Abstract
Stimulation of κ-opioid receptors in the substantia nigra pars reticulata (SNPR) increases the locomotor activity of young rats: an effect blocked by systemic administration of a D2-like receptor agonist. Based on these initial findings, we proposed that: (a) D2-like receptors in the dorsal striatum are responsible for attenuating κ-opioid-induced locomotor activity, and (b) the effects of D2-like receptor stimulation are mediated by the indirect pathway, which extends from the dorsal striatum to the SNPR via the globus pallidus (GP) and subthalamic nucleus (STN). To test the first hypothesis, young rats were given a systemic injection (IP) of saline or the κ-opioid receptor agonist U50,488 on postnatal day (PD) 18. Later in the testing session, rats received bilateral infusions of vehicle or the D2-like receptor agonist R(-)-propylnorapomorphine (NPA) into the dorsal striatum, and the ability of NPA to block U50,488-induced locomotor activity was determined. To test the second hypothesis, rats were given sham or bilateral electrolytic lesions of the GP or STN on PD 16. Two days later, saline- and U50,488-induced locomotor activity was measured after systemic (IP) administration of vehicle or NPA. As predicted, dorsal striatal infusions of NPA attenuated the U50,488-induced locomotor activity of young rats. Contrary to our expectations, bilateral lesions of the GP or STN did not impair NPA’s ability to block U50,488-induced locomotor activity. When considered together, these results suggest that: (a) stimulation of D2-like receptors in the dorsal striatum is sufficient to attenuate the κ-opioid-mediated locomotor activity of young rats; and (b) the indirect pathway does not mediate the effects of D2-like receptor stimulation in this behavioral model.
Keywords: Indirect pathway, D2-like receptors, κ-opioid receptors, dorsal striatum, globus pallidus, subthalamic nucleus
INTRODUCTION
Along with other neurotransmitters, dopamine and dynorphin are critical modulators of basal ganglia functioning (for reviews, see Hauber, 1998; Steiner and Gerfen, 1998; Lévesque et al., 2003). For example, R(-)-propylnorapomorphine (NPA) and quinpirole stimulate D2-like dopamine receptors of the basal ganglia and, as a consequence, increase the locomotor activity of both young and adult rats (Arnt and Hyttel, 1990; Moody and Spear, 1992; Van Hartesveldt et al., 1992, 1994; Koeltzow et al., 2003; Wacan et al., 2006). Stimulation of κ-opioid receptors (i.e., the high affinity receptor for dynorphin) also alters locomotion but, in this case, systemically administered κ-opioid agonists (e.g., U50,488 and U69,593) differentially affect the locomotor activity of young and adult animals. In adult rodents, systemic administration of U50,488 or U69,593 depresses locomotor activity and rearing (Ukai and Kameyama, 1985; Jackson and Cooper, 1988; Leyton and Stewart, 1992), while the same compounds robustly increase the locomotor activity and wall-climbing of young rats and mice (Jackson and Kitchen, 1989; Kehoe and Boylan, 1994; Duke et al., 1997; McDougall et al., 1997, 1999; Karper et al., 2000). The basis for this interesting ontogenetic behavioral difference is uncertain, however it may be due to the age-dependent maturation of neural mechanisms mediating locomotion.
Although systemic administration of dopamine receptor agonists or κ-opioid receptor agonists increase the locomotor activity of young rats, combined administration of NPA and U50,488 does not produce a potentiated locomotor response. Paradoxically, D2-like receptor agonists (NPA or quinpirole) cause a dose-dependent reduction in the U50,488-induced locomotion of young rats (Duke et al., 1997; McDougall et al., 1997, 1999; Nazarian et al., 1999). This effect is not due to the enhanced expression of stereotypic responses, because coadministering NPA and U50,488 reduces stereotypy in young rats (relative to rats treated with NPA alone) (Duke et al., 1997; McDougall et al., 1999). Interestingly, D2-like receptor agonists attenuate κ-opioid-mediated locomotor activity regardless of endogenous dopamine levels (McDougall et al., 1997). The latter result suggests that D2-like agonists modulate the U50,488-induced locomotor activity of young rats by acting at postsynaptic, rather than presynaptic, dopamine receptors.
The neuroanatomical loci for these various psychopharmacological effects are partially understood, with the substantia nigra pars reticulata (SNPR) being the brain region responsible for mediating U50,488’s locomotor activating effects. Evidence for this conclusion is two-fold: First, bilateral administration of U50,488 into the SNPR causes a dose-dependent increase in the locomotor activity of rats on postnatal day (PD) 18; and, second, microinjecting the κ-opioid receptor antagonist nor-binaltorphimine (nor-BNI) into the SNPR completely attenuates the locomotor activating effects of systemically administered U50,488 (Collins et al., 2000). When combined with results from adult rat studies (Herrera-Marschitz et al., 1986; Matsumoto et al., 1988; Thompson and Walker, 1990, 1992), it is also apparent that κ-opioid receptor stimulation increases the locomotor activity of young rats by disinhibiting nondopaminergic (i.e., GABAergic) motor pathways projecting from the SNPR to the ventromedial thalamus and superior colliculus (Zavala et al., 2002).
Less certain are the neural systems responsible for mediating the κ-opioid/dopamine interactions observed in young rats. Specifically, the D2-like receptor population responsible for attenuating κ-opioid-mediated locomotion has not been determined; however, one possibility is that U50,488-induced locomotor activity is modulated by D2-like receptors located in the dorsal striatum. According to this hypothesis, D2-like receptor agonists attenuate U50,488-induced locomotor activity by altering the firing rate of striatopallidal projections (a component of the indirect pathway). The indirect pathway is composed of a series of neurons that project from the dorsal striatum to the globus pallidus (GP) and from there to the subthalamic nucleus (STN) and SNPR (Parent and Hazrati, 1995a, 1995b; Hauber, 1998; Lévesque et al., 2003). Striatopallidal neurons mainly express D2-like receptors and, when stimulated, alter the firing rate of GABAergic neurons in the SNPR (Nakanishi et al., 1987; Le Moine and Bloch, 1995; Surmeier et al., 1996; Centonze et al., 2002; for reviews, see Gerfen, 1992; Surmeier et al., 2007). Thus, D2-like receptors in the dorsal striatum modulate the functioning of the same SNPR GABAergic output neurons that are normally inhibited by κ-opioid receptor agonists.
Based on these findings, we propose the dual hypotheses that: (a) D2-like receptors in the dorsal striatum are responsible for attenuating U50,488-induced locomotor activity, and (b) the effects of D2-like receptor stimulation are mediated by the indirect pathway. To test the first hypothesis, young rats (PD 18) were given a systemic injection of U50,488 followed by bilateral infusions of NPA into the anterodorsal portion of the dorsal striatum. [This striatal subregion was chosen because infusions of NPA into this area cause robust behavioral effects in adult rats (Bordi et al., 1989).] It was predicted that NPA infusions would cause a dose-dependent reduction in U50,488-induced locomotor activity. To test the second hypothesis, young rats were given sham lesions or bilateral lesions of the GP or STN (two components of the indirect pathway) and the ability of NPA to attenuate U50,488-induced locomotor activity was assessed. It was predicted that NPA would decrease U50,488’s locomotor activating effects in sham controls, while leaving the U50,488-induced locomotor activity of GP- or STN-lesioned rats unaffected. In other words, NPA should be ineffective because critical components of the indirect pathway were compromised.
EXPERIMENTAL PROCEDURES
Animals
Subjects were 208 rats of Sprague-Dawley descent (Charles River, Hollister, CA, USA), born and raised at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups at postnatal day (PD) 3 (day of parturition is PD 0). The colony room was maintained at 21–23°C and kept under a 12-h light/dark schedule. Food and water were freely available. Subjects were cared for in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in commercially available (Coulbourn Instruments, Allentown, PA, USA) activity monitoring chambers (25.5 × 25.5 × 41 cm), consisting of acrylic walls, a plastic floor, and an open top. Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine horizontal locomotor activity (distance traveled). Photobeam resolution was 0.76 cm, with the position of each rat being determined every 100 msec.
Drugs
R(-)-propylnorapomorphine hydrochloride (NPA) was dissolved in distilled water containing 0.1% metabisulfite (an antioxidant). Because NPA is prone to rapid oxidation, this compound was freshly prepared prior to testing. (±)-trans-U50,488 methanesulfonate salt (U50,488) was dissolved in saline. Microinjected drugs were administered at a volume of 0.5 µl per side; whereas, systemically administered drugs were injected intraperitoneally (IP) at a volume of 5 ml/kg. In both experiments, U50,488 was administered at a dose of 5 mg/kg. This dose of U50,488 was chosen because previous research, using a 2–10 mg/kg dose range, showed that 5 mg/kg U50,488 produces substantial locomotor activity in preweanling rats (Bolanos et al., 1996; see also Carden et al., 1994; Kehoe and Boylan, 1994; Duke et al., 1997; McDougall et al., 1997). All drugs were purchased from Sigma (St. Louis, MO, USA).
Surgical procedures
On PD 16, anesthesia was induced by isoflurane (2.5–5%) mixed with oxygen. A topical lidocaine solution (1%) was applied to the scalp and ibuprofen (2 mg/kg, IP) was administered. Rats were placed in a Cunningham Neonatal Rat Adapter attached to a standard Kopf stereotaxic apparatus and the scalp was incised to reveal the skull. For the microinjection experiment, two craniotomies were performed and a stainless steel double guide cannula (22 gauge; Plastics One, Roanoke, VA, USA) was implanted in the anterodorsal portion of the dorsal striatum (A/P +6.5, L ±2.4, D/V +4.8). Guide cannulae were fixed in place using cyanoacrylate gel followed by dental cement. Stainless steel stylets (Plastics One) were used to seal the guide cannulae until time of testing. For the lesion experiment, two craniotomies were performed and Teflon-insulated tungsten electrodes (0.2 mm dia.; A–M Systems, Carlsborg, WA, USA) were bilaterally positioned into either the GP (A/P +5.3, L ±2.6, D/V +3.8) or STN (A/P +6.2, L ±1.6, D/V +3.8). Lesions were made by passing anodal constant current (DC Lesion Maker; Grass Technologies, West Warwick, RI, USA) through the exposed tip of an electrode, with the cathode inserted in the anus. GP lesions were produced by applying a 1.5 mA current for 45 s; whereas, STN lesions were produced by applying a 1.5 mA current for 30 s. The identical procedure was used for the sham-lesioned groups, with the exception that no current was applied. In all cases, stereotaxic coordinates are from the developing rat brain atlas of Sherwood and Timiras (1970). Cannula placements and lesions are shown using schematic representations from the rat brain atlas of Paxinos and Watson (1998).
After surgery, rats were allowed to recover away from the dam in a temperature-controlled chamber (30°C). Rats were returned to the litter when fully responsive (≈3 hr), with post-operative monitoring indicating that dams cared for their pups.
Microinjection experiment protocol
On PD 18, rats (N = 64) were placed into the activity chambers for 20 min. Rats were then injected (IP) with saline or 5 mg/kg U50,488 and immediately returned to the chambers for an additional 20 min. Rats were removed from the testing chamber and the stainless steel stylets were replaced by infusion cannulae (Plastics One) which extended 1 mm below the guide cannulae. Hamilton microsyringes (10 µl) attached to a dual infusion pump (World Precision Instruments, Sarasota, FL, USA) were used to bilaterally microinject vehicle or NPA (5, 10, or 20 µg) into the dorsal striatum at a volume of 0.5 µl per side. NPA was delivered at a constant rate over a 60 s period and the infusion cannulae were subsequently left in place for an additional 60 s. After drug infusion, rats were returned to the testing chamber for 40 min. Locomotor activity (distance traveled) was measured during all components of the behavioral testing session.
Lesion experiment protocol
On PD 18, GP-, STN-, and sham-lesioned rats (N = 144) were placed into the activity chambers where locomotor activity (distance traveled) was measured. After 20 min, rats were injected (IP) with saline or 5 mg/kg U50,488 and immediately returned to the chambers. After an additional 20 min, rats were injected (IP) with vehicle or NPA (0.1 or 1 mg/kg) and returned to the testing chamber for 40 min.
Histology
After behavioral testing, rats were given an overdose of sodium pentobarbital and perfused intracardially with 4% paraformaldehyde. Brains were cryoprotected in a 20% sucrose solution, sectioned coronally (70 µm) using a cryostat, and then stained with thionin for assessment of cannula placement and lesion site accuracy. To determine drug dispersion, additional rats received bilateral microinjections of either neutral red or crystal violet and their brains were removed 5 or 30 min later. Examination of coronal sections indicated that dye dispersion from the anterodorsal portion of the dorsal striatum was minimal in all cases.
Data analysis
Analyses of variance (ANOVAs) for repeated measures (5-min time blocks) were used for statistical analysis of distance traveled data. Because of ongoing experimental manipulations, separate ANOVAs were used to analyze time blocks 1–4 (habituation), 5–8 (U50,488 or saline), and 9–16 (NPA or vehicle). For both experiments, higher order interactions were further analyzed by one- and two-way ANOVAs. Post hoc analysis of behavioral data was made using Newman-Keuls tests (P<0.05).
Litter effects were minimized by assigning no more than one subject from a litter to a particular group (for a discussion of litter effects, see Zorrilla, 1997). In experiments with ten or fewer groups, care was taken to ensure that all treatment groups included one rat from each litter. For both experiments, an equal number of male and female rats were assigned to each group. Preliminary statistical analyses showed that distance traveled data did not vary according to sex of the animals, so the sex variable was excluded from subsequent analyses.
Histological assessment of cannula placement and lesion site accuracy was done by observers blind to drug treatment conditions. In terms of the microinjection experiment, 88.5% of the rats had proper cannula placements in the anterodorsal portions of the dorsal striata. Assessment of lesion site accuracy and completeness indicated that 92.5% of the rats had successful bilateral lesions of the STN, while 81.2% of the rats had substantial lesions of the GP. Data from animals with inappropriate cannula placements or electrolytic lesions were not included in the statistical analysis. For this reason, the number of subjects reported per group varied from 7–9.
RESULTS
Microinjection experiment
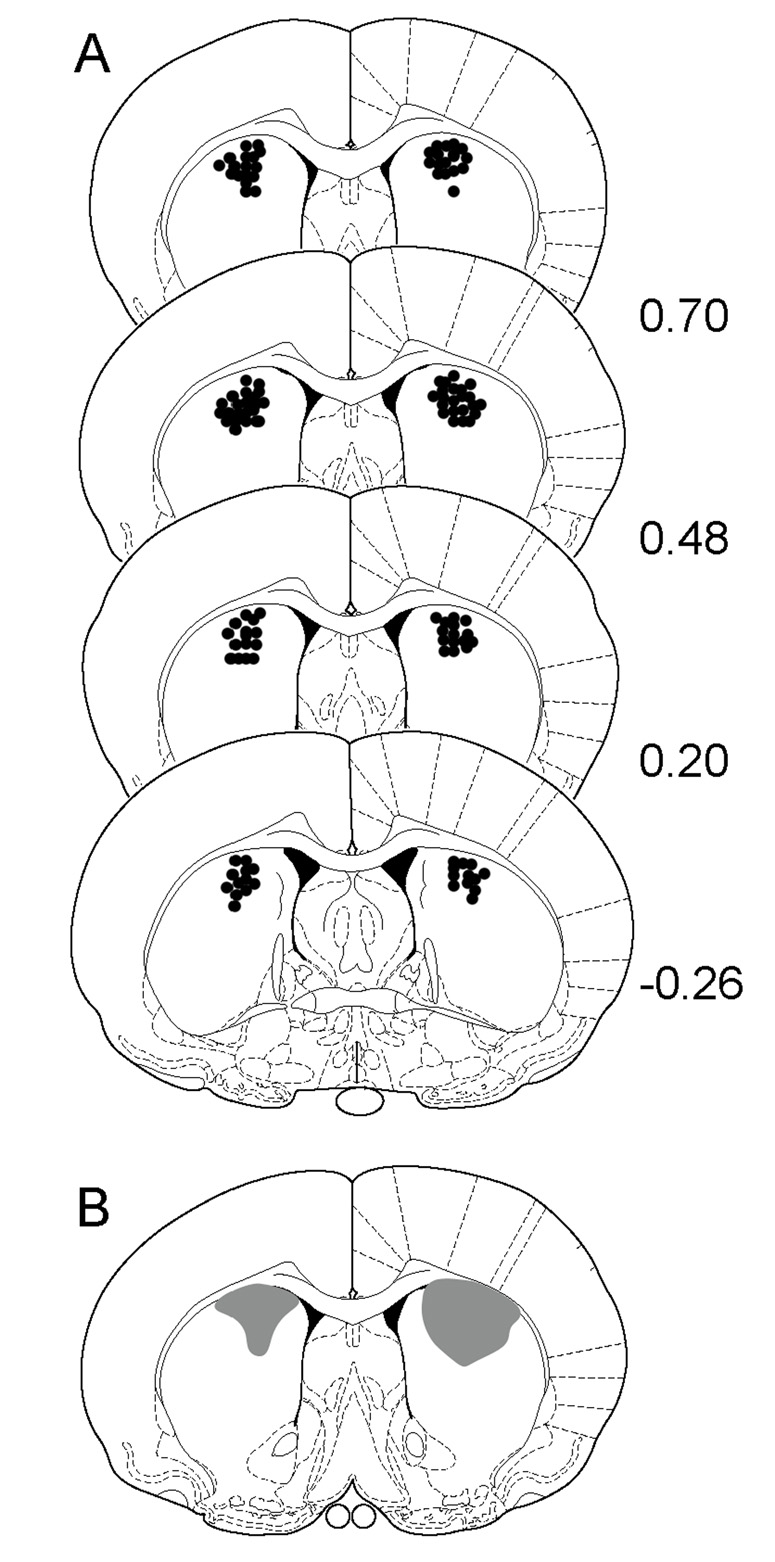
Cannula placements of rats included in the statistical analyses are shown in Fig. 1. Across the first four time blocks (i.e., the habituation phase), rats showed a progressive decline in distance traveled scores (i.e., locomotor activity) (Fig. 2, left panels) [Time effect, F(3, 189)=53.17, P<0.001]. Group differences became apparent when rats were injected (IP) with either saline or U50,488 after the fourth time block (i.e., 20 min into the testing session). Specifically, U50,488-treated rats exhibited greater locomotor activity than saline controls on time blocks 5–8 (Fig. 2, compare middle panels) [Kappa main effect, F(1, 62)=33.28, P<0.001; Kappa × Time interaction, F(3, 186)=10.92, P<0.001]. After vehicle or NPA was bilaterally infused into the dorsal striatum (i.e., 40 min into the testing session), rats in the U50-Vehicle group continued to exhibit more locomotor activity than Sal-Vehicle controls on time blocks 10–16 (Fig. 2, compare right panels) [Kappa × DA × Time interaction, F(21, 392)=5.74, P<0.001].
Fig. 1.
(A) Schematic representations of cannula placements in the anterodorsal portion of the dorsal striatum. (B) Schematic representation showing typical dye (crystal violet) dispersion 5 min (left) and 30 min (right) after infusion. In all cases, numbers on the right indicate distance (mm) from Bregma using coordinates from the rat brain atlas of Paxinos and Watson (1998).
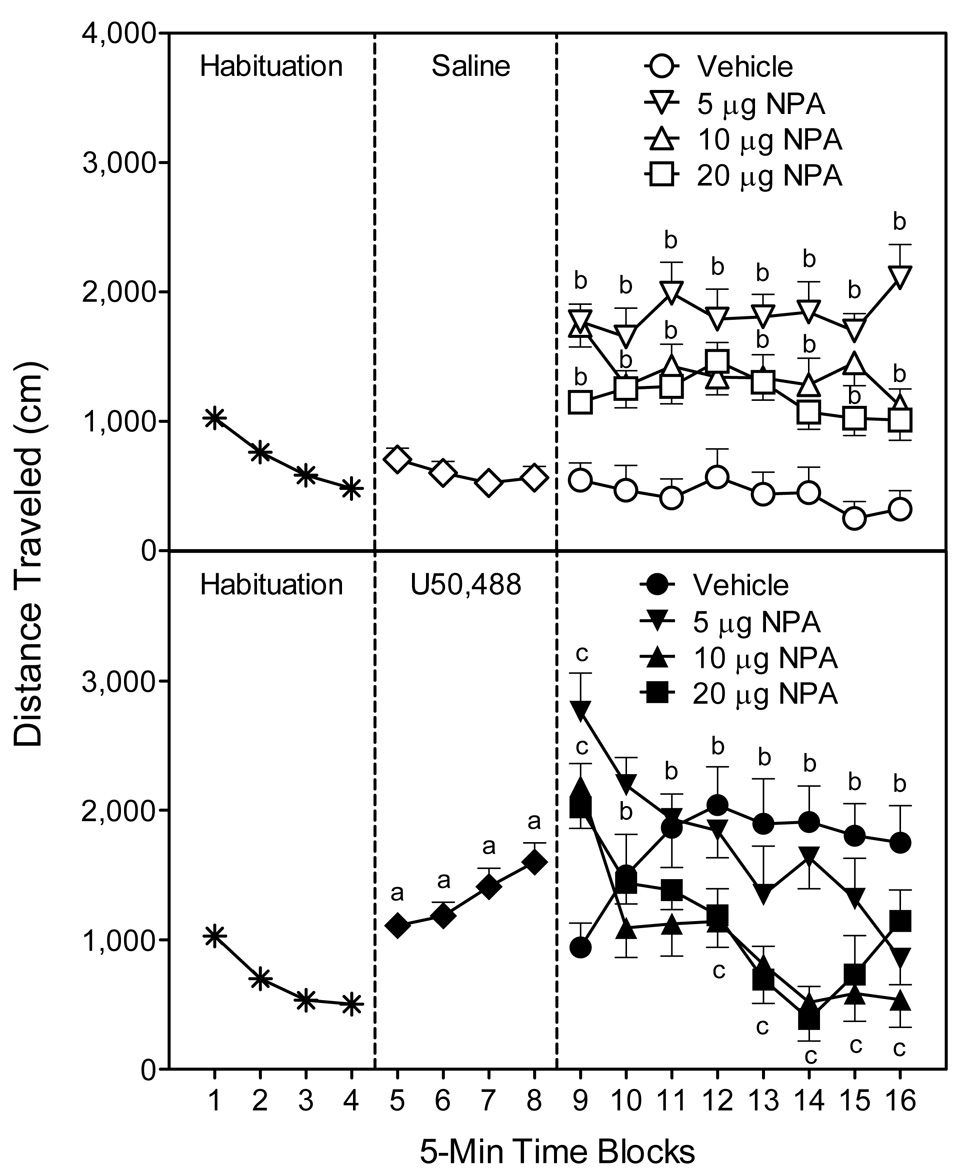
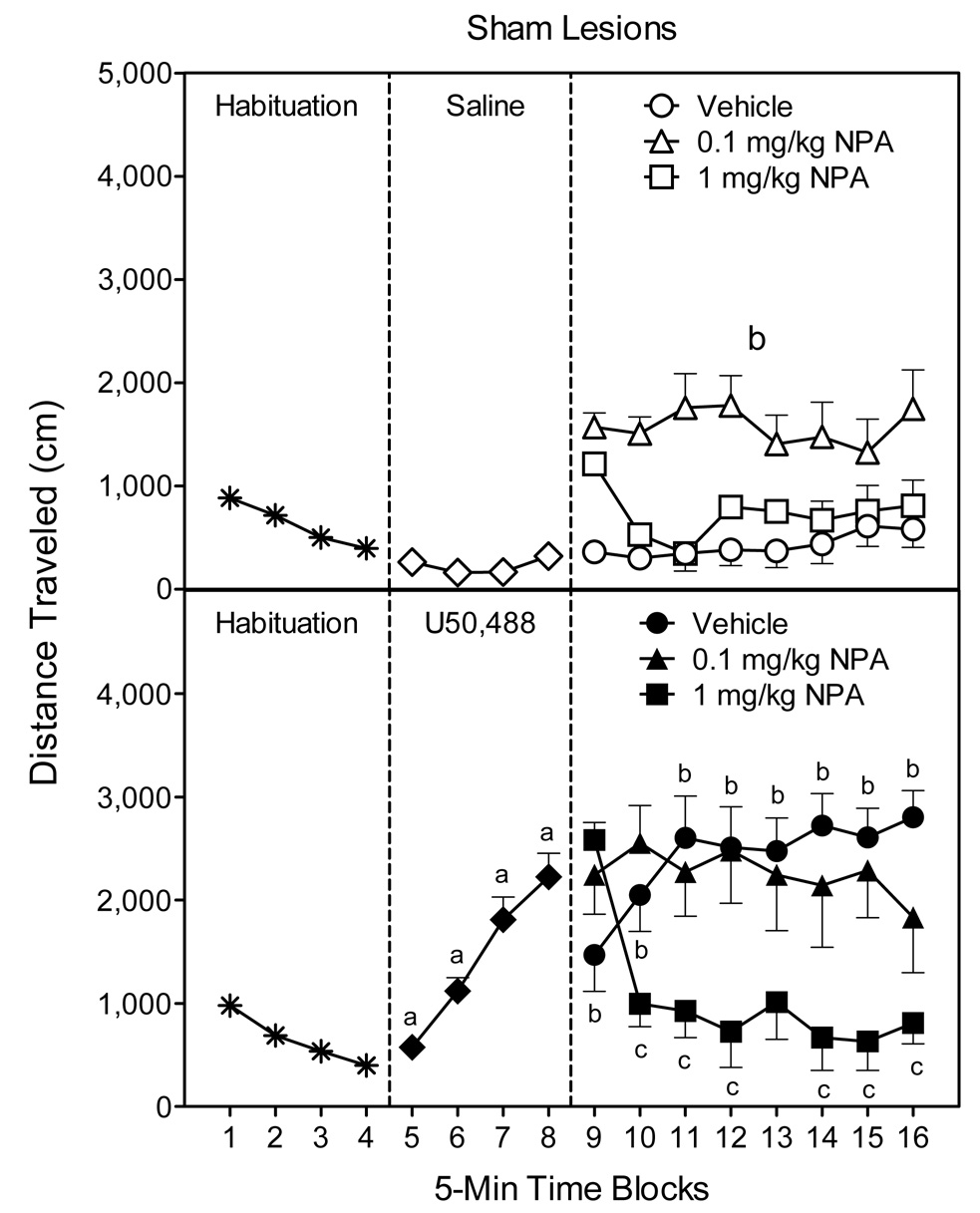
Fig. 2.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) during the 80-min behavioral testing session. Rats were habituated to the apparatus on time blocks 1–4. At the conclusion of time block 4 (indicated by the first dashed line), rats were given an IP injection of saline (upper graph) or 5 mg/kg U50,488 (lower graph). At the conclusion of time block 8 (indicated by the second dashed line), rats received bilateral microinjections of vehicle or NPA (5, 10, or 20 µg) into the anterodorsal portion of the dorsal striatum. aSignificantly different from saline-treated rats (◇). bSignificantly different from saline-treated rats in the vehicle group (○). cSignificantly different from U50,488-treated rats in the vehicle group (●).
Separate statistical analyses were used to assess the effects of NPA in the saline- and U50,488-treated rats. In the saline-treated rats (Fig. 2, upper graph, right panel), all doses of NPA (5, 10, and 20 µg) enhanced locomotion, with the greatest increase in locomotor activity being produced by 5 µg NPA [DA main effect, F(3, 28)=18.35, P<0.001]. Differences between the NPA (5, 10, and 20 µg) and vehicle-treated rats were apparent on all eight time blocks [DA × Time interaction, F(21, 196)=1.88, P<0.05]. In contrast, on only specific time blocks did 5 µg NPA stimulate more locomotor activity than 10 µg NPA (time blocks 11, 14, and 16) or 20 µg NPA (time blocks 9, 11, and 14–16).
Among the U50,488-treated rats (Fig. 2, lower graph, right panel), bilateral infusions of 10 or 20 µg NPA significantly attenuated U50,488-induced locomotor activity on time blocks 12–15 [DA main effect, F(3, 28)=4.65, P<0.01; DA × Time interaction, F(21, 196)=6.57, P<0.001]. A similar effect occurred on time block 16, except that 5 and 10 µg NPA, but not 20 µg NPA, reduced the U50,488-induced locomotor activity of young rats. Interestingly, on time block 9 (immediately after injections) all doses of NPA caused a transient increase in the locomotor activity of U50,488-treated rats that persisted for only 5 min. Whether this transient locomotor effect is related to the injection procedure or some other factor is uncertain.
Lesion experiment
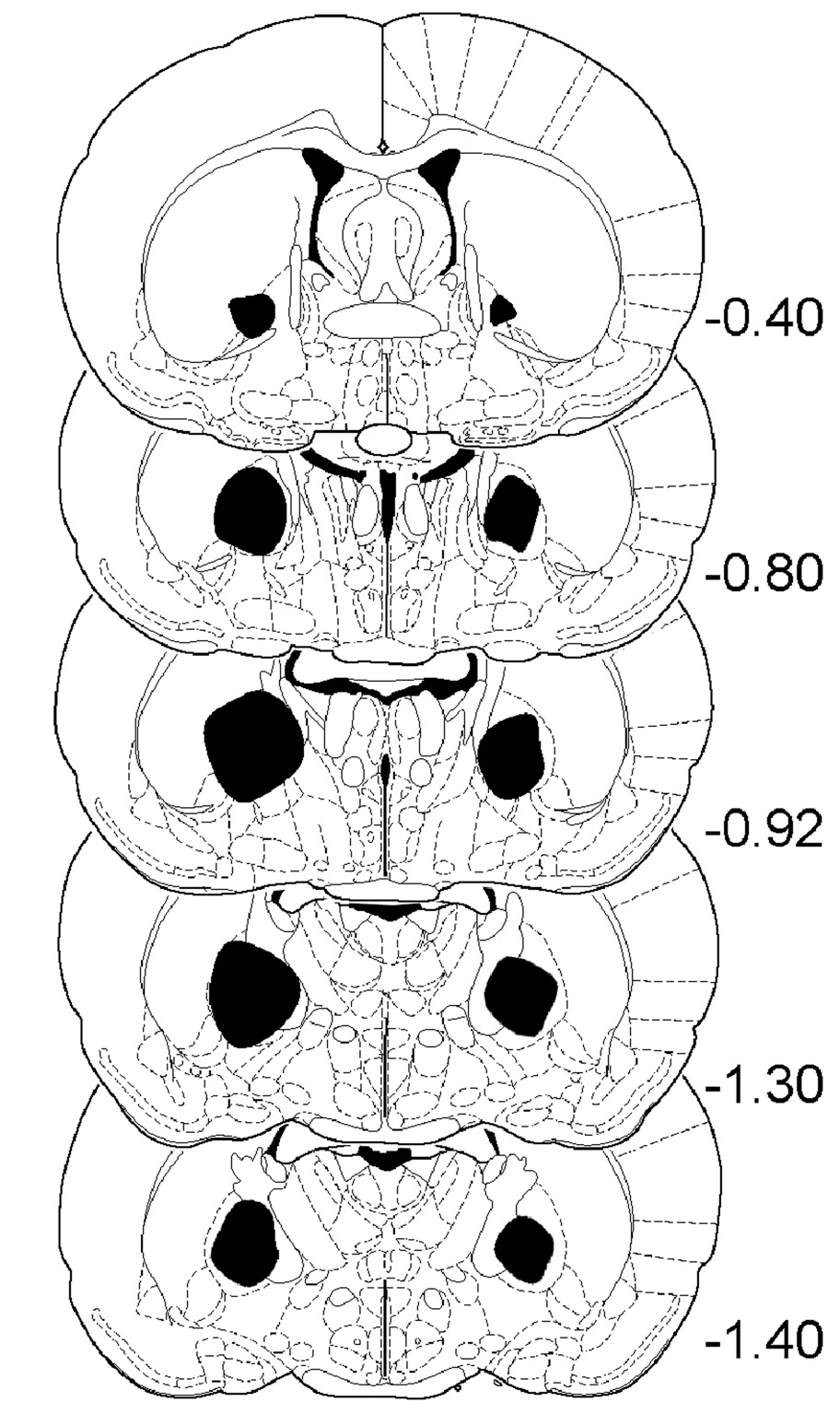
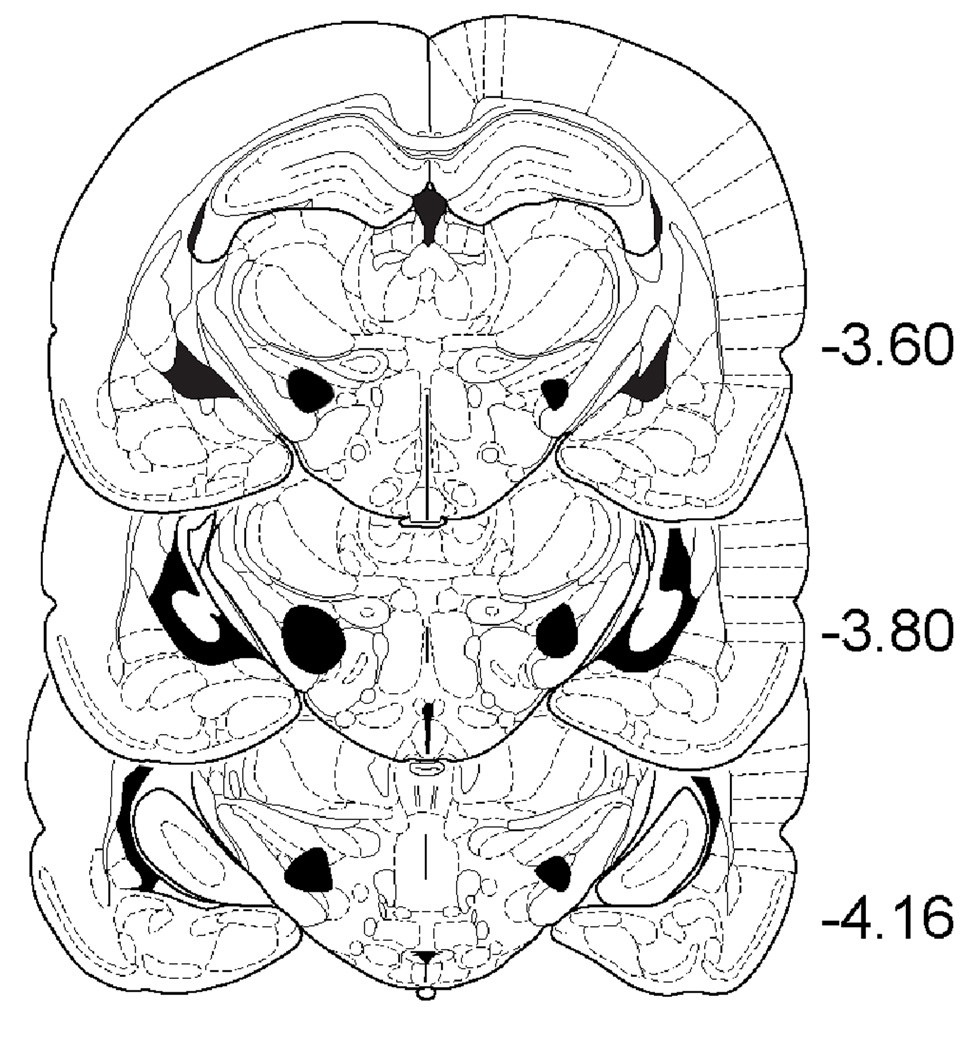
The minimal and maximal extents of GP and STN lesions are shown in Fig. 3 and Fig. 4. GP lesions typically extended from −0.40 through −1.60 (relative to Bregma); whereas STN lesions extended from −3.60 through −4.20.
Fig. 3.
Schematic representations showing the minimal (left side) and maximal (right side) extent of GP lesions. Schematic representations are adapted from the rat brain atlas of Paxinos and Watson (1998), with numbers on the right indicating distance (mm) from Bregma.
Fig. 4.
Schematic representations showing the minimal (left side) and maximal (right side) extent of STN lesions. Schematic representations are adapted from the rat brain atlas of Paxinos and Watson (1998), with numbers on the right indicating distance (mm) from Bregma.
During the habituation phase (i.e., time blocks 1–4), GP- and STN-lesioned rats exhibited significantly greater basal locomotor activity than sham controls (Table 1) [Condition main effect, F(2, 141)=37.16, P<0.001]. Differences between the GP- and sham-lesioned rats were apparent on time blocks 1–4; whereas the STN-lesioned rats exhibited elevated locomotor activity on time blocks 2–4 [Condition × Time interaction, F(6, 423)=16.89, P<0.001]. Because of these differences in basal locomotor activity, drug-induced behavioral effects of the three lesion conditions were assessed separately.
Table 1.
Mean distance traveled scores (±SEM) of sham, globus pallidus (GP), and subthalamic nucleus (STN) lesioned rats during habituation (time blocks 1–4)
| Lesion Condition | Time block | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Total (1–4) | |
| Sham | 933 (±56) | 703 (±51) | 520 (±47) | 398 (±45) | 2554 (±173) |
| Globus Pallidus | 1340* (±95) | 1673* (±108) | 1587* (±101) | 1557* (±113) | 6157* (±349) |
| Subthalamic Nucleus | 886 (±75) | 989* (±84) | 1155* (±95) | 1182* (±111) | 4211* (±323) |
Denotes significantly different from sham-lesioned rats on the same time block (P<0.05).
Sham lesions
Sham controls injected with U50,488 exhibited significantly more locomotor activity than saline-treated rats on time blocks 5–8 (Fig. 5, compare middle panels) [Kappa main effect, F(1, 45)=58.74, P<0.001; Kappa × Time interaction, F(3, 135)=38.97, P<0.001]. On time blocks 9–16 (i.e., after IP injection of vehicle or NPA), rats in the U50-Vehicle group continued to exhibit more locomotor activity than Sal-Vehicle controls (Fig. 5, compare right panels) [Kappa × DA × Time interaction, F(14, 287)=2.65, P<0.001].
Fig. 5.
Mean distance traveled scores (±SEM) of sham lesioned rats (n = 7–8 per group) during the 80-min behavioral testing session. Rats were habituated to the apparatus on time blocks 1–4. At the conclusion of time block 4 (indicated by the first dashed line), rats were given an IP injection of saline (upper graph) or 5 mg/kg U50,488 (lower graph). At the conclusion of time block 8 (indicated by the second dashed line), rats received an IP injection of vehicle or NPA (0.1 or 1 mg/kg). aSignificantly different from saline-treated rats (◇). bSignificantly different from saline-treated rats in the vehicle group (○). cSignificantly different from U50,488-treated rats in the vehicle group (●).
Further analysis of only the saline groups showed that the effects of NPA did not differ significantly according to time block (Fig. 5, upper graph, right panel). Instead, sham-lesioned rats given saline and 0.1 mg/kg NPA had greater locomotor activity than rats in the Sal-Vehicle group [DA main effect, F(2, 20)=13.79, P<0.001]. Rats given the higher dose of NPA (1 mg/kg) did not differ from the Sal-Vehicle controls. Among the U50,488 groups (Fig. 5, lower graph, right panel), the higher dose of NPA (1 mg/kg) caused a reduction in locomotor activity, relative to the U50-Vehicle group, on time blocks 10–12 and 14–16 [DA × Time interaction, F(14, 147)=5.44, P<0.001]. The lower dose of NPA (0.1 mg/kg) did not significantly reduce U50,488-induced locomotor activity on any time block.
Globus pallidus lesions
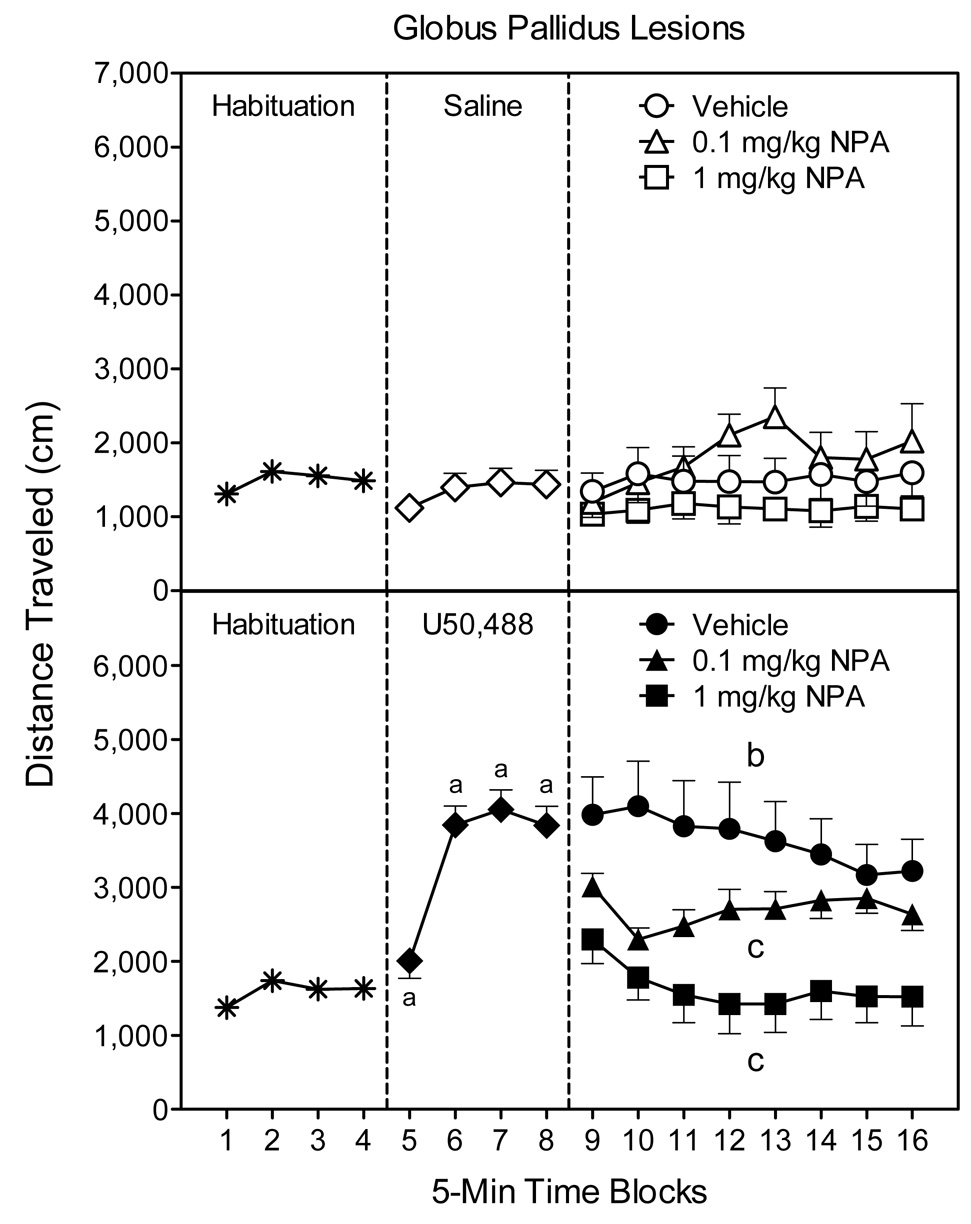
On time blocks 5–8, U50,488 significantly increased the locomotor activity of GP-lesioned rats relative to their saline controls (Fig. 6, compare middle panels) [Kappa main effect, F(1, 46)=55.40, P<0.001; Kappa × Time interaction, F(3,138=28.40, P<0.001]. On time blocks 9–16, the three-way interaction did not reach statistical significance [P>0.05]; however, analysis of the Kappa × DA interaction indicated that rats in the U50-Vehicle group exhibited greater locomotor activity than rats in the Sal-Vehicle group (Fig. 6, compare right panels) [Kappa × DA interaction, F(2, 42)=3.81, P<0.001].
Fig. 6.
Mean distance traveled scores (±SEM) of globus pallidus (GP) lesioned rats (n = 7–9 per group) during the 80-min behavioral testing session. Rats were habituated to the apparatus on time blocks 1–4. At the conclusion of time block 4 (indicated by the first dashed line), rats were given an IP injection of saline (upper graph) or 5 mg/kg U50,488 (lower graph). At the conclusion of time block 8 (indicated by the second dashed line), rats received an IP injection of vehicle or NPA (0.1 or 1 mg/kg). aSignificantly different from saline-treated rats (◇). bSignificantly different from saline-treated rats in the vehicle group (○). cSignificantly different from U50,488-treated rats in the vehicle group (●).
Separate statistical analysis of the saline groups showed that NPA (0.1 or 1 mg/kg) did not significantly affect the locomotor activity of GP-lesioned rats (Fig. 6, upper graph, right panel). Among the U50,488 groups (Fig. 6, lower graph, right panel), both doses of NPA decreased the U50,488-induced locomotor activity of GP-lesioned rats [DA main effect, F(2, 20)=7.03, P<0.01]. This effect was dose-dependent because U50,488-treated rats given 0.1 mg/kg NPA exhibited more locomotor activity than rats injected with the greater dose of NPA (1 mg/kg), but they were less active than rats in the U50-Vehicle group.
Subthalamic nucleus lesions
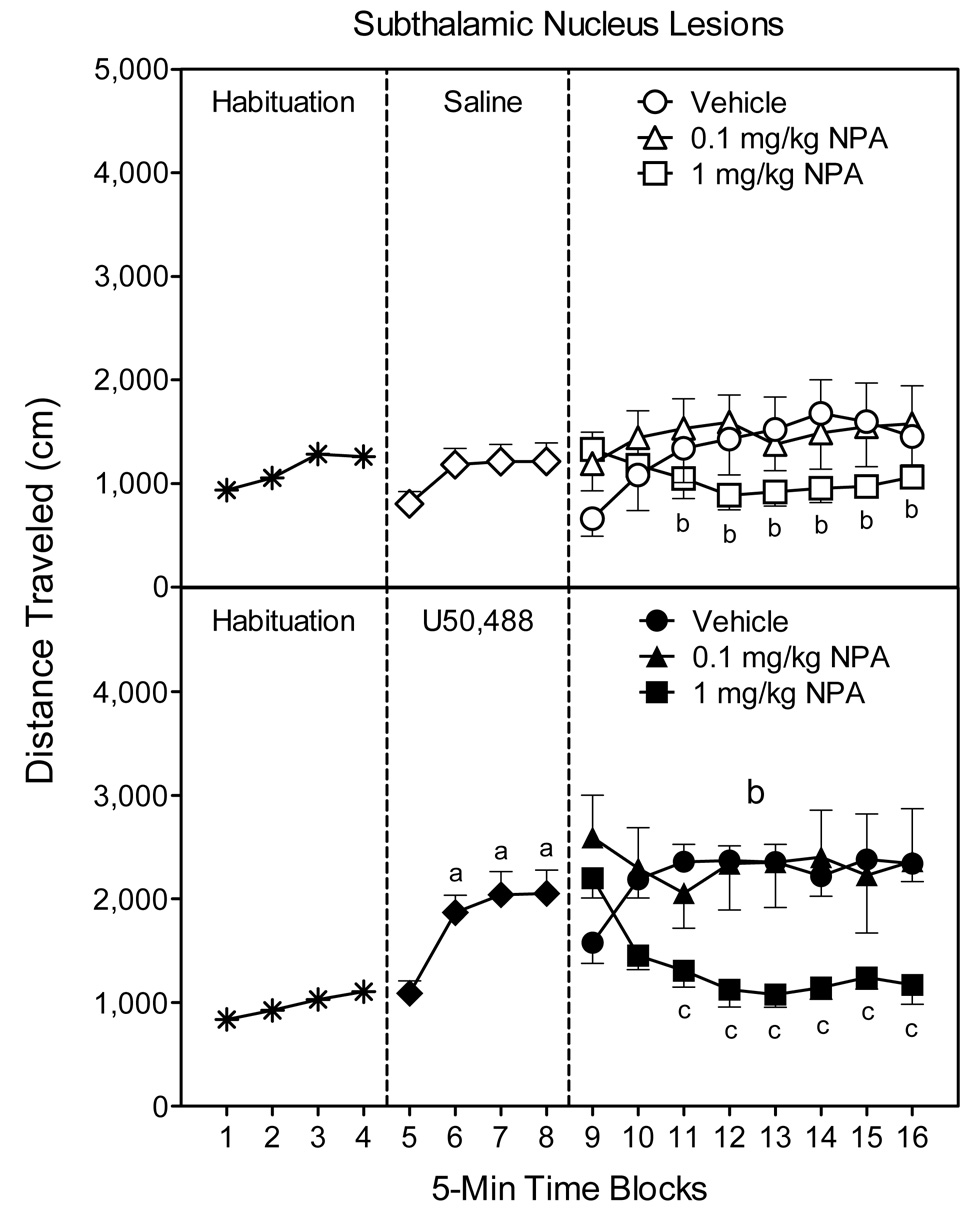
U50,488-treated rats with bilateral lesions of the STN exhibited enhanced locomotor activity on time blocks 6–8 (Fig. 7, compare middle panels) [Kappa main effect, F(1, 47)=8.69, P<0.01; Kappa × Time interaction, F(3, 141)=4.65, P<0.01]. During the subsequent testing phase (time blocks 9–16), rats treated with U50,488 continued to exhibit more locomotor activity than saline controls (Fig. 7, compare right panels) [Kappa main effect, F(1, 43)=13.26, P<0.001]. NPA attenuated the locomotor activity of STN-lesioned rats, but this effect was only apparent after administration of 1 mg/kg NPA and occurred in both the saline- and U50,488-treated rats [DA main effect, F(2, 43)=5.60, P<0.01]. Differences between the NPA- and vehicle-treated rats were apparent on time blocks 11–16 [DA × Time interaction, F(14, 301)=7.01, P<0.001].
Fig. 7.
Mean distance traveled scores (±SEM) of subthalamic nucleus (STN) lesioned rats (n = 7–9 per group) during the 80-min behavioral testing session. Rats were habituated to the apparatus on time blocks 1–4. At the conclusion of time block 4 (indicated by the first dashed line), rats were given an IP injection of saline (upper graph) or 5 mg/kg U50,488 (lower graph). At the conclusion of time block 8 (indicated by the second dashed line), rats received an IP injection of vehicle or NPA (0.1 or 1 mg/kg). aSignificantly different from saline-treated rats (◇). bSignificantly different from saline-treated rats in the vehicle group (○). cSignificantly different from U50,488-treated rats in the vehicle group (●).
DISCUSSION
Unlike what is typically observed in adult rats, κ-opioid receptor stimulation caused a robust increase in the locomotor activity of young rats on PD 18 (see also Jackson and Kitchen, 1989; Duke et al., 1997; McDougall et al., 1997, 1999). U50,488’s locomotor activating effects were apparent in control animals as well as rats given bilateral lesions of the GP or STN. Consistent with past studies (Duke et al., 1997; McDougall et al., 1999; Nazarian et al., 1999), systemic administration of NPA (1 mg/kg, IP) fully attenuated the U50,488-induced locomotor activity of intact animals. As hypothesized this NPA-induced effect is mediated, at least partially, by the dorsal striatum. More specifically, bilateral infusion of 10 or 20 µg NPA into the anterodorsal portion of the dorsal striatum was able to significantly reduce κ-opioid-mediated locomotion (Fig. 2). It remains possible that D2-like receptors in other brain regions can also modify U50,488-induced locomotor activity, but it is now clear that stimulation of dorsal striatal D2-like receptors is sufficient to attenuate the κ-opioid-mediated locomotor activity of rats on PD 18. Although stereotyped behaviors were not systematically assessed in the present study, we have previously shown that combined treatment with NPA and U50,488 does not result in a potentiated stereotypic response. Instead, co-administration of U50,488 (5 mg/kg) and NPA (1 mg/kg) produces less stereotypy than when NPA is given alone (Duke et al., 1997; McDougall et al., 1999) In the microinjection experiment, NPA (10 or 20 µg) was able to both reduce U50,488-induced locomotion and stimulate locomotor activity when given alone (Fig. 2). This pattern of results shows that NPA’s ability to block U50,488-induced locomotor activity cannot be ascribed to the onset of intense stereotypy that masks the locomotor response.
Contrary to our expectations, bilateral lesions of the GP or STN did not impair NPA’s ability to block the U50,488-induced locomotor activity of rats on PD 18. This result was unexpected because we originally hypothesized that the indirect pathway, which extends from the dorsal striatum to the SNPR via the GP and STN, mediates the effects of NPA in this behavioral model. Several explanations for these results are possible: First, a separate population of nonstriatal D2-like receptors, distinct from D2-like receptors located in the dorsal striatum, may co-modulate κ-opioid-mediated locomotor activity. Second, dorsal striatal dopamine receptors may be exclusively responsible for attenuating U50,488-induced locomotion, yet fiber tracts distinct from the indirect pathway may be responsible for mediating this κ-opioid/dopamine interaction. Third, electrolytic lesions of the GP or STN may have been insufficient to disrupt the functioning of the indirect pathway. The first possibility (i.e., that D2-like receptors in nonstriatal regions are capable of attenuating κ-opioid-mediated locomotion) cannot be excluded even though intrastriatal infusions of NPA robustly decreased U50,488-induced locomotor activity (see Fig. 2). The difficulty in ruling out this explanation is two-fold: (a) D2-like receptors are widely distributed in midbrain and forebrain structures of young and adult rats (Boyson et al., 1986; Bouthenet et al., 1991; Schambra et al., 1994; Demotes-Mainard et al., 1996; Tarazi and Baldessarini, 2000), and (b) selective D2-like antagonists (a drug class often used in receptor localization experiments) may be of little probative value because they cause a general reduction in basal and U50,488-induced locomotor activity that is independent of opioid system functioning (Duke et al., 1997; Nazarian et al., 1999).
A more likely explanation is that the critical D2-like receptors are, in fact, exclusively located in the dorsal striatum, but that NPA’s ability to modulate κ-opioid system functioning does not involve the indirect pathway. Instead, the effects of D2-like receptor stimulation may be mediated by the direct pathway (also referred to as the striatonigral tract), which is composed of GABA/dynorphin/substance P containing neurons that project from the dorsal striatum to the SNPR (Hauber, 1998; Steiner and Gerfen, 1998; Lévesque et al., 2003). In adult rats, in situ hybridization studies confirm that striatonigral neurons mainly express D1-like, rather than D2-like receptors (for reviews, see Gerfen, 1992; Surmeier et al., 2007); however, a significant subset of striatonigral neurons appear to express both D1-like and D2-like receptors (Surmeier et al., 1996; Aizman et al., 2000). The behavioral relevance of these striatonigral D2-like receptors is not known, but it is conceivable that they could impact the functioning of the SNPR and κ-opioid-mediated locomotor activity. An important caveat is that ontogenetic studies have not determined the degree of D1- and D2-like receptor co-localization in striatal output neurons. Although speculative, a finding of increased receptor co-localization during postnatal ontogeny would be consistent with the idea that the direct pathway is capable of mediating some of the behavioral effects of NPA.
A third possibility is that bilateral electrolytic lesions of the GP or STN may have been insufficient to disrupt the functioning of the indirect pathway. The major disadvantage of the electrolytic lesioning technique is that it is not possible to selectively target cell bodies or axons, instead electrolytic lesions produce very precise damage to a particular brain area. For ontogenetic research, electrolytic lesions are often appropriate because a full lesion can be made in a very short time frame, whereas chemically-induced lesions take longer for completion. In terms of the present study, care was taken to ensure that only rats with substantial lesions of the GP and STN were included in the statistical analyses. Lesion accuracy was further confirmed by the behavioral data because young rats with GP and STN lesions exhibited elevated amounts of basal locomotion; a finding previously reported in young and adult rats with unilateral or bilateral electrolytic lesions of the GP (Dewar et al., 1983; Thompson et al., 1984; Joel et al., 1998). Importantly, Dewar et al. (1983) reported that unilateral electrolytic lesions of the GP seemed to “completely and permanently disrupt striatal output” (i.e., destroy striatopallidal connections). If true, the inability of NPA to attenuate U50,488-induced locomotor activity in GP-lesioned rats cannot be attributed to an incomplete disruption of the indirect pathway.
At present, it remains uncertain why systemic administration of U50,488 enhances the locomotor activity of preweanling rats, while depressing the locomotor activity of adult rats. One possibility is that ontogenetic changes in GABAergic systems may be responsible for U50,488’s age-dependent effects. Evidence for this possibility is three-fold. First, GABAA receptor stimulation induces excitation, rather than inhibition, in various brain regions of developing mammals (for reviews, see Leinekugel et al., 1999; Ben-Ari, 2002). The switch from excitation to inhibition occurs at around PD 15 in rat hippocampus (Khazipov et al., 2004), thus the time-course of U50,488’s paradoxical locomotor activating effects is approximately similar to the time-course of GABA excitation. Second, dynorphin and GABA are co-released by striatal neurons projecting to the substantia nigra (Hauber, 1998; Steiner and Gerfen, 1998; Lévesque et al., 2003). Third, electrophysiological evidence shows that GABA and dynorphin modulate each other’s actions in the SNPR (Robertson et al., 1987). In sum, transient GABAergic excitation may explain why systemically administered U50,488 induces locomotor activity in preweanling rats; however, it is difficult to reconcile this explanation with studies showing that (a) intranigral infusions of U50,488 increase the locomotor activity of both young and adult rats (Herrera-Marschitz et al., 1986; Matsumoto et al., 1988; Thompson and Walker, 1990, 1992; Collins et al., 2000) and (b) muscimol (a GABAA agonist) affects the locomotor activity of 14-day-old and adult mice similarly (Tirelli et al., 1991). An alternative explanation is that the “balance” between striatal and nigral κ-opioid receptor systems may differ according to age (Collins et al., 2000; Zavala et al., 2002). In adult rats, stimulating presynaptic κ-opioid receptors in the nucleus accumbens and striatum inhibits DA release and decreases locomotion (Di Chiara and Imperato, 1988; Maisonneuve et al., 1994; Gray et al., 1999). Therefore, systemically administered U50,488 may decrease locomotion in adult rats because the behavioral consequences of stimulating κ-opioid receptors in the SNPR is masked or overwhelmed by κ-opioid-induced reductions in striatal and accumbal dopamine release. The opposite relationship may exist in preweanling rats, with the locomotor activating effects of SNPR stimulation overwhelming the potential locomotor inhibiting effects of accumbal or striatal κ-opioid receptors (for a fuller discussion, see Collins et al., 2000).
In addition to assessing the effects of D2-like receptor stimulation on κ-opioid-mediated locomotor activity, the design of these experiments allowed the behavioral effects of NPA to be examined separately from U50,488. In this regard, some interesting ontogenetic differences were apparent. As has been reported before in both young and adult rats, systemic administration of NPA induces relatively more locomotion at lower doses than higher doses, with stereotypic responding becoming more prominent as the dose of NPA increases (Meller et al., 1988; Mestlin and McDougall, 1993; Wacan et al., 2006). Our results are consistent with these past findings, because only 0.1 mg/kg NPA, but not 1 mg/kg NPA, enhanced the locomotor activity of sham-lesioned rats (see Fig. 3, upper graph).
Of more interest, a large number of studies have reported that intrastriatal infusions of NPA (5–40 µg) or apomorphine (5–20 µg) cause stereotypy in adult rats (Dourish et al., 1985; Bordi et al., 1989; Waszczak et al., 2002), while the D2/D3 agonist quinpirole (3–40 µg) causes yawning and behavioral sedation (Bordi and Meller, 1989; Delfs and Kelley, 1990; Canales and Iversen, 1998). In the only study providing potentially conflicting data, Van Hartesveldt et al. (1992) reported that adult rats exhibit a biphasic locomotor response after dorsal striatal quinpirole administration, with moderate doses (10–20 µg) decreasing and a higher dose (40 µg) increasing locomotion. In the current study, infusing NPA into the dorsal striatum caused a dramatic increase in the locomotor activity of young rats, with the 5 µg dose producing greater locomotion than the 10 or 20 µg doses. Our observations suggested that these rats were engaged in forward locomotion with few of the more intense stereotypic behaviors being evident. Although the present results are in partial disagreement with Bordi, et al. (1989) who reported that NPA (5–40 µg) caused only “sporadic” locomotor activity and intense stereotypy, it is likely that the divergent findings may be a consequence of age-dependent differences in drug responsiveness. The neuroanatomical basis for this ontogenetic effect is uncertain, but it may result from age-dependent changes in either the density of D2-like receptors or in the coupling of D1/D2 receptors (see Moody and Spear, 1992; Tarazi and Baldessarini, 2000). In any event, the present results show that intrastriatal infusions of the D2-like agonist NPA have dramatically different effects in young and adult rats, with locomotor activity predominating in young rats (Fig. 2) and stereotypy predominating in adults (Bordi et al., 1989).
In summary, systemic administration of D2-like agonist compounds (i.e., NPA and quinpirole) attenuate the κ-opioid-mediated locomotor activity of young rats. These dopamine-mediated effects appear to be mediated by the dorsal striatum, because infusing NPA into this brain region reduces U50,488-induced locomotor activity. Contrary to our original hypotheses, bilateral lesions of the GP or STN did not block the behavioral effects of D2-like receptor stimulation, thus suggesting that the indirect pathway is not responsible for mediating NPA’s actions. Although speculative, it is possible that the direct pathway, which projects from the dorsal striatum to the SNPR, may mediate NPA-induced effects in this behavioral model.
Acknowledgments
This work was partially supported by a grant (GM073842) from the National Institutes of Health.
Abbreviations
- ANOVA
Analysis of variance
- GP
globus pallidus
- IP
intraperitoneal
- NPA
R(-)-propylnorapomorphine
- nor-BNI
nor-binaltorphimine
- PD
postnatal day
- SNPR
substantia nigra pars reticulata
- STN
subthalamic nucleus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aizman O, Brismar H, Uhlén P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. doi: 10.1038/72929. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Dopamine D-2 agonists with high and low efficacies: differentiation by behavioural techniques. J Neural Transm. 1990;80:33–50. doi: 10.1007/BF01245021. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. Excitatory actions of GABA during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Garmsen GM, Clair MA, McDougall SA. Effects of the κ-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Bordi F, Meller E. Enhanced behavioral stereotypies elicited by intrastriatal injection of D1 and D2 dopamine agonists in intact rats. Brain Res. 1989;504:276–283. doi: 10.1016/0006-8993(89)91368-1. [DOI] [PubMed] [Google Scholar]
- Bordi F, Carr KD, Meller E. Stereotypies elicited by injection of N-propylnorapomorphine into striatal subregions and nucleus accumbens. Brain Res. 1989;489:205–215. doi: 10.1016/0006-8993(89)90852-4. [DOI] [PubMed] [Google Scholar]
- Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res. 1991;564:203–219. doi: 10.1016/0006-8993(91)91456-b. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Molinoff PB. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986;6:3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales JJ, Iversen SD. Behavioural topography in the striatum: differential effects of quinpirole and D-amphetamine microinjections. Eur J Pharmacol. 1998;362:111–119. doi: 10.1016/s0014-2999(98)00752-3. [DOI] [PubMed] [Google Scholar]
- Carden SE, Davachi L, Hofer MA. U50,488 increases ultrasonic vocalizations in 3-, 10-, and 18-day-old rat pups in isolation and the home cage. Dev Psychobiol. 1994;27:65–83. doi: 10.1002/dev.420270107. [DOI] [PubMed] [Google Scholar]
- Centonze D, Picconi B, Baunez C, Borrelli E, Pisani A, Bernardi G, Calabresi P. Cocaine and amphetamine depress striatal GABAergic synaptic transmission through D2 dopamine receptors. Neuropsychopharmacology. 2002;26:164–175. doi: 10.1016/S0893-133X(01)00299-8. [DOI] [PubMed] [Google Scholar]
- Collins RL, Zavala AR, Nazarian A, McDougall SA. κ-Opioid receptors in the substantia nigra pars reticulata mediate the U-50,488-induced locomotor activity of preweanling rats. Dev Brain Res. 2000;119:97–103. doi: 10.1016/s0165-3806(99)00153-4. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Kelley AE. The role of D1 and D2 dopamine receptors in oral stereotypy induced by dopaminergic stimulation of the ventrolateral striatum. Neuroscience. 1990;39:59–67. doi: 10.1016/0306-4522(90)90221-o. [DOI] [PubMed] [Google Scholar]
- Demotes-Mainard J, Henry C, Jeantet Y, Arsaut J, Arnauld E. Postnatal ontogeny of dopamine D3 receptors in the mouse brain: autoradiographic evidence for a transient cortical depression. Dev Brain Res. 1996;94:166–174. doi: 10.1016/0165-3806(96)00041-7. [DOI] [PubMed] [Google Scholar]
- Dewar D, Jenner P, Marsden CD. Lesions of the globus pallidus, entopeduncular nucleus and substantia nigra alter dopamine mediated circling behaviour. Exp Brain Res. 1983;52:281–292. doi: 10.1007/BF00236638. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–1080. [PubMed] [Google Scholar]
- Dourish CT, Cooper SJ, Philips SR. Yawning elicited by systemic and intrastriatal injection of piribedil and apomorphine in the rat. Psychopharmacology. 1985;86:175–181. doi: 10.1007/BF00431705. [DOI] [PubMed] [Google Scholar]
- Duke MA, Meier TL, Bolanos CA, Crawford CA, McDougall SA. Paradoxical effects of kappa opioid stimulation on the locomotor activity and Fos immunoreactivity of the preweanling rat: role of dopamine receptors. Behav Neurosci. 1997;111:1114–1122. doi: 10.1037//0735-7044.111.5.1114. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Gray AM, Rawls SM, Shippenberg TS, McGinty JF. The κ-opioid agonist, U-69593, decreases acute amphetamine-evoked behaviors and calcium-dependent dialysate levels of dopamine and glutamate in the ventral striatum. J Neurochem. 1999;73:1066–1074. doi: 10.1046/j.1471-4159.1999.0731066.x. [DOI] [PubMed] [Google Scholar]
- Hauber W. Involvement of basal ganglia transmitter systems in movement initiation. Prog Neurobiol. 1998;56:507–540. doi: 10.1016/s0301-0082(98)00041-0. [DOI] [PubMed] [Google Scholar]
- Herrera-Marschitz M, Christensson-Nylander I, Sharp T, Staines W, Reid M, Hökfelt T, Terenius L, Ungerstedt U. Striato-nigral dynorphin and substance P pathways in the rat. II. Functional analysis. Exp Brain Res. 1986;64:193–207. doi: 10.1007/BF00238214. [DOI] [PubMed] [Google Scholar]
- Jackson A, Cooper SJ. Observational analysis of the effects of kappa opioid agonists on open field behaviour in the rat. Psychopharmacology. 1988;94:248–253. doi: 10.1007/BF00176854. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Kitchen I. Behavioural effects of selective ε-, κ-, and δ-opioid agonists in neonatal rats. Psychopharmacology. 1989;97:404–409. doi: 10.1007/BF00439459. [DOI] [PubMed] [Google Scholar]
- Joel D, Ayalon L, Tarrasch R, Veenman L, Feldon J, Weiner I. Electrolytic lesion of globus pallidus ameliorates the behavioral and neurodegenerative effects of quinolinic acid lesion of the striatum: a potential novel treatment in a rat model of Huntington’s disease. Brain Res. 1998;787:143–148. doi: 10.1016/s0006-8993(97)01428-5. [DOI] [PubMed] [Google Scholar]
- Karper PE, Nazarian A, Crawford CA, Drago J, McDougall SA. Role of dopamine D1 receptors for κ-opioid mediated locomotor activity and antinociception during the preweanling period: a study using D1 receptor knockout mice. Physiol Behav. 2000;68:585–590. doi: 10.1016/s0031-9384(99)00223-1. [DOI] [PubMed] [Google Scholar]
- Kehoe P, Boylan CB. Behavioral effects of kappa-opioid-receptor stimulation on neonatal rats. Behav Neurosci. 1994;108:418–423. doi: 10.1037//0735-7044.108.2.418. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Khalilov I, Tyzio R, Morozova E, Ben-Ari Y, Holmes GL. Developmental changes in GABAergic actions and seizure susceptibility in the rat hippocampus. Eur J Neurosci. 2004;19:590–600. doi: 10.1111/j.0953-816x.2003.03152.x. [DOI] [PubMed] [Google Scholar]
- Koeltzow TT, Austin JD, Vezina P. Behavioral sensitization to quinpirole is not associated with increased nucleus accumbens dopamine overflow. Neuropharmacology. 2003;44:102–110. doi: 10.1016/s0028-3908(02)00328-3. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Khalilov I, McLean H, Caillard O, Gaiarsa JL, Ben-Ari Y, Khazipov R. GABA is the principal fast-acting excitatory transmitter in the neonatal brain. Adv Neurol. 1999;79:189–201. [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Lévesque M, Bédard A, Cossette M, Parent A. Novel aspects of the chemical anatomy of the striatum and its efferent projections. J Chem Neuroanat. 2003;26:271–281. doi: 10.1016/j.jchemneu.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Leyton M, Stewart J. The stimulation of central κ opioid receptors decreases male sexual behavior and locomotor activity. Brain Res. 1992;594:56–74. doi: 10.1016/0006-8993(92)91029-e. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Archer S, Glick SD. U50,488, a κ opioid receptor agonist, attenuates cocaine-induced increases in extracellular dopamine in the nucleus accumbens of rats. Neurosci Lett. 1994;181:57–60. doi: 10.1016/0304-3940(94)90559-2. [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Brinsfield KH, Patrick RL, Walker JM. Rotational behavior mediated by dopaminergic and nondopaminergic mechanisms after intranigral microinjection of specific mu, delta and kappa opioid agonists. J Pharmacol Exp Ther. 1988;246:196–203. [PubMed] [Google Scholar]
- McDougall SA, Garmsen GM, Meier TL, Crawford CA. Kappa opioid mediated locomotor activity in the preweanling rat: role of pre and postsynaptic dopamine receptors. Psychopharmacology. 1997;133:62–68. doi: 10.1007/s002130050372. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Rodarte-Freeman AL, Nazarian A. Indirect dopamine agonists augment the locomotor activating effects of the κ-opioid receptor agonist U-50,488 in preweanling rats. Dev Psychobiol. 1999;34:183–193. doi: 10.1002/(sici)1098-2302(199904)34:3<183::aid-dev3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Meller E, Bordi F, Bohmaker K. Enhancement by the D1 dopamine agonist SKF 38393 of specific components of stereotypy elicited by the D2 agonists LY 171555 and RU 24213. Life Sci. 1988;42:2561–2567. doi: 10.1016/0024-3205(88)90324-4. [DOI] [PubMed] [Google Scholar]
- Mestlin M, McDougall SA. Ontogenetic differences in the effects of EEDQ on dopamine mediated behaviors. Pharmacol Biochem Behav. 1993;45:797–802. doi: 10.1016/0091-3057(93)90123-b. [DOI] [PubMed] [Google Scholar]
- Moody CA, Spear LP. Ontogenetic differences in the psychopharmacological responses to separate and combined stimulation of D1 and D2 dopamine receptors during the neonatal to weanling age period. Psychopharmacology. 1992;106:161–169. doi: 10.1007/BF02801967. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Electrical membrane properties of rat subthalamic neurons in an in vitro slice preparation. Brain Res. 1987;437:35–44. doi: 10.1016/0006-8993(87)91524-1. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Rodarte-Freeman AL, McDougall SA. Dopaminergic modulation of kappa opioid mediated ultrasonic vocalizations, antinociception, and locomotor activity in the preweanling rat. Behav Neurosci. 1999;113:816–825. doi: 10.1037//0735-7044.113.4.816. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Rev. 1995a;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995b;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain: in stereotaxic coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- Robertson BC, Hommer DW, Skirboll LR. Electrophysiological evidence for a non-opioid interaction between dynorphin and GABA in the substantia nigra of the rat. Neuroscience. 1987;23:483–490. doi: 10.1016/0306-4522(87)90071-6. [DOI] [PubMed] [Google Scholar]
- Schambra UB, Duncan GE, Breese GR, Fornaretto MG, Caron MG, Fremeau RT., Jr Ontogeny of D1A and D2 dopamine receptor subtypes in rat brain using in situ hybridization and receptor binding. Neuroscience. 1994;62:65–85. doi: 10.1016/0306-4522(94)90315-8. [DOI] [PubMed] [Google Scholar]
- Sherwood NM, Timiras P. A stereotaxic atlas of the developing rat brain. Berkeley: University of California Press; 1970. [Google Scholar]
- Steiner H, Gerfen CR. Role of dynorphin and enkephalin in the regulation of striatal output pathways and behavior. Exp Brain Res. 1998;123:60–76. doi: 10.1007/s002210050545. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Walker JM. Inhibitory effects of the κ opiate U50,488 in the substantia nigra pars reticulata. Brain Res. 1990;517:81–87. doi: 10.1016/0006-8993(90)91011-5. [DOI] [PubMed] [Google Scholar]
- Thompson LA, Walker JM. Involvement of the nigrotectal and nigrothalamic pathways in kappa opioid-induced circling. Synapse. 1992;12:189–194. doi: 10.1002/syn.890120303. [DOI] [PubMed] [Google Scholar]
- Thompson R, Harmon D, Yu J. Detour problem-solving behavior in rats with early lesions to the “general learning system.”. Physiol Psych. 1984;12:193–203. [Google Scholar]
- Tirelli E, Jodogne C, Perikel JJ. Adult-like biphasic neurobehavioral changes induced by a GABA-A agonist in infant and weanling mice. Dev Brain Res. 1991;61:207–215. doi: 10.1016/0165-3806(91)90133-4. [DOI] [PubMed] [Google Scholar]
- Ukai M, Kameyama T. Multi-dimensional analyses of behavior in mice treated with U-50,488H, a purported kappa (non-mu) opioid agonist. Brain Res. 1985;337:352–356. doi: 10.1016/0006-8993(85)90074-5. [DOI] [PubMed] [Google Scholar]
- Van Hartesveldt C, Cottrell GA, Potter T, Meyer ME. Effects of intracerebral quinpirole on locomotion in rats. Eur J Pharmacol. 1992;214:27–32. doi: 10.1016/0014-2999(92)90091-h. [DOI] [PubMed] [Google Scholar]
- Van Hartesveldt C, Meyer ME, Potter TJ. Ontogeny of biphasic locomotor effects of quinpirole. Pharmacol Biochem Behav. 1994;48:781–786. doi: 10.1016/0091-3057(94)90346-8. [DOI] [PubMed] [Google Scholar]
- Wacan JJ, Reichel CM, Farley CM, McDougall SA. The partial dopamine D2-like receptor agonist terguride functions as an agonist in preweanling rats after a 5-day reserpine regimen. Psychopharmacology. 2006;185:104–111. doi: 10.1007/s00213-005-0263-5. [DOI] [PubMed] [Google Scholar]
- Waszczak BL, Martin LP, Finlay HE, Zahr N, Stellar JR. Effects of individual and concurrent stimulation of striatal D1 and D2 dopamine receptors on electrophysiological and behavioral output from rat basal ganglia. J Pharmacol Exp Ther. 2002;300:850–861. doi: 10.1124/jpet.300.3.850. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Yoshida ST, Osburn JR, McDougall SA. Paradoxical locomotor activating effects of κ-opioid receptor stimulation in the preweanling rat: role of the ventromedial thalamus and superior colliculus. Dev Brain Res. 2002;139:301–306. doi: 10.1016/s0165-3806(02)00516-3. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]