Abstract
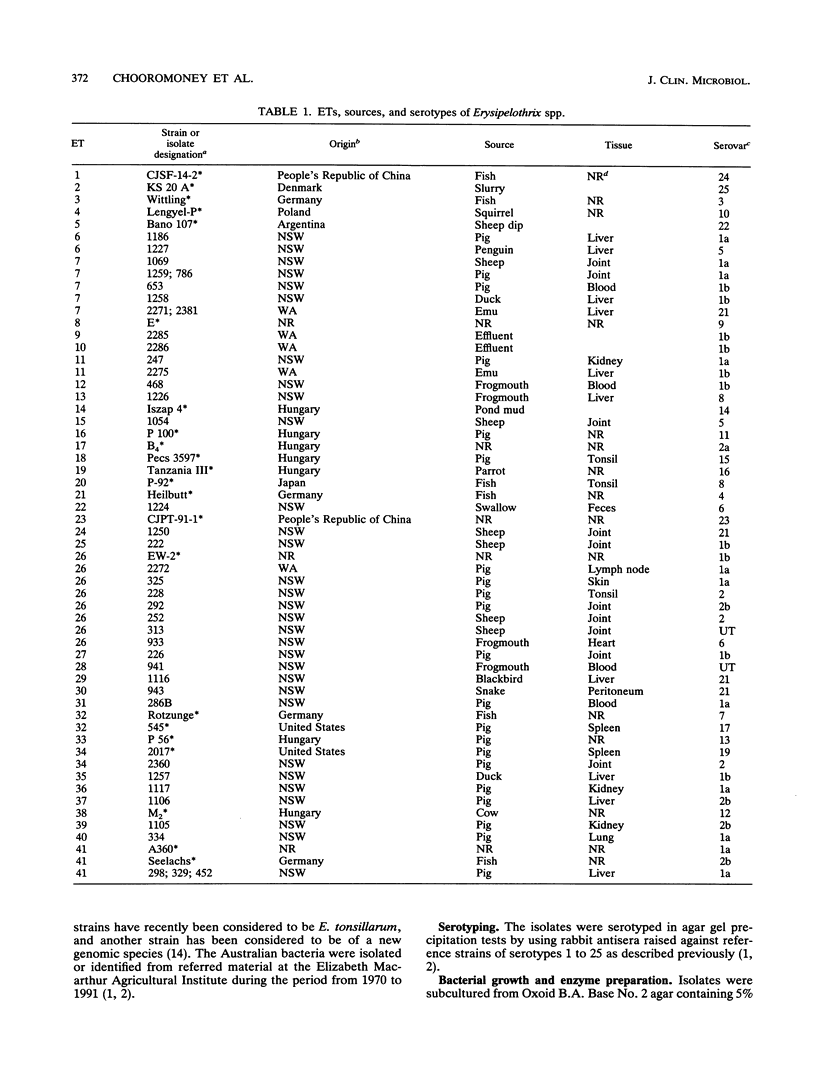
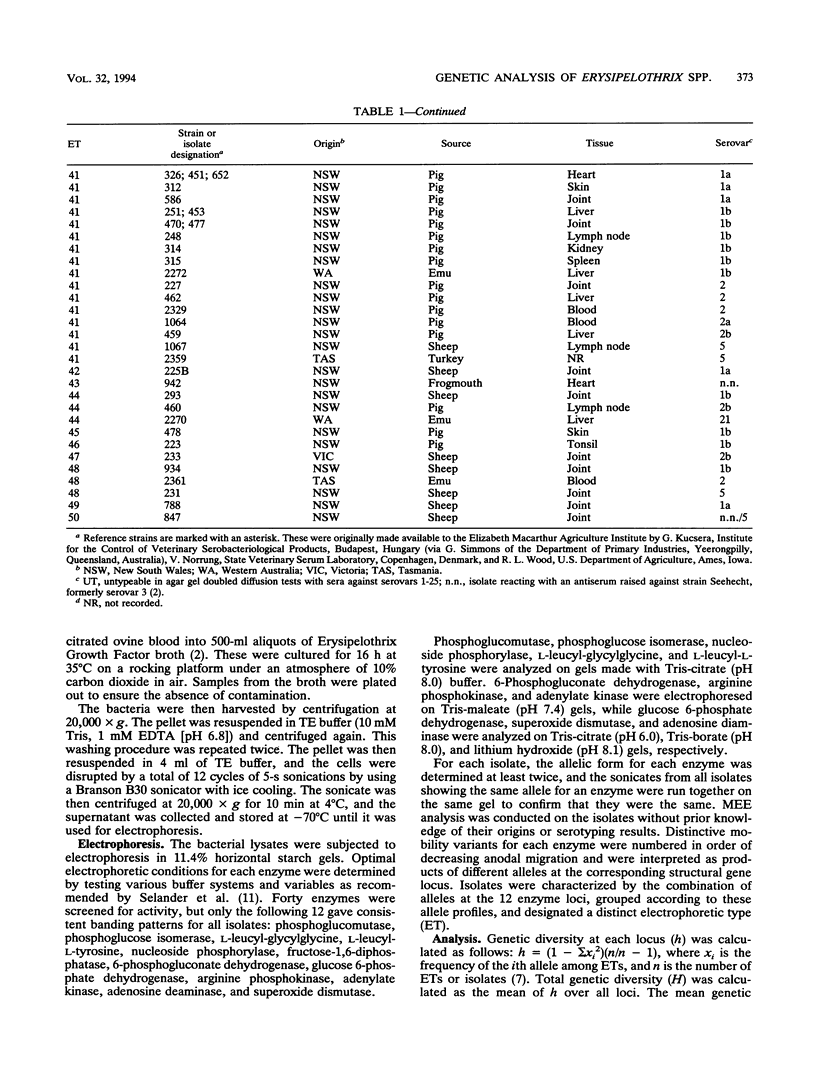
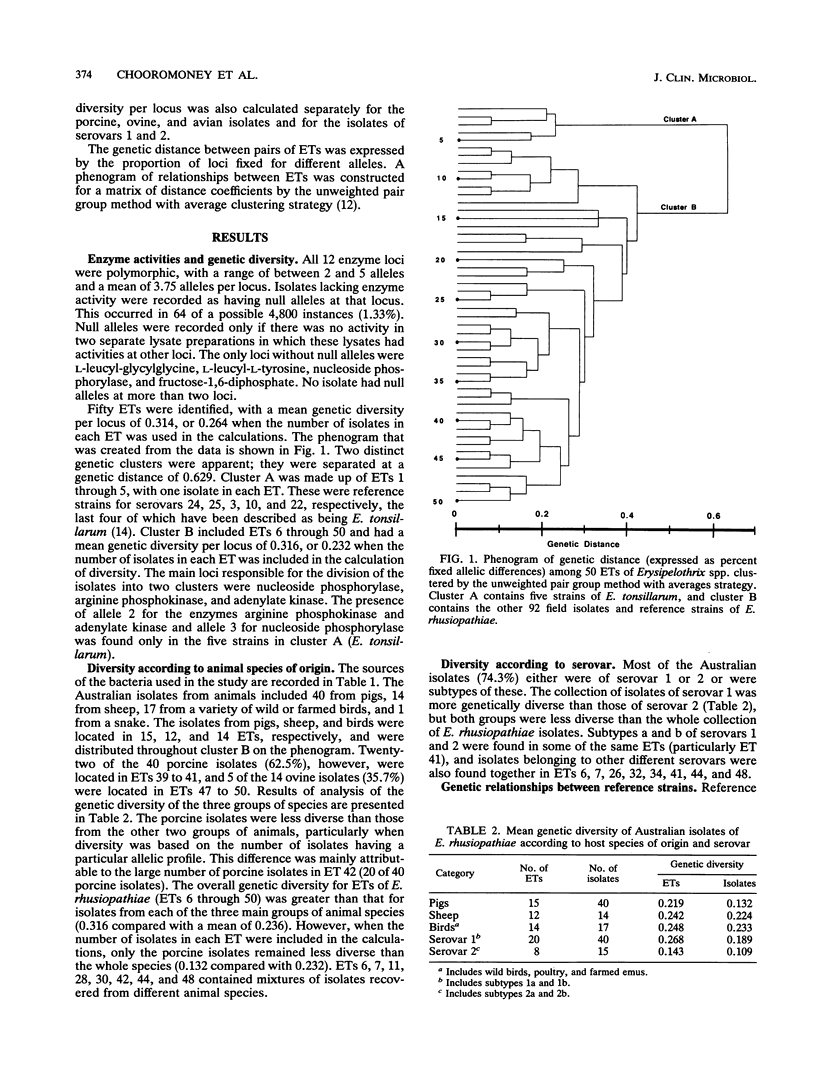
The genetic diversity of 74 Australian field isolates of Erysipelothrix rhusiopathiae and 22 reference strains for serovars of E. rhusiopathiae or Erysipelothrix tonsillarum was examined by multilocus enzyme electrophoresis. Four serovar reference strains of E. tonsillarum (strains KS 20 A, Wittling, Lengyel-P, and Bano 107 for serovars 25, 3, 10, and 22, respectively) were genetically distinct from E. rhusiopathiae. However, the E. tonsillarum reference strain for serovar 14 (Iszap-4) and the reference strain for serovar 13 (Pecs-56), which has been said to represent a new genomic species, were found to cluster with typical isolates and reference strains of E. rhusiopathiae. Our reference strain for serovar 7 (Rotzunge) was also genetically typical of E. rhusiopathiae, thus indicating that these serotype reactivities cannot be relied upon as a means of identifying isolates as E. tonsillarum. Australian field isolates of E. rhusiopathiae were genetically diverse. Those recovered from sheep or birds were more diverse than those isolated from pigs, and isolates of serovar 1 were more diverse than those of serovar 2. The diversity found among isolates of the same serovar and the presence of isolates of different serovars in the same electrophoretic types (ETs) indicated that serotyping of E. rhusiopathiae was unreliable for use as an epidemiological tool. Some ETs contained isolates recovered from different animal species. ET 41 contained 32.2% of the field isolates and two reference strains, indicating that this clone of E. rhusiopathiae is both widespread and commonly associated with disease in various species of animals.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cross G. M., Claxton P. D. Serological classification of Australian strains of Erysipelothrix rhusiopathiae isolated from pigs, sheep, turkeys and man. Aust Vet J. 1979 Feb;55(2):77–81. doi: 10.1111/j.1751-0813.1979.tb15170.x. [DOI] [PubMed] [Google Scholar]
- Eamens G. J., Turner M. J., Catt R. E. Serotypes of Erysipelothrix rhusiopathiae in Australian pigs, small ruminants, poultry, and captive wild birds and animals. Aust Vet J. 1988 Aug;65(8):249–252. doi: 10.1111/j.1751-0813.1988.tb14311.x. [DOI] [PubMed] [Google Scholar]
- Griffiths G. L., Buller N. Erysipelothrix rhusiopathiae infection in semi-intensively farmed emus. Aust Vet J. 1991 Mar;68(3):121–122. doi: 10.1111/j.1751-0813.1991.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Hampson D. J., Blackall P. J., Woodward J. M., Lymbery A. J. Genetic analysis of Actinobacillus pleuropneumoniae, and comparison with Haemophilus spp. Taxon "minor group" and Taxon C. Zentralbl Bakteriol. 1993 Jun;279(1):83–91. doi: 10.1016/s0934-8840(11)80494-9. [DOI] [PubMed] [Google Scholar]
- Nei M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics. 1978 Jul;89(3):583–590. doi: 10.1093/genetics/89.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørrung V., Molin G. A new serotype of Erysipelothrix rhusiopathiae isolated from pig slurry (short communication). Acta Vet Hung. 1991;39(3-4):137–138. [PubMed] [Google Scholar]
- Nørrung V., Munch B., Larsen H. E. Occurrence, isolation and serotyping of Erysipelothrix rhusiopathiae in cattle and pig slurry. Acta Vet Scand. 1987;28(1):9–14. doi: 10.1186/BF03548251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørrung V. Two new serotypes of Erysipelothrix rhusiopathiae. Nord Vet Med. 1979 Nov;31(11):462–465. [PubMed] [Google Scholar]
- Selander R. K., Caugant D. A., Ochman H., Musser J. M., Gilmour M. N., Whittam T. S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986 May;51(5):873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T., Fujisawa T., Tamura Y., Suzuki S., Muramatsu M., Sawada T., Benno Y., Mitsuoka T. DNA relatedness among Erysipelothrix rhusiopathiae strains representing all twenty-three serovars and Erysipelothrix tonsillarum. Int J Syst Bacteriol. 1992 Jul;42(3):469–473. doi: 10.1099/00207713-42-3-469. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Sawada T., Muramatsu M., Tamura Y., Fujisawa T., Benno Y., Mitsuoka T. Serotype, antimicrobial susceptibility, and pathogenicity of Erysipelothrix rhusiopathiae isolates from tonsils of apparently healthy slaughter pigs. J Clin Microbiol. 1987 Mar;25(3):536–539. doi: 10.1128/jcm.25.3.536-539.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood R. L., Haubrich D. R., Harrington R., Jr Isolation of previously unreported serotypes of Erysipelothrix rhusiopathiae from swine. Am J Vet Res. 1978 Dec;39(12):1958–1961. [PubMed] [Google Scholar]


