SUMMARY
The present work investigated sites of ethanol action in ATP-gated P2X receptors (P2XRs) using chimeric strategies that exploited the differences in ethanol response between P2X2R (inhibition) and P2X3R (potentiation). We tested ethanol (10–200mM) effects on ATP- and α,β-methylene-ATP (α,β-meATP)-induced currents in wildtype P2X2, P2X3 and chimeric P2X2/P2X3Rs expressed in Xenopus oocytes using two-electrode voltage-clamp (−70mV). Exchanging ectodomain regions of P2X2 and P2X3Rs reversed wildtype ethanol responses. Substituting back portions of the P2X2R ectodomain at TM interfaces in chimeras that contained the P2X3R ectodomain restored wildtype P2X2R-like ethanol response. Point mutations that replaced non-conserved ectodomain residues at TM interfaces of P2X3Rs with homologous P2X2R residues identified positions that reversed the direction (304) or changed the magnitude (53, 55 and 313) of ethanol response. Homologous substitutions in P2X2Rs did not significantly alter wildtype P2X2R-like ethanol responses. These findings suggest that ectodomain segments at TM interfaces play key roles in determining qualitative and quantitative responses to ethanol of P2X2 and P2X3Rs. Studies that substituted TM regions of P2X3R with respective P2X2R TMs indicate that the TM1, but not the TM2, region plays a role in determining the magnitude of ethanol response. Studies with ATP and α,β-meATP support prior indications that TM regions are important in agonist desensitization and suggest that both ectodomain and TM regions play roles in determining agonist potency and selectivity. Overall, these findings are the first to identify potential targets for ethanol in P2X2 and P2X3Rs and should provide insight into the sites of ethanol action in other P2XRs.
Keywords: Purinergic P2X receptors, Chimeric receptors, Sites of ethanol action, Alcohol, Xenopus oocytes, Electrophysiology
INTRODUCTION
P2XRs constitute a superfamily of ligand-gated ion channels (LGICs) that are becoming increasingly important in various areas of neuroscience. P2XRs are fast acting, cation-permeable ion channels gated by synaptically released extracellular ATP (Khakh, 2001; North, 2002). Currently, seven subtypes of the P2X family of LGICs have been identified (P2X1–P2X7). Messenger RNA or subunit protein for all of the P2XRs have been found in the central nervous system (Khakh, 2001; North, 2002; Rubio and Soto, 2001). P2XR subtypes can form functional ATP-activated homomeric channels (e.g., P2X2, P2X4) and many subtypes can also form functional recombinant heteromeric receptors (e.g., P2X2/3, P2X4/6) when expressed in Xenopus oocytes or mammalian cell lines (Khakh et al, 2001; North and Surprenant, 2000; Ormond et al, 2006). P2XRs are multimeric proteins: a functional P2XR results from the assembly of three subunits (Jiang et al, 2003; Stoop et al, 1999; Torres et al, 1999). All of these subunits are thought to consist of two transmembrane (TM) domains, a large extracellular domain (ectodomain) and intracellular amino- (N) and carboxy (C)-terminals (North, 2002). The TM1 and TM2 membrane spanning domains are involved in ion channel gating and both TM domains are believed to participate in ion pore formation (Burnstock, 2004). The ectodomain contains an ATP-binding site and is a site for regulation of the channel (Chizh and Illes, 2001; Khakh, 2001; North, 2002).
P2XRs are involved in fast synaptic transmission (Khakh, 2001; North and Verkhratsky, 2006), neurotransmitter release (Chizh and Illes, 2001; Deuchars et al, 2001; Hugel and Schlichter, 2002; Khakh, 2001), nociception (Chizh and Illes, 2001; Cockayne et al, 2000; Tsuda et al, 2003) and modulation of long term potentiation (Sim et al, 2006). Moreover, recent investigations suggest that these receptors also play a role in neurodegenerative processes (for review see Franke and Illes, 2006). In addition, purinergic receptors are becoming a focus of investigations as targets for alcohol and other drugs of abuse (Franke and Illes, 2006).
Results from the previous studies support the notion that P2XRs play a role in mediating and/or modulating at least a subset of the cellular and behavioral effects of ethanol. Native P2XRs are sensitive to direct modulation by pharmacologically relevant ethanol concentrations (Li et al, 1998; Li et al, 2000). Moreover, presynaptic P2XRs can modulate the release of neurotransmitters such as GABA, glycine and glutamate (Mori et al, 2001; Papp et al, 2004; Dr. J. Ye of the University of Medicine and Dentistry of New Jersey, personal communication), that are implicated in mediating behavioral effects of ethanol (Davies, 2003; Mihic et al, 1997; Woodward, 2000). Therefore, ethanol modulation of P2XRs may directly and indirectly affect neuronal activity leading to altered behavioral functions.
The site(s)/mechanism(s) of ethanol action in P2XRs have not been characterized. This is partly due to difficulties in carrying out mechanistic studies of native P2XRs in neurons and the lack of specific P2XR antagonists. Thus, the use of recombinant expression systems is particularly important in the study of ethanol action in P2XRs. Using the Xenopus oocyte expression system in combination with the two-electrode voltage electrophysiology, we and others have demonstrated that ethanol reversibly inhibits ATP-activated function of P2X2 and P2X4Rs (Davies et al, 2002; Xiong et al, 2000). In contrast, ATP-gated currents in P2X3Rs are potentiated by ethanol (Davies et al, 2005). More recent work found that mutating a residue in the ectodomain of P2X4Rs changes the magnitude of ethanol inhibition (Xiong et al, 2005), suggesting that the ectodomain of P2XRs may be one important determinant of ethanol action on these receptors.
To identify regions that are important for ethanol action in P2XRs, the present study utilized chimeric strategies that exploited the difference in ethanol sensitivity between P2X2R (inhibition) and P2X3R (potentiation). The results found that regions within the ectodomain near the TM domains and the TM1 region of P2X2 and P2X3Rs are important for the qualitative and quantitative responses to ethanol. In addition, these findings identified regions of P2X2 and P2XRs that play important role in agonist selectivity and desensitization.
METHODS
Isolation of Xenopus Laevis oocytes and cRNA injections
Xenopus oocytes were isolated and maintained as described previously (Davies et al, 2002; Davies et al, 2005). All procedures for the maintenance of Xenopus Laevis frogs and oocyte isolation were approved by The Institutional Animal Care and Use Committee of the University of Southern California. Stage V and VI oocytes were used for cRNA (0.01– 5ng) injections using Nanoject II Nanoliter injection system (Drummond Scientific, Broomall, PA) one day after isolation. Injected oocytes were stored at 16 °C in incubation medium containing (in mM), NaCl 96, KCl 2, MgCl2 1, CaCl2 1, HEPES 5, theophylline 0.6, pyruvic acid 2.5, with 1% horse serum and 0.05 mg/ml gentamycin. Theophylline was included in the incubation medium to slow oocyte maturation after cRNA injections. The oocytes were used in electrophysiological recordings for 3–10 days after cRNA injections. No ATP-induced currents were observed in water injected or uninjected oocytes since native P1 or P2Rs are absent in defolliculated oocytes (King et al, 1996a; King et al, 1996b).
Generation of chimeric P2X2/P2X3 and mutant receptors
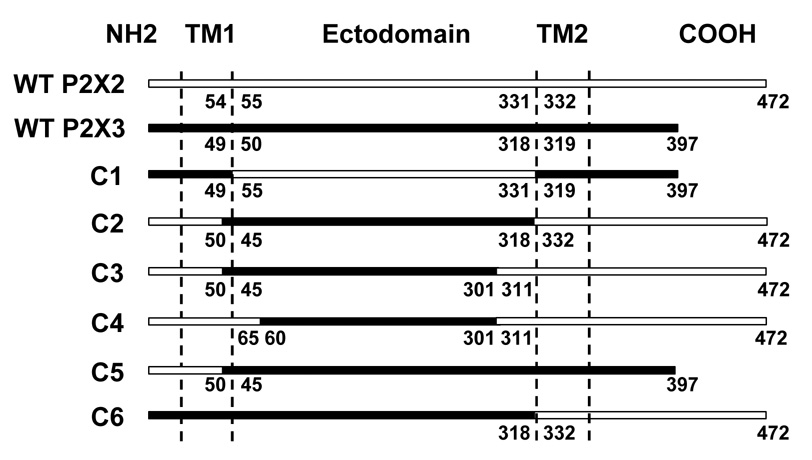
The cDNAs of rat P2X2R (GenBank accession No. U14414) and P2X3R (GenBank accession No. X91167), sub-cloned into pcDNA3 vector (Invitrogen, Carlsbad, CA) were used to create chimeric receptors. Similar restriction sites were introduced into P2X2 and P2X3R cDNAs at selected nucleotides using QuickChange IIXL Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). P2X2 and P2X3R cDNAs were then digested using SnaBI and/or AgeI restriction enzymes, resultant fragments isolated and ligated appropriately to generate chimeric receptors (Fig. 1). Sequence alignment of P2X1-7R subtypes was used for selection of correct junctions of P2X2/P2X3 in chimeric receptors. In chimera C1, the P2X3 ectodomain was replaced with the homologous region of the P2X2R. Chimeras C2, C3, C4 contained different portions of P2X3R ectodomain sequences (respectively 45–318, 60–301 and 45–301) on the backbone of the P2X2R. In chimera C5, the N-terminus and the TM1 domain (the latter two further referred to as TM1 region) of the P2X3R were replaced with the corresponding regions of the P2X2R. The TM2 domain and the C-terminus (the latter two further referred to as TM2 region) of the P2X3R were replaced with the corresponding regions of the P2X2R in the C6 chimera. We followed the sequence alignments published earlier (Khakh and Egan, 2005; Koshimizu et al, 2002) for the borders of the TM and ectodomain regions of P2X2 and P2X3Rs. Mutagenesis was performed to introduce single point mutations using QuickChange IIXL Site-Directed Mutagenesis kit. Validation of chimeric and mutant constructs was performed using automated DNA sequencing (USC/Norris DNA Core Facility, University of Southern California).
Figure 1.
Schematic representation of wildtype P2X2R (empty horizontal bar), wildtype P2X3R (black horizontal bar) and chimeric receptor constructs used in the study. The N- and C-termini, extracellular region and the transmembrane (TM) domains are indicated as NH2, COOH, ectodomain, TM1 and TM2, respectively. Numbers represent the amino acids on the boundaries of the TM domains and the ectodomains in wildtype P2XR sequences and the P2X2/P2X3R junctions in chimeric receptors.
cRNA synthesis
The DNA of wildtype, chimeric or mutant receptors was linearized and transcribed using the mMESSAGE mMACHINE kit (Ambion, Austin, TX) to result in cRNA, which was stored at −70 °C until injection.
Whole-cell voltage-clamp recordings
Two-electrode voltage-clamp recordings of oocytes were performed using a Warner Instruments Model OC-725C oocyte clamp amplifier (Hamden, CT) at Vh=−70mV. An oocyte was placed in a small depression of a 100µl capacity oocyte perfusion chamber (RC-3Z, Warner Instruments, Hamden CT) and impaled with two electrodes pulled from 1.2mm thick-walled filament glass capillaries (WPI, Sarasota, FL) that had a resistance of 0.5-3MΩ when filled with 3M KCl. The oocyte was continuously perfused at a rate of 3–4 ml/min by extracellular Ringer’s solution containing (in mM) 110 NaCl, 2.5 KCl, 10 HEPES and 1.8 BaCl2, pH 7.5, using a peristaltic pump (Rainin Instrument, Oakland, CA). Ca2+ in the solution was replaced with Ba2+ to prevent the activation of Ca2+-dependent Cl− channels (Khakh et al, 1999). Currents were recorded using a strip-chart recorder (Barnstead/ Thermolyne, IA). All experiments were performed at room temperature (20–23 °C).
Experimental procedures
Ethanol modulation was tested by the application of ethanol in combination with ATP or α,β-methylene-ATP (α,β-meATP) for 20–30sec. Effective submaximal concentrations of agonist (EC5–10 furthered referred to as EC10) were used in the current studies. We have previously shown that the use of EC10 can maximize the effects of ethanol and minimize receptor desensitization (Davies et al, 2002; Davies et al, 2005). Ethanol was co-applied with the agonists. We found similar responses to ethanol regardless of whether ethanol was applied before the agonist (i.e. pre-application) or with the agonist (i.e. co-application) (Davies et al, 2005). After a sufficient washout time (5–10min) the cells were challenged with the same EC10 of the agonist to ensure validity of the response to ethanol and to asses any change in the baseline level. The highest concentration of ATP and α,β-meATP used for all receptors was 100µM since it induced maximal response from all receptors studied and allowed longer viability of an oocyte and sufficient testing time. The washout time between agonist applications was at least 5–15 min to allow complete resensitization of the receptors (Davies et al, 2002; Davies et al, 2005). Ethanol did not affect the resting membrane currents in oocytes expressing P2XRs in the absence of agonists as well as in uninjected oocytes. The action of ethanol in wildtype and chimeric receptors was concentration-dependent and reversible.
Data analysis
Data are obtained from several batches of oocytes of at least 3 different frogs and are expressed as mean ± SEM. Ethanol effects are presented as percentage change of peak currents evoked by ATP or α,β-meATP EC10 alone. Ethanol concentration-response data were fitted to a concentration-response curve by using the following logistic equation: I = Imax * [ethanol]/([ethanol] +(EC50)), where I/Imax is the percentage of the maximum obtainable response, EC50 is the concentration producing a half-maximal response.
To generate ATP and α,β-meATP dose-response curves, peak amplitudes evoked by 100µM concentration of both agonists were taken as 100% response and this was used to normalize all the currents generated by lower agonist concentrations. Sigmoidal nonlinear regression fit (I = Imin + (Imax−Imin)/1+10^((LogEC50)−[agonist])*nH)), where Imin and Imax are respectively the minimum and maximum obtainable responses, EC50 is the concentration producing a half-maximal response and nH is the Hill slope of ATP or α,β-meATP concentration responses, was used to estimate the EC50 values and Hill coefficients.
GraphPAD Prism software (San Diego, CA) was used for data analysis and curve fitting. Statistical analysis was performed using One-way ANOVA and significance set at P < 0.05.
Materials
Adenosine 5′-triphosphate disodium salt, α,β-methyleneadenosine 5′-triphosphate lithium salt, ethanol (190 proof, USP), collagenase were purchased from Sigma Co. (St. Louis, MO, USA). All other chemicals were of reagent grade.
RESULTS
Agonist properties of wildtype and chimeric receptors
Activation by ATP and α,β-meATP
Maximum agonist-induced currents (Imax), EC50 and Hill slope values for ATP and α,β-meATP concentration response curves for wildtype and chimeric P2XRs are shown in Table 1. ATP EC50 values for wildtype P2X2 and P2X3Rs were not significantly different. As expected, P2X3Rs, but not P2X2Rs, were sensitive to α,β-meATP (Khakh et al, 2001; North, 2002).
Table 1.
Imax, EC50 and Hill slope values for ATP- and α,β-meATP-concentration response curves of wildtype and chimeric receptors. Data shown are mean ± SEM of at least 5 experiments per data point. Imax represent peak currents generated by 100µM ATP or α,β-meATP. EC50 and Hill slope values were determined from the agonist concentration response curves. Imax were used to normalize responses to different agonist concentrations.
| Receptor | ATP | α,β-meATP | ||||
|---|---|---|---|---|---|---|
| Imax, nA | EC50, µM | Hill slope | Imax | EC50, µM | Hill slope | |
| WT P2X2 | 8301 ± 703 | 5.3 ± 0.6 | 2.2 ± 0.2 | 36 ± 32.08 | ND | ND |
| WT P2X3 | 9588 ± 1200 | 4.1 ± 0.53 | 0.9 ± 0.05 | 6975 ± 1535 | 2.5 ± 0.7 | 0.83 ± 0.08 |
| C1 | 10286 ± 1423 | 6.2 ± 0.8 | 1.5 ± 0.18 | 8350 ± 1434 | 13.8 ± 0.7# | 2.2 ± 0.08 |
| C2 | 9065 ± 925 | 0.34 ± 0.02* | 1.5 ± 0.12 | 5914 ± 625 | 0.9 ± 0.05 | 1.50 ± 0.1 |
| C3 | 7438 ± 1743 | 0.65 ± 0.07* | 1.29 ± 0.44 | 4983 ± 603 | 2.3 ± 0.4 | 1.2 ± 0.15 |
| C4 | 7237 ± 630.4 | 0.49 ± 0.01* | 0.74 ± 0.11 | 5200 ± 1143 | 0.14 ± 0.02 | 0.8 ± 0.04 |
| C5 | 6995 ± 1427 | 0.71 ± 0.06* | 1.6 ± 0.14 | 7920 ± 1691 | 2.3 ± 0.56 | 1.5 ± 0.11 |
| C6 | 7688 ± 657.5 | 0.23 ± 0.04* | 1.08 ± 0.14 | 5755 ± 866 | 0.24 ± 0.04 | 1.4 ± 0.14 |
ND – not defined. EC50 cannot be determined because wildtype P2X2Rs are insensitive to α, β-meATP.
significantly different compared to ATP EC50 values of wildtype P2X2 and P2X3Rs (P < 0.05).
significantly different compared to α,β-meATP EC50 values of wildtype P2X3Rs (P < 0.05).
All chimeras tested produced functional responses in Xenopus oocytes. ATP Imax values in chimeras were not significantly different from those of the wildtype receptors (Table 1). ATP EC50 of chimera C1, which consisted of the P2X2R ectodomain combined with P2X3R TM1 and TM2 regions (Fig. 1), did not significantly differ from those of wildtype receptors (Table 1). In contrast, ATP EC50 values for chimeras C2–C6, which consisted of various segments of the P2X3R ectodomain combined with P2X2R TM1 and/or TM2 regions (Fig. 1), were significantly lower as compared to the wildtype receptors (Table 1).
Chimeras containing the P2X3R ectodomain with one or two TM regions derived from the P2X2R (C2–C6) had similar sensitivity to α,β-meATP as wildtype P2X3Rs (i.e., Imax and EC50 values in Table 1). These data are consistent with the notion that the ectodomain region of P2XRs controls agonist selectivity (Koshimizu et al, 2002; Zemkova et al, 2004). However, chimera C1, which contained the ectodomain from P2X2Rs, also showed sensitivity to α,β-meATP (Table 1), although the EC50 was 7 times higher than that of the wildtype P2X3Rs (Table 1). Our findings suggest that there are complex interactions between the ectodomain and TM regions of P2XRs that affect sensitivity to α,β-meATP.
Agonist desensitization
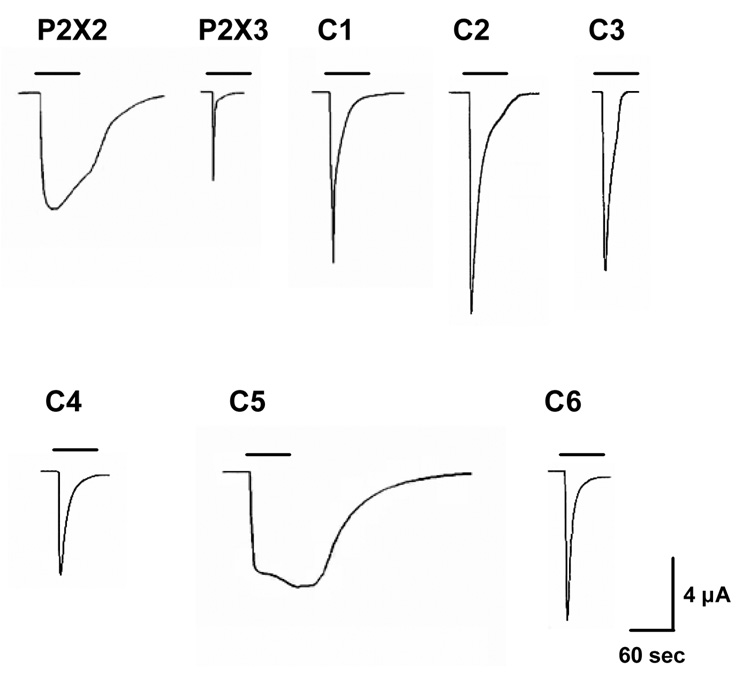
As expected (North, 2002; Werner et al, 1996), P2X2Rs desensitized slowly in response to ATP in contrast to P2X3Rs (Fig. 2). We found that chimera C1 both TM regions of which were derived from the P2X3R demonstrated faster desensitization compared to that of the wildtype P2X2R (Fig. 2). On the other hand, chimeras C2–C4 that contained both of the TM regions of the P2X2R showed slower desensitization as compared to that of the wildtype P2X3R. We found that chimera C5 containing the TM1 region of the P2X2R desensitized much slower as compared to that of the wildtype P2X3R (Fig. 2). The desensitization of the C5 chimera was similar to that of wildtype P2X2Rs. In contrast, chimera C6, that had the TM1 region derived from the P2X3R, showed faster desensitization (Fig. 2). Together, these findings suggest that the TM regions, and especially the TM1 region, play a role in controlling the rate of agonist desensitization of P2X2Rs and P2X3Rs. We recognize that conclusions regarding the role of different P2XR regions in desensitization kinetics of P2X2 and P2X3R subtypes are limited due to the relatively slow nature of the oocyte perfusion system. The desensitization rates of wildtype P2X2 and P2X3Rs are largely different and thus, the changes in desensitization among the different chimeras relative to those of the wildtype receptors should be viewed as qualitative rather than quantitative. Further studies using fast agonist-jump protocols in mammalian cells would provide a finer level of P2XR kinetic properties and would strengthen our conclusions.
Figure. 2.
Desensitization characteristics of wildtype P2X2R, P2X3R and chimeric C1–C6 receptors: Figure shows representative tracings of currents induced by 100µM ATP during 60sec of agonist application. Vertical scale bar represents 4 µA; horizontal scale bar represents 60 sec.
Ectodomain regions near the TM domains determine qualitative and quantitative responses to ethanol of P2X2 and P2X3Rs
1. Ectodomain swap between P2X2 and P2X3Rs
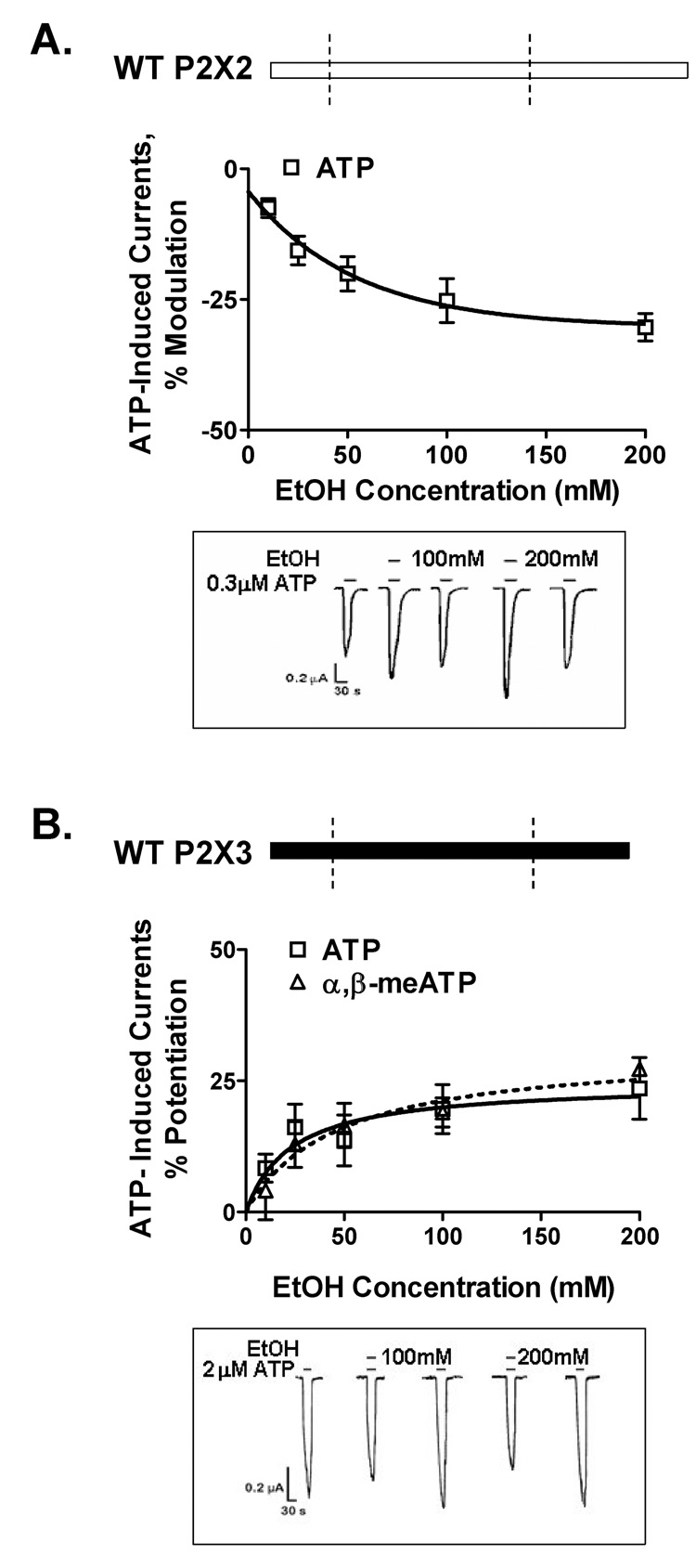
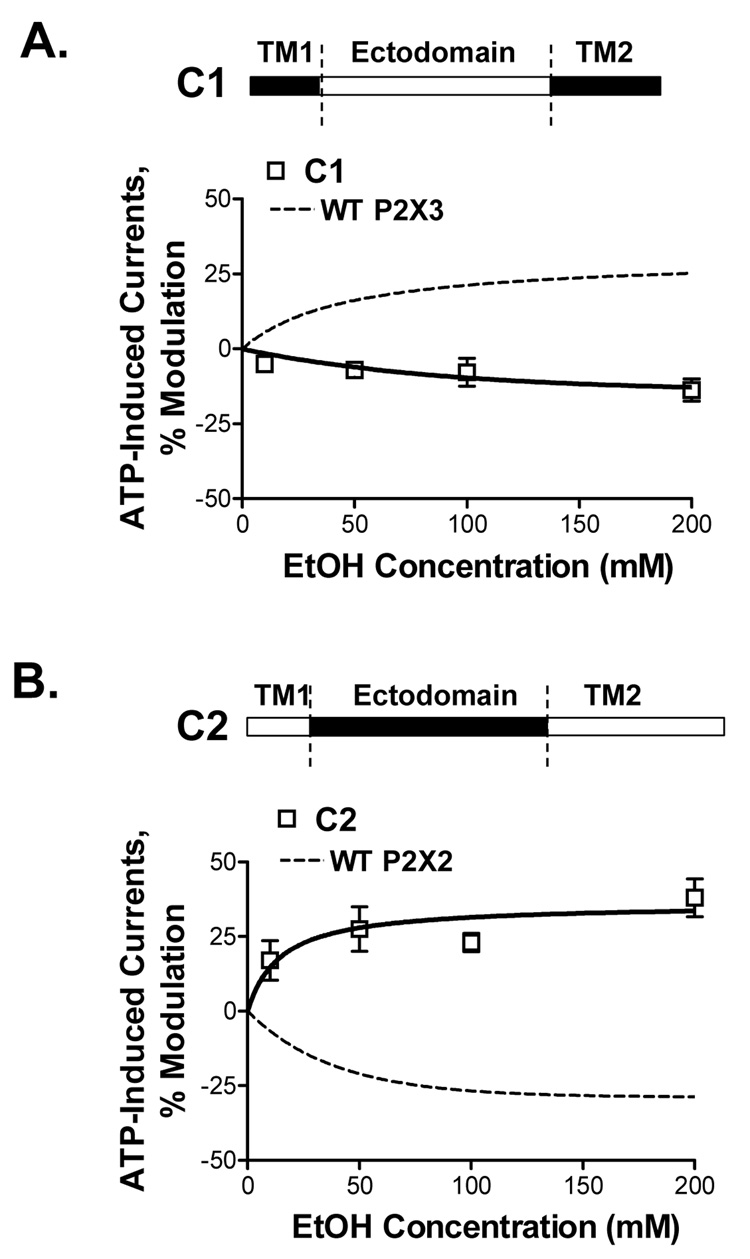
Ethanol (10–200mM) inhibits ATP-activated currents in wildtype P2X2Rs and potentiates ATP-gated currents of P2X3R (Fig. 3A,B; Davies et al, 2005). To investigate the role of the ectodomain in potentiating effect of ethanol in P2X3Rs, we tested chimera C1 in which the P2X3R ectodomain was replaced with the homologous P2X2R region (Fig. 4A, schematic). Replacing the ectodomain reversed ethanol’s potentiation in the wildtype P2X3R to inhibition in the C1 chimera (Fig. 4A). Ethanol inhibition of chimera C1 was comparable to that of the wildtype P2X2R. The findings from chimera C1 support an important role for the P2X3R ectodomain in determining the qualitative response to ethanol.
Figure 3. Ethanol effects in wildtype P2X2 and P2X3Rs.
(A,B) Schematic representations of wildtype P2X2R (empty bar) and P2X3R (black bar) are shown. (A) Ethanol inhibits ATP-gated function of wildtype P2X2Rs. (B) Ethanol potentiates ATPand α,β-meATP-gated function of wildtype P2X3Rs. (A,B) Lower insets - representative tracings of ATP EC10-induced currents: effect of 100mM and 200mM ethanol. The horizontal bars depict when ATP (lower bar) and ethanol (upper bar) were applied to the oocyte. Vertical scale bars are 0.2 µA, horizontal scale bars represent 30 sec. Data shown are mean ± SEM of 3–9 experiments per data point. Currents were generated by EC10 of ATP (1–2µM and 0.07–0.5µM respectively for P2X2 and P2X3Rs) and α,β-meATP (0.05–0.2µM for the wildtype P2X3R). On this and following figures the receptor construct is shown on the upper panel, empty bars represent the P2X2R, black bars - the P2X3R.
Figure 4. Ethanol effects on ATP-activated currents in C1 and C2 chimeric receptors.
(A,B) Schematics of C1 and C2 chimeras are depicted (empty bar – P2X2R, black bar - P2X3R sequences). (A) Ethanol inhibits ATP-gated function of chimera C1. Ethanol response of this chimera is opposite of that of the wildtype P2X3R (represented by a dashed line). (B) Ethanol potentiates ATP-activated currents in chimera C2. This effect is reversed compared to that of the wildtype P2X2R (represented by a dashed line). Currents were generated by EC10 of ATP (0.8–1.2µM and 0.05–0.2µM for respectively C1 and C2 chimeras). Data are presented as mean ± SEM of 3–9 oocytes per data point.
To explore the role of the ectodomain in ethanol’s effect in P2X2Rs, we next tested chimera C2, in which the P2X2R ectodomain was replaced with the P2X3R ectodomain (Fig. 4B, schematic). Replacing the ectodomain reversed the ethanol response in the presence of ATP from inhibition in the wildtype P2X2R to potentiation in the C2 chimera (Fig. 4B). The degree of ethanol potentiation of ATP-gated function of this chimera was comparable to that of the wildtype P2X3Rs. The findings from the C2 chimera support a role for the P2X2R ectodomain in determining the qualitative response to ethanol.
Taken together, the findings from chimeras C1 and C2 suggest that the ectodomains of P2X3 and P2X2Rs determine the qualitative response to ethanol (either potentiation in P2X3Rs or inhibition in P2X2Rs).
2. Ectodomain – TM interface
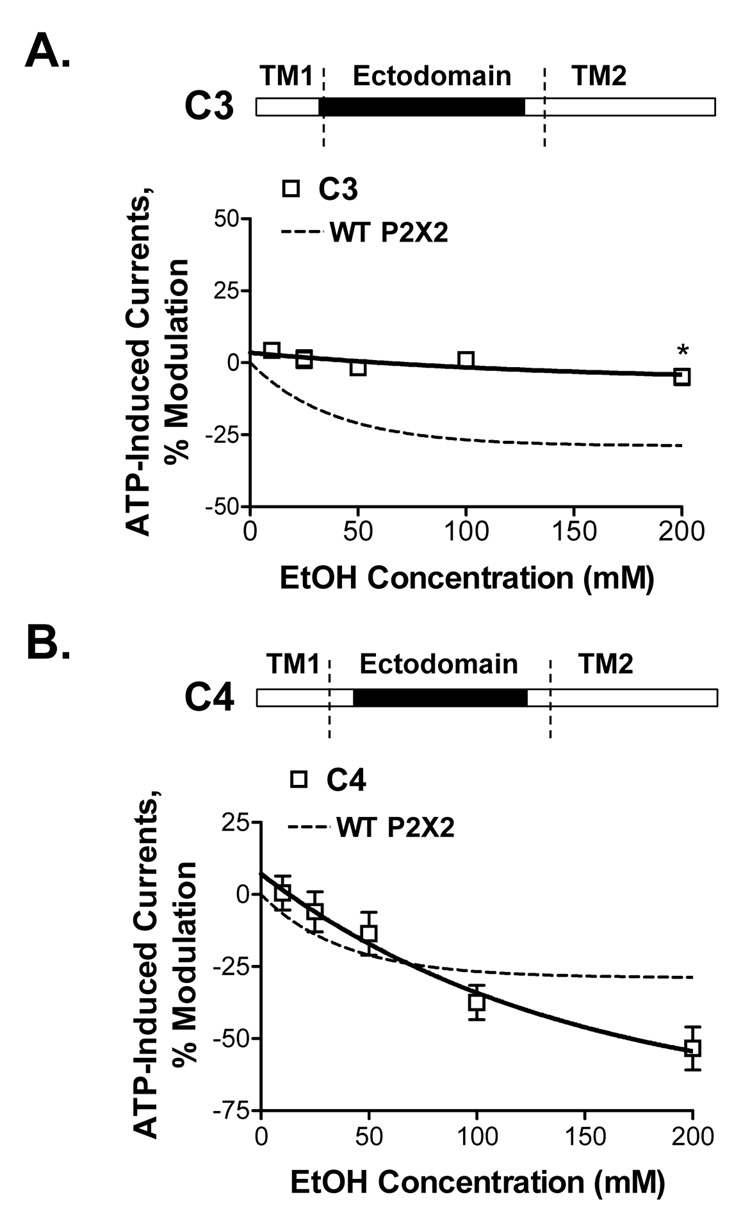
To investigate the role of sub-regions within the P2X3R ectodomain in ethanol potentiation of P2X3Rs, we created chimeric receptors that contained different lengths of P2X3R ectodomain with the rest of the sequences derived from the wildtype P2X2R. We tested the response to ethanol in chimera C3 in which ectodomain segment of the P2X2R, excluding the region near the TM2 domain, was replaced by homologous P2X3R ectodomain segment (Fig. 5A, schematic). Ethanol did not significantly affect ATP-induced currents in this chimera (Fig. 5A). This suggests that the P2X3R ectodomain region near the TM2 domain of the receptor is necessary for P2X3R-like ethanol potentiation. These results also suggest that the P2X2R ectodomain segment near the TM2 domain is not sufficient for P2X2R-like ethanol inhibition.
Figure 5. Ethanol effects on ATP-activated currents in C3 and C4 chimeric receptors.
(A,B) Schematics of C3 and C4 chimeras are shown (empty bar – P2X2R, black bar - P2X3R sequences). (A) There is no effect of ethanol on ATP-activated currents in the chimera C3. (B) Ethanol inhibition of ATP-activated currents in C4 chimera is similar to that of the wildtype P2X2R. Currents were generated by EC10 of ATP (0.1–0.25µM and 0.005–0.01µM respectively for C3 and C4 chimeras). Data are presented as mean ± SEM of 3–12 oocytes per data point.
Next, we tested ethanol sensitivity of chimera C4 in which an ectodomain segment of the P2X2R was replaced with the homologous P2X3R ectodomain segment with the exception of the regions near both the TM1 and TM2 domains (Fig. 5B, schematic). The presence of both of P2X2R ectodomain regions near the TM1 and TM2 domains in this chimera was sufficient to reverse the P2X3R-like ethanol potentiation of chimera C2 to P2X2R-like ethanol inhibition (Fig. 5B).
Taken together, findings from chimeras C2–C4 provide evidence that the ectodomain regions near the TM domains of P2X2 and P2X3Rs are important in determining qualitative and quantitative responses to ethanol.
3. P2X3R point mutations
Based on the results obtained with chimeric receptors C1–C4, we used sequence alignment to identify specific residues within the ectodomain regions (segments near the TM domains) that may play a role in determining the response to ethanol of wildtype P2X3Rs. Using site-directed mutagenesis, we replaced non-conserved residues in the identified ectodomain regions of the P2X3R with the homologous P2X2R residues and tested these mutants for ethanol sensitivity. Mutating Leu 304 to Ile in the P2X3R ectodomain region near the TM2 domain reversed the ethanol response from potentiation to inhibition in the P2X3 L304I mutant (Table 2). Furthermore, P2X3R ectodomain mutants D53E, A55G near the TM1 domain and N313S mutant near the TM2 domain showed significantly less ethanol potentiation compared to that of wildtype P2X3Rs (Table 2). These mutations did not significantly alter the EC50 and Hill slope values compared to those of wildtype P2X3Rs (Table 2). Ethanol’s effects in other P2X3R ectodomain mutants (V51D, I56P, R52S, Y306H, N308Q and I314L) did not significantly differ from that of wildtype P2X3Rs (P>0.2, n=5–7 for each mutant). Taken together, data from point mutants add key evidence that ectodomain residues near the TM1 domain (positions 53 and 55) and the TM2 domain (positions 304 and 313) are important for the action of ethanol in P2X3Rs.
Table 2.
Response to 100mM ethanol and agonist properties for the point mutants of wildtype P2X3Rs. Data shown are mean ± SEM of 3–10 experiments per data point. In ethanol experiments currents were generated by EC10 of ATP (0.1–0.5µM, 0.08–0.2µM, 0.1–0.2µM, 0.05–0.1µM, and 0.1–0.2µM respectively for wildtype and D53E, A55G, L304I and N313S P2X3 mutant receptors).
| Receptor | % Potentiation, 100mM Ethanol |
ATP-concentration Response | |
|---|---|---|---|
| EC50, µM | Hill slope | ||
| WT P2X3 | 23.3 ± 5.4 | 4.5 ± 0.84 | 0.77 ± 0.07 |
| P2X3 D53E | 6.8 ± 3.4* | 2.0 ± 0.37 | 1.02 ± 0.10 |
| P2X3 A55G | 6.3 ± 3.8* | 2.2 ± 0.69 | 0.81 ± 0.01 |
| P2X3 L304I | −5.0 ± 0.55* | 1.6 ± 0.44 | 0.93 ± 0.14 |
| P2X3 N313S | 3.0 ± 1.2* | 3.4 ± 0.45 | 0.74 ± 0.03 |
P < 0.05 compared to ethanol effects in wildtype P2X3Rs.
4. P2X2R point mutations
We next tested whether ectodomain residues at the TM domain interfaces play a role in ethanol sensitivity of wildtype P2X2Rs. Using sequence homology, we identified positions in P2X2Rs (59, 61, 317 and 326) that are homologous to those demonstrated to play a role in ethanol action in P2X3Rs (53, 55, 304 and 313). The residues at these positions were individually substituted to the homologous residues of the P2X3Rs and tested for ethanol sensitivity. We found that the effect of 100mM ethanol on ATP-induced currents in E59D, G61A, I317L and S326N P2X2R mutants was not significantly different from that in the wildtype P2X2R (Table 3). These data suggest that sites for ethanol action in P2X2Rs and P2X3Rs may differ.
Table 3.
Response to 100mM ethanol and agonist properties for wildtype P2X2 and the corresponding point mutants. There was no significant difference in ethanol sensitivity between wildtype and mutant receptors (P > 0.05 compared to the ethanol effect in wildtype P2X2Rs). Data shown are mean ± SEM of 5–10 experiments per data point. In ethanol experiments currents were generated by EC10 of ATP (1.5–2.0µM, 0.4–0.5µM, 1.0–1.5µM, 1.3–1.5µM and 1.0–1.5µM respectively for wildtype and E59D, G61A, I317L and S326N mutant P2X2 receptors).
| Receptor | % Inhibition, 100mM Ethanol |
ATP-concentration Response | |
|---|---|---|---|
| EC50, µM | Hill slope | ||
| WT P2X2 | 13.3 ± 1.6 | 6.3 ± 0.12 | 2.3 ± 0.15 |
| P2X2 E59D | 14.8 ± 2.2 | 1.4 ± 0.06 | 1.2 ± 0.14 |
| P2X2 G61A | 16.8 ± 0.3 | 5.2 ± 0.64 | 1.3 ± 0.03 |
| P2X2 I317L | 11.8 ± 2.8 | 5.2 ± 0.35 | 1.7 ± 0.09 |
| P2X2 S326N | 13.6 ± 2.3 | 2.6 ± 0.16 | 1.9 ± 0.17 |
The TM1 region of P2X3Rs determines the quantitative response to ethanol
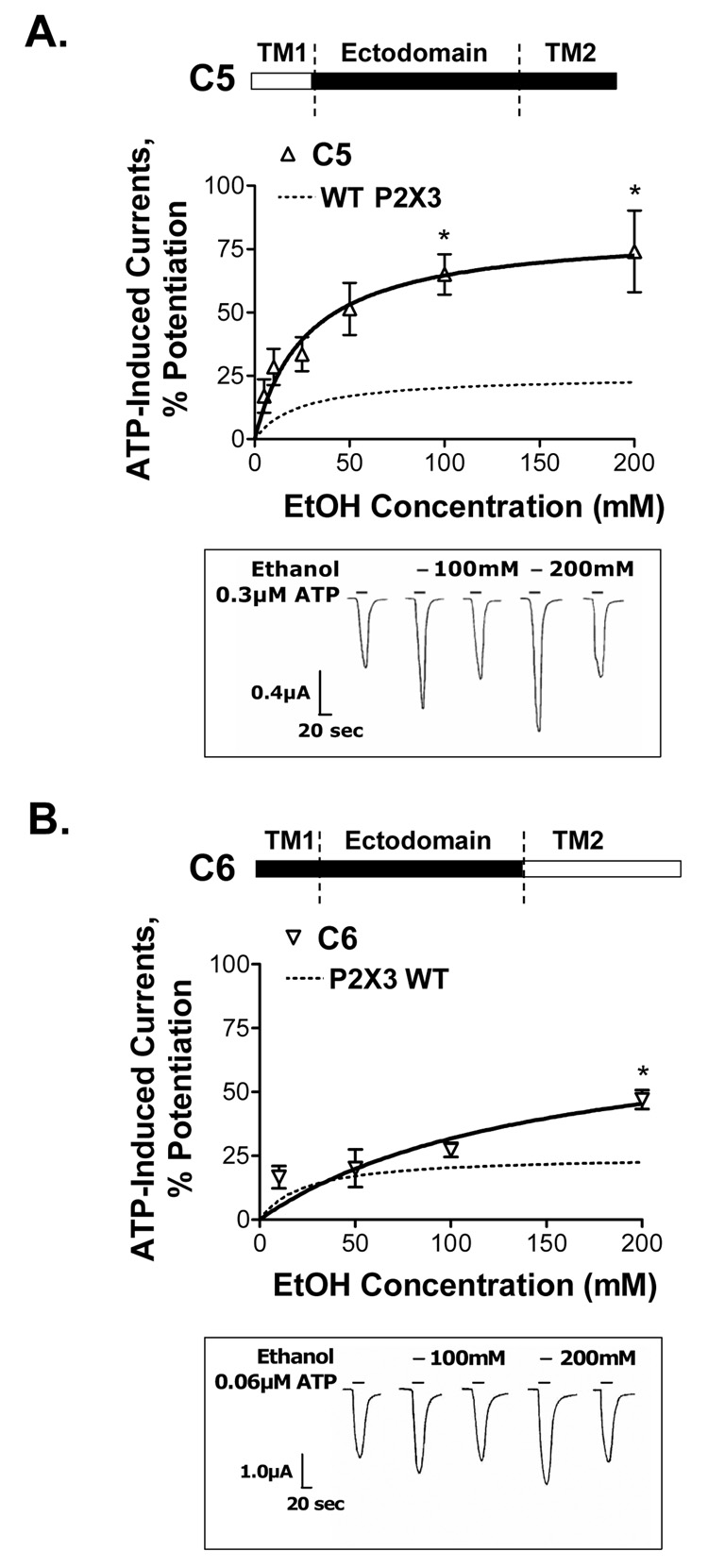
To investigate the role of the TM1 region in the ethanol response of P2X3Rs, we replaced this region with the corresponding region from the P2X2R (Fig. 6A, C5 chimera schematic). Ethanol potentiated ATP-activated currents in the C5 chimera. The degree of ethanol potentiation was approximately 3-fold greater compared to that of wildtype P2X3Rs (Fig. 6A). These data suggest that the TM1 region of wildtype P2X3Rs regulates the magnitude of ethanol potentiation in P2X3Rs.
Figure 6. Ethanol effects on ATP-activated currents in C5 and C6 chimeric receptors.
(A,B) Schematics of C5 and C6 chimeras are depicted (empty bar – P2X2R, black bar - P2X3R sequences). (A) Ethanol significantly potentiates ATP-gated function of C5 chimera. The degree of ethanol potentiation is significantly higher compared to that of the wildtype P2X3R (represented by dotted line). (B) Ethanol potentiaes ATP-gated currents in chimera C6. The degree of ethanol potentiation is significantly different from that of wildtype P2X3R at 200mM ethanol. (A,B) Lower insets - representative tracings of ATP EC10-induced currents: effect of 100mM and 200mM ethanol. The horizontal bars depict when ATP (lower bar) and ethanol (upper bar) were applied to the oocyte. (A) Vertical scale bar is 0.4 µA, horizontal scale bar represents 20 sec. (B) Vertical scale bar represents 1.0 µA, horizontal scale bar is 20 sec. (A,B) Data are presented as mean ± SEM of 3–12 oocytes per data point. Currents were generated by EC10 of ATP (0.1–0.3µM and 0.02–0.06µM respectively for C5 and C6 chimeras). * − P < 0.05 compared to wildtype P2X3Rs.
To investigate the role of the TM2 region in the ethanol sensitivity of the P2X3Rs, we replaced this region of the wildtype P2X3R with the corresponding P2X2R region (Fig. 6B, C6 chimera schematic). Ethanol also potentiated ATP-induced currents in this receptor (Fig. 6B), but in contrast to the C5 chimera, there was no significant difference in the degree of ethanol potentiation between C6 chimera and wildtype P2X3Rs at 10–100mM ethanol concentrations (Fig. 6B). At 200mM concentration of ethanol, the degree of potentiation by ethanol was significantly greater compared to that of the wildtype P2X3R. These data suggest that the TM2 region of P2X3Rs does not affect the magnitude of ethanol potentiation at pharmacologically relevant concentrations of ethanol.
Taken together, these findings implicate the TM1, but not the TM2, region in controlling the magnitude of ethanol potentiation in wildtype P2X3R.
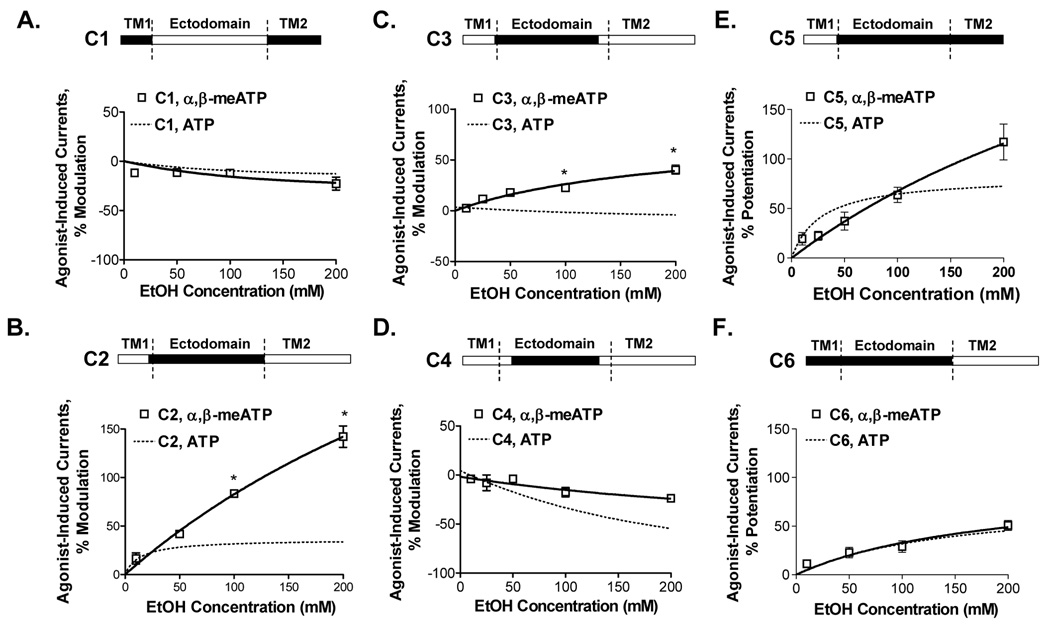
Response to ethanol in the presence of α,β-meATP
To investigate possible differences in ethanol response based on the agonist used, we tested the effects of ethanol on wildtype and chimeric C1–C6 receptors in the presence of α,β-meATP. Ethanol responses in the presence of α,β-meATP were similar to those in the presence of ATP in wildtype P2X3Rs (Fig. 3B) and in chimeras C1, C4, C5 and C6 (Fig. 7). We found significant difference in the magnitude of ethanol response in the presence of α,β-meATP versus ATP in chimeras C2 and C3 (Fig. 7). Overall, the effects of ethanol in the chimeric receptors in the presence of α,β-meATP were similar to those in the presence of ATP and thus support the conclusions regarding the role of the ectodomain and TM1 region in determining the effects of ethanol in P2XRs.
Figure 7. Ethanol effects on α,β-meATP-activated currents in C1–C6 chimeras.
(A–F) Schematics of chimeras are shown (empty bar – P2X2R, black bar - P2X3R sequences). For comparisons, data of ethanol effects on ATP-induced currents are shown by dotted lines. Ethanol responses in the presence of α,β-meATP and ATP were similar in chimeras C1, C4, C5 and C6 (Fig. 7A,D,E,F). Ethanol responses in chimeras C2 and C3 in the presence of α,β-meATP and ATP demonstrated quantitative differences (Fig. 7B,C). Currents were generated by EC10 of α,β-meATP (0.8–1.0µM, 0.1–0.15µM, 0.2–0.5µM, 0.005–0.01µM, 0.4–0.6µM and 0.02–0.05µM respectively for C1, C2, C3, C4, C5 and C6 chimeras). Data are presented as mean ± SEM of 3–12 oocytes per data point. * − P < 0.05 compared to data generated in the presence of ATP.
DISCUSSION
The present study utilized a chimeric strategy that exploited differences in ethanol modulation between P2X2Rs (ethanol inhibition) and P2X3Rs (ethanol potentiation) in order to identify regions involved in ethanol action. Based, in part on previous studies (Weight et al, 1999; Xiong et al, 2005), the current work tested the hypothesis that the ectodomain plays a major role in determining the effect of ethanol on P2XR function. Our findings demonstrate that the ectodomain is important for the qualitative response to ethanol of P2X2Rs (inhibition) and P2X3Rs (potentiation). Moreover, we identified segments within the ectodomain of P2X2 and P2X3Rs that are important for the qualitative and quantitative responses to ethanol of these receptors. These segments are localized at the ectodomain – TM domain interface of P2X2 and P2X3Rs.
Follow-up studies using point mutations of the non-conserved residues in the ectodomain-TM interface identified positions in the P2X3R ectodomain segments near the TM domains that are important for the qualitative and quantitative responses to ethanol of wildtype P2X3Rs. Notably, a single mutation of L304 near the TM2 domain of P2X3Rs changed the ethanol response of the wildtype P2X3R from potentiation to inhibition suggesting that this residue is a key determinant of the qualitative response to ethanol of P2X3Rs. In addition, two residues located at the ectodomain-TM1 domain interface (D53 and A55) and one - at the ectodomain-TM2 domain interface (N313) were important in determining the magnitude of the ethanol effect. Presently we do not know if these sites represent interaction sites for alcohol or if they are transduction sites that couple ATP binding to channel opening. Future studies utilizing mutational analysis and n-chain alcohols should allow us to begin to address these issues.
While we were able to identify residues that play a role in ethanol modulation of P2X3Rs, our mutagenesis studies showed that the homologous positions in P2X2Rs (i.e. 59, 61, 317 and 326) do not appear to be important in determining the inhibitory response of ethanol in P2X2Rs. However, these latter findings do not completely eliminate ectodomain regions near the TM domains as potential targets for ethanol action in P2X2Rs as there are other non-conserved residues within these regions. Future studies will explore the role of these other non-conserved residues in the action of ethanol in P2X2Rs.
The mechanism(s) that underlie ethanol action in P2XRs are unknown at the present time. The current understanding of the trimeric assembly of the functional P2XRs suggests that the N- and C-terminal ends of the ectodomains of the same or neighboring subunits are closely localized (Jiang et al, 2003; North, 2002). Based on this structural arrangement, we speculate that specific interactions could occur between several of the amino acid residues in the ectodomain regions at the TM domain interfaces. It is possible that the residues identified in this study as important for mediating ethanol response (i.e., D53, A55, L304, N313) are involved in the formation of a structural element in P2X3Rs that is sensitive to ethanol. Preliminary studies from our group on P2X4Rs found that a mutation of a position homologous to N313 in P2X3Rs (D331 in PX2X4Rs) significantly reduced ethanol inhibition of these receptors (unpublished data). Recent work from others has identified another position in the ectodomain region of P2X4R (H241) that appeared to play a role in ethanol sensitivity (Xiong et al, 2005). Future investigations will be necessary to determine whether changes in ethanol sensitivity for positions D331 and H241 of P2X4Rs are linked to the same structural ethanol-sensitive region in P2X4Rs or if the two positions differ in their roles in ethanol sensitivity. Taken together, these findings are consistent with the notion that a common, ethanol sensitive region within the ectodomain exist in several ethanol-sensitive P2XR subtypes. Our studies also begin to identify residues within these regions that play an important role in the action of ethanol in P2XRs.
The current study also provides insight into the role of the TM1 and TM2 regions in ethanol modulation of P2X3Rs. The findings indicate that the TM1, but not TM2, region of P2X3Rs is important in determining the magnitude of ethanol potentiation. Therefore, the interaction between the TM1 and ectodomain regions of respectively P2X2 and P2X3Rs in a single P2XR subunit as well as between subunits of homomeric receptors appears to provide additional regulation for the receptor, thus affecting the magnitude of ethanol response. These interactions may also be relevant to ethanol effects in heteromeric P2X2/P2X3Rs. The presence of such heteromers is demonstrated in certain neurons like dorsal root ganglion (Khakh, 2001; North, 2002) and trigeminal ganglion afferents (Staikopoulos et al., 2007).
Studies on ethanol effects that are mediated by P2XRs in the central and peripheral nervous systems are still in early stages. It has been shown that P2X2 and P2X3Rs are expressed in the spinal and central somatosensory systems (Khakh, 2001; North, 2002; Staikopoulos et al., 2007). Studies using P2X2 and P2X3R double-knockout animals implicate P2X2 and P2X3Rs in central nociceptive mechanisms (Cockayne et al., 2005). Recent studies show that presynaptic P2XRs are involved in ethanol-induced synaptic transmission in ventral tegmental area of dopaminergic system (Dr. J. Ye of the University of Medicine and Dentistry of New Jersey, personal communication). Therefore, identification of the sites of ethanol action in P2X2 and P2X3Rs in recombinant expression systems is valuable for further in vivo characterization of these receptors and understanding of their role in ethanol effects in native neurons.
The findings from chimeric receptors also increase our understanding of the regions involved in agonist selectivity of P2XRs. It has been reported that residues participating in agonist binding are localized in the ectodomain of P2XRs (Jiang et al, 2000; Roberts and Evans, 2004). Previous findings from chimeric studies suggest that the ectodomain controls the agonist selectivity of P2XRs. Particularly, the sensitivity to α,β-meATP has been attributed to the ectodomain of P2X3Rs (Koshimizu et al, 2002; Zemkova et al, 2004). In addition, it was suggested that the structure of TM1 domain is important for ATP and α,β-meATP potency/selectivity of P2XRs (Haines et al, 2001). Our findings from chimeric receptors add support for these notions and suggest that both the ectodomain and the TM regions play important roles in regulating agonist potency and/or selectivity of P2XRs.
The present findings provide evidence that the TM regions (TM domains with the intracellular termini) regulate the rate of agonist desensitization of P2X2 and P2X3Rs. A role for intracellular termini in the agonist desensitization of P2X2 and P2X4Rs has been suggested previously (Boue-Grabot et al, 2000; Koshimizu et al 2002; Fountain and North, 2006; Smith et al, 1999). It is still poorly understood which regions regulate fast desensitization of P2X3Rs. Previous studies found that combination of P2X3R ectodomain with P2X2R TM regions in chimeras results in accelerated rates of desensitization compared to that of wildtype P2X2Rs implicating a role for P2X3R ectodomain in agonist desensitization (Koshimizu et al, 2002). In contrast, it was suggested that the interaction between both TM domains controls the rate of agonist desensitization of P2X3Rs (Werner et al, 1996). Our findings from chimeras C1–C4 add support for the latter notion suggesting that the TM regions play a key role in the rate of desensitization of agonist-induced currents in P2X3Rs. Furthermore, findings from chimeras C1, C5 and C6 suggest that the TM1 region has an important role in desensitization of both wildtype P2X2Rs and P2X3Rs.
In conclusion, utilizing a chimeric strategy and point mutations we identified regions/residues that are important for ethanol and agonist action in P2X2 and P2X3Rs. Findings from the specific mutations within each receptor suggest that some of the sites of ethanol action may differ between the two receptor subtypes. More detailed studies are needed to characterize the structural and functional requirements of these regions/residues and to determine possible mechanisms that underlie the effects of ethanol in P2XRs. Additionally, chimeras should serve as useful tools for studies of receptor properties such as fast desensitization, that are, in part, difficult in wildtype P2XRs.
ACKNOWLEDGEMENTS
We thank Dr. Iain Chessell at GlaxoSmithKline for supplying the cDNAs used in this study; Miriam Fine and Sacha Kuo for technical assistance and Daya Perkins for editorial input. Support: NIAAA/NIH AA03972 (RLA), AA013890 (DLD), AA013922 (DLD), F31 AA017029-01 (MP), AA09986 (JJW), and the USC School of Pharmacy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Boue-Grabot E, Archambault V, Seguela P. A protein kinase C site highly conserved in P2X subunits controls the desensitization kinetics of P2X(2) ATP-gated channels. J. Biol. Chem. 2000;275:10190–10195. doi: 10.1074/jbc.275.14.10190. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Introduction: P2 receptors. Curr. Top. Med. Chem. 2004;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- Chizh BA, Illes P. P2X receptors and nociception. Pharmacol. Rev. 2001;53:553–568. [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berso A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Dunn PM, Zhong Y, Rong W, Hamilton SG, Knight GE, Ruan HZ, Ma B, Yip P, Nunn P, McMahon SB, Burnstock G, Ford AP. P2X2 knockout mice and P2X2/P2X3 double knockout mice reveal a role for the P2X2 receptor subunit in mediating multiple sensory effects of ATP. J. Physiol. 2005;567:621–639. doi: 10.1113/jphysiol.2005.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DL, Kochegarov AA, Kuo ST, Kulkarni AA, Woodward JJ, King BF, Alkana RL. Ethanol differentially affects ATP-gated P2X(3) and P2X(4) receptor subtypes expressed in Xenopus oocytes. Neuropharmacol. 2005;49:243–253. doi: 10.1016/j.neuropharm.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Davies DL, Machu TK, Guo Y, Alkana RL. Ethanol sensitivity in ATP-gated P2X receptors is subunit dependent. Alcohol. Clin. Exp. Res. 2002;26:773–778. [PubMed] [Google Scholar]
- Davies M. The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J. Psychiatry Neurosci. 2003;28:263–274. [PMC free article] [PubMed] [Google Scholar]
- Deuchars SA, Atkinson L, Brooke RE, Musa H, Milligan CJ, Batten TF, Buckley NJ, Parson SH, Deuchars J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J. Neurosci. 2001;21:7143–7152. doi: 10.1523/JNEUROSCI.21-18-07143.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke H, Illes P. Involvement of P2 receptors in the growth and survival of neurons in the CNS. Pharmacol.Therap. 2006;109:297–324. doi: 10.1016/j.pharmthera.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Fountain SJ, North RA. A C-terminal lysine that controls human P2X4 receptor desensitization. J. Biol. Chem. 2006;281:15044–15049. doi: 10.1074/jbc.M600442200. [DOI] [PubMed] [Google Scholar]
- Haines WR, Migita K, Cox JA, Egan TM, Voigt MM. The first transmembrane domain of the P2X receptor subunit participates in the agonist-induced gating of the channel. J. Biol. Chem. 2001;276:32793–32798. doi: 10.1074/jbc.M104216200. [DOI] [PubMed] [Google Scholar]
- Hugel S, Schlichter R. Presynaptic P2X receptors facilitate inhibitory GABAergic transmission between cultured rat spinal cord dorsal horn neurons. J. Neurosci. 2002;20:2121–2130. doi: 10.1523/JNEUROSCI.20-06-02121.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang LH, Rassendren F, Surprenant A, North RA. Identification of amino acid residues contributing to the ATP-binding site of a purinergic P2X receptor. J. Biol. Chem. 2000;275:34190–34196. doi: 10.1074/jbc.M005481200. [DOI] [PubMed] [Google Scholar]
- Jiang LH, Kim M, Spelta V, Bo X, Surprenant A, North RA. Subunit arrangement in P2X receptors. J. Neurosci. 2003;23:8903–8910. doi: 10.1523/JNEUROSCI.23-26-08903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat. Rev. Neurosci. 2001;2:165–174. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Bao XR, Labarca C, Lester HA. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nat. Neurosci. 1999;2:322–330. doi: 10.1038/7233. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Burnstock G, Kennedy C, King BF, North RA, Seguela P, Voigt M, Humphrey PA. International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- Khakh BS, Egan TM. Contribution of transmembrane regions to ATP-gated P2X2 channel permeability dynamics. J. Biol. Chem. 2005;280:6118–6129. doi: 10.1074/jbc.M411324200. [DOI] [PubMed] [Google Scholar]
- King BF, Pintor J, Wang S, Ziganshin AU, Ziganshina LE, Burnstock G. A novel P1 purinoceptor activates an outward K+ current in follicular oocytes of Xenopus laevis. J. Pharmacol. Exp. Ther. 1996a;276:93–100. [PubMed] [Google Scholar]
- King BF, Wang S, Burnstock G. P2 purinoceptor-activated inward currents in follicular oocytes of Xenopus laveis. J. Physiol.(Lond) 1996b;494:17–28. doi: 10.1113/jphysiol.1996.sp021472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimizu TA, Ueno S, Tanoue A, Yanagihara N, Stojilkovic SS, Tsujimoto G. Heteromultimerization modulates P2X receptor functions through participating extracellular and C-terminal subdomains. J. Biol. Chem. 2002;277:46891–46899. doi: 10.1074/jbc.M205274200. [DOI] [PubMed] [Google Scholar]
- Li C, Peoples RW, Weight FF. Ethanol-induced inhibition of a neuronal P2X purinoceptor by an allosteric mechanism. Br. J. Pharmacol. 1998;123:1–3. doi: 10.1038/sj.bjp.0701599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xiong K, Weight FF. Ethanol inhibition of adenosine 5'-triphosphate-activated current in freshly isolated adult rat hippocampal CA1 neurons. Neurosci. Lett. 2000;295:77–80. doi: 10.1016/s0304-3940(00)01586-x. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Mori M, Heuss C, Gähwiler BH, Gerber U. Fast synaptic transmission mediated by P2X receptors in CA3 pyramidal cells of rat hippocampal slice cultures. J. Physiol. 2001;535:115–123. doi: 10.1111/j.1469-7793.2001.t01-1-00115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol.Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- North RA, Verkhratsky A. Purinergic transmission in the central nervous system. Pflugers Arch. Eur. J. Physiol. 2006;452:479–485. doi: 10.1007/s00424-006-0060-y. [DOI] [PubMed] [Google Scholar]
- Ormond SJ, Barrera NP, Qureshi OS, Henderson RM, Edwardson JM, Murrell-Lagnado RD. An uncharged region within the N terminus of the P2X6 receptor inhibits its assembly and exit from the endoplasmic reticulum. Mol. Pharmacol. 2006;69:1692–1700. doi: 10.1124/mol.105.020404. [DOI] [PubMed] [Google Scholar]
- Papp L, Vizi ES, Sperlagh B. Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor−/− mice. Neuroreport. 2004;15:2387–2391. doi: 10.1097/00001756-200410250-00017. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Soto F. Distinct localization of P2X receptors at excitatory postsynaptic specializations. J. Neurosci. 2001;21:641–653. doi: 10.1523/JNEUROSCI.21-02-00641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JA, Evans RJ. ATP binding at human P2X1 receptors. Contribution of aromatic and basic amino acids revealed using mutagenesis and partial agonists. J. Biol. Chem. 2004;279:9043–9055. doi: 10.1074/jbc.M308964200. [DOI] [PubMed] [Google Scholar]
- Sim JA, Chaumont S, Jo J, Ulmann L, Young MT, Cho K, Buell G, North RA, Rassendren F. Altered hippocampal synaptic potentiation in P2X4 knock-out mice. J. Neurosci. 2006;26:9006–9009. doi: 10.1523/JNEUROSCI.2370-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FM, Humphhpey PP, Murrell-Lagnado R. Identification of amino acids within the P2X2 receptor C-terminus that regulate desensitization. J. Physiol. 1999;520:91–99. doi: 10.1111/j.1469-7793.1999.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staikopoulos V, Sessle BJ, Furness JB, Jennings EA. Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neurosci. 144:208–216. doi: 10.1016/j.neuroscience.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop R, Thomas S, Rassendren F, Kawashima E, Buell G, Surprenant A, North R. Contribution of individual subunits to the multimeric P2X2 receptor: Estimates based on methanethiosulfonate block at T336C. Mol. Pharmacol. 1999;56:973–981. doi: 10.1124/mol.56.5.973. [DOI] [PubMed] [Google Scholar]
- Torres GE, Egan TM, Voigt MM. Hetero-oligomeric assembly of P2X receptor subunits. Specificities exist with regard to possible partners. J. Biol. Chem. 1999;274:6653–6659. doi: 10.1074/jbc.274.10.6653. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Weight FF, Li C, Peoples RW. Alcohol action on membrane ion channels gated by extracellular ATP (P2X receptors) Neurochem. Int. 1999;35:143–152. doi: 10.1016/s0197-0186(99)00056-x. [DOI] [PubMed] [Google Scholar]
- Werner P, Seward E, Buell G, North RA. Domains of P2X receptors involved in desensitization. Proc. Natl. Acad. Sci. USA. 1996;93:15485–15490. doi: 10.1073/pnas.93.26.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ. Ethanol and NMDA receptor signaling. Crit. Rev. Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- Xiong K, Hu X-Q, Stewart RR, Weight FF, Li C. The mechanism by which ethanol inhibits rat P2X4 receptors is altered by mutation of histidine 241. Br. J. Pharmacol. 2005;145:576–586. doi: 10.1038/sj.bjp.0706192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Li C, Weight FF. Inhibition by ethanol of rat P2X4 receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 2000;130:1394–1398. doi: 10.1038/sj.bjp.0703439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemkova H, He M-L, Koshimizu T, Stojilkovic SS. Identification of ectodomain regions contributing to gating, deactivation, and resensitization of purinergic P2X receptors. J. Neurol. Sci. 2004;24:6968–6978. doi: 10.1523/JNEUROSCI.1471-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]