Abstract
Wounding of epidermal layers triggers multiple coordinated responses to damage. We show here that the Caenorhabditis elegans ortholog of the tumor suppressor death-associated protein kinase, dapk-1, acts as a previously undescribed negative regulator of barrier repair and innate immune responses to wounding. Loss of DAPK-1 function results in constitutive formation of scar-like structures in the cuticle, and up-regulation of innate immune responses to damage. Overexpression of DAPK-1 represses innate immune responses to needle wounding. Up-regulation of innate immune responses in dapk-1 requires the TIR-1/p38 signal transduction pathway; loss of function in this pathway synergizes with dapk-1 to drastically reduce adult lifespan. Our results reveal a previously undescribed function for the DAPK tumor suppressor family in regulation of epithelial damage responses.
Keywords: antimicrobial peptide, epidermis, innate immunity, wound repair, cuticle
The epidermis forms an outer protective barrier against environmental damage and pathogens for most animals. Epidermal responses to physical wounding have been extensively characterized in vertebrates and insects (1–3). Repair of a barrier epithelium such as the skin involves several coordinated processes: choreographed movement of cells at the wound edge, leading to reepithelialization (4); scab formation followed by synthesis of new external extracellular matrix (5, 6); and activation of local cutaneous innate immune defenses such as the expression of antimicrobial peptides (AMPs) that can defend against opportunistic infection at the wound site (7–9).
Despite the differing structures of epidermal layers in different animals, recent molecular genetic studies suggest that epidermal wound healing pathways are evolutionarily conserved. Activation of JNK and AP-1 transcription factors appears central to promoting cell motility at the leading edge of an epidermal wound (10). In both insects and vertebrates, transcription factors of the Grainyhead family are activated in response to wound signals via an ERK kinase pathway and promote transcription of extracellular matrix cross-linking enzymes (11, 12). Less is currently known about the pathways that induce innate immune responses to sterile wounding; in human skin, the EGFR pathway has been implicated in local activation of AMPs (13). As well as acting as antibiotics at the wound site, some AMPs may directly promote reepithelialization (14), linking these 2 arms of the wound response.
Like other immune responses, cutaneous responses to damage must be tightly regulated to prevent chronic responses to transient stimuli (15). Negative regulatory pathways prevent innate immune responses to infection or wounds from developing into pathological reactions (15); defects in such negative regulation can underlie chronic skin inflammatory diseases such as atopic dermatitis (16). Loss of barrier repair functions can also result in chronic inflammation (e.g., loss of AP-1 function blocks reepithelialization; see ref. 17); and has been implicated in psoriasis (18), suggesting an intimate link between wound repair and regulation of innate immunity.
The Caenorhabditis elegans epidermis allows wound repair processes to be studied in the context of a simple epithelium that secretes an external collagenous cuticle. As in other animals, the nematode skin is likely to have an active role in preventing organismal damage from physical or biological challenges. We recently showed that laser or puncture wounding of C. elegans activates epidermal innate responses via the Toll-interleukin 1 receptor (TIR) domain protein TIR-1 and a p38 MAPK cascade (19). Here, we identify a new negative regulator of epidermal damage responses, the C. elegans ortholog of the tumor suppressor death-associated protein kinase, dapk-1. Loss of DAPK-1 function results in constitutive formation of scar-like structures in the cuticle, and up-regulation of antimicrobial gene expression. Transient overexpression of DAPK-1 represses the transcriptional response to puncture wounding. We show that up-regulation of innate immune responses, but not barrier repair, in dapk-1 mutants requires the TIR-1/p38 MAPK pathway, and that dapk-1 mutants depend on this innate immune pathway for adult survival. Our results reveal a previously undescribed role for the DAPK tumor suppressor in negative regulation of epithelial damage responses.
Results
In genetic screens for mutants displaying progressive defects in epidermal morphogenesis, we identified multiple alleles of dapk-1, which encodes the C. elegans member of the calcium-calmodulin activated DAPK family [supporting information (SI) Fig. S1]; dapk-1 mutations form an allelic series (Table S1), in which the strongest allele, ju4, results in a missense alteration (S179L) in the peptide-binding ledge of the DAPK-1 kinase domain. RNA interference phenocopied these dapk-1 epidermal phenotypes (data not shown), indicating that these mutations result in loss of DAPK-1 function.
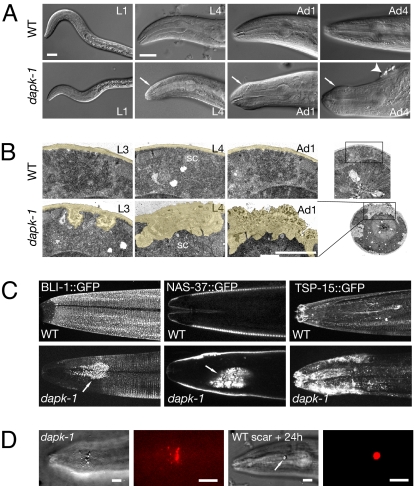
dapk-1 mutants appeared morphologically normal until mid larval development. Beginning in the L3 stage, dapk-1(ju4) mutants displayed striking and progressive defects in morphology of the epidermis and cuticle in specific body regions, especially in the nose, tail, vulva, and the dorsal midline in the region of the posterior pharyngeal bulb (Fig. 1A). Cuticle in these regions became up to 5–10 times thicker than the wild type, at the expense of underlying epidermis; this thickened cuticle appeared refractile under differential interference contrast (DIC) microscopy, and had aberrant ultrastructure with inclusions of electron-dense material (Fig. 1B). By using transgenic markers, we found that these regions of thickened cuticle accumulated collagens and other cuticle components (Fig. 1C). The thickened cuticle of dapk-1 mutants also accumulated proteins such as TSP-15, a component of the epithelial apical membrane, suggesting a breakdown of epithelial-cuticle integrity. Other epidermal compartments such as subapical adherens junctions appeared normal (data not shown), suggesting dapk-1 mutants have specific defects in synthesis or accumulation of apically secreted proteins. The C. elegans cuticle is not normally autofluorescent; in contrast, the regions of cuticle thickening in dapk-1 contained autofluorescent aggregates (Fig. 1D).
Fig. 1.
Progressive hypertrophy of cuticle structure resembling the epidermal wound response displayed by dapk-1 mutants. (A) Nomarski DIC micrographs showing single wild-type and dapk-1(ju4) mutant animals from L1 to adult. The dapk-1 morphology defect (white arrow) becomes progressively more severe as the animals age; ≈30% of dapk-1 mutant adults also develop refractile encrustations or blisters at the dorsal midline (arrowhead); cuticle abnormalities also occur in the tail and vulval regions. (B) Electron micrographs of sections of L3, L4, and adult lateral epidermis and cuticle (head region, level of anterior pharyngeal bulb) in wild type and dapk-1(ju4). The cuticle is colored yellow; sc, seam cell. Note expansion of cuticle layer in dapk-1. (C) dapk-1 adults locally accumulate cuticle collagen markers BLI-1::GFP, a component of the strut layer, and COL-19::GFP (data not shown); other cuticle components (NAS-37::GFP) and apical epidermal membrane proteins (TSP-15::GFP) also accumulate in the head region. Images are confocal projections or sections (NAS-37::GFP) of lateral head. (D) Autofluorescence of cuticle in unwounded dapk-1 and cuticle scar in wild type 24 h after needle wounding; Nomarski DIC and Rhodamine filters. (Scale bars, 5 μm.)
The areas of thickened cuticle and autofluorescent aggregates in dapk-1 mutants resemble the scars caused by needle or laser wounding of the C. elegans epidermis (Fig. 1D) (19). These similarities, and the progressive nature of the dapk-1 epidermal defects, suggested that dapk-1 mutants might have weakened epidermal layers that undergo breakage and scarring in response to mechanical stress caused by movement. However, inhibition of movement using the unc-54 mutation (Table S2) or by levamisole (data not shown) did not suppress the epidermal defects of dapk-1 mutants. Also, other mutants known to have fragile epidermal layers (itermediate filaments/ifb-1, plectin/vab-10; see refs. 20 and 21) do not display scar-like structures (data not shown). These findings suggest that the scar-like structures of dapk-1 mutants are not a secondary result of a fragile epidermis. The scar-like areas appear to be structurally weak, because they occasionally rupture in dapk-1 adults and in assays of cuticle fragility. Because DAPK has been implicated in regulation of endocytosis (22) and can phosphorylate syntaxin (23), we tested whether mutation or RNA interference of genes involved in cuticle secretion or endocytosis could enhance or suppress a weak dapk-1 phenotype. Double mutants with the cuticle secretion gene che-14 (24) showed additive interactions with dapk-1 (Fig. S2A). Inhibition of genes involved in cuticle secretion (e.g., sec-23; see ref. 25) or endocytosis neither enhanced nor suppressed dapk-1 defects (Fig. S2B). These results suggest that the cuticle hypertrophy in dapk-1 mutants is not due to a primary defect in cuticle secretion or epidermal endocytosis.
DAPK regulates apoptosis and autophagy in mammalian cells (26), and C. elegans dapk-1 mutants have defects in apoptotic cell death (R.-H. Chen, J.-Y. Chen, and Y.-C.W., unpublished work) and autophagy (27). If the epidermal defects of dapk-1 mutants reflected defective apoptosis or autophagy, other mutants defective in these processes should show similar phenotypes. However, mutations that eliminate apoptosis (e.g., ced-3) or autophagy (bec-1) neither phenocopied nor suppressed the epidermal defects of dapk-1 mutants, suggesting these defects are not caused by misregulation of cell death or autophagy (Fig. S3; Table S2).
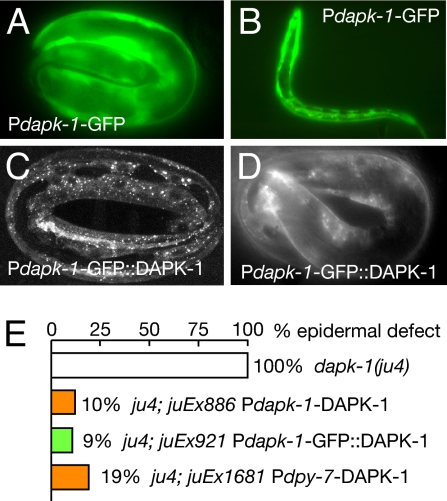
dapk-1 transcriptional reporters were expressed in multiple tissues, including the epidermis, from late embryogenesis onwards (Fig. 2 A and B). To determine whether DAPK-1 acts cell autonomously in epidermal development, we expressed DAPK-1 specifically in epidermal cells by using the promoter of the cuticle collagen dpy-7 (28), and found that such transgenes rescued dapk-1 epidermal defects (Fig. 2E and Table S3). Within epidermal cells, functional GFP::DAPK-1 localized to cytoplasmic puncta of unknown identity (Fig. 2 C and D). These results suggest that DAPK-1 acts independently of cell death pathways and autonomously in the epidermis.
Fig. 2.
DAPK-1 is expressed and functions within the epidermis to control epidermal development. (A and B) dapk-1 transcriptional reporters (2.7-kb promoter) were expressed in epidermal cells from late embryonic (3-fold) stage onwards, as well as in muscles and neurons (data not shown). (C and D) Functional GFP::DAPK-1 localizes to cytoplasmic puncta in embryonic epidermis; images of juEx921 (C) and rescued ju4; juEx921 (D). (E) Quantitation of transgenic rescue of dapk-1(ju4) epidermal morphology phenotypes; equivalent rescue was observed for dapk-1(gk219) (Table S3).
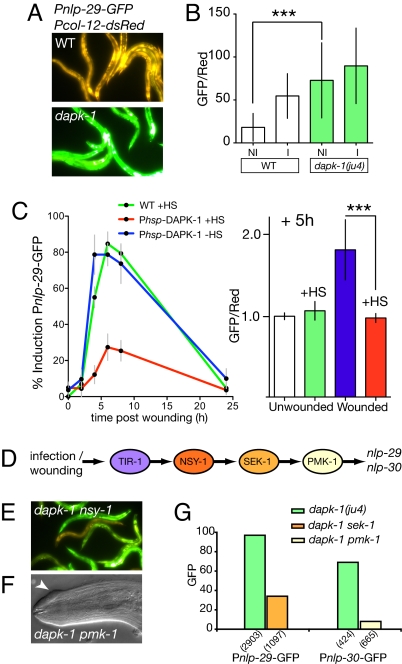
The similarities between the dapk-1 phenotype and the effects of wounding led us to test whether loss of dapk-1 also affected epidermal innate immune responses to wounding. We found that dapk-1 mutants constitutively up-regulated transgenic reporters for several epidermal AMP genes, compared with unwounded controls (Fig. 3 A and B, and Fig. S4A). These AMP genes are induced by infection by the epidermis-penetrating fungus Drechmeria coniospora and by sterile wounding (19, 29). By using oligonucleotide microarrays, we found that transcripts for multiple epidermal AMPs were up-regulated in dapk-1 relative to the wild type (Fig. S4C; Table S4). The dapk-1 mutant transcriptional profile overlapped strikingly with that of animals infected with Drechmeria (30) (19/303 genes in common with the top 419 Drechmeria-up-regulated genes; see Fig. S4C and Table S4), but not with those of animals infected with bacterial pathogens (0/303 were up-regulated in 2 or more bacterial infections) (31). Some epidermal AMPs, including nlp-29 but not nlp-30, are also induced by osmotic stress and are constitutively up-regulated in osmotic stress response mutants such as dpy-9 (30). However, dapk-1 mutants did not inappropriately induce other transcriptional responses to osmotic stress such as gpdh-1 (Fig. S4B) (32). This finding suggests that the constitutive induction of AMP expression in dapk-1 mutants is not due to a defect in regulation of epidermal osmotic stress response. Epidermal specific expression of DAPK-1 in dapk-1(ju4) mutants was sufficient to restore Pnlp-29-GFP expression to normal levels (data not shown), indicating dapk-1 acts autonomously in the epidermis to repress innate immune responses. To address whether nlp-29 up-regulation was a direct or indirect consequence of the loss of DAPK-1 function, we tested whether transient overexpression of DAPK-1 could suppress innate immune responses to needle wounding. We found that heat shock-induced overexpression of DAPK-1 immediately after wounding strongly suppressed induction of Pnlp-29-GFP (Fig. 3C), suggesting DAPK-1 can acutely repress innate immune responses to damage.
Fig. 3.
DAPK-1 negatively regulates epidermal innate immune responses via the TIR-1/p38 MAPK cascade and independently of epidermal integrity. (A) Photographs of frIs7 Pnlp-29-GFP/Pcol-12-dsRed in young adult wild type and dapk-1(ju4). (B) Biosort quantitation of frIs7 GFP/dsRed fluorescence ratios in L4 and adult wild-type and dapk-1(ju4), uninfected (NI), and Drechmeria infected (I); mean ± SD; n > 100, for each condition; ju4 is significantly different from wild type (P < 0.001, Mann–Whitney test). (C) Pnlp-29-GFP (frIs7) induction by needle wounding is suppressed by heat shock induced expression of DAPK-1. frIs7; Phsp-16-DAPK-1(juEx1933) animals raised at 20 °C were wounded at 0 h and then transferred to 35 °C for 2 h. Mean and SEM for 3 experimental groups (n > 9, for each group) are shown. By using worm sorting at 5 h post wounding, we found significantly reduced Pnlp-29-GFP induction after heat shock (P < 0.0001, Mann–Whitney test); GFP/Red ratio is normalized to unwounded non-heat-shocked frIs7; juEx1933 (n > 18, per condition). (D) The p38 innate immune response pathway in C. elegans epidermis (19). (E) nlp-29 induction in dapk-1 mutants depends on the TIR-1/MAPK pathway; dapk-1 nsy-1 double mutants show reduced nlp-29-GFP expression, compared with dapk-1. (F) pmk-1 does not suppress dapk-1 morphogenetic defects (arrow; Table S2). (G) Biosort quantitation of Pnlp-29-GFP in dapk-1 sek-1 and Pnlp-30-GFP in dapk-1 pmk-1 shows suppression, compared with dapk-1 single mutants; frIs7 expression in the double mutants is higher than that of mapk single mutants (data not shown), suggesting that dapk-1 mutants may also activate AMP transcription in parallel to the TIR-1/MAPK cascade.
Fungal infection and sterile wounding stimulate transcription of nlp-29 and nlp-30 via the TIR domain adaptor protein TIR-1, which activates the NSY-1/SEK-1/PMK-1 p38 MAPK cascade in the epidermis (Fig. 3D) (19, 29). We tested whether the constitutive activation of AMP transcription in dapk-1 also depended on the TIR-1/PMK-1 cascade. AMP transcription was significantly reduced in dapk-1 double mutants with tir-1 or with mutations in genes of the MAPK cascade (collectively mapk; see Fig. 3 E and G); tir-1 or mapk mutations did not suppress the morphological and cuticle fragility phenotypes of dapk-1 mutants (Fig. 3F and Fig. S5C). To test whether AMP up-regulation in dapk-1 mutants was caused by cuticle fragility of the scar-like areas, we took advantage of an extragenic suppressor of dapk-1, found in an unrelated screen. The sydn-1(ju541) mutation affects neuronal development (Y. Dai and Y. Jin, personal communication), and was fortuitously found to suppress dapk-1(ju4) epidermal and cuticle fragility phenotypes (Fig. S5 A and C). However, such genetic suppression of dapk-1 morphological defects did not block Pnlp-29-GFP up-regulation (Fig. S5B), suggesting the activation of innate immune responses in dapk-1 mutants is not a consequence of morphological defects.
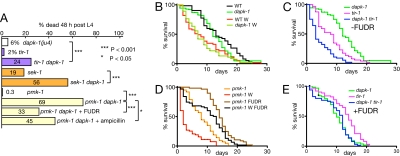
In the course of this analysis, we found that dapk-1 double mutants with tir-1 or other components of the PMK-1 MAPK pathway frequently died within early adulthood (Fig. 4A). Although dapk-1 single mutants had normal lifespan (Fig. 4B), dapk-1 tir-1 double mutants displayed synergistic reduction in lifespan (Fig. 4C). We hypothesized that a defective innate immune response might be particularly deleterious to dapk-1 mutants, as such animals would be more vulnerable to bacterial infection via the skin due to their epidermal defects. To test this model, we examined the effects of epidermal wounding on lifespan. Under our conditions, needle wounding of the wild type or of dapk-1 resulted in an ≈1.8-fold reduction of median lifespan (13 to 7 days; see Fig. 4B), whereas wounding of immunocompromised animals caused a 4-fold reduction of lifespan (8 to 2 days for tir-1 and pmk-1; see Fig. 4 C and D). This effect of wounding on immunocompromised strains was suppressed by inhibiting bacterial proliferation by using the DNA synthesis inhibitor 5-fluoro-2-deoxy uridine (FUDR) (Fig. 4D). This result suggests that wounding can reduce lifespan in 2 ways: physical damage, and (in immunocompromised strains) opportunistic infection via the wound site by Escherichia coli OP50 or other environmental microorganisms. E. coli OP50 is pathogenic to older C. elegans (33, 34), and can be pathogenic if introduced across the epidermis by injection (35); dapk-1 mutants appear to have normal wound responses; however, their epidermal defects render them susceptible to infection and, thus, dependent on the epidermal innate immune pathway for adult viability. In support of this hypothesis, growth on FUDR suppressed the synergistic reduction in lifespan of dapk-1 tir-1 double mutants (Fig. 4E).
Fig. 4.
Synergistic premature adult lethality of dapk-1 with immunocompromised mutants. (A) dapk-1 double mutants with tir-1 or mapk mutants die within 48 h of L4 stage (n > 200, for each genotype); dapk-1; pmk-1 premature lethality is partly suppressed by growth on ampicillin, carbenicillin (data not shown), or FUDR (n > 50, for each). Comparisons use Fisher exact test. (B) dapk-1 lifespan is not significantly different from the wild type (median 12 days unwounded, 7 days wounded). (C) Synergism of dapk-1 and tir-1: dapk-1; tir-1 (median 3 days) is significantly different from tir-1 (median 8 days) (P < 0.001). (D) The lifespan of pmk-1 is reduced 4-fold by wounding (P < 0.001); wounding on FUDR reduces pmk-1 lifespan by only 1.25-fold (P = 0.06 between pmk-1 wounded and unwounded on FUDR). (E) The synergistic reduction in lifespan of dapk-1; tir-1 double mutants is suppressed by FUDR; lifespan of dapk-1 tir-1 FUDR+ is not different from dapk-1 alone (P = 0.9).
Discussion
Loss of function in the C. elegans DAP kinase ortholog DAPK-1 results in an unusual progressive epidermal defect that appears to reflect constitutive activation of cutaneous responses to damage. Several lines of evidence suggest that dapk-1 mutants are not merely defective in epidermal integrity or cuticle secretion. First, other epidermal fragility mutants do not resemble dapk-1 in phenotype, and RNAi of cuticle secretion or endocytosis genes failed to enhance or suppress dapk-1 defects. Suppression of movement fails to suppress dapk-1 phenotypes, whereas genetic suppression of dapk-1 morphological and cuticle fragility defects by the sydn-1 mutation does not abrogate its up-regulation of innate immune pathways. Last, transient overexpression of DAPK-1 blocked a transcriptional response to wounding. These findings suggest that DAPK-1 directly represses epidermal responses to damage.
DAPK was initially identified in functional screens for genes acting in IFN-mediated cell death (36). Numerous studies have placed DAPK in a network of pathways regulating apoptosis and autophagy (26). Unexpectedly, although dapk-1 mutants display defects in autophagy (27) and apoptosis (R.-H. Chen, J.-Y. Chen, and Y.-C.W., unpublished work), we find no evidence that these processes are involved in the epidermal function of DAPK. These observations raise the possibility that DAPK family members have additional roles beyond their functions in cell death. Indeed, DAPK has recently been shown to function as a negative regulator of T cell activation via NF-κB (37) and as a negative regulator of inflammatory gene expression in monocytes (38), suggesting a conserved role for DAPK as a negative regulator of various immune responses.
Although the upstream triggers of damage responses in C. elegans epidermis are not known, calcium signals often have this role (39). As a member of the calcium-calmodulin regulated kinase family, DAPK-1 could be directly activated by such signals; if the action of DAPK-1 is to limit wound healing responses, its activity must presumably be delayed relative to other pathways that promote acute responses to epidermal damage. We speculate that an initial calcium transient caused by wounding or infection activates the TIR-1/MAPK cascade via as yet unknown signaling pathways. Because the increased nlp-29 expression of dapk-1 mutants depends on TIR-1, DAPK-1 likely inhibits some step upstream of TIR-1 in this cascade. Last, the identification of a role for a well-known tumor suppressor in epidermal damage responses is highly intriguing, given the long-noted similarities between wound healing and tumorigenesis (40). Further analysis of the mechanism of DAPK function in C. elegans could shed light on both these processes.
Materials and Methods
Genetics and Phenotypic Quantitation.
The wild-type strain used is Bristol N2 (41). All strains were maintained at 25 °C on NGM agar plates under standard conditions, unless stated. The following mutations were used: atgr-18(gk378), bec-1(ok700), ced-2(e1752), ced-3(n717), che-14(e1960), nsy-1(ky397), pmk-1(km25), sek-1(km4), unc-51(e369), unc-54(e190), sydn-1(ju541), and tir-1(tm3036) (19). We used the following transgenes: cgEx198 [BLI-1::GFP] (J. Crew and J. Kramer, personal communication), kaIs12 [COL-19::GFP] (42), oxEx587 [NAS-37::GFP] (43), imEx1 [TSP-15::GFP] (44), kbIs5 [Pgpdh-1-GFP] (32), frIs7 [Pnlp-29-GFP + Pcol-12-dsRed] (19), and juEx1384 [Pnlp-30-GFP + Pcol-12-dsRed].
We isolated dapk-1 alleles ju4 and ju469 in screens for epidermal-defective mutants induced by ethyl methanesulfonate (EMS) or diepoxyoctane, respectively; ju557 was isolated in a screen for EMS-induced mutations that failed to complement ju4; gk219 was isolated by the C. elegans knockout consortium. Epidermal morphology defects were quantitated in complete broods of 2–6 animals raised at 15, 20, and 25 °C and scored as 1–2 day-old adults.
dapk-1 Molecular Biology and Transgenes.
The dapk-1 transcriptional GFP reporter was generated by subcloning a 2.7-kb genomic fragment into vector pPD95.75, yielding pCZ764 (transgenic lines juEx927 and juEx928). Isolation of the dapk-1 cDNA is described in SI Methods. To express full-length DAPK-1 under control of the 2.7-kb promoter, we digested the DAPK-1 cDNA clone pCZ763 with PstI and inserted the 2.7-kb dapk-1 promoter to produce the Pdapk-1-DAPK-1 construct pCZ767; pCZ767 was injected at 2 ng/μL into ju4 and gk219 with SUR-5::GFP as coinjection marker to generate arrays juEx886–889 and juEx890–892, respectively. To generate GFP-tagged DAPK-1, we partially digested pCZ767 with EcoR I and inserted GFP (amplified from pPD95.75) into an EcoR I site in the 5′ UTR to create pCZ770 Pdapk-1-GFP::DAPK-1; pCZ770 was injected at 1.5 ng/μL into ju4 and gk219 with Pttx-3-RFP as coinjection marker to generate transgenes juEx921–923 and juEx924–926, respectively. To express DAPK-1 in the epidermis, we amplified the 305 bp dpy-7 promoter (28), and cloned it into pCZ763 to create Pdpy-7-DAPK-1 (pCZ766); pCZ766 was injected at 1 ng/μL into wild-type animals with Pttx-3-RFP as coinjection marker to generate transgenes juEx1681 and juEx1682. dapk-1 cDNA was cloned into the hsp-16 vector pPD49.78 (Fire lab vector kit) to create pCZ763; pCZ763 was injected at 3 ng/μL with the coinjection marker Pttx-3-RFP to create juEx1933.
Electron Microscopy.
dapk-1(ju4) animals were grown at 25 °C and prepared for electron microscopy as previously described (20). We sectioned 1 L3, 1 L4, and 2 adults from the tip of the nose to the anterior end of the gonad. Images in Fig. 1 are from the region of the anterior pharyngeal bulb.
Pnlp-29-GFP Scoring and Worm Sorting.
Fluorescent worm sorting by using the Copas Biosort was performed as described (19), on synchronized populations grown at 20 °C. For quantitative analysis, we selected events with TOF 250–800 and GFP 15–250. To score frIs7 induction in the heat-shock time course (Fig. 3C), we wounded young adults and scored fluorescence by using a Leica MZFLIII fluorescence dissection microscope and long-pass GFP filter. Each animal was scored as uninduced (orange or yellow-orange) or induced (yellow, yellow-green, and green) at each time point to estimate the percentage induction in a population. Photomicrographs in Fig. 3 and Fig. S4 were taken by using a Zeiss Axioplan imaging microscope and Axiocam color camera, 40× objective, Zeiss GFP long-pass filters and 250 ms exposure time. Images were not processed further.
Lethality, Lifespan, and Wounding Experiments.
Premature lethality was measured by picking L4s and scoring viability 48 h later. Aging experiments were performed at 20 °C; n > 50 for each condition. We picked L4s to individual plates and transferred them until no further progeny were produced. Animals were scored daily until death, defined as failure to respond to touch. Kaplan–Meier survival curves were compared by using the Mantel-Cox test (Graphpad Prism). We wounded animals with single stabs of a microinjection needle to the posterior body 24 h after the L4 stage, as previously described (19). To prevent bacterial proliferation in lifespan assays, we transferred day 1 adults to plates containing 50 μg/mL FUDR immediately before wounding. Suppression of premature lethality was also tested by using 50 μg/mL ampicillin or 50 mM carbenicillin, as described (33).
Supplementary Material
Acknowledgments.
We thank Y. Dai and Y. Jin for sharing unpublished results on sydn-1; J. Crew and J. Kramer (Northwestern University, Chicago) for BLI-1::GFP; O. Zugasti (California Institute for Regenerative Medicine, San Francisco) for the nlp-30 array; our laboratories for advice and comments; Y. Duverger for worm sorting, by using facilities of the Marseille-Nice genopole. Some strains were provided by the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis) funded by the National Institutes of Health National Center for Research Resources. The Ewbank lab is a Fondation pour la Recherche Médicale Équipe Labellisée and is supported by Institut National de la Santé et de la Recherche Médicale, the Centre National de la Recherche Scientifique, and the Agence Nationale pour la Recherche. This work was supported by National Institutes of Health Grant GM54657 (to A.D.C.). Y.-C.W. was supported by the National Science Council, Taiwan. Collaborative work was supported by United States National Science Foundation Award OISE 0726131 (to A.D.C. and J.J.E.) and a French Foreign Ministry award from the France Berkeley Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809339106/DCSupplemental.
References
- 1.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 2.Galko MJ, Krasnow MA. Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol. 2004;2:E239. doi: 10.1371/journal.pbio.0020239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wigglesworth VB. Wound healing in an insect (Rhodnius prolixus Hemiptera) J Exp Biol. 1937;14:364–381. [Google Scholar]
- 4.Wood W, et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 5.Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- 6.Ting SB, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308:411–413. doi: 10.1126/science.1107511. [DOI] [PubMed] [Google Scholar]
- 7.Schmid P, et al. An intrinsic antibiotic mechanism in wounds and tissue-engineered skin. J Invest Dermatol. 2001;116:471–472. doi: 10.1046/j.1523-1747.2001.01279.x. [DOI] [PubMed] [Google Scholar]
- 8.Nizet V, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 9.Stramer B, et al. Gene induction following wounding of wild-type versus macrophage-deficient Drosophila embryos. EMBO Rep. 2008;9:465–471. doi: 10.1038/embor.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schafer M, Werner S. Transcriptional control of wound repair. Annu Rev Cell Dev Biol. 2007;23:69–92. doi: 10.1146/annurev.cellbio.23.090506.123609. [DOI] [PubMed] [Google Scholar]
- 11.Moussian B, Uv AE. An ancient control of epithelial barrier formation and wound healing. BioEssays. 2005;27:987–990. doi: 10.1002/bies.20308. [DOI] [PubMed] [Google Scholar]
- 12.Jane SM, Ting SB, Cunningham JM. Epidermal impermeable barriers in mouse and fly. Curr Opin Genet Dev. 2005;15:447–453. doi: 10.1016/j.gde.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen OE, et al. Injury-induced innate immune response in human skin mediated by transactivation of the epidermal growth factor receptor. J Clin Invest. 2006;116:1878–1885. doi: 10.1172/JCI28422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carretero M, et al. In vitro and in vivo wound healing-promoting activities of human cathelicidin LL-37. J Invest Dermatol. 2008;128:223–236. doi: 10.1038/sj.jid.5701043. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Ulevitch RJ. Limiting inflammatory responses during activation of innate immunity. Nat Immunol. 2005;6:1198–1205. doi: 10.1038/ni1274. [DOI] [PubMed] [Google Scholar]
- 16.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–266. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zenz R, et al. c-Jun regulates eyelid closure and skin tumor development through EGFR signaling. Dev Cell. 2003;4:879–889. doi: 10.1016/s1534-5807(03)00161-8. [DOI] [PubMed] [Google Scholar]
- 18.Zenz R, et al. Psoriasis-like skin disease and arthritis caused by inducible epidermal deletion of Jun proteins. Nature. 2005;437:369–375. doi: 10.1038/nature03963. [DOI] [PubMed] [Google Scholar]
- 19.Pujol N, et al. Distinct Innate Immune Responses to Infection and Wounding in the C. elegans Epidermis. Curr Biol. 2008;18:481–489. doi: 10.1016/j.cub.2008.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo WM, Goncharov A, Jin Y, Chisholm AD. Intermediate filaments are required for C. elegans epidermal elongation. Dev Biol. 2004;267:216–229. doi: 10.1016/j.ydbio.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Bosher JM, et al. The Caenorhabditis elegans vab-10 spectraplakin isoforms protect the epidermis against internal and external forces. J Cell Biol. 2003;161:757–768. doi: 10.1083/jcb.200302151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelkmans L, et al. Genome-wide analysis of human kinases in clathrin- and caveolae/raft-mediated endocytosis. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 23.Tian JH, Das S, Sheng ZH. Ca2+-dependent phosphorylation of syntaxin-1A by the death-associated protein (DAP) kinase regulates its interaction with Munc18. J Biol Chem. 2003;278:26265–26274. doi: 10.1074/jbc.M300492200. [DOI] [PubMed] [Google Scholar]
- 24.Michaux G, Gansmuller A, Hindelang C, Labouesse M. CHE-14, a protein with a sterol-sensing domain, is required for apical sorting in C. elegans ectodermal epithelial cells. Curr Biol. 2000;10:1098–1107. doi: 10.1016/s0960-9822(00)00695-3. [DOI] [PubMed] [Google Scholar]
- 25.Roberts B, Clucas C, Johnstone IL. Loss of SEC-23 in Caenorhabditis elegans causes defects in oogenesis, morphogenesis, and extracellular matrix secretion. Mol Biol Cell. 2003;14:4414–4426. doi: 10.1091/mbc.E03-03-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bialik S, Kimchi A. The death-associated protein kinases: Structure, function, and beyond. Annu Rev Biochem. 2006;75:189–210. doi: 10.1146/annurev.biochem.75.103004.142615. [DOI] [PubMed] [Google Scholar]
- 27.Kang C, You YJ, Avery L. Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 2007;21:2161–2171. doi: 10.1101/gad.1573107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilleard JS, Barry JD, Johnstone IL. cis regulatory requirements for hypodermal cell-specific expression of the Caenorhabditis elegans cuticle collagen gene dpy-7. Mol Cell Biol. 1997;17:2301–2311. doi: 10.1128/mcb.17.4.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couillault C, et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- 30.Pujol N, et al. Anti-fungal innate immunity in C. elegans is enhanced by evolutionary diversification of antimicrobial peptides. PLoS Pathog. 2008;4:e1000105. doi: 10.1371/journal.ppat.1000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong D, Bazopoulou D, Pujol N, Tavernarakis N, Ewbank JJ. Genome-wide investigation reveals pathogen-specific and shared signatures in the response of Caenorhabditis elegans to infection. Genome Biol. 2007;8:R194. doi: 10.1186/gb-2007-8-9-r194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamitina T, Huang CG, Strange K. Genome-wide RNAi screening identifies protein damage as a regulator of osmoprotective gene expression. Proc Natl Acad Sci USA. 2006;103:12173–12178. doi: 10.1073/pnas.0602987103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garigan D, et al. Genetic analysis of tissue aging in Caenorhabditis elegans: A role for heat-shock factor and bacterial proliferation. Genetics. 2002;161:1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garsin DA, et al. Long-lived C. elegans daf-2 mutants are resistant to bacterial pathogens. Science. 2003;300:1921. doi: 10.1126/science.1080147. [DOI] [PubMed] [Google Scholar]
- 35.Ewbank JJ. Tackling both sides of the host-pathogen equation with Caenorhabditis elegans. Microbes Infect. 2002;4:247–256. doi: 10.1016/s1286-4579(01)01531-3. [DOI] [PubMed] [Google Scholar]
- 36.Deiss LP, Feinstein E, Berissi H, Cohen O, Kimchi A. Identification of a novel serine/threonine kinase and a novel 15-kD protein as potential mediators of the gamma interferon-induced cell death. Genes Dev. 1995;9:15–30. doi: 10.1101/gad.9.1.15. [DOI] [PubMed] [Google Scholar]
- 37.Chuang YT, Fang LW, Lin-Feng MH, Chen RH, Lai MZ. The tumor suppressor death-associated protein kinase targets to TCR-stimulated NF-kappa B activation. J Immunol. 2008;180:3238–3249. doi: 10.4049/jimmunol.180.5.3238. [DOI] [PubMed] [Google Scholar]
- 38.Mukhopadhyay R, et al. DAPK-ZIPK-L13a axis constitutes a negative-feedback module regulating inflammatory gene expression. Molecular cell. 2008;32(3):371–382. doi: 10.1016/j.molcel.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bement WM, Yu HY, Burkel BM, Vaughan EM, Clark AG. Rehabilitation and the single cell. Curr Opin Cell Biol. 2007;19:95–100. doi: 10.1016/j.ceb.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schafer M, Werner S. Cancer as an overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell Bio. 2008;9:628–638. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 41.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thein MC, et al. Caenorhabditis elegans exoskeleton collagen COL-19: An adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Dev Dyn. 2003;226:523–539. doi: 10.1002/dvdy.10259. [DOI] [PubMed] [Google Scholar]
- 43.Davis MW, Birnie AJ, Chan AC, Page AP, Jorgensen EM. A conserved metalloprotease mediates ecdysis in Caenorhabditis elegans. Development. 2004;131:6001–6008. doi: 10.1242/dev.01454. [DOI] [PubMed] [Google Scholar]
- 44.Moribe H, et al. Tetraspanin protein (TSP-15) is required for epidermal integrity in Caenorhabditis elegans. J Cell Sci. 2004;117:5209–5220. doi: 10.1242/jcs.01403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.