DNA association and transcriptional response must determine the actual kinetics of these events (39), the basis for the coordination of the signaling systems rests solely in the cis-regulatory logic. The genomic sequences mediating Blimp1 autorepression, hesC, and notch repression; the AND logic dependence of wnt8 on Blimp1; and dominant HesC repression of delta permit only this sequential outcome. A general feature of this subcircuit is the extremely important role in its logic played by repression: Blimp1 represses the hesC gene, HesC in turn represses the delta gene, and Blimp1 represses the blimp1 gene as well as activating the wnt8 gene.
In summary, we here show experimentally a gene regulatory network subcircuit that (1) causes Delta-Notch signaling first in the NSM and then in the endoderm, (2) prevents delta and notch transcription in the same cells, and (3) ensures exclusive adjacent domains of Wnt8 and Delta signaling. The sea urchin endomesodermal GRN displays many functions mediated by these signal systems in both NSM and endoderm, and now we know how they come to be alternatively and successively expressed.
Materials and Methods
Microinjection and QPCR Measurement of GFP mRNA in Eggs Expressing GFP Constructs.
PCR products were purified with the Qiagen Qiaquick PCR purification kit and microinjected into fertilized S. purpuratus eggs as described (40). Linearized BAC constructs were desalted by drop dialysis into TE buffer on a 0.025 μm VSWP filter (Millipore). Approximately 1500 molecules of the desired reporter construct were injected, along with a 6-fold molar excess of HindIII-digested carrier sea urchin DNA per egg, in a 4 pl volume of 0.12 M KCl. A similar injection solution was made for BAC reporters but with 400 copies of the BAC per 4 pl and no carrier DNA. Embryos were collected at different stages for observation by fluorescence microscopy for qualitative assessment of spatial activity, or for quantitative analysis of transcript prevalence by real-time PCR (QPCR). For high-density cDNA time-course experiments, gametes were harvested from three females and three males, pooled, and cultured at 14.5°C. Three separate samples were removed at 20 min intervals for independent processing and QPCR analysis. Data points represent the average of the three samples. All experimental and control constructs were tested in multiple batches of eggs. Microinjection and measurement of GFP mRNA by QPCR was performed as described (41).
mRNA for microinjection was prepared using full-length cDNA with either Sp6 or T7 polymerase sequences at the 5′ end. The Sp6 or T7 mMessage mMachine kit (Ambion) were used to generate mRNA in a standard reaction at 37°C for 2 h using 0.5 to 1 μg cDNA template. This reaction was followed by the Ambion Poly(A) Tailing Kit reaction according to manufacturer's protocol. Products were assessed by gel electrophoresis and quantified by spectrophotometry before the tailing reaction. Samples were desalted by use of the Qiagen Micro RNeasy columns and stored at −70°C. ΔΔCt was computed by taking the change in cycle number of an internal standard (ubiquitin) mRNA a target gene mRNA in control condition minus the change in cycle number of ubiquitin mRNA and target gene in experimental condition. A ±1.6 cycle difference was considered significant.
Constructs.
Standard PCR and fusion PCR techniques using the High Fidelity PCR Kit (Roche) were used to build constructs. PCR products for all significant reporter constructs were subsequently cloned using the EPICENTRE Copy Control Cloning System in the case of large inserts (>5 kb) or the pGEM-T Easy Vector System from Promega, and confirmed by sequencing.
Binding-site sequences were mutated by PCR, and the resulting constructs were checked by sequencing. The PCR primers were designed with tailed nonpriming sequences, including the mutant form of the candidate transcription factor binding sites. Mutations were designed by swapping A to C, T to G, and vice versa. Primers used for constructs and site-specific mutations are listed in Table S1.
Supplementary Material
Acknowledgments.
We are grateful to Roger Revilla-i-Domingo for whole-mount in situ hybridizations, Christina Theodoris for high-resolution expression time-course measurements, and Julie Hahn for construction of BAC recombinants. J.S. is a Fellow of the California Institute for Regenerative Medicine (CIRM). Research was supported by National Institute of Child Health and Human Development Grant HD-037105 and National Institute of General Medical Sciences Grants GM-075089 and GM-061005.
Footnotes
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Gene Networks in Animal Development and Evolution,” held February 15–16, 2008, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS web site at http://www.nasonline.org/SACKLER_Gene_Networks.
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806442105/DCSupplemental.
References
- 1.Davidson EH. The Regulatory Genome: Gene Regulatory Networks in Development and Evolution. San Diego: Academic; 2006. [Google Scholar]
- 2.Sodergren E, et al. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Materna SC, Howard-Ashby M, Gray RF, Davidson EH. The C2H2 zinc finger genes of Strongylocentrotus purpuratus and their expression in embryonic development. Dev Biol. 2006;300:108–120. doi: 10.1016/j.ydbio.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Howard-Ashby M, et al. Identification and characterization of homeobox transcription factor genes in Strongylocentrotus purpuratus, and their expression in embryonic development. Dev Biol. 2006;300:74–89. doi: 10.1016/j.ydbio.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Howard-Ashby M, et al. Gene families encoding transcription factors expressed in early development of Strongylocentrotus purpuratus. Dev Biol. 2006;300:90–107. doi: 10.1016/j.ydbio.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Tu Q, Brown CT, Davidson EH, Oliveri P. Sea urchin Forkhead gene family: Phylogeny and embryonic expression. Dev Biol. 2006;300:49–62. doi: 10.1016/j.ydbio.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo F, Fernandez-Serra M, Squarzoni P, Archimandritis A, Arnone MI. Identification and developmental expression of the ets gene family in the sea urchin Strongylocentrotus purpuratus. Dev Biol. 2006;300:35–48. doi: 10.1016/j.ydbio.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Oliveri PO, Tu Q, Davidson EH. Global regulatory logic for specification of an embryonic cell lineage. Proc Natl Acad Sci USA. 2008;105:5955–5962. doi: 10.1073/pnas.0711220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherwood DR, McClay DR. Identification and localization of the sea urchin Notch homologue: Insights into vegetal plate regionalization and Notch receptor regulation. Development. 1997;124:3363–3374. doi: 10.1242/dev.124.17.3363. [DOI] [PubMed] [Google Scholar]
- 10.Walton KD, Croce JC, Glenn TD, Wu SY, McClay DR. Genomics and expression profiles of the Hedgehog and Notch signaling pathways in sea urchin development. Dev Biol. 2006;300:153–164. doi: 10.1016/j.ydbio.2006.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McClay DR, Peterson RE, Range RC, Winter-Vann AM, Ferkowicz MJ. A micromere induction signal is activated by beta-catenin and acts through notch to initiate specification of secondary mesenchyme cells in the sea urchin embryo. Development. 2000;127:5113–5122. doi: 10.1242/dev.127.23.5113. [DOI] [PubMed] [Google Scholar]
- 12.Sweet HC, Gehring M, Ettensohn CA. LvDelta is a mesoderm-inducing signal in the sea urchin and can endow blastomeres with organizer-like properties. Development. 2002;129:1945–1955. doi: 10.1242/dev.129.8.1945. [DOI] [PubMed] [Google Scholar]
- 13.Ransick A, Davidson EH. cis-Regulatory processing of Notch signaling input to the sea urchin glial cells missing gene during mesoderm specification. Dev Biol. 2006;297:587–602. doi: 10.1016/j.ydbio.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 14.Peterson RE, McClay DR. A Fringe-modified Notch signal affects specification of mesoderm and endoderm in the sea urchin embryo. Dev Biol. 2005;282:126–137. doi: 10.1016/j.ydbio.2005.02.033. [DOI] [PubMed] [Google Scholar]
- 15.Wikramanayake AH, et al. Nuclear beta-catenin-dependent Wnt8 signaling in vegetal cells of the early sea urchin embryo regulates gastrulation and differentiation of endoderm and mesodermal cell lineages. Genesis. 2004;39:194–205. doi: 10.1002/gene.20045. [DOI] [PubMed] [Google Scholar]
- 16.Minokawa T, Wikramanayake AH, Davidson EH. cis-Regulatory inputs of the wnt8 gene in the sea urchin endomesoderm network. Dev Biol. 2005;288:545–558. doi: 10.1016/j.ydbio.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 17.Davidson EH, et al. A genomic regulatory network for development. Science. 2002;295:1669–1678. doi: 10.1126/science.1069883. [DOI] [PubMed] [Google Scholar]
- 18.Smith J, Theodoris C, Davidson EH. A gene regulatory network subcircuit drives a dynamic pattern of gene expression. Science. 2007;318:794–797. doi: 10.1126/science.1146524. [DOI] [PubMed] [Google Scholar]
- 19.Livi CB, Davidson EH. Expression and function of blimp1/krox, an alternatively transcribed regulatory gene of the sea urchin endomesoderm network. Dev Biol. 2006;293:513–525. doi: 10.1016/j.ydbio.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Smith J, Kraemer E, Liu H, Theodoris C, Davidson EH. A spatially dynamic cohort of regulatory genes in the endomesodermal gene network of the sea urchin embyro. Dev Biol. 2008;313:863–875. doi: 10.1016/j.ydbio.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revilla-i-Domingo R, Oliveri P, Davidson EH. A missing link in the sea urchin embryo gene regulatory network: hesC and the double-negative specification of micromeres. Proc Natl Acad Sci USA. 2007;104:12383–12388. doi: 10.1073/pnas.0705324104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson SR, Turner DL, Weintraub H, Parkhurst SM. Specificity for the hairy/enhancer of split basic helix-loop-helix (bHLH) proteins maps outside the bHLH domain and suggests two separable modes of transcriptional repression. Mol Cell Biol. 1995;15:6923–6931. doi: 10.1128/mcb.15.12.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alifragis P, Poortinga G, Parkhurst SM, Delidakis C. A network of interacting transcriptional regulators involved in Drosophila neural fate specification revealed by the yeast two-hybrid system. Proc Natl Acad Sci USA. 1997;94:13099–13104. doi: 10.1073/pnas.94.24.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giagtzoglou N, Alifragis P, Koumbanakis KA, Delidakis C. Two modes of recruitment of E(spl) repressors onto target genes. Development. 2003;130:259–270. doi: 10.1242/dev.00206. [DOI] [PubMed] [Google Scholar]
- 25.Fisher AL, Caudy M. Groucho proteins: transcriptional corepressors for specific subsets of DNA-binding transcription factors in vertebrates and invertebrates. Genes Dev. 1998;12:1931–1940. doi: 10.1101/gad.12.13.1931. [DOI] [PubMed] [Google Scholar]
- 26.Oliveri P, Carrick DM, Davidson EH. A regulatory gene network that directs micromere specification in the sea urchin embryo. Dev Biol. 2002;246:209–228. doi: 10.1006/dbio.2002.0627. [DOI] [PubMed] [Google Scholar]
- 27.Revilla-i-Domingo R. Cis-Regulatory Analysis of the Sea Urchin Delta Gene: Validating the Architecture of the Gene Regulatory Network Model for Micromere Lineage Specification. Pasadena, CA: California Institute of Technology; 2007. [Google Scholar]
- 28.Thirunavukkarasu K, et al. Regulation of the human ADAMTS-4 promoter by transcription factors and cytokines. Biochem Biophys Res Commun. 2006;345:197–204. doi: 10.1016/j.bbrc.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 29.Robertson AJ, Dickey CE, McCarthy JJ, Coffman JA. The expression of SpRunt during sea urchin embryogenesis. Mech Dev. 2002;117:327–330. doi: 10.1016/s0925-4773(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 30.Tietze K, Oellers N, Knust E. Enhancer of splitD, a dominant mutation of Drosophila, and its use in the study of functional domains of a helix-loop-helix protein. Proc Natl Acad Sci USA. 1992;89:6152–6156. doi: 10.1073/pnas.89.13.6152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oellers N, Dehio M, Knust E. bHLH proteins encoded by the Enhancer of split complex of Drosophila negatively interfere with transcriptional activation mediated by proneural genes. Mol Gen Genet. 1994;244:465–473. doi: 10.1007/BF00583897. [DOI] [PubMed] [Google Scholar]
- 32.Kuo TC, Calame KL. B lymphocyte-induced maturation protein (Blimp)-1, IFN regulatory factor (IRF)-1, and IRF-2 can bind to the same regulatory sites. J Immunol. 2004;173:5556–5563. doi: 10.4049/jimmunol.173.9.5556. [DOI] [PubMed] [Google Scholar]
- 33.Yuh CH, Dorman ER, Howard ML, Davidson EH. An otx cis-regulatory module: A key node in the sea urchin endomesoderm gene regulatory network. Dev Biol. 2004;269:536–551. doi: 10.1016/j.ydbio.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 34.Yu J, Angelin-Duclos C, Greenwood J, Liao J, Calame K. Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol Cell Biol. 2000;20:2592–2603. doi: 10.1128/mcb.20.7.2592-2603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gyory I, Wu J, Fejér G, Seto E, Wright KL. PRDI-BF1 recruits the histone H3 methyltransferase G9a in transcriptional silencing. Nat Immunol. 2004;5:299–308. doi: 10.1038/ni1046. [DOI] [PubMed] [Google Scholar]
- 36.Osipovich O, et al. Targeted inhibition of V(D)J recombination by a histone methyltransferase. Nat Immunol. 2004;5:309–316. doi: 10.1038/ni1042. [DOI] [PubMed] [Google Scholar]
- 38.Lee PY, Nam J, Davidson EH. Exclusive developmental functions of gatae cis-regulatory modules in the Strongylocentrorus purpuratus embryo. Dev Biol. 2007;307:434–445. doi: 10.1016/j.ydbio.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolouri H, Davidson EH. Transcriptional regulatory cascades in development: Initial rates, not steady state, determine network kinetics. Proc Natl Acad Sci USA. 2003;100:9371–9376. doi: 10.1073/pnas.1533293100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rast JP. Transgenic position of the sea urchin embryo. In: Tuan RS, Lo CW, editors. Developmental Biology Protocols, Vol 2 (Methods in Molecular Biology) Vol 136. Totowa, NJ: Humana; 2000. pp. 365–373. [Google Scholar]
- 41.Revilla-i-Domingo R, Minokawa T, Davidson EH. R11: A cis-regulatory node of the sea urchin embryo gene network that controls early expression of SpDelta in micromeres. Dev Biol. 2004;274:438–451. doi: 10.1016/j.ydbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.