INTRODUCTION
The endogenous endothelin (ET) peptides participate in a remarkable variety of pain-related processes. Pain that is elevated by inflammation, by skin incision, by cancer, during a Sickle Cell Disease crisis and by treatments that mimic neuropathic and inflammatory pain are all reduced by local administration of antagonists of endothelin receptors. Many acute effects of endogenously released endothelin can be simulated by local administration of endothelin, which at high concentrations causes pain and at lower concentrations sensitizes the nocifensive reactions to mechanical, thermal and chemical stimuli.
This review covers the topic of ET-1 and pain in five sections. The first section describes the biochemistry, second messenger pathways and receptor coupling that are activated by ET receptors. The second section summarizes the cellular physiological responses to ET receptor activation, emphasizing those neuronal responses that might account for nociceptor activation or sensitization. The third section begins with a description of the pharmacological analysis of receptors involved in pain caused by activation of the different ET receptors by exogenous ET-1, and then segues to a description of the neurophysiological (and vascular) effects in vivo. The final two sections review the evidence for the involvement of endogenous ET-1 and its receptors in pain arising from peripheral inflammation, injury and diseases, including cancer, and finally, the modulation of pain by ET receptors in the CNS. Our goal is to broadly frame the subject of endothelin and pain and to present the generally accepted as well as the disputed concepts, including important unanswered questions.
1. BIOCHEMICAL PROPERTIES
1.A. Structure of endothelins and their receptors
Endothelins are a family of naturally occurring peptides 261, with growth-promoting, vasoactive and nociceptive properties affecting the function of a number of tissues and systems, including somatosensory, respiratory, circulatory, endocrine, urogenital, visual, digestive and central nervous systems 204. To date, there are three known, 21-aminoacid long endothelins in humans (i.e. ET-1, ET-2 and ET-3) produced from as many distinct genes located on chromosomes 6, 1 and 20, respectively 24. Genes for ET-1, ET-2, ET-3 are also present in bovine and porcine tissues, suggesting that endothelins are produced in all mammalian species 111, 208 Endothelins have overlapping tissue distribution with ET-1 being the most widely expressed. 148, 224 Endothelin-1 is synthesized and released by epithelia, 1 and also produced by cardiomyocytes, leukocytes and macrophages, 143 endothelial cells 149.
Endothelin-1’s actions are primarily local, in a paracrine (ET-1 secreted by one cell acts on neighboring cells) or autocrine (ET-1 released acts on the cell producing it) manner. The immediate actions are usually brief, since all endothelins are rapidly degraded by extracellular proteases, the endothelin converting enzymes (ECE’s).53, 109 Endothelins share high homology with sarafotoxins, a family of cardiotoxic peptides from snake. 225
All endothelins and sarafotoxins act via two subtypes of G protein-coupled receptors, termed ETA 5 and ETB, 209 which are encoded by two distinct genes found on chromosomes 4 (4q31.2) and 13 (13q22), respectively. 210 The human ETA and ETB receptor share ~51% amino acid sequence identity, with higher homology in the transmembrane regions 64. In particular, transmembrane domains 2 and 5 have been shown to play a crucial role in subtype-selective ligand binding and peptide docking. 25, 136, 174, 203, 241 Several splice receptors have been variants of ETA26, 96, 158 as well as ETB65, 165, 222 described for which physiological roles remain unconfirmed. The ETA receptor is abundant in vascular smooth muscle cells where its activation elicits an increase of intracellular calcium, leading to a long-lasting contraction. 152 The ETB receptor is predominantly found in endothelial cells where it stimulates the release of nitric oxide (NO) thereby causing the relaxation of vascular smooth muscle cells. 242 However, in cardiac tissue ETA receptors are also found in endothelial cells, where they mediate ET-1’s elevation of intracellular Ca2+ 114 while in the stomach ETB receptors are located on smooth muscle cells and may mediate contraction. 160 Despite these often separate and distinct tissue distributions, 105 ETA and ETB receptors also appear to be co-expressed in a number of cells, including smooth muscle cells, 99 astrocytes, 93 epithelial cells of the choroid plexus 4 and the anterior pituitary 92 and cardiac endothelial cells. 114
ETA and ETB receptors display different ligand selectivity. ETB binds ET-1, ET-2 and ET-3 with about equal affinities whereas ETA binds ET-1>ET-2≫ET-3 210. Specific competitive peptide or non-peptide antagonists of ETA as well as antagonists and agonists of ETB receptors have proven to be important tools to study the function of each receptor type 54.
Some evidence suggests an interaction between separate ETA and ETB receptors expressed in the same cells. In ETA receptor-transfected human Girardi heart cells, which intrinsically express ETB receptors, the stimulation of ETA receptors with ET-1 results in a lowering of the affinity for ETB receptors for both the agonist BQ-3020 and the antagonist BQ-788, indicating intracellular cross-talk between ETA and ETB receptors. 176 We hypothesize that such interactions between ET receptor subtypes could be of particular importance in pathological conditions and may contribute to determining which of the dual roles of ETB receptors, pro- or anti-algesic, is the dominant one (see 3.D., below).
1.B. G Protein coupling and intracellular pathways
The coupling of ET receptors to heterotrimeric G proteins has been extensively studied, mostly using heterologous expression systems, in an attempt to clarify the cellular pathways underlying the different actions of ETs (e.g., rapid intracellular signaling, mitogenesis, cell-growth, etc.). A survey of the literature indicates that both ETA and ETB receptors are able to couple to pertussis toxin-sensitive (Gαi) as well as insensitive (Gαq/11) families of G proteins, although the particular Gα-subunit depends on the cell type. 6, 106, 237 These findings are corroborated by a heterologous expression study of ETA or ETB in Chinese hamster lung fibroblasts (CCL39), reporting that the affinity for various G proteins varies between the ETA and ETB receptors, with Gαi3 interacting to a greater extent with ETA while Gαi1, Gαi2 and Gαq/11 are more likely to interact with ETB.221 Both receptors appear to couple to IP3 production through Gαq, ostensibly activating PLC and PLD 3, as well as to cAMP, through adenylyl cyclase inhibition, 226 or to cGMP 219, 220 through guanylyl cyclase activation by NO. 101 Moreover, ET-1 has been linked to the modulation of a plethora of signaling pathways, including the increase in cytosolic [Ca2+], 133 activation of phospholipase A2, 6 regulation of Na+/H+ exchange, 250 production of NO 232, extracellular-signal regulated kinase 1,2 (ERK1/2) activation, protein kinase C or phosphatidylinositol-3-kinase stimulation 97, 118, 171 and activation of the ras/raf/mitogen-activated protein kinase pathway. 125, 264 The pathways mediating the mitogenic activities of ETA receptors are being unraveled in relation to their role in cancer. 10 They involve protein kinase C as well as phosphatidylinositol-3-kinase, 218 while ETB receptor mediated cell proliferation appears to be mediated by a PKC-independent pathway. 231
Although heterologous expression systems have contributed much to our knowledge of receptor coupling, the association of endothelin receptors with different G proteins and the activation of signaling pathways in the various native cells, in normal and pathological conditions, remains largely unknown. One particular area concerns the ability of Gβγ subunits released by the activation of ET receptors to modulate the activity of effectors such as PLCβ, ACs, ion channels, kinases and components of the synaptic vesicle release machinery, all effects which occur with other GPCRs. 140, 157, 239
1.C. Desensitization, internalization and recycling
Both ET receptor types are desensitized by phosphorylation through the G protein–coupled receptor kinase type 2 (GRK2), 73 subsequently uncoupling the receptor from its G protein. Both ETA and ETB receptors are internalized via clathrin-dependent pathways with very different outcomes. ETA receptors are recycled back to the surface 29, 177, 178 whereas ETB receptors are sorted to the lysosome and degraded. 84, 172 This is seemingly linked, in neuronal cells, with the presence of a postsynaptic density-95/disc-large/zona occludens (PDZ) binding motif in the cytoplasmic carboxyl-terminal region of the ETA receptor that is lacking in the ETB receptor. 178
Clearance of ET-1 from the circulation is mediated largely through ETB receptors expressed on endothelial, lung and kidney cells by a mechanism involving uptake and subsequent degradation by neutral endopeptidase. 29, 59, 75, 119, 173 This is also the case in the central nervous system where the astrocytic ETB receptor is responsible for the elimination of accumulated extracellular ET-1, 94 a feature linked to the ability of ETB to form a much more stable complex with ET-1 than does ETA. 236
2. CELL PHYSIOLOGICAL RESPONSES TO ENDOTHELINS
2.A. Endothelin receptor coupling in pain
ETA receptors have been found on a large proportion of the cell bodies of small diameter sensory neurons (DRGs) which are associated with C- and Aδ-fibers that carry pain impulses. 192 Moreover, activation of these nociceptive fibers by ET-1 is blocked by BQ-123, an ETA receptor antagonist. 81 Together, these studies strongly argue for a selective role of peripheral ETA receptors in the induction of pain through nociceptive fibers.
The receptor pathways involved in pain generation by ET-1 are complex. ETA receptor activation sometimes elicits a rise in intracellular calcium 259, 272 which is probably mediated by Gαq/11, acting through PLC to release Ca+2 from intracellular stores, and also through stimulation of guanylyl cyclase (GC). The latter enzyme converts GTP to cGMP, which can activate protein kinase G, effecting an anti-nociceptive response. 207 (This pathway is complicated, however, since cGMP is also elevated by NO,60 a substance that has a positive role in mediating inflammatory hyperalgesia. 2 In addition, GTP itself enhances the activation of Na+ current in small sensory neurons, 11 in a PKC-dependent way, 12 an effect that would be diminished by the nucleotide’s conversion to cGMP.) From this complex of information it is not possible to determine the overall change in sensory processing that results from cGMP’s elevation by ET-1.
In addition to elevating Ca2+ in peripheral sensory neurons, ET-1 stimulates (Ca2+ - independent) PKC-ε mediated phosphorylation and concomitant functional enhancement of the TRPV1 channels present on nociceptive C-fibers. 188 Potentiation of TRPV1 most likely occurs in concert with a modulatory effect on tetrodotoxin (TTX) -resistant Na+ channels 273 (see 2.B., below), which is also known to be effected by PKC.82 The G protein α- or βγ-subunits mediating these effects are presently unknown, as is the identity of their direct target, although a Phospholipase C enzyme is a probable candidate (see Figure 1).
Figure 1.
Schematic of the actions of ET-1 on a sensory nerve ending and on local keratinocytes in the epidermis. ET-1 is secreted by many different resident and invading cells and binds to receptors on both nerve and skin cells. Activation of the GPCR ETA receptors on nerve cells results in acute changes in ion channels that enhance excitability: delayed rectifier (D-R) type K+ channels are inhibited, TTX-R Na+ channel activation is enhanced, as are TRPV1 channels. Calcium is released rapidly from intracellular stores and in turn activates PKC (and, possibly, PKA) as part of the coupling between ETA activation and its downstream targets. Keratinocytes express ETB receptors and, in addition to releasing ET-1, keratinocytes also release β-endorphin. That opioid, in turn, binds to μ-opiate receptors (MOR) on nerve endings, which are coupled through G-proteins to increase the outward currents through the G protein-coupled inward rectifier K+ channels (GIRK2). The hyperpolarization of the nerve’s membrane by this action lowers excitability by moving the resting potential away from threshold and by lowering the membrane resistance. This same GIRK may also be a target of NK1 receptors, activated by Substance P, but in this case the K+ channels close, the membrane depolarizes and the nerve becomes more excitable. (Dashed lines show speculative pathways; Circled + or − depict stimulatory or inhibitory effects.) After ref. 135.
ETB receptors appear to be mainly expressed in DRG satellite cells and ensheating Schwann cells 192 where it is thought that they induce the synthesis and release of prostaglandin E2, a compound involved in inflammatory pain. 135 ETB receptors are also found on keratinocytes. Importantly for pain, ETB receptors are known to mediate the release of β-endorphin from keratinocytes and thereby produce a local analgesic effect. 129 The signaling pathway responsible for this effect remains to be investigated, although Gs, Gi2 and Gi3 have all been detected in keratinocytes, the major cell type in the skin’s epidermis. 235 In these cells adenylyl cyclase activation is thought to mediate a rise in intracellular calcium 175 along a pathway which might lead to β-endorphin release (see Figure 2).
Figure 2.

Schematic of the signaling pathways initiated by endothelin-1 in keratinocytes. Endogenous production and subsequent release of ET-1 is proposed to activate resident ETA receptors which mediate cell proliferation as well as the release of noxious chemical stimuli such as glutamate and CGRP. ETB receptors on keratinocytes have a dual function: i. Acute stimulation of ET-1 release, followed by slow negative regulation of ET-1 synthesis and secretion, as part of an autoregulatory loop, ii. Acute stimulation of β-endorphin release, as part of the local analgesic action of endothelin-1. (Dashed lines show speculative pathways; Circled + or − depict stimulatory or inhibitory effects.)
2.B. In vitro effects on ion channels and transmitter release
Isolated sensory neurons are stimulated by ET-1 to elevate cGMP 60 and to release neuropeptides and glutamate, actions probably resulting from an ETA-dependent elevation of intracellular [Ca+2]. 60, 272
Primary cultures of keratinocytes also are stimulated by ET-1, with separate studies showing ETB receptor-mediated release of the opioid peptide β-endorphin 129 and of ET-1 itself. 265, 266 The latter comprises an autocrine loop, but with a biphasic dynamic response; immediately upon exposure to ET-1 keratinocytes show elevated intracellular Ca2+, which would be expected to cause an acute increase in the release of many substances, including ET-1 itself, but after prolonged (24 h) exposure to ET-1, its basal release by keratinocytes is suppressed, probably by negative feedback on its synthesis. 266 Without this dampening, counteracting mechanism, the initial release of a small amount of ET-1 would further activate ETB receptors on keratinocytes and continuously stimulate its own release. The β-endorphin released by keratinocytes exerts an analgesic and anti-hyperalgesic action, binding to μ-opiate receptors on the nociceptor nerve endings and opening G protein-sensitive K+ channels, thereby lowering excitability and the generation of action potentials.129
ET-1 biphasically modulates N- and L-type Ca2+ channels in neurons, causing an initial depression followed by a long lasting facilitation. 169 ET-1 also induces neuronal depolarization and non-selective inward cationic currents, both predominantly calcium-mediated, followed by hyperpolarization, perhaps due to Ca+2-activated K+ currents. 233 In addition, ET-1 is capable of increasing [Ca2+] in neurons with an important contribution from store-released Ca2+ ions. 272, 259
It is noteworthy that TRPV1 and other TRP receptors have been identified in keratinocytes 45, 227 which contain neuroactive substances that can be released by stimulation. 102, 227 One scenario for ET-1’s peripheral actions might involve its initial release from directly, externally stimulated keratinocytes and the autocrine activation of ET receptors on the same and adjacent keratinocytes, followed by the subsequent release of substances that would activate epidermal nerve endings, e.g., glutamate and CGRP (see below), amplified by the modulation of TRPV1 (and possibly, other TRPs) in both keratinocytes and neurons (Figure 2).
As noted above, the soma and axons of sensory neurons contain functional ETA receptors, visualized by immunocytochemistry, 192 and causing excitatory actions. ET-1 enhances excitation of isolated sensory nerve soma, particularly of small and medium diameter cells, at least in part by altering the activation of voltage-gated Na+ channels. 273 This action is prominent for the slowly inactivating, tetrodotoxin-resistant (TTX-R) Na+ channels that occur in nociceptive cells, e.g. Nav1.8 and Nav1.9. The TTX-sensitive channels, which are expressed predominantly in large sensory neurons but are also present in nociceptors, are not altered by ET-1. The effect on TTX-R is a “negative shift” in the membrane potential-dependence of channel opening, to increase the probability of a Na+ channel opening at potentials positive to the resting potential and effecting an increase in membrane excitability. Such increased excitability also occurs in vivo when ET-1 is injected around nerve (see 3.F., below).
Very similar effects on TTX-R of sensory neurons follow their exposure to inflammatory mediators such as PGE2 66, 82 and 5-HT, 39 and further studies of these sensitizing agents indicate that they act through PKC- and PKA-linked pathways. 82 Furthermore, in unpublished results from our laboratory we have observed that ET-1, like NGF 270, suppresses the outward delayed rectifier K+ current, IK, in most sensory neurons, an action that will enhance the pro-excitatory effects of TTX-R’s activation shifts. It is likely that ET-1 uses similar or identical pathways to modulate these same channels, and others, e.g. TRPV1 (see above), allowing the confluence of pathways from several types of receptors to effect changes in, e.g., TRPV1, TTX-R and IK, in order to sensitize nociceptive neurons.
3. EFFECTS OF EXOGENOUS ENDOTHELINS IN PAIN
3.A. ETA receptor activation triggers acute nociception
There appears to be no tonic, resting contribution of ET-1 to physiological pain. Injection of antagonists to ETA or ETB receptors has no effect on the nocifensive responses to noxious thermal or mechanical stimulation. 7,86, 163 Exogenous endothelin-1, however, elicits pain when injected into several species of mammals at different peripheral locations: verbally reported pain when injected into the human forearm, 51, 91, 126 overt nociceptive behavior when injected into the knee joint of dogs or rats, as well as the peritoneal cavity and hindpaw foot pad of mice and rats 55, 56, 70, 81, 156, 186, 195, 196 or applied epineurally onto or injected intraneurally into the rat sciatic nerve. 52,68 The peptide also sensitizes the human forearm to mechanical and cold stimulation 70, 91 the rat hindpaw to noxious and innocuous mechanical forces, 14, 43, 49, 70, 162 and the mouse hindpaw to noxious mechanical stimulation, 7 formalin or capsaicin 184–186 and heat. 156
A single i.p. injection of ET-1, ET-2, or ET-3 into mice reliably elicits an abdominal constriction response within 15 min, similar to the effects of acetylcholine (ACh) or phenyl-p-quinone (PpQ).196, 197 The percent of mice responding was dose-dependent, with ET-3 significantly less potent than ET-1 and ET-2, and the response was blocked by morphine administered to the CNS, evidence of its nociceptive nature. 195 Administration of the ETA receptor antagonist BQ-123, i.p., 5 min before ET, blocked the effect of ET-1 and of ET-2, but did not prevent the constrictions induced by ET-3, nor did it inhibit similar behavioral effects produced by Ach or PpQ, establishing the ETA receptor requirement and the pain-related nature of this response.
Subsequently, ET-1, unlike sarafotoxin S6 (ETB agonist), caused nociception when injected into the knee-joint of naive rats, 55, 56 an effect that was halved by systemic bosentan, a mixed ETA/ETB antagonist or local intra-articular injection of the ETA antagonist BQ-123, but was unaffected by the ETB antagonist BQ-788. Thus, ETA receptors also initiate nociception in response to ET-1 injected into the rat knee-joint.
When applied epineurally onto the exposed rat sciatic nerve, ET-1 produced unilateral hindpaw flinching, an effect blocked by preemptive i.p. morphine, and completely prevented, or reversed, by an epineural ETA antagonist, with no effect from an ETB antagonist. 52
Activation of distal nerve endings by subcutaneous (s.c.) injection of ET-1 into the rat plantar hindpaw induced both flinching behavior, in awake rats, and impulse firing in nociceptor afferents recorded from anesthetized rats. 81 The flinching developed within minutes after injection, and was strongly inhibited by BQ-123, similar to that from neuronal application (see above). Recordings from single, physiologically characterized sensory fibers showed that almost all nociceptive C-fibers Aδ-fibers were excited by ET-1 injected into their receptive fields. In contrast, the large majority of “light touch” Aβ-fibers (9 of 12) did not respond to ET-1. BQ-123, when co-injected with ET-1, blocked ET-1-induced activation in all C- and Aδ-fibers tested. Therefore, nociceptive fibers are selectively excited through ETA with the same doses of s.c. ET-1 that cause pain behavior when injected into the rat hindpaw.
Recent results confirm that exogenous ET-1 is capable of evoking acute pain in humans. Spontaneous pain in response to ET-1 was found to develop rapidly after intradermal injection of ET-1 at high concentrations (10−7 and 10−6 M) into the volar aspect of the forearm of healthy males, where it decreased gradually, respectively ending at 30 and 60 min after ET-1 administration. 91
3.B. ETA receptor mediates hyperalgesia
The nature of the response to exogenous ET-1 (acute nociception vs. sensitization/hyperalgesia) seems to depend on the concentration of the peptide. Endothelin-1 injected into the footpad of rats 14, 49, 70 and mice 7, 156 at 30 nM-10 μM induced hyperalgesia and sensitization at the site of injection, rather than the overt pain behavior produced by higher doses, 100–500 μM. 81
Different test species may also produce differences in the detected pain response. An enhanced responsiveness to mechanical stimulation, evoked by exogenous ET-1 in rodents’ plantar footpad, appeared as a slowly developing and prolonged reduction in the force withdrawal threshold of the injected paw. In rats such dose-dependent allodynia reached its maximum in tens of minutes 14 or a few hours,162 and then lasted for 3 – 8 hours. This hyper-responsiveness, unlike overt nociception, was only partially attenuated by ETA receptor antagonists. In mice, ET-1 injected s.c. into the paw also produced tactile allodynia, but this was completely prevented the local ETA antagonist BQ-123. 7 Thermal hyperalgesia induced by s.c. injection of ET-1 in mice (0.8–8 μM, i.pl.), manifested as a unilateral decrease in paw withdrawal latency in response to heat stimuli, was inhibited solely and completely by an ETA receptor antagonist. 156
The behavioral sensitivity produced by the ETA receptor was reflected in BQ-123’s prevention of the amplification of Fos immunoreactivity in the nociceptive-processing outermost laminae of the spinal cord, in species pre-treated with ET-1 and then subjected to thermal stimulation. To the extent that such immediate early gene expression is indicative of cellular activation that may precede longer duration neural plasticity, the blockade of peripheral ETA receptors may provide a pre-emptive treatment to minimize central sensitization and long-lasting pain.
3.C. ET-1 potentiates effects of other algogens
Exogenous ET-1 enhances the nociceptive actions of other algogens, administered into the knee-joint of dogs or rats 55, 56, 70 or into plantar skin in mice. 185, 186 When injected intra-articularly (i.a.), ET-1 potentiated the effects of PGE2 injected i.a. 1.5 or 3 h later. Incapacitation in dogs, caused by elevated joint pain, was produced by PGE2 given, after ET-1, at the usually ineffective dose of 1 μg/joint. 70 The role of specific ET receptors in this process has not been determined.
In studies of the rat knee-joint (see 3.a., above), low doses of ET-1 (15 or 30 pmol/joint), that did not cause incapacitation per se, potentiated the effect of subsequently given carrageenan on hindpaw lifting. Systemic (i.v.) treatment with the non-selective ET receptor antagonist bosentan, given 15 min before joint stimulation, did not affect paw elevation in naïve animals in response to carrageenan or to lipo-polysaccharide (LPS), but reduced by ~50% the ET-1 -enhanced response to LPS 55. No such inhibitory action was seen with BQ-788, showing that potentiation of inflammation-induced incapacitance in joints by ET-1 involves only ETA receptors.
Endothelin-1 induces sensitization to the nociception caused by formalin and capsaicin injections in mice.184–186 Intraplantar formalin induced a “classic” nocifensive response evaluated by the amount of time spent licking the injected paw, both in an early, phase 1, period of activity (0–10 min after injection) and in the phase 2 period (from 15 min to 60–90 min). Both phases of nociception were significantly potentiated by simultaneous injection of formalin with either ET-1 or ET-3, but the first phase was unaffected by the ETB agonist sarafotoxin S6c. Pre-treatment with the mixed ETA/ETB receptor antagonist bosentan attenuated the potentiation by ET-1 of both phases. From these data viewed together, the contribution of the ETA receptor to the first phase of the formalin effect appears to be more relevant than that of the ETB receptor. 184
With regard to capsaicin’s actions, local injection of ET-1 30 min prior to ipsilateral injection of capsaicin (0.1 μg/paw) increased the amount of time spent licking the injected paw, with a maximum ~2.5-fold increase. 185 Importantly, the selective ETB receptor agonists S6c and IRL-1620 did not change the capsaicin response. ETA receptor blockade fully prevented the potentiation, whereas blockade of ETB receptors was ineffective.
Altogether, exogenous ET-1 enhanced the algesic inflammatory actions of PGE2 and LPS in experimental articular pain in dogs and rats, and potentiated capsaicin-, and likely the first phase of formalin-induced pain in plantar skin in mice, all via the ETA receptor.
3.D. ETB receptor mediates both pro-nociceptive and anti-nociceptive effects of ET-1
Pro-nociceptive effects via ETB receptor
Certain pro-nociceptive effects of exogenous ET-1 are also mediated by ETB receptors (Figure 3). Injection of ETB receptor agonists i.p. into mice rapidly elicited abdominal constrictions which, unlike those from ET-1, were unaffected by an ETA receptor antagonist, implying a role for ETB receptors. 196
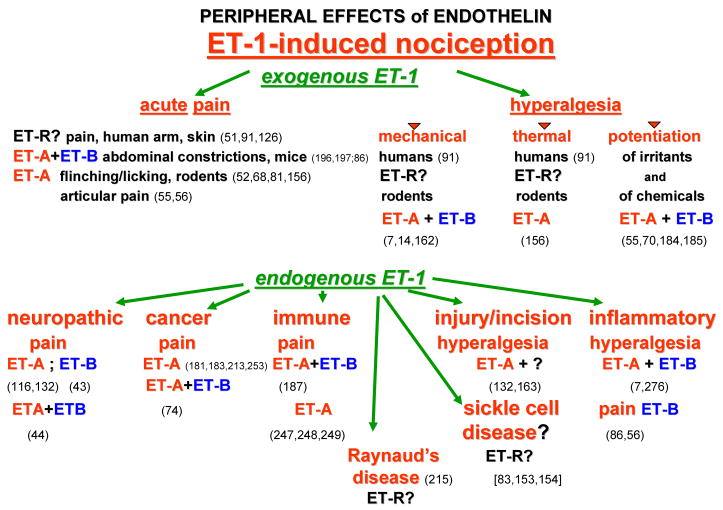
Figure 3.
A diagrammatic display of the documented ways in which ET-1 causes pain and hyperalgesia. The upper section lists the manner of pain caused by exogenous ET-1, and the lower section lists the various disease states wherein ETs and their ET receptors are thought to contribute to pain and hyperalgesia. Question marks indicate the conditions where the role of ETs is not established. Numbers in parentheses refer to citations in References.
The ETB receptor, along with ETA, also partially mediates mechanical hyperalgesia from exogenous ET-1. In mice, BQ-788 completely prevented the decrease in paw withdrawal threshold from ET-1 7. In rats, however, BQ-788 only partially reduced mechanical allodynia (S-D rats, von Frey hairs), as did BQ-123, suggesting involvement of both receptor subtypes. 14 Mechanical hyperalgesia induced by a lower ET-1 concentration (1.5 μM, in Wistar rats, tested with von Frey dynamic aesthesiometer), was equally partially inhibited by either of the antagonists, BQ-788 or BQ-123. 162 In contrast, the mechanical hyper-nociception from lower concentrations of ET-1, 30–300 nM, in rats is purely ETB receptor-dependent, since it was inhibited only by BQ-788 and not by BQ-123. 49
Again it appears that different concentrations of exogenous ET-1 may activate different receptor subtypes to produce different behavioral effects. Such differences in response could arise from coupling between receptor sub-types such that activation of one influences the coupling of another (see 1.B., above). Alternatively, the difference could reflect different ET-1 distributions that are differentially affected by ET-1 diffusing in the skin; after s.c. injection thosecells or axon regions in the more superficial skin, e.g. the epidermis, are reached and affected only by the higher concentrations of ET-1, but the deeper structures are affected by both high and low concentrations. This latter explanation is consistent with the very high concentrations of exogenous ET-1, and its receptor antagonists, compared to their KDs determined in vitro, that are required for induction of acute nociception or its inhibition 68,81,128 and with the very slow responses that occur when much lower concentrations are used, e.g., 3 h to peak mechanical allodynia from 1.5 μM ET-1162 vs. 20 min from 10 μM ET-1.14
Selective activation of the ETB receptor potentiated the second phase of formalin-induced nociception in mice, to about the same extent as was seen with ET-1 per se, indicating that this potentiation, in contrast to that of the first phase, is largely mediated by ETB.184 Capsaicin-induced hypernociception, however, remained unaffected by ETB receptor activation, 186 showing the selective actions of ETB modulation between different chemical algogens (see 3.C., above). In contrast, the ETB receptor does not appear to mediate heat algesia; the thermal nocifensive response was equivalent between wild-type and homozygous ETB knockout mice. 86 Consistent with this, blockade of ETB receptors by BQ-788 was ineffective against ET-1-induced thermal hyperalgesia (hot plate test) in wild-type mice. 156
Anti-nociceptive effects involving ETB receptors
In contrast to the above-listed pro-nociceptive effects, ETB receptor activation has also been shown to provide an anti-hyperalgesic/anti-nociceptive action. 128, 129, 185, 186, 187 These apparently opposite effects may arise because of different ET-1 doses and/or different local conditions such as inflammation (see 4.A, below). For example, hyperalgesia from capsaicin-injected into the mouse hindpaw was enhanced by only a narrow range of ET-1 concentrations. 185 Ten pmol/paw of ET-1 gave the maximum enhancement, above which this potentiation decreased and was absent at 30 pmol/paw (given intraplantar 30 min beforehand). However, pretreatment with ET-1 plus a local ETB receptor antagonist BQ-788 (10 nmol/paw) unmasked a significant hyperalgesic response at that otherwise ineffective, higher dose of ET-1.186 This finding suggested that at high exogenous ET-1 doses an anti-hyperalgesic action of ET-1 was occurring via ETB receptors, a notion that was supported by results showing that co-injection of selective agonists of ETB receptors could abolish the ETA-mediated potentiation. Thus, simultaneous activation of ETB and ETA receptors by exogenous ET-1 limited the hyperalgesic effect of the peptide. 186 It is likely that this ETB mediated inhibition represents the analgesic mechanism resulting from release of β-endorphin from adventitial tissues, such as skin, and its anti-hyperalgesic actions through μ-opiate receptors (see 2.B., above).128, 129
3. E. Peripheral vasoconstriction in ET-1’s actions
Endothelins were first and foremost recognized as potent vasoconstrictive agents. Vascular constriction was documented in almost every organ and tissue, including peripheral nerve 274 and skin, 28, 47 yet the potential physiological importance of this action has been largely overlooked in recent work. ET-1 application to rat peripheral nerve had been shown to cause conduction block in vivo but not in isolated nerve in vitro; 274 the conduction block corresponded to physiological deficits and to a reduction in blood flow in the circulation supplying the nerve, suggesting that local ischemia from ET-1-induced vasconstriction had secondary consequences for neural physiology, and thus for behavior. The importance of this effect may differ among different tissues, where the type of blood vessels, their neural control and their importance for neuronal activity may differ. 199, 200 At present it seems prudent to not dismiss a role for ET-1-induced vasoconstriction, potentially leading to local ischemia, in the overall actions of ET-1 on intact organisms.
3. F. In vivo neurophysiological actions of ET-1
Peripheral nerve in vivo can be activated by ET-1, applied at the trunk of peripheral axons 68 or at cutaneous endings. 81 Action potentials appeared in the absence of acute electrical or physiological stimulation in functionally identified nociceptors, within a minute or so after injection of subcutaneous ET-1 (e.g. 200 μM). Such neurophysiological actions, which occur almost exclusively in almost all cutaneous C- and A-delta nociceptors, are totally prevented by ETA antagonists, 81 and also strongly suppressed by agonists of ETB receptors, 129 consistent with the ETB-mediated release of β-endorphin from keratinocytes (see 2.B., 3.D., above). There is virtually no stimulation of low threshold mechano-receptive Aβ-fibers by ET-1.
The peripheral nerve fiber types activated by ET-1 differ depending on the site of application. Pain behavior from high concentrations of ET-1 applied directly to rat sciatic nerve could be prevented by the non-selective conduction block caused by the proximal application of lidocaine to the sciatic nerve, which also blocked pain responses to toe pinch. However, application of TTX at the same proximal site, at doses that also blocked the response to toe pinch, had no effect on ET-1-induced pain. 107 Earlier studies of impulses in functionally identified sciatic fibers had shown that such doses of TTX failed to block only a small population of axons, the slowest conducting C-fibers, which were almost unanimously unresponsive to noxious cutaneous stimulation. 80 These are probably identical to the ones stimulated by activation of ETA receptors in peripheral nerve. In contrast, subcutaneous injection of high concentrations of ET-1 in the paw led to pain behavior that was partially reduced by TTX at the proximal sciatic nerve (and totally abolished by lidocaine). This difference in TTX susceptibility dependent on the locus of administered ET-1 requires that different fiber types are activated at these different locations, and therefore the mechanism of impulse initiation and the cellular distribution of impulse-generating ETA receptors must also differ. Perhaps ETA receptors at cutaneous nerve terminals in the paw are located on more types of fibers, or even other cell types, than those in the nerve axons of the trunk.
In vivo behavioral studies have identified a number of receptors, signaling enzymes and pathways involved in ET-1-induced hypernociception. For example, mechano-sensitization appears to engage local, cutaneous NMDA and CGRP1 receptors, and may result from an ET-1-enhanced release of glutamate and/or CGRP, e.g., from peripheral nerve endings, replicating in nerve terminals the ET-1-enhanced release of glutamate and CGRP seen in isolated sensory nerves (see 2.B., above). Consistent with this, tactile allodynia after s.c. plantar injection of ET-1 (10 μM) in rats was virtually abolished by local injection of the NMDA receptor inhibitors (+)MK-801 or D-AP5 (5 mM).130 In contrast, hindpaw flinching caused by high concentrations of ET-1 was not affected by either of these antagonists, although it was suppressed by blockade of the CGRP1 receptor. It thus appears that overt pain behavior, in response to high concentrations of exogenous ET-1 and mediated by ETA receptors, is coupled through the neuropeptide-dependent reactions, while mechano-sensitivity, from low [ET-1], and mediated by both ETA and ETB receptors, is modulated by both NMDA and CGRP1 receptors. Perhaps activation of the ETB receptor leads to the release of glutamate and causes tactile allodynia, whereas overt pain behavior requires both glutamate and CGRP, which are released by ETA receptor activation.
Taken together, these findings suggest that in skin ET-1 increases the level of free neurotransmitter/neuropeptides to sensitize nociceptive afferents, subserving tactile allodynia. Because sensory neurons also express receptors for CGRP and glutamate, this ET-1-enhanced autocrine release would increase the subsequent neuronal responses, causing a gain in a positive feedback cycle for sensory activation in the periphery.
3. G. Intracellular pathways mediating endothelins’ peripheral actions
The intracellular pathways that couple ET receptor activation to changes in pain behavior are challenging to identify. This is, at least in part, due to the complexity of the integrated peripheral responses, which involve multiple cell types, including keratinocytes in skin, endothelial cells that modify blood flow and thereby indirectly alter local physiology, 274 the autonomic fibers that modify the skin micro-environment and can modulate sensory afferent sensitivity, 115, 211 and the variety of nociceptive and tactile nerve fibers themselves, generating impulses whose integration in the spinal and supra-spinal parts of the CNS results in the eventual behavior. By examining only the behavioral outcome it is therefore impossible to know which of these, often interactive, processes is the target of an inhibitor of an intracellular pathway, so the following must be interpreted cautiously.
Several downstream signaling pathways appear to be targets of ET receptor activation in vivo, leading to pro-nociceptive responses. As indicated by behavioral studies, these include cyclic AMP (cAMP), various isoforms of Protein Kinase C and mitogen activated protein kinases (MAP kinases). 49, 162 Hypernociception could be induced by ET-1, as well as by ET-2, or ET-3, and can be inhibited by antagonists of ETB and ETA receptors. 14, 162 When rats were pre-treated with a phosphodiesterase inhibitor, which will elevate resting and stimulated levels of intracellular cAMP, mechanical hypernociception induced by ET-1 was more than doubled. On the other hand, hypernociception was not affected by the PKA inhibitor H89, implying that cAMP must be stimulating some other target, e.g., Epac. 42, 76 In contrast, ET-1-induced hyperalgesia was lessened by i.pl. pre-treatment with inhibitors of PKC, staurosporine or calphostin C. Allowing for the possibility of non-selective actions of these inhibitors at the doses required to have an effect in skin, these data nevertheless suggest that endothelins, acting through ETB or ETB receptors, induce mechanical hypernociception in the rat hindpaw that depends on the activity of PKCs and can be enhanced, although not directly driven, by elevated cAMP.
MAP kinases appear to contribute to hyperalgesia from ET-1162. Inhibitors of p38 MAPKinase, extracellular signal-regulated kinases (ERK1/2) and c-Jun N-terminal kinase (JNK), as well as those of phospholipase C (PLC) and PKC, locally injected 15 min before ET-1, caused long-lasting reductions of the mechanical hyperalgesia. A smaller inhibition occurred at 4 h after ET-1 injection from an inhibitor of Phospholipase A2, PACOCF3. Thus, mechanical hyperalgesia triggered by ET-1 in the rat hind paw appears to include contributions from several signaling pathways.162. Whether all of the MAP kinases are activated in the same cell types, or in different neurons, or even in keratinocytes, etc., and whether they act in parallel or sequentially, seems to be an important unanswered question related to the complex, interacting pathways that contribute to nociceptive sensitivity.
Evidence for the involvement of bioactive lipids produced by the cyclo-ogenase (COX) enzymes in ET-1 nociceptive signaling is contradictory. Endothelin-1 potentiates the pain and sensitization from pro-inflammatory mediators, e.g., PGE2, 70 or agents inducing inflammation, such as carrageenan, 55 or formalin (the second phase) 184 (see 3.C., above). Despite the known potentiation by bioactive lipids in the inflammatory processes that were also enhanced by ET-1, broadly acting cyclo-oxygenase inhibitors such as indomethacin and ibuprofen did not block the ET-1-induced nociceptive writhing in mice. Even traditional non-steroidal anti-inflammatory drugs were 30-fold (aspirin) to > 100-fold (ibuprofen, naproxen, tolmetin) less potent in antagonizing ET-1 responses than in antagonizing abdominal constriction from Ach197. On the other hand, a PGE2 signaling cascade, effected downstream by endogenous ET-1, mediates IL-15-, IL-18-, and IL-33–dependent inflammatory hyper-nociception in mice 247–249 (see 4.A., below). Perhaps the only clear conclusion from this collection of results is that there is no single, linear sequential pathway that connects the reactions that drive ET-1’s modulation of hyperalgesia to the reactions that involve COX-derived products, e.g. prostaglandins and thromboxanes.
4. ROLE OF ENDOGENOUS ET-1 IN PAIN FROM INJURY AND DISEASE
Elevated tissue or plasma concentrations of ET-1 occur in a variety of pathological states, e.g., in metastasized prostate and breast cancer cells 167, following cutaneous injury 1 or ischemic injury related to acute respiratory distress syndrome, sepsis, and disseminated intravascular coagulation 57, 112, 252. In many of these pathologies ET-1 contributes to pain.
4.A. Inflammatory pain
Inflammation releases substances that excite or sensitize primary afferent nerve fibers and cause pain/hyperalgesia, 131, 212 and ET-1 is significantly over-secreted in inflammatory conditions. 194 Carrageenan injected into peripheral tissues rapidly increased local and plasma ET-1 levels 17 and chronic constriction of the rat sciatic nerve, with a substantial contribution from local inflammation, 151 caused thermal and mechanical hyperalgesia and elevated both ET-1 and ETA at the injury site. 132 Inflammation of the rat hind paw led to elevated TRPV1 expression in sciatic nerve, 190 providing an increase in one of the critical receptor molecules with which elevated ET-1 interacts to promote hyperalgesia (see 2.B., above).
Endogenously released ET-1 mediates nociception in inflammatory models in vivo. The nociceptive response (abdominal constriction) to i.p. injection of pro-inflammatory phenyl-p-quinone (PpQ), or ET-1 in mice was desensitized by ET-1 pre-injection.197 Both ETA and ETB receptor subtypes contributed positively to pain from intraperitoneal acute inflammation in mice.196
Abdominal constrictions from i.p. phenylbenzoquinone (PBQ) had a reduced intensity in heterozygous (+/−) ETB knockout mice that was 80% less than in wild type controls, and such constrictions were totally absent in the homozygous (−/−) knockouts. 86 Abdominal constrictions from PBQ recorded over 5–15 min after i.p. injection were strongly inhibited in wild-type mice by a systemically delivered ETB receptor-selective antagonist, but unaffected by a systemic ETA receptor antagonist. 86
In a different inflammatory model, wild-type (+/+) ETB-receptor mice had a marked inflammatory response to topical arachidonic acid (AA), but the (+/−), and (−/−) mice had significantly reduced fluid phase responses, measured by edema and by secreted metalloproteinase activity (37 and 65% inhibition, respectively) 86. The cellular phase of the inflammatory response, marked by neutrophil infiltration, was correspondingly reduced in the (+/−) and (−/−) mice (by 51 and 65%, respectively). Topical administration of an ETB receptor antagonist on (+/+) mice inhibited AA-induced swelling (39%), whereas an ETA receptor antagonist was ineffective. Collectively, these results implicate pendogenous ET-1 acting through the ETB receptor in mediation of both inflammatory pain and cutaneous inflammatory cellular responses in mice.
Endothelin-1 levels in synovial fluid were elevated in rheumatoid arthritis (RA), osteoarthritis and gout, and plasma levels of ET-1 in active RA exceeded the values in non-active RA, whereas ET-1-like immunoreactivity in synovial fluid was found at levels several-fold higher than those in plasma 159. In a rat model of experimental arthritis, endogenously-released ET-1 mediated pain in the inflamed knee-joint via both ETA and ETB receptors, with the higher contribution from the ETB receptor 56. Thus, when ET-1 was injected into a “primed” rat joint (wherein 300 μg of carrageenan had been injected 72 h beforehand, to initiate inflammation and sensitize the joint), the pain and incapacitation re-stimulated by ET-1 was equi-sensitive to inhibition of ETA or ETB receptors (each causing approximately = 80% inhibition). When LPS (lipo-polysaccharide) was used to induced hyperalgesia in the primed joint, the pain and incapacitation responses were inhibited ~50% (maximally) by either the non-selective ET receptor antagonist bosentan or by ETB receptor-selective BQ-788, but were unaffected by BQ-123. Thus, unlike the predominantly ETA mediated nociception induced by exogenous ET-1 in the naive joint 55, 56, both ETA and ETB receptors contributed to the stimulation of nociception by ET-1 in the carrageenan-primed (pre-inflamed) joint, ETB receptors account for the LPS-induced nociception in the primed joint, dependent to a large extent on release of endogenous ET-1. The pro-nociceptive role of ETB receptors was confirmed by the fact that its highly selective agonist, sarafotoxin S6c, when injected 72 h after priming with carrageenan, increased the pain-indicating incapacitation.
Surprisingly, the same agonist for the ETB receptor produced an anti-nociceptive effect when it was given 24 h before either the initial injection of carrageenan into the naïve joint, or re-stimulation of the primed joint with any of the following: carrageenan, ET-1, or S6c 50. Apparently ETB activation exerts a prophylactic action, inhibiting the development of inflammatory (carrageenan-induced) pain, and ETB receptor-operated mechanisms limit the priming effect of carrageenan to nociception evoked by subsequent inflammatory insult. These findings dramatically illustrate the dual roles of ETB receptors, pro- and anti-nociceptive on the same inflammatory conditions, depending on the order in which the processes occur. There may be an inflammation-induced shift in the expression or the coupling of ETB receptors that explains this duality of action.
ET-1 is derived from various cells in skin: keratinocytes, 244 vascular endothelial cells, 262 immune cells 61, 217 and mast cells. 62 Sensory afferents themselves 72, 77, 234 and satellite cells of DRG 124 contain ET-1. Thus, both cells of the skin and those that innervate it may release ET-1 in normal and pathological conditions.
Local inflammation in skin clearly involves ET-1 and its receptors. Thermal hyperalgesia in rats during acute inflammation following s.c. complete Freund’s adjuvant (CFA, 20 μg/paw) was partially (~50%) attenuated by an ETA receptor antagonist 276. A similar degree of inhibition occurred with an ETB receptor antagonist. In contrast, ETB receptor activation with the highly selective agonist IRL-1620, given before and with CFA, almost totally reversed maximum thermal hyperalgesia; the latter actions of IRL-1620 were reversed by co-administration of BQ-788, naloxone or antiserum against β-endorphin confirming an opiate-dependence of ETB receptor-linked peripheral analgesia in the inflamed skin (see 3.D., above). These experiments thus revealed pro-and anti-nociceptive roles of ETB receptors at the acute inflammation site.
Cutaneous ETA receptors participate in thermal hyperalgesia, in both acute (induced by carrageenan, 3.5 h before testing) and chronic (induced by CFA, 1 week before testing) inflammation in mice 7. When mice were injected s.c. (intraplantar, i.pl.) with carrageenan or CFA the paw withdrawal latency (PWL; unilateral hot plate test) significantly decreased, with the decrease during acute inflammation being half as much as at chronic inflammation. Pre-treatment with BQ-123 (i.pl.), before inflammation, completely blocked the thermal hyperalgesia measured 90 min after this ETA antagonist was administered, in both inflammatory conditions, whereas identically given BQ-788 was without effect. Unlike this exclusively ETA-dependent thermal hyperalgesia, mechanical allodynia (Randall and Selitto paw pressure test) induced by the same inflammation stimulus involved both ETA and ETB receptors.
In summary, the ETA receptor always appears to promote inflammatory pain/hyperalgesia, but ETB receptor activation is capable of providing opposing actions, likely depending on the inflammatory state and other factors, the species, procedure, even the type of skin, glabrous or hairy, etc. These mechanisms, dynamically regulating the roles of the ETB receptor in different pathological conditions, remain an intriguing area for future investigation.
4. B. Injury associated pain
Damage to the structure of tissues most often leads to inflammation. Although at first it may appear that the same cellular and molecular responses are involved in all injury- and inflammation-related pain states, recent evidence indicates that the neurobiology of different pain states differ from each other in important ways. 30, 146, 147 Nevertheless, there are some events common to all injury. Overproduction of ET-1 is a characteristic of injured skin, 110, 121, 194 and sites of tissue injury also have elevated inflammatory cytokines, and other substances that are released from broken resident cells, e.g. ATP, or imported by inflammatory cells, such as neutrophils, that have bold enzymatic, e.g., oxidative, activity. After UV damage the ET-1 level in skin peaked in 1–2 days 1 and elevated ET-1 was also found after local administration of cytokines TNF-α or IL-1α. 1, 110, 229 Local, transient inflammatory response at a wound resulted in a robust activation of primary afferent nociceptors in the skin 229 that far outlasts the incision-induced injury discharge, and may spread well beyond the region of cutaneous injury, probably through central sensitization in the spinal cord.
The complex reactions to incision are likely to involve responses to local injury and inflammation, including those that trigger wound healing, recruit immune cells, release cytokines and other cellular activators, and also trigger changes in the expression of genes in the sensory neurons (DRG) and activate second order sensory neurons and various types of glia in the spinal cord. 117, 198, 240, 254 These complex factors render the overall response after incisions different from that from inflammation alone.
The specific molecules responsible for sensitizing nociceptors after incision have been investigated in studies using different experimental animal models. A rat model of post-incisional pain 58 has been used to examine the contribution of endogenous ET-1 and ET receptors subtypes to injury-induced pain.163 Mechanical hyperesthesia from an incision through the hairy skin of the rat developed next to the wound (primary responses) and at a distance (secondary responses, involving spinal circuits). Control incisions showed both primary tactile allodynia and hyperalgesia, and a weaker secondary hyperesthesia, peaking 3–4 h after surgery and lasting at least 72 h. Primary allodynia, but not hyperalgesia, was suppressed by an ETA receptor antagonist given locally just before surgery. 163 The antagonist had no effect on mechano-responsiveness of intact skin. This suppression of post-incisional primary allodynia by local ETA antagonist disappeared in 24 h. Secondary responses to incision, however, both allodynia and hyperalgesia, were inhibited even more strongly by these local ETA antagonists, and this inhibition remained effective for at least 24 h. These results are evidence that while the endogenous basal level of ET-1 does not modulate mechano-sensitivity of intact skin, ET-1 released from hairy skin by incision activates nociceptors to cause primary allodynia and to sensitize spinal circuits through central sensitization.
Post-incisional hyperalgesia, paralleled by local tissue acidosis, 228, 256 appeared more resistant than allodynia to ETA receptor antagonism and is likely less dependent on endogenous ET-1. The important clinical consequence is the indication that local blockade of ETA receptors in the immediate peri-operative period may be able to prevent the later development of central sensitization that supports prolonged and persistent post-operative pain.
4.C. ET-1 in acute pain of sickle cell disease
In patients with sickle cell disease, hemoglobin-S (Hgb-S)-containing erythrocytes have a reduced deformability that can greatly increase the viscosity of blood and even result in mechanical blockage of the microcirculation, creating ischemic tissue and leading to the occurrence of painful crises, an episode of severe pain lasting several days.
ET-1 levels are elevated in the plasma of sickle cell patients (see Ref. 89). Levels of circulating ET-1, along with other inflammatory mediators, have been measured in asymptomatic sickle cell disease, and also, during and after a pain crisis. 83 Highly elevated levels of ET-1 were found in symptomatic sickle cell patients during acute pain crises (130.9 ± 23.1 pg/ml) compared with healthy African-American controls (0.535 ± 0.508 pg/ml). These levels decreased, but never to normal values, 1 to 3 weeks after patient discharge from the hospital (post-crisis, 23.69 ± 9.52 pg/ml). Interestingly, the plasma level of PGE2 was also elevated during a crisis, ~2.6-fold, but it remained elevated afterwards, ~2-fold, compared to healthy controls. The other inflammatory mediators that were measured (TNF-a, IL-b, IL-6, IL-8, and IL-10) were neither elevated in asymptomatic sickle cell disease nor during an acute vaso-occlusive crisis. ET-1 appears to contribute to both the prolonged vasospasm and to inflammation in acute painful sickle cell crisis, and ET-1 was suggested to play a key role in the cycle of ischemia and inflammation that initiates and sustains pain at crisis. The elevated level of PGE2 was linked to the down-regulatory effects of PGE2 on immune cell function, which could contribute to the increased susceptibility to infection observed in patients with sickle cell disease. 83
The age-sensitivity in rats of acute nociception and mechanical hyperalgesia in response to exogenous ET-1 suggests that there may be greater pain to stimuli that release endogenous endothelins in younger versus older (and possibly male versus female) mammals 153. Such differences may be relevant to painful vaso-occlusive episodes that occur most dramatically in children with sickle cell disease. 13, 19, 113 Importantly, exposure to ET-1 in infancy may result in sex-dependent modulation of ET-1-induced pain later in life (sensitization in males and desensitization in females), correlating with changes in ETB receptor expression. 154 Further developments should help to define a beneficial approach for treatment of repetitive painful vaso-occlusive episodes.
4. D. Endothelin-1 role in immune-mediated hypernociception
One of the most important roles of endothelins, in general, is their influence on the immune system and contribution to the inflammatory diseases that involve immune components. Among complex disorders with a strong immunological component are asthma, rheumatoid arthritis 137, type 1 (autoimmune) diabetes 251, autoimmune pancreatitis 123, 142, autoimmune prostatitis 161, 255, autoimmune fatigue syndrome 238, retroperitoneal fibrosis with associated autoimmune diseases 32. Erythema nodosum, a painful disorder of the subcutaneous fat, is the most common type of panniculitis 214, and psoriatic arthritis, a chronic, autoimmune, seronegative inflammatory arthritis characterized by varying degrees of axial and peripheral arthritis 145. All of these are associated with pain.
Elevated ET-1 is pathogenic in asthma, and may cause some of the pain associated with this disease.191 A similar argument can be made for the pain-associated immune disease - Henoch-Schonlein purpura (HSP) - one of the common types of vasculitis disorders seen in childhood. It is characterized by abdominal pain and arthritis, along with a rash and renal complications. Cytokines have been implicated in its pathogenesis, and ET-1 levels were found significantly higher in HSP patients during the acute phase compared with the control group and the HSP patients in the remission phase 164.
An immune-mediated Type I hypersensitivity reaction can be induced by i.pl. ovalbumin (OVA, 0.3–1 μg) challenge in mice sensitized to this antigen 14 days beforehand, and utilized to examine the involvement of ET-1. Sensitized mice exhibited greater total licking time in response to intraplantar OVA injection than controls. Nocifensive behavior induced by this OVA challenge was inhibited by systemic morphine or local depletion of mast cells, or by systemic pre-treatment with a number of ETA and ETB receptor selective antagonists or agonists. ETB receptor activation induced nocifensive responses only in OVA-sensitized mice. Compound 48/80 (a synthetic polyamine, inducing degranulation of mast cells) potentiated the nociception induced by i.pl. capsaicin (0.1 μg/paw), and this potentiation was inhibited by local BQ-123, showing a pro-nociceptive role for ETA receptors, whereas blockade of ETB receptors by BQ-788 was ineffective. Therefore, non-immune activation of mast cells could also trigger an ET-dependent change in nocifensive responsiveness. The authors concluded that mast cell- and ET-dependent nociception, that was evoked by immune-mediated Type I hypersensitivity reactions, is mediated locally by both types of ET receptors in mice. In agreement with this pro-nociceptive role of both ET receptors are the responses to systemic treatment with dual ETA/ETB or selective ETA receptor antagonists, which inhibited the nocifensive response to the antigen.
A cascade of cytokines constitutes a link between inflammatory stimuli and release of the final mediators that directly sensitize nociceptors. Rheumatoid arthritis (RA) is characterized by a T helper 1 (Th1) inflammatory immune response in joints. 15, 189 In the pathogenesis of RA, for which a major symptom is mechanical allodynia, cytokines IL-12, IL-15, and IL-18 play a key role by means of elevated IFN-γ production. 15, 31, 69, 120, 141, 189, 205, 230 This signaling cascade, effected downstream by endogenous ET-1, is intimately involved in cytokine-dependent inflammatory hypernociception in mice.247–249
To elucidate the pathophysiology of RA, experimental models were established in which an antigen challenge (intraplantar injection of OVA, on day 21(D21)) was mounted in previously immunized mice (subcutaneously injected OVA, on D 0, D 7 and D14). Ovalbumin induced a Th1 inflammatory response with early neutrophil influx followed by mononuclear cell infiltration and similar features of joint inflammation to those in RA patients 38, 243. Of relevance to pain, the ovalbumin challenge evoked IFN-γ and ET-1 production, both of which were inhibited in immunized IL-18−/− mice.
Signaling pathways revealed in the OVA-induced model for RA in mice, wherein IL-15 or IL-18 triggers the sequential productions of IFN-γ → ET-1 → PGE2, 247, 248 or IL-33 triggers the cascade TNFα → IL-1β → IFN-γ → ET-1 → PGE, 249 suggest that ET receptors as well as the cytokines could be targets for the treatment of antigen-induced inflammatory pain.
4.E. ET-1 in neuropathic pain of peripheral origin
Neuropathic pain, a common disorder that originates from pathology of the peripheral nervous system, is presented by patients with various diseases, including diabetes, infection (herpes zoster), “channelopathies” (inherited diseases caused by mutations in genes coding for ion channel subunits or the proteins that regulate them, and which include a wide range of neurologic diseases, including periodic paralysis, congenital myasthenic syndromes, malignant hypothermia, etc.16, 37), small-fiber neuropathy (a peripheral nerve disease in older people, who develop burning pain in their feet 71), as well as autoimmune diseases. In addition, neuropathic pain frequently develops after nerve compression or nerve trauma. Neuropathic pain reflects both peripheral and central nervous system sensitization, and abnormal impulse activity may arise from the intact afferent neurons that share the receptive field or the common nerve trunk with injured axons. 37
Only a few studies of intact animals link ET-1 to neuropathic pain. One is the investigation of tactile allodynia in the streptozotocin-induced diabetic rat model of neuropathic pain. 116 Tactile allodynia, the perception of pain in response to normally non-painful stimulation, is a common complication of diabetic neuropathy. Diabetic neuropathy is associated with decreased neurovascular blood flow and concomitant endoneurial hypoxia resulting in impaired nerve conduction 35, 36, 245. Early degeneration of small nerve fibers in diabetes leads to symptoms ranging from hyperalgesia to loss of pain and temperature sensation (for review see Ref. 251). The abnormal activation of ETA receptors in peripheral nerves has been implicated in diabetes-induced reductions in peripheral neurovascularization and concomitant endoneurial hypoxia 258, 275. Increased nociception in streptozotocin-induced diabetic rats is not attributable to streptozotocin neurotoxicity 34, but derives, indirectly, from hyperglycemia 108. Rats injected with streptozotocin, after 8–12 weeks developed stabilized, high blood glucose levels, evidence of their diabethic condition, along with tactile allodynia 116. The lower withdrawal thresholds that characterized this tactile allodynia were about doubled by morphine (8 mg/kg, i.p.), indicating the nociceptive nature of the response. A novel ETA receptor antagonist, ABT-627 (Atrasentan®), approved for clinical use, was administered systemically, either acutely (i.p.) or chronically (p.o., given in the drinking water for 7 days) to these rats. When injected intraperitonealy, ABT-627 reversed the allodynia by 40–50%, but only for 0.5–2-h after injection. Chronic ABT-627 treatment (10 mg/kg/day, p.o.), similarly to its acute effect, resulted in a ~40% increase in tactile allodynia thresholds. In contrast, an ETB receptor-selective antagonist injected i.p. did not significantly alter tactile allodynia. Combined i.p. administration of this ETA antagonist and the ETB receptor antagonist produced an acute relief of tactile allodynia that was significantly less than that from the ETA receptor inhibitor ABT-627 alone. Selective blockade of ETA receptors, resulting in an attenuation of tactile allodynia in the streptozotocin-treated rats, is evidence of endogenous ET-1 mediating diabetic neuropathy via ETA receptors. In our interpretation, the reduced effectiveness of ETA receptor blockade by coincident ETB receptor blockade clearly indicates that anti-nociceptive actions derive from endogenous ET-1 through ETB receptors (see 3.D., above).
The just-stated hypothesis is confirmed by another recent study. Reduced expression of ETB receptors (by ~30–35%) was revealed in sciatic nerves (with no changes in DRGs) from rats with experimental chronic diabetes, and these diabetic animals also exhibited both an increase in the withdrawal responses to “high threshold” stimuli (mechanical hyperalgesia) and to light touch (tactile allodynia) 18. Mechanical hyperalgesia and tactile allodynia in ETB receptor-deficient transgenic rats appeared similar to those observed in diabetic rats. These results suggest that decreased expression of ETB receptors in peripheral nerve may contribute to the development of neuropathic pain in chronic diabetes, and that ETB receptors contribute to anti-nociception in that situation. The mechanistic basis for these behavioral changes is not apparent, however, since ETB receptors have been localized on the Schwann cells but not the axons of peripheral nerve 192, and do not have an obvious function in impulse conduction at this location.
ETA receptors mediate neuropathic pain in the rat sciatic nerve chronic constriction injury model (CCI). Endothelin-1 and ETA receptor are locally up-regulated at the site of chronic constriction (nerve ligation) in rats with neuropathic pain behavior 5 days after surgical manipulation, and systemic delivery of the ETA receptor antagonist ABT-627 acutely reduced thermal hyperalgesia and completely reversed mechanical hyperalgesia in those animals.132
Contrasting the role of ETA receptors in driving diabetic and nerve-injury induced neuropathy, ETB receptors appear to trigger trigeminal neuropathy 43. Sustained bilateral mechanical allodynia in a rat model of trigeminal neuropathic pain results from unilateral constriction of the infraorbital nerve (ION). Mechanical allodynia on the face 12–15 days after nerve constriction, that was abolished for up to 90 min by subcutaneous morphine, was unchanged by systemic bosentan (10 mg/kg, iv) or ABT-627 (10 mg/kg, i.v.). In contrast, systemic delivery of the ETB receptor antagonist A-192621 resulted in a ~60% reversal of allodynia, evidence of ETB receptors positive contribution. However, co-injection of A-192621 with ABT-627 (co-inhibition of ETA and ETB receptors, mimicking the actions of bosentan) also did not modify ION injury-induced mechanical allodynia. Control injection of ET-1 into the upper lip of naive rats caused ipsilateral mechanical allodynia, although the effected receptor type was not identified. These data show that endogenous ET-1 contributes to orofacial mechanical allodynia via ETB receptors, but in order to engage the anti-allodynic effect of ETB receptor blockade, functional ETA receptors are required. Whether these receptors are present on the same cells, e.g., neurons, where converging intracellular signaling pathways allow cross-modulation, or are present on different cell types, which are coupled by diffusible substances released by ET receptor activation, is an interesting question.
Furthermore, orofacial cold hyperalgesia also occurs after chronic constriction of the infraorbital nerve. 44 This symptom, which peaks at 4–6 days after surgery, appeared to lack an inflammatory component (was unaffected by indomethacin and celecoxib), but was ameliorated by systemically administered antagonists of ETA and of ETB receptors. These inhibitions, reaching 65–90%, lasted for 1–2.5 hours. Bosentan (the dual ETA/ETB receptor antagonist) abolished this injury-induced cold hyperalgesia, for up to 6 h. Thus, the cold allodynia after ION injury involves ET receptors in a different way than that for mechanical hypersensitivity from the same injury, suggesting that a single mechanism is not common to all types of hypernociception, and that peripheral sensory neurons of different modality can be modulated very differently by endogenous endothelins.
4.F. Endothelin-1 in Raynaud’s phenomenon
Raynaud’s phenomenon (RP), a painful condition that occurs in response to cold or stress, is characterized by episodic ischemia of digits of the hands or feet. Primary RP, without tissue loss, may precede by years the onset of visceral and/or cutaneous sclerosis. Secondary Raynaud’s phenomenon, following tissue loss or damage, eventually develops in 95% of patients with systemic sclerosis (SSc) and other collagen or vascular diseases and reflects the microvascular damage of distal extremities, complicated by functional disability and digital ulcerations.46,193
Endothelin-1 appears to be crucial in the pathogenesis of RP and in the development of skin alterations in SSc. 257 Bosentan, a non-selective ET receptor antagonist, prevented digital ulcers in SSc,134 and when used in a small number of patients with severe RP (associated with pre-scleroderma and systemic sclerosis, independent of digital ulcers), it significantly decreased pain. This treatment also lessened Raynaud’s disease activity, judging from the number and severity of vasospastic attacks and reflected by improved thermoregulation in all patients after a 16-week treatment. 215 These findings may point to a therapeutic use of ET receptor antagonists in severe RP, and perhaps in delaying the onset and suppressing the symptoms of early stages of this disease.
4. G. The Endothelin Axis in cancer pain
Endothelin-1 is involved in the growth and progression of many types of tumors. These include prostatic, ovarian, renal, pulmonary, colorectal, cervical, breast, bladder, endometrial carcinomas, bone metastases, melanoma and others, where ET and ET-receptors, collectively are referred as the “ET axis”. The activation of ET receptors contributes to cell proliferation, escape from apoptosis, neo-vascularization, invasion and metastatic spread (see reviews 10, 40, 166, 223). Endothelin-1 activates a network of intracellular events resulting in tumorigenesis. The ETA receptor mediates ET-1’s effects in prostate, ovarian, breast, renal, bladder, cervical cancer and bone malignancies, 8,40, 88, 100, 182, 202, 223 while both ETA and ETB receptor subtypes are presumably responsible for ET-1’s actions in colon cancer and Kaposi’s sarcoma. 180, 201 Endothelins also modulate the trafficking, differentiation and activation of tumor-infiltrating immune cells. 10, 85
Over 70% of patients with advanced breast or prostate carcinoma present with metastatic disease, and bone is a common site for metastasizing prostate and breast cancer 79. Endothelin-1, via ETA receptors, stimulates mitogenesis in osteoblasts leading to new bone formation 87, 88, 263. Tumor-produced ET-1 enhances osteoblast proliferation, decreases osteoclastic bone resorption and osteoclast motility, the events resulting in osteoblastic metastases 263, while osteoblasts and endothelial cells in the bone of the host are secreting certain factors (e.g., IL-1, TNF-a and transforming growth factor-β) which in turn stimulate more ET-1 production by the metastatic cancer cells 40. Thus, the ET axis serves a critical role in a positive feedback loop that can accelerate the growth of tumors in bone.
Bone cancer is often associated with severe pain. Contemporary experimental models of bone cancer pain utilize a direct injection of cancer cells into calcaneus, humerus, or the medulla of the femur. 33, 103, 155, 181, 206, 216, 253, 271 The first of two studies of ET-1’s contribution to pain from bone tumors used a murine fibrosarcoma model. 33, 253 Fibrosarcoma-bearing mice presented signs of ongoing, resting (spontaneous) pain along with hyperalgesic responses to mechanical and cold stimuli. Tactile allodynia, tested with a very weak von Frey hair, was evident at a time before any bone destruction and then advanced, in parallel with progressing bone destruction. Cold hyperalgesia appeared at a later time. Systemic morphine inhibited both mechanical and cold hyperalgesia.
Increased levels of ET-1 or ET-2 (but not ET-3) were found in perfusates collected from the tumor sites of these hyperalgesic mice. Injection of the ETA receptor antagonist BQ-123 into the tumor site partially attenuated mechanical hyperalgesia, although systemic (i.p.) delivery was ineffective. An antagonist of the ETB receptor, BQ-788, was much less effective at reducing hyperalgesia. These findings suggest that endogenous ET-1 makes a partial contribution to fibrosarcoma-induced pain, primarily through tumor-associated ETA receptors.
In addition, hyperalgesia was present but significantly less after subcutaneous tumor implantation that did not involve bone. Mice with calcaneous inoculation of melanoma cells (G3.26, a B6 subclone) developed neither ongoing pain, nor hyperalgesia nor bone destruction, nor did ET-1 levels increase in the collected perfusates.253 From this it appears that engagement of the ET axis depends both on the type of cancer and on the anatomical location of tumors.
When ET-1 was injected into a tumor-bearing paw it induced enhanced acute nociception, both in mice with sarcoma inoculated into bone (calcaneous) 253 and in mice with subcutaneous inoculation of androgen-independent human prostate cancer cells (PPC-1 into the plantar hind paw. 269 This suggests that primary afferents can be sensitized or activated by ET-1 released from tumors. Systemic ETA receptor antagonists YM598 and ABT-627 inhibited this ET-1-induced potentiation of nociception, 268 probably by acting at receptors around the tumor.
In the other study of ET-1 in bone cancer pain, osteolytic (2472) sarcoma cells were injected into the medullary space of the mouse femur, and the injection site was sealed to prevent leakage of cells outside the bone. 181 These sarcoma-bearing mice exhibited ongoing pain and touch-evoked hyperesthesia. Immunohistochemistry revealed high levels of ET-1 in cultured 2472 sarcoma cells, but the levels of ETA and ETB receptors appeared low or undetectable. On the other hand, ~21% of small and medium diameter and 5% of large diameter DRG neurons expressed ETA receptors and essentially every non-myelinating Schwann cell expressed ETB receptors. Acute systemic delivery of the ETA receptor antagonist ABT-627 strongly suppressed the indices of ongoing and movement-evoked pain. In contrast, the ETB receptor antagonist A-192621 (i.p.) enhanced both spontaneous and palpation-induced guarding, although without altering the flinching behavior. Chronic administration of ABT-627 (p.o. in drinking water, over PIDs 6–14) inhibited both ongoing and movement-evoked pain behavior in bone cancer. Such chronic delivery of this ETA receptor antagonist also partially reduced c-Fos expression in dorsal horn laminae I–II, where it is a marker of sensitization of primary afferent terminals, 104, 170 and in deeper loci, laminae III–VI, where dynorphin expression was also reduced. Neither tumor growth nor bone destruction were influenced by chronic ABT-627.
These studies identify a triggering role for ETA receptors in sarcoma cancer pain. The chronic, systemic administration of ABT-627 was shown to be effective against sarcoma cancer pain, even at later stages of tumor development, unlike the earlier results with systemic BQ-123. 253 Whether this difference in effectiveness is due to receptor affinity or to pharmacokinetic factors of distribution or biotransformation is not obvious. The anti-nociceptive actions of ETB receptors (likely present on Schwann cells), present in this model of sarcoma cancer pain, could be explained by the ET-1 clearance function of ETB receptors, or by some anti-nociceptive mechanism resembling the one found for skin. 129 The latter mechanism might have more support since ET-1 clearance mechanisms are decreased in cancer cells with ETA receptor–mediated growth and progression, e.g., prostate cancer cell lines. 167, 179, 246
Murine models of other types of cancer pain have been developed, with some similarities for ET involvement. 213 Recently, either of two skin tumors that differed significantly in production of ET-1, squamous cell carcinoma (SCC, HSC-3 cell line) or melanoma (WM 164 cell line), were inoculated into the plantar hind paw of female mice, and indices of pain, tumor volume and ET-1 content were compared. 183 Tactile allodynia correlated with tumor volume, with a stronger correlation in the melanoma group. However, despite significantly greater tumor volumes in the melanoma group, tactile allodynia was more pronounced in the SCC group. The expression of both ET-1 mRNA and ET-1 protein were higher in the SCC tumor compared to the melanoma tumor. BQ-123 injected into the mid-plantar paw, at the site of greatest tumor development on PID 32, was effective against tactile allodynia in the SCC model only. The results indicate that the intensity of cancer pain more likely depends on a tumor’s ET-1 content rather than its size, and that ETA receptors mediate pain in squamous cell carcinoma, but not in melanoma cancer in skin. Whether this distinction remains when these tumors metastasize to other regions, such as bone, remains to be tested.
Inoculation of B16-BL6 cells, a highly invasive and metastatic murine melanoma, into the plantar region of the mouse hindpaw resulted in a progressing mechanical tactile allodynia from PID 11 until PID 16–20, 74 which is opposite to the effects described previously for implantation of the melanoma G3.26, B6 cell line. 253 Intraplantar BQ-123 inhibited tumor-induced allodynia (PID15 –18), while BQ-788 was without effect. An intraplantar injection of ET-1 elicited acute nocifensive licking that was greater in the melanoma-bearing hind paw than in controls, and was inhibited by the ETA antagonist BQ-123. In naive mice, an intraplantar injection of tumor extract, prepared from the paw-grown tumor, evoked mechanical allodynia which was reduced, although only partially, by local inhibitors of both ETA and ETB receptors. The levels of mRNA coding for the ETA, but not the ETB, receptor were increased in the ipsilateral DRGs (where both neuronal and glial cells were present). Immunochemical staining showed that the cultured melanoma cells expressed an ET, probably ET-1, and that ET content increased with the tumor mass over the time after inoculation. These results suggest that endogenous ET-1 contributes to melanoma-induced hyperalgesia, and that in melanoma ETB receptors are contributing to pain, along with ETA receptors.
The findings in this study are in agreement with the role of ETB receptors that has been proposed for melanoma. ETB receptors are over-expressed in melanoma cells and correlated with tumor progression. ETB receptors probably mediate all of ET-1’s tumor promoting effects. Thus, BQ-788 affects apoptosis most effectively in metastatic melanoma cells lines 138, and an orally delivered, non-peptide ETB antagonist, A-192621, inhibited melanoma growth in vivo in nude mice 9. Bosentan, a dual ETA/ETB receptor antagonist, stabilized the cancer in patients with stage IV metastatic melanoma. 127 We suggest that the ETB receptor, playing a leading role in the tumorigenesis of melanoma, effects pain-related signaling, as well. The latter is worth further investigation.
Given the expanding evidence for a role of the ET-1 axis in initiation and progression of malignancies, the use of ET receptor inhibitors is an obvious approach to cancer treatment. Promising results have been obtained with ETA receptor antagonists in pre-clinical models, i.e. inhibiting tumor cell proliferation, invasiveness, or angiogenesis, as well as attenuating osteoblastic and pain-related responses to tumor. Among the ETA antagonists that have been tested, ABT-627 (Atrasentan) is an orally bioavailable, highly receptor-specific and rapidly absorbed antagonist. 40 In a Phase III clinical trial that included 809 men with metastatic hormone-refractory prostate cancer (HRPC), Atrasentan suppressed the increase of prostate-specific antigen and the progression of bone turnover and of pain. However, enthusiasm for further developments of Atrasentan treatment was dampened by the fact that significant improvement in survival or time to cancer progression was not demonstrated. 41, 139 Although disease stabilization or reversal is the most desired goal of such therapy, improvement in the quality of life for patients with advanced disease is an important achievement. That various ET receptor antagonists, alone or in combination, have been able to achieve substantial relief of cancer-associated pain in many pre-clinical tests, shows that they deserve further studies and clinical evaluation. Also, a combination of ET receptor blockade with inhibition of other “pro-algesic” receptors, 79 such as TRPV1, could present an effective multi-target approach in pain treatment.
5. PAIN MODULATION BY ENDOTHELINS IN THE CNS
In contrast to its generally pro-nociceptive actions in the periphery, ET-1 inhibits nociception when administered centrally. The administration of ET-1 into the lateral ventricle (i.c.v.) and into the periaqueductal gray area (PAG) produced a significant increase in hot plate and tail flick latencies. 48, 168 Spinal (i.t.) administration of ET-1 also produced a dose-dependent antinociceptive effect in both the tail flick test in mice 122 and in the formalin test in rats. 260 The latter suppression of acute nociception by i.t. ET-1, appearing as a dose-dependent decrease in phases 1 and 2 of formalin-induced flinching behavior, was antagonized by an ETA selective antagonist. In contrast, ETB activation by i.t. agonists enhanced phase 2 flinching, indicating that ET receptor subtypes might have opposing roles in spinal nociceptive processing. 260
The pathways and cellular events underlying central ET-1-induced antinociception are not clearly identified yet. ET-1 injected i.c.v. increased hot plate latencies in mice, like the effect of morphine, but this antinociception was naloxone-insensitive and was antagonized by the calcium entry blocker cinnarizine, given pre-emptively i.p. 168. These results, along with the antinociception produced by the L-type calcium channel activator BAY K 8644, 27 led to a suggestion that the CNS actions of ET-1 might be due to mechanisms involving an increased influx of extracellular Ca2+ into cells. In cultured rat cerebellar granule neurons, however, ET-1 inhibited the L-type Ca2+ current, via ETA.98 This inhibition of Ca2+ current was neither due to a change in cell membrane potential nor because of Ca2+-dependent inactivation of Ca2+ channels, but resulted from a decrease in the frequency of L-type Ca2+-channel opening. 98 It is noteworthy that ET-1’s effects on Ca2+ channels in central neurons are opposite to those on peripheral neurons (see 2. A., B., above); whether this difference is related to the opposite effects on pain caused by ET-1 in these different locations is an interesting consideration.
NMDA receptors are likely implicated in certain of the central antinociceptive effects of ET-1. Stimulation of the peri-aqueductal gray region with ET-1 resulted in enhanced hot plate latencies, an antinociception that was prevented by the NMDA receptor antagonist 2-APV, but not by an antagonist of the AMPA-kaianate type of glutamate receptor, injected into the same area prior to ET-1. 48 On the other hand, NMDA-mediated nociception can be inhibited via activation of protease-activated receptor-1 (PAR-1) sites by intrathecal thrombin, using a pathway involving ET-1 and its ETA receptor subtype. Thrombin generates a tethered ligand for activating PAR-1, whose activation potentiates NMDA receptor activity in hippocampal neurons. 78 Intrathecally injected thrombin, or selective PAR-1 agonists, however, inhibit nocifensive writhing behavior induced in mice by either i.t. NMDA or by inter-abdominal acetic acid (which behavior is NMDA-mediated), although thrombin has no such effect on hot plate or tail flick latencies, which are independent of NMDA. 67 These anti-nociceptive actions were prevented by inhibition of ETA, but not by ETB receptors, indicating an essential role for ET-1 in central antinociception, opposite of the circumstance in the periphery. The source of the pain-inhibiting endogenous ET-1 is not known, although endothelin is produced by both neuronal and non-neuronal cells, including glial, 144 and thrombin has been shown to release ET-1 from cultured astrocytes. 63 Endothelial cells of cerebral microvessels produce and release ET-1 through the adventitial side of the cell to the extracellular space of the CNS. 267 Such non-neuronal ET-1 in the CNS may reach ET receptors on neurons in a paracrine manner or by way of CSF, and affect transmission in pain pathways.
ET-1 of neuronal origin may also participate in pain-inhibiting actions in the CNS. It has been shown that neuron-specific ET-1-knockout mice are hyper-responsive to radiant heat and mechanical stimulation, and to hyperalgesia from intraplantar carrageenan-evoked inflammation or after spinal nerve ligation. The same mice have less stress-induced analgesia in a swim-stress model, compared to wild-type mice. 95 These changes suggest that a basal release of ET-1 from neurons likely affects baseline pain thresholds, and that additional ET-1 released during persistent pain states acts to further suppress pain, and to contribute to stress-induced analgesia. In fact, neuronal ET-1 in normal mice was increased in the hypothalamus after induction of peripheral inflammation with intraplantar carrageenan. 95 Interestingly, [ET-1] measured in other parts of the CNS, midbrain, medulla and spinal cord, all involved in endogenous pain inhibitory systems, was unchanged.
Interactions of ET-1 with opioid receptors indicate another central pathway for endogenous modulation of pain. Antinociceptive, naloxone-sensitive effects of intrathecally administered ET-1 on the tail flick latencies in mice were inhibited by intrathecal verapamil, an L-type Ca2+ channel blocker, and by the δ-opioid receptor-selective antagonist naltrindole, but not by antagonists of μ- or κ-opioid receptors 122. ET-1 may thus mediate a Ca2+ entry-dependent release of endogenous opiates acting on δ-opioid receptors. Importantly, although ET-1’s peripheral actions can be complicated by its potent vasoconstrictive actions (see above), ET-1 does not affect spinal blood flow, since the highest dose of ET-1 used for intrathecal microinjections was ~30-fold lower than the dose just able to increase spinal cord microvascular resistance. 90
In contrast to the antinociception from ET-1 itself, centrally administered (i.c.v.) antagonists of ETA potentiated morphine analgesia on tail-flick latencies in rats and mice. 20, 21, 150 Stimulation of ETB in the CNS did not affect the actions of morphine. 22 While chronic morphine treatment results in functional uncoupling of μ-opioid receptors from their G-proteins, antagonists of ETA receptors enhanced the coupling of G-proteins to opioid receptors (as shown in vitro in neuroblastoma cells), an effect that has been proposed as a mechanism for potentiation and restoration of morphine anti-nociception in morphine-tolerant animals. 23 It is tempting to speculate that hyper-nociceptive input elevates the release of endogenous ET-1 in the CNS which in turn prevents opiate-induced desensitization of specific opiate receptors, thereby promoting the inherent anti-nociception.
CONCLUSIONS
Endothelin-1 and its receptors are implicated in many forms of long-lasting, elevated pain, from inflammation, injury and diseases that affect peripheral tissues, while normal physiological pain is unaffected. Many different ion channels and transmitter pathways appear to be altered by this “endothelin axis”, suggesting that diverging signaling pathways, such as MAPKinases, account for these broad effects. Early therapeutic results, e.g. in cancer pain, encourage the further development of drugs targeted at endothelin receptors for the prevention or relief of elevated pain.
Figure 4.
A diagram of the cases where ETs and the ETB receptors cause analgesia or anti-hyperalgesia. The three sections list: upper-effects of exogenous ET-1; middle-ETB’s selective activation by local agonists; lower – evidence for anti-hyperalgesic actions from endogenously released ET. Numbers in parentheses refer to citations listed in References.
Acknowledgments
The authors acknowledge the inspiration and intellectual drive of Dr. Gudarz Davar, who initiated the studies of ET-1 and pain in these laboratories. Thanks to Mr. James Bell for help with the figures. AK and GS receive salary support from USPHS Grant R-01 CA080153. JPM receives salary support from the French Centre National de la Recherche Scientifique.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahn GY, Butt KI, Jindo T, Yaguchi H, Tsuboi R, Ogawa H. The expression of endothelin-1 and its binding sites in mouse skin increased after ultraviolet B irradiation or local injection of tumor necrosis factor alpha. J Dermatol. 1998;25:78–84. doi: 10.1111/j.1346-8138.1998.tb02354.x. [DOI] [PubMed] [Google Scholar]
- 2.Aley KO, McCarter G, Levine JD. Nitric oxide signaling in pain and nociceptor sensitization in the rat. J Neurosci. 1998;18:7008–7014. doi: 10.1523/JNEUROSCI.18-17-07008.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambar I, Sokolovsky M. Endothelin receptors stimulate both phospholipase C and phospholipase D activities in different cell lines. Eur J Pharmacol. 1993;245:31–41. doi: 10.1016/0922-4106(93)90166-7. [DOI] [PubMed] [Google Scholar]
- 4.Angelova K, Puett D, Narayan P. Identification of endothelin receptor subtypes in sheep choroid plexus. Endocrine. 1997;7:287–293. doi: 10.1007/BF02801321. [DOI] [PubMed] [Google Scholar]
- 5.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 6.Aramori I, Nakanishi S. Coupling of two endothelin receptor subtypes to differing signal transduction in transfected Chinese hamster ovary cells. J Biol Chem. 1992;267:12468–12474. [PubMed] [Google Scholar]
- 7.Baamonde A, Lastra A, Villazon M, Bordallo J, Hidalgo A, Menendez L. Involvement of endogenous endothelins in thermal and mechanical inflammatory hyperalgesia in mice. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:245–251. doi: 10.1007/s00210-003-0841-1. [DOI] [PubMed] [Google Scholar]
- 8.Bagnato A, Cirilli A, Salani D, Simeone P, Muller A, Nicotra MR, Natali PG, Venuti A. Growth inhibition of cervix carcinoma cells in vivo by endothelin A receptor blockade. Cancer Res. 2002;62:6381–6384. [PubMed] [Google Scholar]
- 9.Bagnato A, Rosano L, Spinella F, Di Castro V, Tecce R, Natali PG. Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res. 2004;64:1436–1443. doi: 10.1158/0008-5472.can-03-2344. [DOI] [PubMed] [Google Scholar]
- 10.Bagnato A, Rosano L. The endothelin axis in cancer. Int J Biochem Cell Biol. 2008 doi: 10.1016/j.biocel.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol. 2003;548:373–382. doi: 10.1113/jphysiol.2003.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker MD. Protein kinase C mediates up-regulation of tetrodotoxin-resistant, persistent Na+ current in rat and mouse sensory neurones. J Physiol. 2005;567:851–867. doi: 10.1113/jphysiol.2005.089771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballas SK, Smith ED. Red blood cell changes during the evolution of the sickle cell painful crisis. Blood. 1992;79:2154–2163. [PubMed] [Google Scholar]
- 14.Balonov K, Khodorova A, Strichartz GR. Tactile allodynia initiated by local subcutaneous endothelin-1 is prolonged by activation of TRPV-1 receptors. Exp Biol Med (Maywood) 2006;231:1165–1170. [PubMed] [Google Scholar]
- 15.Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, Petersen LJ, Beurskens FJ, Schuurman J, van de Winkel JG, Parren PW, Gracie JA, Jongbloed S, Liew FY, McInnes IB. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–2692. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]
- 16.Bernard G, Shevell MI. Channelopathies: a review. Pediatr Neurol. 2008;38:73–85. doi: 10.1016/j.pediatrneurol.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Bertelli A, Clerico A, Chicca A, Giovannini L, Gorio A, Romano MA. Role of endothelin-1 in carrageenin-induced inflammation. Int J Tissue React. 1992;14:225–230. [PubMed] [Google Scholar]
- 18.Berti-Mattera LN, Gariepy CE, Burke RM, Hall AK. Reduced expression of endothelin B receptors and mechanical hyperalgesia in experimental chronic diabetes. Exp Neurol. 2006;201:399–406. doi: 10.1016/j.expneurol.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Beyer JE, Simmons LE, Woods GM, Woods PM. A chronology of pain and comfort in children with sickle cell disease. Arch Pediatr Adolesc Med. 1999;153:913–920. doi: 10.1001/archpedi.153.9.913. [DOI] [PubMed] [Google Scholar]
- 20.Bhalla S, Matwyshyn G, Gulati A. Potentiation of morphine analgesia by BQ123, an endothelin antagonist. Peptides. 2002;23:1837–1845. doi: 10.1016/s0196-9781(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 21.Bhalla S, Matwyshyn G, Gulati A. Endothelin receptor antagonists restore morphine analgesia in morphine tolerant rats. Peptides. 2003;24:553–561. doi: 10.1016/s0196-9781(03)00110-4. [DOI] [PubMed] [Google Scholar]
- 22.Bhalla S, Matwyshyn G, Gulati A. Central endothelin-B receptor stimulation does not affect morphine analgesia in rats. Pharmacology. 2004;72:20–25. doi: 10.1159/000078628. [DOI] [PubMed] [Google Scholar]
- 23.Bhalla S, Ciaccio N, Wang ZJ, Gulati A. Involvement of endothelin in morphine tolerance in neuroblastoma (SH-SY5Y) cells. Exp Biol Med (Maywood) 2006;231:1152–1156. [PubMed] [Google Scholar]
- 24.Bloch KD, Hong CC, Eddy RL, Shows TB, Quertermous T. cDNA cloning and chromosomal assignment of the endothelin 2 gene: vasoactive intestinal contractor peptide is rat endothelin 2. Genomics. 1991;10:236–242. doi: 10.1016/0888-7543(91)90505-9. [DOI] [PubMed] [Google Scholar]
- 25.Boivin S, Tessier S, Aubin J, Lampron P, Detheux M, Fournier A. Identification of a binding domain of the endothelin-B receptor using a selective IRL-1620-derived photoprobe. Biochemistry. 2004;43:11516–11525. doi: 10.1021/bi049246x. [DOI] [PubMed] [Google Scholar]
- 26.Bourgeois C, Robert B, Rebourcet R, Mondon F, Mignot TM, Duc-Goiran P, Ferre F. Endothelin-1 and ETA receptor expression in vascular smooth muscle cells from human placenta: a new ETA receptor messenger ribonucleic acid is generated by alternative splicing of exon 3. J Clin Endocrinol Metab. 1997;82:3116–3123. doi: 10.1210/jcem.82.9.4209. [DOI] [PubMed] [Google Scholar]
- 27.Bourson A, Moser PC, Gower AJ, Mir AK. Central and peripheral effects of the dihydropyridine calcium channel activator BAY K 8644 in the rat. Eur J Pharmacol. 1989;160:339–347. doi: 10.1016/0014-2999(89)90089-7. [DOI] [PubMed] [Google Scholar]
- 28.Brain SD, Tippins JR, Williams TJ. Endothelin induces potent microvascular constriction. Br J Pharmacol. 1988;95:1005–1007. doi: 10.1111/j.1476-5381.1988.tb11731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bremnes T, Paasche JD, Mehlum A, Sandberg C, Bremnes B, Attramadal H. Regulation and intracellular trafficking pathways of the endothelin receptors. J Biol Chem. 2000;275:17596–17604. doi: 10.1074/jbc.M000142200. [DOI] [PubMed] [Google Scholar]
- 30.Brennan TJ. Frontiers in translational research: the etiology of incisional and postoperative pain. Anesthesiology. 2002;97:535–537. doi: 10.1097/00000542-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24:207–212. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burkhardt Soares S, Fehr A, Brandt AS, Roth S. [Retroperitoneal fibrosis] Aktuelle Urol. 2007;38:221–231. doi: 10.1055/s-2007-959247. [DOI] [PubMed] [Google Scholar]
- 33.Cain DM, Wacnik PW, Eikmeier L, Beitz A, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve in a model of cancer pain in the mouse. Pain Med. 2001;2:15–23. doi: 10.1046/j.1526-4637.2001.002001015.x. [DOI] [PubMed] [Google Scholar]
- 34.Calcutt NA, Chaplan SR. Spinal pharmacology of tactile allodynia in diabetic rats. Br J Pharmacol. 1997;122:1478–1482. doi: 10.1038/sj.bjp.0701538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cameron NE, Cotter MA, Low PA. Nerve blood flow in early experimental diabetes in rats: relation to conduction deficits. Am J Physiol. 1991;261:E1–8. doi: 10.1152/ajpendo.1991.261.1.E1. [DOI] [PubMed] [Google Scholar]
- 36.Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes. 1997;46 (Suppl 2):S31–37. doi: 10.2337/diab.46.2.s31. [DOI] [PubMed] [Google Scholar]
- 37.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canetti C, Silva JS, Ferreira SH, Cunha FQ. Tumour necrosis factor-alpha and leukotriene B(4) mediate the neutrophil migration in immune inflammation. Br J Pharmacol. 2001;134:1619–1628. doi: 10.1038/sj.bjp.0704403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardenas LM, Cardenas CG, Scroggs RS. 5HT increases excitability of nociceptor-like rat dorsal root ganglion neurons via cAMP-coupled TTX-resistant Na(+) channels. J Neurophysiol. 2001;86:241–248. doi: 10.1152/jn.2001.86.1.241. [DOI] [PubMed] [Google Scholar]
- 40.Carducci MA, Jimeno A. Targeting bone metastasis in prostate cancer with endothelin receptor antagonists. Clin Cancer Res. 2006;12:6296s–6300s. doi: 10.1158/1078-0432.CCR-06-0929. [DOI] [PubMed] [Google Scholar]
- 41.Carducci MA, Saad F, Abrahamsson PA, Dearnaley DP, Schulman CC, North SA, Sleep DJ, Isaacson JD, Nelson JB. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer. 2007;110:1959–1966. doi: 10.1002/cncr.22996. [DOI] [PubMed] [Google Scholar]
- 42.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta Biochim Biophys Sin (Shanghai) 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chichorro JG, Zampronio AR, Rae GA. Endothelin ET(B) receptor antagonist reduces mechanical allodynia in rats with trigeminal neuropathic pain. Exp Biol Med (Maywood) 2006;231:1136–1140. [PubMed] [Google Scholar]
- 44.Chichorro JG, Zampronio AR, Souza GE, Rae GA. Orofacial cold hyperalgesia due to infraorbital nerve constriction injury in rats: reversal by endothelin receptor antagonists but not non-steroidal anti-inflammatory drugs. Pain. 2006;123:64–74. doi: 10.1016/j.pain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J Biol Chem. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- 46.Cooke JP, Marshall JM. Mechanisms of Raynaud’s disease. Vasc Med. 2005;10:293–307. doi: 10.1191/1358863x05vm639ra. [DOI] [PubMed] [Google Scholar]
- 47.Crossman DC, Brain SD, Fuller RW. Potent vasoactive properties of endothelin 1 in human skin. J Appl Physiol. 1991;70:260–266. doi: 10.1152/jappl.1991.70.1.260. [DOI] [PubMed] [Google Scholar]
- 48.D’Amico M, Berrino L, Maione S, Filippelli A, de Novellis V, Rossi F. Endothelin-1 in periaqueductal gray area of mice induces analgesia via glutamatergic receptors. Pain. 1996;65:205–209. doi: 10.1016/0304-3959(95)00178-6. [DOI] [PubMed] [Google Scholar]
- 49.da Cunha JM, Rae GA, Ferreira SH, Cunha Fde Q. Endothelins induce ETB receptor-mediated mechanical hypernociception in rat hindpaw: roles of cAMP and protein kinase C. Eur J Pharmacol. 2004;501:87–94. doi: 10.1016/j.ejphar.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 50.Daher JB, Souza GE, D’Orleans-Juste P, Rae GA. Endothelin ETB receptors inhibit articular nociception and priming induced by carrageenan in the rat knee-joint. Eur J Pharmacol. 2004;496:77–85. doi: 10.1016/j.ejphar.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Dahlof B, Gustafsson D, Hedner T, Jern S, Hansson L. Regional haemodynamic effects of endothelin-1 in rat and man: unexpected adverse reaction. J Hypertens. 1990;8:811–817. doi: 10.1097/00004872-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. Neuroreport. 1998;9:2279–2283. doi: 10.1097/00001756-199807130-00025. [DOI] [PubMed] [Google Scholar]
- 53.Davenport AP, Kuc RE. Cellular expression of isoforms of endothelin-converting enzyme-1 (ECE-1c, ECE-1b and ECE-1a) and endothelin-converting enzyme-2. J Cardiovasc Pharmacol. 2000;36:S12–14. doi: 10.1097/00005344-200036051-00006. [DOI] [PubMed] [Google Scholar]
- 54.Davenport AP. International Union of Pharmacology. XXIX Update on endothelin receptor nomenclature. Pharmacol Rev. 2002;54:219–226. doi: 10.1124/pr.54.2.219. [DOI] [PubMed] [Google Scholar]
- 55.De-Melo JD, Tonussi CR, D’Orleans-Juste P, Rae GA. Effects of endothelin-1 on inflammatory incapacitation of the rat knee joint. J Cardiovasc Pharmacol. 1998;31 (Suppl 1):S518–520. doi: 10.1097/00005344-199800001-00149. [DOI] [PubMed] [Google Scholar]
- 56.De-Melo JD, Tonussi CR, D’Orleans-Juste P, Rae GA. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain. 1998b;77:261–269. doi: 10.1016/S0304-3959(98)00098-0. [DOI] [PubMed] [Google Scholar]
- 57.Druml W, Steltzer H, Waldhausl W, Lenz K, Hammerle A, Vierhapper H, Gasic S, Wagner OF. Endothelin-1 in adult respiratory distress syndrome. Am Rev Respir Dis. 1993;148:1169–1173. doi: 10.1164/ajrccm/148.5.1169. [DOI] [PubMed] [Google Scholar]
- 58.Duarte AM, Pospisilova E, Reilly E, Mujenda F, Hamaya Y, Strichartz GR. Reduction of postincisional allodynia by subcutaneous bupivacaine: findings with a new model in the hairy skin of the rat. Anesthesiology. 2005;103:113–125. doi: 10.1097/00000542-200507000-00018. [DOI] [PubMed] [Google Scholar]
- 59.Dupuis J, Goresky CA, Fournier A. Pulmonary clearance of circulating endothelin-1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol. 1996;81:1510–1515. doi: 10.1152/jappl.1996.81.4.1510. [DOI] [PubMed] [Google Scholar]
- 60.Dymshitz J, Vasko MR. Endothelin-1 enhances capsaicin-induced peptide release and cGMP accumulation in cultures of rat sensory neurons. Neurosci Lett. 1994;167:128–132. doi: 10.1016/0304-3940(94)91044-8. [DOI] [PubMed] [Google Scholar]
- 61.Ehrenreich H, Anderson RW, Ogino Y, Rieckmann P, Costa T, Wood GP, Coligan JE, Kehrl JH, Fauci AS. Selective autoregulation of endothelins in primary astrocyte cultures: endothelin receptor-mediated potentiation of endothelin-1 secretion. New Biol. 1991;3:135–141. [PubMed] [Google Scholar]
- 62.Ehrenreich H, Burd PR, Rottem M, Hultner L, Hylton JB, Garfield M, Coligan JE, Metcalfe DD, Fauci AS. Endothelins belong to the assortment of mast cell-derived and mast cell-bound cytokines. New Biol. 1992;4:147–156. [PubMed] [Google Scholar]
- 63.Ehrenreich H, Costa T, Clouse KA, Pluta RM, Ogino Y, Coligan JE, Burd PR. Thrombin is a regulator of astrocytic endothelin-1. Brain Res. 1993;600:201–207. doi: 10.1016/0006-8993(93)91374-2. [DOI] [PubMed] [Google Scholar]
- 64.Elshourbagy NA, Lee JA, Korman DR, Nuthalaganti P, Sylvester DR, Dilella AG, Sutiphong JA, Kumar CS. Molecular cloning and characterization of the major endothelin receptor subtype in porcine cerebellum. Mol Pharmacol. 1992;41:465–473. [PubMed] [Google Scholar]
- 65.Elshourbagy NA, Adamou JE, Gagnon AW, Wu HL, Pullen M, Nambi P. Molecular characterization of a novel human endothelin receptor splice variant. J Biol Chem. 1996;271:25300–25307. doi: 10.1074/jbc.271.41.25300. [DOI] [PubMed] [Google Scholar]
- 66.England S, Bevan S, Docherty RJ. PGE2 modulates the tetrodotoxin-resistant sodium current in neonatal rat dorsal root ganglion neurones via the cyclic AMP-protein kinase A cascade. J Physiol. 1996;495 (Pt 2):429–440. doi: 10.1113/jphysiol.1996.sp021604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fang M, Kovacs KJ, Fisher LL, Larson AA. Thrombin inhibits NMDA-mediated nociceptive activity in the mouse: possible mediation by endothelin. J Physiol. 2003;549:903–917. doi: 10.1113/jphysiol.2002.036384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fareed MU, Hans G, Atanda A, Strichartz G, Davar G. Pharmacological characterization of acute pain behavior produced by application of endothelin-1 to rat sciatic nerve. J Pain. 2000:46–53. [Google Scholar]
- 69.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 70.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S220–222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 71.Fink E, Oaklander AL. Small-fiber neuropathy: answering the burning questions. Sci Aging Knowledge Environ. 2006;pe7:2006. doi: 10.1126/sageke.2006.6.pe7. [DOI] [PubMed] [Google Scholar]
- 72.Franco-Cereceda A, Rydh M, Lou YP, Dalsgaard CJ, Lundberg JM. Endothelin as a putative sensory neuropeptide in the guinea-pig: different properties in comparison with calcitonin gene-related peptide. Regul Pept. 1991;32:253–265. doi: 10.1016/0167-0115(91)90019-d. [DOI] [PubMed] [Google Scholar]
- 73.Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- 74.Fujita M, Andoh T, Saiki I, Kuraishi Y. Involvement of endothelin and ET(A) endothelin receptor in mechanical allodynia in mice given orthotopic melanoma inoculation. J Pharmacol Sci. 2008;106:257–263. doi: 10.1254/jphs.fp0072051. [DOI] [PubMed] [Google Scholar]
- 75.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 76.Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci. 2008;28:7991–8002. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Giaid A, Gibson SJ, Ibrahim BN, Legon S, Bloom SR, Yanagisawa M, Masaki T, Varndell IM, Polak JM. Endothelin 1, an endothelium-derived peptide, is expressed in neurons of the human spinal cord and dorsal root ganglia. Proc Natl Acad Sci U S A. 1989;86:7634–7638. doi: 10.1073/pnas.86.19.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gingrich MB, Junge CE, Lyuboslavsky P, Traynelis SF. Potentiation of NMDA receptor function by the serine protease thrombin. J Neurosci. 2000;20:4582–4595. doi: 10.1523/JNEUROSCI.20-12-04582.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goblirsch MJ, Zwolak PP, Clohisy DR. Biology of bone cancer pain. Clin Cancer Res. 2006;12:6231s–6235s. doi: 10.1158/1078-0432.CCR-06-0682. [DOI] [PubMed] [Google Scholar]
- 80.Gokin A, Strichartz G. Differential activity-dependence and TTX-sensitivity of pharmacologically characterized rat sciatic c-fibers recorded in vivo. Soc for Neurosci Ann Mtg Abstracts. 2000;928:13. [Google Scholar]
- 81.Gokin AP, Fareed MU, Pan HL, Hans G, Strichartz GR, Davar G. Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats. J Neurosci. 2001;21:5358–5366. doi: 10.1523/JNEUROSCI.21-14-05358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92:2551–2555. [PubMed] [Google Scholar]
- 84.Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279:27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- 85.Grimshaw MJ. Endothelins and hypoxia-inducible factor in cancer. Endocr Relat Cancer. 2007;14:233–244. doi: 10.1677/ERC-07-0057. [DOI] [PubMed] [Google Scholar]
- 86.Griswold DE, Douglas SA, Martin LD, Davis TG, Davis L, Ao Z, Luttmann MA, Pullen M, Nambi P, Hay DW, Ohlstein EH. Endothelin B receptor modulates inflammatory pain and cutaneous inflammation. Mol Pharmacol. 1999;56:807–812. [PubMed] [Google Scholar]
- 87.Guise TA, Yin JJ, Mohammad KS. Role of endothelin-1 in osteoblastic bone metastases. Cancer. 2003;97:779–784. doi: 10.1002/cncr.11129. [DOI] [PubMed] [Google Scholar]
- 88.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 89.Hammerman SI, Kourembanas S, Conca TJ, Tucci M, Brauer M, Farber HW. Endothelin-1 production during the acute chest syndrome in sickle cell disease. Am J Respir Crit Care Med. 1997;156:280–285. doi: 10.1164/ajrccm.156.1.9611085. [DOI] [PubMed] [Google Scholar]
- 90.Han SP, Chen XL, Westfall TC, Knuepfer MM. Characterization of the depressor effect of intrathecal endothelin in anesthetized rats. Am J Physiol. 1991;260:H1685–1691. doi: 10.1152/ajpheart.1991.260.5.H1685. [DOI] [PubMed] [Google Scholar]
- 91.Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: a behavioral study. Neurosci Lett. 2007;418:117–121. doi: 10.1016/j.neulet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 92.Harada N, Himeno A, Shigematsu K, Sumikawa K, Niwa M. Endothelin-1 binding to endothelin receptors in the rat anterior pituitary gland: possible formation of an ETA-ETB receptor heterodimer. Cell Mol Neurobiol. 2002;22:207–226. doi: 10.1023/a:1019822107048. [DOI] [PubMed] [Google Scholar]
- 93.Hasselblatt M, Kamrowski-Kruck H, Jensen N, Schilling L, Kratzin H, Siren AL, Ehrenreich H. ETA and ETB receptor antagonists synergistically increase extracellular endothelin-1 levels in primary rat astrocyte cultures. Brain Res. 1998;785:253–261. doi: 10.1016/s0006-8993(97)01368-1. [DOI] [PubMed] [Google Scholar]
- 94.Hasselblatt M, Lewczuk P, Loffler BM, Kamrowski-Kruck H, von Ahsen N, Siren AL, Ehrenreich H. Role of the astrocytic ET(B) receptor in the regulation of extracellular endothelin-1 during hypoxia. Glia. 2001;34:18–26. doi: 10.1002/glia.1036. [DOI] [PubMed] [Google Scholar]
- 95.Hasue F, Kuwaki T, Kisanuki YY, Yanagisawa M, Moriya H, Fukuda Y, Shimoyama M. Increased sensitivity to acute and persistent pain in neuron-specific endothelin-1 knockout mice. Neuroscience. 2005;130:349–358. doi: 10.1016/j.neuroscience.2004.09.036. [DOI] [PubMed] [Google Scholar]
- 96.Hatae N, Aksentijevich N, Zemkova HW, Kretschmannova K, Tomic M, Stojilkovic SS. Cloning and functional identification of novel endothelin receptor type A isoforms in pituitary. Mol Endocrinol. 2007;21:1192–1204. doi: 10.1210/me.2006-0343. [DOI] [PubMed] [Google Scholar]
- 97.He S, Dibas A, Yorio T, Prasanna G. Parallel signaling pathways in endothelin-1-induced proliferation of U373MG astrocytoma cells. Exp Biol Med (Maywood) 2007;232:370–384. [PubMed] [Google Scholar]
- 98.Held B, Pocock JM, Pearson HA. Endothelin-1 inhibits voltage-sensitive Ca2+ channels in cultured rat cerebellar granule neurones via the ET-A receptor. Pflugers Arch. 1998;436:766–775. doi: 10.1007/s004240050700. [DOI] [PubMed] [Google Scholar]
- 99.Henry PJ. Endothelin-1 (ET-1)-induced contraction in rat isolated trachea: involvement of ETA and ETB receptors and multiple signal transduction systems. Br J Pharmacol. 1993;110:435–441. doi: 10.1111/j.1476-5381.1993.tb13829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Herrmann E, Bogemann M, Bierer S, Eltze E, Toma MI, Kopke T, Hertle L, Wulfing C. The role of the endothelin axis and microvessel density in bladder cancer - correlation with tumor angiogenesis and clinical prognosis. Oncol Rep. 2007;18:133–138. [PubMed] [Google Scholar]
- 101.Hirata Y, Emori T, Eguchi S, Kanno K, Imai T, Ohta K, Marumo F. Endothelin receptor subtype B mediates synthesis of nitric oxide by cultured bovine endothelial cells. J Clin Invest. 1993;91:1367–1373. doi: 10.1172/JCI116338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol. 2000;150:1349–1360. doi: 10.1083/jcb.150.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Honore JC, Robert B, Vacher-Lavenu MC, Chapron C, Breuiller-Fouche M, Ferre F. Expression of endothelin receptors in human myometrium during pregnancy and in uterine leiomyomas. J Cardiovasc Pharmacol. 2000;36:S386–389. doi: 10.1097/00005344-200036051-00112. [DOI] [PubMed] [Google Scholar]
- 104.Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- 105.Hori S, Komatsu Y, Shigemoto R, Mizuno N, Nakanishi S. Distinct tissue distribution and cellular localization of two messenger ribonucleic acids encoding different subtypes of rat endothelin receptors. Endocrinology. 1992;130:1885–1895. doi: 10.1210/endo.130.4.1312429. [DOI] [PubMed] [Google Scholar]
- 106.Horstmeyer A, Cramer H, Sauer T, Muller-Esterl W, Schroeder C. Palmitoylation of endothelin receptor A. Differential modulation of signal transduction activity by post-translational modification. J Biol Chem. 1996;271:20811–20819. doi: 10.1074/jbc.271.34.20811. [DOI] [PubMed] [Google Scholar]
- 107.Houck CS, Khodorova A, Reale AM, Strichartz GR, Davar G. Sensory fibers resistant to the actions of tetrodotoxin mediate nocifensive responses to local administration of endothelin-1 in rats. Pain. 2004;110:719–726. doi: 10.1016/j.pain.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 108.Hounsom L, Tomlinson DR. Does neuropathy develop in animal models? Clin Neurosci. 1997;4:380–389. [PubMed] [Google Scholar]
- 109.Hunter AR, Turner AJ. Expression and localization of endothelin-converting enzyme-1 isoforms in human endothelial cells. Exp Biol Med (Maywood) 2006;231:718–722. [PubMed] [Google Scholar]
- 110.Imokawa G, Yada Y, Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992;267:24675–24680. [PubMed] [Google Scholar]
- 111.Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ishibashi M, Saito K, Watanobe K, Uesugi S, Furue H, Yamaji T. Plasma endothelin-1 levels in patients with disseminated intravascular coagulation. N Engl J Med. 1991;324:1516–1517. doi: 10.1056/NEJM199105233242121. [DOI] [PubMed] [Google Scholar]
- 113.Jacob E, Beyer JE, Miaskowski C, Savedra M, Treadwell M, Styles L. Are there phases to the vaso-occlusive painful episode in sickle cell disease? J Pain Symptom Manage. 2005;29:392–400. doi: 10.1016/j.jpainsymman.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 114.Jacques D, Descorbeth M, Abdel-Samad D, Provost C, Perreault C, Jules F. The distribution and density of ET-1 and its receptors are different in human right and left ventricular endocardial endothelial cells. Peptides. 2005;26:1427–1435. doi: 10.1016/j.peptides.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 115.Janig W, Levine JD, Michaelis M. Interactions of sympathetic and primary afferent neurons following nerve injury and tissue trauma. Prog Brain Res. 1996;113:161–184. doi: 10.1016/s0079-6123(08)61087-0. [DOI] [PubMed] [Google Scholar]
- 116.Jarvis MF, Wessale JL, Zhu CZ, Lynch JJ, Dayton BD, Calzadilla SV, Padley RJ, Opgenorth TJ, Kowaluk EA. ABT-627, an endothelin ET(A) receptor-selective antagonist, attenuates tactile allodynia in a diabetic rat model of neuropathic pain. Eur J Pharmacol. 2000;388:29–35. doi: 10.1016/s0014-2999(99)00865-1. [DOI] [PubMed] [Google Scholar]
- 117.Ji RR, Strichartz G. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;reE14:2004. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- 118.Jiang ZY, Zhou QL, Chatterjee A, Feener EP, Myers MG, Jr, White MF, King GL. Endothelin-1 modulates insulin signaling through phosphatidylinositol 3-kinase pathway in vascular smooth muscle cells. Diabetes. 1999;48:1120–1130. doi: 10.2337/diabetes.48.5.1120. [DOI] [PubMed] [Google Scholar]
- 119.Johnstrom P, Fryer TD, Richards HK, Harris NG, Barret O, Clark JC, Pickard JD, Davenport AP. Positron emission tomography using 18F-labelled endothelin-1 reveals prevention of binding to cardiac receptors owing to tissue-specific clearance by ET B receptors in vivo. Br J Pharmacol. 2005;144:115–122. doi: 10.1038/sj.bjp.0706064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Joosten LA, Lubberts E, Helsen MM, van den Berg WB. Dual role of IL-12 in early and late stages of murine collagen type II arthritis. J Immunol. 1997;159:4094–4102. [PubMed] [Google Scholar]
- 121.Kadono S, Manaka I, Kawashima M, Kobayashi T, Imokawa G. The role of the epidermal endothelin cascade in the hyperpigmentation mechanism of lentigo senilis. J Invest Dermatol. 2001;116:571–577. doi: 10.1046/j.1523-1747.2001.01296.x. [DOI] [PubMed] [Google Scholar]
- 122.Kamei J, Hitosugi H, Kawashima N, Misawa M, Kasuya Y. Antinociceptive effects of intrathecally administered endothelin-1 in mice. Neurosci Lett. 1993;153:69–72. doi: 10.1016/0304-3940(93)90079-z. [DOI] [PubMed] [Google Scholar]
- 123.Kamisawa T, Satake K. Clinical management of autoimmune pancreatitis. Adv Med Sci. 2007;52:61–65. [PubMed] [Google Scholar]
- 124.Kar S, Chabot JG, Quirion R. Quantitative autoradiographic localisation of [125I]endothelin-1 binding sites in spinal cord and dorsal root ganglia of the rat. Neurosci Lett. 1991;133:117–120. doi: 10.1016/0304-3940(91)90071-z. [DOI] [PubMed] [Google Scholar]
- 125.Kasuya Y, Abe Y, Hama H, Sakurai T, Asada S, Masaki T, Goto K. Endothelin-1 activates mitogen-activated protein kinases through two independent signalling pathways in rat astrocytes. Biochem Biophys Res Commun. 1994;204:1325–1333. doi: 10.1006/bbrc.1994.2608. [DOI] [PubMed] [Google Scholar]
- 126.Katugampola R, Church MK, Clough GF. The neurogenic vasodilator response to endothelin-1: a study in human skin in vivo. Exp Physiol. 2000;85:839–846. [PubMed] [Google Scholar]
- 127.Kefford R, Beith JM, Van Hazel GA, Millward M, Trotter JM, Wyld DK, Kusic R, Shreeniwas R, Morganti A, Ballmer A, Segal E, Nayler O, Clozel M. A phase II study of bosentan, a dual endothelin receptor antagonist, as monotherapy in patients with stage IV metastatic melanoma. Invest New Drugs. 2007;25:247–252. doi: 10.1007/s10637-006-9014-7. [DOI] [PubMed] [Google Scholar]
- 128.Khodorova A, Fareed MU, Gokin A, Strichartz GR, Davar G. Local injection of a selective endothelin-B receptor agonist inhibits endothelin-1-induced pain-like behavior and excitation of nociceptors in a naloxone-sensitive manner. J Neurosci. 2002;22:7788–7796. doi: 10.1523/JNEUROSCI.22-17-07788.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khodorova A, Navarro B, Jouaville LS, Murphy JE, Rice FL, Mazurkiewicz JE, Long-Woodward D, Stoffel M, Strichartz GR, Yukhananov R, Davar G. Endothelin-B receptor activation triggers an endogenous analgesic cascade at sites of peripheral injury. Nat Med. 2003;9:1055–1061. doi: 10.1038/nm885. [DOI] [PubMed] [Google Scholar]
- 130.Khodorova A, Vasko MR, Ritcher JA, Strichartz G. NMDA receptors favor tactile allodynia induced by injection of low concentrations of endothelin-1 into the rat’s hindpaw. Soc for Neurosci Ann Mtg Abstracts. 2006;245:23. [Google Scholar]
- 131.Kidd BL, Urban LA. Mechanisms of inflammatory pain. Br J Anaesth. 2001;87:3–11. doi: 10.1093/bja/87.1.3. [DOI] [PubMed] [Google Scholar]
- 132.Klass M, Hord A, Wilcox M, Denson D, Csete M. A role for endothelin in neuropathic pain after chronic constriction injury of the sciatic nerve. Anesth Analg. 2005;101:1757–1762. doi: 10.1213/01.ANE.0000180766.74782.7E. [DOI] [PubMed] [Google Scholar]
- 133.Koizumi S, Kataoka Y, Niwa M, Yamashita K, Taniyama K, Kudo Y. Endothelin increased [Ca2+]i in cultured neurones and slices of rat hippocampus. Neuroreport. 1994;5:1077–1080. doi: 10.1097/00001756-199405000-00014. [DOI] [PubMed] [Google Scholar]
- 134.Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E, Rich E, Carpentier P, Molitor J, Seibold JR, Hsu V, Guillevin L, Chatterjee S, Peter HH, Coppock J, Herrick A, Merkel PA, Simms R, Denton CP, Furst D, Nguyen N, Gaitonde M, Black C. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50:3985–3993. doi: 10.1002/art.20676. [DOI] [PubMed] [Google Scholar]
- 135.Koyama Y, Mizobata T, Yamamoto N, Hashimoto H, Matsuda T, Baba A. Endothelins stimulate expression of cyclooxygenase 2 in rat cultured astrocytes. J Neurochem. 1999;73:1004–1011. doi: 10.1046/j.1471-4159.1999.0731004.x. [DOI] [PubMed] [Google Scholar]
- 136.Krystek SR, Jr, Patel PS, Rose PM, Fisher SM, Kienzle BK, Lach DA, Liu EC, Lynch JS, Novotny J, Webb ML. Mutation of peptide binding site in transmembrane region of a G protein-coupled receptor accounts for endothelin receptor subtype selectivity. J Biol Chem. 1994;269:12383–12386. [PubMed] [Google Scholar]
- 137.Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22:1–12. doi: 10.2165/00019053-200422001-00002. [DOI] [PubMed] [Google Scholar]
- 138.Lahav R, Suva ML, Rimoldi D, Patterson PH, Stamenkovic I. Endothelin receptor B inhibition triggers apoptosis and enhances angiogenesis in melanomas. Cancer Res. 2004;64:8945–8953. doi: 10.1158/0008-5472.CAN-04-1510. [DOI] [PubMed] [Google Scholar]
- 139.Lalich M, McNeel DG, Wilding G, Liu G. Endothelin receptor antagonists in cancer therapy. Cancer Invest. 2007;25:785–794. doi: 10.1080/07357900701522588. [DOI] [PubMed] [Google Scholar]
- 140.Li J, Xiang B, Su W, Zhang X, Huang Y, Ma L. Agonist-induced formation of opioid receptor-G protein-coupled receptor kinase (GRK)-G beta gamma complex on membrane is required for GRK2 function in vivo. J Biol Chem. 2003;278:30219–30226. doi: 10.1074/jbc.M302385200. [DOI] [PubMed] [Google Scholar]
- 141.Liew FY, McInnes IB. The role of innate mediators in inflammatory response. Mol Immunol. 2002;38:887–890. doi: 10.1016/s0161-5890(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 142.Lin LF, Huang PT, Ho KS, Tung JN. Autoimmune chronic pancreatitis. J Chin Med Assoc. 2008;71:14–22. doi: 10.1016/S1726-4901(08)70067-4. [DOI] [PubMed] [Google Scholar]
- 143.Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation. 2000;102:2434–2440. doi: 10.1161/01.cir.102.19.2434. [DOI] [PubMed] [Google Scholar]
- 144.MacCumber MW, Ross CA, Snyder SH. Endothelin in brain: receptors, mitogenesis, and biosynthesis in glial cells. Proc Natl Acad Sci U S A. 1990;87:2359–2363. doi: 10.1073/pnas.87.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Manadan AM, Sequeira W, Block JA. The treatment of psoriatic arthritis. Am J Ther. 2006;13:72–79. doi: 10.1097/00045391-200601000-00012. [DOI] [PubMed] [Google Scholar]
- 146.Mantyh PW. A mechanism based understanding of cancer pain. Pain. 2002;96:1–2. doi: 10.1016/s0304-3959(01)00482-1. [DOI] [PubMed] [Google Scholar]
- 147.Mantyh PW, Clohisy DR, Koltzenburg M, Hunt SP. Molecular mechanisms of cancer pain. Nat Rev Cancer. 2002;2:201–209. doi: 10.1038/nrc747. [DOI] [PubMed] [Google Scholar]
- 148.Masaki T. Endothelins: homeostatic and compensatory actions in the circulatory and endocrine systems. Endocr Rev. 1993;14:256–268. doi: 10.1210/edrv-14-3-256. [DOI] [PubMed] [Google Scholar]
- 149.Masaki T. Historical review: Endothelin. Trends Pharmacol Sci. 2004;25:219–224. doi: 10.1016/j.tips.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 150.Matwyshyn GA, Bhalla S, Gulati A. Endothelin ETA receptor blockade potentiates morphine analgesia but does not affect gastrointestinal transit in mice. Eur J Pharmacol. 2006;543:48–53. doi: 10.1016/j.ejphar.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 151.Maves TJ, Pechman PS, Gebhart GF, Meller ST. Possible chemical contribution from chromic gut sutures produces disorders of pain sensation like those seen in man. Pain. 1993;54:57–69. doi: 10.1016/0304-3959(93)90100-4. [DOI] [PubMed] [Google Scholar]
- 152.Maxwell MJ, Goldie RG, Henry PJ. Ca2+ signalling by endothelin receptors in rat and human cultured airway smooth muscle cells. Br J Pharmacol. 1998;125:1768–1778. doi: 10.1038/sj.bjp.0702252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.McKelvy AD, Mark TR, Sweitzer SM. Age- and sex-specific nociceptive response to endothelin-1. J Pain. 2007;8:657–666. doi: 10.1016/j.jpain.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 154.McKelvy AD, Sweitzer SM. Endothelin-1 exposure in infancy, but not in young childhood sensitizes males and desensitizes females to endothelin-1 inuced pain later in development. Soc for Neurosci Ann Mtg Abstracts. 2007;72:6. [Google Scholar]
- 155.Medhurst SJ, Walker K, Bowes M, Kidd BL, Glatt M, Muller M, Hattenberger M, Vaxelaire J, O’Reilly T, Wotherspoon G, Winter J, Green J, Urban L. A rat model of bone cancer pain. Pain. 2002;96:129–140. doi: 10.1016/s0304-3959(01)00437-7. [DOI] [PubMed] [Google Scholar]
- 156.Menendez L, Lastra A, Hidalgo A, Baamonde A. Nociceptive reaction and thermal hyperalgesia induced by local ET-1 in mice: a behavioral and Fos study. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:28–34. doi: 10.1007/s00210-002-0655-6. [DOI] [PubMed] [Google Scholar]
- 157.Mirshahi T, Mittal V, Zhang H, Linder ME, Logothetis DE. Distinct sites on G protein beta gamma subunits regulate different effector functions. J Biol Chem. 2002;277:36345–36350. doi: 10.1074/jbc.M205359200. [DOI] [PubMed] [Google Scholar]
- 158.Miyamoto Y, Yoshimasa T, Arai H, Takaya K, Ogawa Y, Itoh H, Nakao K. Alternative RNA splicing of the human endothelin-A receptor generates multiple transcripts. Biochem J. 1996;313 (Pt 3):795–801. doi: 10.1042/bj3130795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Miyasaka N, Hirata Y, Ando K, Sato K, Morita H, Shichiri M, Kanno K, Tomita K, Marumo F. Increased production of endothelin-1 in patients with inflammatory arthritides. Arthritis Rheum. 1992;35:397–400. doi: 10.1002/art.1780350406. [DOI] [PubMed] [Google Scholar]
- 160.Mizuguchi T, Nishiyama M, Moroi K, Tanaka H, Saito T, Masuda Y, Masaki T, de Wit D, Yanagisawa M, Kimura S. Analysis of two pharmacologically predicted endothelin B receptor subtypes by using the endothelin B receptor gene knockout mouse. Br J Pharmacol. 1997;120:1427–1430. doi: 10.1038/sj.bjp.0701054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Motrich RD, Maccioni M, Riera CM, Rivero VE. Autoimmune prostatitis: state of the art. Scand J Immunol. 2007;66:217–227. doi: 10.1111/j.1365-3083.2007.01971.x. [DOI] [PubMed] [Google Scholar]
- 162.Motta EM, Calixto JB, Rae GA. Mechanical hyperalgesia induced by endothelin-1 in rats is mediated via phospholipase C, protein kinase C, and MAP kinases. Exp Biol Med (Maywood) 2006;231:1141–1145. [PubMed] [Google Scholar]
- 163.Mujenda FH, Duarte AM, Reilly EK, Strichartz GR. Cutaneous endothelin-A receptors elevate post-incisional pain. Pain. 2007;133:161–173. doi: 10.1016/j.pain.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Muslu A, Islek I, Gok F, Aliyazicioglu Y, Dagdemir A, Dundaroz R, Kucukoduk S, Sakarcan A. Endothelin levels in Henoch-Schonlein purpura. Pediatr Nephrol. 2002;17:920–925. doi: 10.1007/s00467-002-0885-3. [DOI] [PubMed] [Google Scholar]
- 165.Nambi P, Wu HL, Ye D, Gagnon A, Elshourbagy N. Characterization of a novel porcine endothelin(B) receptor splice variant. J Pharmacol Exp Ther. 2000;292:247–253. [PubMed] [Google Scholar]
- 166.Nelson J, Bagnato A, Battistini B, Nisen P. The endothelin axis: emerging role in cancer. Nat Rev Cancer. 2003;3:110–116. doi: 10.1038/nrc990. [DOI] [PubMed] [Google Scholar]
- 167.Nelson JB, Chan-Tack K, Hedican SP, Magnuson SR, Opgenorth TJ, Bova GS, Simons JW. Endothelin-1 production and decreased endothelin B receptor expression in advanced prostate cancer. Cancer Res. 1996;56:663–668. [PubMed] [Google Scholar]
- 168.Nikolov R, Maslarova J, Semkova I, Moyanova S. Intracerebroventricular endothelin-1 (ET-1) produces Ca(2+)-mediated antinociception in mice. Methods Find Exp Clin Pharmacol. 1992;14:229–233. [PubMed] [Google Scholar]
- 169.Nishimura T, Akasu T, Krier J. Endothelin modulates calcium channel current in neurones of rabbit pelvic parasympathetic ganglia. Br J Pharmacol. 1991;103:1242–1250. doi: 10.1111/j.1476-5381.1991.tb12331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Noguchi K, Kowalski K, Traub R, Solodkin A, Iadarola MJ, Ruda MA. Dynorphin expression and Fos-like immunoreactivity following inflammation induced hyperalgesia are colocalized in spinal cord neurons. Brain Res Mol Brain Res. 1991;10:227–233. doi: 10.1016/0169-328x(91)90065-6. [DOI] [PubMed] [Google Scholar]
- 171.Obara K, Koide M, Ishikawa T, Tanabe Y, Nakayama K. Protein kinase C delta but not PKC epsilon activity is involved in contractile potentiation by endothelin-1 in the porcine coronary artery. J Cardiovasc Pharmacol. 2000;36:S120–121. doi: 10.1097/00005344-200036051-00038. [DOI] [PubMed] [Google Scholar]
- 172.Oksche A, Boese G, Horstmeyer A, Furkert J, Beyermann M, Bienert M, Rosenthal W. Late endosomal/lysosomal targeting and lack of recycling of the ligand-occupied endothelin B receptor. Mol Pharmacol. 2000;57:1104–1113. [PubMed] [Google Scholar]
- 173.Opgenorth TJ, Wessale JL, Dixon DB, Adler AL, Calzadilla SV, Padley RJ, Wu-Wong JR. Effects of endothelin receptor antagonists on the plasma immunoreactive endothelin-1 level. J Cardiovasc Pharmacol. 2000;36:S292–296. doi: 10.1097/00005344-200036051-00086. [DOI] [PubMed] [Google Scholar]
- 174.Orry AJ, Wallace BA. Modeling and docking the endothelin G-protein-coupled receptor. Biophys J. 2000;79:3083–3094. doi: 10.1016/S0006-3495(00)76543-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Osawa Y, Koizumi H, Fukaya T, Yasui C, Ohkawara A, Ueda T. Adenylate cyclase induces intracellular Ca2+ increase in single human epidermal keratinocytes of the epidermal sheet as measured by digital imaging microscopy using Fura 2-AM. Arch Dermatol Res. 1991;283:91–95. doi: 10.1007/BF00371615. [DOI] [PubMed] [Google Scholar]
- 176.Ozaki S, Ohwaki K, Ihara M, Ishikawa K, Yano M. Coexpression studies with endothelin receptor subtypes indicate the existence of intracellular cross-talk between ET(A) and ET(B) receptors. J Biochem. 1997;121:440–447. doi: 10.1093/oxfordjournals.jbchem.a021608. [DOI] [PubMed] [Google Scholar]
- 177.Paasche JD, Attramadal T, Sandberg C, Johansen HK, Attramadal H. Mechanisms of endothelin receptor subtype-specific targeting to distinct intracellular trafficking pathways. J Biol Chem. 2001;276:34041–34050. doi: 10.1074/jbc.M103243200. [DOI] [PubMed] [Google Scholar]
- 178.Paasche JD, Attramadal T, Kristiansen K, Oksvold MP, Johansen HK, Huitfeldt HS, Dahl SG, Attramadal H. Subtype-specific sorting of the ETA endothelin receptor by a novel endocytic recycling signal for G protein-coupled receptors. Mol Pharmacol. 2005;67:1581–1590. doi: 10.1124/mol.104.007013. [DOI] [PubMed] [Google Scholar]
- 179.Papandreou CN, Usmani B, Geng Y, Bogenrieder T, Freeman R, Wilk S, Finstad CL, Reuter VE, Powell CT, Scheinberg D, Magill C, Scher HI, Albino AP, Nanus DM. Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen-independent progression. Nat Med. 1998;4:50–57. doi: 10.1038/nm0198-050. [DOI] [PubMed] [Google Scholar]
- 180.Peduto Eberl L, Bovey R, Juillerat-Jeanneret L. Endothelin-receptor antagonists are proapoptotic and antiproliferative in human colon cancer cells. Br J Cancer. 2003;88:788–795. doi: 10.1038/sj.bjc.6600810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Peters CM, Lindsay TH, Pomonis JD, Luger NM, Ghilardi JR, Sevcik MA, Mantyh PW. Endothelin and the tumorigenic component of bone cancer pain. Neuroscience. 2004;126:1043–1052. doi: 10.1016/j.neuroscience.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 182.Pflug BR, Zheng H, Udan MS, D’Antonio JM, Marshall FF, Brooks JD, Nelson JB. Endothelin-1 promotes cell survival in renal cell carcinoma through the ET(A) receptor. Cancer Lett. 2007;246:139–148. doi: 10.1016/j.canlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 183.Pickering V, Jay Gupta R, Quang P, Jordan RC, Schmidt BL. Effect of peripheral endothelin-1 concentration on carcinoma-induced pain in mice. Eur J Pain. 2008;12:293–300. doi: 10.1016/j.ejpain.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Piovezan AP, D’Orleans-Juste P, Tonussi CR, Rae GA. Endothelins potentiate formalin-induced nociception and paw edema in mice. Can J Physiol Pharmacol. 1997;75:596–600. [PubMed] [Google Scholar]
- 185.Piovezan AP, D’Orleans-Juste P, Tonussi CR, Rae GA. Effects of endothelin-1 on capsaicin-induced nociception in mice. Eur J Pharmacol. 1998;351:15–22. doi: 10.1016/s0014-2999(98)00281-7. [DOI] [PubMed] [Google Scholar]
- 186.Piovezan AP, D’Orleans-Juste P, Souza GE, Rae GA. Endothelin-1-induced ET(A) receptor-mediated nociception, hyperalgesia and oedema in the mouse hind-paw: modulation by simultaneous ET(B) receptor activation. Br J Pharmacol. 2000;129:961–968. doi: 10.1038/sj.bjp.0703154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Piovezan AP, D’Orleans-Juste P, Frighetto M, Souza GE, Henriques MG, Rae GA. Endothelins contribute towards nociception induced by antigen in ovalbumin-sensitised mice. Br J Pharmacol. 2004;141:755–763. doi: 10.1038/sj.bjp.0705663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Plant TD, Zollner C, Kepura F, Mousa SS, Eichhorst J, Schaefer M, Furkert J, Stein C, Oksche A. Endothelin potentiates TRPV1 via ETA receptor-mediated activation of protein kinase C. Mol Pain. 2007;3:35. doi: 10.1186/1744-8069-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Plater-Zyberk C, Joosten LA, Helsen MM, Sattonnet-Roche P, Siegfried C, Alouani S, van De Loo FA, Graber P, Aloni S, Cirillo R, Lubberts E, Dinarello CA, van Den Berg WB, Chvatchko Y. Therapeutic effect of neutralizing endogenous IL-18 activity in the collagen-induced model of arthritis. J Clin Invest. 2001;108:1825–1832. doi: 10.1172/JCI12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pogatzki-Zahn EM, Shimizu I, Caterina M, Raja SN. Heat hyperalgesia after incision requires TRPV1 and is distinct from pure inflammatory pain. Pain. 2005;115:296–307. doi: 10.1016/j.pain.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 191.Polikepahad S, Moore RM, Venugopal CS. Endothelins and airways--a short review. Res Commun Mol Pathol Pharmacol. 2006;119:3–51. [PubMed] [Google Scholar]
- 192.Pomonis JD, Rogers SD, Peters CM, Ghilardi JR, Mantyh PW. Expression and localization of endothelin receptors: implications for the involvement of peripheral glia in nociception. J Neurosci. 2001;21:999–1006. doi: 10.1523/JNEUROSCI.21-03-00999.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pope JE. The diagnosis and treatment of Raynaud’s phenomenon: a practical approach. Drugs. 2007;67:517–525. doi: 10.2165/00003495-200767040-00003. [DOI] [PubMed] [Google Scholar]
- 194.Rae GA, Henriques MG. Endothelins in inflammation. In: Said S, editor. Pro-inflammatory and anti-inflammatory peptides. New York: Marcel and Dekker; 1998. pp. 163–202. [Google Scholar]
- 195.Raffa RB, Schupsky JJ, Martinez RP, Jacoby HI. Endothelin-1-induced nociception. Life Sci. 1991;49:PL61–65. doi: 10.1016/0024-3205(91)90252-7. [DOI] [PubMed] [Google Scholar]
- 196.Raffa RB, Schupsky JJ, Jacoby HI. Endothelin-induced nociception in mice: mediation by ETA and ETB receptors. J Pharmacol Exp Ther. 1996a;276:647–651. [PubMed] [Google Scholar]
- 197.Raffa RB, Schupsky JJ, Lee DK, Jacoby HI. Characterization of endothelin-induced nociception in mice: evidence for a mechanistically distinct analgesic model. J Pharmacol Exp Ther. 1996b;278:1–7. [PubMed] [Google Scholar]
- 198.Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- 199.Rendell MS, Finnegan MF, Healy JC, Lind A, Milliken BK, Finney DE, Bonner RF. The relationship of laser-Doppler skin blood flow measurements to the cutaneous microvascular anatomy. Microvasc Res. 1998;55:3–13. doi: 10.1006/mvre.1997.2049. [DOI] [PubMed] [Google Scholar]
- 200.Rendell MS, Johnson ML, Smith D, Finney D, Capp C, Lammers R, Lancaster S. Skin blood flow response in the rat model of wound healing: expression of vasoactive factors. J Surg Res. 2002;107:18–26. doi: 10.1006/jsre.2002.6484. [DOI] [PubMed] [Google Scholar]
- 201.Rosano L, Spinella F, Di Castro V, Nicotra MR, Albini A, Natali PG, Bagnato A. Endothelin receptor blockade inhibits molecular effectors of Kaposi’s sarcoma cell invasion and tumor growth in vivo. Am J Pathol. 2003;163:753–762. doi: 10.1016/S0002-9440(10)63702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rosano L, Di Castro V, Spinella F, Tortora G, Nicotra MR, Natali PG, Bagnato A. Combined targeting of endothelin A receptor and epidermal growth factor receptor in ovarian cancer shows enhanced antitumor activity. Cancer Res. 2007;67:6351–6359. doi: 10.1158/0008-5472.CAN-07-0883. [DOI] [PubMed] [Google Scholar]
- 203.Rose PM, Krystek SR, Jr, Patel PS, Liu EC, Lynch JS, Lach DA, Fisher SM, Webb ML. Aspartate mutation distinguishes ETA but not ETB receptor subtype-selective ligand binding while abolishing phospholipase C activation in both receptors. FEBS Lett. 1995;361:243–249. doi: 10.1016/0014-5793(95)00164-5. [DOI] [PubMed] [Google Scholar]
- 204.Rubanyi GM, Polokoff MA. Endothelins: molecular biology, biochemistry, pharmacology, physiology, and pathophysiology. Pharmacol Rev. 1994;46:325–415. [PubMed] [Google Scholar]
- 205.Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160:5654–5660. [PubMed] [Google Scholar]
- 206.Sabino MA, Ghilardi JR, Jongen JL, Keyser CP, Luger NM, Mach DB, Peters CM, Rogers SD, Schwei MJ, de Felipe C, Mantyh PW. Simultaneous reduction in cancer pain, bone destruction, and tumor growth by selective inhibition of cyclooxygenase-2. Cancer Res. 2002;62:7343–7349. [PubMed] [Google Scholar]
- 207.Sachs D, Cunha FQ, Ferreira SH. Peripheral analgesic blockade of hypernociception: activation of arginine/NO/cGMP/protein kinase G/ATP-sensitive K+ channel pathway. Proc Natl Acad Sci U S A. 2004;101:3680–3685. doi: 10.1073/pnas.0308382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Saida K, Mitsui Y, Ishida N. A novel peptide, vasoactive intestinal contractor, of a new (endothelin) peptide family. Molecular cloning, expression, and biological activity. J Biol Chem. 1989;264:14613–14616. [PubMed] [Google Scholar]
- 209.Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, Masaki T. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- 210.Sakurai T, Yanagisawa M, Masaki T. Molecular characterization of endothelin receptors. Trends Pharmacol Sci. 1992;13:103–108. doi: 10.1016/0165-6147(92)90038-8. [DOI] [PubMed] [Google Scholar]
- 211.Sawynok J, Esser MJ, Reid AR. Peripheral antinociceptive actions of desipramine and fluoxetine in an inflammatory and neuropathic pain test in the rat. Pain. 1999;82:149–158. doi: 10.1016/S0304-3959(99)00043-3. [DOI] [PubMed] [Google Scholar]
- 212.Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 213.Schmidt BL, Pickering V, Liu S, Quang P, Dolan J, Connelly ST, Jordan RC. Peripheral endothelin A receptor antagonism attenuates carcinoma-induced pain. Eur J Pain. 2007;11:406–414. doi: 10.1016/j.ejpain.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 214.Schwartz RA, Nervi SJ. Erythema nodosum: a sign of systemic disease. Am Fam Physician. 2007;75:695–700. [PubMed] [Google Scholar]
- 215.Selenko-Gebauer N, Duschek N, Minimair G, Stingl G, Karlhofer F. Successful treatment of patients with severe secondary Raynaud’s phenomenon with the endothelin receptor antagonist bosentan. Rheumatology (Oxford) 2006;45(Suppl 3):iii45–48. doi: 10.1093/rheumatology/kel290. [DOI] [PubMed] [Google Scholar]
- 216.Seong J, Park HC, Kim J, Kim UJ, Lee BW. Radiation-induced alteration of pain-related signals in an animal model with bone invasion from cancer. Ann N Y Acad Sci. 2004;1030:179–186. doi: 10.1196/annals.1329.023. [DOI] [PubMed] [Google Scholar]
- 217.Sessa WC, Nasjletti A. Dexamethasone selectively attenuates prostanoid-induced vasoconstrictor responses in vitro. Circ Res. 1990;66:383–388. doi: 10.1161/01.res.66.2.383. [DOI] [PubMed] [Google Scholar]
- 218.Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shraga-Levine Z, Galron R, Sokolovsky M. Cyclic GMP formation in rat cerebellar slices is stimulated by endothelins via nitric oxide formation and by sarafotoxins via formation of carbon monoxide. Biochemistry. 1994;33:14656–14659. doi: 10.1021/bi00253a002. [DOI] [PubMed] [Google Scholar]
- 220.Shraga-Levine Z, Sokolovsky M. cGMP formation in rat atrial slices is ligand and endothelin receptor subtype specific. Circ Res. 1996;78:424–430. doi: 10.1161/01.res.78.3.424. [DOI] [PubMed] [Google Scholar]
- 221.Shraga-Levine Z, Sokolovsky M. Functional coupling of G proteins to endothelin receptors is ligand and receptor subtype specific. Cell Mol Neurobiol. 2000;20:305–317. doi: 10.1023/a:1007010125316. [DOI] [PubMed] [Google Scholar]
- 222.Shyamala V, Moulthrop TH, Stratton-Thomas J, Tekamp-Olson P. Two distinct human endothelin B receptors generated by alternative splicing from a single gene. Cell Mol Biol Res. 1994;40:285–296. [PubMed] [Google Scholar]
- 223.Smollich M, Wulfing P. The endothelin axis: a novel target for pharmacotherapy of female malignancies. Curr Vasc Pharmacol. 2007;5:239–248. doi: 10.2174/157016107781024082. [DOI] [PubMed] [Google Scholar]
- 224.Sokolovsky M. Endothelins and sarafotoxins: physiological regulation, receptor subtypes and transmembrane signaling. Pharmacol Ther. 1992;54:129–149. doi: 10.1016/0163-7258(92)90030-4. [DOI] [PubMed] [Google Scholar]
- 225.Sokolovsky M. Endothelins and sarafotoxins: receptor heterogeneity. Int J Biochem. 1994;26:335–340. doi: 10.1016/0020-711x(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 226.Sokolovsky M, Shraga-Levine Z, Galron R. Ligand-specific stimulation/inhibition of cAMP formation by a novel endothelin receptor subtype. Biochemistry. 1994;33:11417–11419. doi: 10.1021/bi00204a002. [DOI] [PubMed] [Google Scholar]
- 227.Southall MD, Li T, Gharibova LS, Pei Y, Nicol GD, Travers JB. Activation of epidermal vanilloid receptor-1 induces release of proinflammatory mediators in human keratinocytes. J Pharmacol Exp Ther. 2003;304:217–222. doi: 10.1124/jpet.102.040675. [DOI] [PubMed] [Google Scholar]
- 228.Steen KH, Steen AE, Reeh PW. A dominant role of acid pH in inflammatory excitation and sensitization of nociceptors in rat skin, in vitro. J Neurosci. 1995;15:3982–3989. doi: 10.1523/JNEUROSCI.15-05-03982.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Steinhoff M, Stander S, Seeliger S, Ansel JC, Schmelz M, Luger T. Modern aspects of cutaneous neurogenic inflammation. Arch Dermatol. 2003;139:1479–1488. doi: 10.1001/archderm.139.11.1479. [DOI] [PubMed] [Google Scholar]
- 230.Strengell M, Matikainen S, Siren J, Lehtonen A, Foster D, Julkunen I, Sareneva T. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. J Immunol. 2003;170:5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 231.Sugden PH. An overview of endothelin signaling in the cardiac myocyte. J Mol Cell Cardiol. 2003;35:871–886. doi: 10.1016/s0022-2828(03)00153-6. [DOI] [PubMed] [Google Scholar]
- 232.Sullivan JC, Goodchild TT, Cai Z, Pollock DM, Pollock JS. Endothelin(A) (ET(A)) and ET(B) receptor-mediated regulation of nitric oxide synthase 1 (NOS1) and NOS3 isoforms in the renal inner medulla. Acta Physiol (Oxf) 2007;191:329–336. doi: 10.1111/j.1748-1716.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 233.Suzuki T. Endothelin-1-induced depolarization and hyperpolarization in submandibular ganglion neurons. Bull Tokyo Dent Coll. 2004;45:189–192. doi: 10.2209/tdcpublication.45.189. [DOI] [PubMed] [Google Scholar]
- 234.Szolcsanyi J, Oroszi G, Nemeth J, Szilvassy Z, Blasig IE, Tosaki A. Functional and biochemical evidence for capsaicin-induced neural endothelin release in isolated working rat heart. Eur J Pharmacol. 2001;419:215–221. doi: 10.1016/s0014-2999(01)00973-6. [DOI] [PubMed] [Google Scholar]
- 235.Takahashi H, Miyokawa N, Katagiri M, Iizuka H. Expression of guanine nucleotide binding proteins, Gs and Gi, in mRNAs in epidermal keratinocytes. Arch Dermatol Res. 1990;282:392–396. doi: 10.1007/BF00372090. [DOI] [PubMed] [Google Scholar]
- 236.Takasuka T, Akiyama N, Horii I, Furuichi Y, Watanabe T. Different stability of ligand-receptor complex formed with two endothelin receptor species, ETA and ETB. J Biochem. 1992;111:748–753. doi: 10.1093/oxfordjournals.jbchem.a123830. [DOI] [PubMed] [Google Scholar]
- 237.Takigawa M, Sakurai T, Kasuya Y, Abe Y, Masaki T, Goto K. Molecular identification of guanine-nucleotide-binding regulatory proteins which couple to endothelin receptors. Eur J Biochem. 1995;228:102–108. doi: 10.1111/j.1432-1033.1995.tb20236.x. [DOI] [PubMed] [Google Scholar]
- 238.Tan EM, Sugiura K, Gupta S. The case definition of chronic fatigue syndrome. J Clin Immunol. 2002;22:8–12. doi: 10.1023/a:1014248301721. [DOI] [PubMed] [Google Scholar]
- 239.Tang W, Tu Y, Nayak SK, Woodson J, Jehl M, Ross EM. Gbetagamma inhibits Galpha GTPase-activating proteins by inhibition of Galpha-GTP binding during stimulation by receptor. J Biol Chem. 2006;281:4746–4753. doi: 10.1074/jbc.M510573200. [DOI] [PubMed] [Google Scholar]
- 240.Tanga FY, Raghavendra V, DeLeo JA. Quantitative real-time RT-PCR assessment of spinal microglial and astrocytic activation markers in a rat model of neuropathic pain. Neurochem Int. 2004;45:397–407. doi: 10.1016/j.neuint.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 241.Tessier S, Boivin S, Aubin J, Lampron P, Detheux M, Fournier A. Transmembrane domain V of the endothelin-A receptor is a binding domain of ETA-selective TTA-386-derived photoprobes. Biochemistry. 2005;44:7844–7854. doi: 10.1021/bi0500933. [DOI] [PubMed] [Google Scholar]
- 242.Tirapelli CR, Casolari DA, Yogi A, Montezano AC, Tostes RC, Legros E, D’Orleans-Juste P, de Oliveira AM. Functional characterization and expression of endothelin receptors in rat carotid artery: involvement of nitric oxide, a vasodilator prostanoid and the opening of K+ channels in ETB-induced relaxation. Br J Pharmacol. 2005;146:903–912. doi: 10.1038/sj.bjp.0706388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Tsuboi R, Sato C, Shi CM, Nakamura T, Sakurai T, Ogawa H. Endothelin-1 acts as an autocrine growth factor for normal human keratinocytes. J Cell Physiol. 1994;159:213–220. doi: 10.1002/jcp.1041590204. [DOI] [PubMed] [Google Scholar]
- 245.Tuck RR, Schmelzer JD, Low PA. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984;107 (Pt 3):935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- 246.Usmani BA, Harden B, Maitland NJ, Turner AJ. Differential expression of neutral endopeptidase-24.11 (neprilysin) and endothelin-converting enzyme in human prostate cancer cell lines. Clin Sci (Lond) 2002;103 (Suppl 48):314S–317S. doi: 10.1042/CS103S314S. [DOI] [PubMed] [Google Scholar]
- 247.Verri WA, Jr, Cunha TM, Parada CA, Wei XQ, Ferreira SH, Liew FY, Cunha FQ. IL-15 mediates immune inflammatory hypernociception by triggering a sequential release of IFN-gamma, endothelin, and prostaglandin. Proc Natl Acad Sci U S A. 2006;103:9721–9725. doi: 10.1073/pnas.0603286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Verri WA, Jr, Cunha TM, Parada CA, Poole S, Liew FY, Ferreira SH, Cunha FQ. Antigen-induced inflammatory mechanical hypernociception in mice is mediated by IL-18. Brain Behav Immun. 2007;21:535–543. doi: 10.1016/j.bbi.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 249.Verri WA, Jr, Guerrero AT, Fukada SY, Valerio DA, Cunha TM, Xu D, Ferreira SH, Liew FY, Cunha FQ. IL-33 mediates antigen-induced cutaneous and articular hypernociception in mice. Proc Natl Acad Sci U S A. 2008;105:2723–2728. doi: 10.1073/pnas.0712116105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Vigne P, Ladoux A, Frelin C. Endothelins activate Na+/H+ exchange in brain capillary endothelial cells via a high affinity endothelin-3 receptor that is not coupled to phospholipase C. J Biol Chem. 1991;266:5925–5928. [PubMed] [Google Scholar]
- 251.Vinik AI. Advances in diabetes for the millennium: new treatments for diabetic neuropathies. MedGenMed. 2004;6:13. [PMC free article] [PubMed] [Google Scholar]
- 252.Voerman HJ, Stehouwer CD, van Kamp GJ, Strack van Schijndel RJ, Groeneveld AB, Thijs LG. Plasma endothelin levels are increased during septic shock. Crit Care Med. 1992;20:1097–1101. doi: 10.1097/00003246-199208000-00005. [DOI] [PubMed] [Google Scholar]
- 253.Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21:9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Wieseler-Frank J, Maier SF, Watkins LR. Immune-to-brain communication dynamically modulates pain: physiological and pathological consequences. Brain Behav Immun. 2005;19:104–111. doi: 10.1016/j.bbi.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 255.Wiygul RD. Prostatitis: epidemiology of inflammation. Curr Urol Rep. 2005;6:282–289. doi: 10.1007/s11934-005-0025-2. [DOI] [PubMed] [Google Scholar]
- 256.Woo YC, Park SS, Subieta AR, Brennan TJ. Changes in tissue pH and temperature after incision indicate acidosis may contribute to postoperative pain. Anesthesiology. 2004;101:468–475. doi: 10.1097/00000542-200408000-00029. [DOI] [PubMed] [Google Scholar]
- 257.Worda M, Sgonc R, Dietrich H, Niederegger H, Sundick RS, Gershwin ME, Wick G. In vivo analysis of the apoptosis-inducing effect of anti-endothelial cell antibodies in systemic sclerosis by the chorionallantoic membrane assay. Arthritis Rheum. 2003;48:2605–2614. doi: 10.1002/art.11179. [DOI] [PubMed] [Google Scholar]
- 258.Xu QG, Cheng C, Sun H, Thomsen K, Zochodne DW. Local sensory ganglion ischemia induced by endothelin vasoconstriction: vulnerability of diabetic neurons and microvessels. Neuroscience. 2003;122:897–905. doi: 10.1016/s0306-4522(03)00520-7. [DOI] [PubMed] [Google Scholar]
- 259.Yamamoto H, Kawamata T, Ninomiya T, Omote K, Namiki A. Endothelin-1 enhances capsaicin-evoked intracellular Ca2+ response via activation of endothelin a receptor in a protein kinase Cepsilon-dependent manner in dorsal root ganglion neurons. Neuroscience. 2006;137:949–960. doi: 10.1016/j.neuroscience.2005.09.036. [DOI] [PubMed] [Google Scholar]
- 260.Yamamoto T, Shimoyama N, Asano H, Mizuguchi T. Analysis of the role of endothelin-A and endothelin-B receptors on nociceptive information transmission in the spinal cord with FR139317, an endothelin-A receptor antagonist, and sarafotoxin S6c, an endothelin-B receptor agonist. J Pharmacol Exp Ther. 1994;271:156–163. [PubMed] [Google Scholar]
- 261.Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988;6:S188–191. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- 262.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 263.Yin JJ, Mohammad KS, Kakonen SM, Harris S, Wu-Wong JR, Wessale JL, Padley RJ, Garrett IR, Chirgwin JM, Guise TA. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc Natl Acad Sci U S A. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yogi A, Callera GE, Montezano AC, Aranha AB, Tostes RC, Schiffrin EL, Touyz RM. Endothelin-1, but not Ang II, activates MAP kinases through c-Src independent Ras-Raf dependent pathways in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2007;27:1960–1967. doi: 10.1161/ATVBAHA.107.146746. [DOI] [PubMed] [Google Scholar]
- 265.Yohn JJ, Morelli JG, Walchak SJ, Rundell KB, Norris DA, Zamora MR. Cultured human keratinocytes synthesize and secrete endothelin-1. J Invest Dermatol. 1993;100:23–26. doi: 10.1111/1523-1747.ep12349932. [DOI] [PubMed] [Google Scholar]
- 266.Yohn JJ, Smith C, Stevens T, Morelli JG, Shurnas LR, Walchak SJ, Hoffman TA, Kelley KK, Escobedo-Morse A, Yanagisawa M, et al. Autoregulation of endothelin-1 secretion by cultured human keratinocytes via the endothelin B receptor. Biochim Biophys Acta. 1994;1224:454–458. doi: 10.1016/0167-4889(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 267.Yoshimoto S, Ishizaki Y, Kurihara H, Sasaki T, Yoshizumi M, Yanagisawa M, Yazaki Y, Masaki T, Takakura K, Murota S. Cerebral microvessel endothelium is producing endothelin. Brain Res. 1990;508:283–285. doi: 10.1016/0006-8993(90)90407-3. [DOI] [PubMed] [Google Scholar]
- 268.Yuyama H, Koakutsu A, Fujiyasu N, Tanahashi M, Fujimori A, Sato S, Shibasaki K, Tanaka S, Sudoh K, Sasamata M, Miyata K. Effects of selective endothelin ET(A) receptor antagonists on endothelin-1-induced potentiation of cancer pain. Eur J Pharmacol. 2004;492:177–182. doi: 10.1016/j.ejphar.2004.04.016. [DOI] [PubMed] [Google Scholar]
- 269.Yuyama H, Sonoda R, Shibasaki K, Fujimori A, Sudoh K, Sasamata M, Miyata K. Effect of single oral administration of YM598, a novel selective endothelin ETA receptor antagonist, on blood pressure in normotensive and hypertensive rats. Vascul Pharmacol. 2004;41:27–34. doi: 10.1016/j.vph.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 270.Zhang YH, Vasko MR, Nicol GD. Ceramide, a putative second messenger for nerve growth factor, modulates the TTX-resistant Na(+) current and delayed rectifier K(+) current in rat sensory neurons. J Physiol. 2002;544:385–402. doi: 10.1113/jphysiol.2002.024265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zhao C, Wacnik PW, Tall JM, Johns DC, Wilcox GL, Meyer RA, Raja SN. Analgesic effects of a soy-containing diet in three murine bone cancer pain models. J Pain. 2004;5:104–110. doi: 10.1016/j.jpain.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 272.Zhou QL, Strichartz G, Davar G. Endothelin-1 activates ET(A) receptors to increase intracellular calcium in model sensory neurons. Neuroreport. 2001;12:3853–3857. doi: 10.1097/00001756-200112040-00050. [DOI] [PubMed] [Google Scholar]
- 273.Zhou Z, Davar G, Strichartz G. Endothelin-1 (ET-1) selectively enhances the activation gating of slowly inactivating tetrodotoxin-resistant sodium currents in rat sensory neurons: a mechanism for the pain-inducing actions of ET-1. J Neurosci. 2002;22:6325–6330. doi: 10.1523/JNEUROSCI.22-15-06325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zochodne DW, Ho LT, Gross PM. Acute endoneurial ischemia induced by epineurial endothelin in the rat sciatic nerve. Am J Physiol. 1992;263:H1806–1810. doi: 10.1152/ajpheart.1992.263.6.H1806. [DOI] [PubMed] [Google Scholar]
- 275.Zochodne DW, Cheng C. Diabetic peripheral nerves are susceptible to multifocal ischemic damage from endothelin. Brain Res. 1999;838:11–17. doi: 10.1016/s0006-8993(99)01670-4. [DOI] [PubMed] [Google Scholar]
- 276.Zou S, Ren K, Dubner R, Khodorova A, Davar G. Endothelin receptor mechanisms of adjuvant-induced hyperalgesia in rats. Soc for Neurosci Ann Mtg Abstracts. 2003;588:16. [Google Scholar]