Abstract
Host dendritic cells (DCs) play a critical role in initiating graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL), and separation of GVL from GVHD remains a major challenge in the treatment of hematologic malignancies by allogeneic hematopoietic cell transplantation (HCT). Here, we show that preconditioning with anti-CD3 monoclonal antibody before conditioning with total body irradiation (TBI) prevents GVHD but retains GVL in a HCT model of major histocompatibility complex (MHC)–mismatched C57BL/6 donor to BALB/c host. Prevention of GVHD is associated with inhibition of donor T-cell expression of homing and chemokine receptors, and inhibition of GVHD target tissue expression of chemokines. Furthermore, inhibition of donor T-cell expression of gut homing α4β7 and chemokine receptor (CCR)9 by anti-CD3 preconditioning results from a reduction of CD103+ DCs in draining mesenteric lymph nodes (LNs), which is associated with down-regulation of DC expression of CCR7, a receptor required for tissue DC migration to draining LNs. These results indicate that anti-CD3 preconditioning reduces not only tissue release of chemokines but also prevents tissue DC migration to draining LNs and subsequently reduces the capacity of DCs of draining LNs to imprint donor T-cell tissue tropism. Therefore, modulation of host DCs by anti-CD3 preconditioning before HCT represents a new approach for separating GVL from GVHD.
Introduction
In allogeneic hematopoietic cell transplantation (HCT), both graft-versus-host disease (GVHD) and graft-versus-leukemia (GVL) activity are predominantly mediated by donor T cells in bone marrow grafts.1–4 Donor T cells are activated in host lymphoid tissues and then migrate to epithelial GVHD target tissues (ie, gut, liver, lung, and skin) to mediate GVHD.5–7 Studies have shown that inhibition of donor T-cell migration to GVHD target tissues prevents GVHD but retains GVL activities in lymphohematologic tissues.8,9
Alloreactive T cells are activated by host antigen-presenting cells (APCs), especially dendritic cells (DCs), in secondary lymphoid tissues.10,11 It has been proposed that, during activation, host DCs in draining lymph nodes (LNs) induce donor T cells to express homing and chemokine receptors (CCRs) that medicate tissue-specific migration.12It has been shown that DCs in mesenteric LNs (MLNs) induce T-cell expression of α4β7 receptors and CCR9 that mediate T-cell migration to gut tissues,13,14 because DCs in MLNs are able to metabolize vitamin A into retinoic acid (RA) that induces T cells to up-regulate α4β7 and CCR9.15 Similarly, DCs in peripheral LNs induce T-cell expression of E-selectin ligand (E-Lig), P-selectin ligand (P-Lig), CCR4, and CCR10 that mediate T-cell migration to skin tissue,14,16,17 because DCs in peripheral LNs are able to metabolize vitamin D3 to an active form that induces T cells to up-regulate CCR1018 T-cell expression of chemokine receptors as well as GVHD target tissue release of chemokines has been shown to play critical roles in the control of donor T-cell migration to GVHD target tissues.19–26
It has been proposed that CD103+ DCs in lamina propria (LP) capture antigens locally and then migrate via afferent lymph to draining MLN, where they activate naive T cells and induce expression of gut tissue-specific homing and chemokine receptors.27 It has also been indicated that DC migration from LP to MLN and from dermis to peripheral LN (PLN) both requires DC expression of CCR7,27,28 and MLN and PLN DCs reciprocally induce T-cell gut and skin tissue tropism.14 Consistently, DCs in host draining LNs have been shown to induce donor T-cell expression of gut and skin homing receptors,29 although donor T cells can still infiltrate GVHD target tissues in recipients deficient in MLNs and PLNs.30
In addition, host DCs in tissues may attract activated donor T cells to GVHD target tissues, because depletion of APCs in liver were shown to markedly reduced activated donor T-cell migration into liver.31 It has been proposed that tissue inflammatory chemokines attract donor T-cell migration to GVHD target tissues after total body irradiation (TBI) conditioning,26 and the chemokines are secreted by tissue macrophages and tissue DCs as well as infiltrated donor T cells.32 However, it is not yet clear which cells are the initial ones in chemokine release. It was reported that, in the case of viral infection, plasmacytoid DCs initiate the complex chemokine and cytokine network.33Therefore, plasmacytoid DCs in GVHD target tissues may also play an initial role in chemokine release after TBI conditioning.
Modulation of the tissue distribution of DCs has been suggested to regulate immune responses. For example, intravenous injection of lipopolysaccharide (LPS) led to a massive migration of DCs from tissues to LN and spleen,34 and blockade of LPS was shown to ameliorate GVHD.35 In contrast, intravenous injection of anti-CD3 was shown to cause DC migration into spleen and suppress allogeneic skin graft rejection.36 We have also reported that conditioning of allogeneic recipients with anti-CD3 prevents GVHD but retains GVL activity in recipients who had not been irradiated by confining donor T cells to the host lympho-hematologic tissues,9 but it is not yet clear whether this results from modulation of host DC tissue distribution. Our results in the current study indicate that anti-CD3 preconditioning can modulate host DC subset tissue distribution and inhibit donor T-cell migration to GVHD target tissues.
Methods
Mice
C57BL/6 and BALB/c mice were purchased from NCI Laboratories (Frederick, MD). All animals were maintained in a pathogen-free room at the City of Hope Research Animal Facility. Male mice 8 to 12 weeks old were used in the current studies. Animal use protocols were approved by the institutional review committee at the Beckman Research Institute.
Conditioning of recipients and HCT
Production of anti-CD3 monoclonal antibody (mAb; 145-2C11) was described in our previous publication.9 Recipient BALB/c mice were injected intravenously with anti-CD3 (5 μg/g) on day −9 and were given sublethal TBI (800 rads) on day 0. Then the recipients received transplanted donor T cell–depleted bone marrow (TCD-BM) cells (5 × 106) and whole spleen cells (2.5-5 × 106). For GVL experiments, Luc+ B-cell leukemia/lymphoma 1 (BCL1) cells (0.5 × 106) were injected intraperitoneally at the same time when donor bone marrow (BM) and spleen cells were injected intravenously. In vivo imaging of tumor growth was previously described.9 The recipients were monitored daily for survival and every 5 days for body weight changes and clinical signs of GVHD. The clinical scoring system was previously described.26,37
Cell preparation
CD11+ DCs in LP, liver, LN, and spleen were collected as previously described.27,38 CD103+ and CD103− CD11+ DC subsets (purity > 99%) were isolated with flow cytometric sorting after magnetic enrichment of CD11c+ DCs. Mononuclear cells (MNCs) from liver and gut were processed and collected as previously described.39 MNCs from skin were collected as follows: back skin (3 × 3 cm2) were cut into small pieces and digested in RPMI containing 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid; Irvine Scientific, Santa Ana, CA), 0.01% DNase (Sigma-Aldrich, St Louis, MO), 0.27% collagenase (Sigma-Aldrich), and 1000 U hyaluronidase at 37°C for 1 hour. Skin MNCs were then isolated by Lymphocyte M (Accurate Chemical & Scientific, Westbury, NY).
Flow cytometric analysis
The following anti–mouse mAbs were purchased from BD Biosciences Pharmingen (San Jose, CA), eBioscience (San Diego, CA), and R&D Systems (Minneapolis, MN): TCRβ (H57-597), CD4 (RM4-5), CD8α (53-6.7), CD11b/Mac-1 (M1/70), H-2b (AF6-88.5), H-2d (34-2-12), CD11c (HL3), α4β7 (DATK32), CD103 (M290), CCR9 (CW-1.2), CCR4 (1G1), CCR10 (248918), CCR5 (HM-CCR5), CXCR3 (1C6/CXCR3), PDCA-1 (JF05-1C2.4.1), E-selectin/Fc chimera, P-selectin/Fc chimera, and anti–IFN-γ (2E2). Fluorescence-activated cell sorting (FACS) was performed with a 4-laser MoFlo Immunocytometry System (Dako, Glostrup, Denmark), and data were analyzed with FlowJo software (TreeStar, Ashland, OR), as described previously.9,40 The FoxP3 staining kit was purchased from eBioscience.
Quantification of chemokine expression by real-time RT-PCR
Isolation of total tissue RNA and synthesis of first strand cDNA were described previously.37,41 mRNA was quantified by real-time quantitative PCR using Applied Biosystems 7300 Fast Real-Time PCR System (Applied Biosystems, Forest City, CA). The primers for chemokines were previously described in the following publications: Ccl3-5,42 Ccl17,43 Ccl22,43 Ccl25,44 Ccl27,45 Ccl28,45 and Cxcl9-11.37 Relative expression levels of genes were normalized within each sample to the housekeeping gene GAPDH (glyceraldehyde 3-phosphate dehydrogenase) and were presented relative to the expression in recipients of syngeneic transplants, in which BALB/c recipients that had been irradiated were injected with 5 × 106 syngeneic TCD-BM cells as previous described.37
Mixed leukocyte reaction and in vitro induction of donor T-cell expression of homing and chemokine receptors
Sorted CD4+, CD8+, or CD4+/CD8+ T cells (2 × 105) from spleen of donor C57BL/6 mice were cultured with CD11c+ DCs (105) from BABL/c host in a U-bottom 96-well plate for 4 days. T-cell homing and chemokine receptor expression was measured by flow cytometry, and T-cell proliferation was measured by 3H-thymidine (3H-TdR; Amersham, Buckinghamshire, United Kingdom) incorporation, which were previously described.9
GVHD histopathology and scoring
Colon, liver, and skin specimens were fixed in formalin before embedding in paraffin blocks. Tissue sections were stained with hematoxylin and eosin (H&E) as described previously.9 Assessment of tissue damage was performed based on scoring system previously described.37,46,47
Statistical analysis
Comparison of survival groups was analyzed using the log-rank test with Prism version 4.0 software (GraphPad, La Jolla, CA). Comparison of 2 means was analyzed using the unpaired 2-tailed Student t test.
Results
Anti-CD3 preconditioning prevented GVHD but retained GVL in recipients conditioned with TBI
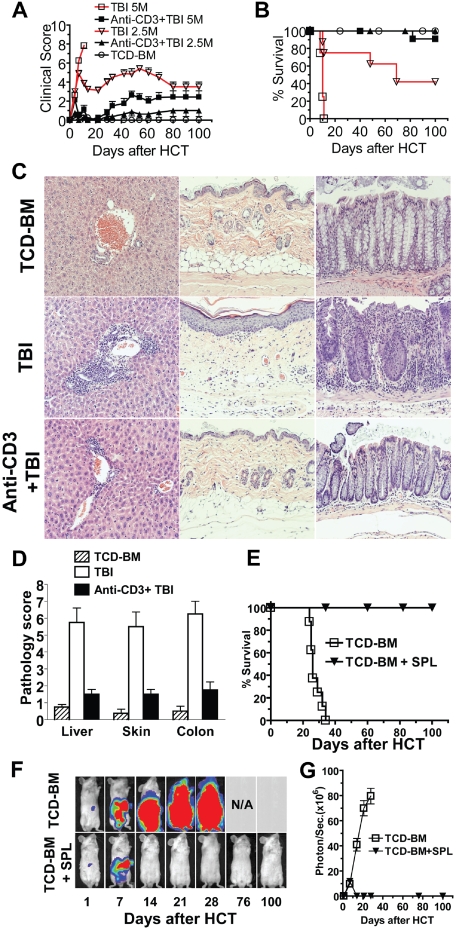
Our recent report showed that anti-CD3 conditioning allowed donor CD8+ T cells to mediate GVL without causing clinical GVHD in recipients who had not been irradiated.9 In the current study, we tested whether anti-CD3 preconditioning could separate GVL from GVHD in recipients conditioned with TBI. Accordingly, recipient BALB/c mice were injected with anti-CD3 (5 μg/g) or phosphate-buffered saline (PBS) as preconditioning. Nine days after anti-CD3 injection, the mice were conditioned with TBI. At this time point, serum anti-CD3 was not detectable by blocking assay, and the host T-cell receptor αβ+ (TCRαβ+) cells were also not detectable in blood. Six hours after TBI, the recipients were injected intravenously with TCD-BM (5 × 106) and spleen cells (2.5-5 × 106) from C57BL/6 donor mice. The recipients were monitored daily for clinical GVHD, including body weight, posture, diarrhea, and survival. We found that, while 5 × 106 donor spleen cells induced severe clinical GVHD in control recipients without anti-CD3 preconditioning, and all recipients died by 15 days after HCT, the same dose of donor cells induced only moderate clinical GVHD, and 91% (11/12) of the recipients survived for more than 100 days (P < .01, Figure 1A,B). Similarly, 2.5 × 106 donor spleen cells induced severe GVHD in the control recipients, and only 42% (5/12) of recipients survived for more than 100 days after HCT. In contrast, the same dose of donor cells induced minimal clinical GVHD in recipients preconditioned with anti-CD3, and all recipients survived for more than 100 days (Figure 1A,B). Therefore, anti-CD3 preconditioning markedly reduced clinical GVHD.
Figure 1.
Anti-CD3 preconditioning separated GVL from GVHD in recipients conditioned with TBI. BALB/c mice were preconditioned with anti-CD3 on day −9. The mice were conditioned with 800 rads sublethal TBI on day 0. Six hours later, the mice were injected intravenously with TCD-BM cells (5 × 106) and spleen cells (2.5 or 5 × 106) from C57BL/6 donors. There were 12 mice in each group combined from 3 replicate experiments. (A) Clinical score. (B) Survival percentage. (C,D) Liver, skin, and colon tissues from the recipients conditioned with TBI with or without anti-CD3 preconditioning were evaluated for tissue inflammation and damage 60 days after HCT. A representative histopathology and the mean plus or minus SE of 6 recipients in each group are shown. (E-G) BALB/C recipients that were preconditioned with anti-CD3 were injected intravenously with BCL1 cells transfected with luciferase (Luc+) and donor TCD-BM and spleen cells (2.5 × 106). There were 8 mice in each group combined from 2 replicate experiments. The survival percentage, representative photographs of in vivo BLI of Luc+ BCL1 cells, and the intensity (photo/second) of BLI are shown.
In additional experiments, we compared the histopathology of liver, skin, and colon of the recipients with or without anti-CD3 preconditioning 60 days after injection of 2.5 × 106 donor spleen cells. We observed that anti-CD3 preconditioning markedly reduced infiltration and tissue damage in liver, skin, and colon (P < .01, Figure 1C,D). Taken together, these results indicate that anti-CD3 preconditioning prevents induction of acute GVHD.
Furthermore, we tested whether anti-CD3 preconditioning could retain GVL while preventing GVHD. Accordingly, luciferase transfected (Luc+) BCL1 leukemia/lymphoma cells (0.5 × 106) were coinjected with donor TCD-BM (5 × 106) and spleen cells (2.5 × 106) into recipients preconditioned with anti-CD3 6 hours after TBI conditioning. The control recipients were injected with TCD-BM and Luc+BCL1 cells only. The recipients were monitored for survival daily and for tumor growth using in vivo bioluminescent imaging (BLI) weekly. We found that Luc+ BCL1 tumor cells grew rapidly in recipients given TCD-BM without donor spleen cells, and killed the recipients 30 to 40 days after HCT (Figure 1E-G). In contrast, after a transient growth, Luc+ BCL1 tumor cells were eliminated in the recipients that received transplants of both TCD-BM and spleen cells, and all recipients survived for more than 100 days with little clinical GVHD (P < .01, Figure 1E-G). These results indicate that anti-CD3 preconditioning prevents GVHD but retains GVL activity.
Anti-CD3 preconditioning reduced donor T helper 1 cell differentiation and donor T cell infiltration of GVHD target tissues
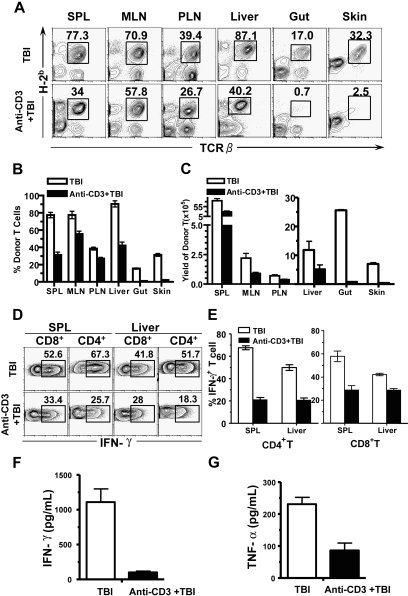
We and others previously reported that donor T-cell expansion in recipients conditioned with TBI reached first peak 5 days after HCT.7,9 Therefore, we compared the percentage and yield of donor T cells in lymphoid and GVHD target tissues (liver, gut, and skin) in the recipients with or without anti-CD3 preconditioning, 5 days after injection of donor TCD-BM and spleen cells (5 × 106). We found that the percentage and the yield of donor T cells in the spleen, MLNs, and PLNs of the recipients with anti-CD3 preconditioning were approximately 2-fold lower than those in the control recipients (P < .01, Figure 2A-C). In contrast, the percentage and yield of donor T cells in skin and gut of the recipients preconditioned with anti-CD3 was more than 15-fold lower than those of the control recipients (P < .01, Figure 2A-C). These results indicate that anti-CD3 preconditioning markedly inhibits donor T-cell migration into GVHD target tissues such as gut and skin in TBI-conditioned recipients.
Figure 2.
Anti-CD3 preconditioning inhibited donor T-cell infiltration of GVHD target tissues. Five days after injection of donor TCD-BM and spleen cells (5 × 106), the percentage and yield of donor T cells in spleen, MLNs, PLNs, liver, gut, and skin of the recipients with or without anti-CD3 preconditioning were compared. There were 4 recipients in each group. (A) A representative FACS pattern. Mononuclear cells from different tissues were stained with anti-TCRαβ versus anti-H-2b (donor MHCI), and the donor-type T cells were gated. (B) Mean plus or minus SE of the donor T-cell percentage among total mononuclear cells of 4 recipients. (C) Mean plus or minus SE of the yield of donor T cells in different tissues. (D) A representative intracellular IFN-γ staining pattern of the gated H-2b+CD4+ or H-2b+CD8+ T cells. The IFN-γ+ cells were gated. (E) Mean plus or minus SE of the percentage of donor IFN-γ+CD4+ or CD8+ cells of 4 examined recipients. (F,G) Mean plus or minus SE of serum IFN-γ and TNF-α levels of 6 examined recipients.
Interestingly, the percentage and yield of donor T cells in liver of the recipients with anti-CD3 preconditioning were only approximately 2-fold lower than those of the control recipients, although the difference was significant (P < .01, Figure 2A-C). This was markedly different from that of skin and gut tissues, but similar to that of spleen (Figure 2A-C). These differences may be attributed to the ability of donor T cells to directly enter spleen and liver by blood circulation. Despite the moderate difference in donor T-cell yield from liver of recipients with or without anti-CD3 preconditioning early after HCT, the clinical acute GVHD of the recipients from the 2 groups was markedly different (Figure 1). Since interferonγ (IFN-γ)–producing T helper 1 (Th1) and T cytotoxic type 1 (Tc1) cells were reported to play an important role in mediating acute GVHD target tissue damage,37,48 we compared the percentage of the IFN-γ+ donor CD4+ and CD8+ T cells in spleen and liver of the recipients. We found that anti-CD3 preconditioning reduced the percentage of IFN-γ+ cells among total CD4+ and CD8+ T cells by approximately 2-fold in the spleen and liver relative to those in control recipients (P < .01, Figure 2D,E). Consistently, anti-CD3 preconditioning reduced the serum levels of Th1 cytokine IFN-γ and tumor necrosis factor α (TNF-α) by 2- to 5-fold (P < .01, Figure 2F,G). These results indicate that anti-CD3 preconditioning leads to a significant reduction in donor Th1 differentiation.
Anti-CD3 preconditioning inhibited up-regulation of chemokine receptors by donor T cells as well as chemokine release by GVHD target tissues in recipients conditioned with TBI
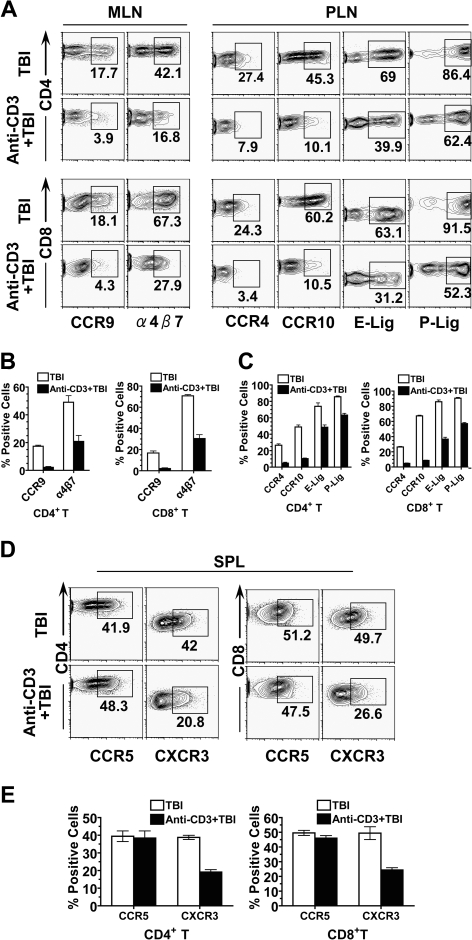
It has been reported that homing and chemokine receptors expressed by donor T cells as well as chemokines released by GVHD target tissues play a critical role in donor T-cell migration into GVHD target tissues.12,19,26 It has also been proposed that donor T cell expression of homing and chemokine receptors is induced by host DCs in LNs12,29; homing receptor α4β7 receptor and CCR9 mediate donor T cell migration into gut13,14; homing receptor E-Lig, P-Lig, and CCR4 and CCR10 mediate donor T-cell migration into skin tissues.14,16,17 Therefore, we first compared donor T-cell expression of homing and chemokine receptors in MLNs and PLNs of recipients with or without anti-CD3 preconditioning. We found that anti-CD3 preconditioning reduced the percentage of α4β7+ CD4+ and CD8+ T cells in MLNs by more than 2-fold and reduced the percentage of CCR9+ CD4+ and CD8+ T cells by more than 4-fold (P < .01, Figure 3A,B). Similarly, anti-CD3 preconditioning reduced the percentage of E-Lig+ or P-Lig+ CD4+ and CD8+ T cells in PLNs by approximately 2-fold and reduced the percentage of CCR4+ or CCR10+ CD4+ and CD8+ T cells by approximately 5-fold (P < .01, Figure 3A,B). These results indicate that anti-CD3 preconditioning inhibits donor T-cell up-regulation of homing and chemokine receptors in host draining LNs.
Figure 3.
Anti-CD3 preconditioning inhibited donor T-cell expression of homing and chemokine receptors. Five days after HCT, donor T-cell expression of gut homing α4β7 receptor and CCR9 in MLNs, donor T-cell expression of skin homing E-Lig, P-Lig, CCR4, and CCR10 in PLNs, and donor T-cell expression of non–tissue-specific CCR5 and CXCR3 in spleen were compared. There were 4 recipients in each group. (A) A representative FACS pattern of CCR9 and α4β7 receptor by gated H-2b+CD4+ or H-2b+CD8+ donor T cells from MLN, as well as a representative FACS pattern of CCR4, CCR10, E-Lig, and P-Lig of donor CD4+ or CD8+ T cells from PLN. (B) Mean plus or minus SE of CCR9+ or α4β7+ cells among donor CD4+ or CD8+ T cells from MLN. (C) Mean plus or minus SE of CCR4+, CCR10+, E-Lig+, or P-Lig+ cells among donor CD4+ or CD8+ T cells from PLN. (D) A representative FACS pattern of CCR5 and CXCR3 by gated H-2b+CD4+ or H-2b+CD8+ donor T cells from spleen. (E) Mean plus or minus SE of CCR5+ or CXCR3+ cells among gated donor CD4+ or CD8+ T cells from spleen of 4 recipients.
In addition, CCR5 and CXCR3 have been reported to be expressed by Th1 cells and mediate T-cell infiltration of nonspecific GVHD target tissues.20,21,49 Therefore, we compared donor T-cell expression of CCR5 and CXCR3 in spleen of recipients with or without anti-CD3 preconditioning. We found that, although there was no significant difference in the percentage of donor CCR5+ CD4+ and CD8+ T cells, there was a 2-fold reduction in the percentage of CXCR3+ CD4+ and CD8+ T cells in recipients preconditioned with anti-CD3 (Figure 3D,E). These results indicate that anti-CD3 preconditioning can also inhibit donor T-cell expression of some non–tissue-specific chemokine receptors.
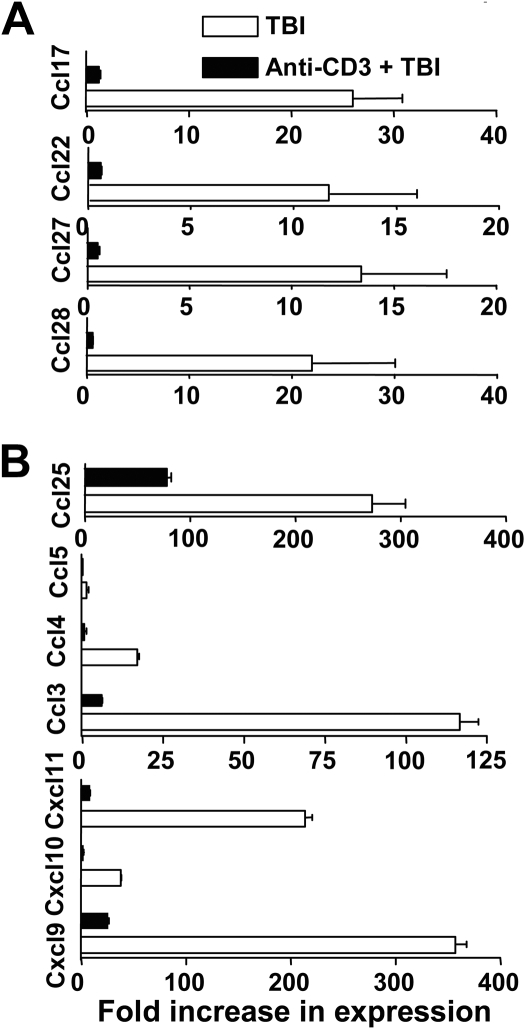
Next, we compared the GVHD target tissue expression of chemokines, including skin tissue expression of CCL17 and CCL22 (CCR4 ligand), and CCL27 and CCL28 (CCR10 ligand), the gut tissue expression of CCL25 (CCR9 ligand), and non–tissue-specific chemokines CCL3-5 (CCR5 ligand) and CXCL9-11 (CXCR3 ligand). We found that anti-CD3 preconditioning reduced the skin tissue expression of CCL17, CCL22, CCL27, and CCL28 by more than 10-fold and reduced gut tissue expression of CCL25 by more than 3-fold (P < .01, Figure 4). In addition, anti-CD3 preconditioning reduced the gut, skin, and liver tissue expression of CCL3-5 and CXCL9-11 by more than 10-fold (P < .01; Figure 4B and Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). These results indicate that anti-CD3 preconditioning inhibits the release of inflammatory chemokines in GVHD target tissues trigged by TBI conditioning.
Figure 4.
Anti-CD3 preconditioning inhibited GVHD target tissue expression of chemokines. Expression of chemokine mRNA at day 5 after HCT in various tissues (including skin and colon) of recipients conditioned with TBI and with or without anti-CD3 preconditioning was measured by real-time RT-PCR. (A) Expression of Ccl17, Ccl22, Ccl27, and Ccl28 by skin tissues. (B) Expression of Ccl25, Ccl3-5, and CXCL9-11 by colon tissue. Data are presented relative to the expression in syngeneic control recipients. Mean plus or minus SE of 4 recipients in each group is shown.
Anti-CD3 preconditioning reduced CD103+ DCs in MLNs and down-regulated MLN DC capacity in imprinting donor T-cell expression of gut homing and chemokine receptors
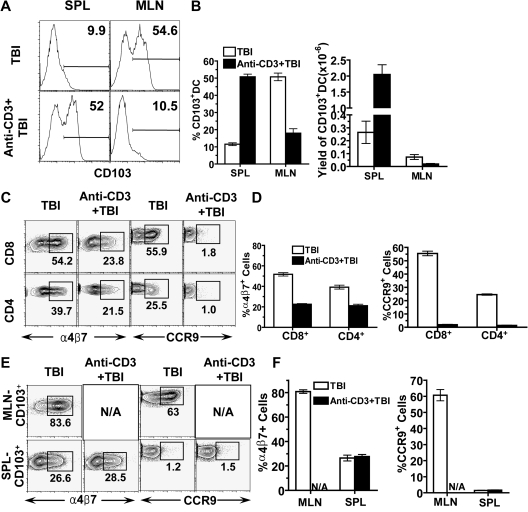
We observed a marked reduction of α4β7+ CCR9+ donor T cells in MLNs of recipients preconditioned with anti-CD3 (Figure 3), and it was reported that CD103+ DCs in MLN induced T-cell expression of α4β7 receptor and CCR9.27 Therefore, we evaluated the effect of anti-CD3 preconditioning on the percentage and yield of CD103+ DCs in MLNs as well as the capacity of MLN DCs in inducing donor T-cell expression of gut homing α4β7 receptor and CCR9. We found that anti-CD3 preconditioning reduced the percentage and yield of CD103+ DCs in MLN by approximately 5-fold, but increased the percentage and yield of CD103+ DCs in spleen by more than 5-fold (P < .01, Figure 5A,B). These results indicate that anti-CD3 preconditioning reduces CD103+ DCs in MLN.
Figure 5.
Anti-CD3 preconditioning reduced CD103+ DCs in MLN and reduced MLN DC capacity to induce donor T-cell expression of α4β7 receptor and CCR9. Spleen and MLN cells of BALB/c mice with or without anti-CD3 preconditioning were harvested and enriched for CD11c+ DCs by micromagnetic beads. The CD11c+-enriched cells were further analyzed with flow cytometry or used for in vitro culture. (A) A representative FACS pattern of CD103 expression among CD11c+ DCs. (B) Mean plus or minus SE of CD103+ cells among CD11c+ DCs and the yield of CD103+CD11c+ DCs in spleen and MLNs of 4 mice with or without anti-CD3 preconditioning. (C) Sorted CD4+/CD8+ T cells (0.2 × 106) from C57BL/6 spleen were cocultured with enriched CD11c+ DCs (0.1 × 106) from the MLNs of host BALB/c mice with or without anti-CD3 preconditioning for 4 days. Thereafter, donor CD4+ or CD8+ T cells were analyzed for the expression of α4β7 receptor and CCR9. One representative of 4 replicate experiments is shown. (D) Mean plus or minus SE of the percentage of α4β7+ or CCR9+ cells among donor CD4+ or CD8+ T cells in the culture of the 4 experiments. (E) Sorted donor CD8+ T cells (0.2 × 106) were cocultured with CD103+ DCs (0.05 × 106) from MLN and spleen of the host mice, and then donor CD8+ T-cell expression of α4β7 receptor and CCR9 were analyzed. The α4β7+ or CCR9+ CD8+ T cells were gated. One representative of 4 replicate experiments is shown. (F) Mean plus or minus SE of the α4β7+ receptor or CCR9+ cells among donor CD8+ T cells of the 4 experiments.
Next, we compared the capacity of MLN DCs to induce donor T-cell expression of α4β7 receptor and CCR9 in vitro as described previously.27 We observed that CD11c+ DCs from MLN of BALB/c mice without anti-CD3 preconditioning induced approximately 50% of donor CD8+ T cells to express α4β7 receptor and CCR9, and induced 40% and 25% of donor CD4+ T cells to express α4β7 receptor and CCR9, respectively. In contrast, anti-CD3 preconditioning reduced the capacity of DCs to induce donor CD4+ and CD8+ T-cell expression of α4β7 receptor by 2-fold and almost completely abrogated the capacity of DCs to induce donor CD4+ and CD8+ T cell expression of CCR9 (P < .01, Figure 5C,D). These results indicate that the marked reduction of CD103+ DCs in MLNs after anti-CD3 preconditioning leads to marked reduction of the capacity of MLN DCs to induce donor T-cell expression of α4β7 receptor and CCR9.
Because anti-CD3 preconditioning markedly increased the percentage and yield of CD103+ DCs in spleen (Figure 5A,B), we compared the ability of spleen and MLN CD103+ DCs to induce donor T-cell expression of α4β7 receptor and CCR9. Accordingly, CD103+ DCs were sorted from MLN and spleen of control mice or mice that had been anti-CD3-preconditioned. There was not a sufficient number of CD103+ DCs in the MLNs of mice conditioned with anti-CD3 available for experiments. The sorted CD103+ DCs were cocultured with sorted donor CD8+ T cells. We found that CD103+ DCs from MLNs of the control mice without anti-CD3 preconditioning induced more than 80% or 60% of donor CD8+ T cells to express α4β7 receptor or CCR9, respectively. In contrast, CD103+CD11c+ DCs from the spleen of the same mice induced 3-fold fewer α4β7+ and 50-fold fewer CCR9+ donor CD8+ T cells (P < .01, Figure 5E,F). Similarly, CD103+ DCs from spleen of mice preconditioned with anti-CD3 failed to induce donor CD8+ T cell expression of CCR9 (Figure 5E,F). These results indicate that CD103+ DCs in MLN but not in spleen can efficiently induce donor T cells to up-regulate both α4β7 receptor and CCR9. We should point out that, although MLN DCs have been reported to induce CCR9+FoxP3+ regulatory T (Treg) cells when cocultured with ovalbumin-specific transgenic CD4+ T cells,50,51 host MLN DCs induce alloreactive donor CD4+ and CD8+ T cells to express only CCR9 but not FoxP3 (data not shown).
Reduction of CD103+ DCs in MLNs by anti-CD3 preconditioning was associated with down-regulation of CCR7 on CD103+ DCs
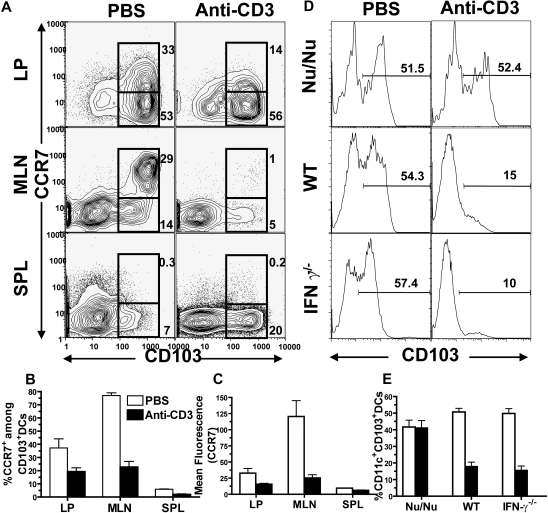
It was proposed that CD103+ DCs migration from LP to MLN tissue was dependent on their expression of CCR7.27 Therefore, we compared the CCR7 expression by CD103+ DCs in LP and MLNs with or without anti-CD3 preconditioning. We found that, consistent with a previous report,27 most (> 85%) of the CD11c+ DCs from LP were CD103+, which was approximately 2- or 15-fold higher than that in MLNs or spleen, respectively (P < .01, Figure 6A). We also found that CCR7 expression levels on CD103+ DCs in LP were variable, and approximately 30% of the CD103+ DCs stained positive for CCR7. Interestingly, the percentage of CCR7+ DCs among CD103+ DCs in MLN tissue was 2-fold higher than that in LP, and their expression levels of CCR7 was 4-fold higher than that in LP (P < .01, Figure 6A-C). These results indicate that CD103+ DCs with high-level expression of CCR7 are enriched in MLNs.
Figure 6.
Anti-CD3 preconditioning down-regulated CCR7 expression by CD103+ DCs in intestine LP and MLN, and this effect required anti-CD3 activation of host T cells. (A) Nine days after anti-CD3 preconditioning, CD11c+ DCs from LP, MLN, and spleen of the BALB/c mice with or without preconditioning were analyzed with flow cytometry. The gated CD11c+ DCs are shown in CD103 versus CCR7. The percentage of CCR7+CD103+ or CCR7−CD103+ cells among total DCs is shown next to the gating boxes. One representative of 4 replicate experiments is shown. The mean plus or minus SE of the percentage of total CD103+ DCs among total CD11c+ DCs in different tissues before and after anti-CD3 preconditioning is 85.4 (± 2.9) versus 72.9 (± 6.5), LP; 57.2 (± 1.8) versus 8.4 (± 0.8), MLN; and 7.1 (± 0.8) versus 28.9 (± 3.1), spleen. (B) Mean plus or minus SE of CCR7+ cells among CD103+ DCs. (C) Mean plus or minus SE of CCR7 expression level (mean fluorescence) by CD103+ DCs. (D) T cell–deficient Nu/Nu mice and IFN-γ−/− mice as well as wild-type mice were preconditioned with anti-CD3 or PBS. Nine days later, the MLN cells were enriched with CD11c+ DCs, and the percentage of CD103+ DCs among total CD11c+ DCs was measured. One representative of 4 examined mice in each group is shown. (E) Mean plus or minus SE of the percentage of CD103+ DCs in MLN of 4 recipients with or without anti-CD3 preconditioning.
After anti-CD3 preconditioning, the percentage of CCR7+CD103+ DCs and their CCR7 expression levels in LP were reduced by more than 2-fold (P < .01, Figure 6A-C). Accordingly, the percentage of CCR7+CD103+ DCs among total CD11c+ DCs or among residual CD103+ DCs in MLNs was reduced by approximately 30-fold or 4-fold, respectively, and their CCR7 expression levels were reduced by 5-fold (P < .01, Figure 6A-C). There was no increase of CCR7+CD103+ DCs in spleen, although the CCR7−CD103+ DCs were increased by 4-fold (P < .01, Figure 6A-C). These results indicate that reduction of CD103+ DCs in MLNs after anti-CD3 preconditioning is associated with down-regulation of CCR7 expression by CD103+ DCs in LP. This also indicates that anti-CD3 preconditioning may prevent CD103+ DC migration from LP to MLNs.
Reduction of CD103+ DCs in MLNs by anti-CD3 preconditioning required activation of host T cells
To test whether anti-CD3 activation of host T cells was necessary for reduction of CD103+ DCs in MLN, we compared the percentage of CD103+ DCs in MLN of wild-type BALB/c and T cell–deficient BALB/c nu/nu mice before and after anti-CD3 preconditioning. We found that, while anti-CD3 preconditioning always markedly reduced the percentage of CD103+ DCs in MLNs of wild-type mice, it resulted in little change in BALB/c nu/nu mice (Figure 6D,E). These results indicate that anti-CD3 activation of host T cells is required for reduction of CD103+ DCs in MLNs.
Because reduction of CD103+ DCs in MLNs after anti-CD3 preconditioning was associated with down-regulation of CCR7 expression by the CD103+ DCs (Figure 6A-C), and anti-CD3 preconditioning led to an elevation of serum IFN-γ,52 a cytokine that was reported to regulate chemokine receptor expression,49 we compared the CD103+ DC percentage in MLN of IFN-γ−/− mice with or without anti-CD3 preconditioning. We found that anti-CD3 preconditioning still markedly reduced the percentage of CD103+ DCs in MLNs of IFN-γ−/− mice (P < .01, Figure 6D,E). These results indicate that IFN-γ is not required for reduction of CD103+ DCs in MLN by anti-CD3 preconditioning.
Discussion
We have demonstrated here that anti-CD3 preconditioning resulted in separation of GVL from GVHD in recipients conditioned with TBI. The GVHD prevention resulted from reduction of donor T-cell migration to GVHD target tissues, which was associated with inhibition of donor T-cell expression of homing and chemokine receptors as well as inhibition of GVHD target tissue expression of chemokines.
Chemokine receptors expressed by donor T cells and chemokines released by GVHD target tissues play a critical role in alloreactive T-cell migration to GVHD target tissues.19,26 Anti-CD3 preconditioning markedly reduced donor T-cell expression of α4β7 receptor and CCR9 in MLNs, and markedly reduced T-cell expression of P-Lig, E-Lig, CCR4, and CCR10 in PLNs. Anti-CD3 preconditioning also markedly reduced the expression of CCL25 (CCR9 ligand) in gut, and reduced the expression of CCL17 and CCL22 (CCR4 ligand), and CCL27 and CCL28 (CCR10 ligand) in skin. In addition, the expression of other chemokines that regulate Th1 cell migration into inflammatory tissues, including CCL3-5 (CCR5 ligands) and CXCL9-11 (CXCR3 ligands), were also markedly reduced in gut and skin tissues after anti-CD3 preconditioning. Therefore, it is not surprising that anti-CD3 preconditioning prevents donor T-cell infiltration of gut and skin tissues.
The reduction of donor T-cell expression of gut homing and chemokine receptors by anti-CD3 preconditioning resulted from reduction of CD103+ DCs in draining MLNs. It was reported that CD103+ DCs in MLNs induced T cells to express gut homing α4β7 receptor and chemokine receptor CCR9.27 Consistently, we observed that anti-CD3 preconditioning markedly reduced the percentage and yield of CD103+ DCs in MLNs and subsequently led to a marked reduction in the capacity of MLN DCs to induce donor T-cell expression of α4β7 receptor and CCR9. It is of interest that only the CD103+ DCs from MLNs but not those from spleen were able to induce donor T-cell expression of α4β7 receptor and CCR9, especially CCR9. This result was consistent with our observation that reduction of CD103+ DCs in MLN led to a reduction of α4β7+ or CCR9+ donor T cells in the recipient, even when there was an increase of CD103+ DCs in the spleen. It is not yet clear why the CD103+ DCs from the MLNs can but those from the spleen cannot induce donor T-cell expression of α4β7 receptor and CCR9. This may be due to the difference in their capacity to produce RA, because addition of RA to the culture led to the induction of donor T-cell expression of CCR9 by spleen DCs as well as by MLN DCs from mice preconditioned with anti-CD3 (Figure S2). It was reported that RA is required for induction of T-cell expression of gut homing receptors, and MLN DCs were able to secret RA by metabolizing vitamin A.15
The reduction of CD103+ DCs in MLNs was associated with down-regulation of CCR7 expression by CD103+ DCs in intestine LP after anti-CD3 preconditioning. It was reported that CD103+ DCs in LP carried the orally administered ovalbumin peptide to MLNs and induced the antigen-specific OT-II T cells to express α4β7 receptor and CCR9,27 and the CD103+ DC migration from intestinal LP to draining MLNs was CCR7-dependent.27 We found that the marked reduction of CD103+ DCs in MLNs or marked increase of CD103+ DCs in spleen after anti-CD3 preconditioning was associated with a significant reduction of CCR7 expression of CD103+ DCs in intestinal LP. These changes were dependent on anti-CD3 activation of host T cells, although IFN-γ was not required.
Anti-CD3 preconditioning markedly reduced chemokine expression in GVHD target tissues such as gut, skin, and liver in recipients conditioned with TBI. The mechanisms are not yet clear. It may result from the reduction of serum levels of IFN-γ and TNF-α as well as the reduction of some DC subsets such as plasmacytoid DCs in the tissues, because it was reported that plasmacytoid DC cells were the initiators of a complex chemokine and cytokine network,33 and because we observed a marked reduction of plasmacytoid DC antigen (PDCA)-1+ plasmacytoid DCs in liver tissues after anti-CD3 preconditioning (Figure S3 bottom row).
Taken together, we hypothesize that anti-CD3 preconditioning activates host T cells and leads to the secretion of cytokines that modulate host DC subset tissue redistribution. First, the cytokines down-regulate CCR7 on CD103+ DCs in intestinal LP, which prevents CD103+ DC migration from LP to draining MLN, although they are able to enter blood circulation and end up in the spleen. In addition, anti-CD3 preconditioning may also prevent DC migration from skin to PLN, since CCR7 was also required for DC migration from skin to PLN.28 Subsequently, the DCs in MLN and PLN lose the capacity to imprint donor T-cell gut and skin tissue tropism. In addition, anti-CD3 preconditioning also results in the reduction of PDCA-1+ plasmacytoid DCs and CD11b+ myeloid DCs in the GVHD target tissues such as liver (Figure S3), which may subsequently reduce tissue release of chemokines. Therefore, anti-CD3 preconditioning down-regulates donor T-cell expression of chemokine receptors on the one hand and down-regulates chemokine release by GVHD target tissues on the other hand. Accordingly, donor T-cell infiltration of GVHD target tissues is prevented, although they are still activated and able to kill host hematologic cells and tumor cells in lympho-hematologic tissues.
We should point out that other mechanisms may also contribute to the prevention of GVHD by anti-CD3 preconditioning. For example, a recent report showed that anti-CD3 treatment induced expansion of FoxP3+ Treg cells in anti-CD3–treated mice.53 Consistently, we also observed a 3-fold increase in donor FoxP3+CD4+ T cells in recipients preconditioned with anti-CD3 (Figure S4). The increased donor Treg cells may be the major contributor to the inhibition of donor T-cell proliferation and Th1 differentiation in the recipients preconditioned with anti-CD3. Although host DCs were reported to dictate donor T-cell activation and differentiation,3 sorted DCs from recipients with or without anti-CD3 preconditioning showed similar capacity in stimulating donor T-cell proliferation and differentiation into Th1 (Figure S5). Donor Treg cells have been reported to inhibit Th1 differentiation and prevent GVHD.4,40,54 However, we should point out that inhibition of donor T-cell expansion in lymphoid tissues by Treg cells or by potential residual anti-CD3 does not appear to be the major factor in GVHD prevention, because the reduction of donor T-cell yield in lymphoid tissues was only approximately 2-fold, but the reduction of donor T-cell infiltration in gut and skin was more than 15-fold.
In summary, this is the first report showing that modulation of host DC subset tissue distribution before HCT (ie, prevention of tissue DC migration to draining LN) leads to confinement of donor T cells to lympho-hematologic tissues and separation of GVL from GVHD. It is of interest that, although anti-CD3 preconditioning modulated host DCs and prevented GVHD, preconditioning with antithymocyte globulin (ATG), a clinically used reagent for reduced intensity conditioning (RIC), did not reduce CCR7+CD103+ DCs in host MLN or prevent GVHD in recipients who had been conditioned with TBI (Figures S6,S7). This may explain why patients conditioned with ATG-based RIC still developed severe GVHD.55,56
We are aware that, in previous reports, anti-CD3 or anti-CD3 (Fab)2 prevented GVHD and also reduced GVL activity when administered after HCT.57 We should point out that our studies are different from the previous ones, because in our studies anti-CD3 was administered 9 days before HCT, and anti-CD3 was not detectable in the serum of recipients when donor T cells were injected. In this case, anti-CD3 had little direct effect on donor T-cell activation or GVL activity.
We should also point out that there is a concern about cytokine storm triggered by anti-CD3 when intact anti-CD3 is used, although the anti-CD3 dose used in our studies is the same as that used clinically for the prevention of organ graft rejection.58 It was reported that using anti-CD3 (Fab)2 avoided cytokine storm,59 and we also recently reported that the histone deacetylase inhibitor SAHA ameliorated the cytokine storm triggered by intact anti-CD3.53 We have recently observed that preconditioning with anti-CD3 (Fab)2 or with anti-CD3 and SAHA modulated host DCs, similar to preconditioning with intact anti-CD3 alone (Figure S6). Therefore, we expect that preconditioning with the clinically applicable Fc receptor (FCR)–non-binding anti-CD360 will modulate host DCs and separate GVL from GVHD. In conclusion, modulation of HCT recipients with CD3-specific antibodies before HCT may represent a new approach for the separation of donor T cell–mediated GVL from GVHD.
Supplementary Material
Acknowledgments
We thank James Young for critical reading of the manuscript. We thank Lucy Brown and her staff at the City of Hope (COH) Flow Cytometry Facility, and Sofia Loera and her staff at the COH Anatomic Pathology Laboratory for their excellent technical assistance. We also thank Dr Richard Ermel and his staff at the COH Research Animal Facility for providing excellent animal care.
This work was supported by grants from the Marcus Foundation (S.F.) and the National Institutes of Health (R01 AI066008; D. Zeng).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.L. and Y.C. designed and performed research, analyzed data, and wrote the manuscript; W.H., D. Zhao, T.Y., C.L., C.Z., and I.T. performed research; F.K. and S.F. reviewed manuscript; and D. Zeng designed research, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Defu Zeng, MD, The Beckman Research Institute, Gonda Building, R2017, City of Hope National Medical Center, 1500 East Duarte Road, Duarte, CA 91010; e-mail: dzeng@coh.org.
References
- 1.Reddy P, Maeda Y, Liu C, Krijanovski OI, Korngold R, Ferrara JL. A crucial role for antigen-presenting cells and alloantigen expression in graft-versus-leukemia responses. Nat Med. 2005;11:1244–1249. doi: 10.1038/nm1309. [DOI] [PubMed] [Google Scholar]
- 2.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 4.Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol. 2007;25:139–170. doi: 10.1146/annurev.immunol.25.022106.141606. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara J, Antn J. The pathophysiology of graft-vs.-host disease. In: Blume KG, Forman SJ, Appelbaum FR, editors. Thomas' hematopoietic cell transplantation. Malden, MA: Blackwell Publishing Ltd; 2004. pp. 353–368. [Google Scholar]
- 6.Panoskaltsis-Mortari A, Price A, Hermanson JR, et al. In vivo imaging of graft-versus-host-disease in mice. Blood. 2004;103:3590–3598. doi: 10.1182/blood-2003-08-2827. [DOI] [PubMed] [Google Scholar]
- 7.Beilhack A, Schulz S, Baker J, et al. In vivo analyses of early events in acute graft-versus-host disease reveal sequential infiltration of T cell subsets. Blood. 2005;106:1113–1122. doi: 10.1182/blood-2005-02-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YM, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J Clin Invest. 2003;111:659–669. doi: 10.1172/JCI16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang C, Lou J, Li N, et al. Donor CD8+ T cells mediate graft-versus-leukemia activity without clinical signs of graft-versus-host disease in recipients conditioned with anti-CD3 monoclonal antibody. J Immunol. 2007;178:838–850. doi: 10.4049/jimmunol.178.2.838. [DOI] [PubMed] [Google Scholar]
- 10.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 11.Duffner UA, Maeda Y, Cooke KR, et al. Host dendritic cells alone are sufficient to initiate acute graft-versus-host disease. J Immunol. 2004;172:7393–7398. doi: 10.4049/jimmunol.172.12.7393. [DOI] [PubMed] [Google Scholar]
- 12.Sackstein R. A revision of Billingham's tenets: the central role of lymphocyte migration in acute graft-versus-host disease. Biol Blood Marrow Transplant. 2006;12:2–8. doi: 10.1016/j.bbmt.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Tietz W, Allemand Y, Borges E, et al. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol. 1998;161:963–970. [PubMed] [Google Scholar]
- 17.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigmundsdottir H, Pan J, Debes GF, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 19.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wysocki CA, Burkett SB, Panoskaltsis-Mortari A, et al. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J Immunol. 2004;173:845–854. doi: 10.4049/jimmunol.173.2.845. [DOI] [PubMed] [Google Scholar]
- 21.Duffner U, Lu B, Hildebrandt GC, et al. Role of CXCR3-induced donor T cell migration in acute GVHD. Exp Hematol. 2003;31:897–902. doi: 10.1016/s0301-472x(03)00198-x. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrandt GC, Corrion LA, Olkiewicz KM, et al. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J Immunol. 2004;173:2050–2059. doi: 10.4049/jimmunol.173.3.2050. [DOI] [PubMed] [Google Scholar]
- 23.Varona R, Cadenas V, Gomez L, Martinez AC, Marquez G. CCR6 regulates CD4+ T cell-mediated acute graft-versus-host disease responses. Blood. 2005;106:18–26. doi: 10.1182/blood-2004-08-2996. [DOI] [PubMed] [Google Scholar]
- 24.Terwey TH, Kim TD, Kochman AA, et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood. 2005;106:3322–3330. doi: 10.1182/blood-2005-05-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaksch M, Remberger M, Mattsson J. Increased gene expression of chemokine receptors is correlated with acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11:280–287. doi: 10.1016/j.bbmt.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Chakraverty R, Cote D, Buchli J, et al. An inflammatory checkpoint regulates recruitment of graft-versus-host reactive T cells to peripheral tissues. J Exp Med. 2006;203:2021–2031. doi: 10.1084/jem.20060376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johansson-Lindbom B, Svensson M, Pabst O, et al. Functional specialization of gut CD103+ dendritic cells in the regulation of tissue-selective T cell homing. J Exp Med. 2005;202:1063–1073. doi: 10.1084/jem.20051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohl L, Mohaupt M, Czeloth N, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 29.Kim TD, Terwey TH, Zakrzewski JL, et al. Organ-derived dendritic cells have differential effects on alloreactive T cells. Blood. 2008;111:2929–2940. doi: 10.1182/blood-2007-06-096602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beilhack A, Schulz S, Baker J, et al. Prevention of acute graft-versus-host disease by blocking T cell entry to secondary lymphoid organs. Blood. 2008;111:2919–2928. doi: 10.1182/blood-2007-09-112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Shlomchik WD, Joe G, et al. APCs in the liver and spleen recruit activated allogeneic CD8+ T cells to elicit hepatic graft-versus-host disease. J Immunol. 2002;169:7111–7118. doi: 10.4049/jimmunol.169.12.7111. [DOI] [PubMed] [Google Scholar]
- 32.Mapara MY, Leng C, Kim YM, et al. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol Blood Marrow Transplant. 2006;12:623–634. doi: 10.1016/j.bbmt.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Decalf J, Fernandes S, Longman R, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007;204:2423–2437. doi: 10.1084/jem.20070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turnbull EL, Yrlid U, Jenkins CD, Macpherson GG. Intestinal dendritic cell subsets: differential effects of systemic TLR4 stimulation on migratory fate and activation in vivo. J Immunol. 2005;174:1374–1384. doi: 10.4049/jimmunol.174.3.1374. [DOI] [PubMed] [Google Scholar]
- 35.Cooke KR, Gerbitz A, Crawford JM, et al. LPS antagonism reduces graft-versus-host disease and preserves graft-versus-leukemia activity after experimental bone marrow transplantation. J Clin Invest. 2001;107:1581–1589. doi: 10.1172/JCI12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muraille E, Andris F, Pajak B, et al. Downregulation of antigen-presenting cell functions after administration of mitogenic anti-CD3 monoclonal antibodies in mice. Blood. 1999;94:4347–4357. [PubMed] [Google Scholar]
- 37.Yi T, Zhao D, Lin CL, et al. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008;112:2101–2110. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drakes ML, Czinn SJ, Blanchard TG. Isolation and purification of colon lamina propria dendritic cells from mice with colitis. Cytotechnology. 2004;46:151–161. doi: 10.1007/s10616-005-2552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng D, Hoffmann P, Lan F, Huie P, Higgins J, Strober S. Unique patterns of surface receptors, cytokine secretion, and immune functions distinguish T cells in the bone marrow from those in the periphery: impact on allogeneic bone marrow transplantation. Blood. 2002;99:1449–1457. doi: 10.1182/blood.v99.4.1449. [DOI] [PubMed] [Google Scholar]
- 40.Zhao D, Zhang C, Yi T, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood. 2008;112:2129–2138. doi: 10.1182/blood-2008-02-140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Huang X, Yi T, Yen Y, Moore DD, Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 42.Murai M, Yoneyama H, Harada A, et al. Active participation of CCR5(+)CD8(+) T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J Clin Invest. 1999;104:49–57. doi: 10.1172/JCI6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beaty SR, Rose CE, Jr, Sung SS. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol. 2007;178:1882–1895. doi: 10.4049/jimmunol.178.3.1882. [DOI] [PubMed] [Google Scholar]
- 44.Stenstad H, Svensson M, Cucak H, Kotarsky K, Agace WW. Differential homing mechanisms regulate regionalized effector CD8alphabeta+ T cell accumulation within the small intestine. Proc Natl Acad Sci U S A. 2007;104:10122–10127. doi: 10.1073/pnas.0700269104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hieshima K, Kawasaki Y, Hanamoto H, et al. CC chemokine ligands 25 and 28 play essential roles in intestinal extravasation of IgA antibody-secreting cells. J Immunol. 2004;173:3668–3675. doi: 10.4049/jimmunol.173.6.3668. [DOI] [PubMed] [Google Scholar]
- 46.Cooke KR, Kobzik L, Martin TR, et al. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation: I. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 47.Kaplan DH, Anderson BE, McNiff JM, Jain D, Shlomchik MJ, Shlomchik WD. Target antigens determine graft-versus-host disease phenotype. J Immunol. 2004;173:5467–5475. doi: 10.4049/jimmunol.173.9.5467. [DOI] [PubMed] [Google Scholar]
- 48.Blazar BR, Carreno BM, Panoskaltsis-Mortari A, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171:1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 49.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 50.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 51.Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li N, Zhao D, Kirschbaum M, et al. HDAC inhibitor reduces cytokine storm and facilitates induction of chimerism that reverses lupus in anti-CD3 conditioning regimen. Proc Natl Acad Sci U S A. 2008;105:4796–4801. doi: 10.1073/pnas.0712051105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perruche S, Zhang P, Liu Y, Saas P, Bluestone JA, Chen W. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat Med. 2008;14:528–535. doi: 10.1038/nm1749. [DOI] [PubMed] [Google Scholar]
- 54.Zeng D, Lan F, Hoffmann P, Strober S. Suppression of graft-versus-host disease by naturally occurring regulatory T cells. Transplantation. 2004;77:S9–S11. doi: 10.1097/01.TP.0000106475.38978.11. [DOI] [PubMed] [Google Scholar]
- 55.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–763. [PubMed] [Google Scholar]
- 56.Giralt S. Reduced-intensity conditioning regimens for hematologic malignancies: what have we learned over the last 10 years? Hematology Am Soc Hematol Educ Program. 2005:384–389. doi: 10.1182/asheducation-2005.1.384. [DOI] [PubMed] [Google Scholar]
- 57.Johnson BD, McCabe C, Hanke CA, Truitt RL. Use of anti-CD3 epsilon F(ab′)2 fragments in vivo to modulate graft-versus-host disease without loss of graft-versus-leukemia reactivity after MHC-matched bone marrow transplantation. J Immunol. 1995;154:5542–5554. [PubMed] [Google Scholar]
- 58.Webster AC, Pankhurst T, Rinaldi F, Chapman JR, Craig JC. Monoclonal and polyclonal antibody therapy for treating acute rejection in kidney transplant recipients: a systematic review of randomized trial data. Transplantation. 2006;81:953–965. doi: 10.1097/01.tp.0000215178.72344.9d. [DOI] [PubMed] [Google Scholar]
- 59.Blazar BR, Jenkins MK, Taylor PA, et al. Anti-CD3 epsilon F(ab′)2 fragments inhibit T cell expansion in vivo during graft-versus-host disease or the primary immune response to nominal antigen. J Immunol. 1997;159:5821–5833. [PubMed] [Google Scholar]
- 60.Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.