Abstract
Many insects possess a sexual communication system that is vulnerable to chemical espionage by parasitic wasps. We recently discovered that a hitch-hiking (H) egg parasitoid exploits the antiaphrodisiac pheromone benzyl cyanide (BC) of the Large Cabbage White butterfly Pieris brassicae. This pheromone is passed from male butterflies to females during mating to render them less attractive to conspecific males. When the tiny parasitic wasp Trichogramma brassicae detects the antiaphrodisiac, it rides on a mated female butterfly to a host plant and then parasitizes her freshly laid eggs. The present study demonstrates that a closely related generalist wasp, Trichogramma evanescens, exploits BC in a similar way, but only after learning. Interestingly, the wasp learns to associate an H response to the odors of a mated female P. brassicae butterfly with reinforcement by parasitizing freshly laid butterfly eggs. Behavioral assays, before which we specifically inhibited long-term memory (LTM) formation with a translation inhibitor, reveal that the wasp has formed protein synthesis-dependent LTM at 24 h after learning. To our knowledge, the combination of associatively learning to exploit the sexual communication system of a host and the formation of protein synthesis-dependent LTM after a single learning event has not been documented before. We expect it to be widespread in nature, because it is highly adaptive in many species of egg parasitoids. Our finding of the exploitation of an antiaphrodisiac by multiple species of parasitic wasps suggests its use by Pieris butterflies to be under strong selective pressure.
Keywords: egg parasitoid, memory, phoresy, Pieris, Trichogramma
A wide variety of animals exploit the sexual communication system of their prey or hosts (1–3). Eavesdropping on sex pheromone signals from virgin host insects has been shown for several parasitic wasp species (4, 5). In contrast, antiaphrodisiac pheromones, signaling that mating has taken place, have almost completely been neglected in research on chemical espionage to date. Antiaphrodisiacs have evolved in males of a wide range of insects to enhance their postmating success by reducing the attractiveness of females to conspecific mates (6–15). The use of an antiaphrodisiac is favored in males when they compete for females. Mated females benefit by not being harassed by other males, thereby increasing their investment in oviposition (O) (16). However, using an antiaphrodisiac may incur fitness costs to both males and females when it is exploited by natural enemies. To egg parasitoids (parasitic wasps that parasitize eggs of other insects), antiaphrodisiacs are direct indicators of future host egg availability, whereas sex pheromones represent indirect information. Such tiny wasps that have limited control over flight direction would derive an adaptive benefit by exploiting an antiaphrodisiac, even more so when it is followed by mounting the mated adult female host and transportation (phoresy) by the latter. Phoresy is defined as the transport of certain organisms on the bodies of others for purposes other than direct parasitism of the transporting individual(s) (17). Several arthropod species are known to be phoretic (18, 19), including egg parasitoids (20–23).
Recently, we verified the existence of such a strategy by using the tiny (± 0.5-mm long) wasp Trichogramma brassicae and one of its hosts, the Large Cabbage White butterfly Pieris brassicae. The gregarious butterfly P. brassicae lays clutches of 20–50 eggs on wild and cultivated Brassica species (24). Male P. brassicae butterflies synthesize an antiaphrodisiac, benzyl cyanide (BC), that is transferred to the females during mating within their ejaculate (11). Female T. brassicae wasps were shown to spy on BC innately, to find mated P. brassicae females marked with this pheromone, and using them as a transport vehicle to find and parasitize the freshly laid eggs of butterflies (25). The prevalence of this sophisticated strategy in nature is still unknown. Host location by chemical espionage on an antiaphrodisiac in combination with transportation on an adult mated female host may have evolved frequently in parasitic wasps and imposes constraints on the evolution of sexual communication in hosts. After confirming the espionage-and-ride strategy in T. brassicae, follow-up bioassays were performed in the laboratory with its close relative Trichogramma evanescens. This generalist wasp is known to parasitize eggs of a wide range of Lepidoptera (e.g., Cabbage White butterflies) (26). It became evident that naive T. evanescens wasps neither respond to odors of mated P. brassicae females nor specifically mount them (27). However, parasitic insects can learn to associate chemical cues with the presence of hosts or food (28–30). Associative learning is certainly expected in generalist parasitoids as an innate response to a specific host cue would result in a decreased probability to find eggs of other potential host species (29).
In this study, we investigated whether T. evanescens can associatively learn to exploit the antiaphrodisiac pheromone BC of P. brassicae, and ride on a mated female butterfly. The wasps were exposed to an operant conditioning procedure with positive reinforcement, where approaching and mounting a mated female butterfly on the odorous stimulus is followed by reinforcement through parasitizing a clutch of freshly laid butterfly eggs. This “rewarding hitch-hiking (H) experience” is hypothesized to mimic a successful ride with a mated female butterfly in the field. Also, we were interested in memory formation after learning. The dynamics of memory formation are likely to be adapted to the foraging requirements and ecological constraints of an animal in nature (31–33). Information acquired during a learning event can be stored in temporally distinct memory forms that are remarkably similar across different animal taxa (34). Early phase, short-term memory (STM) can be inhibited by anesthesia. Besides STM, 2 different forms of longer lasting memory have been described that are both resistant to anesthesia. Long-term memory (LTM) requires gene-expression and/or protein synthesis, whereas anesthesia-resistant memory (ARM) does not (35). With a few known exceptions, LTM is generally formed only after repeated learning events spaced in time (33, 36–41). We hypothesized that LTM acquisition already after a single rewarding H experience is especially adaptive for T. evanescens. A few limited opportunities to hitch-hike with a mated female of a gregarious butterfly species (like P. brassicae) should be enough to lay all of the eggs a female wasp produces during her rather short lifespan (27, 42). Here, we tested whether T. evanescens wasps have formed protein synthesis-dependent LTM at 1 and 24 h after 1 rewarding H experience.
Results
Associative Learning.
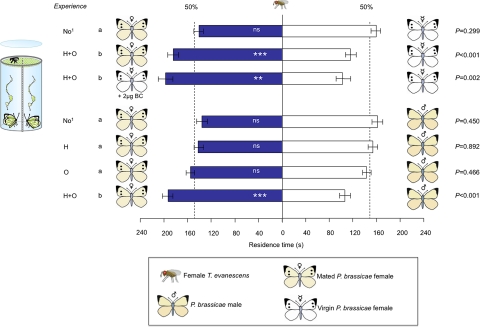
In 2-choice olfactory bioassays, naive female wasps and wasps that only had either a H or an O experience did not discriminate between the odors of mated females and males of P. brassicae (P = 0.450, P = 0.892, and P = 0.466, respectively, Wilcoxon's matched pairs signed-ranks test; see Fig. 1). In contrast, 1 h after a rewarding H experience (H+O) with a mated female butterfly, wasps were significantly arrested by the scent of mated female butterflies, when tested against the scent of male and virgin female butterflies (P < 0.001, Wilcoxon's matched pairs signed-ranks test; see Fig. 1). H+O-experienced wasps were also arrested by the odor of virgin female butterflies treated with 2 μg of synthetic BC when solvent-treated virgin females were offered as alternative (P = 0.002, Wilcoxon's matched pairs signed-ranks test; see Fig. 1). An H+O experience significantly shifted the odor preference of wasps toward mated female butterfly odors [general linear model (GLM), F6,273 = 8.80; P < 0.001]. These data indicate that only H+O-experienced wasps learned to associate the antiaphrodisiac BC of mated female P. brassicae butterflies with the presence of suitable host eggs.
Fig. 1.
Response of differently experienced T. evanescens wasps to odors of adults of the Large Cabbage White butterfly P. brassicae. Mean residence time (± SEM.) in the 2 odor fields of a 2-chamber olfactometer; n = 40 wasps tested per experiment; H, wasps were given an H experience 1 h before experiment; O, wasps were given an O experience 1 h before experiment; H+O, wasps were given an H experience followed by an O experience 1 h before experiment. Asterisks indicate significant differences within a choice test (Wilcoxon matched pairs signed-ranks test); **, P < 0.01; ***, P < 0.001; ns, not significant; a and b indicate significant differences between choice tests; 1, data of naive wasps are in agreement with previous work where another Dutch T. evanescens strain was used (27).
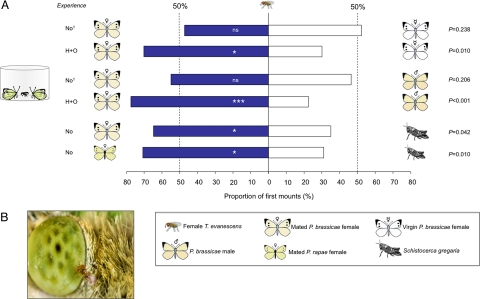
In subsequent behavioral 2-choice trials, we investigated whether T. evanescens wasps mount mated P. brassicae females in response to the antiaphrodisiac BC after an H+O experience. Naive wasps did not discriminate in climbing onto mated female, virgin female, and male butterflies (P = 0.238 and P = 0.206, respectively, binomial test; see Fig. 2). They do prefer to climb onto P. brassicae and Pieris rapae butterflies when nonhosts (L3 and L4 instars of the desert locust Schistocerca gregaria) were offered as alternatives (P = 0.042 and P = 0.010, respectively, binomial test; see Fig. 2). However, wasps that received an H+O experience 1 h before the bioassays significantly preferred to climb onto mated female butterflies when offered against virgin female and male butterflies (P = 0.010 and P < 0.001, respectively, binomial test; see Fig. 2). Here, an H+O experience significantly shifted the preference wasps toward mounting mated female butterflies (χ2 = 8.80; P = 0.003).
Fig. 2.
Mounting behavior of T. evanescens. (A) Proportion of first mounts of naive and experienced T. evanescens wasps on adults of the Large Cabbage White butterfly P. brassicae, the Small Cabbage White butterfly P. rapae, and young instars of the desert locust S. gregaria. Experienced wasps were given an H experience followed by an O experience (H+O) 1 h before the experiment; n = 40 climbing wasps tested per combination. (B) Female Trichogramma wasp of ≈0.5 mm touching the eye of a mated P. brassicae female (credits: Nina E. Fatouros, www.bugsinthepicture.com). Asterisks indicate significant differences within a choice test (binomial test); *, P < 0.05; ***, P < 0.001; ns, not significant; 1, data of naive wasps are in agreement with previous work where another Dutch T. evanescens strain was used (27).
Memory Formation.
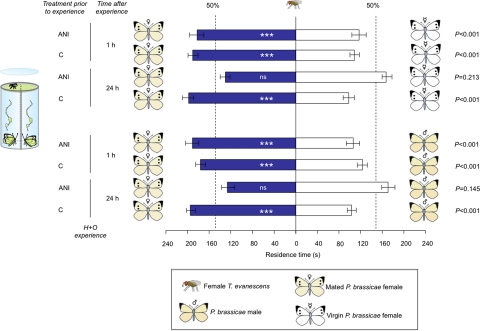
The observed 1-h memory retention of T. evanescens for the odors of mated female P. brassicae butterflies, and virgin P. brassicae females painted with the antiaphrodisiac BC after an H+O experience (Fig. 1), is probably based on STM. To test whether T. evanescens forms protein synthesis-dependent LTM formation, we fed wasps the translation-inhibitor anisomycin (ANI) in a sucrose solution, or only a sucrose solution before a rewarding H experience. ANI treatment and time after the H+O experience together significantly affected the odor preference of wasps (GLM, F1,157 = 24.85; P < 0.001), whereas it did not matter whether mated female butterflies were offered against either male or virgin female butterflies (GLM, F1,157 = 0.21; P = 0.644). ANI treatment did not affect 1-h memory retention, because wasps that were fed sucrose with ANI 1 h before the experience also had a significant preference for mated female butterfly odors, similar to the control wasps that had been offered sucrose only (Fig. 3). Sucrose-fed wasps also had the same odor preference 24 h after an H+O experience. In contrast, wasps that were fed an ANI-containing sucrose solution did not discriminate between the odors of mated female, male, and virgin female butterflies 24 h after the same experience (Fig. 3). Consequently, these data demonstrate that T. evanescens has formed protein synthesis-dependent LTM 24 h after 1 H+O-learning event.
Fig. 3.
Effects of the translation-inhibitor ANI on the response of T. evanescens wasps to odors of adults of the Large Cabbage White butterfly P. brassicae at 1 h and 24 h after a rewarding H experience (H+O). Mean residence time (± SEM.) in 2 odor fields in a 2-chamber olfactometer; n = 40 wasps tested per experiment; ANI, wasps were fed sucrose plus ANI before experience; C, control wasps were fed only sucrose before experience. Asterisks indicate significant differences within a choice test (Wilcoxon matched pairs signed-ranks test); ***, P < 0.001; ns, not significant.
Discussion
Our data demonstrate that T. evanescens learns to exploit a butterfly antiaphrodisiac, and to ride on a mated female butterfly, after a rewarding H experience with the latter. In contrast, it does not learn after either only an H or only an O experience. Obviously, the wasp learns to associate its response to the odors of a mated female butterfly labeled with an antiaphrodisiac, with an O into host eggs. Associative learning is especially expected in parasitic wasps that attack a wide range of host species. Trichogramma wasps are widely considered as being true generalists (43), and have indeed been shown to associatively learn to respond to plant volatiles and female sex pheromones of moths (44, 45). However, in contrast to T. evanescens, its close relative T. brassicae does show an innate response to the antiaphrodisiac BC of P. brassicae (25). We hypothesize that the difference in response to the same host cue is caused by differences in the actual host range of both wasps in the field. P. brassicae can be a common or high-ranked host in the field for T. brassicae (25), but not for the T. evanescens strains used in the present study and in previous work (27). Future studies need to further characterize the natural host range of both species, especially for the populations from which the laboratory strains originate.
The present study represents a step toward elucidating the mechanisms underlying memory formation in egg parasitoids. We showed that T. evanescens acquired protein synthesis-dependent LTM at 24 h, but not at 1 h, after 1 learning event. Early phase memory at 1 h after learning is probably based on STM. Protein synthesis-dependent LTM acquisition after a single learning event has only been shown in 2 larval parasitoids, Cotesia glomerata and Lariophagus distinguendus (33, 41). Obviously, even animals as tiny as Trichogramma spp. are able to form this type of LTM, despite its high energetic costs (46). It remains to be investigated up to how long the protein synthesis-dependent form of LTM is consolidated, and whether it coexists with other traces of longer lasting memory as is known from Cotesia parasitic wasps and Drosophila flies (33, 36). In Trichogramma wasps, preference for a certain host can persist for at least 5 days after an O experience in that host (47), whereas other learning effects appear to be extremely short lived. The response of Trichogramma maidis to a combination of maize, host egg, and host pheromone odor, for example, only lasted for several minutes after an O experience in the presence of that odor combination (48).
The sophisticated combination of chemical espionage on an antiaphrodisiac with phoretic transportation on an adult mated female host is expected to be widespread in nature as it is highly adaptive in numerous species of egg parasitoids. In many generalist species, the espionage-and-ride strategy may only be acquired after learning. Both associative learning and phoresy are widespread phenomena in parasitic wasps (18, 23, 28–30). Recently, we have shown that the antiaphrodisiac of P. brassicae, BC, can also indirectly lure parasitic wasps. When deposited onto Brussels sprouts plants by female P. brassicae butterflies during egg deposition, BC elicits an indirect plant defense that recruits T. brassicae wasps to the eggs (49). The recruitment of different Trichogramma wasp species indirectly via induced plant defense and directly by facilitating phoretic behavior might put the use of BC by the butterflies under even stronger selective pressure than previously assumed (25, 49). Future studies should investigate whether natural variation in the use of this sexual communication signal is correlated with the egg mortality inflicted by parasitic wasps.
Materials and Methods
Insects.
P. brassicae L. and P. rapae L. (Lepidoptera: Pieridae) were reared on Brussels sprouts plants (Brassica oleracea L. var. gemmifera cv. Cyrus) in a climate room (21 ± 1 °C, 50–70% r.h., L16:D8). Virgin females were obtained by sexing in the pupal phase. Mated females and males were taken as copulating pairs (25, 27). Larval instars (L3 and L4) of the desert locust S. gregaria Forskal (Orthoptera: Acrididae) (with approximately the same size as the butterflies that they were tested against) were obtained from a culture at the Laboratory of Entomology, Wageningen University, The Netherlands. T. evanescens Westwood (Hymenoptera: Trichogrammatidae) (iso-female strain GD011) originated from a P. rapae egg collected in 2006 in a cabbage field in Wageningen, The Netherlands. Since then, it was reared in eggs of the moth Ephestia kuehniella under laboratory conditions (25 ± 1 °C, 50–70% rh, L16:D8). Only mated, 2-days-old female wasps were used in the bioassays.
Associative Learning.
To test whether the observed experience-induced preference changes are the result of associative learning, and not sensitization, we tested the following wasps: N, naive wasps; O, wasps that were only given an O experience 1 h before a bioassay in a <24-h old group of P. brassicae eggs deposited on Brussels sprouts leaves; H, wasps that were only given an H experience by mounting a mated female P. brassicae butterfly, remaining on it during a short simulated flight (the butterfly was relocated with a pair of weak forceps); and, last, descending it, 1 h before a bioassay, and H+O wasps that were given a rewarding H experience 1 h or 24 h before a bioassay, i.e., an H experience followed by O into a <24-h old group of P. brassicae eggs deposited on Brussels sprouts leaves. All experiences were conducted in a plastic container (9 cm high, 13.5 cm in diameter) covered with a glass Petri dish. An H experience lasted from 1 to 5 min. O lasted until a wasp had parasitized a few eggs, up to a maximum of 10 min after the first leaf contact.
Memory Formation.
To inhibit the formation of protein synthesis-dependent LTM (but not STM), 1 day-old naive wasps were deprived of food [honey diluted in water (3:1)] and then fed overnight on 30 μL of a 10 mM solution of the translation-inhibitor ANI (Sigma) in a 2% sucrose solution, until the start of a rewarding H experience (33). Control wasps were given the same treatment, except that the sucrose solution did not contain ANI.
Response to Butterfly Odors.
In a 2-chamber olfactometer, 2 adult P. brassicae butterflies per chamber were introduced as odor source (25, 27). The time spent by the wasps in 1 of the 2 odor fields was observed for 300 s. Each day 10–15 wasps were tested, until a total of 40 wasps per combination was reached. The olfactometer was rotated 180° after every third insect to compensate for any unforeseen asymmetry in the setup. After each third wasp tested, the butterflies were replaced with new ones.
Response to Butterfly Antiaphrodisiac.
In a similar 2-choice olfactory bioassay, the response of wasps toward the antiaphrodisiac BC of mated P. brassicae females was tested. A virgin female was painted with 10 μL of 0.2 μg/μL BC (Aldrich, purity 99%) solution in hexane and tested against a virgin female treated with 10 μL of hexane only (25). The butterflies were replaced with newly painted ones after each third wasp tested.
Mounting on Butterfly.
Mounting behavior of T. evanescens wasps was investigated in 2-choice bioassays conducted in a plastic container (9 cm high, 13.5 cm in diameter) covered with a glass Petri dish; 2 adult butterflies were placed in the arena after cooling down in a 4 °C-refrigerator to decrease their mobility. A wasp was introduced in between the butterflies and continuously observed until it climbed onto 1 of the 2 butterflies. When a wasp did not mount a butterfly within 300 s, a “no response” was recorded. After each third wasp, the butterflies were replaced. For each combination, 40 climbing wasps were investigated.
Statistical Analysis.
To compare the residence times of wasps in the 2 odor fields within the olfactory bioassays, a Wilcoxon matched pairs signed-ranks test was used. For the comparison of residence times of wasps that received different treatments, GLM procedures were used in SAS version 8.02. The time spent in the odor field of mated females was used as dependent variable, because distributions of residence times in the 2 odor fields were linearly related, and bioassays had the same duration (300 s). For the data on associative learning, a test of homogeneity of treatment groups was carried out, followed by multiple comparisons of least square means of treatment groups. Significance level was determined after Tukey adjustment of α-values to correct for type I error. When analyzing data of memory formation, a factorial design was considered, by which presence/absence of ANI treatment was tested at both time points after an H+O experience (1 h/24 h), for both mated female butterflies tested against male and virgin female butterflies. Nonsignificant interactions were omitted from the final model.
To determine whether preferences of wasps to climb onto a butterfly in the 2-choice mounting bioassays were significantly different from a nonpreference situation, a 2-tailed binomial test was used. When analyzing the differences in the choice distributions of wasps mounting a butterfly across the treatments, a Generalized Linear Model with a logit-link function and binomial distribution of error variance (PROC GENMOD, SAS) was used. Only wasps that mounted a butterfly were considered in the bioassay, and wasps that had not made a choice within 300 s were excluded from the analysis. A choice of wasp for the butterfly was considered the dependent variable with binary coding, and the probability of mounting a mated female butterfly was modeled with experience levels and combinations of alternative butterflies (either a virgin female or a male butterfly) as factors. Significant differences were based on likelihood-ratio statistics. All tests were carried out at α = 0.05 by using SAS version 8.02.
Acknowledgments.
We thank Marion Munneke, Silja Tribuhl, and Joop Woelke for assistance with the experiments; and Leo Koopman, Frans van Aggelen, and André Gidding for rearing the insects at the Laboratory of Entomology, Wageningen University, Wageningen, The Netherlands. This work was supported by The Netherlands Organisation for Scientific Research/Earth and Life Sciences (NWO/ALW) Veni Grant 86305020 (to M.E.H.), Deutsche Forschungsgemeinschaft Grant FA 824/1-11 (to N.E.F.), and People's Republic of China State Administration of Foreign Experts Affairs Grant CG2007440005 (to M.-H.Q.).
Footnotes
The authors declare no conflict of interest.
References
- 1.Vinson SB. In: Insect Communication. Lewis T, editor. London: Academic; 1984. pp. 325–348. [Google Scholar]
- 2.Stowe MK, Turlings TCJ, Loughrin JH, Lewis WJ, Tumlinson JH. The chemistry of eavesdropping, alarm and deceit. Proc Natl Acad Sci USA. 1995;92:23–28. doi: 10.1073/pnas.92.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zuk M, Kolluru GR. Exploitation of sexual signals by predators and parasitoids. Quart Rev Biol. 1998;73:415–438. [Google Scholar]
- 4.Powell W. In: Pheromones of Non-Lepidopteran Insects Associated With Agricultural Plants. Hardie J, Minks AK, editors. Wallingford, United Kingdom: Centre for Agricultural Bioscience International Publishing; 1999. pp. 405–427. [Google Scholar]
- 5.Fatouros NE, Dicke M, Mumm R, Meiners T, Hilker M. Foraging behavior of egg parasitoids exploiting chemical information. Behav Ecol. 2008;19:677–689. [Google Scholar]
- 6.Happ GM. Multiple sex pheromones of the mealworm beetle, Tenebrio molitor L. Nature. 1969;222:180–181. doi: 10.1038/222180a0. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert LE. Postmating female odor in Heliconius butterflies: A male-contributed antiaphrodisiac? Science. 1976;193:419–420. doi: 10.1126/science.935877. [DOI] [PubMed] [Google Scholar]
- 8.Kukuk P. Evidence for an antiaphrodisiac in the sweat bee Lasioglossum zephyrum (Dialictus) Science. 1985;227:656–657. doi: 10.1126/science.3969557. [DOI] [PubMed] [Google Scholar]
- 9.Andersson J, Borg-Karlson A-K, Wiklund C. Sexual cooperation and conflict in butterflies: A male-transferred anti-aphrodisiac reduces harassment of recently mated females. Proc R Soc London Ser B. 2000;267:1271–1275. doi: 10.1098/rspb.2000.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simmons LW. Sperm Competition and its Evolutionary Consequences in the Insects. Princeton and Oxford: Princeton Univ Press; 2001. [Google Scholar]
- 11.Andersson J, Borg-Karlson A-K, Wiklund C. Antiaphrodisiacs in Pierid butterflies: A theme with variation! J Chem Ecol. 2003;29:1489–1499. doi: 10.1023/a:1024277823101. [DOI] [PubMed] [Google Scholar]
- 12.Zhang QH, Aldrich JR. Male-produced anti-sex pheromone in a plant bug. Naturwissenschaften. 2003;90:505–508. doi: 10.1007/s00114-003-0466-8. [DOI] [PubMed] [Google Scholar]
- 13.Andersson J, Borg-Karlson A-K, Wiklund C. Sexual conflict and anti-aphrodisiac titre in a polyandrous butterfly: Male ejaculate tailoring and absence of female control. Proc R Soc London Ser B. 2004;271:1765–1770. doi: 10.1098/rspb.2003.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kyriacou CP. Sex, flies and acetate. Nature. 2007;446:502–504. doi: 10.1038/446502a. [DOI] [PubMed] [Google Scholar]
- 15.Schulz S, Estrada C, Yildizhan S, Bopprž M, Gilbert LE. An antiaphrodisiac in Heliconius melpomene butterflies. J Chem Ecol. 2008;34:82–93. doi: 10.1007/s10886-007-9393-z. [DOI] [PubMed] [Google Scholar]
- 16.Forsberg J, Wiklund C. Mating in the afternoon: Time-saving in courtship and remating by females of a polyandrous butterfly Pieris napi. Behav Ecol Sociobiol. 1989;25:349–356. [Google Scholar]
- 17.Howard LO. Concerning phoresy in insects. Entomol News. 1927;38:145–147. [Google Scholar]
- 18.Clausen CP. Phoresy among entomophagous insects. Ann Rev Entomol. 1976;21:343–368. [Google Scholar]
- 19.Saul-Gershenz LS, Millar JG. Phoretic nest parasites use sexual deception to obtain transport to their host's nest. Proc Natl Acad Sci USA. 2006;103:14039–14044. doi: 10.1073/pnas.0603901103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arakaki N, Wakamura S, Yasuda T. Phoretic egg parasitoid, Telenomus euproctidis (Hymenoptera: Scelionidae), uses sex pheromone of Tussock moth Euproctis taiwana (Lepidoptera: Lymantriidae) as a kairomone. J Chem Ecol. 1996;22:1079–1085. doi: 10.1007/BF02027946. [DOI] [PubMed] [Google Scholar]
- 21.Vinson SB. The general host selection behavior of parasitoid Hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Cont. 1998;11:79–96. [Google Scholar]
- 22.Bruni R, Sant'Ana J, Aldrich JR, Bin F. Influence of host pheromone on egg parasitism by scelionid wasps: Comparison of phoretic and nonphoretic parasitoids. J Insect Behav. 2000;12:165–173. [Google Scholar]
- 23.Fatouros NE. Berlin: Free University; 2006. Parasitic Wasps on Butterfly Expedition: Foraging Strategies of Egg and Larval Parasitoids Exploiting Infochemicals of Brussel sprouts and their Pieris Hosts. PhD thesis. [Google Scholar]
- 24.Feltwell J. Large White Butterfly: The Biology, Biochemistry and Physiology of Pieris brassicae (Linnaeus) The Hague-Boston-London: Dr. W. Junk Publishers; 1982. [Google Scholar]
- 25.Fatouros NE, Huigens ME, van Loon JJA, Dicke M, Hilker M. Chemical communication: Butterfly anti-aphrodisiac lures parasitic wasps. Nature. 2005;433:704. doi: 10.1038/433704a. [DOI] [PubMed] [Google Scholar]
- 26.Pak GA, Kaskens JWM, de Jong EJ. Behavioural variation among strains of Trichogramma spp.: Host-species selection. Entomol Exp Appl. 1990;56:91–102. [Google Scholar]
- 27.Fatouros NE, Bukovinszkine'Kiss G, Dicke M, Hilker M. The response specificity of Trichogramma egg parasitoids towards infochemicals during host location. J Insect Behav. 2006;20:53–65. [Google Scholar]
- 28.Turlings TCJ, Wäckers FL, Vet LEM, Lewis WJ, Tumlinson JH. In: Insect Learning. Papaj DR, Lewis AC, editors. New York: Chapman and Hall; 1993. pp. 51–78. [Google Scholar]
- 29.Vet LEM, Lewis WJ, Cardž RT. In: Chemical Ecology of Insects 2. Cardé RT, Lewis WJ, editors. New York: Chapman and Hall; 1995. pp. 65–101. [Google Scholar]
- 30.Steidle JLM, van Loon JJA. Dietary specialization and infochemical use in carnivorous arthropods: Testing a concept. Entomol Exp Appl. 2003;108:133–148. [Google Scholar]
- 31.Menzel R. Memory dynamics in the honeybee. J Comp Physiol A. 1999;185:323–340. [Google Scholar]
- 32.Menzel R. In: Gognitive Ecology of Pollination: Animal Behavior and Floral Evolution. Chittka L, Thompson JD, editors. Cambridge: Cambridge Univ Press; 2001. pp. 21–40. [Google Scholar]
- 33.Smid HM, et al. Species-specific acquisition and consolidation of long-term memory in parasitic wasps. Proc R Soc London Ser B. 2007;274:1539–1546. doi: 10.1098/rspb.2007.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubnau J. Neurogenetic dissection of conditioned behavior: Evolution by analogy or homology? J Neurogenet. 2003;17:295–326. doi: 10.1080/01677060390441859. [DOI] [PubMed] [Google Scholar]
- 35.Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Curr Biol. 2005;15:R700–R713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in the Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 37.Bailey CH, Bartsch D, Kandel ER. Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA. 1996;93:13445–13452. doi: 10.1073/pnas.93.24.13445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menzel R. Searching for the memory trace in a minibrain, the honeybee. Learn Mem. 2001;8:53–62. doi: 10.1101/lm.38801. [DOI] [PubMed] [Google Scholar]
- 39.Igaz LM, Vianna MRM, Medina JH, Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulton D, Kemenes I, Andrew RJ, Benjamin PR. A single time-window for protein-synthesis dependent long-term memory formation after one-trial appetitive conditioning. Eur J Neurosci. 2005;21:1347–1358. doi: 10.1111/j.1460-9568.2005.03970.x. [DOI] [PubMed] [Google Scholar]
- 41.Collatz J, Müller C, Steidle JLM. Protein synthesis-dependent long-term memory induced by one single associative trial in the parasitic wasp Lariophagus distinguendus. Learn Mem. 2006;13:263–266. doi: 10.1101/lm.192506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doyon J, Boivin G. The effect of development time on the fitness of female Trichogramma evanescens. J Insect Sci. 2005;5:1–5. doi: 10.1093/jis/5.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wajnberg E, Hassan SA. Biological Control With Egg Parasitoids. Wallingford, United Kingdom: Centre for Agricultural Bioscience International Publishing; 1995. [Google Scholar]
- 44.Kaiser L, Pham-Delegue MH, Masson C. Behavioural study of plasticity in host preferences of Trichogramma maidis (Hym.: Trichogrammatidae) Physiol Entomol. 1989;14:53–60. [Google Scholar]
- 45.Schšller M, Prozell S. Response of Trichogramma evanescens to the main sex pheromone component of Ephestia spp. and Plodia interpunctella, (Z,E)-9,12-tetra-decadenyl acetate (ZETA) J Stor Prod Res. 2002;38:177–184. [Google Scholar]
- 46.Mery F, Kawecki TJ. A cost of long-term memory in Drosophila. Science. 2005;308:1148. doi: 10.1126/science.1111331. [DOI] [PubMed] [Google Scholar]
- 47.Bjorksten TA, Hoffmann AA. Persistence of experience effects in the parasitoid Trichogramma nr. brassicae. Ecol Entomol. 1998;23:110–117. [Google Scholar]
- 48.Kaiser L, Pham-Delegue MH, Bakchine E, Masson C. Olfactory responses of Trichogramma maidis Pint. et. Voeg.: Effects of chemical cues and behavioral plasticity. J Insect Behav. 1989;2:701–712. [Google Scholar]
- 49.Fatouros NE, et al. Male-derived butterfly anti-aphrodisiac mediates induced indirect plant defense. Proc Natl Acad Sci USA. 2008;105:10033–10038. doi: 10.1073/pnas.0707809105. [DOI] [PMC free article] [PubMed] [Google Scholar]