Abstract
Neonatal exposures to organophosphates that are not acutely symptomatic or that produce little or no cholinesterase inhibition can nevertheless compromise the development and later function of critical neural pathways, including serotonin (5HT) systems that regulate emotional behaviors. We administered parathion to newborn rats on postnatal days (PN) 1-4 at doses spanning the threshold for detectable cholinesterase inhibition (0.1 mg/kg/day) and the first signs of loss of viability (0.2 mg/kg/day). In adolescence (PN30), young adulthood (PN60) and full adulthood (PN100), we measured radioligand binding to 5HT1A and 5HT2 receptors, and to the 5HT transporter in the brain regions comprising all the major 5HT projections and 5HT cell bodies. Parathion caused a biphasic effect over later development with initial, widespread upregulation of 5HT1A receptors that peaked in the frontal/parietal cortex by PN60, followed by a diminution of that effect in most regions and emergence of deficits at PN100. There were smaller, but statistically significant changes in 5HT2 receptors and the 5HT transporter. These findings stand in strong contrast to previous results with neonatal exposure to a different organophosphate, chlorpyrifos, which evoked parallel upregulation of all three 5HT synaptic proteins that persisted from adolescence through full adulthood and that targeted males much more than females. Our results support the view that the various organophosphates have disparate effects on 5HT systems, distinct from their shared property as cholinesterase inhibitors, and the targeting of 5HT function points toward the importance of studying the impact of these agents on 5HT-linked behaviors.
Keywords: Organophosphate pesticides, Parathion, Serotonin Receptors, Serotonin Transporter, Sex-Selective Neurotoxicity
INTRODUCTION
It is increasingly evident that organophosphate pesticides damage the developing brain at exposures below the threshold for overt signs of intoxication and even below that required for cholinesterase inhibition, the biomarker used for risk assessment [12,14,22,23,30,36-38,46-48,63]. Numerous studies have detailed how organophosphates disrupt the basic patterns of neural cell replication and differentiation, alter axonogenesis and synaptogenesis, and discoordinate the development of neural circuits, ultimately producing widespread behavioral deficits [7,9,10,12,18,38-40,46-48,56,65]. Because of the initial focus on cholinergic actions, many reports of the effects of organophosphate exposure have concerned the targeting of acetylcholine systems and cognitive/learning deficits related to these pathways [15,16,19,21,22,26,27,41,46-48,63]. However, recent research indicates that organophosphates target serotonin (5HT) systems to an even greater extent, contributing to adverse outcomes related to emotional and social behaviors [1-5,33,42,43,51,53-55,57,58,62]. Indeed, evidence is now accumulating that relate organophosphate exposures to depression and suicide [8,20,25,28].
Because organophosphates cause developmental neurotoxicity through mechanisms beyond their shared property as cholinesterase inhibitors, the various members of this pesticide class could differ in their impact on 5HT systems. In recent studies, we showed that exposures of neonatal rats on postnatal days (PN) 1-4 to three different organophosphates, chlorpyrifos, diazinon and parathion, at doses spanning the threshold for detectable but nonsymptomatic cholinesterase inhibition, produced dissimilar initial effects on 5HT systems, as monitored on PN5 [4,52,58]. Notably, parathion was entirely distinct, eliciting deficits in 5HT1A receptor expression, whereas the other two organophosphates produced increases. In subsequent work, we showed some basic similarities in the long-term effects of chlorpyrifos and diazinon on 5HT systems, but also some significant disparities that emerged between adolescence and adulthood [3,5,51,55], contributing to divergent effects on emotional behaviors [1,45]. Accordingly, in the present study, we evaluated the long-term effects of neonatal parathion exposure, again conducting studies from adolescent through adult stages. We gave parathion on PN1-4, an exposure window identified in our earlier work with chlorpyrifos as a peak of sensitivity for disrupting 5HT systems [2,4,5,54]. We focused on two parathion treatments spanning the maximum tolerated dose, 0.1 mg/kg/day, which produces 10% cholinesterase inhibition [58], well below the 70% inhibition required for the symptoms of cholinergic hyperstimulation [13], and 0.2 mg/kg/day, just past the threshold for the first signs of systemic toxicity in neonates [50]. Our measurements focused on three 5HT synaptic proteins known to be highly affected by developmental exposure to chlorpyrifos [3-5,55] or diazinon [51,58], the 5HT1A and 5HT2 receptors, and the presynaptic 5HT transporter (5HTT). The two receptors play major roles in 5HT-related mental disorders, particularly depression [6,17,66,67], and the transporter, which regulates the synaptic concentration of 5HT, is the primary target for antidepressant drugs [29,34,35]. We evaluated effects in all the brain regions comprising the major 5HT projections (frontal/parietal cortex, temporal/occipital cortex, hippocampus, striatum) as well as those containing 5HT cell bodies (midbrain, brainstem). The study design and assays were all identical to those in our previous work on chlorpyrifos and diazinon [1,5,51,55,58], so as to foster comparison of the outcomes of exposure to the three different organophosphates.
METHODS
Animal treatments
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Institutional Animal Care and Use Committee and in accordance with all federal and state guidelines. Timed-pregnant Sprague–Dawley rats were housed in breeding cages, with a 12 h light–dark cycle and free access to food and water. On the day after birth, all pups were randomized and redistributed to the dams with a litter size of 10 (5 males, 5 females) to maintain a standard nutritional status. Because of its poor water solubility, parathion was dissolved in dimethylsulfoxide to provide consistent absorption [50,58,64] and was injected subcutaneously in a volume of 1 ml/kg once daily on postnatal days (PN) 1-4; control animals received equivalent injections of the dimethylsulfoxide vehicle, which does not itself produce developmental neurotoxicity [64]. Doses of 0.1 and 0.2 mg/kg/day were chosen because they straddle the threshold for detectable cholinesterase inhibition and the first signs of impaired viability [50,58]: the low dose produces 5-10% inhibition without mortality, whereas the higher dose elicits 5-10% mortality. The PN1-4 regimen was chosen because it represents a peak period for sensitivity of 5HT systems to the developmental neurotoxicity of chlorpyrifos [46-48] and because the systemic toxicity and cholinesterase inhibition in response to parathion have already been characterized for this treatment window [50,58]. Accordingly, both the toxicodynamic effects and treatment window parallel those used in our prior studies with chlorpyrifos and diazinon [1,5,51,55,58]. Randomization of pup litter assignments within treatment groups was repeated at intervals of several days up until weaning, coordinated with weighing of the animals and changes of cage bedding, and in addition, dams were rotated among litters to distribute any maternal caretaking differences randomly across litters and treatment groups. Each treatment group comprised 12 litters and all pups within a reconstituted litter belonged to the same treatment group to ensure that dams did not discriminate between control and treated pups in the maternal caretaking or nursing behaviors. Offspring were weaned on PN21.
On PN30, 60 and 100, one male and one female were selected from each litter of origin and were decapitated. The cerebellum (including flocculi) was removed and the midbrain/brainstem was separated from the forebrain by a cut rostral to the thalamus. The striatum and hippocampus were then dissected from these larger divisions and the midbrain and brainstem were divided from each other. The cerebral cortex was divided down the midline and then further sectioned into anterior and posterior regions (frontal/parietal cortex and temporal/occipital cortex, respectively). The cerebellum, which is sparse in 5HT projections, was reserved for future studies; also, the midbrain, hippocampus and striatum were not evaluated on PN30 because these regions were utilized in another study of acetylcholine biomarkers [49]. Tissues were frozen with liquid nitrogen and stored at -45° C.
Assays
All of the assay methodologies used in this study have appeared in previous papers [5,50,53,55], so only brief descriptions will be provided here. Tissues were thawed and homogenized (Polytron, Brinkmann Instruments, Westbury, NY) in ice-cold 50 mM Tris (pH 7.4), and the homogenates were sedimented at 40,000 × g for 15 min. The pellets were washed by resuspension (Polytron) in homogenization buffer followed by resedimentation, and were then dispersed with a homogenizer (smooth glass fitted with Teflon pestle) in the same buffer. An aliquot was assayed for measurement of membrane protein [59].
Two radioligands were used to determine 5HT receptor binding: 1 nM [3H]8-hydroxy-2-(di-n-propylamino)tetralin for 5HT1A receptors, and 0.4 nM [3H]ketanserin for 5HT2 receptors. Binding to the presynaptic 5HT transporter was evaluated with 85 pM [3H]paroxetine. For 5HT1A receptors and the 5HT transporter, specific binding was displaced by addition of 100 μM 5HT; for 5HT2 receptors, we used 10 μM methylsergide for displacement. The overall strategy was to examine binding at a single ligand concentration in preparations from all regions in every animal, focusing on a concentration above the Kd but below full saturation. We can thus detect changes that originate either in altered Kd or Bmax but can not distinguish between the two possible mechanisms, albeit that a change in Kd would seem highly unlikely. This strategy was necessitated by the amount of tissue available for each determination and technical limitations engendered by the requirement to measure binding in three treatment groups at three different ages in multiple brain regions, with at least six animals for each sex. Thus, there were hundreds of separate membrane preparations, each of which had to be evaluated for binding of three different ligands.
Data analysis
Data were compiled as means and standard errors. Because we evaluated binding parameters for multiple proteins all related to 5HT synapses, the initial comparisons were conducted by a global ANOVA (data log-transformed because of heterogeneous variance among ages, regions and the different protein measures) incorporating all the variables and measurements so as to avoid an increased probability of type 1 errors that might otherwise result from multiple tests of the same data set. Where we identified interactions of treatment with the other variables, data were then subdivided for lower-order ANOVAs to evaluate treatments that differed from the corresponding control. Where permitted by the interaction terms, individual groups that differed from control in a given region at a given age were identified with Fisher’s Protected Least Significant Difference Test; however, where only main treatment effects were present (without interactions), we present the main effect without subsequent lower-order tests of individual values. Significance was assumed at the level of p < 0.05. For interactions at p < 0.1, we also examined whether lower-order main effects were detectable after subdivision of the interactive variables [60]. The p < 0.1 criterion for interaction terms was not used to assign significance to the effects but rather to identify interactive variables requiring subdivision for lower-order tests of the main effects of parathion, the variable of chief interest [60].
For convenience, the results are presented as the percent change from control values but statistical comparisons were conducted only on the original data. Although not shown here, the control values for each variable were quite similar to those published in our previous reports [5,51,53,55].
Materials
Animals were obtained from Charles River (Raleigh, NC) and parathion was purchased from Chem Service (West Chester, PA). PerkinElmer Life Sciences (Boston, MA) was the source for [3H]8-hydroxy-2-(di-n-propylamino)tetralin (specific activity, 135 Ci/mmol), [3H]ketanserin (63 Ci/mmol) and [3H]paroxetine (19.4 Ci/mmol). Methylsergide was obtained from Sandoz Pharmaceuticals (E. Hanover, NJ) and all other chemicals came from Sigma Chemical Co. (St. Louis, MO).
RESULTS
Because only three of the six regions were analyzed at all three ages, there were two global ANOVAs conducted on the data set. The first evaluated all measurements at all ages for the frontal/parietal cortex, temporal/occipital cortex and brainstem and the second evaluated the two ages (PN 60, PN100) for which we had all six regions. The ANOVAs indicated interactions of treatment × region (p < 0.006), treatment × protein measure (p < 0.003), treatment × age × sex (p < 0.003), treatment × age × region (p < 0.0001), treatment × sex × region (p < 0.003), treatment × region × measure (p < 0.0001), treatment × age × sex × region (p < 0.04), treatment × age × region × measure (p < 0.0001), treatment × sex × region × measure (p < 0.0001), and treatment × age × sex × region × measure (p < 0.05). We subdivided the data by age and sex, and evaluated treatment effects and interactions of treatment with region and measure in lower-order tests so as to facilitate comparison of the present results with our prior, parallel studies of chlorpyrifos and diazinon [1,5,51,55,58].
We similarly conducted global ANOVAs for effects on brain region weight and body weight, both of which showed no significant differences (data not shown). However, in the preweaning period, across the entire cohort of animals (a greater number than those used for the neurochemical studies presented here), we did find a significant reduction in body weight (p < 0.005 for treatment, p < 0.003 for treatment × age), with the main effect confined to the group receiving 0.2 mg/kg (p < 0.0001), representing approximately a 5% decrease from control values. We also evaluated large numbers of animals through 22 weeks postpartum, well beyond the period studied here, and found small, later-emerging body weight deficits at either dose in females, again amounting to about 5% (data not shown). These weight data were compiled from cohorts of animals utilized in this and two other studies [24,49].
On PN30, there were significant elevations in 5HT1A receptors in both males (Figure 1A) and females (Figure 1B). With each sex considered separately, significant effects were seen only at the higher parathion dose; however, the higher-order test (treatment, region, sex) indicated a main effect of both doses of parathion, without a significant interaction with either region or sex. The effects on 5HT2 receptors were distinctly smaller and in the opposite direction. Females showed a significant decrement in the temporal/occipital cortex in the groups receiving either the low or high dose of parathion but again, the higher-order ANOVA indicated a main treatment effect (p < 0.02) across both sexes (no treatment × sex interaction), with regional distinctions (treatment × region, p < 0.03) and with significant reductions in both the frontal/parietal cortex (p < 0.05) and the temporal/occipital cortex (p < 0.02). In contrast to the receptor measurements, there were no significant treatment-related differences for 5HTT binding.
Figure 1.
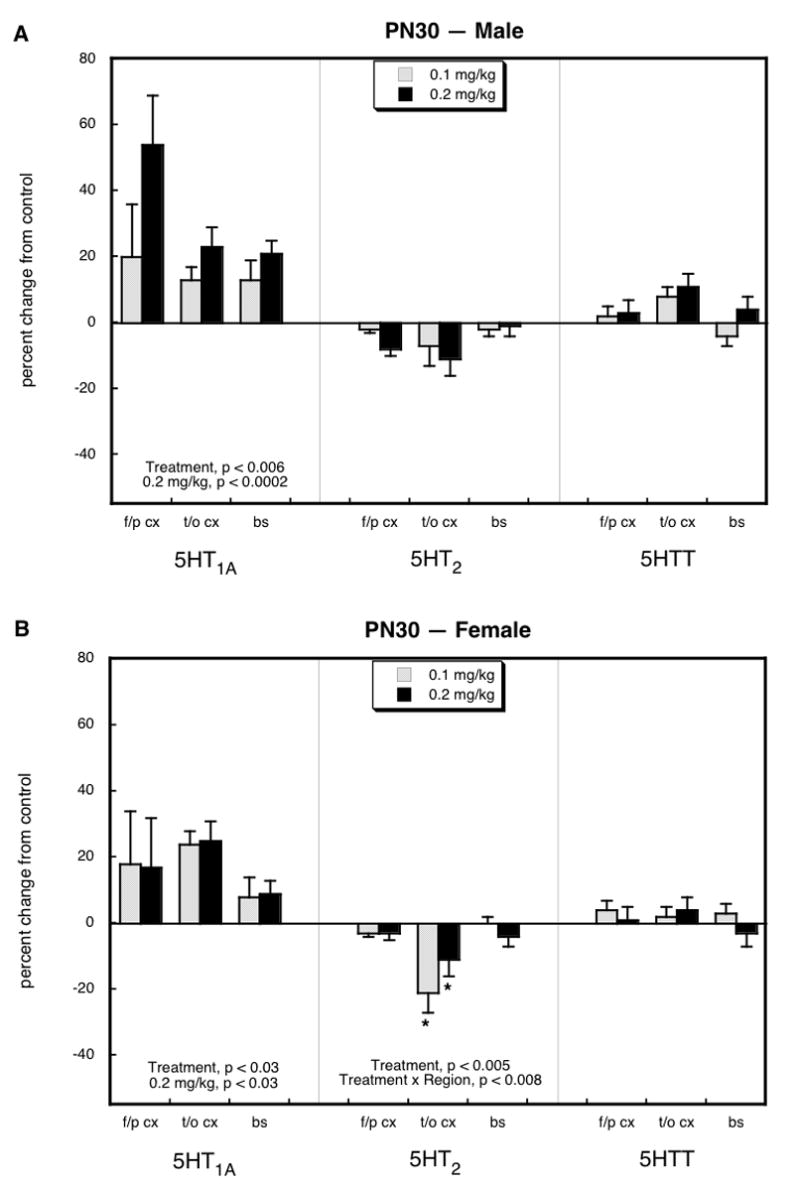
Effects of neonatal parathion exposure on 5HT synaptic proteins on PN30 in males (A) and females (B). Data represent means and standard errors obtained from at least 6 animals in each treatment group for each sex. Global ANOVA (treatment, region, sex, protein measure) indicates a significant main treatment effect (p < 0.005 for all treatments, p < 0.05 for 0.1 mg/kg vs. control, p < 0.0001 for 0.2 mg/kg vs. control) and an interaction of treatment × measure (p < 0.0001). Lower-order ANOVAs for each subdivision appear within the panels. Where there was no interaction of treatment × region, only main treatment effects are reported; where there was an interaction of treatment × region, asterisks denote individual values that differ from the corresponding control. Abbreviations: f/p cx = frontal/parietal cortex; t/o cx = temporal/occipital cortex; bs = brainstem.
A different pattern emerged by PN60, in young adulthood. In males (Figure 2A), we still saw a large increase in 5HT1A receptors in the frontal/parietal cortex but the increases were no longer evident in the temporal/occipital cortex and brainstem. Indeed, the brainstem, as well as the hippocampus, displayed significant decrements, with an especially large loss of receptors in the latter region. In the striatum, we saw a small but statistically significant increase in 5HT1A receptors. Again, the effects on 5HT2 receptors and the 5HTT site were much less notable, with small decrements (brainstem 5HT2 receptors and 5HTT, hippocampus 5HTT) or increments (hippocampus 5HT2 receptors, frontal/parietal cortex 5HTT). In young adult females (Figure 2B), we likewise still saw a significant elevation in 5HT1A receptors in the frontal/parietal cortex, of even greater magnitude than had been seen on PN30. However, again the earlier increase was lost in the temporal/occipital cortex and brainstem, and indeed, the brainstem, midbrain, hippocampus and striatum all showed uniform deficits of 20-40%. We also saw a small increase in 5HT2 receptors and the 5HTT site in the striatum.
Figure 2.
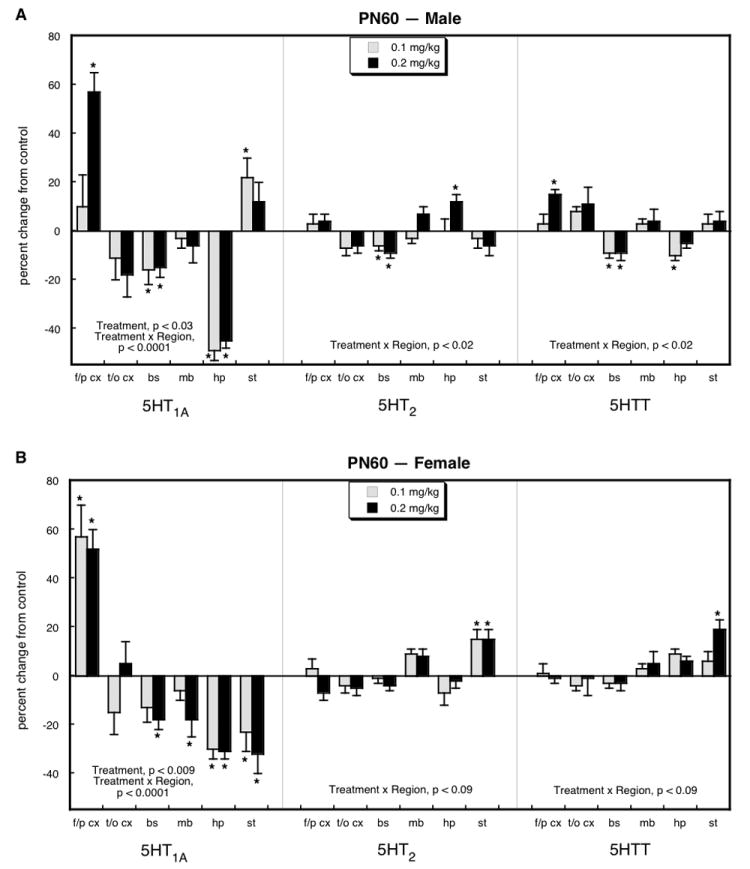
Effects of neonatal parathion exposure on 5HT synaptic proteins on PN60 in males (A) and females (B). Data represent means and standard errors obtained from at least 6 animals in each treatment group for each sex. Global ANOVA (treatment, region, sex, protein measure) indicates interactions of treatment × sex (p < 0.05), treatment × measure (p < 0.06), treatment × sex × region (p < 0.005), treatment × region × measure (p < 0.0001) and treatment × sex × region × measure (p < 0.0001). Lower-order ANOVAs for each subdivision appear within the panels. Asterisks denote individual values that differ from the corresponding control. Abbreviations: f/p cx = frontal/parietal cortex; t/o cx = temporal/occipital cortex; bs = brainstem; mb = midbrain; hp = hippocampus; st = striatum.
By PN100, the promotional effect of neonatal parathion exposure on 5HT1A receptors in the frontal/parietal cortex disappeared entirely, and instead, both males (Figure 3A) and females (Figure 3B) displayed significant reductions that extended also to the temporal/occipital cortex. Males showed significant increases in 5HT1A receptors in the striatum and smaller elevations for 5HT2 receptors in the frontal/parietal cortex and for the 5HTT site overall. Females showed more extensively distributed deficits, including 5HT1A receptors (brainstem, striatum), 5HT2 receptors (frontal/parietal cortex, temporal/occipital cortex) and the 5HTT (frontal/parietal cortex, temporal/occipital cortex, brainstem); hippocampal 5HT1A receptors were also slightly but significantly increased in females.
Figure 3.
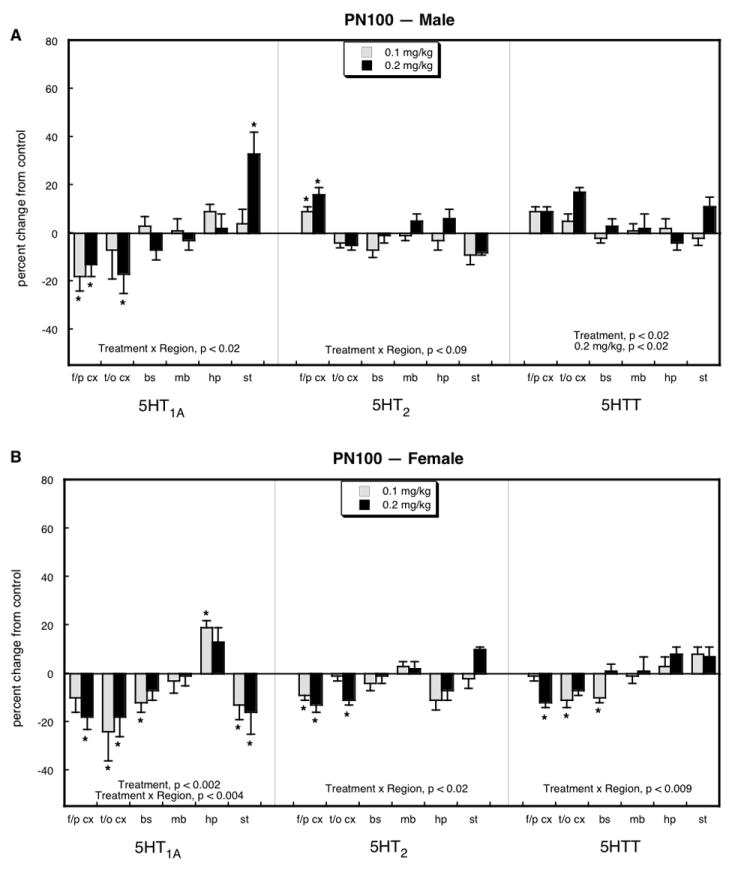
Effects of neonatal parathion exposure on 5HT synaptic proteins on PN100 in males (A) and females (B). Data represent means and standard errors obtained from at least 6 animals in each treatment group for each sex. Global ANOVA (treatment, region, sex, protein measure) indicates interactions of treatment × sex (p < 0.01), treatment × region (p < 0.03), treatment × sex × region (p < 0.07), treatment × region × measure (p < 0.003) and treatment × sex × region × measure (p < 0.004). Lower-order ANOVAs for each subdivision appear within the panels. Where there was no interaction of treatment × region, only main treatment effects are reported; where there was an interaction of treatment × region, asterisks denote individual values that differ from the corresponding control. Abbreviations: f/p cx = frontal/parietal cortex; t/o cx = temporal/occipital cortex; bs = brainstem; mb = midbrain; hp = hippocampus; st = striatum.
DISCUSSION
In our earlier work with neonatal chlorpyrifos exposure, we noted immediate upregulation of forebrain and brainstem 5HT receptors in association with the initial neural cell damage [4,11]. Afterwards, there was a period of transition in which different regional patterns and sex-selectivity emerged [2,5,55], indicating that the net effect on 5HT systems represents not only the primary injury but also the adaptive changes and plasticity in response to that damage. Accordingly, although both sexes share similar initial effects [4,11] the inherently greater neural plasticity of the female brain [31,32,61] results in smaller persistent effects than those seen in males [5,55]. The maximum tolerated dose for neonatal parathion exposure is an order of magnitude lower than for chlorpyrifos [50] and consequently, this organophosphate results in much less initial neural injury, since effects on brain development are unrelated to systemic toxicity [50]. Accordingly, parathion does not show the immediate, global upregulation of 5HT receptors and the 5HTT site as seen with chlorpyrifos; indeed, there are selective changes in the opposite direction, notably a decrease in 5HT1A receptors in both males and females [58].
Based on the major differences in the initial effects of neonatal chlorpyrifos or parathion exposure on 5HT systems, it is therefore not surprising that the long-term alterations seen here for parathion were entirely distinct from those of chlorpyrifos, differing not only in temporal sequence but also in sex-selectivity and in targeting of specific receptors and brain regions. With chlorpyrifos exposure, there is global upregulation of all three 5HT synaptic proteins evident by young adulthood and persisting for many months later, with a strong selectivity for males [5,55]. In contrast, as seen here, parathion evoked upregulation of 5HT1A receptors in adolescence, but with the transition to young adulthood (PN60), the effect persisted in only two regions in males (frontal/parietal cortex, striatum) and one region in females (frontal/parietal cortex), whereas most of the other regions displayed deficits. Further, parathion showed slight 5HT2 downregulation and no effect on 5HTT binding. By later stages of adulthood (PN100), the 5HT1A receptor upregulation caused by neonatal parathion treatment disappeared even in the region that had shown the greatest initial increases (frontal/parietal cortex), replaced by significant deficits. Whereas upregulation was still seen in the male striatum, and an increase also emerged in the female hippocampus, all the other regions showed either no significant change or downregulation. Again, the greatest effects were confined to 5HT1A receptors, with smaller effects on 5HT2 receptors and the 5HTT site.
In the larger sense, then, the long-term consequences of neonatal parathion exposure on 5HT synaptic markers are not only distinctly different from those of chlorpyrifos [5,55] but are far smaller in magnitude and less persistent, as well as being more restricted in terms of receptor subtypes and sex-selectivity. In that regard, parathion’s effects bear greater similarity to those of diazinon, which similarly has a smaller effect than that of chlorpyrifos and primarily targets 5HT1A receptors in males [51]. However, diazinon evokes persistent downregulation of this subtype, rather than showing the time-dependent shifts seen here for parathion, so the main point again is that each of the organophosphates produces unique changes in the development of 5HT synaptic parameters. If these neurochemical findings correspond to functional deficits in 5HT circuits, we would expect to find disparities in the effects of parathion directed toward emotional behaviors, just as identified previously in studies comparing chlorpyrifos to diazinon [1,45]; comparable studies with parathion are underway.
Our results thus reinforce two important concepts for the developmental neurotoxicity of organophosphates. First, exposures that are at or below the threshold for detectable cholinesterase inhibition or signs of systemic toxicity [50,58], and certainly well below the 70% cholinesterase inhibition required for observable intoxication [13], nevertheless compromise the development of 5HT systems that are critical to emotion, appetite and sleep patterns [1,34,35,44], expanding the scope of synaptic and behavioral targets that need to be considered in evaluating the outcomes and safety of early-life organophosphate exposure. Second, the longstanding assumption that all organophosphates produce the same effects is clearly incorrect, and accordingly, each agent needs to be evaluated separately, incorporating relevant neurochemical and functional endpoints in addition to their impact on cholinesterase activity.
Acknowledgments
This research was supported by NIH ES10356. We thank Bethany Bodwell for technical assistance. The authors declare they have no competing financial interests. Theodore Slotkin and Frederic Seidler have provided expert witness testimony on behalf of government agencies, corporations and individuals.
Abbreviations
- 5HT
5-hydroxytryptamine, serotonin
- 5HTT
5HT transporter
- ANOVA
analysis of variance
- PN
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005;203:134–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, Chen JJ, Mann JJ. Serotonin-1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 7.Barone S, Das KP, Lassiter TL, White LD. Vulnerable processes of nervous system development: a review of markers and methods. Neurotoxicology. 2000;21:15–36. [PubMed] [Google Scholar]
- 8.Beseler C, Stallones L, Hoppin JA, Alavanja MCR, Blair A, Keefe T, Kamel F. Depression and pesticide exposures in female spouses of licensed pesticide applicators in the Agricultural Health Study Cohort. J Occup Environ Med. 2006;48:1005–1013. doi: 10.1097/01.jom.0000235938.70212.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betancourt AM, Burgess SC, Carr RL. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicol Sci. 2006;92:500–506. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- 10.Betancourt AM, Carr RL. The effect of chlorpyrifos and chlorpyrifos-oxon on brain cholinesterase, muscarinic receptor binding, and neurotrophin levels in rats following early postnatal exposure. Toxicol Sci. 2004;77:63–71. doi: 10.1093/toxsci/kfh003. [DOI] [PubMed] [Google Scholar]
- 11.Campbell CG, Seidler FJ, Slotkin TA. Chlorpyrifos interferes with cell development in rat brain regions. Brain Res Bull. 1997;43:179–189. doi: 10.1016/s0361-9230(96)00436-4. [DOI] [PubMed] [Google Scholar]
- 12.Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 13.Clegg DJ, van Gemert M. Determination of the reference dose for chlorpyrifos: proceedings of an expert panel. J Toxicol Environ Health. 1999;2:211–255. doi: 10.1080/109374099281179. [DOI] [PubMed] [Google Scholar]
- 14.Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eskenazi B, Rosas LG, Marks AR, Bradman A, Harley K, Holland N, Johnson C, Fenster L, Barr DB. Pesticide toxicity and the developing brain. Basic Clin Pharmacol Toxicol. 2008;102:228–236. doi: 10.1111/j.1742-7843.2007.00171.x. [DOI] [PubMed] [Google Scholar]
- 17.Fujita M, Charney DS, Innis RB. Imaging serotonergic neurotransmission in depression: hippocampal pathophysiology may mirror global brain alterations. Biol Psychiat. 2000;48:801–812. doi: 10.1016/s0006-3223(00)00960-4. [DOI] [PubMed] [Google Scholar]
- 18.Gupta RC. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol Mech Meth. 2004;14:103–143. doi: 10.1080/15376520490429175. [DOI] [PubMed] [Google Scholar]
- 19.Icenogle LM, Christopher C, Blackwelder WP, Caldwell DP, Qiao D, Seidler FJ, Slotkin TA, Levin ED. Behavioral alterations in adolescent and adult rats caused by a brief subtoxic exposure to chlorpyrifos during neurulation. Neurotoxicol Teratol. 2004;26:95–101. doi: 10.1016/j.ntt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Jaga K, Dharmani C. The interrelation between organophosphate toxicity and the epidemiology of depression and suicide. Rev Environ Health. 2007;22:57–73. doi: 10.1515/reveh.2007.22.1.57. [DOI] [PubMed] [Google Scholar]
- 21.Jett DA, Navoa RV, Beckles RA, McLemore GL. Cognitive function and cholinergic neurochemistry in weanling rats exposed to chlorpyrifos. Toxicol Appl Pharmacol. 2001;174:89–98. doi: 10.1006/taap.2001.9198. [DOI] [PubMed] [Google Scholar]
- 22.Landrigan PJ. Pesticides and polychlorinated biphenyls (PCBs): an analysis of the evidence that they impair children’s neurobehavioral development. Mol Genet Metab. 2001;73:11–17. doi: 10.1006/mgme.2001.3177. [DOI] [PubMed] [Google Scholar]
- 23.Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Godbold JH, et al. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107(suppl 3):431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lassiter TL, Ryde IT, MacKillop EA, Brown KK, Levin ED, Seidler FJ, Slotkin TA. Exposure of neonatal rats to parathion elicits sex-selective reprogramming of metabolism and alters the response to a high-fat diet in adulthood. Environ Health Perspect. 2008 doi: 10.1289/ehp.11673. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee WJ, Alavanja MCR, Hoppin JA, Rusiecki JA, Kamel F, Blair A, Sandler DP. Mortality among pesticide applicators exposed to chlorpyrifos in the agricultural health study. Environ Health Perspect. 2007;115:528–534. doi: 10.1289/ehp.9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, Slotkin TA. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicol Teratol. 2002;24:733–741. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- 27.Levin ED, Addy N, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Dev Brain Res. 2001;130:83–89. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- 28.London L, Flisher AJ, Wesseling C, Mergler D, Kromhout H. Suicide and exposure to organophosphate insecticides: cause or effect? Am J Ind Med. 2005;47:308–321. doi: 10.1002/ajim.20147. [DOI] [PubMed] [Google Scholar]
- 29.Maes M, Meltzer H. The serotonin hypothesis of major depression. In: Bloom FE, Kupfer DJ, Bunney BS, Ciaranello RD, Davis KL, Koob GF, Meltzer HY, Schuster CR, Shader RI, Watson SJ, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 933–944. [Google Scholar]
- 30.May M. Disturbing behavior: neurotoxic effects in children. Environ Health Perspect. 2000;108:A262–A267. doi: 10.1289/ehp.108-a262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McEwen BS. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 2002;23:921–939. doi: 10.1016/s0197-4580(02)00027-1. [DOI] [PubMed] [Google Scholar]
- 32.Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. J Neurobiol. 1999;40:602–619. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Moreno M, Canadas F, Cardona D, Sunol C, Campa L, Sanchez-Amate MC, Flores P, Sanchez-Santed F. Long-term monoamine changes in the striatum and nucleus accumbens after acute chlorpyrifos exposure. Toxicol Lett. 2008;176:162–167. doi: 10.1016/j.toxlet.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Nemeroff CB. The neurobiology of depression. Sci Am. 1998;278(6):42–49. doi: 10.1038/scientificamerican0698-42. [DOI] [PubMed] [Google Scholar]
- 35.Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol. 2002;17:S1–S12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- 36.Perera FP, Rauh V, Whyatt RM, Tang D, Tsai WY, Bernert JT, Tu YH, Andrews H, Barr DB, Camann DE, et al. A summary of recent findings on birth outcomes and developmental effects of prenatal ETS, PAH, and pesticide exposures. Neurotoxicology. 2005;26:573–587. doi: 10.1016/j.neuro.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Physicians for Social Responsibility. Pesticides and Children. Editoin. Physicians for Social Responsibility; Washington DC: 1995. [Google Scholar]
- 38.Pope CN. Organophosphorus pesticides: do they all have the same mechanism of toxicity? J Toxicol Environ Health. 1999;2:161–181. doi: 10.1080/109374099281205. [DOI] [PubMed] [Google Scholar]
- 39.Qiao D, Seidler FJ, Padilla S, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: What is the vulnerable period? Environ Health Perspect. 2002;110:1097–1103. doi: 10.1289/ehp.021101097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qiao D, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Fetal chlorpyrifos exposure: adverse effects on brain cell development and cholinergic biomarkers emerge postnatally and continue into adolescence and adulthood. Environ Health Perspect. 2003;111:536–544. doi: 10.1289/ehp.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rauh VA, Garfinkel R, Perera R, Andrews H, Hoepner L, Barr D, Whitehead D, Tang D, Whyatt RM. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ricceri L, Markina N, Valanzano A, Fortuna S, Cometa MF, Meneguz A, Calamandrei G. Developmental exposure to chlorpyrifos alters reactivity to environmental and social cues in adolescent mice. Toxicol Appl Pharmacol. 2003;191:189–201. doi: 10.1016/s0041-008x(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 43.Ricceri L, Venerosi A, Capone F, Cometa MF, Lorenzini P, Fortuna S, Calamendrei G. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicol Sci. 2006;93:105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- 44.Risch CS, Nemeroff CB. Neurochemical alterations of serotonergic neuronal systems in depression. J Clin Psychiat. 1991;53(suppl 10):3–6. [PubMed] [Google Scholar]
- 45.Roegge CS, Timofeeva OA, Seidler FJ, Slotkin TA, Levin ED. Developmental diazinon neurotoxicity in rats: later effects on emotional response. Brain Res Bull. 2008;75:166–172. doi: 10.1016/j.brainresbull.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–151. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107(suppl 1):71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- 49.Slotkin TA, Bodwell BE, Ryde IT, Levin ED, Seidler FJ. Exposure of neonatal rats to parathion elicits sex-selective impairment of acetylcholine systems in brain regions during adolescence and adulthood. Environ Health Perspect. 2008 doi: 10.1289/ehp.11451. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environ Health Perspect. 2006;114:746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slotkin TA, Ryde IT, Levin ED, Seidler FJ. Developmental neurotoxicity of low-dose diazinon exposure of neonatal rats: effects on serotonin systems in adolescence and adulthood. Brain Res Bull. 2008;75:640–647. doi: 10.1016/j.brainresbull.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slotkin TA, Seidler FJ. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res Bull. 2007;73:301–309. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod Toxicol. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 56.Slotkin TA, Seidler FJ, Fumagalli F. Exposure to organophosphates reduces the expression of neurotrophic factors in neonatal rat brain regions: similarities and differences in the effects of chlorpyrifos and diazinon on the fibroblast growth factor superfamily. Environ Health Perspect. 2007;115:909–916. doi: 10.1289/ehp.9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slotkin TA, Seidler FJ, Ryde IT, Yanai J. Developmental neurotoxic effects of chlorpyrifos on acetylcholine and serotonin pathways in an avian model. Neurotoxicol Teratol. 2008 doi: 10.1016/j.ntt.2008.02.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environ Health Perspect. 2006;114:1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 60.Snedecor GW, Cochran WG, editors. Statistical Methods. Editoin. Iowa State University Press; Ames, Iowa: 1967. [Google Scholar]
- 61.Suzuki S, Brown CM, Wise PM. Mechanisms of neuroprotection by estrogen. Endocrine. 2006;29:209–215. doi: 10.1385/ENDO:29:2:209. [DOI] [PubMed] [Google Scholar]
- 62.Timofeeva OA, Roegge CS, Seidler FJ, Slotkin TA, Levin ED. Persistent cognitive alterations in rats after early postnatal exposure to low doses of the organophosphate pesticide, diazinon. Neurotoxicol Teratol. 2008;30:38–45. doi: 10.1016/j.ntt.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weiss B, Amler S, Amler RW. Pesticides. Pediatrics. 2004;113:1030–1036. [PubMed] [Google Scholar]
- 64.Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- 65.Yanai J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann NY Acad Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]
- 66.Yatham LN, Liddle PF, Dennie J, Shiah IS, Adam MJ, Lane CJ, Lam RW, Ruth TJ. Decrease in brain serotonin-2 receptor binding in patients with major depression following desipramine treatment: a positron emission tomography study with fluorine-18-labeled setoperone. Arch Gen Psychiat. 1999;56:705–711. doi: 10.1001/archpsyc.56.8.705. [DOI] [PubMed] [Google Scholar]
- 67.Yatham LN, Liddle PF, Shiah IS, Scarrow G, Lam RW, Adam MJ, Zis AP, Ruth TJ. Brain serotonin-2 receptors in major depression: a positron emission tomography study. Arch Gen Psychiat. 2000;57:850–858. doi: 10.1001/archpsyc.57.9.850. [DOI] [PubMed] [Google Scholar]


