Summary
Using a systems approach to mine spontaneously resolving inflammatory exudates, novel families of lipid- derived mediators were identified in animal systems that control both the duration and magnitude of acute inflammation. These new families were coined the resolvins and protectins because they posses potent bioactions and novel chemical structures. The mapping of these new resolution circuits has already provided new avenues for appreciating the molecular basis of many inflammatory diseases. This presentation/minireview gives recent advances from our studies on resolvin and protectin biosynthesis and the actions of these novel mediators. These previously unappreciated families of lipid derived mediators were originally isolated from murine models of acute inflammation captured during the natural spontaneous resolution phase. They are biosynthesized from omega-3 fatty acids and possess potent anti-inflammatory, pro-resolving and anti-fibrotic in vivo actions. These new families of endogenous pro-resolving and anti-inflammatory agonists were also used as biotemplates to design potent mimetics/analogs which were used to confirm each of their structures and specific functions. Moreover, together the identification of these mediators indicate that resolution is an active process at the tissue level in vivo as well as constitute a new genus of anti-inflammatories with a previously unknown pro-resolving mechanism of action.
Introduction
Current anti-inflammatory therapies are directed towards the inhibition of specific enzymes and/or antagonism of specific receptors. The widely used cyclooxygenase inhibitors and anti-tumor necrosis factor α (TNF-α) are examples of this approach that are used with the goal of blocking the production of pro-inflammatory chemical mediators. Recent research efforts in the author’s laboratory have uncovered new mechanisms that terminate or resolve the local acute inflammatory response. These are active processes and help establish that the resolution that was once considered a passive process is actually an active process at the tissue level in vivo; for recent reviews see refs. [1, 2]. Thus, rather than targeting inhibition, the focus of research in this laboratory is now addressing the potential use of agonists to stimulate key endogenous regulatory points within the control of mechanisms that naturally resolve inflammation. This approach has the potential to open a new appreciation of inflammatory disease mechanisms as well as a new frontier in molecular pharmacology. The essential fatty acids, in particular omega-3, are precursors to a new genus of lipid mediators that are both pro-resolving and anti-inflammatory and appear to play a physiological role in terminating inflammation. This ISSFAL presentation/mini review highlights the formation and actions of these novel endogenous chemical mediators.
The Ideal Outcome is Complete Resolution and Tissue Homeostasis
Inflammation can have different meanings that depend on the clinical setting and the training of the clinician. In general, an acute inflammatory reaction in response to infection or unwanted tissue damage is rigorously characterized at the gross level by the cardinal signs of inflammation (heat, redness, swelling and pain), and in experimental settings in vivo the temporal relationships are well established, namely, edema, the accumulation of leukocytes, specifically polymorphonuclear neutrophils [PMN], followed by accumulation of monocytes and macrophages [3]. These events in spontaneously resolving reactions are coupled with release of factors that prevent further or excessive trafficking of leukocytes allowing for resolution [4, 5]. Early in the inflammatory response, pro-inflammatory mediators such as prostaglandins and leukotrienes play an important role [6, 7]. The progression from an acute inflammation to chronic inflammation as in many widely occurring human diseases such as arthritis, periodontal disease and cardiovascular disease, to name a few, is widely viewed as an excess of pro-inflammatory mediators [8]. Although mononuclear cells can sometimes contribute to pro-inflammatory responses, they are also critical in wound healing, tissue repair and remodeling in a non-inflammatory, non-phlogistic manner [3]. Hence, it is possible that problems associated with mounting the endogenous pro-resolving circuits and compounds de novo could underlie some of the aberrant mechanisms in chronic inflammation.
Complete resolution of an acute inflammatory response and the local tissues return to homeostasis is necessary for ongoing health. Removal of leukocytes from tissues involved in the inflammatory response without leaving remnants of the host defenses and combat between leukocytes, invading microbes, and/or other initiators of inflammation is an ideal outcome. In my laboratory we’ve focused on the question “How is the acute inflammatory response regulated? ” It was widely believed and argued that simple dilution of pro-inflammatory mediators was enough to turn off or “burn out” inflammation, with the subsequent responses ending naturally or passively [3]. New evidence from the author’s laboratory and collaborators now indicates that resolution of inflammation and the return to homeostasis is not passive, but an actively regulated process (Figure 1) [1].
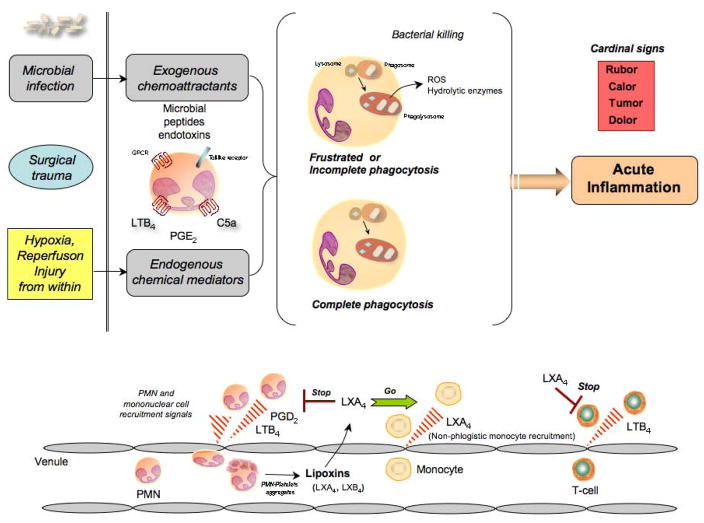
Figure 1.
Lipid mediators in acute inflammation. In response to injury or infection, the acute inflammatory reaction is normally a protective mechanism, initiated by neutrophils summoned to the site to engulf and destroy invading microbes. In this process, neutrophil-derived pro-inflammatory mediators, such as leukotrienes and prostaglandins, and the unintentional release of hydrolytic enzymes and reactive oxygen and free radicals can enhance this process potentially leading to chronic inflammation. At contained exudate sites, within the resolution phase neutrophils promote resolution when they change phenotype (lower panel) to generate protective mediators that are derived from polyunsaturated fatty acids (PUFA).
Pro-resolving Lipid Mediators and Endogenous Anti-inflammation
Pro-resolving lipid mediators are a novel genus of endogenous chemical mediators, including lipoxin, resolvins, and protectins, that are involved in acute inflammation [1]. They are actively biosynthesized in the resolution phase of acute inflammation and are potent agonists that control the duration and magnitude of inflammation (Figure 1). They are also potent chemo-attractants, but via a non-inflammatory mechanism: for example, lipoxins activate mononuclear cell infiltration without stimulating release of pro-inflammatory chemokines or activation of pro-inflammatory gene pathways and products [1]. They also stimulate the uptake of apoptotic PMNs [9] and activate endogenous antimicrobial defense mechanisms [10] as well as clearance on mucosal surfaces [11]. Together these dual actions are agonistic; via acting on separate cell populations they stimulate resolution of inflammation (Table 1).
Table 1.
Dual Actions of Novel Anti-Inflammatory and Pro-Resolving Lipid Mediators
| Agonists | |
|---|---|
| Counter-Regulate | Promote Resolution |
| Stop signals | Stimulate |
|
|
Lipoxins
Lipoxins, specifically lipoxin A4 (LXA4) and LXB4, are anti-inflammatory and were the first pro-resolving mediators recognized as their appearance signals the resolution of acute contained inflammation [12]. Lipoxins are lipoxygenase-derived eicosanoids, derived enzymatically from arachidonic acid (AA), an omega-6 fatty acid that is released and mobilized during inflammation [6]. In human systems, they are made, for example, during cell–cell interactions involving mucosal, i.e., epithelial cells of the gastrointestinal tract or bronchial tissue interactions with leukocytes and within the vasculature platelet–leukocyte interactions are a main source [1]. Aspirin has an unexpected impact with in resolution. In humans, aspirin “jump-starts” this process via its ability to trigger the endogenous biosynthesis of lipid mediators [13, 14]. For example, aspirin affects lipoxin generation, leading to the production of mediators known as aspirin-triggered lipoxins (ATLs) by the cyclooxygenase-2 (COX-2) pathway [15]. Aspirin, via acetylation of COX-2, does not inhibit the activity of the enzyme in the same way as a selective COX-2 inhibitor. Instead, the acetylation changes the chirality of the enzyme’s products and instead generates 15R-HETE, a precursor to 15-epi-lipoxins [reviewed in Ref. 1]. Aspirin thus has the unique ability to initiate resolution of inflammation by stimulating the early formation of mediators that would normally be made later in the time course of an inflammatory response.
During local contained inflammation, the first line of host defense, namely the neutrophils, die at the site and can undergo apoptosis as well as necrotic cell death. As part of resolution, lipoxins signal macrophages to enhance their take up of the remains of these cells [9, 16]. Lipoxins are highly potent anti-inflammatory mediators that are formed and act in picogram to nanogram amounts with human tissues and in animal disease models (Tables 1 and 2). They have the specific pro-resolution actions of limiting PMN recruitment, chemotaxis, and adhesion to the site of inflammation [see 17 and references within]. They are essentially a braking signal for PMN-mediated tissue injury (Figure or Table 2). The 5-lipoxygenase (5-LO) pathway, for example, generates leukotrienes that are primarily pro-inflammatory mediators. Topical application of leukotriene B4, a potent pro-inflammatory mediator and neutrophil chemoattractant, on the ears of mice ears stimulates rapid and significant PMN infiltration [18]. Neutrophils rapidly, within minutes, infiltrate the ear tissue and, when in high flux, excessive PMN infiltration can lead to local tissue damage and increased vascular permeability causing leakage. When metabolically stable LXA4 (aspirin-triggered) analogs were applied topically to these ears, they rapidly ‘stopped’ PMN infiltration as well as reduced vascular permeability [18]. To date, at least four generations of stable lipoxin analogs have now been introduced that show promise in a number of different in vivo settings [19–22]. Thus, the endogenous anti-inflammatory and pro-resolving mediators were used as biotemplates to design and produce novel agonists and potentially a new genus of therapeutics [23].
Table 2.
Lipoxin A4 (LXA4) and Aspirin-triggered Lipoxin A4 (ATL) and Their Stable Analogs in Animal Systems
| Disease Model | Action(s) | References |
|---|---|---|
| Dermal inflammation (ear tissue) | Reduce neutrophil recruitment into ear skin and prevent vascular permeability | [18, 39, 45, 46] |
| Dorsal air pouch (skin) | Block TNF-α induced leukocyte recruitment | [47] |
| Oral inflammation (murine model) | Block P. gingivalis elicited PMN infiltration and lower PGE2 and COX-2 levels within exudates | [48] |
| Peritonitis | Reduce neutrophil recruitment and regulate chemokine/cytokine production | [30, 39] |
| Inhibit vascular leakage | [49] | |
| Promote phagocytosis of apoptotic neutrophil by macrophage | [16] | |
| Promote lymphatic removal of microbial particles (zymosan) | [44] | |
| Colitis (inflammatory bowel disease) | Attenuate proinflammatory gene expression and reduce severity of colitis Inhibit weight loss, inflammation and immune dysfunction |
[50] [51] |
| Kidney | Reduce proteinuria, IL-1β, IL-6 and proliferation score of mesangial cells | [52] |
| Antagonizes LTD4-induced reduction of glomerular rate | [53] | |
| Protects in ischemic acute renal failure | [54] | |
| Lung pleuritis | Shorten duration of pleural exudation | [55] |
| Asthma | Inhibit airway hyper-responsiveness and pulmonary inflammation | [56, 57] |
| Modulates airway obstruction in humans | [58] | |
| Cystic Fibrosis | Decrease neutrophilic inflammation, pulmonary and bacterial burden | [59, 60] |
| Sepsis | Attenuate LPS-induced acute lung injury and acid-induced lung injury | [61–63] |
| Reperfusion injury | Reduce second organ injury and PMN infiltration | [39, 64] |
| Detachment of adherent leukocytes in mesenteric ischemia-reperfusion | [65] | |
| Angiogenesis | Reduce angiogenic phenotype: endothelial cell proliferation and migration | [66] |
| Periodontitis (oral inflammation in rabbit) | Reduce microbe-initiated, neutrophil- mediated tissue damage as well as bone class destruction | [67] |
| Eye | Accelerate cornea re-epithelialization, limit sequelae of thermal injury, i.e. neovascularization, opacity | [29] |
| Reduce corneal angiogenesis, inhibiting inflammatory cytokines and VEGF/VEGFR2 expression | [68] | |
| Multi-organ graft-vs.- host disease | Protect against graft-vs.-host diseases | [69] |
| Pain reduction | Prolong paw withdraw latency, reduce hyperalgesic index and paw edema | [70] |
| Edema | Stop histamine, PAF and prostaglandin stimulated edema | [71] |
Novel Mediators from EPA and DHA
Resolvins
Resolvins and protectins are two families of new compounds identified in the resolution of inflammation using a systems approach with LC-MS-MS and complete structural elucidation of the bioactive mediators and related compounds [4, 5, 14, 24, 25]. The term resolvins or resolution-phase interaction products are endogenous compounds biosynthesized from the major omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), they are E series (RvE) and D series (RvD) resolvins, respectively [5]. Resolvins are also produced by the COX-2 pathway, in the presence of aspirin and are generated in an aspirin triggered form as well. A growing body of evidence indicates that resolvins possess potent anti-inflammatory and immunoregulatory actions that include blocking the production of pro-inflammatory mediators and regulating the trafficking of leukocytes cells and mediators to sites of inflammation [reviewed in Ref. 2]. Specifically, resolvins stop PMN infiltration in vivo and transmigration [5, 26]. They also reduce cytokine expression by isolated microglia cells [24]. Moreover, we recently established the stereochemistry and actions of RvD1 and AT-RvD1, RvE1 and PD1 (vide infra) as well as demonstrated the stereoselective basis for their potent actions as well as enzymatic inactivation [14, 25, 26]. In a recent set of experiments, we compared the actions of RvD1 and the 17R-aspirin triggered (AT)-RvD1 (0–1000 nM) on human PMN transendothelial migration [26]. Both RvD1 and AT-RvD1 stopped PMN transmigration in a concentration-dependent manner (P < 0.001), and were not statistically different from one another at the concentrations tested. The potency of these compounds is noteworthy giving a ~50% reduction in PMN transmigration observed with concentrations as low as 10 nM. The actions of RvD1 have been studied in several inflammatory disease models: they are potent and stereoselective (Table 3 and references cited within).
Table 3.
Resolvins and Protectins in Complex Disease Animal Models*
| Disease model | Species | Action(s) | References |
|---|---|---|---|
| Resolvin E1 | |||
| Periodontitis | Rabbit | Reduces neutrophil infiltration; prevents connective tissue and bone loss; promotes healing of diseased tissues; regenerates of lost soft tissue and bone | [42, 43] |
| Peritonitis | Mouse | Stops neutrophil recruitment; regulates chemokine/cytokine production | [14, 30, 44] |
| Promotes lymphatic removal of phagocytes | |||
| Dorsal air pouch | Mouse | Stops neutrophil recruitment | [4] |
| Retinopathy | Mouse | Protects against neovascularization | [37] |
| Colitis | Mouse | Decreases neutrophil recruitment and proinflammatory gene expression; improves survival; reduces weight loss | [41] |
| Resolvin D1 | |||
| Peritonitis | Mouse | Stops neutrophil recruitment | [24, 26] |
| Dorsal skin air pouch | Mouse | Stops neutrophil recruitment | [5, 24] |
| Kidney ischemia- reperfusion | Mouse | Protects from ischemia-reperfusion-induced kidney damage and loss of function; regulates macrophages and protects from fibrosis | [72] |
| Retinopathy | Mouse | Protects against neovascularization | [37] |
| Protectin D1 | |||
| Peritonitis | Mouse | Stops neutrophil recruitment; regulates chemokine/cytokine production | [14, 30, 44, 73] |
| Promotes lymphatic removal of phagocytes | |||
| Regulates T-cell migration | |||
| Asthma | Mouse | Protects from lung damage, airway inflammation and airway hyperresponsiveness | [74] |
| Human | Protectin D1 is generated in humans and appears to be diminished in asthmatics | [74] | |
| Kidney ischemia- reperfusion | Mouse | Protects from ischemia-reperfusion-induced kidney damage and loss of function; regulates macrophages and is anti-fibrotic | [72] |
| Retinopathy | Mouse | Protects against neovascularization | [37] |
| Ischemic stroke | Rat | Stop leukocyte infiltration, inhibits NF-κB and cyclooxygenase-2 induction | [28] |
| Alzheimer’s disease | Human | Diminished protectin D1 production in human Alzheimer’s disease | [75] |
The actions of each of the main resolvins and protectins, i.e., RvE1, RvD1 and PD1, were confirmed with compounds prepared by total organic synthesis (see text and cited references for further details).
Protectins
Protectins are also produced and biosynthesized from the precursor DHA via a separate new pathway. Protectins are distinguished by the presence of their conjugated triene containing structure [25]. The name “protectins” was coined from the observed anti-inflammatory [24] and protective actions in neural tissues and systems. The prefix neuroprotectin gives the tissue address to the production and local actions of these such as neuroprotectin D1 [25, 27]. Like resolvins, the protectins stop PMN infiltration [24, 25]. They are biosynthesized by and act on glial cells and reducing cytokine expression [24]. NPD1 reduces retinal and corneal injury [27], stroke damage [28], and improves corneal wound healing in mouse models [29] (see Table 3 and references cited within).
Resolution of Inflammation in vivo
In order to study resolution using a systems biology approach, the murine dorsal air pouch system was particularly helpful in yielding a contained exudate [4, 5]. This system permitted direct analysis of exudate, in terms of lipidomics (bioaction products, as well as metabolites), proteomics, and cellular composition, by monitoring leukocyte trafficking. Importantly, it permitted the direct comparison of when and where different mediators are produced and activated during resolution, namely temporal spatial differential analyses [1, 30]. In this context, we used the murine dorsal air pouch to determine the formation and roles of endogenous LXA4 in the resolution of acute inflammation [31]. Upon initiation of inflammation with TNF-α, there was a typical acute-phase response denoted by rapid PMN infiltration preceded by both local prostaglandins and leukotrienes. Unexpectedly, the eicosanoids then underwent what we’ve termed a “class switch.” As the exudate evolve, the eicosanoids profiles switched and the lipid mediators made within that milieu changed with time [31]. Leukotrienes (potent chemo-attractants) were deactivated and the transcriptional regulation of enzymes needed for production of lipoxins was activated. This in turn attracted mononuclear cells and stimulated macrophages to take up apoptotic neutrophils within the contained inflammatory exudate site. These results suggested a dual action in that specific eicosanoids namely lipoxins actively reduced the entry of neutrophils into the site of inflammation while accelerating uptake of apoptotic cells of neutrophils [1].
Within the inflammatory exudate, arachidonate-derived eicosanoids changed from initial production of prostaglandins and leukotrienes to lipoxins, which halted further recruitment of neutrophils. This class switch was driven in part by COX-derived prostaglandins E2 and D2, which regulate transcription of enzymes involved in lipoxin biosynthesis [31]. Along with Sir John Savill, we coined “alpha signals omega,” the beginning signals the end [32] because the appearance of lipoxins within inflammatory exudates were concomitant with spontaneous resolution of inflammation [31], and these chemical mediators were non-inflammatory stimulators of monocyte recruitment and enhance macrophage phagocytosis of apoptotic PMNs [9, 33].
DHA is important in neural function [34] as mentioned supra. It is also one of the major polyunsaturated fatty acids (PUFA) found in the retina along with AA [34] In addition, EPA is found in the retinal vascular endothelium [35]. In order to determine whether EPA, DHA, and AA regulate retinal vaso-obliteration and neovascularization in vivo, with we studied an oxygen-induced retinopathy murine model to compare the effects of omega-3 and -6 PUFAs on retinal angiogenesis in wild-type mice with mice that overexpressing the C. elegans fat-1 gene [36]. This gene converts omega-6 PUFA into omega-3 resulting in elevated tissue levels of omega-3 PUFA within the fat-1 overexpressing mice. A protective effect against pathological angiogenesis was found in the retina when there was a lower ratio of omega-6:omega-3 PUFA [37]. Wild-type mice lacking the fat-1 transgene had more extensive vaso-obliteration and more severe retinal neovascularization compared with fat-1 mice [37]. Of interest, in mice fed omega-3 PUFA, there were markers of biosynthesis of neuroprotectin D1 (NPD1) and RvE1 in the retinal tissues. In mice without omega-3 PUFA supplementation, administration of either RvD1, RvE1, or NPD1 each gave protection from vaso-obliteration and neovascularization. These fat-1 mice are also protected from colitis [38].
Pro-resolving mediators are protective in peritonitis [25, 26, 30, 39]. In one study, zymosan A was injected into mice to induce peritonitis [25] and PD1 was protective. To assess whether PD1 could reduce leukocyte infiltration, doses as low as 1 ng were injected. At 4 hours, peritoneal lavages were collected and analyzed. PD1 had a potent action, blocking >90% of further leukocyte infiltration, specifically stopping PMN migrating and infiltration into the site in vivo [25]. When both PD1 and RvE1 were injected together to determine whether their actions were synergistic or additive, RvE1 (10 ng) significantly reduced PMN infiltration although the response was less than that obtained with PD1 (10 ng). In combination, the reduction was even greater which suggests that there was an additive effect of protectin D1 and resolvin E1 in vivo in murine peritonitis. Inflammatory bowel disorders, like colitis, are characterized by a relapsing inflammatory process due to mucosal damage and abnormal mucosal responses [40]. Using a well-studied experimental colitis model, RvE1 protects against bowel inflammation in mice challenged with an intrarectal antigenic hapten, 2,4,6-trinitrobenzene sulfonic acid (TNBS), to induce colitis [41]. RvE1 was administered prior to the induction of colitis in one group, which were compared with mice that received TNBS alone. With treatment of as little as 1 microgram of RvE1 per mouse, there was dramatic reduction in mortality, weight loss, and less severe histologic display of colitis, namely reduction in the associated inflammatory cells, such as PMN, and lymphocytes compared with mice with TNBS-induced colitis. RvE1 is also protective in periodontal disease in rabbits, where it appears to stimulate regeneration [42, 43]. Thus, pro-resolving mediators display anti-inflammatory and anti-fibrotic actions in several widely used laboratory models of inflammation (see Tables 2 and 3).
In summation, the acute inflammatory response is normally a self-limited protective mechanism, initiated by neutrophils in response to injury or infection. Excessive inflammatory responses can lead to chronic disorders. Neutrophil-derived pro-inflammatory mediators, including leukotrienes and prostaglandins, can amplify this process. Within contained inflammatory exudates, we found that neutrophils can change phenotypes to generate protective mediators, derived from fatty acids, to promote resolution. There is an active catabasis to return tissues to a homeostatic health state to return from the inflammatory battle [30]. These protective mediators include the arachidonic acid derived lipoxins as well as omega-3 EPA-derived resolvin E-series, and DHA-derived resolvin-D series and protectins. These findings provide evidence that the resolution of acute inflammation is not passive, but an actively regulated response. Of interest, the production of pro-resolving lipid mediators, such as lipoxins, protectins and resolvins, when administered in vivo can accelerate this process and the return to tissue homeostasis [44].
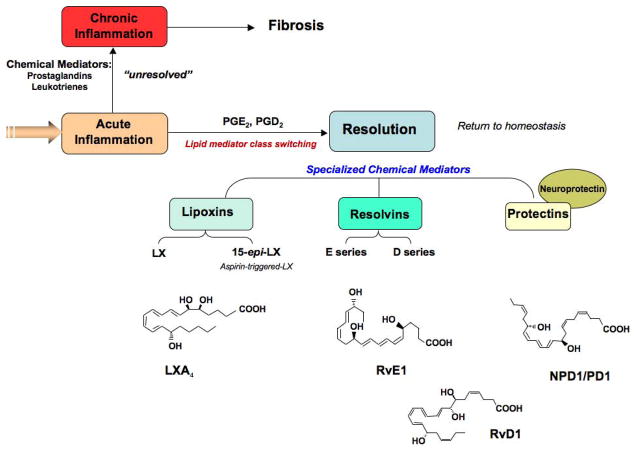
Figure 2.
Biosynthesis of specialized mediators in resolution of acute inflammation. Local mediators include the lipoxins (LX) derived from arachidonic acid (AA); resolvins derived from omega-3 PUFAs eicosapentaenoic acid (EPA; resolvins E series) and docosahexaenoic acid (DHA; resolvins D series); and protectins enzymatically derived from DHA. The structures of each of these key mediators have been confirmed by total organic synthesis (see text for details).
Acknowledgments
The author acknowledges the support of NIH grants P50-DE016191 (Specialized Center for Oral Inflammation and Resolution), GM038765 and DK074448.
The author thanks Mary H. Small for expert assistance with manuscript preparation and the members of his laboratory and collaborators for their expertise and efforts in the reports reviewed herein. I also thank Dr. Nan Chiang of this Center for expert help with preparation of the illustrations used herein.
Abbreviations
- COX-2
cyclooxygenase 2
- EPA
eicosapentaenoic acid
- DHA
docosahexaenoic acid
- HETE
hydroxyeicosatetraenoic acid
- LT
leukotriene
- LX
lipoxin
- LXA4
5S, 6R,15S-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid
- 15-epi-LXA4
5S,6R,15R-trihydroxy-7,9,13-trans-11-cis-eicosatetraenoic acid
- PUFA
polyunsaturated fatty acid
- PMN
polymorphonuclear leukocytes
- Resolvins
structurally unique and potent bioactive local mediators; E series from EPA and D series from DHA
- RvD1
resolvin D1, 7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E,19Z-docosahexaenoic acid
- AT-RvD1
aspirin-triggered-resolvin D1, 7S,8,17R-trihydroxy-docosa-4Z,9E,11E,13Z,15E,19Z-hexaenoic acid
- RvE1
resolvin E1, 5S,12R,18R-trihydroxy-6Z,8E,10E,14Z,16E-eicosapentaenoic acid
- Protectins
potent protective mediators biosynthesized from DHA that contain conjugated triene structures [1]
- PD1/NPD1
protectin D1/neuroprotectin D1, 10R,17S-dihydroxy-docosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Serhan CN. Resolution phase of inflammation: novel endogenous anti-inflammatory and proresolving lipid mediators and pathways. Annu Rev Immunol. 2007;25:101–137. doi: 10.1146/annurev.immunol.25.022106.141647. [DOI] [PubMed] [Google Scholar]
- 2.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: pro-resolving lipid mediators and their potential therapeutic impact. Nat Rev Immunol. 2008 doi: 10.1038/nri2294. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotran RS, Kumar V, Collins T. Robbins Pathologic Basis of Disease. Saunders; Philadelphia: 1999. p. 1425. [Google Scholar]
- 4.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192:1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 7.Samuelsson B. Leukotrienes: mediators of immediate hypersensitivity reactions and inflammation. Science. 1983;220:568–575. doi: 10.1126/science.6301011. [DOI] [PubMed] [Google Scholar]
- 8.Van Dyke TE, Serhan CN. A novel approach to resolving inflammation. Sci Am Oral and Whole Body Health. 2006:42–45. [Google Scholar]
- 9.Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164:1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 10.Canny G, Levy O, Furuta GT, et al. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc Natl Acad Sci USA. 2002;99:3902–3907. doi: 10.1073/pnas.052533799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell EL, Louis NA, Tomassetti SE, et al. Resolvin E1 promotes mucosal surface clearance of neutrophils: a new paradigm for inflammatory resolution. FASEB J. 2007;21:3162–3170. doi: 10.1096/fj.07-8473com. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN. Special Issue on Lipoxins and Aspirin-Triggered Lipoxins. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3–4):139–321. doi: 10.1016/j.plefa.2005.05.002. guest ed. [DOI] [PubMed] [Google Scholar]
- 13.Chiang N, Bermudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers anti-inflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92:9475–9479. doi: 10.1073/pnas.92.21.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell S, Thomas G, Harvey K, et al. Lipoxins, aspirin-triggered epi-lipoxins, lipoxin stable analogues, and the resolution of inflammation: stimulation of macrophage phagocytosis of apoptotic neutrophils in vivo. J Am Soc Nephrol. 2002;13:2497–2507. doi: 10.1097/01.asn.0000032417.73640.72. [DOI] [PubMed] [Google Scholar]
- 17.Serhan CN. Lipoxin biosynthesis and its impact in inflammatory and vascular events. Biochim Biophys Acta. 1994;1212:1–25. doi: 10.1016/0005-2760(94)90185-6. [DOI] [PubMed] [Google Scholar]
- 18.Takano T, Clish CB, Gronert K, Petasis N, Serhan CN. Neutrophil-mediated changes in vascular permeability are inhibited by topical application of aspirin-triggered 15-epi-lipoxin A4 and novel lipoxin B4 stable analogues. J Clin Invest. 1998;101:819–826. doi: 10.1172/JCI1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN, Maddox JF, Petasis NA, et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 20.Guilford WJ, Parkinson JF. Second-generation beta-oxidation resistant 3-oxa-lipoxin A4 analogs. Prostaglandins Leukot Essent Fatty Acids. 2005;73:245–250. doi: 10.1016/j.plefa.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Petasis NA, Keledjian R, Sun Y-P, et al. Design and synthesis of benzo-lipoxin A4 analogs with enhanced stability and potent anti-inflammatory properties. Bioorg Med Chem Lett. 2008 doi: 10.1016/j.bmcl.2008.1001.1013. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan TP, Vallin KS, Shah ST, et al. Aromatic lipoxin A4 and lipoxin B4 analogues display potent biological activities. J Med Chem. 2007;50:5894–5902. doi: 10.1021/jm060270d. [DOI] [PubMed] [Google Scholar]
- 23.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br J Pharmacol. 2008;153:S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. J Biol Chem. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- 25.Serhan CN, Gotlinger K, Hong S, et al. Anti-inflammatory actions of neuroprotectin D1/protectin D1 and its natural stereoisomers: assignments of dihydroxy-containing docosatrienes. J Immunol. 2006;176:1848–1859. doi: 10.4049/jimmunol.176.3.1848. [DOI] [PubMed] [Google Scholar]
- 26.Sun YP, Oh SF, Uddin J, et al. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. J Biol Chem. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- 27.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 29.Gronert K, Maheshwari N, Khan N, Hassan IR, Dunn M, Laniado Schwartzman M. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280:15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 30.Bannenberg GL, Chiang N, Ariel A, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol. 2005;174:4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 31.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 32.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 33.Maddox JF, Serhan CN. Lipoxin A4 and B4 are potent stimuli for human monocyte migration and adhesion: selective inactivation by dehydrogenation and reduction. J Exp Med. 1996;183:137–146. doi: 10.1084/jem.183.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bazan NG. Supply, uptake, and utilization of docosahexaenoic acid during photoreceptor cell differentiation. Nestle Nutrition Workshop Series. 1992;28:121–133. [Google Scholar]
- 35.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- 37.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hudert CA, Weylandt KH, Wang J, et al. Transgenic mice rich in endogenous n-3 fatty acids are protected from colitis. Proc Natl Acad Sci USA. 2006;103:11276–11281. doi: 10.1073/pnas.0601280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bannenberg G, Moussignac RL, Gronert K, et al. Lipoxins and novel 15-epi-lipoxin analogs display potent anti-inflammatory actions after oral administration. Br J Pharmacol. 2004;143:43–52. doi: 10.1038/sj.bjp.0705912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumberg RS, Strober W. Prospects for research in inflammatory bowel disease. JAMA. 2001;285:643–647. doi: 10.1001/jama.285.5.643. [DOI] [PubMed] [Google Scholar]
- 41.Arita M, Yoshida M, Hong S, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc Natl Acad Sci U S A. 2005;102:7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hasturk H, Kantarci A, Ohira T, et al. RvE1 protects from local inflammation and osteoclast mediated bone destruction in periodontitis. FASEB J. 2006;20:401–403. doi: 10.1096/fj.05-4724fje. [DOI] [PubMed] [Google Scholar]
- 43.Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179:7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 44.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN. Aspirin-triggered 15-epi-lipoxin A4 and LXA4 stable analogs are potent inhibitors of acute inflammation: Evidence for anti-inflammatory receptors. J Exp Med. 1997;185:1693–1704. doi: 10.1084/jem.185.9.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schottelius AJ, Giesen C, Asadullah K, et al. An aspirin-triggered lipoxin A4 stable analog displays a unique topical anti-inflammatory profile. J Immunol. 2002;169:7063–7070. doi: 10.4049/jimmunol.169.12.7063. [DOI] [PubMed] [Google Scholar]
- 47.Clish CB, O’Brien JA, Gronert K, Stahl GL, Petasis NA, Serhan CN. Local and systemic delivery of a stable aspirin-triggered lipoxin prevents neutrophil recruitment in vivo. Proc Natl Acad Sci USA. 1999;96:8247–8252. doi: 10.1073/pnas.96.14.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A4 analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: A role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–4768. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- 49.Chiang N, Takano T, Arita M, Watanabe S, Serhan CN. A novel rat lipoxin A4 receptor that is conserved in structure and function. Br J Pharmacol. 2003;139:89–98. doi: 10.1038/sj.bjp.0705220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gewirtz AT, Collier-Hyams LS, Young AN, et al. Lipoxin A4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- 51.Fiorucci S, Wallace JL, Mencarelli A, et al. A beta-oxidation-resistant lipoxin A4 analog treats hapten-induced colitis by attenuating inflammation and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:15736–15741. doi: 10.1073/pnas.0404722101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu SH, Wu XH, Liao PY, Dong L. Signal transduction involved in protective effects of 15(R/S)-methyl-lipoxin A4 on mesangioproliferative nephritis in rats. Prostaglandins Leukot Essent Fatty Acids. 2007;76:173–180. doi: 10.1016/j.plefa.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 53.Katoh T, Takahashi K, DeBoer DK, Serhan CN, Badr KF. Renal hemodynamic actions of lipoxins in rats: a comparative physiological study. Am J Physiol. 1992;263:F436–442. doi: 10.1152/ajprenal.1992.263.3.F436. [DOI] [PubMed] [Google Scholar]
- 54.Leonard MO, Hannan K, Burne MJ, et al. 15-epi-16-(para-fluorophenoxy)-lipoxin A4-methyl ester, a synthetic analogue of 15-epi-lipoxin A4, is protective in experimental ischemic acute renal failure. J Am Soc Nephrol. 2002;13:1657–1662. doi: 10.1097/01.asn.0000015795.74094.91. [DOI] [PubMed] [Google Scholar]
- 55.Bandeira-Melo C, Serra MF, Diaz BL, et al. Cyclooxygenase-2-derived prostaglandin E2 and lipoxin A4 accelerate resolution of allergic edema in Angiostrongylus costaricensis-infected rats: relationship with concurrent eosinophilia. J Immunol. 2000;164:1029–1036. doi: 10.4049/jimmunol.164.2.1029. [DOI] [PubMed] [Google Scholar]
- 56.Levy BD, De Sanctis GT, Devchand PR, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A4. Nat Med. 2002;8:1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 57.Levy BD, Lukacs NW, Berlin AA, et al. Lipoxin A4 analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21:3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145:1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 59.Karp CL, Flick LM, Park KW, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 60.Starosta V, Ratjen F, Rietschel E, Paul K, Griese M. Anti-inflammatory cytokines in cystic fibrosis lung disease. Eur Respir J. 2006;28:581–587. doi: 10.1183/09031936.06.00071405. [DOI] [PubMed] [Google Scholar]
- 61.Jin SW, Zhang L, Lian QQ, et al. Posttreatment with aspirin-triggered lipoxin A4 analog attenuates lipopolysaccharide-induced acute lung injury in mice: the role of heme oxygenase-1. Anesth Analg. 2007;104:369–377. doi: 10.1213/01.ane.0000252414.00363.c4. [DOI] [PubMed] [Google Scholar]
- 62.Wu SH, Wu XH, Lu C, Dong L, Chen ZQ. Lipoxin A4 inhibits proliferation of human lung fibroblasts induced by connective tissue growth factor. Am J Respir Cell Mol Biol. 2006;34:65–72. doi: 10.1165/rcmb.2005-0184OC. [DOI] [PubMed] [Google Scholar]
- 63.Fukunaga K, Kohli P, Bonnans C, Fredenburgh LE, Levy BD. Cyclooxygenase 2 plays a pivotal role in the resolution of acute lung injury. J Immunol. 2005;174:5033–5039. doi: 10.4049/jimmunol.174.8.5033. [DOI] [PubMed] [Google Scholar]
- 64.Chiang N, Gronert K, Clish CB, O’Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104:309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scalia R, Gefen J, Petasis NA, Serhan CN, Lefer AM. Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: Role of P-selectin. Proc Natl Acad Sci USA. 1997;94:9967–9972. doi: 10.1073/pnas.94.18.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fierro IM, Kutok JL, Serhan CN. Novel lipid mediator regulators of endothelial cell proliferation and migration: Aspirin-triggered-15R-lipoxin A4 and lipoxin A4. J Pharmacol Exp Ther. 2002;300:385–392. doi: 10.1124/jpet.300.2.385. [DOI] [PubMed] [Google Scholar]
- 67.Serhan CN, Jain A, Marleau S, et al. Reduced inflammation and tissue damage in transgenic rabbits overexpressing 15-lipoxygenase and endogenous anti-inflammatory lipid mediators. J Immunol. 2003;171:6856–6865. doi: 10.4049/jimmunol.171.12.6856. [DOI] [PubMed] [Google Scholar]
- 68.Jin Y, Arita M, Zhang Q, Serhan C, Dana R. Aspirin-triggered lipoxin agonist (ATLa) inhibits corneal angiogenesis (abstract). Association for Research in Vision and Ophthalmology Annual Meeting; Fort Lauderdale. May 6–10, 2007. [Google Scholar]
- 69.Devchand PR, Schmidt BA, Primo VC, et al. A synthetic eicosanoid LX-mimetic unravels host-donor interactions in allogeneic BMT-induced GvHD to reveal an early protective role for host neutrophils. FASEB J. 2005;19:203–210. doi: 10.1096/fj.04-2565com. [DOI] [PubMed] [Google Scholar]
- 70.Svensson CI, Zattoni M, Serhan CN. Lipoxins and aspirin-triggered lipoxin stop inflammatory pain processing. J Exp Med. 2007;204:245–252. doi: 10.1084/jem.20061826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Menezes-de-Lima O, Jr, Kassuya CA, Nascimento AF, Henriques MG, Calixto JB. Lipoxin A4 inhibits edema in mice: Implications for the anti-edematogenic mechanism induced by aspirin. Prostaglandins Oth Lipid Mediat. 2006;80:123–135. doi: 10.1016/j.prostaglandins.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 72.Duffield JS, Hong S, Vaidya V, et al. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- 73.Ariel A, Li PL, Wang W, et al. The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem. 2005;280:43079–43086. doi: 10.1074/jbc.M509796200. [DOI] [PubMed] [Google Scholar]
- 74.Levy BD, Kohli P, Gotlinger K, et al. Protectin D1 is generated in asthma and dampens airway inflammation and hyper-responsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lukiw WJ, Cui JG, Marcheselli VL, et al. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]