Abstract
Mucosal tolerance induction generally requires multiple or large antigen (Ag) doses. Since M cells have been implicated as being important for mucosal tolerance induction and because reovirus attachment protein sigma 1 (pσ1) is capable of binding microfold (M) cells, we postulated that targeting a model Ag to M cells via pσ1 could induce a state of unresponsiveness. Accordingly, a genetic fusion between ovalbumin (OVA) and the M cell ligand, reovirus pσ1, termed OVA-pσ1, was developed to enhance tolerogen uptake. When applied nasally, not parenterally, as little as a single dose of OVA-pσ1 failed to induce OVA-specific Abs even in the presence of adjuvant. Moreover, the mice remained unresponsive to peripheral OVA challenge, unlike mice given multiple nasal OVA doses that rendered them responsive to OVA. observed unresponsiveness to OVA-pσ1 could be adoptively transferred using cervical lymph node (CLN) CD4+ T cells, which failed to undergo proliferative or delayed-type hypersensitivity (DTH) responses in recipients. discern the cytokines responsible as a mechanism for this unresponsiveness, restimulation assays revealed increased production of regulatory cytokines, IL-4, IL-10, and TGF-β, with greatly reduced IL-17 and IFN-γ. induced IL-10 was derived predominantly from forkhead box P3 (FoxP3)+ CD25+CD4+ T cells. FoxP3+ CD25+CD4+ T cells were induced in OVA-pσ1-dosed IL-10 deficient (IL-10−/−) mice, and despite showing increased TGF-β synthesis, these mice were responsive to OVA. These data demonstrate the feasibility of using pσ1 as a mucosal delivery platform specifically for low-dose tolerance induction.
Keywords: Th1/Th2 cells, tolerance/suppression, mucosa, vaccination
Introduction
Mucosal tolerance is a dose-dependent process (1–3) requiring either high doses or multiple administrations of Ag typically applied orally. The former mode induces tolerance by clonal anergy/deletion of effector cells, whereas the latter, based on repeated low-dose administration, causes active suppression of effector cells (3–7). Such suppression occurs via activation of specific regulatory cells, among which CD25+CD4+ T regulatory (Treg)3 cells have been best described (2, 6). Although the phenotypic characteristics of Treg cells are still being investigated, currently, this naturally anergic peripheral CD25+CD4+ T cell population is thought to normally constitute not more than 5–10 percent of total peripheral CD4+ T cells (8, 9). Specific Treg cells are known to express the nuclear forkhead box P3 (FoxP3) transcription factor and suppress the immune response in an IL-10- and/or TGF-β-dependent fashion (10).
Peyer’s patches (PPs) are thought to be required for induction of oral tolerance (1, 6, 11) based upon the findings that treatment of female mice with soluble lymphotoxin-β receptor-Ig fusion protein during gestation results in the disruption of the peripheral lymph nodes (LNs) and PPs in their offspring, leaving the mesenteric, sacral, and cervical LNs intact (12). Offspring of such PP-null mice subjected to a high dose OVA oral tolerance regimen do not respond to peripheral OVA challenge (11). The PPs (1, 6, 11) and an analogous structure in the upper respiratory tract, the nasopharyngeal - associated lymphoid tissue (NALT; 13, 14), actively facilitate immunity or unresponsiveness by luminal Ag sampling (2, 14, 15) via a specialized epithelium containing microfold (M) cells (16). Ags are subsequently transported from the luminal surface via M cells, which localize in the follicle-associated epithelium (FAE) to the subepithelial dome area for eventual presentation to mucosal B and T cells (15, 17). A number of pathogens, such as reovirus and Salmonella, specifically target this specialized FAE to infect the host (18–22). Reovirus infects the host via its cell adhesin, protein sigma one (pσ1), that is responsible for reovirus attachment to M cells (14, 18–20). Previous studies demonstrated binding of recombinant fusion pσ1 to NALT, suggesting that a pσ1-based vehicle can be applied for genetic vaccination of mucosal tissues (14, 23). A modified version of pσ1 obtained by chemically conjugating pσ1 to poly-L-lysine significantly enhanced immunity to the encoded DNA vaccine following nasal administration (14, 24). Due to these targeting capabilities, we queried if recombinant pσ1 could ferry soluble proteins to mucosal tissues, as well.
To test this possibility, a cDNA encoding for the model Ag, OVA, was cloned 5’ to pσ1 as a histidine-tag fusion protein and termed OVA-pσ1. We demonstrated here that mice nasally immunized with the OVA-pσ1 fusion protein become tolerogenic to OVA and resistant to peripheral challenge as a result of the generation of Ag-specific Treg cells, which, in turn, actively suppress effector T cells. Immunization with OVA-pσ1 significantly increased the numbers of IL-4-producing CD25−CD4+ T cells, together with IL-10-producing Treg cells, which inhibited the proliferation of OVA-specific CD4+ T cells in vivo and successfully suppressed production of proinflammatory cytokines. In the absence of IL-10, OVA-pσ1-mediated tolerance was lost. Thus, these studies show that nasal pσ1-Ag delivery is an effective means to induce systemic tolerance.
Materials and Methods
Preparation of OVA-pσ1
Protein σ1 was recloned without its fusion maltose-binding protein partner (23) to allow expression in the yeast Pichia pastoris vector pPICB bearing an histag carboxy terminus for protein purification (Invitrogen Corp., Carlsbad, CA), referred to as pσ1. To obtain the fusion protein OVA-pσ1, OVA was amplified with appropriate pairs of primers from an OVA cDNA. The 5′ primer encoded an EcoRI site and an ATG initiation codon embedded into an optimal Kozak’s sequence. The 3′ primer provided a SalI site. The PCR product was gel-purified and cloned into an intermediate topocloning vector. The insert was excised by cutting with EcoRI and SalI and gel-purified again, resulting in EcoRI and SalI ends. The upstream primer for pσ1 contained a SalI site designed to frame the OVA SalI end; the downstream primer contained a KpnI primer designed to frame the fused protein to the histag present in the P. pastoris expression vector pPICB. The PCR products were gel-purified and cloned into a topocloning vector. The inserts were then excised by cutting with SalI and KpnI and gel-purified again, thus having SalI and KpnI ends. Finally, the yeast expression vector, pPICB, was cut with EcoRI and KpnI. A tripartite ligation was set up to join together these components: 1) the “Antigen” (OVA) as an EcoRI-SalI fragment; 2) the “Transporter” (pσ1), as a SalI-KpnI fragment; and 3) the vector cut with EcoRI and KpnI. The junction between the “passenger Ag” and the “transporter” featured a flexible linker (Gly-Arg-Pro) to minimize steric hindrance between the components. The resulting construct was sequenced and expressed in the yeast P. pastoris, according to the manufacturer’s directions (Invitrogen Corp.). Recombinant proteins were extracted from yeast cells by a bead-beater (Biospec Products, Bertlesville, OK) and purified on a Talon metal affinity resin (BD Biosciences, Palo Alto, CA), according to manufacturer’s instructions. Proteins were assessed for purity and quality by Coomassie-stained polyacrylamide gels and by Western blot analysis using a polyclonal rabbit anti-pσ1 (produced in-house) or a polyclonal rabbit anti-OVA Ab (Sigma-Aldrich, St. Louis, MO). All recombinant proteins migrated as a single band with the expected MW.
Mice
Female BALB/c and C57BL/6N mice (Frederick Cancer Research Facility, National Cancer Institute, Frederick, MD) were used throughout this study. DO11.10 and IL-10−/− breeder pairs were obtained from The Jackson Laboratory (Bar Harbor, ME) to establish our colonies. All mice were maintained in the Montana State University (MSU) Animal Resources Center under pathogen-free conditions in individually ventilated cages under HEPA-filtered barrier conditions and were fed sterile food and water ad libitum. The mice were free of bacterial and viral pathogens, as determined by antibody screening and histopathologic analysis of major organs and tissues. All animal studies were approved by the MSU Institutional Animal Care and Use Committee.
Immunizations, tolerance induction, and OVA-specific challenge
For tolerance induction, mice (5 mice/group) were nasally dosed up to three times, as described in the text, with 50 – 100 μg of OVA-pσ1 alone or in combination with cholera toxin (CT; List Biologicals, Campbell, CA): 5 μg of CT for the initial dose and 2.5 μg with each boost (25). OVA-pσ1 was administered nasally in a volume of no more than 20 μl/dose up to four times a day, with no less than 2.5 hour intervals between each administration. Mice were nasally dosed with OVA or OVA-pσ1 plus 25 μg of CpG oligodeoxynucleotide cDNA (CpG-ODN) (Sigma-Aldrich), TCCATGACGTTCCTGACGTT (26). As a tolerance control, a group of age-matched mice received a single oral 25 mg dose of OVA (Grade V, Sigma-Aldrich) in a 200 μl of saline (17). To stimulate anti-OVA immunity, mice were nasally dosed with 100 μg OVA (10 mg/ml) with or without adjuvant (CT or ODN). For peripheral challenge, mice were given a subcutaneous (s.c.) injection at 1:1 ratio of 100 μg OVA in IFA (Sigma-Aldrich) for a total volume of 100 μl per mouse.
Sample collections and Ab ELISA
Serum (saphenous vein) and fecal samples were collected weekly from each mouse. Fecal extractions (4, 14, 25) and vaginal washes (25) were performed, as previously described. OVA-specific endpoint Ab titers were measured by ELISA, as previously described (17), using purified OVA (Grade V) as coating Ag. Specific reactivity to OVA was determined using HRP conjugates of goat anti-mouse IgG-, and IgA-specific Abs (1.0 μg/ml; Southern Biotechnology Associates, Birmingham, AL), and ABTS (Moss Inc., Pasadena, CA) enzyme substrate. The absorbences were measured at 415 nm on a Kinetics Reader model ELx808 (Bio-Tek Instruments). Endpoint titers were expressed as the reciprocal dilution of the last sample dilution, giving an absorbence of 0.1 OD units above the OD415 of negative controls after 1 h incubation (4, 25).
Measurement of delayed-type hypersensitivity (DTH) responses
To measure OVA-specific DTH responses in vivo (17), 10 μg of OVA were injected into the left ear pinna, and PBS alone (20 μl) was administered to the right ear pinna as a control. Ear swelling was measured 24 h later with an electronic digital caliper (World Precision Instruments, Sarasota, FL). The DTH response was calculated as the increase in ear swelling after OVA injection following subtraction of swelling in the control site injected with PBS.
Isolation of CD4+ T cells and T cell ELISPOT
Lymphocytes were isolated from mesenteric lymph nodes (MLNs), head and neck LNs (HNLNs), and spleens. LNs and spleens were obtained and lymphoid cells isolated, as previously described (4, 27). Splenic mononuclear cell suspensions were subjected to Lympholyte-M (Accurate Chemical & Scientific Corporation, Westbury, N.Y.) density gradient centrifugation, and CD4+ T cells were isolated by negative selection (Dynal Mouse CD4 Negative Isolation Kit, Invitrogen). An aliquot of 2 × 106 CD4+ T cells were cultured for 72 h (37° C, 5 % CO2) with an equal number of splenic feeder cells (T cell-depleted, mitomycin C-treated) in the presence or absence of 1 mg/ml OVA (17) or 1 μg/ml of OVA323–329 peptide. For T cell ELISPOT analysis, CD4+ T cells were added to cytokine-coated, nitrocellulose-bottom microtiter plates (Millipore, MutliScreen, MA). For detection of IFN-γ, IL-2, IL-4, IL-10, IL-13, and IL-17, the plates were coated with 5 μg/ml of purified mAbs (BD Pharmingen, San Diego, CA), and the reaction was detected using 0.5 μg/ml of appropriate biotinylated mAbs (BD Pharmingen). For TGF-β1 ELISPOT, an anti-TGF-β1 mAb (10 μg/ml; Clone 1D11; R&D Systems; Minneapolis, MN) was used for coating, and a biotinylated chicken anti-human TGF-β1 Ab (5.0 μg/ml; R&D Systems) was used for detection. The color reaction was developed using a HRP-conjugated goat anti-biotin Ab (Vector Laboratories) and AEC reagent (Moss, Inc.) and enumerated, as previously described (14, 27).
FACS analysis
Lymphocytes from CLNs, HNLNs, MLNs, PPs, and spleens from different immunization groups following OVA plus IFA challenge were cultured at 5 × 106 cells/ml in medium alone or in the presence of OVA (1 mg/ml). Cells were then stained for FACS analysis with Abs to cell surface molecules: FITC-anti-mouse CD4 (GK1.5; BD Pharmingen), Streptavidin-PE-Cy5 (BD Pharmingen) for biotinylated chicken anti-hTGF-β1 IgY (R&D Systems), and PE-Cy5- or APC-anti-mouse CD25 (both clones PC61) (eBioscience). Intracellular staining was performed following standard protocols with 2 % paraformaldehyde and 0.2 % saponin to stain with PE or APC-anti-mouse/rat FoxP3 (clone FJK-16s; eBioscience) or PE-rat anti-mouse IL-10 (BD Pharmingen), as previously described (27). Fluorochrome-conjugated rat IgG2a (clone eBR2a), IgG2b (clone A95-1), and IgG1 (clone R3-34) were used as isotype control Abs. FACS analysis was performed on FACSCaliburTM (BD Bioscience). At least 50,000 events were collected for each sample.
Adoptive transfer of CD4+ T cell subsets
Adoptive transfer presented in Fig. 2A: aliquots of 1 × 107 CD4+ T cells isolated from spleens of naïve DO11.10 TCR transgenic (Tg) mice were adoptively transferred via i.v. injection into naïve BALB/c mice, which, after 24 h, were dosed nasally with sterile PBS (sPBS), 400 μg OVA, or 80 μg OVA-pσ1 or i.m. (tibialis anterior muscle) with 400 μg of OVA. Three days later, total CLN CD4+ T cells (2 × 105) isolated by cell-sorting were adoptively transferred into naïve BALB/c mice, which, 24 h later, were challenged s.c. with 100 μg of OVA in IFA. CD4+ T cells were isolated from the CLNs and spleens 5 days later and subjected to an in vitro proliferation assay.
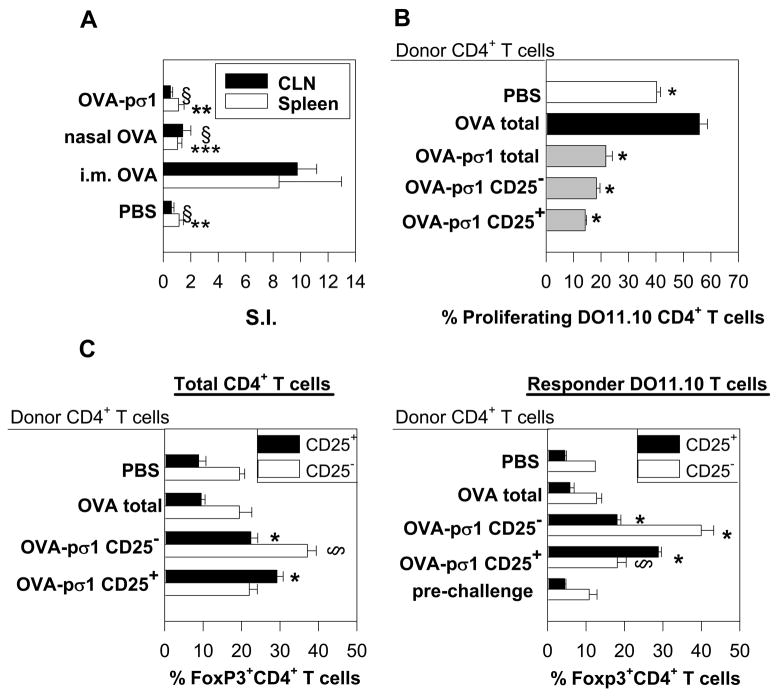
FIGURE 2.
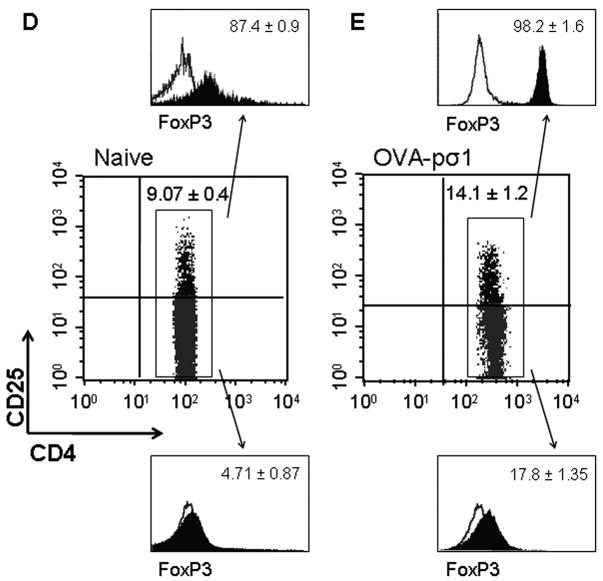
OVA-pσ1-induced unresponsiveness is mediated by CD4+ T cells. (A) CD4+ T cells isolated from CLNs and spleens of mice adoptively transferred with CLN-derived CD4+ T cells from OVA-pσ1- or OVA-dosed mice were cultured in vitro without or with 1 mg of OVA for 5 days. 3H-TdR incorporation was measured and expressed as a stimulation index (SI). For CLN, § p ≤ 0.001 versus i.m. OVA; for spleen** p = 0.003, ***p = 0.006 vs. i.m. OVA, calculated by Student’s t test. (B, C) BALB/c mice were adoptively transferred with Vybrant-labeled DO11.10 Tg CD4+ T (responder) cells and with the designated T cell population (donor CD4+ T cells) isolated from OVA-pσ1- or OVA-dosed mice. (B) In vivo suppression of proliferation of DO11.10 Tg CD4+ T cells following challenge with OVA was measured by FACS. * p ≤ 0.001 vs. mice given CD4+ T cells from OVA-dosed mice, calculated by Student’s t test. (C) Lymphocytes pooled from HNLNs, MLNs, and spleens from recipient mice were stained with anti-CD4, anti-CD25, and anti-FoxP3 mAbs and analyzed by FACS. The mean percentage (± SD) of FoxP3+ CD25−CD4+ T cells and FoxP3+ CD25+CD4+ T cells as a (left panel) proportion of total CD4+ T cells, or (right panel) DO11.10 CD4+ T cells is depicted. * p ≤ 0.001 vs. CD25+CD4+ T cells from mice given CD4+ T cells from OVA-dosed mice; § p ≤ 0.001, ** p ≤ 0.05 vs. CD25−CD4+ T cells from mice given CD4+ T cells from OVA-dosed mice, calculated by Student’s t test. (D, E) FACS analysis was performed on lymphocytes isolated from C57BL/6 mice (D) naïve or (E) nasally dosed with OVA-pσ1 three times at weekly intervals. Depicted are percentages of unstimulated Treg cells isolated from HNLNs. Percentage of FoxP3+ Treg cells (upper filled histogram) and FoxP3+ CD25−CD4+ T cells (lower filled histograms) as proportion of CD4+ T cells are plotted versus isotype control for FoxP3 (empty histogram). Results are presented as an average ± SD of 5 animals per tissue.
Adoptive transfer experiments are presented in Figs. 2B, C, and 3: naïve BALB/c mice were adoptively transferred with Vybrant CM-Dil (Molecular Probes, Eugene, OR) -labeled DO11.10 CD4+ T cells (1 × 107) isolated from naïve OVA-Tg mice and with 6 × 105 of one of the following T cell subsets: CD25+CD4+, CD25−CD4+ or total CD4+ T cells from OVA-pσ1-dosed mice or with total CD4+ T cells from OVA-dosed mice. Control mice received Tg DO11.10 CD4+ T cells and PBS (positive proliferation control). All recipients were challenged 24 h later with OVA, as described above, and four days later, HNLNs, MLNs, and spleens were evaluated by FACS for proliferation of total and Vybrant+ CD4+ T cells.
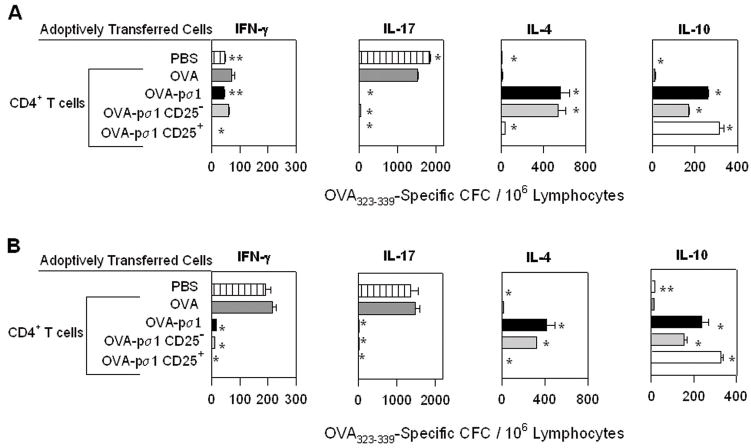
FIGURE 3.
OVA-pσ1-induced tolerance is dependent upon increased production of anti-inflammatory/regulatory cytokines and depression of proinflammatory cytokines. CD4+ T cells were isolated from (A) HNLNs and (B) spleens of recipient mice given the various CD4+ T cell subsets plus responding Tg CD4+ T cells in Fig. 2B and C. To evaluate differences in cytokine production by the responder (DO11.10) CD4+ T cells influenced to become tolerogenic or immunogenic, CD4+ T cells, CD4+ T cells were isolated from recipient mice and subjected to T cell ELISPOT assay after in vitro re-stimulation with OVA323–339 peptide. Results are depicted as cytokine-forming cells (CFC) per 106 CD4+ T cells corrected for unstimulated responses. Adoptive transfer of OVA-pσ1-specific CD25+CD4+ T cells or CD25− CD4+ T cells significantly enhanced numbers of IL-10- and IL-4-producing CD4+ T cells, respectively, in recipient mice with concomitant reductions in IFN-γ and IL-17 CFC responses. Adoptive transfer of OVA-stimulated CD4+ T cells showed enhanced IFN-γ and IL-17 CFCs with little to no IL-4 or IL-10 CFCs. * p < 0.001, ** p ≤ 0.05 vs. mice given CD4+ T cells from OVA-dosed mice, calculated by Student’s t test.
In vitro T cell assays
Cell-sorted CD4+ T cells isolated from CLNs, HNLNs, MLNs and spleens were cultured, as described elsewhere (27). Cells were pulsed with 3H-TdR (0.5 μCi/well), and 3H-TdR incorporation by proliferating CD4+ T cells (triplicate cultures) was measured and expressed as a stimulation index (SI) (27). To assess cytokine production by Treg cells and effector T cells, CD25+CD4+ and CD25−CD4+ T cells (2 × 105) were stimulated in vitro with anti-CD3 mAb-coated wells (10 μg/ml; BD Pharmingen) and a soluble anti-CD28 mAb (5 μg/ml; BD Pharmingen) for 5 days (final volume of 300 μl in a 48-well plate). Capture ELISA was used to quantify triplicate sets of samples to measure cytokine production (27).
Cytokine secretion by DO11.10 T cells cultured for 24 h or 72 h with or without OVA, OVA-pσ1, OVA + pσ1, or pσ1 stimulation was assessed by cytokine ELISA assay. Cytokine ELISA was conducted, as previously described (27), to determine the levels of IFN-γ, IL-4, IL-10, IL-17 in each sample.
Apoptosis assay
Lymphocytes isolated from spleens of naïve DO11.10 mice were cultured with or without stimulation for 24 h or 72 h in 37° C with 5 % CO2. Cells were stimulated with one of the following per 1 ml of culture: 1 mg OVA, OVA-pσ1, pσ1, 1 mg OVA + 1 mg pσ1, 50 μg OVA-pσ1, 50 μg pσ1, or 1 mg OVA + 50 μg pσ1. Percentage of apoptosis of CD4+ T cells was determined by FACS based on the co-staining of CD4+ T cells with Cy-7-AAD (BD Pharmingen), and PE-Annexin V (BD Pharmingen) according to the manufacturer’s instructions.
Statistical analysis
One-way analysis of variance (ANOVA) (p < 0.05) followed by a post hoc Tukey test was applied to evaluate statistical differences between groups in Fig. 1F, Fig. 4, and Fig. 7A. The remaining groups were analyzed by a Student’s t-test to evaluate statistical differences between groups or treatments in performed experiments, and p values < 0.05 are indicated.
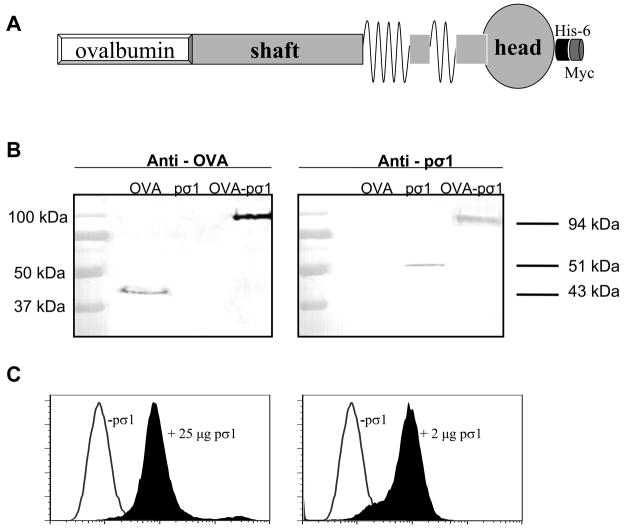
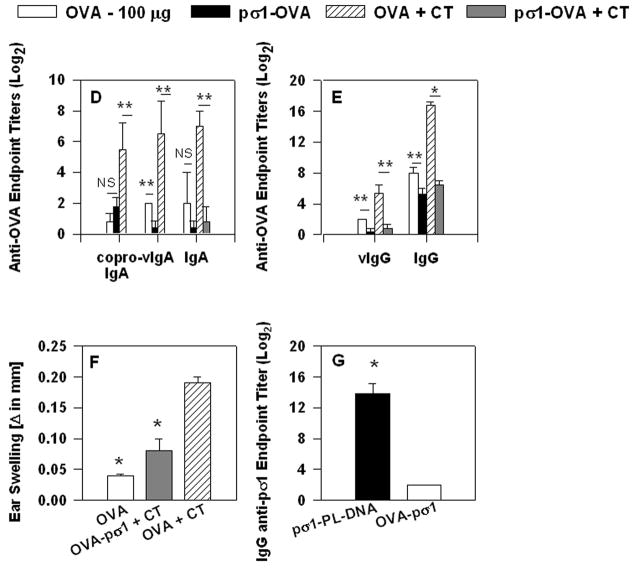
FIGURE 1.
Nasal administration of OVA-pσ1 suppresses OVA-specific immune responses. (A) Schematic representation of OVA-pσ1 protein: tolerogen, OVA and pσ1 (shaft and head), 6 histidine-tag, and Myc Ag-tag (components are not drawn to scale). (B) Western immunoblot of OVA-pσ1, pσ1, and OVA probed with mouse IgG anti-OVA mAb (left panel), or rabbit anti-pσ1 polyclonal Ab (right panel). (C) Recombinant pσ1 binds to L cells. L cells were incubated with 2 μg (left panel) or 25 μg (right panel) of his/myc tagged pσ1, followed by an addition of a biotinylated anti-pσ1 mAb and streptavidin-PE to detect pσ1 by FACS; thus, his/myc tag on pσ1's C terminus does not interfere with L cell binding. (D, E, F) BALB/c mice were nasally dosed with OVA or OVA-pσ1, with or without CT. (D, E) Serum, vaginal, and fecal samples collected on day 28 post-immunization (p.i.) were analyzed for IgA and IgG OVA-specific titers by ELISA. Mean of 10 mice ± SEM is depicted. * p < 0.001, ** p < 0.05 for OVA-pσ1- vs. OVA-, and OVA-pσ1 + CT- vs. OVA + CT- dosed mice, calculated by Student’s t test. (F) DTH reactions to OVA were measured at day 35 p.i. Mean ± SEM of 6 mice per group. Statistical significance between groups was calculated by one-way ANOVA followed by post hoc Tukey test. * p < 0.05 vs. OVA + CT; OVA vs. OVA-pσ1 were not significantly different. (G) Anti-pσ1 Ab ELISA was performed on serum samples collected from BALB/c mice dosed nasally with OVA-pσ1 or pσ1-poly-L-lysine-DNA. Mice dosed with pσ1-PL-DNA, but not OVA-pσ1, showed the pσ1-specific IgG response. * p < 0.001 for OVA-pσ1 vs. pσ1-poly-L-lysine-DNA was calculated by Student’s t test.
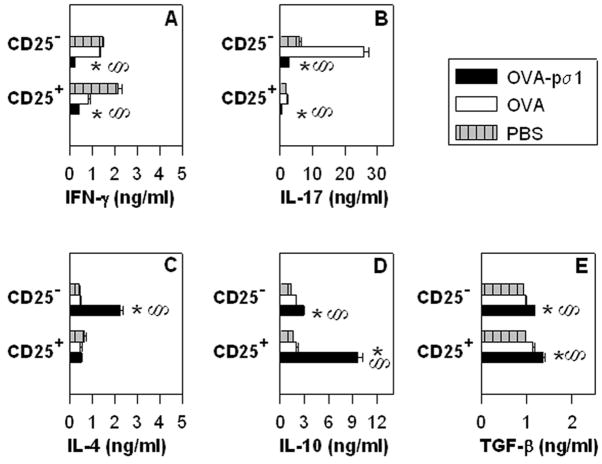
FIGURE 4.
OVA-pσ1-induced tolerance is mediated by IL-10-producing CD25+CD4+ T cells and supported by IL-4-producing CD25−CD4+ T cells. BALB/c mice were adoptively transferred with 1 x 107 DO11.10 Tg CD4+ T cells and 24 h later nasally dosed with 50 μg OVA-pσ1 or OVA only. Mice were challenged s.c. on d 3 after transfer and sacrificed 4 d later. CD25+CD4+ T cells and CD25−CD4+ T cells were isolated from HNLNs, MLNs, and spleens of these mice and cultured in vitro. Presence of (A, B) proinflammatory and (C, D, E) regulatory cytokines in cultured supernatants was measured by ELISA. CD25+CD4+ T cells from OVA-pσ1-dosed mice produced significantly more (D) IL-10, whereas the majority of (C) IL-4 was secreted by CD25−CD4+ T cells. Mean ± SEM of 5 mice per group is shown. Statistical significance for cytokine -producing CD25− or CD25+ CD4+ T cells was calculated by one-way ANOVA followed by a post hoc Tukey test. § p < 0.05 for OVA-pσ1- versus PBS-dosed mice, * p < 0.05 for OVA-pσ1- versus OVA-dosed mice.
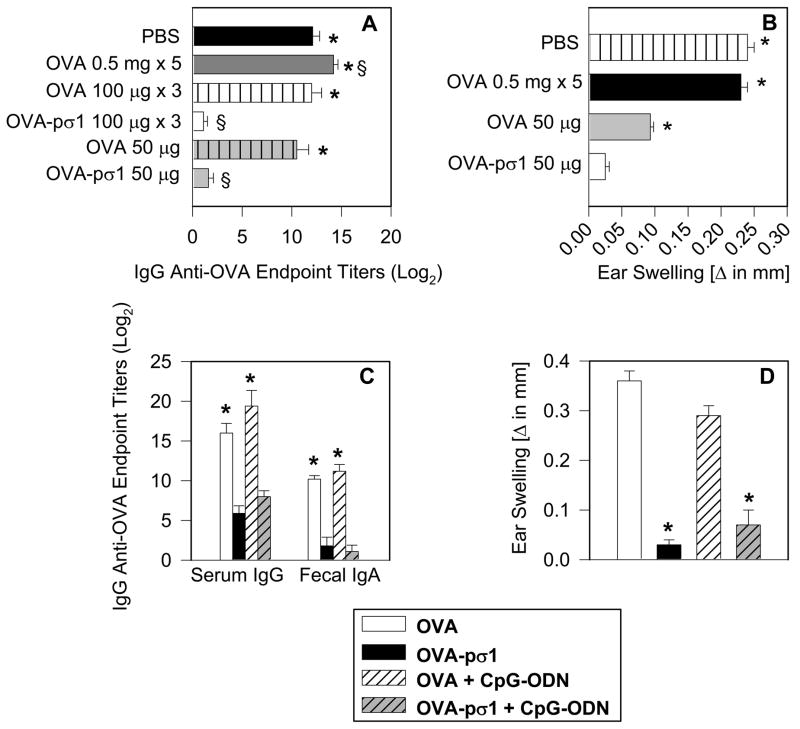
FIGURE 7.
A single nasal dose of OVA-pσ1 is sufficient to induce tolerance to OVA. (A, B) C57BL/6 mice were given OVA-pσ1, OVA, or sterile PBS and were subsequently challenged s.c. with OVA/IFA. (A) Data depict serum IgG anti-OVA Ab titers at 18 d post-challenge (28 d p.i.). Statistical significance was calculated by one-way ANOVA followed by post hoc Tukey test. * p < 0.05 versus mice nasally given 50 μg of OVA-pσ1, § p < 0.05 versus. mice given nasally 50 μg of OVA. (B) OVA-specific DTH responses were measured at day 15 p.i. (day 5 post-challenge). A single 50 μg dose of OVA-pσ1, but not OVA alone, was sufficient for tolerance induction. * p < 0.001 for OVA-pσ1-dosed vs. control mice, calculated by Student’s t test. Data are representative of three separate experiments. (C, D) BALB/c mice were nasally given 100 μg of OVA-pσ1 or OVA, with or without 25 μg of CpG. Ten days p.i., mice were challenged s.c. with OVA/IFA. Data depict (C) plasma IgG and fecal IgA anti-OVA Ab titers 18 d post-challenge (28 d p.i.), and (D) OVA-specific DTH responses at day 15 p.i. (day 5 post-challenge). Statistical significance * p < 0.001 for OVA-pσ1 versus OVA-dosed mice, or for OVA-pσ1 + CpG versus. OVA + CpG -dosed mice, calculated by Student’s t test.
Results
Nasal immunization with OVA-pσ1 induces B and T cell unresponsiveness to OVA
Our past studies (14, 23) have shown that chemically modified pσ1 can be successfully used for mucosal delivery of DNA vaccines. To further exploit pσ1 as a mucosal vaccine delivery platform, OVA cDNA was genetically fused 5′ to the pσ1 gene so as not to disrupt pσ1′s binding capacity. The resulting fusion protein was termed OVA-pσ1 (Fig. 1A). This modified OVA retained its Ag integrity when detected using an anti-OVA mAb (Fig. 1B), and the pσ1 portion retained its binding activity to L cells (Fig. 1C). To test its immunogenicity, OVA-pσ1 was applied nasally with or without the potent mucosal adjuvant CT (Fig. 1D, E, and F). BALB/c mice dosed with OVA-pσ1 or OVA-pσ1 plus CT were unresponsive, as evident by the depressed or lack of OVA-specific IgG and IgA Abs, when compared to mice given OVA alone or OVA plus CT (Fig. 1D and E). Even immunization with OVA alone, which is a poor mucosal immunogen, elicited greater Ag-specific IgG Ab titers than did OVA-pσ1-dosed mice (Fig. 1E). To test unresponsiveness to OVA challenge, mice dosed with OVA, OVA plus CT, or OVA-pσ1 and CT as mucosal adjuvant were peripherally challenged with OVA. Results showed significantly lower OVA-specific DTH responses by OVA and OVA-pσ1 plus CT-dosed mice when compared to mice given OVA plus CT as mucosal adjuvant (Fig. 1F). To discern whether OVA-pσ1-induces tolerance to pσ1, serum samples collected from BALB/c mice nasally dosed with OVA-pσ1 or with DNA complexes with pσ1-poly-L-lysine were tested by Ab ELISA. Virtually no pσ1-specific IgG Ab titers were detected in mice dosed with OVA-pσ1 compared to mice given DNA complexes with pσ1-poly-L-lysine, which exhibited IgG titers in excess of 213 (Fig. 1G). Thus, OVA-pσ1 tolerizes the host to pσ1- and OVA-specific B and T cell responses, even in the presence of co-administered CT, and these results show the feasibility of using pσ1 as a mucosal vaccine delivery platform for induction of mucosal tolerance, rather than immunity.
Tolerance can be adoptively transferred with OVA-specific CD4+ T cells
Using CD4+ T cells isolated from OVA-transgenic (Tg) DO11.10 TCR mice, we hypothesized that OVA-pσ1 would induce tolerance in an OVA-specific fashion. Splenic CD4+ T cells isolated from DO11.10 Tg mice were adoptively transferred into naïve BALB/c mice. Twenty four h after adoptive transfer, the recipient mice were dosed nasally with 400 μg of OVA to induce tolerance (28) or with only 80 μg of OVA-pσ1. Control mice received an intramuscular (i.m.) injection of 400 μg of OVA as a positive immunized control group (28) or nasal sPBS (negative control group). Since Treg cells are highly enriched in CLNs (28), CD4+ T cells were isolated from recipient CLNs and were adoptively transferred 3 d after immunization into a second group of naïve recipient BALB/c mice, which, 24 h later, were challenged s.c. with OVA. Five days post-peripheral challenge, the second recipient mice were evaluated for tolerance induction by an in vitro OVA-specific proliferation assay (Fig. 2A). Contrary to the i.m. immunized group, CD4+ T cells originating from mice dosed nasally with OVA-pσ1 or with a high dose of OVA failed to proliferate following in vitro stimulation with OVA (Fig. 2A). The CD4+ T cells originated from the i.m. OVA-dosed mice showed a ~5-fold increase in OVA-specific proliferation. Thus, these data show that OVA-pσ1 can stimulate OVA-specific tolerance via CD4+ T cells.
Dosing with OVA-pσ1 induces FoxP3+ Treg cells
To define the possible regulatory T cells induced by tolerization with OVA-pσ1, FACS analysis was performed. Mice given nasal OVA-pσ1 revealed a significant induction of FoxP3+ CD25+CD4+ T cells (Fig. 2E), even following a peripheral OVA challenge (Tables I and II). More than a 40 % increase in FoxP3+ CD25+CD4+ T cells in HNLNs (Fig. 2E) and in MLNs (data not shown) was observed in mice given nasal OVA-pσ1, when compared to naïve mice (Fig. 2D and E -upper histograms). In a similar fashion, OVA-pσ1-dosed mice, but not naïve mice, showed a > 60 % increase in FoxP3+ CD25−CD4+ T cells (Fig. 2D and E - lower histograms). The effector function of Treg cells is often associated with secretion of regulatory cytokines, such as IL-10 and/or TGF-β1. Subsequent FACS analysis confirmed that increased Treg cells in OVA-pσ1-dosed mice expressed significantly more IL-10, but not TGF-β1, when compared with PBS- dosed mice (Table I). These results further suggest that tolerance induced by OVA-pσ1 is supported by Treg cells, which may act in an IL-10-dependent manner.
Table I.
Nasal immunization with OVA-pσ1 induces IL-10-secreting CD25+CD4+ Treg cells
| Immunization Groupa | OVA-pσ1b | sPBSb |
|---|---|---|
| % CD25+CD4+ Treg | 15.4 ± 4.5c | 9.7 ± 3.1 |
| % IL-10+ Treg | 63.2 ± 6.4d | 45.1 ± 6.6 |
| % of TGF-β+ Treg | 49.4 ± 3 | 46 ± 3.2 |
C57BL/6 mice were given 100 μg of OVA-pσ1 or PBS nasally on days 0, 1, and 2, and were challenged s.c. on day 10 p.i. with OVA in IFA.
FACS analysis was performed on lymphocytes isolated from HNLNs and in vitro stimulated for 72 h with 1 mg OVA. Mean ± SEM of 5 mice per group is presented. Mice nasally dosed
with OVA-pσ1 showed significant increases in OVA-specific Treg cells that were IL-10+.
Statistical significance was calculated by Student’s t test.
Differences between OVA-pσ1- versus PBS-dosed mice. p = 0.031.
Differences between OVA-pσ1- versus PBS-dosed mice. p = 0.008.
Table II.
Nasal immunization with OVA-pσ1-induces FoxP3+ CD25+ and FoxP3+ CD25− CD4+ T cells
| Immunization Groupa | OVA-pσ1b | OVA oralb | OVA nasalb | sPBSb |
|---|---|---|---|---|
|
% FoxP3+ CD25+CD4+ T cells
| ||||
| CLN | 19.15 ± 1.13c, d | 11.6 ± 1.07 | 6.92 ± 0.8 | 5.92 ± 1.44 |
| HNLN | 18.04 ± 1.5c, d | 11.98 ± 1.71 | 7.29 ± 1.4 | 7.5 ± 1.03 |
| MLN | 17.46 ± 2.8d | 17.45 ± 0.5 | 8.07 ± 1.01 | 7.3 ± 1.4 |
| PP | 18.35 ± 1.48c, d | 14.45 ± 1.4 | 6.91 ± 0.9 | 6.7 ± 1.84 |
| Spleen | 15.95 ± 2.69c, d | 9.49 ± 2.57 | 5.56 ± 1.16 | 7.33 ± 2.08 |
|
| ||||
|
% FoxP3+ CD25− CD4+ T cells
| ||||
| CLN | 22 ± 1.42c, d | 16.7 ± 2.4 | 14 ± 2.55 | 14.5 ± 3.39 |
| HNLN | 21.88 ± 2.71c, d | 15.6 ± 1.5 | 14.13 ± 1.05 | 10.5 ± 1.14 |
| MLN | 19.83 ± 2.4c, d | 10.02 ± 1.7 | 13 ± 0.95 | 12.9 ± 1 |
| PP | 19.93 ± 2.16c, d | 12.03 ± 3.36 | 12.05 ± 1.64 | 12.25 ± 2.19 |
| Spleen | 21.73 ± 2.81c, d | 10.5 ± 1.42 | 10.2 ± 3.01 | 9.62 ± 3.51 |
C57BL/6 mice were nasally dosed with 100 μg OVA-pσ1, OVA or PBS, or orally with 25 mg OVA and challenged, as described in Table I.
FACS analysis was performed on lymphocytes isolated from CLNs, HNLNs, MLNs, PPs, and spleens and in vitro stimulated for 72 h with 1 mg of OVA. Mean ± SD of 5 mice per group is presented. Mice nasally dosed with OVA-pσ1 showed significant increases in OVA-specific FoxP3+ CD25+CD4+ T cells and FoxP3+ CD25− CD4+ T cells.
Statistical significance was calculated by Student’s t test.
p < 0.04, differences between OVA-pσ1- versus OVA oral-dosed mice.
p < 0.005, differences between OVA-pσ1- versus i.n. OVA- and PBS-dosed mice.
OVA-specific Treg, as well as CD25−CD4+ T cells, suppress in vivo proliferation of Ag-specific effector CD4+ T cells
The combined evidence from the adoptive transfer study and from the phenotypic analysis suggests that OVA-pσ1 induces Ag-specific Treg cells. To investigate the ability of OVA-pσ1-induced Treg cells to inhibit proliferation of OVA-specific effector CD4+ T cells in vivo, naïve BALB/c mice were adoptively transferred with CD4+, CD25+CD4+, or CD25−CD4+ T cells isolated from mice given nasal OVA-pσ1 or with CD4+ T cells from mice given only nasal OVA. All recipient mice simultaneously received Vybrant-labeled OVA-specific CD4+ T cells isolated from Tg DO11.10 mice. Recipient mice were s.c. challenged with OVA in IFA 24 h after adoptive transfer, and 4 d later, Tg CD4+ T cells were evaluated by flow cytometry for in vivo proliferation (Fig. 2B). Proliferation of the Tg CD4+ T cells was significantly reduced in mice receiving OVA-pσ1-specific Treg cells, since only 15 % of these cells underwent cell division (Fig. 2B). In contrast, adoptively transferred CD4+ T cells from OVA-immunized mice, allowed expansion of the Tg CD4+ T cells, as evidenced by > 55 % of them undergoing proliferation (Fig. 2B). Significant suppression of the Tg CD4+ T cell proliferation was also obtained using CD25−CD4+ and total CD4+ T cells from OVA-pσ1-dosed mice (p < 0.001), but to a lesser extent (20 % proliferation). This study shows that OVA-pσ1-derived Treg cells significantly inhibited the proliferation of Tg CD4+ T cells greater than OVA-pσ1-derived CD25− (p = 0.009) or OVA-pσ1-derived total CD4+ T cells (p = 0.001). Collectively, these results suggest that OVA-pσ1-induced tolerance is mediated by CD25+CD4+ T cells and, interestingly, may also be contributed in part by CD25−CD4+ T cells, which inhibited the proliferation of OVA-specific effector T cells.
In an effort to define the mechanism by which OVA-pσ1-derived CD4+ T cells inhibit proliferation of these Tg CD4+ T cells, we analyzed the phenotypes of the CD4+ T cells isolated from the OVA-challenged recipients. Mice receiving OVA-pσ1-derived CD25−CD4+ T cells showed significant increases in FoxP3+ CD25−CD4+ T cells (~ 40 %) and elevated numbers of FoxP3+ Treg cells (~ 20 %; Fig. 2C -left panel). On the other hand, mice adoptively transferred with OVA-pσ1-derived CD25+CD4+ T cells showed even greater increases in FoxP3+ Treg cells (~ 30 %), but FoxP3+ CD25−CD4+ T cells in these mice were not elevated and, in fact, were equivalent with control CD4+ T cells from OVA-immunized or PBS-dosed mice (Fig. 2C -left panel). To determine if adoptively transferred OVA- or OVA-pσ1-derived CD4+ T cells induce CD4+ regulatory T cells among the responder DO11.10 CD4+ T cell subset, the percentages of DO11.10 FoxP3+ CD25−CD4+ and FoxP3+ CD25+CD4+ T cells in mice given OVA- or OVA-pσ1-derived CD4+ T cells were evaluated. FACS analysis revealed that prior to the adoptive transfer, the DO11.10 CD4+ T cell population contained ~ 5 % Treg cells and ~ 10 % FoxP3+ CD25−CD4+ T cells (Fig. 2C - right panel). These FoxP3+ CD25+CD4+ T cells expanded in mice given OVA-pσ1-derived (both CD25+ and CD25−) CD4+ T cells, although the greatest increase, by nearly 5-fold, was in mice given OVA-pσ1-derived CD25+CD4+ T cells (Fig. 2C - right panel). In contrast, expansion of DO11.10 FoxP3+ CD25+CD4+ T cells was not observed in mice dosed with PBS or adoptively transferred with OVA-derived CD4+ T cells. Interestingly, more than a 3-fold increase in Tg FoxP3+ CD25−CD4+ T cells were observed in mice co-transferred with OVA-pσ1-derived CD25−CD4+ T cells. By examining the expansion of Tg FoxP3+ CD25−CD4+ T cells in mice given OVA-pσ1-derived CD25+CD4+ T cells, it was found that these FoxP3+ CD25−CD4+ T cells expanded to a lesser extent. No increases in FoxP3+ CD25−CD4+ T cells were observed in mice given OVA- or PBS-dosed recipients given Tg CD4+ T cells (Fig. 2C - right panel). This evidence suggests that OVA-pσ1-induced FoxP3+ CD25−CD4+ T cells do contribute to OVA-pσ1-mediated inhibition of effector T cell proliferation, possibly via expansion of either CD4+ T cell subset or via conversion of FoxP3+ T cells (29).
OVA-pσ1-induced tolerance is mediated by increased regulatory cytokine production
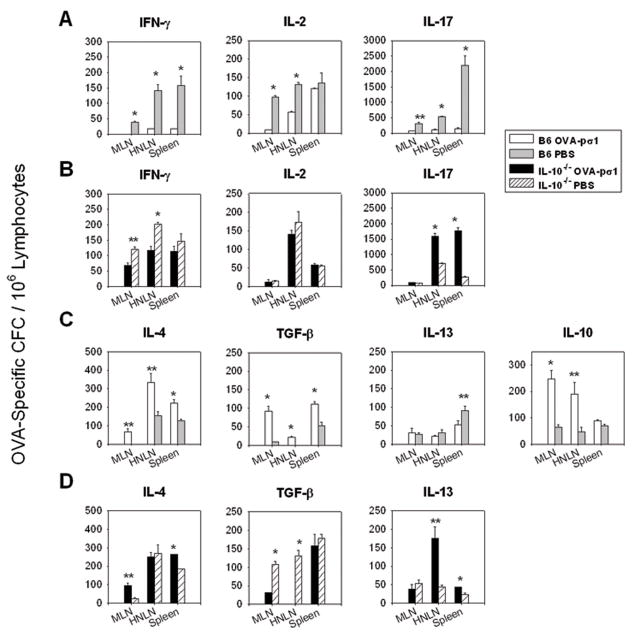
To investigate the specific mechanisms of the observed tolerance, cytokine secretion by CD4+ T cells was measured. Total CD4+ T cells were isolated from recipient HNLNs and spleens given Tg CD4+ T cells and various CD4+ T cell subsets, as described in the adoptive transfer studies in Fig. 2B and C. After in vitro restimulation with OVA323–339 peptide, the cytokine profiles of the responding OVA-specific CD4+ T cells were analyzed. Unlike controls, mice receiving OVA-pσ1-derived (CD25+, CD25−, and total CD4+) T cells failed to induce IL-17-secreting CD4+ T cells and showed no or reduced IFN-γ-secreting CD4+ T cells in all tested lymphoid tissues (Fig. 3A and B). Instead, these mice showed greater anti-inflammatory responses, as evidenced by significant increases in the numbers of IL-10- and IL-4-producing CD4+ T cells. Adoptive transfer of Treg cells resulted in a 15-fold enhancement in IL-10-producing CD4+ T cells, whereas recipient mice given CD25−CD4+ T cells from OVA-pσ1-dosed mice showed a 10-fold enhancement IL-4-producing CD4+ T cells when compared to recipient mice given CD4+ T cells from OVA-vaccinated or PBS-dosed mice (Fig. 3).
To evaluate the specific cytokine profiles for CD25+ and CD25− T cell subsets from OVA-pσ1-dosed mice, BALB/c mice adoptively transferred with DO11.10 Tg CD4+ T cells were given a single nasal dose of OVA-pσ1, OVA, or PBS. Recipient mice were then challenged with OVA/IFA on day 3 post-immunization (p.i.) and sacrificed on day 7 p.i. Induction of tolerance was confirmed in OVA-pσ1-dosed mice by the absence of an OVA-specific DTH response (data not shown). CD25+CD4+ and CD25−CD4+ T cells from pooled HNLNs, MLNs, and spleens were stimulated with plate-bound anti-CD3 and soluble anti-CD28 mAbs. Analysis of cytokines produced by cultured cells revealed that the majority of IL-10 from OVA-pσ1-dosed mice was derived from Treg cells (Fig. 4D), whereas almost all of the IL-4 was produced by CD25-CD4+ T cells (Fig. 4C). These data further confirm that OVA-pσ1-induced tolerance depends largely upon IL-10-producing Treg cells and is further supported Th2 cells. Although TGF-β1 was secreted by both CD25+ and CD25−CD4+ T cells, this cytokine was not strikingly elevated in OVA-pσ1-dosed mice when compared to OVA- and PBS-dosed mice (Fig. 4E), further implying that TGF-β1 may have a lesser role for OVA-pσ1-induced tolerance. Contrary to control mice, production of proinflammatory cytokines, IFN-γ (Fig. 4A) and IL-17 (Fig. 4B), was significantly diminished by both Treg and CD25−CD4+ T cell subsets.
IL-10 is necessary for induction of OVA-pσ1-mediated tolerance
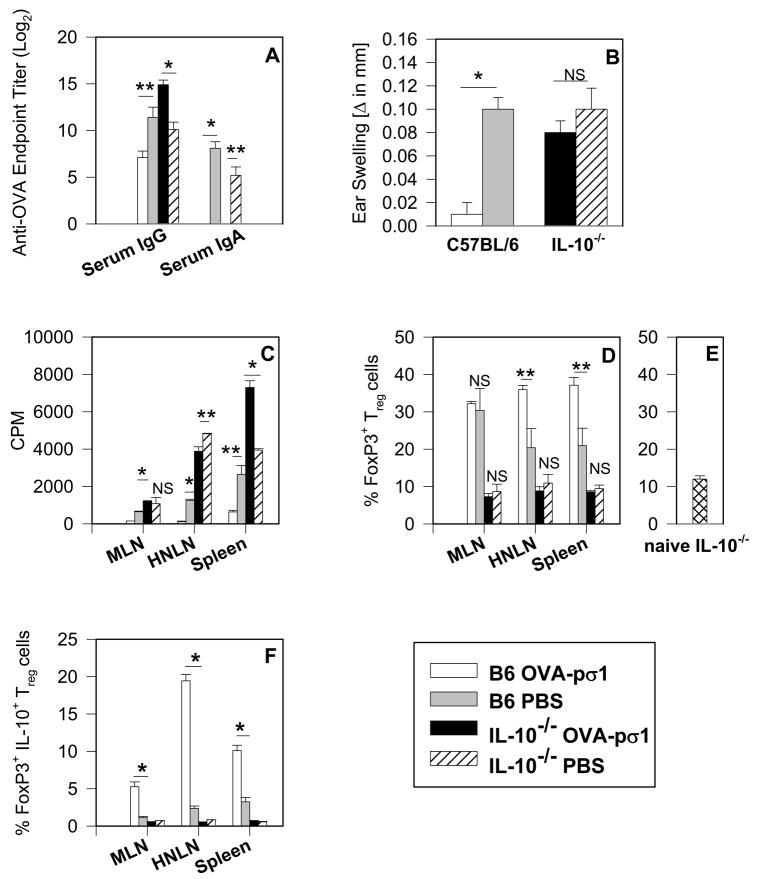
Results thus far have shown that mucosal administration of OVA-pσ1 induces secretion of regulatory cytokines, IL-4, IL-10, and TGF-β, although the most pronounced increase was observed in the production of IL-10. Since past studies have shown that IL-10 is not responsible for tolerance induction (30–32), we queried whether tolerance could be induced in IL-10−/− mice given nasal OVA-pσ1. C57BL/6 mice given nasal OVA-pσ1 were unresponsive to OVA, as evidenced by the low plasma IgG and mucosal IgA anti-OVA Ab titers and a lack of DTH responses, when compared with PBS-primed mice (Fig. 5A and B). In contrast, OVA-pσ1-dosed IL-10−/− mice became immune to OVA after peripheral challenge, as did PBS-primed IL-10−/− mice (Fig. 5A and B). CD4+ T cells isolated from HNLNs, MLNs, and spleens of IL-10−/− mice dosed with OVA-pσ1 showed significant increases in OVA-specific T cell proliferation when compared to the same cells obtained from tolerized C57BL/6 mice. As evidenced in OVA-pσ1-dosed C57BL/6 mice, these had at least three-fold less CD4+ T cell proliferative responses to OVA in HNLNs and spleens than their PBS-dosed littermates (Fig. 5C). Interestingly, FACS analysis of FoxP3+ Treg cells revealed no differences in Treg cell numbers between PBS- and OVA-pσ1-dosed IL-10−/− mice (Fig. 5D), and, in fact, they resembled baseline levels of CD25+CD4+ T cells in these mice (Fig. 5E). In contrast, the HNLNs and spleens of OVA-pσ1-dosed C57BL/6 mice contained significantly more OVA-specific Treg cells than the HNLNs and spleens of both PBS-dosed C57BL/6 mice and OVA-pσ1-dosed IL-10−/− mice, the latter result suggesting that Treg cells in IL-10−/− mice were simply not induced (Fig. 5D). Additionally, almost 50 % of the FoxP3+ Treg cells in HNLNs and spleens of OVA-pσ1-dosed C57BL/6 mice showed intracellular expression of IL-10 (Fig. 5F), confirming the supportive role of IL-10 in OVA-pσ1-induced tolerance. Upon OVA restimulation, analysis of cytokine-secreting CD4+ T cells from C57BL/6 (Fig. 6A and C) and IL-10−/− mice (Fig. 6B and D) was performed. As expected, OVA-pσ1-dosed C57BL/6 mice showed a significant increase in CD4+ T cells secreting regulatory cytokines IL-10 and TGF-β1 in HNLNs and MLNs, and IL-4-producing cells in all tested tissues when compared with PBS-dosed mice (Fig. 6C). Numbers of CD4+ T cells producing IL-13 were not significantly different between immunized and control mice (Fig. 6C).
FIGURE 5.
IL-10 is required for induction and maintenance of OVA-pσ1-mediated tolerance. IL-10−/− and C57BL/6 (IL-10+/+) (B6) mice were given 100 μg of OVA-pσ1 or PBS nasally on days 0, 1, and 2 and were then challenged s.c. on day 10 p.i. with OVA in IFA. (A) OVA-specific ELISA was performed on serum samples collected from OVA-pσ1- and PBS-dosed C57BL/6 and IL-10−/− mice on day 17 p.i. OVA-pσ1-dosed IL-10−/− mice elicited a serum IgG anti-OVA response. (B) OVA-specific DTH responses measured on day 15 p.i. OVA-pσ1-dosed IL-10−/− mice were responsive to OVA. (C) CD4+ T cells isolated from HNLNs, MLNs, and spleens of OVA-pσ1- or PBS-dosed C57BL/6 (IL-10+/+) and IL-10−/− mice were measured for their proliferative responses after in vitro stimulation with OVA. CD4+ T cells from IL-10−/− mice dosed with OVA-pσ1 or PBS, and PBS-dosed C57BL/6 mice proliferated in response to OVA. Depicted values are corrected for 3H-TdR uptake by unstimulated cells. (D) Percentages of FoxP3+ CD25+CD4+ T cells were measured by FACS after in vitro stimulation with OVA, and depicted are values corrected for unstimulated cells. IL-10−/− mice failed to show induction of Treg cells independent of treatment. The mean of two experiments ± SEM per group is shown. * p < 0.001, ** p < 0.05 for OVA-pσ1- vs. PBS-dosed C57BL/6 or IL-10−/− mice; NS, not significant, calculated by Student’s t test. (E) Depicted is the percentage of CD25+CD4+ T cells for the combined MLNs, PPs, and spleens from 5 naïve IL-10−/− mice. Results determined by FACS show the mean ± SD. (F) Percentages of FoxP3+IL-10+ Treg cells, as a proportion of CD25+CD4+ T cells, were measured by FACS. Treg cells induced in C57BL/6 mice given nasally OVA-pσ1 expressed IL-10, whereas significantly less IL-10 was expressed by PBS-dosed C57BL/6 mice. Average ± SEM of 4 mice is shown. * p < 0.001 for OVA-pσ1-versus PBS dosed C57BL/6 mice, calculated by Student’s t test; NS = not significant.
FIGURE 6.
Immunization of IL-10−/− mice with OVA-pσ1 increases IL-17- and IFN-γ producing CD4+ T cells with concomitant increases in anti-inflammatory cytokines, (IL-4 and IL-13) responses when compared to similarly treated C57BL/6 mice. C57BL/6 (IL-10+/+) (B6) and IL-10−/− mice were immunized and challenged, as described in Fig. 5 legend. CD4+ T cells isolated from HNLNs, MLNs, and spleens were restimulated with OVA, and cytokine-specific T cell ELISPOT was performed. CFC/106 CD4+ T cells in (A, C) C57BL/6 and (B, D) IL-10−/− mice are depicted. For OVA-pσ1-dosed C57BL/6 mice, CD4+ T cells producing anti-inflammatory cytokines in MLNs and HNLNs were significantly elevated when compared with (C) PBS-dosed mice. The numbers of CD4+ T cells producing (A) proinflammatory cytokines were significantly reduced in OVA-pσ1-dosed mice versus PBS-dosed C57BL/6 mice. OVA-pσ1-dosed IL-10−/− mice showed significant increases when compared with PBS-dosed mice in (B, D) IL-4-, IL-13-, and IL-17-producing CD4+ T cells, but not TGF-β-producing CD4+ T cells. IFN-γ- and IL-17-producing CD4+ T cells were significantly elevated in IL-10−/− mice given OVA-pσ1 when compared to OVA-pσ1-dosed C57BL/6 mice (A, B). Depicted is the mean of two experiments ± SEM. * p < 0.001, ** p < 0.05 for OVA-pσ1- versus PBS-dosed C57BL/6 or IL-10−/− mice, calculated by Student’s t test.
Production of proinflammatory cytokines, IFN-γ, IL-2, and IL-17 (Fig. 6A), was significantly inhibited in all tested tissues of OVA-pσ1-dosed C57BL/6 mice. IL-10−/− mice revealed that, although the IL-4 and IL-13 responses were significantly more elevated in OVA-pσ1- than PBS-dosed mice (Fig. 6D), TGF-β-secreting CD4+ T cells were significantly depressed, except in the spleen (Fig. 6D). No significant differences in numbers of IL-2-producing CD4+ T cells were observed between OVA-pσ1- and PBS-dosed IL-10−/− mice. Consistent with an antagonistic relationship between IFN-γ and IL-17 (33), there was a significant decrease in IFN-γ and a concomitant increase in the IL-17 in OVA-pσ1-dosed IL-10−/− mice when compared to PBS-dosed mice (Fig. 6B).
OVA-pσ1- mediated tolerance can be induced after a single dose
Since the pσ1-based approach offers the possibility of inducing tolerance while using significantly less Ag, the persistence of OVA-pσ1-induced tolerance was investigated after single dose. C57BL/6 mice were nasally given either 100 μg per dose of OVA, OVA-pσ1, or sterile PBS for three consecutive days, or a single 50 μg dose of OVA-pσ1 or OVA alone. Additionally, a group of C57BL/6 mice was given 0.5 mg of OVA nasally for 5 consecutive days. Ten days p.i., all mice were challenged with OVA by the s.c. route. One day prior to challenge, no OVA-specific serum IgG Ab responses in either immunization group were detected (data not shown). By day 18 post-challenge (day 28 p.i.), mice given OVA or PBS revealed significantly elevated OVA-specific Ab titers in both mucosal (not shown) and systemic immune compartments, and these responses were sustained (Fig. 7A). Moreover, we were unable to induce tolerance to OVA, even in mice dosed nasally with as much as 2.5 mg of OVA alone. In contrast, virtually no OVA-specific serum-IgG Abs (Fig. 7A) nor DTH responses (Fig. 7B) were observed in OVA-challenged mice given a single or three nasal doses of OVA-pσ1. These data show that mice given OVA-pσ1 nasally are tolerized to OVA and that this tolerance can be induced by substantially lower doses of pσ1-delivered Ag.
While OVA-pσ1 can induce tolerance to OVA, even in the presence of CT, chemically modified pσ1 is found to be immunogenic. Mucosal delivery of DNA complexed to poly-L-lysine-modified pσ1 results in enhanced transgene-specific immune responses (14). To determine whether there is an adjuvant effect by the plasmid cDNA that can overcome the OVA-pσ1-induced tolerance, BALB/c mice were given a single nasal dose of OVA or OVA-pσ1 alone, or co-administered with CpG-ODN. In contrast to mice dosed with OVA or OVA + ODN, mice dosed with OVA-pσ1 alone or OVA-pσ1 + ODN showed significantly reduced OVA-specific serum and mucosal Ab (Fig. 7C) and DTH responses (Fig. 7D). Therefore, the tolerogenic effect of this pσ1-based delivery system cannot be overridden by co-administration of the immune-modulatory molecule CpG-ODN.
Mucosal administration of OVA-pσ1 is necessary for induction of tolerance
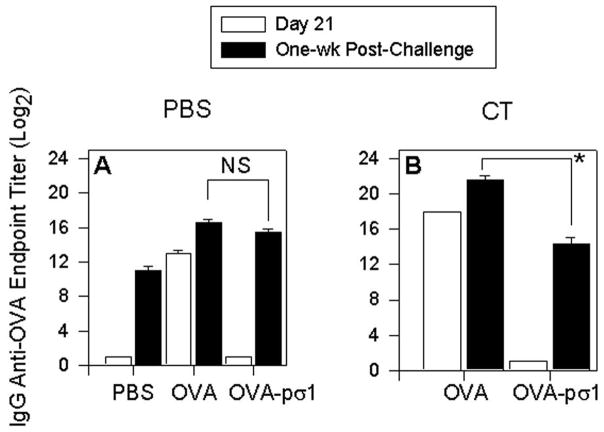
We have shown here that mucosal administration of OVA-pσ1 induces a low-dose tolerance to OVA. In an effort to determine if the pσ1-mediated tolerance can be induced via a non-mucosal route, mice were i.m. immunized on days 0, 7, and 14 with OVA-pσ1 without or with CT and compared to mice similarly immunized with OVA (Fig. 8). On day 21 (one week after the last immunization), mice were evaluated for serum IgG anti-OVA Ab titers by ELISA. As expected, OVA-immunized responded well, but naïve and OVA-pσ1-immunized mice remained OVA unresponsive (Fig. 8A and B - open bars). All mice were subsequently challenged on day 28 with OVA in IFA, and one week later, serum from individual mice was collected and measured for IgG anti-OVA titers. Even though, mice dosed with OVA-pσ1 + CT (Fig. 8B) showed reduced Ab titers relative to OVA + CT-dosed mice, these anti-OVA Ab titers were greater than those obtained in the OVA-challenged, naïve mice (Fig. 8A). Thus, these results show that induction of the optimal tolerance to OVA via the use of pσ1 requires mucosal, not parenteral, administration of OVA-pσ1 (Fig. 8A and B - black bars).
FIGURE 8.
Parenteral immunization with OVA-pσ1 induces anti-OVA Ab response following OVA challenge. C57BL/6 mice were i.m. immunized with (A) PBS, 50 μg OVA, or OVA-pσ1, or (B) with 50 μg OVA or OVA-pσ1 plus 1 μg CT, on days 0, 7, and 14. On day 21, sera were collected and analyzed for the presence of anti-OVA IgG Abs by ELISA (open bars), and showed unresponsiveness by PBS-, OVA-pσ1-, and OVA-pσ1 + CT-dosed mice. On day 28, mice were s.c. challenged with OVA in IFA. Sera collected from mice one week post-challenge (day 35) revealed the presence of IgG anti-OVA Abs (black bars) in all tested groups, although the anti-OVA response was higher in mice dosed with OVA + CT compared to OVA-pσ1 + CT-dosed mice. NS = not significant; * p < 0.001 for OVA vs. OVA-pσ1 and OVA + CT vs. OVA-pσ1 + CT -dosed mice, calculated by Student’s t test.
OVA-pσ1 induces apoptosis of OVA-Tg CD4+ T cells in vitro
Mucosal delivery of OVA-pσ1 triggers the regulatory T cell response and secretion of anti-inflammatory cytokines. In contrast, parenteral administration of OVA-pσ1 induces anti-OVA Ab response after s.c. challenge with OVA. To evaluate if pσ1 has a direct impact on CD4+ T cell populations, or if the induction of tolerance relies solely on the pσ1‘s interaction with the mucosal epithelium, an in vitro study was performed. Lymphocytes isolated from naïve DO11.10 Tg mice were cultured with high- (1 mg/ml) or low-dose (0.05 mg/ml) of OVA-pσ1 or pσ1 and compared to in vitro cultures stimulated with OVA or OVA + pσ1. After 24 or 72 h, cells and culture supernatants were harvested and evaluated for CD4+ T cell apoptosis by FACS, and cytokine production by ELISA, respectively. Only about 13 % of Tg CD4+ T cells underwent apoptosis in response to in vitro stimulation with OVA for up to 72 h. In contrast, stimulation with pσ1, OVA-pσ1, or OVA + pσ1 induced much greater apoptosis of Tg CD4+ T cells after 24 and 72 h (Table III), and the extent of this apoptosis was time- and dose- dependent. Less than 31 % of these cells died after 24 h of culture with a low-dose (0.05 mg/ml) stimulation, whereas 1 mg/ml of OVA-pσ1, pσ1 or OVA + pσ1 doubled the number of apoptotic cells at this time point (Table III). After 72 h, low-dose pσ1 exposure resulted in ~ 54 – 62 % of Tg CD4+ T cell undergoing apoptosis unlike high-dose treatment that resulted in ~ 80 % apoptosis (Table III). Concomitant cytokine analysis of cultured supernatants revealed increased production of IL-4 and IL-10 by the cells stimulated for 24 h with 1 mg/ml OVA-pσ1 or 72 h with 0.05 mg/ml OVA-pσ1 (Table IV). Consistently, these OVA-pσ1-stimulated cells showed significant reduction in inflammatory cytokines IFN-γ and IL-17 at both time points, when compared to OVA-stimulated cells. Cells cultured with a high-dose of OVA-pσ1 for 72 h failed to produce any cytokines, presumably due to apoptosis of 80 % of these cells (Table III). Interestingly, cells incubated with unconjugated pσ1 and OVA mimicked the inflammatory response of OVA-stimulated cells, showing elevated IL-17 and IFN-γ secretion after 24 h, and even after 72 h, the production of IL-10 and IL-4 by these cells was significantly reduced in relation to OVA-stimulated cells (Table IV). These results offer an alternative mechanism in which pσ1 may induce apoptosis of OVA-responsive CD4+ T cells if intact pσ1 survives delivery beyond the initial cell binding to the mucosal epithelium or M cells.
Table III.
Apoptosis of Tg DO11.10 CD4+ T cells
| Treatment a | % Apoptosis of DO11.10 Tg CD4+ T Cells b | ||||
|---|---|---|---|---|---|
| Ag | Dose | 24 hours c | P1d | 72 hours e | P2f |
| media | 7.06 ± 0.6 | 10.37 ± 0.92 | |||
| OVA | 1 mg/ml | 10.96 ± 2.82 | 12.73 ± 6.53 | ||
| OVA-pσ1 | 1 mg/ml | 67.35 ± 7.97 | < 0.001 | 83.80 ± 7.10 | < 0.001 |
| OVA-pσ1 | 0.05 mg/ml | 30.99 ± 3.98 | < 0.001 | 54.41 ± 11.6 | 0.003 |
| pσ1 | 1 mg/ml | 77.82 ± 4.82 | < 0.001 | 86.52 ± 11.8 | < 0.001 |
| pσ1 | 0.05 mg/ml | 32.43 ± 7.26 | 0.005 | 62.58 ± 10.6 | < 0.001 |
| OVA + pσ1 | 1 mg/ml, 1 mg/ml | 77.56 ± 8.42 | < 0.001 | 80.34 ± 8.44 | < 0.001 |
| OVA + pσ1 | 1mg/ml, 0.05 mg/ml | 29.83 ± 9.86 | 0.025 | 62.29 ± 10.13 | < 0.001 |
Lymphocytes were isolated from spleens of naïve DO11.10 Tg mice. Cells were stimulated in vitro with designated concentrations of Ags and analyzed by FACS.
Apoptosis was determined by co-staining with Annexin-V and 7-AAD.
Mean ± SD of three to four replicates are presented after 24 and 72 h of culture.
p-values for DO11.10 CD4+ T cells stimulated with OVA versus OVA-pσ1, pσ1, or OVA + pσ1 for 24 or 72 hours.
Table IV.
Cytokine production by in vitro cultured DO11.10 lymphocytes
| Treatment a | Cytokine Secretion after 24 Hours b | ||||
|---|---|---|---|---|---|
| Ag | Dose | IL-4 (ng/ml) d | IL-10 (ng/ml) e | IL-17 (ng/ml) f | IFN-γ (ng/ml) g |
| OVA | 1 mg/ml | 0.58 ± 0.03 | 0.66 ± 0.04 | 5.59 ± 0.54 | 4.06 ± 0.52 |
| OVA-pσ1 | 1 mg/ml | 1.20 ± 0.1 § | 2.76 ± 0.14 * | 0.37 ± 0.11 * | 0.31 ± 0.08 § |
| OVA-pσ1 | 0.05 mg/ml | 1.67 ± 0.2 § | 4.73 ± 0.29 * | 0 ± 0 * | 0 ± 0 * |
| pσ1 | 1 mg/ml | 0 ± 0 * | 0.26 ± 0.04 § | 0.63 ± 0.17 * | 0.22 ± 0.07 § |
| pσ1 | 0.05 mg/ml | 0 ± 0 * | 0 ± 0 * | 0.58 ± 0.14 * | 0 ± 0 * |
| OVA + pσ1 | 1 mg/ml, 1 mg/ml | 0 ± 0 * | 0.56 ± 0.02 | 4.17 ± 0.6 | 2.6 ± 0.45 |
| OVA + pσ1 | 1mg/ml, 0.05 mg/ml | 0 ± 0 * | 0.42 ± 0.17 | 1 ± 0.14 § | 0.97 ± 0.08 § |
|
| |||||
| Cytokine Secretion after 72 Hours c
|
|||||
| OVA | 1 mg/ml | 2.21 ± 0.13 | 1.82 ± 0.12 | 6.22 ± 0.46 | 10.1 ± 0.98 |
| OVA-pσ1 | 1 mg/ml) | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * | 0 ± 0 * |
| OVA-pσ1 | 0.05 mg/ml | 3.54 ± 0.15 § | 2.2 ± 0.03 § | 0.52 ± 0.13 * | 0.38 ± 0.03 * |
| pσ1 | 1 mg/ml | 0 ± 0 * | 0 ± 0 * | 0.58 ± 0.14 * | 0.72 ± 0.05 * |
| pσ1 | 0.05 mg/ml | 0 ± 0 * | 0.29 ± 0.02 * | 0 ± 0 * | 0.94 ± 0.09 * |
| OVA + pσ1 | 1 mg/ml, 1 mg/ml | 0 ± 0 * | 0.37 ± 0.19 § | 0.72 ± 0.18 * | 0.77 ± 0.2 * |
| OVA + pσ1 | 1mg/ml, 0.05 mg/ml | 1.22 ± 0.18 * | 0.46 ± 0.16 § | 2.87 ± 0.47 § | 2.3 ± 0.29 § |
Lymphocytes were isolated from spleens of naïve DO11.10 Tg mice.
Cultured supernatants from cells stimulated in vitro with designated concentrations of Ags were analyzed by cytokine ELISA.
cells were cultured with or without Ag for 24 or 72 hours.
Cytokine concentration was measured in triplicates and is depicted as an average ± SEM. Presented cytokine concentration is corrected over the cytokine production by unstimulated cells.
Statistical significance was calculated by Student’s t test;
p ≤ 0.001
p < 0.05 for the differences between cells stimulated with OVA vs. OVA-pσ1, pσ1 or OVA + pσ1 - stimulated cells, for each time point.
In summary, these studies show that OVA-pσ1 can readily accomplish tolerance with a single dose and stimulate the production of FoxP3+ Treg cells producing IL-10, and tolerance induction is IL-10 -dependent.
Discussion
We previously showed that the reovirus adhesin, pσ1, can enhance the efficacy of mucosal DNA vaccination (14). In this work, we investigated if a genetic fusion between pσ1 and a model Ag (OVA) would have the same effect. Unlike the previous findings, it was learned that when pσ1 was fused to a protein Ag, tolerance was induced. In fact, tolerance could be accomplished even with a single dose of OVA-pσ1 delivered mucosally. In contrast, parenteral delivery of OVA-pσ1 failed to induce tolerance to OVA. This pσ1-based mucosal tolerance even resisted co-treatment with potent mucosal adjuvants (CT and ODN), and tolerance was not broken after peripheral challenge with OVA. In contrast to OVA-pσ1, nasal administration of OVA alone, which is known to be a poor mucosal immunogen (34), elicited greater Ag-specific Ab responses than did OVA-pσ1. Moreover, the mechanism for this pσ1-induced tolerance was CD4+ T cell-dependent and could be adoptively transferred into naïve mice. Adoptive transfer of CLN-derived CD4+ T cells from mice tolerized with OVA-pσ1 significantly inhibited OVA-specific proliferation of CD4+ T cells in vitro. In subsequent analysis, it was revealed that tolerization via OVA-pσ1 induced significant increases in FoxP3+ CD25+CD4+ T cells, as well as an elevation in FoxP3+ CD25−CD4+ T cells. Adoptive transfer of OVA-pσ1-primed CD25+CD4+ or CD25−CD4+ T cells significantly inhibited Ag-specific proliferation of OVA-Tg CD4+ T cells in vivo. This suppression was due to increased production of IL-10 by OVA-pσ1-induced Treg cells, as evidenced by cytokine and FACS analyses, as well as by the lack of OVA-specific tolerance in OVA-pσ1-dosed IL-10−/− mice. Additionally, it was learned from the in vitro studies that OVA-pσ1 induces apoptosis of OVA-responsive CD4+ T cells in a time- and a dose- dependent manner, offering an additional potential mechanism for pσ1's action if it can survive delivery beyond the initial cell binding to the mucosal epithelium or M cells.
Induction of peripheral tolerance to a number of mucosally delivered Ags has been demonstrated previously using both high- and low-dose tolerization regimen (2, 7, 17, 28, 35); however, we propose that fusion of pσ1 to a protein Ag can significantly improve both mucosal and systemic unresponsiveness to this Ag. Previously, we have shown that chemically modified pσ1 with poly-L-lysine significantly enhances immunity to delivered DNA (14), and because of this modification, Abs to pσ1 are induced, but not to the unmodified pσ1. Thus, genetic fusion of an Ag to pσ1 is instrumental to stimulate tolerance. When OVA-pσ1 was co-administered with potent mucosal adjuvants, CT or CpGODN, the results provided further evidence that immunity to OVA was impaired. This finding was surprising since it is well established that CT given mucosally always induces Ag-specific Abs (36). Although the mechanism by which OVA-pσ1-induced tolerance overrides the immunostimulatory effects of CT is unclear, it is plausible that OVA-pσ1 resists co-treatment with CT due to the pσ1 adhesive properties which, in turn, may act on different receptors than CT-B. Nonetheless, pσ1-induced tolerance to OVA remained effective. Another mucosal immune modulator, the unmethylated CG dinucleotides CpG-containing motifs (CpGODN), was also shown to enhance immunity to OVA after oral administration (26). Here it was shown nasal co-administration of OVA-pσ1 plus CpGODN also resulted in tolerance. In contrast to mice given OVA plus CpGODN, OVA-pσ1 plus CpGODN-dosed mice lacked OVA-specific DTH responses and showed limited OVA-specific systemic and mucosal Ab titers, suggesting that the presence of a plasmid cDNA does not affect tolerogenic properties of genetically modified pσ1.
Despite the reported feasibility to induce low-dose nasal tolerance with OVA (28, 37), a sustainable tolerance by a variety of nasal OVA doses (0.05 – 2.5 mg) could not be induced, but a single oral 25 mg dose of OVA was found effective. In contrast, as little as a single 50 μg nasal dose of OVA-pσ1 was sufficient to induce OVA-specific tolerance that was long-lasting and resisted peripheral challenge with OVA in IFA, showing that OVA-pσ1 is at least a 1000-fold more efficient on a molar basis.
Previous studies have shown that the CT-B subunit could be adapted for mucosal tolerance induction (38–41). In those reports, efficiency of Ag-specific tolerance was improved by its conjugation to CT-B, as evidenced by reduced Ag-specific Ab and DTH responses following mucosal delivery. Many of these studies depended upon chemically coupling the tolerogen, which produces a heterogeneous population of carrier-conjugates and may limit only a fraction of these to bind the intended cell surface receptor (42). Additionally, CT-B is also commonly used as a mucosal adjuvant (43, 44) because it lacks the toxic moieties associated with native CT (45). To circumvent these potential barriers for using CT-B to induce tolerance, a genetic fusion between CT-B and proteolipid protein (PLP) immunodominant peptide was done to ameliorate the clinical manifestations of EAE (42). Although multiple doses were required, nasal administration of this CT-B-PLP peptide fusion protein was effective for inducing tolerance.
Regulatory T cells consist of a phenotypically diverse group bearing a variety of cell surface receptors, but they commonly share their ability to suppress T cell function (46–48). Induction and maintenance of low-dose mucosal tolerance has been associated with activation of Treg cells acting in an Ag-specific fashion (6, 7, 49–51). Evidence provided in this study shows that OVA-pσ1-induced tolerance is mediated by Ag-specific CD4+ T cells, since adoptive transfer of these OVA-pσ1-primed CD4+ T cells conferred unresponsiveness in naïve recipients to in vivo peripheral OVA challenge and inhibited OVA-specific CD4+ T cell proliferation. Studies have also found that low-dose mucosal tolerance is mediated by Treg cells that express the FoxP3 transcription factor (5, 47, 52–54). Consistent with these findings, nasal administration of OVA-pσ1 significantly increased the numbers of Treg cells, in which > 97 % were FoxP3+. Along these lines, expression of FoxP3 was also induced in CD25−CD4+ T cells following tolerization with OVA-pσ1, although the intensity of FoxP3 staining was ~50-fold less than that observed for the CD25+CD4+ T cells. A low level intensity of FoxP3 and its transient expression on activated human CD25− T cells has been previously reported (55). These FoxP3-expressing CD25− T cells are not capable of inhibiting cytokine production nor proliferation, and expression of FoxP3 on these cells is not correlated with the induction of a Treg cell phenotype (55). In contrast to that study, adoptive transfer of OVA-pσ1-primed CD25+ or CD25− T cells significantly inhibited in vivo proliferation of OVA-Tg CD4+ T cells. Additionally, FACS analysis of lymphocytes isolated from mice adoptively transferred with OVA-pσ1-derived CD25− T cells showed significant enrichment in FoxP3+CD25−CD4+ T cells. A significant increase in FoxP3 expression in DO11.10 responder CD4+ T cells (both CD25+ and CD25−) was observed in mice adoptively transferred with OVA-pσ1-derived CD25+ or CD25− CD4+ T cells, suggesting that OVA-pσ1-derived CD4+ T cells induce FoxP3+ expression by the responder CD4+ T cells. This finding is not surprising, since tolerance induction can occur via conversion of CD25− to FoxP3+ CD25+CD4+ T cells, as reported by others (29, 56), and these FoxP3-expressing CD25−CD4+ T cells, as well as converted CD25+CD4+ T cells, are potent inhibitors of CD4+ T cell expansion in vivo (29). Thus, the OVA-pσ1-induced CD25− T cells also have regulatory properties, as evident by being able to suppress proliferation of Tg CD4+ T cells.
Examination of OVA323–339-specific CD4+ T cell cytokine production in recipient mice adoptively transferred with either total CD25− or CD25+ CD4+ T cells from OVA-pσ1-primed mice revealed greatly reduced numbers of CD4+ T cells secreting the proinflammatory cytokines, IFN-γ and IL-17, and instead produced IL-4 and IL-10. These primarily segregated with IL-4 coming from recipients given OVA-pσ1-primed CD25−CD4+ T cells and with IL-10 from recipients given OVA-pσ1-primed Treg cells. The increased production of IL-4 may play a beneficial role in OVA-pσ1-mediated tolerance, perhaps, via the induction of a Th2-type immune bias, which was previously shown to support mucosal tolerance (57, 58). Additionally, IL-4 can also facilitate induction of Treg cells from naïve, peripheral CD25−CD4+ T cells, as recently demonstrated in vitro by others (57). The role for IL-10 in maintenance of tolerance is well established (30–32), even though its role in the induction of both mucosal and peripheral unresponsiveness remains controversial (59–61). One report has shown that the early production of IL-10 by CD4+ T cells is important for induction of high-dose oral tolerance (59). Here, we demonstrated that IL-10 is also necessary for induction of a low-dose nasal tolerance mediated by pσ1, since OVA-pσ1-mediated tolerance could not be established in IL-10−/− mice. IL-10−/− mice given OVA-pσ1 nasally developed elevated serum IgG anti-OVA Ab and DTH responses. In contrast to C57BL/6 mice, immunization of IL-10−/− mice with OVA-pσ1 failed to induce Ag-specific Treg cells and failed to inhibit Ag-specific proliferation of CD4+ T cells. Therefore, we conclude that IL-10 is essential for induction and maintenance of pσ1-induced tolerance, and IL-10-producing Treg cells, as well as possibly CD25−CD4+ T cells, mediate the observed pσ1-induced tolerance.
The role of TGF-β1 appears to be less essential for OVA-pσ1-induced tolerance. TGF-β1 was only modestly induced in OVA-pσ1 tolerized BALB/c mice; however, TGF-β1 was induced in OVA-pσ1 tolerized C57BL/6 mice and greatly reduced in OVA-pσ1-dosed IL-10−/− mice. The PBS-dosed, OVA-challenged IL-10−/− mice also showed elevated numbers of TGF-β1-producing CD4+ T cells. This suggests that TGF-β1 may play a supportive role in OVA-pσ1-induced tolerance, and secretion of TGF-β1 in OVA-pσ1-tolerized mice depends upon the presence of IL-10, as previously suggested (62). Alternatively, the production of TGF-β1 may contribute to the conversion of CD25−CD4+ to CD25+CD4+ T cells in tolerized mice, as implicated by recent studies demonstrating that TGF-β1 induces conversion of naïve peripheral CD25−CD4+ T cells into Treg cells by enhancing FoxP3 expression (29, 35, 56). Further evidence for the supportive role of TGF-β1 in OVA-pσ1-mediated tolerance is suggested by the depressed levels of TGF-β1+ CD4+ T cells in the OVA-pσ1-dosed IL-10−/− mice.
We have demonstrated, here and elsewhere (14, 23), that reovirus adhesin, pσ1, can be engineered to induce either immunity or tolerance, although the exact mechanism of pσ1-mediated modulation of an immune response remains undefined. Pσ1, aside from binding M cells in murine NALT (14), interacts with a variety of rodent and human cell types, including murine L929 (L) cells, RFL-6 cells, and Caco-2 cells (23). Additionally, pσ1 binds to mammalian erythrocytes (63), as well as to intestinal epithelial cells (64). It is known that pσ1 has two distinct binding domains (64, 65). One domain, located in pσ1's head structure, has been shown to interact with cells expressing the junctional adhesion molecule 1 (JAM1), whereas a second binding domain in pσ1's tail is thought to be responsible for binding to ubiquitously expressed sialic acid (64). Interaction of pσ1 with host cells may be JAM1-mediated, since some of them, including primary human DCs and epithelial cells, express this molecule (64). A possible mechanism for pσ1-induced modulation of an immune response could be a well-described ability of pσ1 to induce apoptosis (65, 66). Interestingly, the efficiency of pσ1-mediated apoptosis has been linked to the presence of sialic acid binding domain, since pσ1 mutants, unable to bind sialic acid, are insufficient inducers of apoptosis (65). Given these findings, we showed here that OVA-pσ1 and pσ1 trigger apoptosis of OVA-Tg CD4+ T cells in vitro. However, in contrast to pσ1 alone, or unconjugated pσ1 + OVA, only OVA-pσ1 was capable of stimulating these cells to produce increased levels of regulatory cytokines. The means by which pσ1 induces tolerance are still being investigated, and our results suggested that more than one mechanism may be associated with this process. Nonetheless, mucosal delivery of OVA-pσ1 is obviously crucial for the induction of OVA-specific unresponsiveness. It is known that stimulation of mucosal tolerance is dose-dependent, resulting from active suppression, induction of anergy, or clonal deletion of effector cells (2, 5). Results presented here suggest that a low dose of OVA-pσ1 delivered mucosally induces active suppression of anti-OVA immune responses by regulatory CD4+ T cells. While the in vitro studies suggested possible clonal deletion because of the accompanied pσ1-induced apoptosis of OVA-specific effector CD4+ T cells, it remains to be determined if such events occur in vivo following mucosal delivery.
In summary, we showed that pσ1-mediated tolerance to OVA can be established with a minimal amount of tolerogen when genetically fused to pσ1 and when applied mucosally. In some instances, a single nasal administration using ~1000-fold less OVA was sufficient to induce tolerance to OVA. Tolerance induced by OVA-pσ1 resisted co-treatment with CT, as well as peripheral challenge with OVA and IFA. Although IL-4 and TGF-β seemed to have supportive roles, pσ1-delivered tolerance relied largely on activation of specific FoxP3+ Treg cells that acted in an IL-10-dependent manner. The induced tolerance was IL-10-dependent since IL-10−/− mice were unable to undergo tolerance by OVA-pσ1 presumably via the failure to produce Treg cells. The impact by the OVA-pσ1-induced Treg cells was enhanced by regulatory CD25−CD4+ T cells since a subset of these was FoxP3+ and produced predominantly IL-4 in addition to IL-10. These CD25−CD4+ T cells could also inhibit the proliferation of OVA-Tg CD4+ T cells. TGF-β1 appeared to have a supportive role in tolerance induction by OVA-pσ1. Depression of TGF-β1-producing CD4+ T cells in OVA-pσ1-dosed IL-10−/− mice was observed suggesting that the production of this cytokine may be triggered by IL-10. These collective data show that IL-10 is pivotal in OVA-pσ1-induced tolerance. This finding is relevant because of the low-dose of Ag required for tolerance induction and its potential applicability to treat or prevent a variety of autoimmune diseases and allergies. The opportunity to deliver pσ1-based tolerogens via the nasal route offers a safer, easier, and more cost effective alternative for tolerization.
Acknowledgments
We thank Dr. Arnold Stein, Purdue University, for kindly providing the OVA cDNA, Ms. Sue Brumfield for her assistance at MSU’s fermentation facility, and Ms. Nancy Kommers for her assistance in preparing this manuscript.
Footnotes
This work was supported by Public Health Service grants AI-56286, DE-13812, DE-12242, AI-18958, and AG-25873, and in part, by the Montana Agricultural Station and the U.S. Department of Agriculture Formula Funds. The VMB flow cytometry facility was, in part, supported by NIH/NIH/National Center for Research Resources, Centers of Biomedical Excellence P20 RR-020185, and an equipment grant from the M.J. Murdock Charitable Trust.
Disclosures
The authors have no financial conflict of interest.
Abbreviations used in this paper: Ag, antigen; Treg, T regulatory cells; FoxP3, forkhead nuclear transcription factor; PPs, Peyer’s patches; LNs, lymph nodes; NALT, nasopharyngeal-associated lymphoid tissue; M, microfold; FAE, follicle-associated epithelium; pσ1, protein sigma one; CT, cholera toxin; DTH, delayed-type hypersensitivity; MLNs, mesenteric lymph nodes; HNLNs, head and neck lymph nodes; CLNs, cervical lymph nodes; Tg, transgenic; sPBS, sterile PBS; p.i., post-immunization; CFC, cytokine-producing cell.
References
- 1.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 2.Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000;106:935–937. doi: 10.1172/JCI11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao BG, Link H. Mucosal tolerance: A two-edged sword to prevent and treat autoimmune diseases. Clin Immunol Immunopathol. 1997;85:119–128. doi: 10.1006/clin.1997.4432. [DOI] [PubMed] [Google Scholar]
- 4.Jun S, Gilmore W, Callis G, Rynda A, Haddad A, Pascual DW. A live diarrheal vaccine imprints a Th2 cell bias and acts as an anti-inflammatory vaccine. J Immunol. 2005;175:6733–6740. doi: 10.4049/jimmunol.175.10.6733. [DOI] [PubMed] [Google Scholar]
- 5.Faria AM, Weiner HL. Oral tolerance: mechanisms and therapeutic applications. Adv Immunol. 1999;73:153–264. doi: 10.1016/s0065-2776(08)60787-7. [DOI] [PubMed] [Google Scholar]
- 6.Fujihashi K, Kato H, van Ginkel FW, Koga T, Boyaka PN, Jackson RJ, Kato R, Hagiwara Y, Etani Y, Goma I, Fujihashi K, Kiyono H, McGhee JR. A revisit of mucosal IgA immunity and oral tolerance. Acta Odontol Scand. 2001;59:301–308. doi: 10.1080/000163501750541174. [DOI] [PubMed] [Google Scholar]
- 7.Weiner HL. Oral tolerance: immune mechanisms and treatment of autoimmune diseases. Immunol Today. 1997;18:335–343. doi: 10.1016/s0167-5699(97)01053-0. [DOI] [PubMed] [Google Scholar]
- 8.Kraus TA, Brimnes J, Muong C, Liu JH, Moran TM, Tappenden KA, Boros P, Mayer L. Induction of mucosal tolerance in Peyer's patch-deficient, ligated small bowel loops. J Clin Invest. 2005;115:2234–2243. doi: 10.1172/JCI19102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiter R, Pfeffer K. Impaired germinal center formation and humoral immune response in the absence of CD28 and interleukin-4. Immunology. 2002;106:222–228. doi: 10.1046/j.1365-2567.2002.01405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faria AM, Maron R, Ficker SM, Slavin AJ, Spahn T, Weiner HL. Oral tolerance induced by continuous feeding: enhanced up-regulation of transforming growth factor-β/interleukin-10 and suppression of experimental autoimmune encephalomyelitis. J Autoimmun. 2003;20:135–145. doi: 10.1016/s0896-8411(02)00112-9. [DOI] [PubMed] [Google Scholar]
- 11.Fujihashi K, Dohi T, Rennert PD, Yamamoto M, Koga T, Kiyono H, McGhee JR. Peyer’s patches are required for oral tolerance to proteins. Proc Natl Acad Sci USA. 2001;98:3310–3315. doi: 10.1073/pnas.061412598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rennert PD, Browining JL, Hochman PS. Selective disruption of lymphotoxin ligands reveals a novel set of mucosal lymph nodes and unique effects on lymph node cellular organization. Intern Immunol. 1997;9:1627–1639. doi: 10.1093/intimm/9.11.1627. [DOI] [PubMed] [Google Scholar]
- 13.Kiyono H, Fukuyama S. NALT- versus Peyer's-patch-mediated mucosal immunity. Nat Rev Immunol. 2004;4:699–710. doi: 10.1038/nri1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Y, Wang X, Csencsits KL, Haddad A, Walters N, Pascual DW. M cell-targeted DNA vaccination. Proc Natl Acad Sci USA. 2001;98:9318–9323. doi: 10.1073/pnas.161204098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleeton M, Contractor N, Leon F, Wetzel JD, Dermody TS, Kelsall BL. Peyer's patch dendritic cells process viral antigen from apoptotic epithelial cells in the intestine of reovirus-infected mice. J Exp Med. 2004;200:235–245. doi: 10.1084/jem.20041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleeton M, Contractor N, Leon F, He J, Wetzel D, Dermody T, Iwasaki A, Kelsall B. Involvement of dendritic cell subsets in the induction of oral tolerance and immunity. Ann NY Acad Sci. 2004;1029:60–65. doi: 10.1196/annals.1309.008. [DOI] [PubMed] [Google Scholar]
- 17.Kato H, Fujihashi K, Kato R, Yuki Y, McGhee JR. Oral tolerance revisited: prior oral tolerization abrogates cholera toxin-induced mucosal IgA responses. J Immunol. 2001;166:3114–3121. doi: 10.4049/jimmunol.166.5.3114. [DOI] [PubMed] [Google Scholar]
- 18.Wolf JL, Kauffman RS, Finberg R, Dambrauskas R, Fields BN, Trier JS. Determinants of reovirus interaction with the intestinal M cells and absorptive cells of murine intestine. Gastroenterology. 1983;85:291–300. [PubMed] [Google Scholar]
- 19.Mah DCW, Leone G, Jankowski JM, Lee PWK. The N-terminal quarter of reovirus cell attachment protein σ1 possesses intrinsic virion-anchoring function. Virology. 1990;179:95–103. doi: 10.1016/0042-6822(90)90278-y. [DOI] [PubMed] [Google Scholar]
- 20.Turner DL, Duncan R, Lee PW. Site-directed mutagenesis of the C-terminal portion of reovirus protein σ1: evidence for a conformation-dependent receptor binding domain. Virology. 1992;186:219–227. doi: 10.1016/0042-6822(92)90076-2. [DOI] [PubMed] [Google Scholar]
- 21.Barton ES, Chappell JD, Connolly JL, Forrest JC, Dermody TS. Reovirus receptors and apoptosis. Virology. 2001;290:173–180. doi: 10.1006/viro.2001.1160. [DOI] [PubMed] [Google Scholar]
- 22.Kato T, Owen RL. Structure and function of intestinal mucosal epithelium. Chap. 8. In: Mestecky J, Lamm ME, Strober W, Bienenstock J, McGhee JR, Mayer L, editors. Mucosal Immunology. Elsevier-Academic Press; San Diego, CA: 2005. pp. 131–151. [Google Scholar]
- 23.Wu Y, Boysun MJ, Csencsits KL, Pascual DW. Gene transfer facilitated by a cellular targeting molecule, reovirus protein sigma1. Gene Ther. 2000;7:61–69. doi: 10.1038/sj.gt.3301046. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Hone DM, Haddad A, Shata MT, Pascual DW. M cell DNA vaccination for CTL immunity to HIV. J Immunol. 2003;171:4717–4725. doi: 10.4049/jimmunol.171.9.4717. [DOI] [PubMed] [Google Scholar]
- 25.Csencsits KL, Walters N, Pascual DW. Cutting Edge: Dichotomy of homing receptor dependence by mucosal effector B cells: αE versus L-selectin. J Immunol. 2001;167:2441–2445. doi: 10.4049/jimmunol.167.5.2441. [DOI] [PubMed] [Google Scholar]
- 26.Alignani D, Maletti B, Liskovsky M, Rópolo A, Morón G, Pistoresi-Palencia MC. Orally administered OVA/CpG-ODN induces specific mucosal and systemic immune response in young and aged mice. J Leukoc Biol. 2005;77:898–905. doi: 10.1189/jlb.0604330. [DOI] [PubMed] [Google Scholar]
- 27.Ochoa-Repáraz J, Riccardi C, Rynda A, Jun S, Callis G, Pascual DW. Regulatory T cell vaccination without autoantigen protects against experimental autoimmune encephalomyelitis. J Immunol. 2007;178:1791–1799. doi: 10.4049/jimmunol.178.3.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unger WW, Hauet-Broere F, Jansen W, van Berkel LA, Kraal G, Samsom JN. Early events in peripheral regulatory T cell induction via the nasal mucosa. J Immunol. 2003;171:4592–4603. doi: 10.4049/jimmunol.171.9.4592. [DOI] [PubMed] [Google Scholar]
- 29.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells into CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor FoxP3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonnella PA, Waldner HP, Kodali D, Weiner HL. Induction of low-dose oral tolerance in IL-10 deficient mice with experimental autoimmune encephalomyelitis. J Autoimmun. 2004;3:193–200. doi: 10.1016/j.jaut.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Ghoreishi M, Dutz JP. Tolerance induction by transcutaneous immunization through ultraviolet-irradiated skin is transferable through CD4+CD25+ T regulatory cells and is dependent on host - derived IL-10. J Immunol. 2006;176:2635–2644. doi: 10.4049/jimmunol.176.4.2635. [DOI] [PubMed] [Google Scholar]
- 32.Seewaldt S, Alferink J, Förster I. Interleukin-10 is crucial for maintenance but not for developmental induction of peripheral T cell tolerance. Eur J Immunol. 2002;32:3607–3616. doi: 10.1002/1521-4141(200212)32:12<3607::AID-IMMU3607>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 33.Veldhoen M, Stockinger B. TGFβ1, a "Jack of all trades": the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 2006;27:358–361. doi: 10.1016/j.it.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Van der Heijden PJ, Bianchi ATJ, Dol M, Pals JW, Stok W, Bokhout BA. Manipulation of intestinal immune response against ovalbumin by cholera toxin and its B subunit in mice. Immunology. 1991;72:89–93. [PMC free article] [PubMed] [Google Scholar]
- 35.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, Protschka M, Galle PR, Neurath MF, Blessing M. Cutting edge: TGF-β signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+ T cells. J Immunol. 2004;173:6526–6531. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 36.Lycke N. From toxin to adjuvant: basic mechanisms for the control of mucosal IgA immunity and tolerance. Immunol Lett. 2005;97:193–198. doi: 10.1016/j.imlet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Samson JN, van Berkel LA, van Helvoort JM, Unger WW, Jansen W, Thepen T, Mebius RE, Verbeek SS, Kraal G. FcγRIIB regulates nasal and oral tolerance: a role for dendritic cells. J Immunol. 2005;174:5279–5287. doi: 10.4049/jimmunol.174.9.5279. [DOI] [PubMed] [Google Scholar]
- 38.Sun JB, Holmgren J, Czerkinsky C. Cholera toxin B subunit: An efficient transmucosal carrier-delivery system for induction of peripheral immunological tolerance. Proc Natl Acad Sci USA. 1994;91:10795–10799. doi: 10.1073/pnas.91.23.10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun JB, Rask C, Olsson T, Holmgren J, Czerkinsky C. Treatment of experimental autoimmune encephalomyelitis by feeding myelin basic protein conjugated to cholera toxin B subunit. Proc Natl Acad Sci USA. 1996;93:7196–7201. doi: 10.1073/pnas.93.14.7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun JB, Raghavan S, Sjöling Å, Lundin S, Holmgren J. Oral tolerance induction with antigen-conjugated to cholera toxin B subunit generates both FoxP3+ CD25+ and FoxP3−CD25−CD4+ regulatory T cells. J Immunol. 2006;177:7634–7644. doi: 10.4049/jimmunol.177.11.7634. [DOI] [PubMed] [Google Scholar]
- 41.Bergerot I, Ploix C, Petersen J, Moulin V, Rask C, Fabien N, Lindblad M, Mayer A, Czerkinsky C, Holmgren J, Thivolet C. A cholera toxin-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:4610–4614. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuki Y, Byun Y, Fujita M, Izutani W, Suzuki T, Udake S, Fujihashi K, McGhee JR, Kiyono H. Production of recombinant hybrid molecule of cholera toxin-B-subunit and proteolipid-protein-peptide for the treatment of experimental encephalomyelitis. Biotechnol Bioeng. 2001;74:62–69. doi: 10.1002/bit.1095. [DOI] [PubMed] [Google Scholar]
- 43.Yokohama Y, Harabuchi Y. Intranasal immunization with lipoteichoic acid and cholera toxin evokes specific pharyngeal IgA, and systemic IgG responses and inhibits streptococcal adherence to pharyngeal epithelial cells in mice. Int J Pediatr Otohinolaryngol. 2002;63:235–241. doi: 10.1016/s0165-5876(02)00021-6. [DOI] [PubMed] [Google Scholar]
- 44.Lian T, Bui T, Ho RJ. Formulation of HIV-envelope protein with lipid vesicles expressing ganglioside GM1 associated to cholera toxin B enhances mucosal immune responses. Vaccine. 1999;18:604–611. doi: 10.1016/s0264-410x(99)00315-1. [DOI] [PubMed] [Google Scholar]
- 45.Lycke N. ADP-ribosylating bacterial enzymes for the targeted control of mucosal tolerance and immunity. Ann N Y Acad Sci. 2004;1029:193–208. doi: 10.1196/annals.1309.036. [DOI] [PubMed] [Google Scholar]
- 46.Grundstrom S, Cederbom L, Sundstedt A, Scheipers P, Ivars F. Superantigen-induced regulatory T cells display different suppressive functions in the presence or absence of natural CD4+CD25+ regulatory T cells in vivo. J Immunol. 2003;170:5008–5017. doi: 10.4049/jimmunol.170.10.5008. [DOI] [PubMed] [Google Scholar]
- 47.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4+ T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–1894. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shimizu J. Stimulation of CD4+CD25+ regulatory T cells through GITR breaks immunological self-tolerance. Immunological Aging Research Group Tokyo Metropolian Institute of Gerontology. 2002 http://www.tmig.or.jp/topic/topic_01.html.
- 49.Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science. 1994;265:1237–1240. doi: 10.1126/science.7520605. [DOI] [PubMed] [Google Scholar]
- 50.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto K, Kingsley CI, Zhang X, Jabs C, Izikson L, Sobel RA, Weiner HL, Kuchroo VK, Sharpe AH. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J Immunol. 2005;175:7341–7347. doi: 10.4049/jimmunol.175.11.7341. [DOI] [PubMed] [Google Scholar]
- 52.Harber M, Sundstedt A, Wraith D. The role of cytokines in immunological tolerance: potential for therapy. Expert Rev Mol Med. 2000;2000:1–20. doi: 10.1017/S1462399400002143. [DOI] [PubMed] [Google Scholar]
- 53.Lee JH, Wang LC, Lin YT, Yang YH, Lin DT, Chiang BL. Inverse correlation between CD4+ regulatory T-cell population and autoantibody levels in paediatric patients with systemic lupus erythematosus. Immunology. 2006;117:280–286. doi: 10.1111/j.1365-2567.2005.02306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim HW, Hillsamer P, Banham AH, Kim CH. Cutting edge: Direct suppression of B cells by CD4+CD25+ regulatory T cells. J Immunol. 2005;175:4180–4183. doi: 10.4049/jimmunol.175.7.4180. [DOI] [PubMed] [Google Scholar]
- 55.Allan SE, Crome SO, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FoxP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 56.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of FoxP3 induced in T cells by TGF-β. J Immunol. 2007;178:7667–7677. doi: 10.4049/jimmunol.178.12.7667. [DOI] [PubMed] [Google Scholar]
- 57.Skapenko A, Kalden JR, Lipsky PE, Schulze-Koops H. The IL-4 receptor α-chain-binding cytokines, IL-4 and IL-13, induce forkhead box P3-expressing CD25+CD4+ regulatory T cells from CD25−CD4+ precursors. J Immunol. 2005;175:6107–6116. doi: 10.4049/jimmunol.175.9.6107. [DOI] [PubMed] [Google Scholar]
- 58.al Sabbagh A, Miller A, Santos LM, Weiner HL. Antigen-driven tissue-specific suppression following oral tolerance: orally administered myelin basic protein suppresses proteolipid protein-induced experimental autoimmune encephalomyelitis in the SJL mouse. Eur J Immunol. 1994;24:2104–2109. doi: 10.1002/eji.1830240926. [DOI] [PubMed] [Google Scholar]
- 59.Cong Y, Liu C, Weaver CT, Elson CO. Early upregulation of T cell IL-10 production plays an important role in oral tolerance induction. Ann NY Acad Sci. 2004;1029:319–320. doi: 10.1196/annals.1309.037. [DOI] [PubMed] [Google Scholar]
- 60.Maurer M, Seidel-Guyenot W, Metz M, Knop J, Steinbrink K. Critical role of IL-10 in the induction of low zone tolerance to contact allergens. J Clin Invest. 2003;112:432–439. doi: 10.1172/JCI18106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Silva SR, Jacysyn JF, Macedo MS, Faquim-Mauro EL. Immunosuppressive components of Ascaris suum down-regulate expression of co-stimmulatory molecules and function of antigen-presenting cells via an IL-10-mediated mechanism. Eur J Immunol. 2006;36:3227–3237. doi: 10.1002/eji.200636110. [DOI] [PubMed] [Google Scholar]
- 62.Joetham A, Takeda K, Taube C, Miyahara N, Matsubara S, Koya T, Rha YH, Dakahama A, Gelfand EW. Naturally occurring lung CD4+CD25+ T cell regulation of airway allergic response depends on IL-10 induction of TGF-β. J Immunol. 2007;178:1433–1442. doi: 10.4049/jimmunol.178.3.1433. [DOI] [PubMed] [Google Scholar]
- 63.Dermody TS, Nibert ML, Bassel-Duby R, Fields BN. A σ1 region important for hemagglutination by serotype 3 reovirus strains. J Virol. 1990;64:5173–5176. doi: 10.1128/jvi.64.10.5173-5176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mercier GT, Campbell JA, Chappell JD, Stehle T, Dermody TS, Barry MA. A chimeric adenovirus vector encoding reovirus attachment protein σ1 targets cells expressing junctional adhesion molecule 1. Proc Natl Acad Sci USA. 2004;101:6188–6193. doi: 10.1073/pnas.0400542101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Connolly JV, Barton ES, Dermody TS. Reovirus binding to cell surface sialic acid potentiates virus-induced apoptosis. J Virol. 2001;75:4029–4031. doi: 10.1128/JVI.75.9.4029-4039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tyler KL, Squier MK, Rodgers SE, Schneider BE, Oberhaus SM, Grdina TA, Cohen JJ, Dermody TS. Differences in the capacity of reovirus strains to induce apoptosis are determined by the viral attachment protein σ1. J Virol. 1995;69:6972–6979. doi: 10.1128/jvi.69.11.6972-6979.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]