Abstract
The hippocampus is well-known to be critical for trace fear conditioning, but nothing is known about the importance of perirhinal cortex (PR), which has reciprocal connections with hippocampus. PR damage severely impairs delay fear conditioning to ultrasonic vocalizations (USVs) and discontinuous tones (pips), but has no effect on delay conditioning to continuous tones (Kholodar-Smith, Allen, and Brown, in press). Here we demonstrate that trace auditory fear conditioning also critically depends on PR function. The trace interval between the CS offset and the US onset was 16 s. Pre-training neurotoxic lesions were produced through multiple injections of N-methyl-D-aspartate along the full length of PR, which was directly visualized during the injections. Control animals received injections with phosphate-buffered saline. Three-dimensional reconstructions of the lesion volumes demonstrated that the neurotoxic damage was well-localized to PR and included most of its anterior-posterior extent. Automated video analysis quantified freezing behavior, which served as the conditional response. PR-damaged rats were profoundly impaired in trace conditioning to either of three different CSs (a USV, tone pips, and a continuous tone) as well as conditioning to the training context. Within both the lesion and control groups, the type of cue had no effect on the mean CR. The overall PR lesion effect size was 2.7 for cue conditioning and 3.9 for context conditioning. We suggest that the role of PR in trace fear conditioning may be distinct from some of its more perceptual functions. The results further define the essential circuitry underlying trace fear conditioning to auditory cues.
Keywords: ultrasonic vocalizations, parahippocampal, discontiguous information, auditory objects, context conditioning
Several lines of evidence suggest that perirhinal cortex (PR) functions to bind separate stimulus elements together into unitary representations (Lee, Barense, and Graham, 2005; Barense, Bussey, Lee, Rogers, Davies, Saksida, Murray, and Graham, 2005; Buffalo et al., 2006; Graham, Scahill, Hornberger, Barense, Lee, Bussey, and Saksida, 2006; Murray, Bussey, and Saksida, 2007; Kholodar-Smith, Allen, and Brown, in press). First, PR damage severely impairs performance on object-recognition tasks (Murray and Bussey, 1999; Bussey, Saksida, and Murray, 2002; Petrulis and Eichenbaum, 2003; Norman and Eacott, 2004; Lee et al., 2005; Barense et al., 2005; Buffalo et al., 2006; Graham et al., 2006; Taylor, Moss, Stamatakis, and Tyler, 2006; Bartko et al., 2007; Barker and Warburton, 2008). PR is argued to support performance on tasks that require the use of complex conjunctions of the features that compose an object or a scene (Murray et al., 2007; Kholodar-Smith et al., in press).
Second, PR damage severely impairs context conditioning (Corodimas and LeDoux, 1995; Bucci, Phillips, and Burwell, 2000; Bucci, Saddoris, and Burwell, 2002; Lindquist, Jarrard, and Brown, 2004; Kholodar-Smith et al., in press). Context conditioning is commonly understood to entail “configural” or “conjunctive” learning about key stimulus features (Bucci et al., 2000, 2002; Rudy and O'Reilly, 2001; Lindquist et al., 2004; Kholodar-Smith et al., in press). Eichenbaum and colleagues emphasize the role of PR in “stimulus fusion” (Eichenbaum, Schoenbaum, Young, and Bunsey, 1996; Eichenbaum, 1997). Interestingly, rats require one to three minutes to form a representation of a context that can support context conditioning (Fanselow, 1986, 1990; Fanselow, Landeira-Fernandez, DeCola, and Kim, 1994; Wiltgen et al., 2001; Rudy and Matus-Amat, 2005; Landeira-Fernandez, DeCola, Kim, and Fanselow, 2006; Wiltgen, Sanders, Anagnostaras, Sage, and Fanselow, 2006).
Third, PR damage severely impairs delay fear conditioning to discontinuous but not continuous auditory cues (Lindquist et al., 2004; Kholodar-Smith et al., in press). One plausible inference is that, in order for successful conditioning to occur, the successive segments of these discontinuous conditional stimuli (CSs) must be unitized through a PR-dependent binding mechanism (Kholodar-Smith et al., in press; also discussed in Allen, Furtak, and Brown, 2007; Furtak, Allen, and Brown, 2007a). Unitized representations of chunks of sound have been termed “auditory objects” (see Treisman, 1998; Rauschecker, 1998; Goldstone, 2000; Tian, Reser, Durham, Kustov, and Rauschecker, 2001; Griffiths and Warren, 2004; Furtak et al., 2007a; Kholodar-Smith et al., in press). The recognition of both auditory and non-auditory objects may entail similar or identical PR-dependent mechanisms (see Murray et al., 2007).
The present study uses a trace fear conditioning paradigm to explore a different type of temporal gap—a “trace interval” between the CS offset and the US onset. Although the CS and US were not contiguous in space or time, the US was perfectly predictable across the trace interval. The results reveal what may be a novel aspect of PR function, one that seems not to entail unitization.
Materials and Methods
Subjects
Subjects were fifty-six male Sprague-Dawley rats (~320 g weight; Charles River Laboratories, Kingston, NY). Animals were handled for 3 days prior to surgery. Experiments were conducted in accordance with the Society for Neuroscience policies and the Yale Animal Resources Center guidelines on the care and use of animals.
Surgery
After anesthetizing the rat, the temporal bone and the zygomatic arch of the skull were visualized (Furtak et al., 2007a; Kholodar-Smith et al., in press). The trephination was made along the intersection of the two bones, exposing the temporal cortex (TE) and PR. Each rat received ~8 bilateral PR injections (0.07 µL per infusion; 0.05 µL/min; equally spaced at ~0.7 mm) with either 0.01 M phosphate-buffered 0.9% saline (PBS) or 340 mM NMDA (50 mg/mL; Sigma, St. Louis, MO), using a hypodermic (26 gauge) microsyringe at a 45° angle from vertical. Sometimes only 7 injections were made due to the presence of a blood vessel at the intended injection site. Openings on the lateral surface of the skull were covered with a thin layer of bone wax. Rats were given 5–7 days to recover before initiating the behavioral procedures discussed next.
Behavioral Procedures
Two modified Coulbourn chambers (29 cm length × 25.5 cm width × 32 cm height) differed in odorant, lighting, flooring, visual design on the walls, and background noise. Chamber A served as the training and context-testing chamber. Chamber B served as a “shifted” context for testing CS-elicited freezing. A “bat detector” (Mini-3, tuned at 23 kHz; UltrasoundAdvise, London, UK) was positioned inside each sound-attenuating enclosure. Both chambers were equipped with an infra-red camera, which was attached to a video recorder. Video and audio signals that were recorded during cue and context testing were stored for offline analysis of freezing behavior. The latter was done with video-analysis software (see Supplementary Fig. 1 and Supplementary Fig 2) described elsewhere (Bang, Allen, Jones, Boguszewski, and Brown, 2008; Boguszewski, Bang, and Brown, 2007). For cue and context conditioning, respectively, the correlation between machine and human scoring (n = 12 rats) is 0.99 and 0.91 (Bang and Brown, unpublished).
Auditory Stimuli
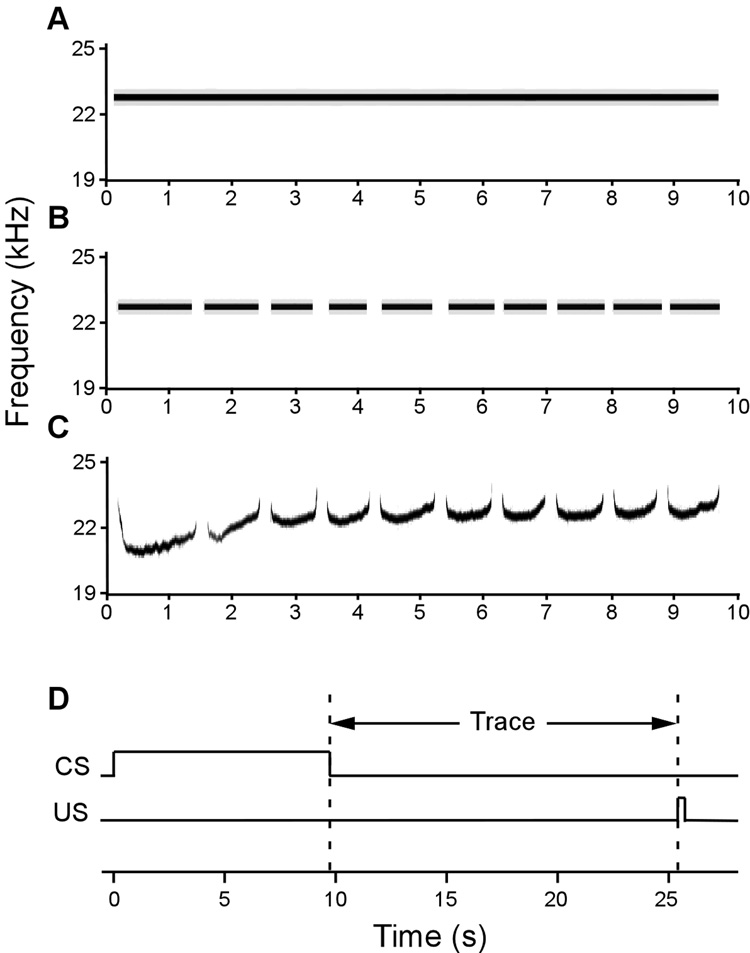
The auditory CSs (Fig. 1) were similar to ones that have previously been shown to elicit widespread firing in rat PR neurons (Allen et al., 2007; Furtak et al., 2007a). A pre-recorded 10-call 23 kHz ultrasonic vocalization (USV; Fig. 1C) was elicited from a naïve rat by an unsignaled foot shock through the grid floor (1 s, 1 mA). Vocalizations were digitally recorded with an RP2.1 (TuckerDavisTechnologies (TDT), Alachua, FL), sampled at 100 kHz (32-bit), and band-pass filtered (18 – 26 kHz). Continuous and discontinuous tone cues (10 ms rise/fall time) were produced with a D/A tone generator (RPvds, TDT) used in conjunction with an RP2.1 (Fig. 1A – B). All three auditory cues were matched in loudness (~ 65dB).
Figure 1. Training paradigm and spectrograms of the three conditional stimuli (CSs) used as cues.
(A) A continuous 23 kHz tone. (B) A discontinuous 23 kHz tone (“pips”) whose frequency and on/off temporal pattern matches the ~23 kHz ultrasonic vocalization (USV). (C) A segment of a ~23 kHz USV recorded from a conspecific. All cues were matched in principle frequency, duration, and average loudness. (D) Training paradigm. Training included 10 trials of 9.7 s CS presentation, followed by a 16 s trace interval, and terminated with a 1 s US footshock presentation.
Conditioning
Subjects were randomly assigned to receive presentations one of three auditory cues (Fig. 1) that were used as conditional stimuli: a continuous tone (“Tone” Group); a pre-recorded USV (“USV” group); and a discontinuous tone, consisting of 10 tonal segments, whose on/off pattern matched the temporal structure of the USV (“Pips” group). By convention, USVs with root frequencies of 18–35 kHz are collectively termed “22 kHz USVs” (Brudzynski, 2005). Among naïve laboratory rats, USVs and Pips are as “neutral” as continuous tones in terms of the unconditional elicitation of freezing behavior (Endres and Fendt, 2007; Bang et al., 2008). Prior to conditioning, subjects were given 2 min to explore the training chamber. During conditioning, subjects received 10 trace pairings of a 9.7 s auditory CS and a 1.0 s foot shock US (0.8 mA) in Chamber A. The trace interval was 16 s and the ISI was 25.7 s. A recent study suggests that hippocampal participation in trace conditioning diminishes with trace intervals less than 15 s (Misane, Tovote, Meyer, Spiess, Ogren, and Stiedl, 2005). The mean inter-trial interval (±SE) was 5 min ± 15 s.
Testing
Freezing responses to the cue (in Chamber B) and context (in Chamber A) were tested 24 and 48 hr later in a counterbalanced order. Context-elicited freezing was measured for 6 minutes. Cue-elicited freezing was measured in massed trials during the 2-min baseline and 6 min of the cue presentation. Freezing was defined as the cessation of all movement except that required for respiration (Blanchard and Blanchard, 1969; Fanselow, 1997). The freezing recognition software converted the cumulative time spent freezing into a percentage score (100% freezing duration ÷ total duration).
Statistics
Group data were analyzed using ANOVAs and t-tests. The family-wise α-error rate was maintained at 0.05. The lesion effect size (d) on freezing was computed as follows (Cohen, 1988):
| (1) |
where the numerator is the difference between the mean freezing of control and lesioned animals and the denominator is the standard deviation of a pooled estimates from control and lesioned animals. In psychological studies, d = 0.8 is conventionally regarded as a “large effect size” (Cohen, 1988).
Histology
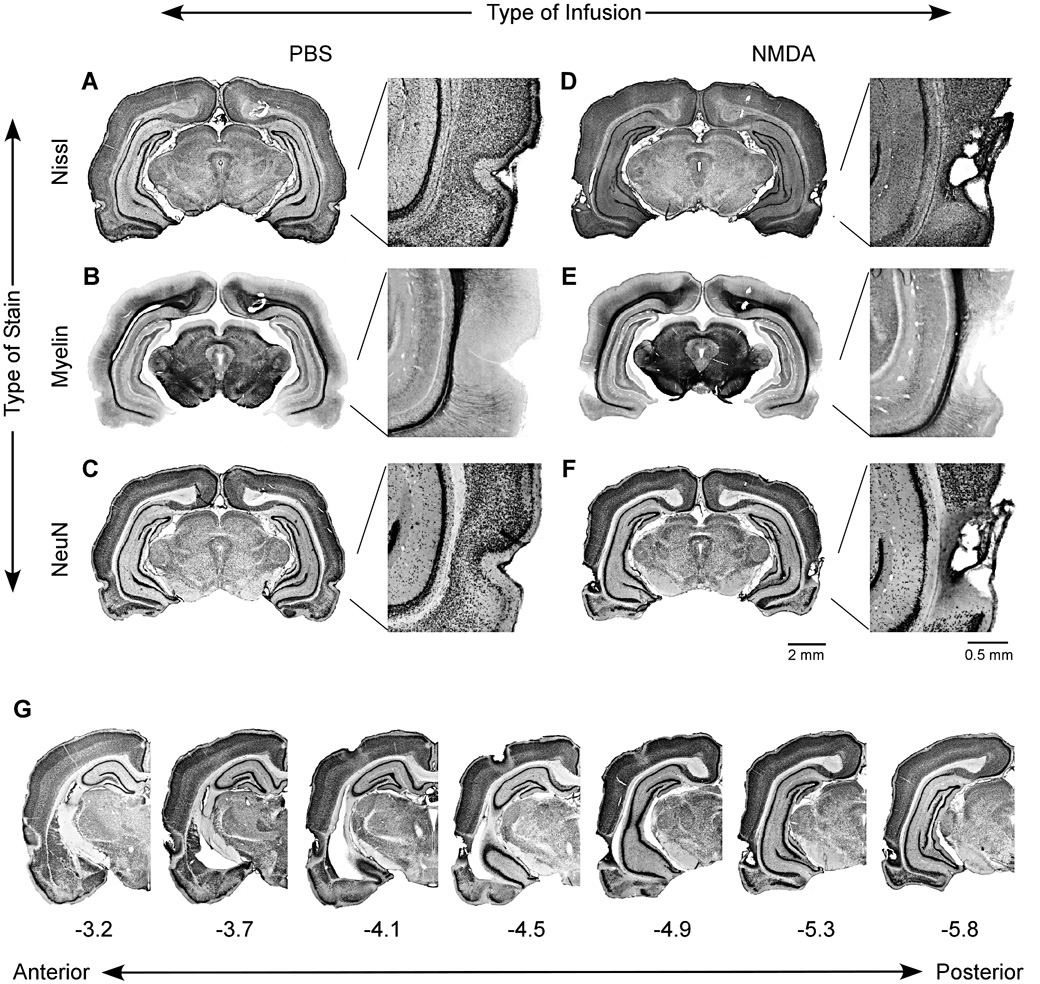
Subjects were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/kg) and perfused intracardially with 0.01 M PBS and 4% paraformaldehyde. Cryoprotected brains were sectioned with a freezing microtome (75 µm) into three sets of immediately-adjacent sections for a Nissl stain, a NeuN stain, and a fiber-specific myelin stain. The conspicuously low level of myelin staining in PR, relative to the adjacent temporal (TE) and entorhinal cortices (EC; Burwell, 2001; Brown and Furtak, 2006), quickly reveals its borders. The Nissl and NeuN stains highlight the distinctive laminar organization of PR around the rhinal fissure. In combination, these stains leave no uncertainty about the borders between PR and adjacent structures.
Lesion Reconstructions
Using a Neurolucida (MicroBrightField, Williston, VT) computer-microscope system, the volume of neurotoxic damage to PR and neighboring regions was estimated using NeuN-stained sections (based on Paxinos and Watson, 1998; Burwell, 2001). The Cavalieri method was used to calculate the percentage damage (by volume) to PR, EC, the ventral hippocampus (vHC), area TE of the temporal cortex (TE1 plus TE2), and the lateral and basolateral subnuclei of the amygdala (LA/BLA).
Results
Histology
None of the 56 rats was excluded based on histology. Figure 2 compares cell damage in sham-operated (Fig. 2A–C) and PR-lesioned rats (Fig. 2D–F). In sham-operated rats, the PR cytoarchitecture was easily discerned in Nissl- (Fig. 2A) and NeuN-stained sections (Fig. 2C) based on its laminar organization around the curvature of the rhinal sulcus. The myelin stain (Fig. 2B) duplicated previous findings of conspicuously levels of PR myelination relative to TE and EC (Burwell, 2001; Brown and Furtak, 2006). This remarkably low level of staining quickly identifies the boarders between PR and adjacent cortex. The multiple NMDA injections caused neurotoxic damage that included areas 35 and 36 along most of the rostro-caudal extent of PR (Fig. 2D – G).
Figure 2. Examples of histology from a sham-operated subject (A–C) and a PR-lesioned subject (D – F).
Coronal slices were reacted using a Nissl stain (A, D), which labels neuronal and glial cell bodies; a gold-chloride stain (B, E), which labels myelin; a NeuN (C, F), which is specific for neurons. The illustrated sections were taken at approximately −5.2 A/P relative to bregma. Multiple injections of PBS resulted in unremarkable damage primarily represented by the needle tracks, while multiple injections of NMDA caused a well-localized neuronal damage. (G) Unilateral images showing the anterior-posterior extent of a representative PR lesion. Brain sections belong to the same subject shown in (D–F).
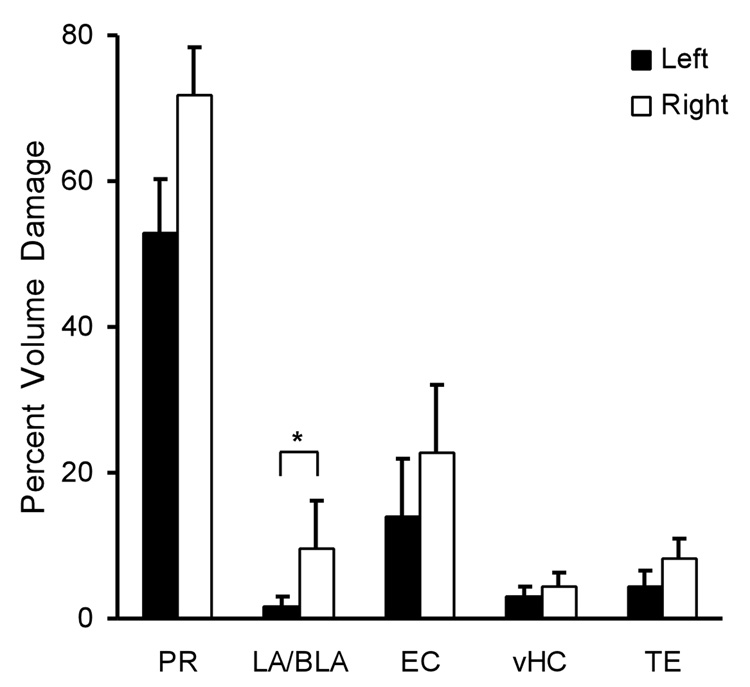
Figure 3 shows the average amount of neurotoxic damage that was sustained in each hemisphere of PR and in adjacent structures. There were no significant differences between hemispheres in the amount of damage to PR, EC, vHC and TE, p > 0.05 (see Fig. 3). There was also no significant difference in the extent of PR damage among the Cue groups: F (2, 24) = 3.17, p = 0.06. The damage to vHC was minor (3.7% volume). For unknown reasons, there was significantly more damage to the right LA/BLA (9.4 ± 6.6%) than the left LA/BLA (1.5 ± 1.5%), t (26) = 2.14, p = 0.04. Because the US was applied bilaterally, the small unilateral amygdala damage is unlikely to have had any behavioral effect (Blair, Huynh, Vaz, Van, Patel, Hiteshi, Lee, and Tarpley, 2005). Finally, there was no significant difference in the effect of PR damage among the Cue groups, F (2, 24) = 1.97, p = 0.16.
Figure 3. Average amount of bilateral damage to PR and adjacent structures caused by neurotoxic lesions.
The amount of damage is given as a percentage of the total volume. PR sustained extensive bilateral damage, whereas damage to adjacent brain regions was limited. In the LA/BLA region there was significantly more damage to the right side than on the left side. Abbreviations: PR, perirhinal cortex; LA/BLA, lateral and basolateral nuclei of amygdala; EC, entorhinal cortex; vHC, ventral hippocampus; TE, temporal cortex. Bars represent the standard error (SE) of the mean.
Cue-Elicited Freezing
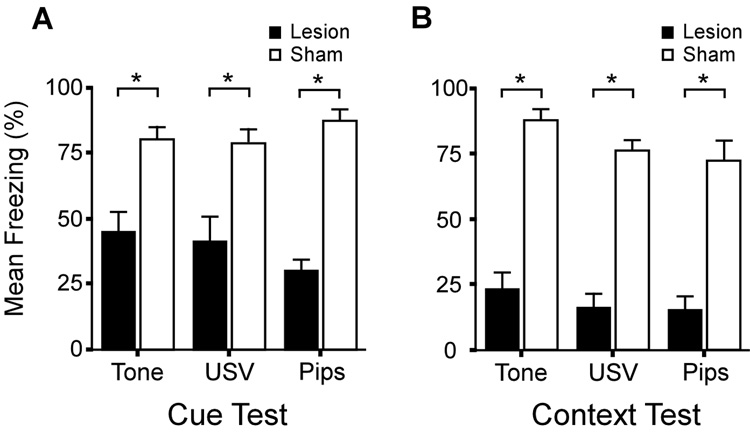
Prior to the cue presentation (during the baseline period), there was no significant effect of Surgery, F (1, 55) = 1.33, p = 0.26; no significant effect of type of Cue, F (2, 55) = 0.32, p = 0.73; and no significant Surgery × Cue interaction, F (2, 55) = 1.37, p = 0.27. During the CS presentation, there was a significant main effect of Surgery, F (1, 50) = 72.23, p < 0.001. Figure 4A shows that freezing during the CS presentation was comparable in the three cue groups. The CS-elicited freezing levels were significantly lower in PR-lesioned rats (39.0 ± 4.3 %) than PBS-infused control animals (82.2 ± 2.7 %), t (52) = 8.48, p < 0.001. There was no main effect of the Cue on freezing levels, F (2, 50) = 0.22, p = 0.80. The mean freezing level among sham-operated rats was 82.24 ± 2.73% and there were no significant differences among Cue groups, F (2, 24) = 0.91, p = 0.42. The mean freezing level among PR-lesioned rats was 39.03 ± 4.29% and there were no significant differences in freezing levels among the Cue groups, F (2, 24) = 1.10, p = 0.35. In addition, there was no significant Surgery × Cue interaction, F (2, 50) = 1.87, p = 0.17. The mean (± SE) lesion effect size (from Eq. 1) on trace cue conditioning was very large (d̅ = 2.7 ± 0.8). The effect sizes for each cue group were as follows: Tone-group (d = 1.9), USV-group (d = 1.7), and Pips-group (d = 4.6).
Figure 4. Effects of the bilateral NMDA lesions on freezing to the cue and training context.
(A) Mean freezing levels to the cue in PR-lesioned subjects (black bars) and sham-operated control animals (white bars). All three cues elicited robust freezing in the sham-operated control animals. By contrast, freezing was significantly reduced to all auditory cues in PR-lesioned subjects. (B) Mean freezing levels to the training context were comparable among the three groups of sham-operated subjects. By contrast, freezing was significantly reduced in the three groups of PR-lesioned subjects. Asterisks denote significant differences between the sham-operated and PR-lesioned subjects. Bars represent the standard error (SE) of the mean.
Context-Elicited Freezing
The effects of PR damage on context-elicited freezing are summarized in Figure 4B. The histograms show comparably severe impairment in all three CS groups. The mean lesion effect size on context conditioning (d̅ = 3.9 ± 0.7) was extremely large. By group, the lesion effect sizes on context conditioning were as follows: Tone-group (d =4.2), USV-group (d = 4.4), and Pips-group (d = 3.0). There was a significant main effect of Surgery on context-elicited freezing, F (1, 50) = 183.87, p < 0.001. Overall, PR-lesioned rats froze significantly less to the context (18.4 ± 5.6 %) than did the PBS-controls (79.3 ± 5.6 %), t (52) = 6.67, p < 0.001. There was no significant effect of Cue on context conditioning, F (2, 50) = 2.63, p = 0.08, and no significant Lesion × Cue interaction, F (2, 50) = 0.28, p = 0.76. Within each Cue group, the pattern of statistical significance was the same. Specifically, there was significantly less freezing in PR-lesioned rats than in PBS-controls in the Tone group, t (16) = 8.65, p < 0.0001; the USV group, t (16) = 9.25, p < 0.0001; and the Pips-group, t (16) = 4.97, p < 0.0001.
Discussion
Brief Summary
The present study explored the role of PR in trace fear conditioning, using three different auditory cues (Fig. 1A–C). In the experimental group, neurotoxic damage was evident along most of the length of PR (Fig. 2). Three-dimensional reconstructions revealed that the volume of neuronal damage was well-localized to PR (Fig. 2, Fig 3). There was relatively little bilateral damage to either vHC or LA/BLA (Fig. 3). PR lesions profoundly impaired trace fear conditioning to all three auditory cues (Fig. 4A; d̅ = 2.7) as well as conditioning the training context (Fig. 4B; d̅ = 3.9). There were no significant differences among the cues in the levels of elicited freezing in either PR-lesioned rats or sham-operated control animals. The new and remarkable finding is that PR damage impaired trace fear conditioning to a continuous tone. The observed deficits in context conditioning and conditioning to discontinuous auditory cues were anticipated (see Kholodar-Smith et al., in press; Lindquist et al., 2004; Bucci et al., 2002, 2002). These and other results are interpreted in terms of dual mnemonic functions of PR.
Trace Fear Conditioning System
Background and contemporary theory
Historically, the dorsal hippocampus (dHC) has been most closely associated with trace conditioning. Damage to the dHC can impair both trace fear and trace eyeblink conditioning (Berger, Rinaldi, Weisz, and Thompson, 1983; Solomon, Vander Schaaf, Thompson, and Weisz, 1986; Moyer, Deyo, and Disterhoft, 1990; Weiss et al., 1996; McEchron, Bouwmeester, Tseng, Weiss, and Disterhoft, 1998; McEchron, Tseng, and Disterhoft, 2000). Recent evidence suggests that the ventral region of the hippocampus (vHC) may be more directly involved in trace conditioning (Rogers, Hunsaker, and Kesner, 2006; Yoon and Otto, 2007). Indeed, the dHC is thought to communicate with the amygdala by means of the vHC (Pitkänen, Pikkarainen, Nurminen, and Ylinen, 2000).
For several days after trace conditioning, HC damage severely impairs memory performance. However, at longer time intervals, HC function is no longer required for retrieval (Kim and Fanselow, 1992; Anagnostaras, Maren, and Fanselow, 1999; also see Moyer, Thompson, and Disterhoft, 1996). One contemporary theory argues that HC-independent memories can emerge from repeated activations and strengthening of the synapses between HC and neocortex and within neocortex (Teyler and DiScenna, 1986; Zola-Morgan and Squire, 1986; Rolls, 1996; Rolls and Kesner, 2006; Paz, Bauer, and Paré, 2007; Teyler and Rudy, 2007; Rudy, 2008).
Cortical fear conditioning pathways
During and after trace fear conditioning, critical interactions are believed to occur between HC, EC, PR, and the prefrontal cortex (PFC; Dash, Hebert, and Runyan, 2004; Paz et al., 2007). The latter has been proposed to be a long-term memory storage site for both trace fear and trace eyeblink conditioning (Powell, Skaggs, Churchwell, and McLaughlin, 2001; Dash et al., 2004; Runyan, Moore, and Dash, 2004; Blum, Hebert, and Dash, 2006; Quinn, Ma, Tinsley, Koch, and Fanselow, 2008). PR sends direct projections to HC as well as indirect projections via EC (Pitkänen et al., 2000; Burwell, 2001; Lavenex, Suzuki, and Amaral, 2004; Furtak, Wei, Agster, and Burwell, 2007b). The CA1 region of HC in turn projects back to PR through the subiculum and EC (Pitkanen et al., 2000).
Both EC and the subiculum project to LA/BLA (Pitkanen et al., 2000; Kerr, Agster, Furtak, and Burwell, 2007), which are “cortex-like” parts of the amygdala (McDonald, 1982; Swanson and Petrovich, 1998). Reciprocal monosynaptic projections connect PR and LA/BLA. The latter projects to the central nucleus of the amygdala (Pitkanen et al., 2000; Furtak et al., 2007b), which in turn controls conditional freezing behavior (Fanselow, 1998; LeDoux, 2000; Choi and Brown, 2003). The only monosynaptic projections from rat HC to the amygdala occur via the subfield CA1 of vHC (Canteras and Swanson, 1992; Pitkanen et al., 2000; Yoon and Otto, 2007). Interestingly, the output from LA/BLA can also control the propagation of activity from PR to EC and HC (Kajiwara, Takashima, Mimura, Witter, and Iijima, 2003), raising the possibility of recursive interactions among these structures.
Functions of PR in trace conditioning
The simplest account of the role of PR in trace fear conditioning is that this “transitional” cortex (Lavenex and Amaral, 2000) serves as a crucial part of the input to and/or output from HC, which houses the essential memory trace (see Wallenstein, Eichenbaum, and Hasselmo, 1998; Shapiro, 2001; Lee and Kesner, 2003; Rudy and O’Reilly, 2001; Rodriguez and Levy, 2001; Rolls and Kesner, 2006; Eichenbaum, Yonelinas, and Ranganath, 2007). However, recent evidence suggests that the role of PR in trace fear conditioning may transcend this purely-passive or non-adaptive conceptualization. A recent neurophysiological study of trace fear conditioning found widespread changes in PR neurons (Allen and Brown, 2008). Some of these firing changes were specific to the trace interval and persisted into the testing sessions 4 and 24 hrs later.
We propose that the critical function of PR in Pavlovian trace conditioning is to enable a representation of the cue in the absence of exteroceptive input. This same PR function is thought to support performance on a delayed non-match to sample (DNMS) tasks. PR damage impairs performance on DNMS tasks in both monkeys (Gaffan and Murray, 1992; Meunier, Bachevalier, Mishkin, and Murray, 1993; Murray and Bussey, 1999) and rats (Otto and Eichenbaum, 1992). Neurophysiological recordings from rat PR have revealed neurons that maintain stimulus-specific activity during the delay period of an odor-guided version of the DNMS task (Young, Otto, Fox, and Eichenbaum, 1997).
In neurophysiological terms, transient sensory memory could be supported by recirculating neuronal activity (Durstewitz, Seamans, and Sejnowski, 2000; Fellous and Sejnowski, 2003) and and/or by endogenous “persistent firing”, which can last for minutes after the termination of the original excitation (Egorov, Unsicker, and von Bohlen und Halbach, 2006). This remarkable single-cell phenomenon is common in LA/BLA (Egorov et al., 2006) and certain layers of PR (Boguszewski, Leung, Zhao, and Brown, 2007; Brown, Zhao, and Leung, 2007) and EC (Egorov, Hamam, Fransén, Hasselmo, and Alonso, 2002; Fransén, Tahvildari, Egorov, Hasselmo, and Alonso, 2006; also see Hasselmo and Stern, 2006), but does not occur HC.
Conclusions
PR damage profoundly impaired both trace fear conditioning and context conditioning. We suggest that the function of PR in trace cue conditioning is different from its role in delay cue and context conditioning. The latter two are consistent with the general hypothesis (see Kholodar-Smith et al., in press), reviewed earlier, that PR supports unitization through “configural” or “conjunctive” learning or “stimulus fusion”. The role of PR in trace conditioning may reflect a different kind of adaptive function, one that is more intimately associated with HC. Seemingly different higher-level functions may turn out to depend on similar cellular mechanisms, such as endogenous persistent firing.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by the National Institutes of Health grant MH58405 and Yale University. We thank Pinki Chakraborty for assisting with the histology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen TA, Brown TH. Simultaneous single-unit recordings from rat perirhinal cortex and dorsomedial prefrontal cortex during trace fear conditioning. Society for Neuroscience abstract. 2008 submitted. [Google Scholar]
- Allen TA, Furtak SC, Brown TH. Single-unit responses to 22 kHz ultrasonic vocalizations in rat perirhinal cortex. Behavioural Brain Research. 2007;182(2):327–336. doi: 10.1016/j.bbr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anagnostaras SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. Journal of Neuroscience. 1999;19(3):1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC. NMDA receptor plasticity in the perirhinal and prefrontal cortices is crucial for the acquisition of long-term object-in-place associative memory. Journal of Neuroscience. 2008;28(11):2837–2844. doi: 10.1523/JNEUROSCI.4447-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang S, Allen TA, Jones LK, Boguszewski P, Brown TH. Asymmetrical stimulus generalization following differential fear conditioning. Neurobiology of Learning and Memory. 2008;90:200–216. doi: 10.1016/j.nlm.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, Murray EA, Graham KS. Functional specialization in the human medial temporal lobe. Journal of Neuroscience. 2005;25(44):10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartko SJ, Winters BD, Cowell RA, Saksida LM, Bussey TJ. Perirhinal cortex resolves feature ambiguity in configural object recognition and perceptual oddity tasks. Learning & Memory. 2007;14(12):821–832. doi: 10.1101/lm.749207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger TY, Rinaldi PC, Weisz DJ, Thompson RF. Single-unit analysis of different hippocampal cell types during classical conditioning of rabbit nictitating membrane response. Journal of Neurophysiology. 1983;50:1197–1219. doi: 10.1152/jn.1983.50.5.1197. [DOI] [PubMed] [Google Scholar]
- Blair HT, Huynh VK, Vaz VT, Van J, Patel RR, Hiteshi AK, Lee JE, Tarpley JW. Unilateral storage of fear memories by the amygdala. Journal of Neuroscience. 2005;25(40):4198–4205. doi: 10.1523/JNEUROSCI.0674-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. Journal of Comparative and Physiological Psychology. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remote memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- Boguszewski PM, Bang S-J, Brown TH. Machine analysis of conditional and unconditional freezing behavior in rats. Society for Neuroscience abstract. 2007 program no. 316.1. [Google Scholar]
- Boguszewski PM, Leung VL, Zhao Y, Brown TH. Persistent-firing neurons in layer II/III of rat perirhinal cortex. Pavlovian Society abstract. 2007 [Google Scholar]
- Brown TH, Furtak SC. Low myelin staining in rat perirhinal cortex and parts of the amygdala. Society for Neuroscience abstract. 2006 program no. 638.17. [Google Scholar]
- Brown TH, Zhao YJ, Leung V. Hebbian plasticity. In: Adelman G, Smith BH, editors. Encyclopedia of Neuroscience. 4rd ed. New York: Elsevier Science; 2007. [Google Scholar]
- Brudzynski SM. Principles of rat communication: quantitative parameters of ultrasonic calls in rats. Behavior Genetics. 2005;35:85–92. doi: 10.1007/s10519-004-0858-3. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Phillips RG, Burwell RD. Contributions of postrhinal and perirhinal cortex to contextual information processing. Behavioral Neuroscience. 2000;114(5):882–894. doi: 10.1037//0735-7044.114.5.882. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Saddoris MP, Burwell RD. Contextual fear discrimination is impaired by damage to the postrhinal or perirhinal cortex. Behavioral Neuroscience. 2002;116(3):479–488. [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning & Memory. 2006;13(5):638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell RD. Borders and cytoarchitecture of the perirhinal and postrhinal cortices in the rat. The Journal of Comparative Neurology. 2001;437:17–41. doi: 10.1002/cne.1267. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. Object memory and perception in the medial temporal lobe: an alternative approach. Current Opinion in Neurobiology. 2005;15(6):730–737. doi: 10.1016/j.conb.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM, Murray EA. Perirhinal cortex resolves feature ambiguity in complex visual discriminations. European Journal of Neuroscience. 2002;15(2):365–374. doi: 10.1046/j.0953-816x.2001.01851.x. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. Journal of Comparative Neurology. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Choi JS, Brown TH. Central amygdala lesions block ultrasonic vocalization and freezing as conditional but not unconditional responses. Journal of Neuroscience. 2003;23(25):8713–8721. doi: 10.1523/JNEUROSCI.23-25-08713.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1988. [Google Scholar]
- Dash PK, Hebert AE, Runyan JD. A unified theory for systems and cellular memory consolidation. Brain Research. Brain Research Reviews. 2004;45(1):30–37. doi: 10.1016/j.brainresrev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK, Sejnowski TJ. Neurocomputational models of working memory. Nature Neuroscience. 2000;3 Suppl:1184–1191. doi: 10.1038/81460. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Hamam BN, Fransén E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature. 2002;420(6912):173–178. doi: 10.1038/nature01171. [DOI] [PubMed] [Google Scholar]
- Egorov AV, Unsicker K, von Bohlen und Halbach O. Muscarinic control of graded persistent activity in lateral amygdala neurons. European Journal of Neuroscience. 2006;24(11):3183–3194. doi: 10.1111/j.1460-9568.2006.05200.x. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Schoenbaum G, Young B, Bunsey M. Functional organization of the hippocampal memory system. Proceedings of the National Academy of Sciences of the U S A. 1996;93(24):13500–13507. doi: 10.1073/pnas.93.24.13500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endres T, Fendt M. Are rats predisposed to learn 22 kHz calls as danger-predicting signals? Behavioural Brain Research. 2007;185(2):69–75. doi: 10.1016/j.bbr.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Associative vs topographical accounts of the immediate shock freezing deficit in rats: Implications for the response selection rules governing species-specific defensive reactions. Learning & Motivation. 1986;17:16–39. [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Animal Learning & Behavior. 1990;18:264–270. [Google Scholar]
- Fanselow M. Species-specific defense reactions: retrospect and prospect. In: Bouton ME, editor. Learning, motivation, and cognition. Washington, DC: American Psychological Association; 1997. pp. 321–341. [Google Scholar]
- Fanselow M. Pavlovian conditioning, negative feedback, and blocking: mechanisms that regulate association formation. Neuron. 1998;20(4):625–627. doi: 10.1016/s0896-6273(00)81002-8. [DOI] [PubMed] [Google Scholar]
- Fanselow MS, Landeira-Fernandez J, DeCola JP, Kim JJ. The immediate-shock deficit and postshock analgesia: Implications for the relationship between the analgesic CR and UR. Animal Learning & Behavior. 1994;22:72–76. [Google Scholar]
- Fellous JM, Sejnowski TJ. Regulation of persistent activity by background inhibition in an in vitro model of a cortical microcircuit. Cerebral Cortex. 2003;13(11):1232–1241. doi: 10.1093/cercor/bhg098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransén E, Tahvildari B, Egorov AV, Hasselmo ME, Alonso AA. Mechanism of graded persistent cellular activity of entorhinal cortex layer v neurons. Neuron. 2006;49(5):735–746. doi: 10.1016/j.neuron.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Furtak SC, Allen TA, Brown TH. Single-unit firing in rat perirhinal cortex caused by fear conditioning to arbitrary and ecological stimuli. Journal of Neuroscience. 2007a;27(45):12277–12291. doi: 10.1523/JNEUROSCI.1653-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furtak SC, Wei SM, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: the perirhinal and postrhinal cortices. Hippocampus. 2007b;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- Gaffan D, Murray EA. Monkeys (Macaca fascicularis) with rhinal cortex ablations succeed in object discrimination learning despite 24-hr intertribal intervals and fail at matching to sample despite double sample presentations. Behavioral Neuroscience. 1992;106(1):30–38. doi: 10.1037//0735-7044.106.1.30. [DOI] [PubMed] [Google Scholar]
- Graham KS, Scahill VL, Hornberger M, Barense MD, Lee AC, Bussey TJ, Saksida LM. Abnormal categorization and perceptual learning in patients with hippocampal damage. Journal of Neuroscience. 2006;26(29):7547–7554. doi: 10.1523/JNEUROSCI.1535-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Stern CE. Mechanisms underlying working memory for novel information. Trends in Cognitive Sciences. 2006;10(11):487–493. doi: 10.1016/j.tics.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiwara R, Takashima I, Mimura Y, Witter MP, Iijima T. Amygdala input promotes spread of excitatory neural activity from perirhinal cortex to the entorhinal-hippocampal circuit. Journal of Neurophysiology. 2003;89(4):2176–2184. doi: 10.1152/jn.01033.2002. [DOI] [PubMed] [Google Scholar]
- Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17(9):697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- Kholodar-Smith DB, Allen TA, Brown TH. Fear conditioning to discontinuous auditory cues requires perirhinal cortical function. Behavioral Neuroscience. 2008 doi: 10.1037/a0012902. in press. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256(5057):675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Landeira-Fernandez J, DeCola JP, Kim JJ, Fanselow MS. Immediate shock deficit in fear conditioning: effects of shock manipulations. Behavioral Neuroscience. 2006;120(4):873–879. doi: 10.1037/0735-7044.120.4.873. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal-neocortical interaction: a hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Intrinsic projections and interconnections. Journal of Comparative Neurology. 2004;472(3):371–394. doi: 10.1002/cne.20079. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotional circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee AC, Barense MD, Graham KS. The contribution of the human medial temporal lobe to perception: bridging the gap between animal and human studies. Quarterly Journal of Experimental Psychology. B, Comparative and Physiological Psychology. 2005;58(3–4):300–325. doi: 10.1080/02724990444000168. [DOI] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Differential roles of dorsal hippocampal subregions in spatial working memory with short versus intermediate delay. Behavioral Neuroscience. 2003;117(5):1044–1053. doi: 10.1037/0735-7044.117.5.1044. [DOI] [PubMed] [Google Scholar]
- Lindquist DH, Jarrard LE, Brown TH. Perirhinal cortex supports delay fear conditioning to rat ultrasonic social signals. Journal of Neuroscience. 2004;24(14):3610–3617. doi: 10.1523/JNEUROSCI.4839-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. Journal of Comparative Neurology. 1982;212(3):293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8(6):638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- McEchron MD, Tseng W, Disterhoft JF. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus. 2000;10(6):739–751. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience. 1993;13(12):5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JR, Brown TH. Impaired trace and contextual fear conditioning in aged rats. Behavioral Neuroscience. 2006;120(3):612–624. doi: 10.1037/0735-7044.120.3.612. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behavioral Neuroscience. 1990;104(2):243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Moyer JRJ, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. Journal of Neuroscience. 1996;16(17):5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misane I, Tovote R, Meyer M, Spiess J, Ogren SO, Stiedl O. Time-dependent involvement of the dorsal hippocampus in trace fear conditioning in mice. Hippocampus. 2005;15(4):418–426. doi: 10.1002/hipo.20067. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Saksida LM. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annual Review of Neuroscience. 2007;30:99–122. doi: 10.1146/annurev.neuro.29.051605.113046. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends in Cognitive Sciences. 1999;3(4):142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Impaired object recognition with increasing levels of feature ambiguity in rats with perirhinal cortex lesions. Behavioural Brain Research. 2004;148(1–2):79–91. doi: 10.1016/s0166-4328(03)00176-1. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Complementary roles of the orbital prefrontal cortex and the perirhinal-entorhinal cortices in an odor-guided delayed-nonmatching-to-sample task. Behavioral Neuroscience. 1992;106(5):762–775. doi: 10.1037//0735-7044.106.5.762. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Paz R, Bauer EP, Paré D. Learning-related facilitation of rhinal interactions by medial prefrontal inputs. Journal of Neuroscience. 2007;27(24):6542–6551. doi: 10.1523/JNEUROSCI.1077-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrulis A, Eichenbaum H. The perirhinal-entorhinal cortex, but not the hippocampus, is critical for expression of individual recognition in the context of the Coolidge effect. Neuroscience. 2003;122(3):599–607. doi: 10.1016/j.neuroscience.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. Annals of the New York Academy of Sciences. 2000;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Powell DA, Skaggs H, Churchwell J, McLaughlin J. Posttraining lesions of the medial prefrontal cortex impair performance of Pavlovian eyeblink conditioning but have no effect on concomitant heart rate changes in rabbits (Oryctolagus cuniculus) Behavioral Neuroscience. 2001;115(5):1029–1038. doi: 10.1037//0735-7044.115.5.1029. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Loya F, Ma QD, Fanselow MS. Dorsal hippocampus NMDA receptors differentially mediate trace and contextual fear conditioning. Hippocampus. 2005;15(5):665–674. doi: 10.1002/hipo.20088. [DOI] [PubMed] [Google Scholar]
- Quinn JJ, Ma QD, Tinsley MR, Koch C, Fanselow MS. Inverse temporal contributions of the dorsal hippocampus and medial prefrontal cortex to the expression of long-term fear memories. Learning & Memory. 2008;15(5):368–372. doi: 10.1101/lm.813608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker JP. Cortical processing of complex sounds. Current Opinion in Neurobiology. 1998;8(4):516–521. doi: 10.1016/s0959-4388(98)80040-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez P, Levy WB. A model of hippocampal activity in trace conditioning: where's the trace? Behavioral Neuroscience. 2001;115(6):1224–1238. doi: 10.1037//0735-7044.115.6.1224. [DOI] [PubMed] [Google Scholar]
- Rogers JL, Hunsaker MR, Kesner RP. Effects of ventral and dorsal CA1 subregional lesions on trace fear conditioning. Neurobiology of Learning and Memory. 2006;86(1):72–81. doi: 10.1016/j.nlm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Rolls ET. A theory of hippocampal function in memory. Hippocampus. 1996;6:601–620. doi: 10.1002/(SICI)1098-1063(1996)6:6<601::AID-HIPO5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79(1):1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Rudy JW. The neurobiology of learning and memory. Sinauer Associates, Inc; 2008. [Google Scholar]
- Rudy JW, Matus-Amat P. The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral Neuroscience. 2005;119(1):154–163. doi: 10.1037/0735-7044.119.1.154. [DOI] [PubMed] [Google Scholar]
- Rudy JW, O'Reilly RC. Conjunctive representations, the hippocampus, and contextual fear conditioning. Cognitive, Affective & Behavioral Neuroscience. 2001;1(1):66–82. doi: 10.3758/cabn.1.1.66. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. Journal of Neuroscience. 2004;24(6):1288–1295. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. Plasticity, hippocampal place cells, and cognitive maps. Archives of Neurology. 2001;58(6):874–881. doi: 10.1001/archneur.58.6.874. [DOI] [PubMed] [Google Scholar]
- Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behavioral Neuroscience. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nature Reviews. Neuroscience. 2007;8(11):872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Petrovich JD. What is the amygdale? Trends in Neuroscience. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Taylor KI, Moss HE, Stamatakis EA, Tyler LK. Binding crossmodal object features in perirhinal cortex. Proceedings of the National Academy of Sciences of the USA. 2006;103(21):8239–8244. doi: 10.1073/pnas.0509704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. The hippocampal memory indexing theory. Behavioral Neuroscience. 1986;100(2):147–154. doi: 10.1037//0735-7044.100.2.147. [DOI] [PubMed] [Google Scholar]
- Teyler TJ, Rudy JW. The hippocampal indexing theory and episodic memory: updating the index. Hippocampus. 2007;17(12):1158–1169. doi: 10.1002/hipo.20350. [DOI] [PubMed] [Google Scholar]
- Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. Functional specialization in rhesus monkey auditory cortex. Science. 2001;292:290–293. doi: 10.1126/science.1058911. [DOI] [PubMed] [Google Scholar]
- Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of the discontiguous events. Trends in Neuroscience. 1998;21(8):317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- Weiss C, Kronforst-Collins MA, Disterhoft JF. Activity of hippocampal pyramidal neurons during trace eye blink conditioning. Hippocampus. 1996;6:192–209. doi: 10.1002/(SICI)1098-1063(1996)6:2<192::AID-HIPO9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Behne NS, Fanselow MS. Sex differences, context preexposure, and the immediate shock deficit in Pavlovian context conditioning with mice. Behavioral Neuroscience. 2001;115(1):26–32. doi: 10.1037/0735-7044.115.1.26. [DOI] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Anagnostaras SG, Sage JR, Fanselow MS. Context fear learning in the absence of the hippocampus. Journal of Neuroscience. 2006;26(20):5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiology of Learning and Memory. 2007;87(4):464–475. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Young BJ, Otto T, Fox GD, Eichenbaum H. Memory representation within the parahippocampal region. Journal of Neuroscience. 1997;17(13):5183–5195. doi: 10.1523/JNEUROSCI.17-13-05183.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Memory impairment in monkeys following lesions limited to the hippocampus. Behavioral Neuroscience. 1986;100(2):155–160. doi: 10.1037//0735-7044.100.2.155. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.