Abstract
Colorectal cancer is the second leading cause of cancer-related deaths in the United States. Understanding the distinct genetic and epigenetic changes contributing to the establishment and growth of metastatic lesions is crucial for the development of novel therapeutic strategies. In a search for key regulators of colorectal cancer metastasis establishment, we have found that the serine/threonine kinase Akt2, a known proto-oncogene, is highly expressed in late-stage colorectal cancer and metastatic tumors. Suppression of Akt2 expression in highly metastatic colorectal carcinoma cells inhibits their ability to metastasize in an experimental liver metastasis model. Overexpression of wild-type Akt1 did not restore metastatic potential in cells with downregulated Akt2, thus suggesting non-redundant roles for the individual Akt isoforms. In contrast, Akt2 overexpression in wild-type PTEN expressing SW480 colorectal cancer cells led to the formation of micrometastases; however, loss of PTEN is required for sustained formation of overt metastasis. Finally, we found that the consequence of PTEN loss and Akt2 overexpression function synergistically to promote metastasis. These results support a role for Akt2 overexpression in metastatic colorectal cancer and establish a mechanistic link between Akt2 overexpression and PTEN mutation in metastatic tumor establishment and growth. Taken together, these data suggest that Akt family members have distinct functional roles in tumor progression and that selective targeting of the PI3K/Akt2 pathway may provide a novel treatment strategy for colorectal cancer metastasis.
Keywords: AKT2, colorectal cancer, metastasis, oncogene, therapeutic target
Colorectal cancer is the third most common cancer diagnosed among men and women and the second leading cause of cancer death in the United States (1). Metastatic or recurrent disease is the most common cause of death in these patients (2). Despite extensive research into the biology of cancer progression, the molecular mechanisms involved in colorectal cancer metastasis are not well characterized. Thus, a thorough understanding of the genetic and epigenetic mechanisms that program metastasis establishment and secondary tumor formation is important for the development and optimal use of novel anticancer therapies.
The phosphatidylinositol 3-kinase (PI3K) signaling pathway contributes to tumor initiation and progression in many types of human malignancies (3). PI3K phosphorylates phosphatidylinositol 4,5-bisphospate (PIP2) at the 3-position of the inositol ring, converting it to phosphatidylinositol 3,4,5-triphosphate (PIP3), a second messenger that is essential for the recruitment of Akt, a survival oncoprotein, to the plasma membrane (4). Activation of Akt, the major downstream effector of PI3K, is frequently observed in human cancers (5). Cancer cells attain constitutive Akt activity through indirect means such as deletion or mutation of the tumor suppressor gene PTEN, a negative regulator of PI3K, overexpression of growth factor receptor tyrosine kinases, or amplification of the catalytic subunit of PI3K (6, 7).
The Akt kinase family is composed of three members, Akt1, Akt2 and Akt3. All three Akt isoforms are structurally homologous and share similar mechanisms of activation but exhibit distinct features. Akt1 and Akt2 are ubiquitously expressed, whereas Akt3 has a more limited tissue distribution (8). Elevated Akt2 expression positively correlates with aggressiveness of cancer and poor survival rates (9). Amplification and overexpression of Akt2 is frequently detected in a number of human tumors, including prostate (10), ovarian (11), breast (9), and pancreatic (12).
Previously, we have determined the distribution of PI3K/Akt pathway component expression in human colorectal adenocarcinomas, and we addressed the function of the p85α regulatory and p110α catalytic subunits of PI3K in colon cancer cell growth using an RNAi approach (13). The goals of our current study were to extend the analysis of Akt isoforms in colorectal carcinoma as well as metastatic tumors and to identify which specific steps in the metastatic process are Akt2 dependent. Here, we demonstrate that Akt2 is overexpressed in metastatic tumors and that suppression of Akt2 expression significantly inhibits metastasis in highly metastatic colorectal cancer cells. Therefore, Akt2 appears to play a critical role in the establishment of metastases in colorectal cancer. Furthermore, concurrent PTEN downregulation and ectopic Akt2 expression led to metastatic phenotype acquisition in colon cancer cells that are non-metastatic. Importantly, our findings suggest a mechanistic link between PTEN deficiency, Akt2 overexpression, and aggressive metastatic phenotype enhancement in colorectal cancer.
Results
Akt1 and Akt2 Expression Is Increased in Colorectal Cancer.
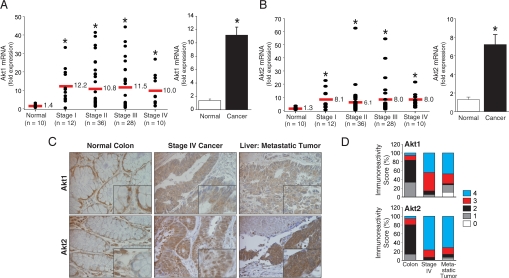
Our initial studies of Akt isoform expression in human colorectal cancers revealed marked Akt2 protein expression in the glandular elements of the cancers (13). To understand further the extent of Akt alterations in the pathogenesis of colon cancer, we used quantitative RT-PCR (qRT-PCR) array to evaluate Akt1 and Akt2 mRNA expression in different stages of colorectal cancer. This study included cDNAs obtained from 86 patients with histopathologically confirmed colorectal cancer representing all stages (stage I, n = 12; stage II, n = 36; stage III, n = 28; stage IV, n = 10) and normal colon samples (n = 10). Comparison of the Akt1 and Akt2 gene expression profiles showed statistically significant mRNA overexpression (8−10-fold; P < 0.05) of both Akt isoforms in stage I through stage IV colorectal cancer samples compared with normal tissue (Figs. 1A and 1B, left panels). The Akt1 and Akt2 mRNA expression levels were similar in all cancer samples, regardless of tumor stage. The results from Akt isoform mRNA expression analysis showed statistically significant increase in Akt1 and Akt2 mRNA expression in colorectal cancers compared with adjacent normal tissue (Figs. 1A and 1B, right panels).
Fig. 1.
Expression of Akt isoforms in human colorectal carcinomas. (A, B, Left) The relative levels of Akt1 or Akt2 mRNA expression, respectively, in normal colon or colorectal cancers of different stages. Bars represent the mean of each group (solid strike). (A, B, Right) Akt1 and Akt2 mRNA expression was significantly increased in colon cancers compared with normal mucosa (*P ≤ 0.05 by Kruskal-Wallis test). (C) Expression of Akt1 or Akt2 in representative colorectal adenocarcinomas and metastatic liver tumors (tissue microarray, ×200 magnification; n = 36 cases; 144 tumor cores, 36 non-neoplastic cores). (D) Distribution of Akt1 or Akt2 immunoreactivity scores in normal mucosa, primary and metastatic tumors.
Next, to gain insight into specific changes associated with colorectal cancer progression, we analyzed tissue samples from 36 patients with metastatic colorectal cancer assessing Akt1 and Akt2 expression in normal mucosa, primary cancer, and liver metastases (Figs. 1C and 1D). Normal cells demonstrated predominantly cellular membrane staining for Akt1 and Akt2 protein (Fig. 1C). Immunodetection of Akt1 or Akt2 revealed intense cytoplasmic staining in a large proportion of tumor cores with some nuclear staining, which is consistent with reports in ovarian, breast, and prostate cancer (14). From a total of 144 tumor cores evaluated for Akt1 immunostaining, only half demonstrated high immunoreactivity scores (score 4) both in primary metastatic colorectal cancer; more than 80% of the matched cases had high Akt2 immunoreactivity in both the primary metastatic colorectal lesions (Fig. 1D). These data demonstrate that Akt2 is overexpressed in primary colon cancers and that Akt2 overexpression profiles of primary colorectal cancers are maintained in their distant metastases.
Akt2 Is Critical for Metastasis of Colorectal Cancer Cells.
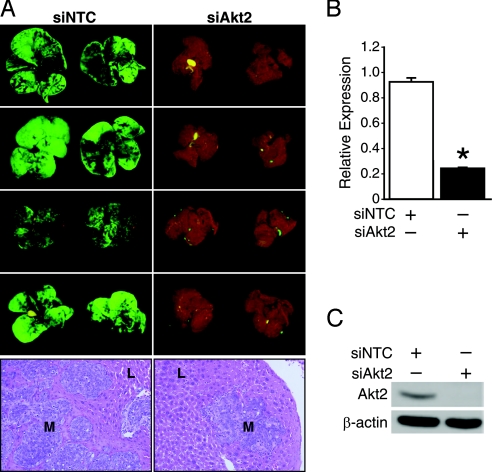
Akt2 expression has been associated with tumor metastasis (9). Because Akt2 overexpression correlates with late-stage colorectal cancer (13) and is maintained in metastatic colorectal cancers, we next assessed the role of Akt2 in the establishment of colorectal cancer metastases. To visualize effectively and identify cancer cells in metastatic locations, we labeled colon cancer cells with green fluorescent protein (GFP) and followed the metastases using Illumatool TLS (13). We tested the metastatic behavior of KM20 cells transfected with either Akt2 siRNA or the non-targeting control (NTC) siRNA. Four weeks after injection of the transfected cells into the spleens of athymic nude mice, animals were killed and livers examined for metastatic lesions. Although cells transfected with NTC siRNA formed numerous macroscopically visible metastases in the livers, cells that were transfected with Akt2 siRNA formed very few metastases (Fig. 2A). Histological analysis confirmed that the number of micrometastatic lesions was also markedly reduced in the livers of the Akt2 siRNA group. As shown in Figs. 2B and 2C, pooled Akt2 siRNA reduced the expression of Akt2 mRNA and protein, respectively. Specifically, the Akt2 siRNA suppressed the expression of Akt2 protein to a level that was undetectable by immunoblotting. A non-targeting siRNA pool was also introduced into KM20 cells without reduction of Akt2 expression.
Fig. 2.
Suppression of Akt2 expression inhibits the ability of KM20 colon cancer cells to establish liver metastases. (A) KM20 cells were transfected with NTC or Akt2 siRNA pool in vitro and injected intrasplenically 56 hours later. Animals were monitored individually for metastatic tumor growth and killed 30 days after intrasplenic inoculation. Illustration of macroscopic foci of GFP-positive colon cancer cells that metastasized to liver; representative photographs of the diaphragmal and visceral liver surfaces are shown. L, normal liver; M, metastasis. (B, C) KM20 cells were transfected with pooled NTC or Akt2 siRNA. After 56 hours of incubation, specific knockdown of Akt2 was confirmed by qRT-PCR or Western blot analysis, respectively. β-actin serves as a loading control.
To exclude the possibility that the observed effect was due to nonspecific suppression of off-target genes by a mixture of Akt2 siRNA sequences, we further tested whether a single Akt2 siRNA sequence could also suppress the ability of KM20 cells to metastasize. Consistent with the pooled siRNA data, transfection of single-sequence Akt2 siRNA reduced the formation of KM20 liver metastatic nodules ([supporting information (SI) Fig. S1A]). Specific Akt2 knockdown was confirmed by qRT-PCR (Fig. S1B) and Western blot (Fig. S1C) analysis. Together, these results suggest that continued Akt2 expression is essential for efficient execution of the metastatic program.
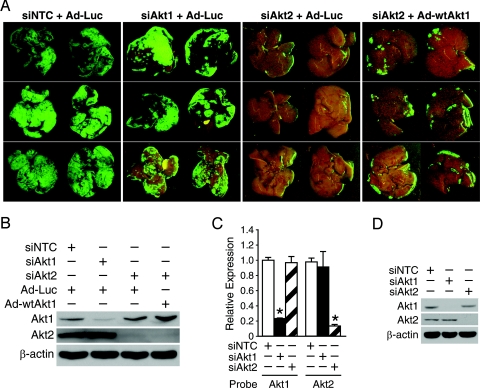
To confirm the significance of Akt2 expression in colorectal cancer metastasis, we next examined whether Akt1 knockdown resulted in a phenotype similar to Akt2 knockdown and, furthermore, whether Akt1 overexpression could rescue the phenotype resulting from knockdown of Akt2. Cells transfected with NTC or Akt1 siRNA formed numerous macroscopically visible metastases in the liver. Furthermore, introducing Akt1 into cells with Akt2 siRNA did not rescue the effects of Akt2 knockdown on metastasis establishment (Fig. 3A). Specific downregulation of Akt1 or Akt2 expression by siRNA in KM20 cells or, conversely, wild-type Akt1 ectopic expression was confirmed by Western blot (Fig. 3B). RNAi specificity was confirmed by demonstrating knockdown of Akt1 and Akt2 expression using the respective siRNA, without notable reduction in the expression of the other isoform (Figs. 3C and 3D). Together, these data establish the functional link between Akt2 and colorectal cancer metastasis and indicate that Akt2 is a major target of PI3K for colorectal cancer metastasis establishment.
Fig. 3.
Akt2 is essential for colorectal cancer metastasis establishment. (A) KM20 cells were transiently transfected with SMARTpool Akt1, Akt2, or NTC siRNA in vitro, infected with control or wild-type Akt1 adenovirus and injected intrasplenically at 56 hours posttransfection. Illustration of macroscopic foci of GFP-positive colon cancer cells that metastasized to liver; representative photographs of the diaphragmal and visceral liver surfaces are shown. (B) Expression of Akt1 and Akt2 proteins was examined by immunoblotting in KM20 cells transiently expressing control or wild-type Akt1 adenovirus. β-actin serves as a loading control. (C, D) KM20 cells were transfected with pooled Akt1, Akt2, or NTC siRNA. After 56 hours of incubation, specific knockdown was confirmed by qRT-PCR or Western blot analysis, respectively. β-actin serves as a loading control.
Akt2 Overexpression Enhances Metastatic Phenotype in PTEN-Deficient Colorectal Cancer Cells.
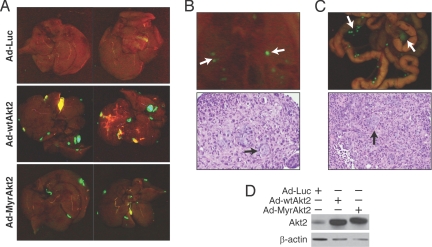
We next determined whether a correlation existed between Akt2 overexpression and enhancement of the metastatic phenotype. To address this question, poorly metastatatic KM12C colon cancer cells, derived from a Dukes' B cancer (15), were infected with wild-type Akt2 (Ad-wtAkt2), constitutively active Akt2 (Ad-MyrAkt2), or luciferase (Ad-Luc) adenoviruses and injected intrasplenically 24 hours later. We found that transient Akt2 overexpression resulted in survival and growth of micrometastases and subsequent macroscopic tumor formation in liver (Fig. 4A), diaphragmal lymph nodes (Fig. 4B), and lymph nodes in the mesentery of the colon (Fig. 4C); ectopic Akt2 expression was confirmed by Western blot (Fig. 4D). Interestingly, overexpression of wild-type Akt2 resulted in increased metastatic phenotype compared with constitutively active form of Akt2, suggesting that translocation to cytosol and nucleus is essential for metastatic phenotype enhancement, as many of the substrates of Akt are proteins that function in the nucleus (16). Therefore, these findings suggest that Akt2 overexpression results in a metastatic phenotype enhancement and further indicates that Akt2 overexpression is critical for colorectal cancer progression.
Fig. 4.
Akt2 overexpression increases metastases in the poorly metastatic KM12C colon cancer cells. (A) KM12C cells were infected with control, wild-type Akt2 and constitutively active Akt2 adenovirus and injected intrasplenically (n = 3 mice per group). Illustration of macroscopic foci of GFP-positive colon cancer cells that metastasized to liver; representative photographs of the diaphragmal and visceral liver surfaces are shown. (B, C) Representative photographs of metastatic tumor growth in diaphragmal (B) and mesenteric lymph nodes (C) are shown. H&E staining confirmed metastasis in lymph nodes. (D) Detection of ectopic Akt2 by immunoblot in KM12C cells expressing the indicated transgene. β-actin serves as a loading control.
Next, we determined whether a correlation existed between Akt2 overexpression and acquisition of the metastatic phenotype by transiently overexpressing Akt2 in the tumorigenic but non-metastatic colorectal cancer cell line SW480. Cells were infected with Ad-wtAkt2, Ad-MyrAkt2, or Ad-Luc adenoviruses and injected intrasplenically 24 hours later. One and 4 weeks after injection, mice were killed (n = 3 per group) and livers examined for metastatic lesions. None of the animals had macroscopically evident metastases. To investigate sequential steps of metastasis formation in the liver, hematoxylin and eosin (H&E)−stained histological sections were analyzed. A subset of SW480 cells with wild-type or constitutively active Akt2 overexpression formed micrometastases by week 1. However, by week 4, no micrometastases were noted (Fig. S2A). These results indicate that overexpression of Akt2 is sufficient for the establishment of metastatic tumors in liver; however, other contributing factors are necessary to sustain metastatic tumor growth.
To define the molecular mechanisms involved in loss of micrometastases in SW480 cells and Akt2-mediated colorectal cancer metastatic phenotype enhancement in KM12C cells, we analyzed the expression level and activation status of relevant targets of the PI3K/Akt cascade in KM12C and SW480 cells. PTEN expression analysis revealed PTEN deficiency in the KM12C cell line. As expected, phosphorylation of Akt at Ser 473 was highly elevated as result of PTEN deficiency (Fig. S2B). Wild-type PTEN introduction resulted in a significant decrease of phosphorylated Akt levels, as shown in Fig. S2C. Analysis of tissue samples from patients with metastatic colorectal cancer for PTEN expression in normal mucosa, primary cancer, and liver metastases demonstrated intense epithelial staining in normal colonic mucosal samples. PTEN staining pattern in stage IV tumor specimens was diffusely weak or demonstrated a heterogeneous pattern of variable intensity; weak or negative staining was noted in metastatic tumors (Fig. S3A). Based on our scoring criteria, loss of PTEN expression was noted in ≈83% of the metastatic tumor specimens (Fig. S3B). Interestingly, strong PTEN staining was observed in the connective tissue stroma of the stage IV tumor specimens. Together, these data led us to speculate that concurrent PTEN deficiency with Akt2 overexpression would increase micrometastasis establishment and preferentially favor persistence and growth of metastases.
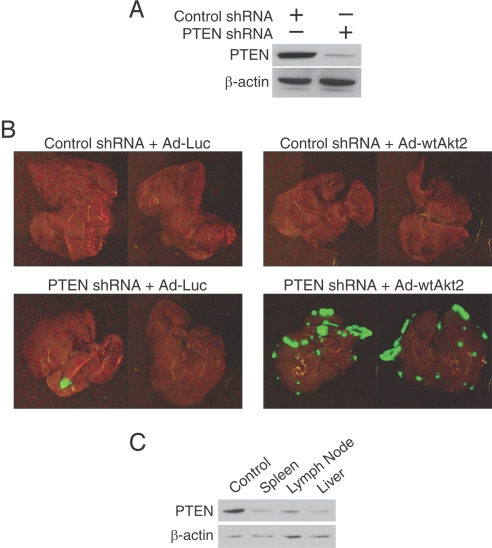
To evaluate our hypothesis, we introduced a short hairpin RNA (shRNA) specific for the endogenous allele of tumor suppressor PTEN into SW480 cells. Stable shRNA-mediated knockdown of PTEN was established, and a mixture of multiple clones was selected for further experiments. The ability of this shRNA sequence to suppress PTEN expression was confirmed by Western blot analysis (Fig. 5A); NTC shRNA did not alter PTEN expression. To determine whether loss of PTEN expression affected the ability of SW480 cells to metastasize, we examined the metastatic behavior of the SW480 tumor cells expressing either PTEN shRNA or the control shRNA after transient Akt2 overexpression. Four weeks after intrasplenic inoculation, mice were killed and livers examined for metastases. None of the animals in the control shRNA group had macroscopically evident metastases (Fig. 5B). Although tumors expressing PTEN shRNA formed macroscopically visible metastases in the liver, a low metastatic incidence was observed (n = 1–2 metastases per animal). Concurrent Akt2 overexpression and PTEN downregulation significantly increased metastatic incidence compared with PTEN downregulated cells only (≈10-fold). To determine whether PTEN expression was suppressed by shRNA in vivo, primary tumors, lymph node metastases, and metastatic liver tumors formed by SW480 PTEN knockdown cells were collected. We observed suppression of PTEN in isolated cells 1 month after initial implantation (Fig. 5C). Taken together, these results provide strong evidence that metastatic colorectal cancer cells develop an increased reliance on Akt2 overexpression for metastases establishment and PI3K activation for metastatic tumor growth.
Fig. 5.
Role of PI3K activation in micrometastasis growth. (A) Expression of PTEN protein was examined by immunoblotting in SW480 cells stably expressing NTC or PTEN shRNA. (B) SW480 cells stably expressing NTC or PTEN shRNA were infected with control adenovirus or wild-type Akt2 and injected intrasplenically (n = 3 mice per group). Illustration of macroscopic foci of GFP-positive colon cancer cells that metastasized to liver; representative photographs of the diaphragmal and visceral liver surfaces are shown. (C) Expression of PTEN protein was examined by immunoblotting of tumor cells recovered from primary tumors, lymph node metastases, and metastatic liver tumors. β-actin serves as a loading control.
Discussion
Akt is at the center of several major signaling pathways (8). Aberrant Akt2 activation plays important roles in sustaining processes important to malignancy (11). The molecular consequences of Akt2 activation have been shown, and a number of Akt2-regulated genes identified that could play a role in cancer cell invasion (14). However, considerably less is known about the biological consequences of Akt2 activation during metastatic colonization.
Experimental metastasis assays have certain advantages and disadvantages that should be considered and balanced depending on the specific aim of each experiment. Implantation of highly metastatic tumor cells into the spleens of immunocompromised mice results in the rapid and efficient formation of metastases to the liver (15). This model is ideal for studying many aspects of metastasis formation, as it recapitulates the pattern of colorectal cancer metastasis in humans and allows study of the ability of a tumor cell to form distant colonies. A major disadvantage of the experimental metastasis models is that only late stages of metastasis formation can be studied. The early stages, including local invasion at the site of the primary tumor, interaction with stromal and immunological microenvironment, and gain of access to lymphatics or blood vessels, are bypassed by direct injection of tumor cells into the spleen. Using this approach, we found that the later steps in hematogenous metastasis to the liver, including cell survival in the circulation, extravasation, and survival of individual extravasated cells, were completed with high efficiency by colon cancer cells expressing Akt2. The oncogenic potential of Akt2 identified in this study suggests that the frequent amplification and up-regulation of this kinase in colorectal cancer plays an important role during metastatic colonization.
Akt2 is a predominant isoform in ovarian, breast, and pancreatic cancers, whereas Akt1 expression has mostly been detected in gastric cancer (11, 17). Previous studies have shown that overexpression of Akt isoforms is a much more frequent event than their gene amplification in human malignancies, suggesting transcriptional regulation of Akt during tumor development (12). Our results from Akt isoform mRNA expression analysis showed a statistically significant increase in Akt1 and Akt2 mRNA expression in colorectal cancers compared with adjacent normal tissue. We also demonstrate that the overexpression of Akt2 in metastatic colorectal cancer is a more frequent event than Akt1. Of interest, a surprising observation in our analysis was that the proportion of advanced stage colorectal cancer cases with high immunoreactivity score for Akt2 was higher than that of Akt1. The apparent differences between Akt1 and Akt2 expression with respect to their oncogenic activity and involvement in human malignancy suggest that the signal transduction pathways of these two closely related proteins may differ and that Akt2 may play a more specific role than Akt1 in colorectal cancer progression.
Deregulation of the PI3K pathway is a common occurrence in tumors (5, 9). Which of these downstream signaling molecules are actually responsible for transducing the PI3K signal that leads to metastatic phenotype enhancement in colorectal cancer is still unknown. It is imperative to understand the nature of the dysregulated molecular mechanisms underlying the progression of colorectal cancer, as it will provide a rational foundation to explore new treatment modalities and to stratify patients that may benefit from novel molecular-based therapies. The limited clinical response to treatment of metastatic colorectal cancer with epidermal growth factor receptor (EGFR) therapies suggest that, during tumor progression, metastatic lesions acquire a number of genetic and epigenetic alterations that result in the persistent activation of growth-promoting pathways (18). Candidates for such mutations are a loss of function in the tumor suppressor PTEN or mutations/amplification of the catalytic subunit of PI3K (6, 7). Previous studies have shown that colon cancer cells, after targeted deletion of wild-type pik3ca gene, which encodes the p110α PI3K catalytic subunit, demonstrated constitutive PI3K and Akt activation during stress conditions, resulting in attenuation of apoptosis and enhanced tumor formation (6). PTEN inactivation appears to influence metastatic ability by promoting cell proliferation while suppressing apoptosis at the secondary metastatic site (7). Results from our study suggest that colorectal cancer cells required Akt2 overexpression and PTEN inactivation during separate steps of the metastatic process. Activated Akt2 appears to influence the metastatic phenotype by promoting extravasation at the secondary metastatic sites, whereas PTEN deficiency preferentially favors persistence and growth of metastases.
PI3K/Akt inhibitors are under development as anti-cancer therapy or have been approved for treatment of certain human cancers (19). A number of drugs in clinical use or preclinical evaluation, originally developed as non-PI3K pathway inhibitors, have been demonstrated to directly or indirectly target PI3K signaling. These include mammalian target of rapamycin (mTOR), inhibitors of epidermal growth factor receptor (EGFR, HER2/neu, c-Kit, PDGFR), and BCR–ABL (20). The theoretical advantage associated with Akt inhibition, compared with downstream inhibition (e.g., mTOR), is that the PI3K/Akt pathway can be more effectively inhibited when targeting Akt directly and might also be less susceptible to the unknown effects of feedback loops than the inhibition of downstream branches (20). For these reasons, a number of isoform-selective Akt inhibitors are currently under development (19, 21). Whether more selective Akt inhibitors will demonstrate wider therapeutic indices is unknown. Collectively, our current observations support the emerging notion that PI3K and, in particular, its downstream effector Akt2 may represent a suitable target for colorectal cancer treatment, as activation of PI3K/Akt pathway is a common event in most colorectal cancers.
Materials and Methods
Cell Lines, Transfection, and Adenovirus Infection.
The human colon cancer cell lines KM12C and KM20 were provided by Dr. Isaiah J. Fidler (M. D. Anderson Cancer Center, Houston, TX). The poorly metastatic KM12C cell line was established from a primary colorectal carcinoma classified as Dukes' B2 (i.e., stage II). The KM20 cell line was derived from a resected surgical specimen of a Dukes' D (i.e., stage IV) primary colon cancer (15). The human colon cancer cell line SW480 was purchased from American Type Culture Collection (Manassas, VA). Tissue culture media and reagents were obtained from Life Technologies, Inc. (Grand Island, NY).
Pool siRNA duplexes (four sequences in the coding region of a gene) for Akt1 and Akt2 or single Akt2 sequence (5′-GUACUUCGAUGAUGAAUUU) were designed and synthesized by customer siRNA Design from Dharmacon (Lafayette, CO). NTC siRNA pool, whose sequences did not match any known mouse or human gene, was used as a negative control. EGFP-N1 vector was purchased from Clontech, Mountain View, CA. GIPZ NTC, whose sequence did not match any known human or mouse gene, or PTEN (5′-CCCTATATTTATCCAAACATTA-3′) shRNAmyr vectors were purchased from Open Biosystems, Huntsville, AL. Plasmid DNA and siRNA were delivered into cells using the electroporation method as described previously (13). Polyclonal stable populations were obtained after selection with G418 sulfate (0.25 mg ml−1; EGFP-N1) or with puromycin (4 μg ml−1, GIPZ shRNAmir, Open Biosystems). Cells were tested routinely for mycoplasma contamination with the nested-PCR and fluorescent staining simultaneously; all tests were negative for contamination.
Adenoviral vectors expressing the constitutively active form of Akt2 (Ad-MyrAkt2), wild-type Akt2 (Ad-wtAkt2), wild-type Akt1 (Ad-wtAkt1), wild-type PTEN (Ad-wtPTEN) and luciferase (Ad-Luc) under the control of the cytomegalovirus promoter were obtained from Vector Biolabs (Philadelphia, PA). To construct the constitutively active mutant of Akt2 the c-Src myristoylation sequence was fused in frame to the N terminus of the HA-Akt2 (wild-type) coding sequence. When targeted to membranes with N-terminal myristoylation, Akt2 becomes constitutively active (4). For infection, recombinant adenovirus was diluted in cell culture media containing 2% FBS and added to subconfluent cells for 6 h at a multiplicity of infection of 40. After this, the medium was replaced with fresh complete culture medium, and the cells were incubated for an additional 18 h.
TissueScan Oncology Panel, RNA Isolation, and qRT–PCR.
Total RNA was purified using the RNeasy Kit including an optional DNase treatment according to the manufacturer's instructions (Qiagen; Valencia, CA). A commercial panel of cDNAs covering four disease stages and normal tissues was purchased from OriGene Technologies, Inc. (Rockville, MD); the data from the array was normalized to the 18s rRNA and calibrated relative to normal tissue sample.
Immunoblotting, Tissue Microarrays, and Immunohistochemistry.
Immunoblotting was performed as described previously (13). Antibodies against PTEN, pAkt (Ser-473), Akt1 and Akt2 were purchased from Cell Signaling Technologies (Beverly, MA) and used according to the manufacturer's directions. The pAkt antibody recognizes phosphorylated Akt1 and Akt2. Neither the Akt1 nor Akt2 specific antibodies cross reacted with recombinant Akt2 or Akt1 in Western blot analyses, respectively. Mouse monoclonal anti-β-actin antibody was obtained from Sigma-Aldrich (St. Louis, MO).
Tissue microarray (TMA) with matching liver metastases A203 (II) and A203 (III) were purchased from ISUABXIS through Accurate Chemical & Scientific Corp (Westbury, NY). Each metastasis array consisted of the 18 matched cases: normal colon, primary colon cancer and liver metastases. Immunohistochemistry was performed as described previously (13). For negative controls, primary antibody was omitted from the above protocol. The number of positive cells was visually evaluated in each core by a pathologist (Dr. S-Y X) and the staining intensity of Akt1, Akt2 or PTEN classified using a five-grade scale, with 0 indicating the absence of immunostaining or less than 10% of stained cells; 1, between 10% and 25%; 2, between 25% and 50%; 3, 50% to 75%; and 4, 75% to 100% of cells stained (22).
Athymic Nude Mice and Metastasis Model.
Male athymic nudenu/nu (4–6 weeks old) mice were obtained from Harlan Sprague–Dawley (Indianapolis, IN) and housed in clean, pathogen-free rooms in an environment with controlled temperature (27 °C), humidity, and a 12 h light/dark cycle. All animal experiments were approved by the Institutional Animal Care and Use Committee at University of Texas Medical Branch and were conducted in accordance with guidelines issued by the National Institutes of Health for the care of laboratory animals. Tumor cells were injected intrasplenically by methods previously described (15). Animals were examined by total-body fluorescence imaging (23) during the course of tumor development. Selective excitation of GFP was produced by using an Illumatool TLS system with an excitation band-pass filter at 470 nm, and emitted fluorescence was collected through a long-pass filter at 515 nm (Lightools Research, Encinitas, CA). Strong yellow autofluorescence of the bile in the gallbladder was also noted in some of the livers, as previously described by Hara et al. (24).
Isolation of Tumor Cells from Spleen, Lymph Node, and Liver.
Tissue was minced and enzymatically dissociated with collagenase (type IV, 1 mg ml−1), hyaluronidase (0.1 mg ml−1) and DNase (20 mg ml−1) (Sigma-Aldrich, St. Louis, MO) for 2 h at 37 °C (15). The cell suspension was dispersed with an 18-gauge needle, filtered through 70 μm sieve, washed in serum-free medium and plated in media plus puromycin (4 μg ml−1).
Statistical Analysis.
Relative expression of Akt1 or Akt2 mRNA in human normal mucosa or colorectal cancer samples was analyzed using the Kruskal-Wallis test at the 0.05 level of significance.
Supplementary Material
Acknowledgments.
The authors thank Kathleen O'Connor, Tianyan Gao, and Titilope Ishola for critical review of the manuscript, Karen Martin for manuscript preparation, and Tatsuo Uchida for advice on statistical analysis. This work was supported by grants RO1CA104748, RO1DK48498, PO1DK35608, and RO1DK61470 from the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810715105/DCSupplemental.
References
- 1.Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Bengmark S, Hafstrom L. The natural history of primary and secondary malignant tumors of the liver. I. The prognosis for patients with hepatic metastases from colonic and rectal carcinoma by laparotomy. Cancer. 1969;23:198–202. doi: 10.1002/1097-0142(196901)23:1<198::aid-cncr2820230126>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 3.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 4.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 5.Brugge J, Hung MC, Mills GB. A new mutational AKTivation in the PI3K pathway. Cancer Cell. 2007;12:104–107. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Samuels Y, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Simpson L, Parsons R. PTEN: Life as a tumor suppressor. Exp Cell Res. 2001;264:29–41. doi: 10.1006/excr.2000.5130. [DOI] [PubMed] [Google Scholar]
- 8.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. PNAS. 2001;98:10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bellacosa A, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64:280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 10.Graff JR, et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275:24500–24505. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 11.Yuan ZQ, et al. Frequent activation of AKT2 and induction of apoptosis by inhibition of phosphoinositide-3-OH kinase/Akt pathway in human ovarian cancer. Oncogene. 2000;19:2324–2330. doi: 10.1038/sj.onc.1203598. [DOI] [PubMed] [Google Scholar]
- 12.Altomare DA, et al. Frequent activation of AKT2 kinase in human pancreatic carcinomas. J Cell Biochem. 2003;88:470–476. doi: 10.1002/jcb.10287. [DOI] [PubMed] [Google Scholar]
- 13.Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted molecular therapy of the PI3K pathway: Therapeutic significance of PI3K subunit targeting in colorectal carcinoma. Ann Surg. 2006;243:833–842. doi: 10.1097/01.sla.0000220040.66012.a9. discussion 843–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arboleda MJ, et al. Overexpression of AKT2/protein kinase B{beta} leads to up-regulation of {beta}1 integrins, increased invasion, and metastasis of human breast and ovarian cancer cells. Cancer Res. 2003;63:196–206. [PubMed] [Google Scholar]
- 15.Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988;48:1943–1948. [PubMed] [Google Scholar]
- 16.Andjelkovic M, et al. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- 17.Han Z, et al. Akt1/protein kinase B alpha is involved in gastric cancer progression and cell proliferation. Dig Dis Sci. 2008;53:1801–1810. doi: 10.1007/s10620-007-9824-2. [DOI] [PubMed] [Google Scholar]
- 18.Varghese HJ, et al. Activated Ras regulates the proliferation/apoptosis balance and early survival of developing micrometastases. Cancer Res. 2002;62:887–891. [PubMed] [Google Scholar]
- 19.Momota H, Nerio E, Holland EC. Perifosine inhibits multiple signaling pathways in glial progenitors and cooperates with temozolomide to arrest cell proliferation in gliomas in vivo. Cancer Res. 2005;65:7429–7435. doi: 10.1158/0008-5472.CAN-05-1042. [DOI] [PubMed] [Google Scholar]
- 20.Hennessy BT, Smith DL, Ram PT, Lu Y, Mills GB. Exploiting the PI3K/AKT pathway for cancer drug discovery. Nat Rev Drug Discov. 2005;4:988–1004. doi: 10.1038/nrd1902. [DOI] [PubMed] [Google Scholar]
- 21.Rhodes N, et al. Characterization of an Akt kinase inhibitor with potent pharmacodynamic and antitumor activity. Cancer Res. 2008;68:2366–2374. doi: 10.1158/0008-5472.CAN-07-5783. [DOI] [PubMed] [Google Scholar]
- 22.Molinolo AA, et al. Dissecting the Akt/mammalian target of rapamycin signaling network: Emerging results from the head and neck cancer tissue array initiative. Clin Cancer Res. 2007;13:4964–4973. doi: 10.1158/1078-0432.CCR-07-1041. [DOI] [PubMed] [Google Scholar]
- 23.Yang M, et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci USA. 2000;97:1206–1211. doi: 10.1073/pnas.97.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hara M, et al. A mouse model for studying intrahepatic islet transplantation. Transplantation. 2004;78:615–618. doi: 10.1097/01.tp.0000128838.54074.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.