Abstract
Purpose
To test the hypothesis that retinopathy of prematurity (ROP) affects the cone photoreceptors less than the rod photoreceptors.
Methods
Electroretinogram (ERG) responses to a 1.8 log unit range of red flashes on a white, rod saturating background were recorded from 42 subjects with a history of preterm birth and ROP (28 untreated; 6 treated) or no ROP (N=8). The sensitivity (SCONE) and saturated amplitude (RCONE) of the cone photoresponse were calculated by fit of a model of the activation of cone phototransduction to the a-waves. The cone-driven b-wave amplitude was evaluated as a function of stimulus intensity. SCONE and RCONE were compared to the rod response parameters (SROD, RROD) recorded from the same preterm subjects. Responses in the former preterms were compared to those in control subjects.
Results
The values of SCONE and RCONE in the former preterms overlapped broadly with those in the control subjects. The shapes of the b-wave stimulus/response functions did not differ between preterms and controls. The relative value of SCONE was significantly greater than that of SROD.
Conclusions
ROP has less effect on the cone than on the rod photoresponses suggesting that cones are more resistant to the ROP disease process. The similar shape of the b-wave stimulus/response function in preterms and controls is evidence that ROP does not alter the balance of ON and OFF signals in the cone pathway.
The sensitivity of rod mediated vision and of the rod photoresponse is low in infants and children with a history of retinopathy of prematurity (ROP).1-3 In rat models of ROP, oxygen levels that are too high or too low have adverse effects on the structure and function of the immature rods4-8, and early rod dysfunction predicts the abnormal retinal vasculature5 which is the hallmark used by clinicians to diagnose ROP. Less is known about the role of cones in ROP. Children’s cone mediated visual functions, including acuity and color vision, are affected by ROP9, 10, and the cone driven multifocal ERG responses of the central retina are attenuated in older children with a history of mild ROP.11 The effects of ROP on cone and cone driven function in the peripheral retina are unknown.
Cone ERG responses to full-field stimuli are relatively more mature than are rod ERG responses in healthy 4- and 10- week old infants.12 This is in keeping with the earlier anatomic development of the cones than rods.13 Primate cones differentiate earlier than rods, and peripheral cone outer segments mature earlier than rod outer segments.14-16 We reasoned that the greater maturity of infants’ cones, as well as the structure of the cones17, would offer relative protection from the adverse events that induce ROP. In the present study, we compared full-field cone and rod ERG responses in the same subjects to test the hypothesis that ROP has less effect on cones than on rods.
Methods
Subjects
Forty-two subjects with a history of preterm birth were studied. All had been monitored in the newborn intensive care nursery by experienced pediatric ophthalmologists using indirect ophthalmoscopy following schedules for examination similar to those used in the multi-center treatment trials.10, 18, 19 Gestational age at birth ranged from 23 to 32 (median 27) weeks and birth weight from 490 to 1850 (median 815) grams. The subjects were categorized by ROP history: treated ROP, untreated ROP, or no ROP. None had active ROP at the time of the ERG test. The treated subjects (N=6) had severe ROP that required ablation of the peripheral avascular retina at preterm ages; none had a retinal detachment. In these subjects, the median estimated area of residual retina was 80% (range 75% to 90%) of the total retinal area at age of test.20 In those categorized as untreated (N=28), mild ROP had been documented but resolved spontaneously without treatment. Eight subjects never developed ROP.
Nineteen subjects were tested as infants at median age 10 (range 7 to 11) weeks post-term. Term is at 40 weeks gestation. Twenty-three other subjects were tested at median age 13 (range 5 to 23) years. In normal subjects, both cone and rod ERG responses to full-field stimuli are completely mature by age one year.12, 21 Previously reported term born 10 week old (N=28) and mature (N=13) control subjects provided data for comparison.12 The rod responses of 11 of the 42 preterm subjects have been reported.2
This study conformed to the tenets of the Declaration of Helsinki and was approved by the Children’s Hospital Committee on Clinical Investigation. Informed consent was obtained from the parents of the infants and children, assent from the older children, and consent from those 18 years and older.
General ERG procedure
Parents stayed with infants and children throughout the procedure. The pupil was dilated with cyclopentolate 1%, and the subject was dark-adapted for 30 minutes. Then, under dim red light, proparacaine 0.5% was instilled, and a bipolar Burian-Allen electrode was placed on the cornea. A ground electrode was placed on the skin over the ipsilateral mastoid.
Thirty-seven subjects were tested using a Compact 4 system (Nicolet, Madison, WI) and five using an Espion system (Diagnosys, Lowell, MA). Despite differences between the two systems in the spectral bandwidth of the stimuli (described below) and in data acquisition (2,564 Hz digitization rate for the Nicolet; 2,000 Hz for the Espion), rod and cone photoresponse parameters in adult control subjects obtained using the Espion system (N=7) did not differ significantly from those obtained previously using the Nicolet system (N=13).12, 21 Therefore, the data obtained using the two systems have been combined.
Responses were differentially amplified (bandpass 1 to 1,000 Hz), displayed, digitized, and stored for analysis. A voltage window was used to reject responses contaminated by artifacts. Two to 16 responses were averaged in each stimulus condition. The inter-stimulus interval ranged from 2 to 60 seconds and was selected so that subsequent b-wave amplitudes were not attenuated.21
Cone ERG
After 3 to 5 minutes of adaptation to a steady, white, rod-saturating background (∼ +3 log phot td), responses were recorded to a 1.8 log unit range (+1.4 to +3.2 log phot td s) of full-field, brief (<3 ms), red stimuli, incremented in 0.3 log unit steps.. In the Nicolet system, a Wratten 29 filter (λ>610 nm) was used; in the Espion system, a 630 nm LED (half bandwidth 30 nm) was used. Cone photoresponse parameters were derived from the a-wave as described below. On records such as shown in Figure 1, the trough to peak amplitude and implicit time of the b-wave were measured and examined as a function of log flash intensity.
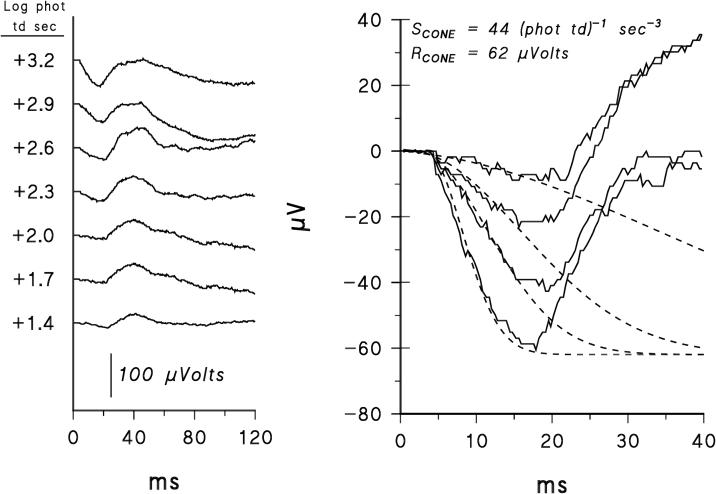
Figure 1.
Left panel. Sample ERG records from a 10-week old infant with a history of mild, untreated ROP. Responses to a 1.8 log unit range of red flashes on a steady white background are shown. Right panel. The first 40 ms of the records. The dashed lines represent Eq. 1 fit to these records; for clarity, responses to only four of the seven intensities are shown. The values of SCONE and RCONE are close to the median values in the 19 preterm infants.
Fit of a model of the activation of cone phototransduction22, 23 was restricted to the first 11 ms of the response to reduce post-receptor contamination.12, 22, 24-27 This model incorporates a low pass exponential filter to represent the capacitance of the cone membrane28, 29 30 by numerical convolution of the filter output with the delayed Gaussian function used to model the rod response.31, 32 The cone model29 is:
| (Eq. 1) |
where I is the flash in phot td s, SCONE a sensitivity parameter ((phot td)-1 s-3), td a brief delay (ms), RCONE the saturated response amplitude (μVolts), and τ the time constant of the low pass filter (ms). The symbol * represents the convolution operation. In the present study, as in the study of normal infants’ cone responses12, τ was fixed at 1.8 ms and td at 3 ms. Goodness of fit of the model (Eq. 1) to the a-wave was evaluated by the RMS errors.
Rod ERG
Responses to full-field, brief (<3 ms), blue stimuli ranging from those that evoked a small b-wave (<15 μVolts) to those saturating the a-wave were recorded. In the Nicolet system, a Wratten 47B filter (λ <510 nm) was used; in the Espion system, a 470 nm LED (half bandwidth 30 nm) was used. The rod photoresponse parameters (SROD and RROD) were calculated by fit of the Hood and Birch33 formulation of the Lamb & Pugh31, 32 model to the a-waves. The equation is
| (Eq. 2) |
where I is the flash in scot td s, SROD ((scot td)-1 s-3) a sensitivity parameter, RROD the saturated response amplitude (μVolts), and td a brief delay (ms). All three parameters, (SROD, RROD, and td) were free to vary. 2
Calibrations
Stimuli were measured with a detector and appropriate photopic or scotopic filter (IL 1700, International Light, Newburyport, MA) placed at the position of the subject’s cornea. Retinal illuminance varies directly with area of the pupil and transmissivity of the ocular media and inversely with the square of the posterior nodal distance.34 We used direct estimation of each subject’s dilated pupil and published estimates of ocular media density35, 36 and axial length of infant and mature eyes37-39 to make this calculation. In summary, equal intensity stimuli produce approximately equal retinal illuminance in 10 week old infant and mature subjects.34, 40-42 For both the Nicolet and Espion systems, the maximum intensity red stimulus produced a retinal illuminance of approximately +3.2 log phot td s; the maximum intensity blue stimulus produced an illuminance of approximately +3.6 log scot td s.
Analyses
The values of SCONE and RCONE and the b-wave stimulus/response functions in the former preterms were compared to those of normal term born 10-week old infants or mature controls.12 Each subject’s SCONE and SROD values were expressed as proportion of the normal mean for age to facilitate comparison of cone and rod sensitivity.12, 21 Comparisons between groups and between cone and rod response parameters were made using Student’s t-test. For both infants and mature subjects, the photopic b-wave amplitude and implicit time were evaluated for variation with stimulus intensity and group (former preterm, control) using analysis of variance. Cone and rod response parameters were evaluated for significant variation with ROP category (treated, untreated, none) using analysis of variance. For the former preterms with treated ROP, the response parameters were evaluated for possible relation to residual retina using Spearman rank order correlation. For all statistical tests, the level of significance was chosen as p < 0.01.
Results
Sample cone ERG records from a former preterm infant with a history of untreated ROP are shown in Figure 1. Also shown is the fit of the model of the cone photoresponse (Eq. 1) to the a-waves. The model describes reasonably well the leading edge of the a-wave. The RMS errors did not vary significantly with age and did not differ significantly between the former preterm and control subjects.
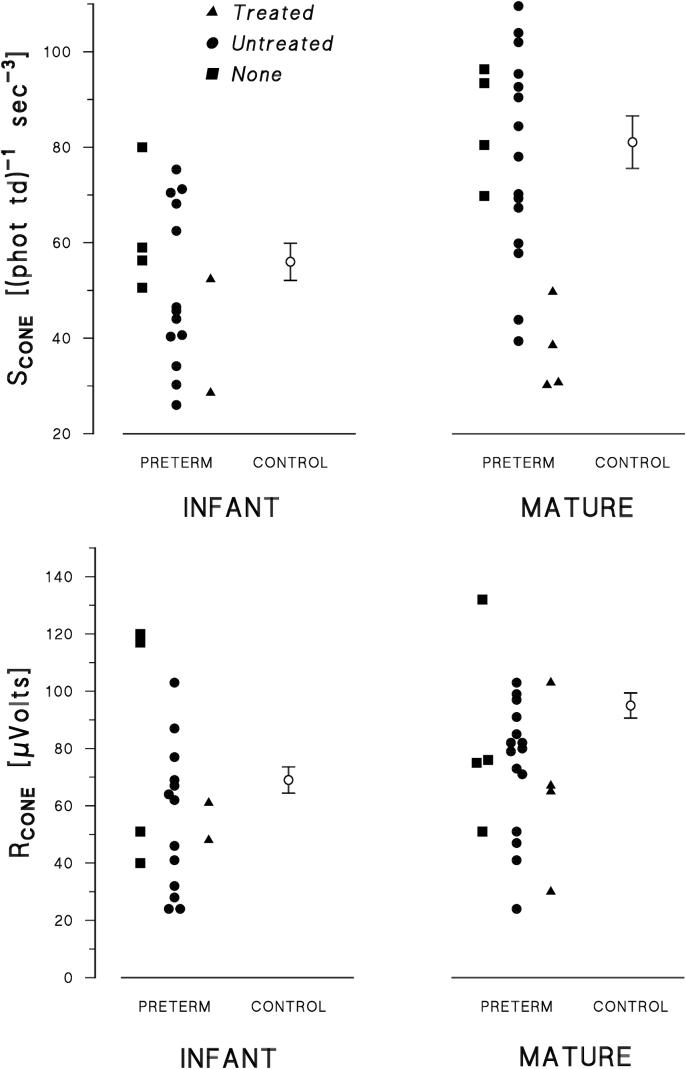
The values of the cone photoresponse parameters, SCONE and RCONE, in all former preterm subjects and the mean values in control subjects are shown in Figure 2. SCONE and RCONE in the former preterms were broadly distributed about the mean control values. The means and standard errors are summarized in Table 1. SCONE and RCONE did not differ significantly between former preterms and controls in either age group. All SCONE and RCONE values in those with treated ROP (Fig. 2) are below average with one exception. Neither parameter was correlated with the estimated area of retina remaining after treatment (SCONE: Spearman rho = 0.717; p = 0.109; RCONE: Spearman rho = 0.717; p = 0.109). Similarly, the rod response parameters (Table 1) in those with treated ROP were not correlated with estimated area of residual retina (SROD: Spearman rho = -0.359; p = 0.485; RROD: Spearman rho = 0.837; p = 0.038).
Figure 2.
Values of SCONE (upper panels) and RCONE (lower panels) in former preterm subjects (N=42), grouped by age at test. Different symbols indicate each ROP category: treated, untreated, none. In each panel, the mean (±SEM) for the term born controls12 is also shown.
Table 1.
Summary of Activation Parameters
| INFANT* | MATURE† | |||||||
|---|---|---|---|---|---|---|---|---|
| Mean (SEM) | Mean (SEM) | |||||||
| Preterm | Control‡ | t | p§ | Preterm | Control‡ | t | p§ | |
| CONES | ||||||||
| SCONE (phot td )-1 sec-3 | 52 (3.8) | 56 (3.9) | -0.76 | 0.45 | 72 (5.2) | 81 (5.5) | -1.19 | 0.24 |
| RCONE (μVolts) | 62 (6.8) | 69 (4.6) | -0.91 | 0.37 | 76 (5.9) | 96 (4.4) | -2.27 | 0.03 |
| RODS | ||||||||
| SROD (scot td )-1 sec-3 | 31 (3.5) | 41 (3.0) | -2.19 | 0.03 | 71 (5.1) | 90 (2.9) | -2.71 | <0.01 |
| RROD (μVolts) | 184 (12.8) | 167 (8.7) | 1.19 | 0.24 | 271 (17.5) | 389 (21.3) | -4.35 | <0.01 |
df for all tests = 45
df for all tests = 34
data from Hansen & Fulton12
level of significance for all tests: p<0.01.
The shapes of the b-wave stimulus/response functions in the former preterms are similar to those in age appropriate controls (Fig. 3). For both groups of infants, there was a monotonic increase in amplitude with stimulus intensity, whereas in the older subjects, whether former preterm or control, a photopic hill43-47 was seen with the peak at ∼ +2.3 log photopic td s. The absence of a photopic hill in healthy infants has been recognized.12, 48 At the +2.3 log photopic td s stimulus, in both infants and older subjects, the amplitude of the b-wave response in former preterms was about the same proportion of that in controls (infants, 0.76; older subjects, 0.87). For the infants, the b-waves were significantly smaller in former preterms than controls (F=17.1, df=1,322, p<0.01), with the greatest difference at stimulus intensities ≥ +2.3 log photopic td s. Among the mature subjects, b-wave amplitudes did not differ significantly between former preterms and controls (F=2.09; df=1, 236; p = 0.15). Analysis of variance showed no significant interactions, consistent with the impression that the shapes of the b-wave stimulus/response functions did not differ between former preterms and controls in either age group. The implicit times of the b-wave responses (data not shown) did not vary significantly with stimulus intensity (+1.4 to +3.2 log photopic td s) or group (former preterms, controls) in either age group (infants: F = 6.10; df 1, 322, p = 0.02; mature subjects: F = 0.54; df 1, 245; p = 0.46).
Figure 3.
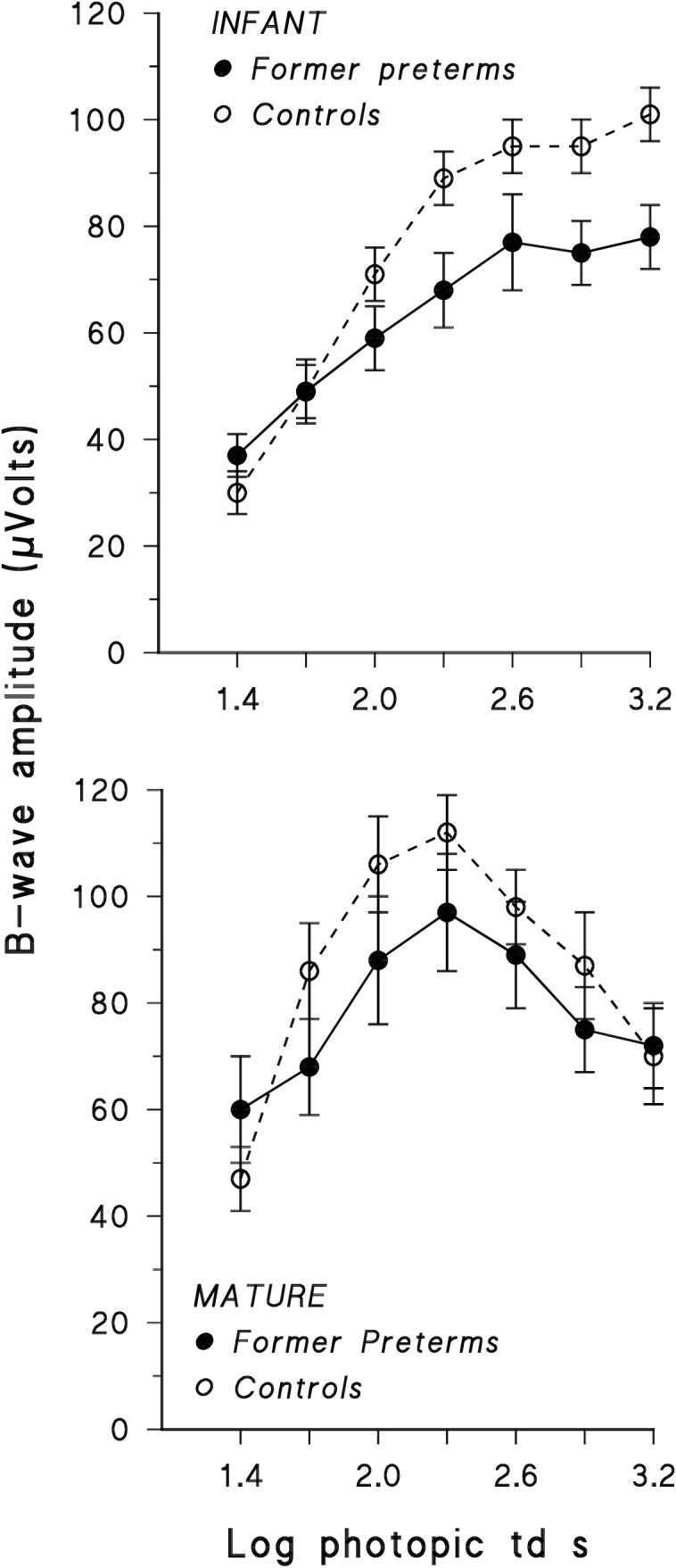
B-wave stimulus/response functions in former preterms compared to those in age appropriate control subjects.12 The means ( ±SEM) are shown.
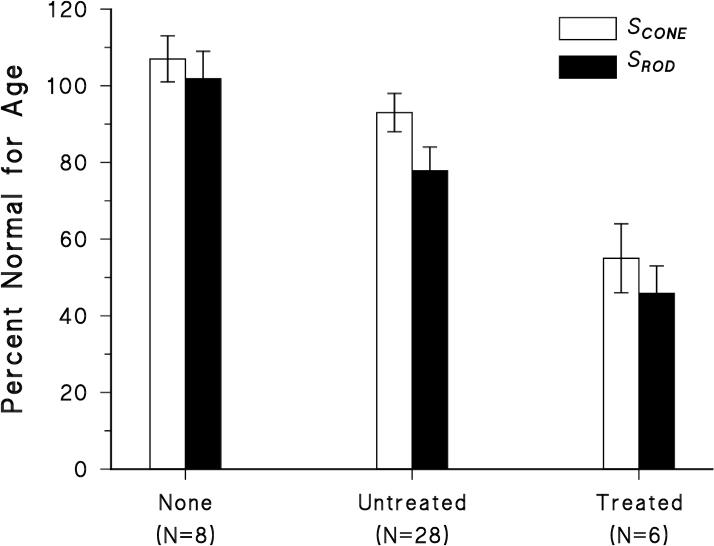
When expressed as proportion of normal mean for age, the mean relative value of SCONE was significantly larger than SROD (t=2.68; df=41; p<0.01). The mean relative values of RCONE and RROD did not differ significantly (t=-0.246; df=41; p = 0.814). In Figure 4, the sensitivity of the photoreceptor responses, SCONE and SROD, expressed as percent of the normal mean for age, is compared for each ROP category. SCONE varied significantly with ROP category (F = 5.81; df 2,41; p < 0.01) as did SROD (F = 5.81; df 2,41; p < 0.01).
Figure 4.
Cone (SCONE) and rod (SROD) photoresponse sensitivity, expressed as percent of normal mean for age, displayed by ROP category: none; untreated; treated. Error bars represent ±SEM.
Discussion
In subjects with a history of preterm birth, the sensitivity of the cones is higher than that of the rods (Fig. 4). The data show only minimal dysfunction of the cones in those with mild, untreated ROP and somewhat greater dysfunction in those who had more severe ROP that required treatment (Figs. 2 and 4). We suspect that cellular dysfunction, rather than loss of cells or area of responsive retina, underlies the deficits in sensitivity because the magnitude of the deficits was not accounted for by loss of retinal area. Similarly, the attenuation of the rod response parameters in these subjects and others21 was not correlated with area of retina remaining after treatment.
The shapes of the b-wave stimulus/response functions are similar in former preterms and controls (Fig. 3). The cone pathways include both ON and OFF bipolar cells, each contributing their relative strengths and timing to determine the shape of the observed b-wave function in the mature49 and immature12 retina. Thus, our results suggest that the combining of the ON and OFF signals in the cone pathways is not altered by ROP.
Although the shapes of the functions are similar in the former preterms and controls, the amplitudes of the b-wave response to full-field stimuli are mildly attenuated in the former preterms (Fig. 3). Post-receptor responses of the central retina to multifocal stimulation were significantly attenuated11, but it could not be determined if the relative contributions of ON and OFF signals were altered. In view of the b-wave responses to full-field stimuli (Fig. 3), it is unlikely that the relative ON and OFF contributions are differentially affected by ROP. In experimental ROP, neural changes accompany abnormal retinal vascularization5, 50, and in our own recent high resolution OCT observations of adolescents and young adults with a history of mild ROP, the abnormal intraretinal capillaries encroach on the neurons in the central retina.51 Although the neurovascular abnormality does not appear to discriminate between ON and OFF neurons, we suspect it has a role in attenuating the post-receptor activity that is represented in the ERG b-wave.
At least two explanations for the lower vulnerability of cones than rods (Fig. 4) warrant consideration. First, earlier maturation may protect the cones. It is the immature photoreceptors that appear particularly vulnerable to retinal oxygen levels that are too high or too low.8 Second, cones appear more resistant to pathological processes. Compared to rods, cones have twice as many mitochondria and approximately three times the surface area of mitochondrial cristae.17 Thus, the cones are equipped for greater aerobic ATP production, and this, Perkins et al.17 theorize, protects against metabolic insults and apoptosis. As a corollary, they postulate that therapeutic interventions that support mitochondrial energy production may be beneficial in many photoreceptor diseases.17 Furthermore, cones, in contrast to rods, have the capability of utilizing endogenous glycogen, affording protection against the adverse effects of hypoxia and attendant hypoglycemia.52 The data from the subjects represented in Figure 4 and additional subjects2 indicate that ROP affects the rods, and it is known that in some patients, ROP has a progressive, degenerative course.53 Thus, therapies that support mitochondrial energy production may be beneficial in ROP and possibly even have a role in preventing ROP, because it is rod sensitivity that predicts the vascular abnormalities in rat models of ROP.5
Acknowledgments
Supported by National Eye Institute Grant EY 10597
Footnotes
Disclosure: A. B. Fulton, None; R. M. Hansen, None; A. Moskowitz, None
References
- 1.Barnaby AM, Hansen RM, Moskowitz A, Fulton A. Investigative Ophthalmology and Visual Science. Vol. 47. 2007. Development of scotopic thresholds in retinopathy of prematurity. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fulton AB, Hansen RM, Petersen RA, Vanderveen DK. The rod photoreceptors in retinopathy of prematurity: An electroretinographic study. Archives of Ophthalmology. 2001;119:499–505. doi: 10.1001/archopht.119.4.499. [DOI] [PubMed] [Google Scholar]
- 3.Hansen RM, Fulton AB. Background adaptation in children with a history of mild retinopathy of prematurity. Investigative Ophthalmology and Visual Science. 2000;40:320–324. [PubMed] [Google Scholar]
- 4.Fulton AB, Reynaud X, Hansen RM, Lemere CA, Parker C, Williams TP. Rod photoreceptors in infant rats with a history of oxygen exposure. Investigative Ophthalmology and Visual Science. 1999;40:168–174. [PubMed] [Google Scholar]
- 5.Akula JD, Hansen R, Martinez-Perez ME, Fulton A. Rod photoreceptor function predicts blood vessel abnormalities inretinopathy of prematurity. Investigative Ophthalmology and Visual Science. 2007;48:4351–4359. doi: 10.1167/iovs.07-0204. [DOI] [PubMed] [Google Scholar]
- 6.Liu K, Akula JD, Falk C, Hansen RM, Fulton AB. The retinal vasculature and function of the neural retina in a rat model of retinopathy of prematurity. Investigative Ophthalmology and Visual Science. 2006;47:2639–2647. doi: 10.1167/iovs.06-0016. [DOI] [PubMed] [Google Scholar]
- 7.Maslim J, Valter K, R. E, Hollander H, Stone J. Tissue oxygen during a critical developmental period controls the death and survival of photoreceptors. Investigative Ophthalmology and Visual Science. 1997;38:1667–1677. [PubMed] [Google Scholar]
- 8.Wellard J, Lee D, Valter K, Stone J. Photoreceptors in the rat retina are specifically vulnerable to both hypoxia and hyperoxia. Visual Neuroscience. 2005;22:501–507. doi: 10.1017/S0952523805224112. [DOI] [PubMed] [Google Scholar]
- 9.Dobson V, Quinn GE, Abramov I, et al. Color vision measured with pseudoisochromatic plates at five-and-a-half years in eyes of children from the CRYO-ROP study. Invest Ophthalmol Vis Sci. 1996;37:2467–74. [PubMed] [Google Scholar]
- 10.Early Treatment For Retinopathy Of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Archives of Ophthalmology. 2003;121:1684–1694. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- 11.Fulton AB, Hansen RM, Moskowitz A, Barnaby AM. Multifocal ERG in subjects with a history of retinopathy of prematurity. Doc Ophthalmol. 2005;111:7–13. doi: 10.1007/s10633-005-2621-3. [DOI] [PubMed] [Google Scholar]
- 12.Hansen RM, Fulton AB. Development of the cone ERG in infants. Investigative Ophthalmology and Visual Science. 2005;46:3458–3462. doi: 10.1167/iovs.05-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- 14.Dorn EM, Hendrickson L, Hendrickson AE. The appearance of rod opsin during monkey retinal development. Investigative Ophthalmology and Visual Science. 1995;36:2634–2651. [PubMed] [Google Scholar]
- 15.Hendrickson AE, Drucker D. The development of parafoveal and midperipheral retina. Behavioural Brain Research. 1992;19:21–32. doi: 10.1016/s0166-4328(05)80191-3. [DOI] [PubMed] [Google Scholar]
- 16.Hendrickson AE. The morphologic development of human and monkey retina. In: Albert DM, Jakobiec FA, editors. Principles and Practice of Ophthalmology: Basic Sciences. WB Saunders Co.; Philadelphia: 1994. pp. 561–577. [Google Scholar]
- 17.Perkins GA, Ellisman MH, Fox DA. Three-dimensional analysis of mouse rod and cone mitochondrial cristae architecture: bioenergetic and functional implications. Molecular Vision. 2003;9:60–73. [PubMed] [Google Scholar]
- 18.Committee for the Classification of Retinopathy of Prematurity An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- 19.Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: three-month outcome. Archives of Ophthalmology. 1988;106:471–479. doi: 10.1001/archopht.1988.01060130517027. [DOI] [PubMed] [Google Scholar]
- 20.Robb RM. Increase in retinal surface area during infancy and childhood. J Ped Ophthalmol Strab. 1982;19:16–20. doi: 10.3928/0191-3913-19820701-06. [DOI] [PubMed] [Google Scholar]
- 21.Fulton AB, Hansen RM. The development of scotopic sensitivity. Investigative Ophthalmology and Visual Science. 2000;41:1588–1596. [PubMed] [Google Scholar]
- 22.Freidburg C, Allen CP, Mason PJ, Lamb TD. Contributions of cone photoreceptors and post-receptoral mechanisms to the human photopic electroretinogram. Journal of Physiology. 2004;556:819–834. doi: 10.1113/jphysiol.2004.061523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hood DC, Birch DG. Phototransduction in human cones measured using the a-wave of the ERG. Vision Research. 1995;35:2801–10. doi: 10.1016/0042-6989(95)00034-w. [DOI] [PubMed] [Google Scholar]
- 24.Robson JG, Frishman LJ. Photoreceptor and bipolar cell contributions to the cat electroretinogram: A kinetic model for the early part of the flash response. J Opt Soc Am. 1996;13:613–622. doi: 10.1364/josaa.13.000613. [DOI] [PubMed] [Google Scholar]
- 25.Bush RA, Sieving PA. A proximal retinal component in the primate photopic ERG a-wave. Investigative Ophthalmology and Visual Science. 1994;35:635–645. [PubMed] [Google Scholar]
- 26.Jamison JA, Bush RA, Lei B, Sieving PA. Characterization of the rod photoresponse isolated from the dark adapted primate ERG. Visual Neuroscience. 2001;18:445–455. doi: 10.1017/s0952523801183112. [DOI] [PubMed] [Google Scholar]
- 27.Bradshaw K. Contribution of post-receptoral cells to the a-wave of the human photopic electroretinogram. Vision Research. 2007 doi: 10.1016/j.visres.2007.07.021. doi:10.1016/j.visres.2007.07.21. [DOI] [PubMed] [Google Scholar]
- 28.Cideciyan AV, Jacobson SG. An alternative phototransduction model for human rod and cone ERG a-waves: normal parameters and variation with age. Vision Res. 1996;36:2609–2621. doi: 10.1016/0042-6989(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 29.Hood DC, Birch DG. Phototransduction in human cones measured using the a-wave of the ERG. Vision Research. 1995;35:2801–2810. doi: 10.1016/0042-6989(95)00034-w. [DOI] [PubMed] [Google Scholar]
- 30.Hood DC, Birch DG. Human cone receptor activity: the leading edge of the a-wave and models of receptor activity. Vis Neurosci. 1993;10:857–871. doi: 10.1017/s0952523800006076. [DOI] [PubMed] [Google Scholar]
- 31.Pugh EN, Jr., Lamb TD. Amplification and kinetics of the activation steps in phototransduction. Biochimica et Biophysica Acta. 1993;1141:111–149. doi: 10.1016/0005-2728(93)90038-h. [DOI] [PubMed] [Google Scholar]
- 32.Lamb TD, Pugh EN., Jr. A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. Journal of Physiology. 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hood DC, Birch DG. Rod phototransduction in retinitis pigmentosa: Estimation and interpretation of parameters derived from the rod a-wave. Investigative Ophthalmology and Visual Science. 1994;35:2948–2961. [PubMed] [Google Scholar]
- 34.Hansen RM, Fulton AB. Development of scotopic retinal sensitivity. In: Simons K, editor. Early Visual Development, Normal and Abnormal. Oxford University Press; New York: 1993. pp. 130–142. [Google Scholar]
- 35.Hansen RM, Fulton AB. Psychophysical estimates of ocular media density of human infants. Vision Res. 1989;29:687–690. doi: 10.1016/0042-6989(89)90031-x. [DOI] [PubMed] [Google Scholar]
- 36.Werner JS. Development of scotopic sensitivity and the absorption spectrum of the human ocular media. J Optical Soc Am. 1982;72:247–258. doi: 10.1364/josa.72.000247. [DOI] [PubMed] [Google Scholar]
- 37.Larsen JS. The saggital growth of the eye. IV. Ultrasonic measurements of the axial length of the eye from birth to puberty. Acta Ophthalmologica. 1971:873–886. doi: 10.1111/j.1755-3768.1971.tb05939.x. [DOI] [PubMed] [Google Scholar]
- 38.Achiron R, Kreiser D, Achiron A. Axial growth of the fetal eye and evaluation of the hyaloid artery in utero ultrasonographic study. Prenatal Diagnosis. 2000;20:894–899. doi: 10.1002/1097-0223(200011)20:11<894::aid-pd949>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 39.Denis D, Burguiere O, Burillon C. A biometric study of the eye, orbit and face in 205 normal human fetuses. Investigative Ophthalmology and Visual Science. 1998;39:2232–2238. [PubMed] [Google Scholar]
- 40.Brown AM, Dobson V, Maier J. Visual acuity of human infants at scotopic, mesopic and photopic luminances. Vision Res. 1987;27:1845–1858. doi: 10.1016/0042-6989(87)90113-1. [DOI] [PubMed] [Google Scholar]
- 41.Hansen RM, Fulton AB, Harris SJ. Background adaptation in human infants. Vision Research. 1986;26:771–779. doi: 10.1016/0042-6989(86)90092-1. [DOI] [PubMed] [Google Scholar]
- 42.Malcolm CA, Hamilton R, McCulloch DL, Montgomery C, Weaver LT. Scotopic electroretinogram (ERG) in term infants born of mothers supplemented with docosahxaenoic acid (DHA) during pregnancy. Investigative Ophthalmology and Visual Science. 2003;44:3685–3691. doi: 10.1167/iovs.02-0767. [DOI] [PubMed] [Google Scholar]
- 43.Lachapelle P, Rufiange M, Dembinska O. A physiological basis for definition of the ISCEV ERG standard flash (SF) based on the photopic hill. Documenta Ophthalmologica. 2001;102:157–62. doi: 10.1023/A:1017505616163. [DOI] [PubMed] [Google Scholar]
- 44.Peachey NS, Alexander KR, Derlacki DJ, Fishman GA. Light adaptation and the luminance-response function of the cone electroretinogram. Documenta Ophthalmologica. 1992;79:363–269. doi: 10.1007/BF00160949. [DOI] [PubMed] [Google Scholar]
- 45.Rufiange M, Rousseau S, Dembinska O, Lachapelle P. Cone-dominated ERG luminance-response function: the Photopic Hill revisited. Documenta Ophthalmologica. 2002;104:231–48. doi: 10.1023/a:1015265812018. [DOI] [PubMed] [Google Scholar]
- 46.Rufiange M, Dassa J, Dembinska O, et al. The photopic ERG luminance-response function (photopic hill): method of analysis and clinical application. Vision Research. 2003;43:1405–12. doi: 10.1016/s0042-6989(03)00118-4. [DOI] [PubMed] [Google Scholar]
- 47.Wali N, Leguire LE. The photopic hill: a new phenomenon of the light adapted electroretinogram. Documenta Ophthalmologica. 1992;80:335–45. doi: 10.1007/BF00154382. [DOI] [PubMed] [Google Scholar]
- 48.Garon M-L, Hamilton R, McCulloch DL, et al. Analysis of the photopic hill: Testing the Glasgow model. Investigative Ophthalmology and Visual Science. 2007;48 E-Abstract 529. [Google Scholar]
- 49.Ueno S, Kondo M, Yasurhiro N, Terasaki H, Miyake Y. Luminance dependence of neural components that underlies the primate photopic electroretinogram. Investigative Ophthalmology and Visual Science. 2004;45:1033–1040. doi: 10.1167/iovs.03-0657. [DOI] [PubMed] [Google Scholar]
- 50.Downie LE, Pianta MJ, Vingrys AJ, Wilkinson-Berka JL, Fletcher EL. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J Comp Neurol. 2007;504:404–17. doi: 10.1002/cne.21449. [DOI] [PubMed] [Google Scholar]
- 51.Ferguson RD, Hammer DX, Ustun TE, et al. Adaptive optics, Fourier domain OCT imaging for developmental disorders of the retina. 6th International Workshop on Adaptive Optics for Industry and Medicine, National University of Ireland,Galway. 2007 [Google Scholar]
- 52.Nihira M, Anderson K, Gorin FA, Burns MS. Primate rod and cone photoreceptors may differ in glucose accessibility. Investigative Ophthalmology and Visual Science. 1995;36:1259–70. [PubMed] [Google Scholar]
- 53.Hirose T, Katsumi O, Arai M, Mehta M, Faria J, Vitral N. Pigmentary degeneration of the retina secondary to retinopathy of prematurity; Paper presented at the International Society of Clinical Electrophysiology of Vision Symposium; Nagoya Japan. 2003. [Google Scholar]