Abstract
Cyclooxygenase (COX-1/COX-2)-catalyzed eicosanoid formation plays a key role in inflammation-associated diseases. Natural forms of vitamin E are recently shown to be metabolized to long-chain carboxychromanols and their sulfated counterparts. Here we find that vitamin E forms differentially inhibit COX-2-catalyzed prostaglandin E2 in IL-1β-stimulated A549 cells without affecting COX-2 expression, showing the relative potency of γ-tocotrienol ≈ δ-tocopherol > γ-tocopherol ≫ α- or β-tocopherol. The cellular inhibition is partially diminished by sesamin, which blocks the metabolism of vitamin E, suggesting that their metabolites may be inhibitory. Consistently, conditioned media enriched with long-chain carboxychromanols, but not their sulfated counterparts or vitamin E, reduce COX-2 activity in COX-preinduced cells with 5 μM arachidonic acid as substrate. Under this condition, 9′- or 13′-carboxychromanol, the vitamin E metabolites that contain a chromanol linked with a 9- or 13-carbon-length carboxylated side chain, inhibits COX-2 with an IC50 of 6 or 4 μM, respectively. But 13′-carboxychromanol inhibits purified COX-1 and COX-2 much more potently than shorter side-chain analogs or vitamin E forms by competitively inhibiting their cyclooxygenase activity with Ki of 3.9 and 10.7 μM, respectively, without affecting the peroxidase activity. Computer simulation consistently indicates that 13′-carboxychromanol binds more strongly than 9′-carboxychromanol to the substrate-binding site of COX-1. Therefore, long-chain carboxychromanols, including 13′-carboxychromanol, are novel cyclooxygenase inhibitors, may serve as anti-inflammation and anticancer agents, and may contribute to the beneficial effects of certain forms of vitamin E.
Keywords: cancer, inflammation, PGE2, tocopherol, tocotrienol
Cyclooxygenases (COX-1 and COX-2) catalyze the conversion of arachidonic acid (AA) to prostaglandin H2 (PGH2), the common precursor to prostaglandins and thromboxanes that are important lipid mediators for regulation of many physiological and pathophysiological responses (1). COXs are bifunctional enzymes that carry out two sequential activities—i.e., the cyclooxygenase activity, which leads to the formation of prostaglandin G2 (PGG2), and the peroxidase activity, which reduces PGG2 to PGH2 (2). COX-1 is constitutively expressed in many tissues, including platelets where thromboxanes are generated by this enzyme to promote platelet aggregation. COX-2 is often induced under acute/chronic inflammatory conditions and is mainly responsible for the generation of proinflammatory eicosanoids, including prostaglandin E2 (PGE2) (3). COX inhibitors, which are nonsteroidal anti-inflammatory drugs (NSAIDs), have been used for the relief of fever, pain, and inflammation (4). Chronic inflammation has been identified as a significant factor in the development of cancer (5). It is well established that NSAIDs are effective chemoprevention agents for cancer (6), although their long-term use has been questioned due to the associated gastrointestinal side effects and increased risk of cardiovascular diseases (7, 8).
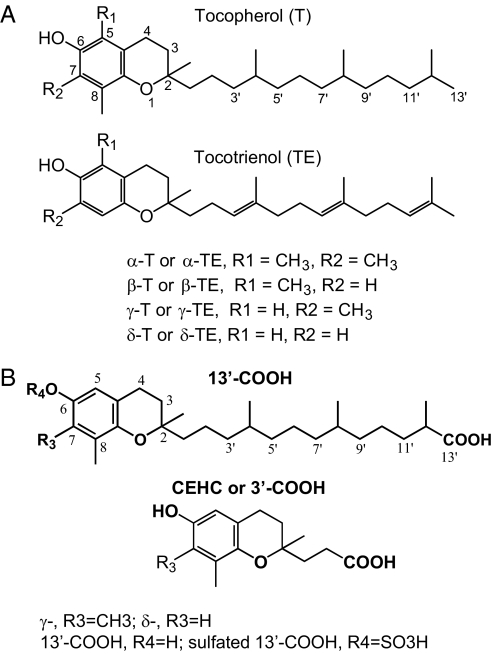
Vitamin E comprises eight lipophilic antioxidants: α-, β-, γ- and δ-tocopherol (α-, β-, γ-, and δ-T) and α-, β-, γ-, and δ-tocotrienol (α-, β-, γ-, and δ-TE) (Fig. 1A). Among them, α-T, the predominate form of vitamin E in tissues, has drawn the most attention and been extensively studied in animals and in human clinical trails (9–11). However, studies by us and others indicate that compared with α-T, other forms of vitamin E appear to have superior bioactivities that are important to disease prevention and/or therapy (12). For instance, γ-T, the major form of vitamin E in U.S. diets, inhibits COX-2-catalyzed PGE2 to a greater extent than αT in lipopolysaccharide-activated macrophage and interlukin-1β (IL-1β)-treated epithelial cells (13). In a rat inflammation model, γ-T but not α-T inhibits proinflammatory eicosanoid and attenuates inflammation-induced damage (14). We also documented that γ-T, in contrast to α-T, inhibits the growth and induces death of cancer cells but has no effect on normal epithelial cells (15).
Fig. 1.
(A) α-, β-, γ-, and δ-tocopherol, and α-, β-, γ-, and δ-tocotrienol. (B) metabolites of γ-T and δ-T: 13′-carboxychromanol (13′-COOH), sulfated 13′-COOH, and CEHC (or 3′-carboxychromanol).
The reasons for the different bioactivity between α-T and other forms of vitamin E are not well understood, but may be, in part, rooted in their distinct metabolism. α-T is well retained in tissues because it is preferentially protected by α-tocopherol transfer protein from being degraded (12, 16). However, other forms of vitamin E, including γ-T, are readily metabolized by cytochrome P450-mediated ω-hydroxylation and oxidation of the 13′-carbon on the hydrophobic side chain to generate 13′-carboxychromanol (Fig. 1B), which is further degraded by β-oxidation to form the terminal urinary-excreted metabolite CEHC (2-(β-carboxyethyl)-6-hydroxychroman, or 3′-carboxychromanol) (Fig. 1B) (12, 16). Recently, we and others reported that vitamin E forms are metabolized to long-chain carboxychromanols, i.e., 9′-, 11′-, 13′-carboxychromanol (17, 18), and sulfated 9′-, 11′-, 13′-carboxychromanol, in human lung epithelial A549 cells (17) (Fig. 1B). Importantly, sulfated long-chain carboxychromanols and 13′-carboxychromanol are found in rat plasma and liver subsequent to γ-T supplementation (17). It is not clear, however, whether these metabolites have important biological activities that may account for the beneficial effects of vitamin E. In the present study, we systemically examined the effect of different forms of vitamin E and their metabolites on cyclooxygenase-catalyzed reaction in IL-1β-activated A549 cells and in enzyme assays using purified COX-1 and COX-2.
Results
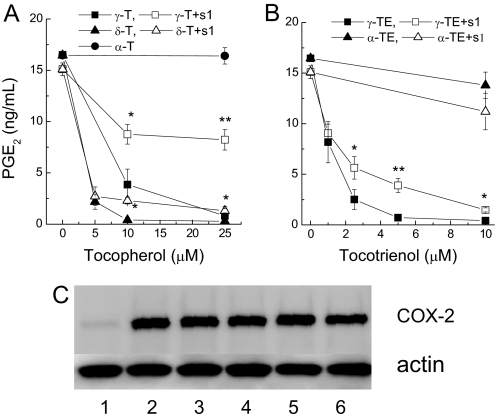
Vitamin E Forms Differentially Inhibited PGE2 Formation in IL-1β-Activated A549 Cells, and Sesamin Partially Diminished the Inhibitory Potency.
Activation of human lung epithelial A549 cells by IL-1β leads to a strong upregulation of COX-2 expression and a marked increase of PGE2 generation (13, 19). In the early stage of the study, we found that when added together with IL-1β for 24 h, vitamin E forms differentially inhibited PGE2 release (Fig. 2). The relative inhibitory potency showed an order of γ-TE ≈ δ-T (IC50 at 1–3 μM) > γ-T (IC50 at 6–7 μM), whereas α-T, β-T (no inhibition at 50 μM), or α-TE (20% inhibition at 20 μM) were much less or not effective at physiologically relevant concentrations. The differential inhibition was not due to differences in cellular accumulation of vitamin E forms. For example, when cellular uptake of γ-T (0.83 ± 0.1 nmoles/106 cells after 10 μM of γ-T was incubated with A549 cells for 18 h) was similar to that of α-T (1.05 ± 0.13 nmoles/106 cells after 25 μM of α-T was incubated with A549 cells), γ-T, but not α-T, potently inhibited PGE2 (Fig. 2).
Fig. 2.
Vitamin E forms differentially inhibited PGE2 in IL-1β-treated A549 cells and the presence of sesamin partially decreased the inhibitory potency (A and B). Vitamin E forms did not affect IL-1β-induced COX-2 expression (C). A549 cells were preincubated with different concentrations of tocopherols (A) and tocotrienols (B) in the presence or absence of 1 μM sesamin (S1) for 15 h and then stimulated with IL-1β (2 ng/ml) for 24 h. PGE2 accumulated in cell culture media was measured by ELISA assays. Results are the averages of three independent experiments and expressed as mean ± SEM. *, P < 0.05 and **, P < 0.01 indicate significant differences of PGE2 in the presence and absence of sesamin. Western blotting (C) showed the effect of vitamin E forms on COX-2 induction with actin as the loading control. Cells were treated with vehicle (lane 1); IL-1β at 2 ng/ml (lane 2); IL-1β and γ-T at 40 μM (lane 3); or δ-T at 40 μM (lane 4), α-TE at 10 μM (lane 5), or γ-TE at 10 μM (lane 6) for 24 h.
To investigate whether the inhibitory action remains in the presence of exogenous AA, cells were incubated with fresh media containing 5 μM AA for 5 min at the end of the chronic IL-1β-treatment experiments. Under this condition, the IC50s of γ-T, δ-T, and γ-TE increased to 25, 10, and 10 μM, respectively. This observation suggests that the inhibitory effect was independent of substrate (AA) availability and appeared to be stronger with endogenous (Fig. 2) than exogenous AA. Results from Western blotting showed that vitamin E forms did not affect IL-1β-induced COX-2 protein expression (Fig. 2C), which is consistent with our previous observations (13). These findings suggest that the inhibitory effect on PGE2 likely stems from their inhibition of COX-2 activity.
Recent studies showed that when chronically incubated with A549 cells, vitamin E forms are metabolized to long-chain carboxychromanols (17, 18) and sulfated carboxychromanols (17). We therefore investigated whether the metabolism of vitamin E affect their inhibition of PGE2. Coincubation with sesamin (SI), which is an inhibitor of tocopherol ω-hydroxylase (20) and blocks vitamin E metabolism in A549 cells (17, 18), significantly diminished the inhibitory potency of γ-T (Fig. 2A). However, sesamin alone at 1 μM did not affect PGE2 generation (data not shown). Sesamin also moderately diminished the inhibitory effect of δ-T and γ-TE (Fig. 2 A and B). Ketoconazole, another cytochrome P450 inhibitor, similarly attenuated the inhibitory action of vitamin E (data not shown). These observations suggest that the inhibition of PGE2 during chronic IL-1β treatment may be, in part, caused by the metabolites generated from vitamin E. However, because sesamin did not completely abolish the inhibitory action, γ-T, δ-T, and γ-TE appear to be capable of inhibiting COX-2 activity in this cellular system. In addition, the observation that sesamin showed more pronounced effect on γ-T than δ-T or γ-TE may be explained by the fact that δ-T and γ-TE appear to be stronger inhibitors than γ-T (Fig. 2 A and B).
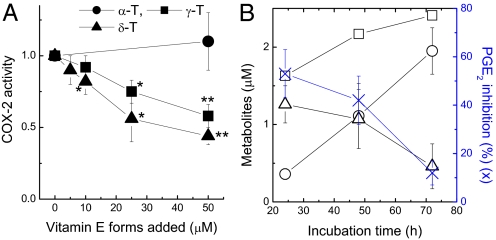
Conditioned Media Enriched with Long-Chain Carboxychromanols, but Not Their Sulfated Counterparts, Inhibited COX-2 Activity in Intact-Cell Assays.
Long-chain carboxychromanols (i.e., 9′-, 11′-, and 13′-COOH) (17, 18) and sulfated long-chain carboxychromanols (i.e., 9′S, 11′S, and 13′S) (Fig. 1B and ref. 17) are accumulated in culture media when vitamin E forms are incubated with confluent A549 cells. To investigate whether these metabolites directly inhibit COX activity, we examined the conditioned media in intact-cell assays where COX-2 was preinduced and 5 μM AA was added as substrate. The conditioned media from γ-T and δ-T, which contained predominantly unconjugated 9′-, 11′-, and 13′-carboxychromanol (17) (supporting information (SI)), dose-dependently inhibited COX-2 activity (Fig. 3A). The media from δ-T were slightly more potent than those from γ-T, likely due to the higher concentrations of the metabolites generated from δ-T (17) (SI). However, conditioned media from α-T did not show any effect, consistent with the fact that α-T is not metabolized to the long-chain metabolites by these cells (17, 18).
Fig. 3.
Conditioned media containing metabolites from γT and δT showed dose-dependent inhibition of COX-2 activity in intact-cell assays (A). Conditioned media were obtained by incubation of confluent A549 cells with α-T, δ-T, or γ-T at 10, 25, and 50 μM for 48 h. Cells with preinduced COX-2 were incubated with conditioned media for 30 min, and added with 5 μM AA for 5 min. Media were collected to measure PGE2 formation. The relative COX activity is expressed as the ratio of PGE2 in the conditioned media to that of vehicle (0.05% DMSO) control media. *, P < 0.05 and **, P < 0.01 indicate significant differences between metabolite- and vehicle-containing media. (B) Inhibition of COX-2 activity (blue cross) correlates with the concentration of unconjugated carboxychromanols (the sum of 9′-, 11′-, and 13′-COOH; open triangle), but not that of sulfated carboxychromanols (the sum of sulfated 9′-, 11′-, and 13′-COOH; open circle) or total metabolites (open square) derived from γ-TE. The conditioned media were obtained by incubation of A549 cells with γ-TE at 20 μM for 24, 48, and 72 h. Metabolites were extracted and measured by HPLC. The conditioned media were then used for the activity assay as described in (A). Results are the averages of three independent experiments (mean ± SD).
Three control experiments were conducted to confirm that the inhibitory effect was executed by the metabolites rather than the precursor vitamins or any nonspecific oxidation products. Specifically, freshly made vitamin E forms (up to 50 μM) did not inhibit COX-2 activity in these intact-cell assays (data not shown), indicating that vitamin E remaining in the media did not contribute to the inhibitory effect. In addition, the media obtained by coincubation of vitamin E and sesamin, or by incubation of vitamin E in cell-free dishes, failed to show any inhibition (data not shown).
To distinguish the inhibitory effect by the carboxychromanols from that of their sulfated counterparts, we took advantage of the observation that 85% metabolites from γ-TE were in the unconjugated form during the first 24 h incubation, whereas more than 90% metabolites became sulfated after 72 h incubation (Fig. 3B). Meanwhile, the amount of total metabolites only slightly increased from 24 to 72 h (Fig. 3B). Using the conditioned media obtained after 24, 48, and 72 h incubation, we found that the inhibitory effect on COX-2 gradually diminished when the metabolites shifted from nonconjugated long-chain carboxychromanols (open triangle) at 24 h to their sulfated derivatives (open circle), which became predominant at 72 h (Fig. 3B). These findings strongly suggest that the carboxychromanols, but not their sulfated counterparts, are responsible for the observed inhibitory effect.
In addition, γ-CEHC has previously been reported to inhibit COX-2 in intact-cell assays (13, 21). Because no γ-CEHC or other short side-chain carboxychromanol (such as 5′- or 7′-carboxychromanol) were generated by A549 cells (17, 18), we reason that the long-chain carboxychromanols are responsible for the inhibitory effect.
13′-Carboxychromanol Strongly Inhibited Purified COX-1 and COX-2.
To directly examine the inhibitory potency of a specific long-chain carboxychromanol on COX activity, we isolated 9′-COOH and 13′-COOH from δ-T-conditioned media because of their relatively high abundance. In the intact-cell assays, isolated 9′-COOH and 13′-COOH inhibited COX-2 with an apparent IC50 of 6 or 4 μM, respectively (Table 1). Ibuprofen and acetaminophen also inhibited COX-2 activity with an IC50 of 5 and 200 μM, respectively (Table 1).
Table 1.
IC50s for inhibiting cyclooxygenases
| IC50 (μM) | COX-2 in A549 cells* | COX-1(ovine)† | COX-2(human)† |
|---|---|---|---|
| 9′-COOH | 6 ± 2 | >20‡ | >20‡ |
| 13′-COOH | 4 ± 2 | 5 ± 2 | 4 ± 2 |
| γ-CEHC (3′-COOH) | 35–70§ | 300 ± 50 | 450 ± 50 |
| α-CMBHC (5′-COOH) | n.d. | 160 ± 40 | 140 ± 40 |
| Ibuprofen | 5 ± 2 | 8 ± 2 | 5 ± 1 |
| Acetaminophen | 200 ± 50 | >250‡ | >250‡ |
The effect of purified 9′-COOH and 13′-COOH on COX activity was assayed in intact cells or using purified ovine COX-1 and human recombinant COX-2 (details described in Materials and Methods). Results were obtained based on two or three independent experiments and expressed as mean ± SD.
*The intact-cell assays were conducted in COX-2 preinduced A549 cells with 5 μM AA for 5 min.
†Assays were conducted with purified enzymes in the presence of 5 μM AA for 2 min.
‡9′-COOH and acetaminophen at 20 and 250 μM (the maximal concentrations tested), respectively, did not show any inhibitory effect on purified COX-1 or COX-2.
n.d., not determined.
Potential inhibition of COX-1 and COX-2 was further examined in purified-enzyme assays where PGF2α and PGE2 were the major end products. Under this condition, only strong COX inhibitors, such as ibuprofen, but not weak inhibitors, such as acetaminophen, are inhibitory (Table 1). 13′-COOH inhibited COX-1 and COX-2 activity with an apparent IC50 of 5 and 4 μM, respectively (Table 1). However, 9′-COOH at 20 μM did not inhibit either enzyme. We were not able to evaluate the effect of 9′-COOH at higher concentrations because of the limited amount of materials. As a comparison, γ-CEHC and α-CMBHC (5′-COOH) showed weak inhibition of COX-1 and COX-2 (Table 1). Consistent with the previous observations (13, 21), unmetabolized vitamin E forms did not inhibit purified COX-1 or COX-2 at 50 μM.
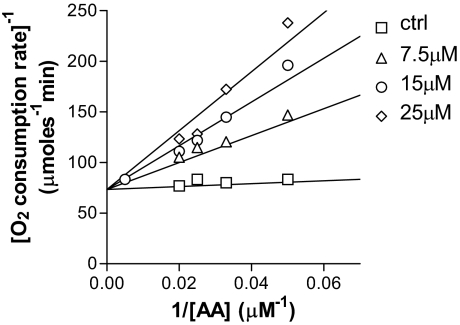
To elucidate the inhibitory mechanism, we investigated the effect of 13′-COOH on the cyclooxygenase and peroxidase activity. Enzyme kinetic studies indicated that 13′-COOH acted as a competitive inhibitor for the cyclooxygenase activity of COX-1 (Fig. 4) and COX-2 with Ki at 3.9 and 10.7 μM, respectively, whereas the Km of AA was 6 μM (COX-1) and 16 μM (COX-2). However, 13′-COOH had no significant impact on the peroxidase activity at concentrations of 30 μM (data not shown).
Fig. 4.
13′-COOH is a competitive inhibitor of the COX-1 cyclooxygenase activity. 13′-COOH (square, 0 μM; triangle, 7.5 μM; circle, 15 μM; diamond, 25 μM) and COX-1 were mixed in 0.1 M Tris (pH 8.0) with 5 mM EDTA, 2 mM phenol, and 1 μM hematin. Reactions were initiated by an injection of AA (6–200 μM). The initial rate of the oxygen consumption during the cyclooxygenase reaction was followed using an oxygen microelectrode. The Km and Vmax of the reactions in the absence and presence of 13′-COOH were calculated using nonlinear regression (GraphPad Prism 3.02). The competitive inhibition is shown by a Lineweaver-Burk plot.
Computer Simulations.
The enzyme kinetic data (Fig. 4) strongly suggest that 13′-COOH competes with arachidonic acid for the binding at the substrate-binding site of the enzyme. To further understand the differential inhibition observed between 9′- and 13′-carboxychromanol, we used computer simulations to analyze their binding to the AA-binding site of COX-1. Based on the procedure that combines docking and averaged scoring over 50 snapshots of a 500 ps MD simulation, we identified a unique energetically-lowest binding mode for 9′-, 13′-COOH, and AA. The identified configuration for AA is in agreement with the X-ray structure (PDB ID code 1DIY). The estimate of the relative free energies of binding among 13′-, 9′-carboxychromanol, and AA is consistent with the results of the enzyme assays (Table 1; Ki vs. Km): 13′-COOH is calculated to bind 0.69 ± 0.24 kcal/mol stronger than AA (99.9% confidence level), whereas 9′-COOH binds 2.72 ± 0.21 kcal/mol weaker (99.9%).
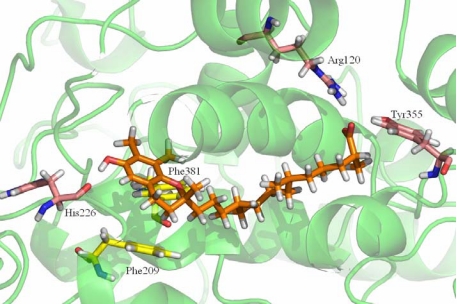
Fig. 5 reveals the detailed interaction between 13′-COOH and the key amino acids at the substrate binding site. Compared with 9′-COOH, the chroman moiety of 13′-carboxychromanol binds deeper into the hydrophobic binding pocket and its conformation is more favorably accommodated by aromatic residues (i.e., Phe-209 and Phe-381; Fig. 5, carbon atoms in yellow). Furthermore, the 6-OH group of 13′-carboxychromanol appears to be engaged in a hydrogen bond with the backbone carbonyl of His-226. This hydrogen bond is, however, not observed when 9′-COOH binds. Like AA, the carboxylic acid group of 13′-COOH appears to form hydrogen bonds with Tyr-355 and Arg-120.
Fig. 5.
Model of 13′-carboxychromanol binding to COX-1 as obtained by docking and MD simulations. Amino acids involved in hydrogen bonding (His-226, Tyr-355, and Arg-120) are represented by purple carbon atoms, and aromatic side chains (Phe-209 and Phe-381) accommodating the chroman moiety are represented by yellow carbon atoms.
Discussion
Cyclooxygenase-catalyzed generation of proinflammatory eicosanoids plays important roles in regulation of inflammatory responses and contributes to the development of chronic diseases such as cancer (5, 6). A major finding of the current study is that long-chain carboxychormanols, which are metabolized from vitamin E forms via ω- and β-oxidation of the hydrophobic side chain, are potent inhibitors of cyclooxygenases. However, the sulfated carboxychromanols, which can also be generated from vitamin E (17), appear to be ineffective. We show that although both 9′-COOH and 13′-COOH inhibit COX-2 activity in intact-cell assays, 13′-COOH is a much more potent inhibitor of COX-1 and COX-2. 13′-COOH acts as a competitive inhibitor for the cyclooxygenase activity but did not appear to inhibit the peroxidase activity. Compared with long-chain carboxychromanols, γ-CEHC, α-CMBHC, and vitamin E forms such as γ-T, δ-T, and γ-TE are weak inhibitors of COX-2.
The observation that 13′-COOH is a more potent inhibitor than vitamin E forms or shorter side-chain carboxychromanols, including 9′-COOH and 3′-COOH (γ-CEHC), indicates that the conversion of 13′-carbon to a carboxylic acid and the length of the side chain are important determining factors for COX inhibition by these chromanol analogs. Enzyme kinetic data showed that 13′-COOH is a competitive inhibitor of COX and therefore competes with AA for binding to the substrate-binding site. It is believed that the carboxylate group of AA forms ion pair or a hydrogen bond with conserved Arg-120 and Tyr-355 at the substrate binding site of COX (22, 23). The importance of these interactions is supported by the observation that site-direct mutagenesis of Arg-120 leads to an increase of the Km for AA binding (24) and renders the enzyme resistant to inhibition by carboxylic acid-containing NSAIDs (25). It is conceivable that the carboxylate group of carboxychromanols may interact similarly with Tyr-355 and the guanidinium group of Arg-120, whereas no such interaction can be formed with vitamin E. Computer simulations consistently indicate that 13′-COOH can form an extended L-shaped conformation to fit in the substrate binding pocket, and its carboxylate group interacts with Arg-120 and Tyr-355 (Fig. 5). Compared with 9′-COOH, the longer side chain linking carboxy and chroman group in 13′-COOH appears to facilitate a more favorable binding by interacting with more hydrophobic amino acids and by facilitating the interaction of the chromanol ring with Phe-209, Phe-381, and His-226 (Fig. 5). Similarly, the longer side chain of 9′-COOH may render a stronger interaction with the enzyme than γ-CEHC and therefore a more potent inhibitory effect. However, sulfated long-chain carboxychromanols may not interact favorably with the majority of hydrophobic amino acids at the AA binding site due to the strong polarity of the sulfate group.
The inhibitory action of 9′-COOH, γ-CEHC, and vitamin E showed discrepancy between cell-culture and enzyme-based assays. Thus, although γ-T, δ-T, and γ-TE reduced PGE2 formation in IL-1β-activated A549 cells, and 9′-COOH or γ-CEHC inhibited COX-2 activity in intact-cell assays, these compounds are much less or not inhibitory to the purified enzymes (Table 1). This selectivity between the cellular and enzyme studies resembles the scenario of weak COX inhibitors (e.g., acetaminophen and salicylate), which have been reported to inhibit COX activity at low micromolar concentrations in cultured cells but are largely ineffective in assays with purified enzymes (19, 26, 27). The observed selectivity has been attributed to the relatively low level of hydroperoxide, such as PGG2, generated in the cellular environments, in contrast with the relatively high and rapid PGG2 formation by purified enzymes (26, 27). We therefore reason that like acetaminophen and salicylate, γ-CEHC, γ-T, δ-T, and γ-TE are weak COX-2 inhibitors that inhibit COX-2 in some cellular environments when the level of hydroperoxide is relatively low. 9′-COOH is also less efficient in enzyme-based assays (Table 1), but its Ki needs to be further determined. These findings suggest that vitamin E forms and short-side chain carboxychromanols may exhibit varied anti-inflammatory effects (28), which are greatly affected by the level of peroxide generated, as well as the concentration of AA and COXs at the site of inflammation (26, 27). However, like ibuprofen, 13′-COOH showed consistent inhibitory action in cells and with purified enzymes, and is therefore likely to be a strong anti-inflammatory agent.
One important implication of our current findings is that the differential bioactivities among vitamin E forms such as α-T and γ-T may, in part, be rooted in their distinct metabolism. Specifically, long-chain carboxychromanols may contribute to in vivo anti-inflammatory effect of γ-T (28). However, compared with γ-T, carboxychromanols generated from α-T are likely to be quantitatively much less important because α-T is protected by α-tocopherol transfer protein (12, 16) and is degraded by tocopherol-ω-hydroxylase to a much less extent (29). To this end, we and others have shown that γ-T inhibits proinflammatory eicosanoids at the inflammatory site and attenuates inflammation-caused damage in various animal models (14, 30–33). Himmelfarb et al. (34) reported that γ-T-enriched, but not α-T-enriched, mixed tocopherols inhibit proinflammatory C-reactive protein and IL-6 in kidney dialysis patients. We recently showed that 13′-COOH is detected in the plasma and liver in rats supplemented with γ-T (17). Our unpublished data revealed that high amounts of 13′-COOH (50–90 nmoles/g) are found in rat feces after γ-T supplementation (Q.J. and H.F., unpublished observation). This suggests that 13′-COOH, a potent inhibitor of cyclooxygenases and potentially accumulated in colon tissues, may contribute to the impressive anticancer effect of mixed tocopherols enriched with γ-T and δ-T on aberrant crypt foci in azoxymethane-induced colon cancer in rodents (35).
Long-chain carboxychromanols and their analogs may be novel and useful anti-inflammatory agents. We have previously shown that γ-CEHC inhibited proinflammatory PGE2 at the site of inflammation in an air-pouch inflammation model in the rat (14). In the same animal model, α-CMBHC also reduced PGE2 by 34% and exudate volume, an inflammation index, by 23% (Q.C. and M. Moreland, unpublished data). Because 13′-COOH is much more potent than γ-CEHC and α-CMBHC in the inhibition of cyclooxygneases, it is conceivable that 13′-COOH may show much stronger anti-inflammatory effect in vivo than these short side-chain analogs. The current study warrants further investigation of in vivo anti-inflammatory activity of long-chain carboxychromanols and their potential use as anticancer agents.
Materials and Methods
Materials.
α-T (99%), γ-T (97%–99%), and δ-T (97%) were purchased from Sigma. γ-CEHC, α-CMBHC (5′-COOH analog, 5-(6-hydroxy-2,5,7,8-tetramethyl-chroman-2-yl)-pentanoic acid, COX-2 antibody, human recombinant or ovine COX-2, and ovine COX-1 were from Cayman Chemicals. α-TE and γ-TE were gifts from BASF. Tissue culture reagents were from Invitrogen. Human recombinant IL-1β, sesamin, ketoconazole, and all other chemicals were from Sigma.
PGE2 Generation by IL-1β Stimulated A549 Cells.
Human lung A549 cells were obtained from American Type Culture Collection and routinely cultured in RPMI-1640 with 10% FBS. Cells (2.5–3 × 105 per well) were seeded and allowed to attach overnight in a 24-well plate. Vitamin E stock solutions were initially made in DMSO and then diluted in 10 mg/ml of fatty acid-free BSA. Confluent cells were incubated in DMEM containing 1% FBS with 0.05% DMSO (control) or vitamin E forms for 14–16 h, and then 2 ng/ml of IL-1β was added for 24 h. PGE2 accumulation in the media was measured by an ELISA from Cayman Chemicals.
COX-2 Activity in the Intact-Cell Assays.
The effect on COX-2 activity was examined in the intact-cell assays as previously described (19). Briefly, COX-2 was preinduced in A549 cells by 0.5 ng/ml of IL-1β for 6 h. Cells were then incubated with fresh medium containing tested compounds for 30 min. The enzyme reaction was initiated by addition of 5 μM AA for 5 min. PGE2 accumulated in the media was measured as an index of COX-2 activity using ELISA.
COX-1 and COX-2 Activity Assay Using Purified Enzymes.
The enzymatic reactions were performed in 0.1 M Tris (pH 8.0) in the presence of 5 mM EDTA, 2 mM phenol, and 1 μM hematin (19). Tested compounds were first incubated with COX-1 or COX-2 at room temperature for 10 min. Enzymatic reactions were initiated by addition of AA at a final concentration of 5 μM for 2 min and stopped by addition of 0.1 M HCl. Stannous chloride in 0.1 M HCl was added to reduce PGG2 and PGH2 to PGF2α. Prostaglandins were extracted by 2.5 vol of ethyl acetate, and the organic layer was dried under N2. PGF2α and PGE2 were quantified using ELISA assays.
Cyclooxygenase and Peroxidase Activity.
The cyclooxygenase activity was measured by monitoring the initial rate of oxygen consumption (nanomoles per minute) in a MT200 glass chamber using a model 1302 oxygen electrode (Warner Instruments, LCC). The standard assay solution contained 0.1 M Tris (pH 8.0), 5 mM EDTA, 2 mM phenol, and 1 μM hematin. Tested compounds were first incubated with ovine COX-1 or COX-2 for 10 min, and the reaction was initiated by an injection of AA (6–200 μM). The peroxidase activity was determined by following N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) oxidation by hydrogen peroxide as previously described (36).
Preparation of Conditioned Media and Quantitation of Vitamin E Metabolites.
Metabolite-containing conditioned media were prepared by incubation of vitamin E forms with confluent A549 cells in DMEM with 1% FBS for 24–72 h. In some experiments, media from incubation of vitamin E in cell-free dishes or coincubation of vitamin E with sesamin were prepared. Conditional media were collected and stored in −20 °C before use. Long-chain carboxychromanols and their sulfated counterparts in culture media were extracted and quantitated by an HPLC assay with fluorescent detection as previously described (17).
Isolation of 9′-COOH and 13′-COOH.
9′- and 13′-carboxychromanol were purified from conditioned media of δ-T using a Supelcosil LC-18-DB Semiprep column (Supelco) by HPLC as previously described (17). The isolated fractions were freeze dried, quantified using HPLC, and stored in −80 °C until use.
Western Blot.
Cells were lysed in Tris-EDTA, 1% SDS, and 1 mM DTT with protease inhibitor cocktails (Sigma), and the resulting solution was heated at 95 °C for 5 min. Proteins (10–25 μg) were loaded on 10% precast SDS-PAGE gels. Resolved proteins were transferred onto a PVDF membrane (Millipore) and probed by antibodies. Membranes were exposed to chemiluminescent reagent (NEN, Life Science Products) and visualized on Kodak film.
Statistical Analysis.
Unpaired student's t tests were used in the statistical analysis.
Molecular Modeling.
13′-carboxychromanol, 9′-carboxychromanol, and AA were docked into the binding pocket of COX-1 (PDB ID code 1IGZ) using AutoDock 4 (37). Molecular dynamic (MD) simulations were run for all docking complexes to investigate the stability of the proposed docking configuration and to reliably predict the relative binding affinities of the compounds. On average, the resulting protein structures differ by 1.5 Å rmsd from the original X-ray structure (PDB ID code 1DIY). Based on the final 500 ps interval of the MD simulation, molecular mechanics/Poisson-Boltzmann surface area (MM/PBSA) method (38) and linear interaction energy (LIE) analysis (39) were applied. Both methods failed (for details, see SI) to reproduce the X-ray structure of COX-1 bound AA as the energetically lowest configuration. In a recent study, Anderson and coworkers (40) showed reliable correlations between calculated and experimental biological activity when using a scoring function averaged over an ensemble of docking complexes. Similar to this concept, 50 snapshots were generated from the final section of the MD simulation, and the average binding affinity between ligand and COX-1 was computed using the ChemScore scoring function (41). More detailed description of the computer simulations is provided in SI.
Supplementary Material
Acknowledgments.
This study was supported by a grant from the National Institutes of Health (R01AT001821) and was also partially supported by NIH Grant P01AT002620.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810962106/DCSupplemental.
References
- 1.Dubois RN, et al. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 2.Rouzer CA, Marnett LJ. Mechanism of free radical oxygenation of polyunsaturated fatty acids by cyclooxygenases. Chem Rev. 2003;103:2239–2304. doi: 10.1021/cr000068x. [DOI] [PubMed] [Google Scholar]
- 3.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 4.Vane JR, Botting RM. Mechanism of action of antiinflammatory drugs. Int J Tissue React. 1998;20:3–15. [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 7.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKellar G, Madhok R, Singh G. The problem with NSAIDs: What data to believe? Curr Pain Headache Rep. 2007;11:423–427. doi: 10.1007/s11916-007-0228-y. [DOI] [PubMed] [Google Scholar]
- 9.Lee IM, Gaziano JM, Buring JE. Vitamin E in the prevention of prostate cancer: Where are we today? J Natl Cancer Inst. 2006;98:225–227. doi: 10.1093/jnci/djj066. [DOI] [PubMed] [Google Scholar]
- 10.Singh U, Devaraj S, Jialal I. Vitamin E, oxidative stress, and inflammation. Annu Rev Nutr. 2005;25:151–174. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 11.Upston JM, Kritharides L, Stocker R. The role of vitamin E in atherosclerosis. Prog Lipid Res. 2003;42:405–422. doi: 10.1016/s0163-7827(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Q, Christen S, Shigenaga MK, Ames BN. γ-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. γ-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. γ-tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci USA. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brigelius-Flohe R, Traber MG. Vitamin E: Function and metabolism. FASEB J. 1999;13:1145–1155. [PubMed] [Google Scholar]
- 17.Jiang Q, Freiser H, Wood KV, Yin X. Identification and quantitation of novel vitamin E metabolites, sulfated long-chain carboxychromanols, in human A549 cells and in rats. J Lipid Res. 2007;48:1221–1230. doi: 10.1194/jlr.D700001-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.You CS, Sontag TJ, Swanson JE, Parker RS. Long-chain carboxychromanols are the major metabolites of tocopherols and tocotrienols in A549 lung epithelial cells but not HepG2 cells. J Nutr. 2005;135:227–232. doi: 10.1093/jn/135.2.227. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell JA, Saunders M, Barnes PJ, Newton R, Belvisi MG. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: Role of arachidonic acid. Mol Pharmacol. 1997;51:907–912. doi: 10.1124/mol.51.6.907. [DOI] [PubMed] [Google Scholar]
- 20.Parker RS, Sontag TJ, Swanson JE. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem Biophys Res Commun. 2000;277:531–534. doi: 10.1006/bbrc.2000.3706. [DOI] [PubMed] [Google Scholar]
- 21.Grammas P, et al. Anti-inflammatory effects of tocopherol metabolites. Biochem Biophys Res Commun. 2004;319:1047–1052. doi: 10.1016/j.bbrc.2004.05.082. [DOI] [PubMed] [Google Scholar]
- 22.Kurumbail RG, Kiefer JR, Marnett LJ. Cyclooxygenase enzymes: Catalysis and inhibition. Curr Opin Struct Biol. 2001;11:752–760. doi: 10.1016/s0959-440x(01)00277-9. [DOI] [PubMed] [Google Scholar]
- 23.Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: Discovery, selectivity and the future. Trends Pharmacol Sci. 1999;20:465–469. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharyya DK, Lecomte M, Rieke CJ, Garavito M, Smith WL. Involvement of arginine 120, glutamate 524, and tyrosine 355 in the binding of arachidonate and 2-phenylpropionic acid inhibitors to the cyclooxygenase active site of ovine prostaglandin endoperoxide H synthase-1. J Biol Chem. 1996;271:2179–2184. doi: 10.1074/jbc.271.4.2179. [DOI] [PubMed] [Google Scholar]
- 25.Mancini JA, Riendeau D, Falgueyret JP, Vickers PJ, O'Neill GP. Arginine 120 of prostaglandin G/H synthase-1 is required for the inhibition by nonsteroidal anti-inflammatory drugs containing a carboxylic acid moiety. J Biol Chem. 1995;270:29372–29377. doi: 10.1074/jbc.270.49.29372. [DOI] [PubMed] [Google Scholar]
- 26.Aronoff DM, Boutaud O, Marnett LJ, Oates JA. Inhibition of prostaglandin H2 synthases by salicylate is dependent on the oxidative state of the enzymes. J Pharmacol Exp Ther. 2003;304:589–595. doi: 10.1124/jpet.102.042853. [DOI] [PubMed] [Google Scholar]
- 27.Boutaud O, Aronoff DM, Richardson JH, Marnett LJ, Oates JA. Determinants of the cellular specificity of acetaminophen as an inhibitor of prostaglandin H(2) synthases. Proc Natl Acad Sci USA. 2002;99:7130–7135. doi: 10.1073/pnas.102588199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sontag TJ, Parker RS. Influence of major structural features of tocopherols and tocotrienols on their omega-oxidation by tocopherol-omega-hydroxylase. J Lipid Res. 2007;48:1090–1098. doi: 10.1194/jlr.M600514-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Devaraj S, Leonard S, Traber MG, Jialal I. Gamma-tocopherol supplementation alone and in combination with alpha-tocopherol alters biomarkers of oxidative stress and inflammation in subjects with metabolic syndrome. Free Radic Biol Med. 2008;44:1203–1208. doi: 10.1016/j.freeradbiomed.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Q, et al. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, et al. Gamma-tocopherol, but not alpha-tocopherol, potently inhibits neointimal formation induced by vascular injury in insulin resistant rats. J Mol Cell Cardiol. 2006;41:544–554. doi: 10.1016/j.yjmcc.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JG, et al. Ozone enhancement of lower airway allergic inflammation is prevented by gamma-tocopherol. Free Radic Biol Med. 2008;43:1176–1188. doi: 10.1016/j.freeradbiomed.2007.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Himmelfarb J, et al. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978–991. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 35.Newmark HL, Huang MT, Reddy BS. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutr Cancer. 2006;56:82–85. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 36.van der Ouderaa FJ, Buytenhek M. Purification of PGH synthase from sheep vesicular glands. Methods Enzymol. 1982;86:60–68. doi: 10.1016/0076-6879(82)86169-7. [DOI] [PubMed] [Google Scholar]
- 37.Morris GM, et al. Automated docking using a lamarckian genetic algorithm and empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 38.Kollman PA, et al. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc Chem Res. 2000;33:889–897. doi: 10.1021/ar000033j. [DOI] [PubMed] [Google Scholar]
- 39.Aqvist J. Calculation of absolute binding free energies for charged ligands and effects of long-range electrostatic interactions. J Comput Chem. 1996;17:1587–1597. [Google Scholar]
- 40.Popov VM, Yee WA, Anderson AC. Towards in silico lead optimization: Scores from ensembles of protein/ligand conformations reliably correlate with biological activity. Proteins. 2007;66:375–387. doi: 10.1002/prot.21201. [DOI] [PubMed] [Google Scholar]
- 41.Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP. Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J Comp Aided Molec. 1997;11:425–445. doi: 10.1023/a:1007996124545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.