Abstract
β-Catenin, a component of the Wnt signaling pathway, is a coactivator of human androgen receptor (hAR) transcriptional activity. Here, we show that Wnt signaling also influences androgen-mediated signaling through its ability to regulate hAR mRNA and protein in prostate cancer (PCa) cells. Three functional LEF-1/TCF binding sites lie within the promoter of the hAR gene as shown by CHIP assays that captured β-catenin-bound chromatin from Wnt-activated LNCaP cells. Chimeric reporter vectors that use the hAR gene promoter to drive luciferase expression confirmed that these LEF-1/TCF binding elements are able to confer robust upregulation of luciferase expression when stimulated by Wnt-1 or by transfection with β-catenin and that dominant-negative TCF or mutations within the dominant TCF-binding element abrogated the response. Semi-quantitative and real time RT-PCR assays confirmed that Wnt activation upregulates hAR mRNA in PCa cells. In contrast, hAR protein expression was strongly suppressed by Wnt activation. The reduction of hAR protein is consistent with evidence that Wnt signaling increased phosphorylation of Akt and its downstream target, MDM2 that promotes degradation of hAR protein through a proteasomal pathway. These data indicate that the hAR gene is a direct target of LEF-1/TCF transcriptional regulation in PCa cells but also show that the expression of the hAR protein is suppressed by a degradation pathway regulated by cross-talk of Wnt to Akt that is likely mediated by Wnt-directed degradation of the B regulatory subunit of protein phosphatase, PP2A.
Keywords: androgen receptor, Wnt signaling, β-catenin, PCDH-PC, Akt, MDM2
Introduction
Prostate cancer (PCa) is a prevalent human tumor that develops and progresses under the influence of androgenic steroids. As in normal prostate cells, androgen action in PCa cells is mediated by a nuclear receptor protein, the human androgen receptor (hAR) that binds androgenic ligands, enters the nucleus and stimulates the transcription of genes having cis-acting androgen response elements within their promoter or regulatory regions (Chang et al., 1995). Androgen depletion, induced by hormonal therapies used to treat advanced PCa patients, transiently suppress disease progression. However, the cancer inevitably recurs in a hormone-refractory form that continues to grow despite the diminished androgen levels in a hormone-treated patient (Miyamoto et al., 2005). In the in vivo setting, hormone refractory PCa cells are known to maintain hAR protein expression and there is a consensus that androgen-mediated gene expression is also sustained despite the deficit in circulating androgen levels (Grossmann et al., 2001). This conundrum has led to extensive research to determine mechanisms through which androgen signaling might be maintained in PCa cells in hormone-treated patients. Various studies reveal that there are likely multiple pathways leading to increased androgen signaling in a low androgen environment involving mechanisms as diverse as hAR gene amplification (Ford et al., 2003), mutations that alter the ligand specificity of the hAR (Tilley et al., 1996) or by association of the hAR protein with coactivators that cooperate to increase transcriptional activity of hAR (Rahman et al., 2004).
One coactivator that markedly influences the transcriptional activity of hAR is β-catenin, a key molecule in the canonical Wnt signaling pathway (Truica et al., 2000; Yang et al., 2002). β-Catenin binds to the activation function 2 region within the N-terminal domain of liganded hAR protein and augments ligand-dependent hAR transcriptional activity in PCa cells (Song et al., 2003). The coactivator function of β-catenin likely involves increased recruitment of p160 coactivator proteins (Li et al., 2004) as well as tertiary proteins, such as histone methyltransferase (Koh et al., 2002). β-Catenin also alters ligand specificity of hAR-mediated transcription, enhancing transcriptional activation by and rostenedione and estradiol and diminishing antagonism by bicalutamide (Truica et al., 2000). Cultured PCa cells in which Wnt signaling is activated by Wnt ligand also show increased hAR-mediated transcriptional effects even in the absence of androgenic ligands (Verras et al., 2004), which implies that the Wnt signaling pathway has additional effects on hAR-mediated signaling aside from those involving interaction of β-catenin with liganded hAR. Here, we describe our work evaluating the ability of Wnt signaling, mediated by β-catenin activated LEF-1/TCF transcription and MDM2-mediated protein degradation, to influence expression of the hAR mRNA and protein in PCa cells. Our results show that the hAR gene is a primary target of LEF-1/TCF transcriptional control and that the Wnt signaling pathway has additional effects that modulate the levels of the hAR-encoded protein through a ubiquitin-mediated degradation process controlled by Akt/Protein kinase B signaling.
Results
Validation of functional LEF-1/TCF binding sites in the 5′ promoter region of the hAR gene
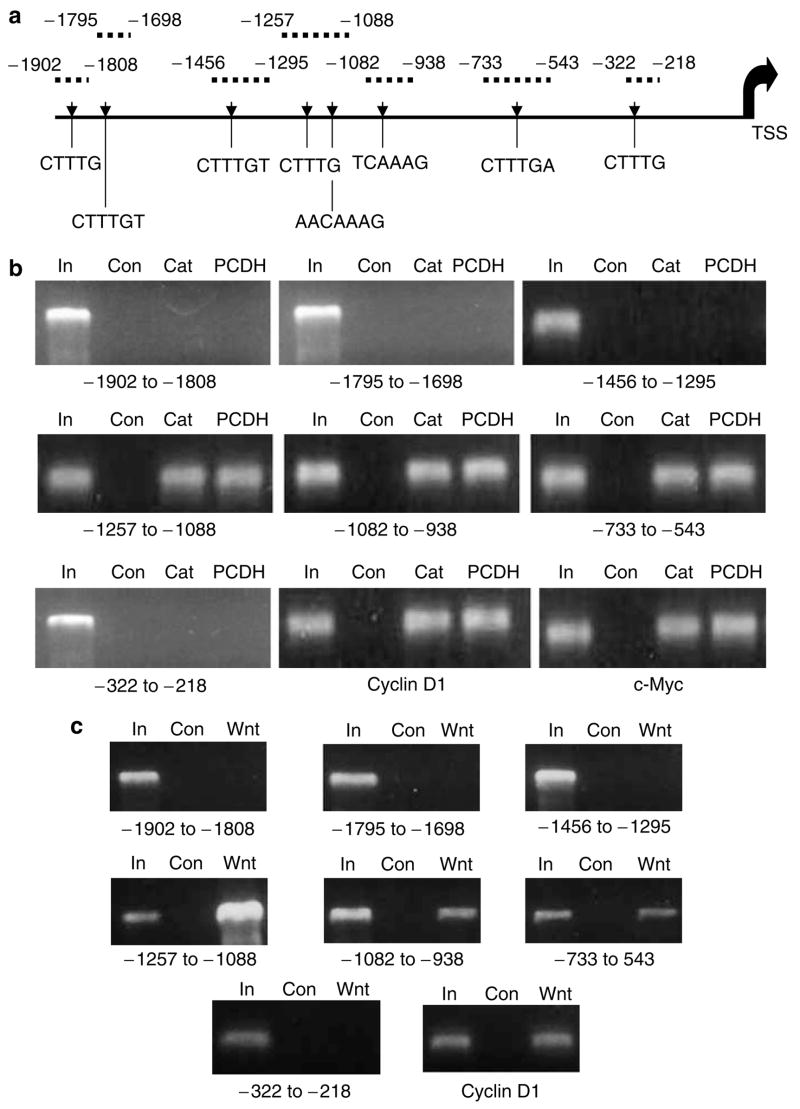
A computerized search of a 2000 bp region immediately 5′ to the transcriptional start site of the hAR gene revealed the presence of eight core (minimal) sequences containing potential LEF-1/TCF binding elements (Figure 1a). A CHIP assay was used to determine whether any of these potential binding elements were occupied by a protein complex that contained β-catenin in control LNCaP cells (transfected with empty vector) or in LNCaP cells with Wnt signaling activated either by transfection with a mutated (stabilized) β-catenin or with protocadherin-PC (PCDH-PC), another gene product known to stimulate LEF-1/TCF-mediated transcription in these cells (Yang et al., 2005). Fixed, sheared chromatin was immunoprecipitated using anti-β-catenin antibody and the immunoprecipitated chromatin was PCR amplified using primer sets that distinguished the various potential binding sites as described in Figure 1a. A sample of DNA extracted from unprecipitated input control LNCaP cells was amplified as a positive control to ensure that each primer set was able to amplify the appropriate sized fragment. Primer sets that amplify known LEF-1/TCF binding regions within the cyclin D1 and c-myc promoters were used as positive controls to ensure that the assay was capable of detecting LEF-1/TCF binding sites within other genes known to be transcriptionally regulated by Wnt signaling. The results of these amplifications (Figure 1b) identified three of the eight potential LEF-1/TCF binding elements within the hAR proximal promoter region as occupied by a protein complex containing β-catenin in Wnt-activated cells. None of these potential LEF-1/TCF binding sites were immunoprecipitated from chromatin obtained from control cells without Wnt activation. This experiment was repeated using a defective recombinant adenovirus that expresses Wnt-1 protein (Ad-Wnt-1) to stimulate Wnt signaling in the LNCaP cells and the results of the CHIP analysis (compared to cells transduced with a Lac Z expressing recombinant adenovirus, Ad-LacZ) were equivalent to that shown by β-catenin or PCDH-PC transfected cells (Figure 1c).
Figure 1.
A CHIP Assay identifies functional LEF-1/TCF binding sites within the proximal promoter of the hAR gene. (a) Scheme identifies relative sites of potential LEF-1/TCF binding sites within the first 2000 bp 5′ upstream of the start of transcription (TSS) of the hAR gene and sites of primer amplification products used to analyse DNA extracted from immunoprecipitated chromatin from cell specimens. (b) Ethidium bromide-stained agarose gel profiles of PCR reaction products from input control DNA (In), β-catenin antibody immunoprecipitated control transfected (empty vector) LNCaP cell chromatin DNA (Con), β-catenin transfected LNCaP cell DNA (Cat) or PCDH-PC transfected LNCaP cell DNA (PCDH). (c) Ethidium bromide-stained agarose gel profiles of PCR reactions products from input control LNCaP DNA (In), β-catenin antibody immunoprecipitated chromatin from 48 h Ad-lac Z transduced LNCaP cells (Con) or from 48 h Ad-Wnt-1 transduced LNCaP cells (Wnt-1). Results show that sheared chromatin within three regions of the hAR promoter were immunoprecipitated by the antibody in β-catenin and PCDH-PC transfected cells as well as the known LEF-1/TCF binding elements within the promoters of the cyclin D1 and c-myc gene but these regions were not immunoprecipitated in control transfected cells.
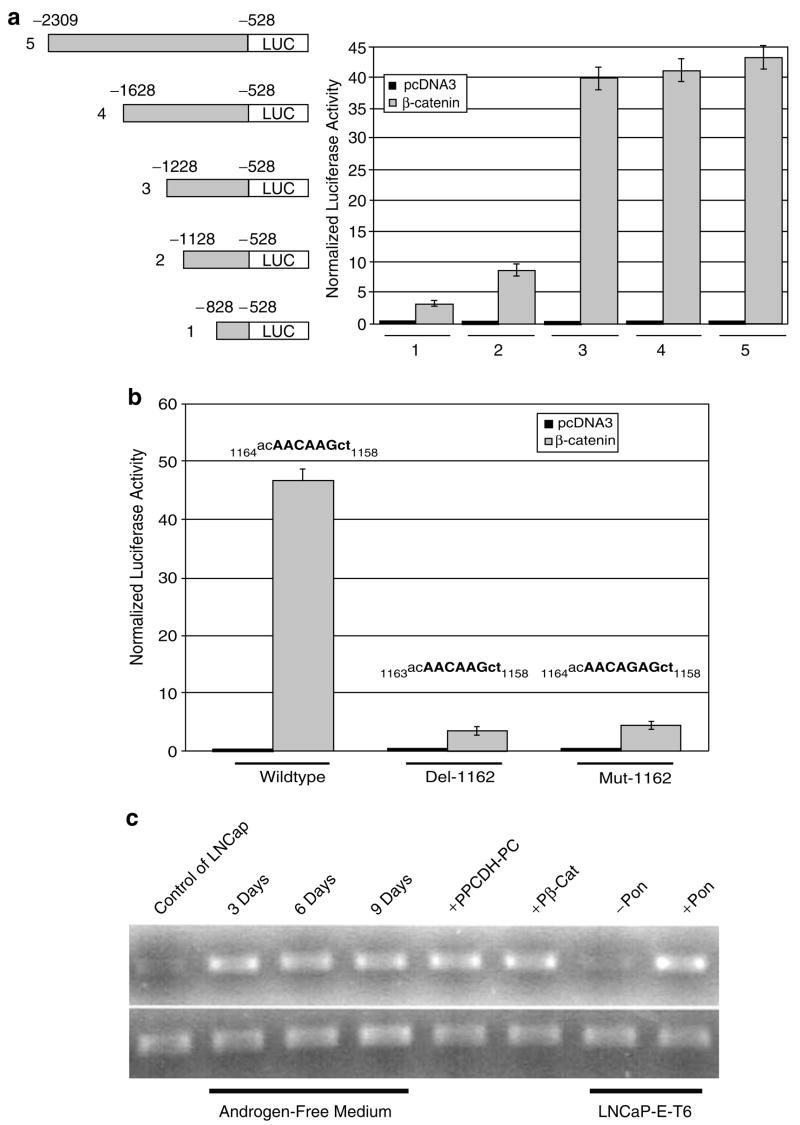
hAR promoter-luciferase reporter fusion vectors demonstrate increased luciferase expression in Wnt-activated LNCaP cells
A series of hAR promoter-luciferase reporter vectors were constructed that contained increasing lengths of the hAR promoter region. These vectors were co-transfected into LNCaP cells along with empty vector (Wnt unstimulated control) or with the β-catenin expression vector (Wnt stimulated). Transfection efficiency was monitored by inclusion of a β-galactosidase (β-gal) reporter vector. Transfected cells were collected 48 h later and luciferase and β-gal activity was measured in the cell extracts. Expression of normalized luciferase was low in all cells co-transfected with empty vector, however, normalized luciferase activity was progressively increased as the length of the hAR promoter was increased in cells co-transfected with the β-catenin expression vector (Figure 2a). Our results indicate that the two more proximal LEF-1/TCF binding elements of the hAR promoter identified in the CHIP assay were weakly, but additively active in promoting luciferase activity in Wnt-stimulated LNCaP cells, whereas the more distal LEF-1/TCF binding element found in the CHIP assay was much more robust in promoting luciferase expression in Wnt-stimulated cells, with levels of luciferase almost 40 times greater than in cells co-transfected with empty vector. Increasing hAR promoter length beyond this did not further increase luciferase activity in Wnt-stimulated cells. Likewise, stimulation of Wnt signaling using the Ad-Wnt-1 adenovirus to transduce cells immediately prior to transfection with the largest hAR promoted luciferase vector (vector #5) showed that this induced luciferase activity more than 40-fold when compared to control, non-Wnt-induced LNCaP cells (Table 1). The ability of Wnt signaling stimulation (by Ad-Wnt-1 or mutated β-catenin) to upregulate luciferase expression from the chimeric hAR reporter vector (#5) was abrogated by co-transfection with a dominant negative TCF (pDN-TCF) expression plasmid but not by empty vector (pCDNA3) (Table 1) or by introducing site-specific mutations into the dominant TCF-binding element (at −1158 to −1163) (Figure 2b) within the hAR promoter, thus confirming that the actions of Wnt signaling in upregulating expression of the reporter from this chimeric vector was dependent upon the activity of TCF transcription factors.
Figure 2.
The promoter of the human androgen receptor gene contains β-catenin sensitive elements that upregulate luciferase expression in chimeric reporter vectors. (a) Chimeric hAR promoter/luciferase reporter vectors with varying amounts of upstream hAR promoter (left) were co-transfected into LNCaP cells along with empty vector (pcDNA3) or β-catenin and normalized luciferase activity was measured after 48 h (right). Results show progressive increase in luciferase as promoter element length is increased. (b) Comparison of normalized luciferase expression from vector 5, above with wild type, deleted (A at −1162) or mutated (G instead of A at −1162) LEF-1/TCF binding site (−1158 to −1164) when co-transfected with empty vector or β-catenin, as indicated. (c) Semiquantitative RT-PCR analysis of hAR (Top) or G3PDH (Bottom) mRNA expression in LNCaP cells or Wnt-activated LNCaP cells (grown in androgen-free medium for 3, 6 or 9 days or transfected with PCDH-PC or β-catenin) or in LNCaP-E-T6 cells (stably transfected with ecdysterone-inducible PCDH-PC expression vector) with or without ponasterone (Pon).
Table 1.
Dominant negative TCF blocks luciferase reporter in Wnt-stimulated LNCaP cells
| Wnt stimulation | Co-transfectiona | (β-gal) normalized luciferase activity |
|---|---|---|
| None | pCDNA3 | 0.14±0.007 |
| Ad-Wnt-1b | pCDNA3 | 45.32±1.97 |
| Ad-Wnt-1b | pDN-TCF | 1.8±0.09 |
| pβ-Catenin | pCDNA3 | 40.24±1.87 |
| pβ-Catenin | pDN-TCF | 0.19±0.07 |
All transfections included the phAR/luciferase vector #5 and β-galactosidase expression vector at 1/10 concentration.
The Ad-Wnt-1 (20 PFU/cell) was adsorbed for 1 h prior to transfection.
Expression of hAR mRNA is induced by Wnt signaling in PCa cells
RNAs extracted from Wnt-stimulated LNCaP cells (induced by transduction with Ad-Wnt-1 or by transfection with by β-catenin or PCDH-PC expression vectors) were reverse transcribed and the expression of hAR and β-actin mRNAs were quantitatively measured using a relative PCR (real-time) assay and compared to control cells (transduced by Ad-lac Z or by an empty expression vector, pCDNA3). Comparison of the hAR/actin mRNA ratio of Ad-Wnt-1 transduced LNCaP cells (at 48 h) to Ad-lac Z transduced cells showed that the ratio was increased by 14.52-fold in the Wnt-1 stimulated cells. Likewise, β-catenin transfected LNCaP cells were compared to empty vector transfected cells and showed an increase of 12.55-fold in the hAR/actin mRNA ratio. Finally, comparison of the hAR/actin mRNA ratio in PCDH-PC-transfected LNCaP cells to control transfected cells revealed an increase of 11.70-fold. A similar assay was performed to assess relative hAR expression in LNCaP cells that were grown for one week in androgen-free medium that were previously shown to have upregulated Wnt signaling activity in conjunction with induced expression of PCDH-PC (Yang et al., 2005). The hAR/actin mRNA ratio of androgen-free cells was 16.45-fold higher than cells maintained in normal medium. This effect was also assessed by a semiquantitative RT–PCR based assay in which amplification products resulting from 32 thermocycles were visualized on an agarose gel (Figure 2c). These latter results confirmed the findings of real time RT-PCR demonstrating that all conditions associated with increased Wnt signaling (culture in androgen-free medium, transfection with β-catenin or PCDH-PC or upregulation of PCDH-PC from a conditional expression vector in stably transfected LNCaP cells (by ponasterone)) were associated with upregulation of hAR mRNA levels. Finally, a real time RT–PCR-based assessment of the hAR/actin mRNA ratio of β-catenin transfected CWR22rv-1 cells (another human PCa cell line with endogenous expression of hAR) showed that the ratio was increased by 11.65-fold compared to control transfected cells, similar to levels in β-catenin or PCDH-PC transfected LNCaP cells. Assessment of the effects of β-catenin transfection on PC-3 or DU145 human PCa cell lines (that do not endogenously express hAR protein) using the real time RT–PCR procedure showed that there was an upregulation of hAR mRNA to a level (more than 10-fold greater than control cells) similar to that of the hAR expressing LNCaP and CWR22rv-1 cells, however the extremely low basal expression of hAR mRNA in the unstimulated cells makes it difficult to determine the significance of the increase.
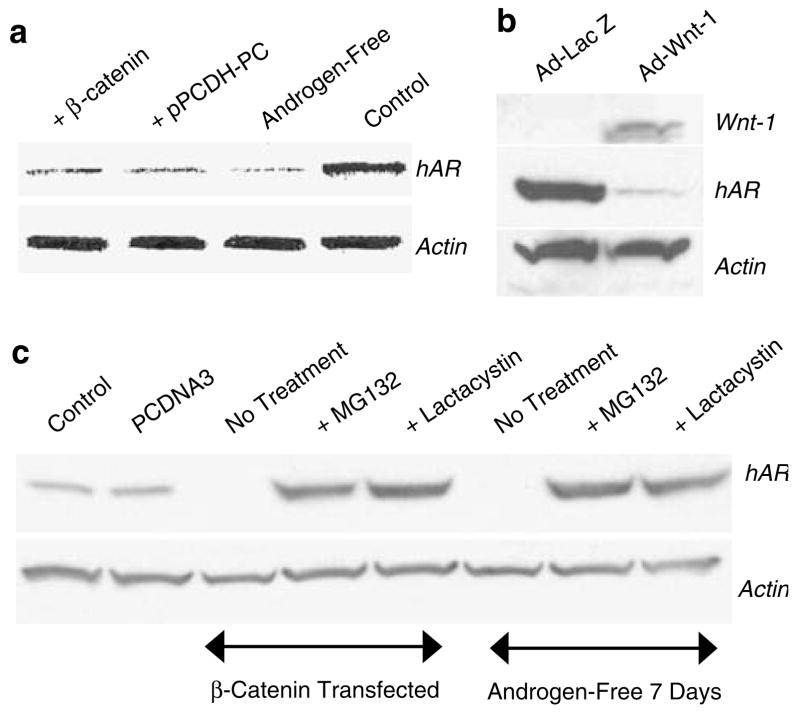
Expression of hAR protein is suppressed by Wnt signaling in LNCaP cells
In contrast to hAR mRNA, which was greatly increased by Wnt signaling in LNCaP cells, expression of hAR protein was reduced by at least 89% as assessed by densitometry of films from Western blot analysis of hAR expression in LNCaP cells transfected with β-catenin or PCDH-PC or in LNCaP cells maintained for 7 days in androgen-free medium (Figure 3a). In a similar manner, LNCaP cells transduced with Ad-Wnt-1 expressed <90% the amount of hAR protein compared to cells transduced with Ad-lac Z at 48 hrs subsequent to transduction (Figure 3b). The suppression of hAR protein levels in Wnt activated LNCaP cells is likely associated with loss of the protein through a ubiquitin-mediated proteasomal degradation process since transient exposure to 2 different proteasome inhibitors, MG132 or lactacystin increases hAR protein in β-catenin transfected cells to levels at least 12.3-fold higher than control-transfected cells (Figure 4c).
Figure 3.
Expression of hAR protein is downregulated in Wnt-activated LNCaP cells by a proteasomal degradation pathway. (a) Western blot shows relative expression of hAR or actin in control LNCaP cells (Control) or in LNCaP cells transfected with β-catenin or PCDH-PC or LNCaP cells grown in androgen-free medium for 7 days. (b) Western blot shows hAR protein is likewise downregulated in LNCaP cells transduced for 48 h with Ad-Wnt-1 but not from cells transduced with Ad-Lac Z. (c) Expression of hAR protein in Wnt-activated cells (β-catenin transfected or cultured in androgen-free medium for 7 days) is restored to levels commensurate with elevated hAR mRNA levels when Wnt-stimulated cells were treated with proteasome inhibitors, MG132 or lactacystin.
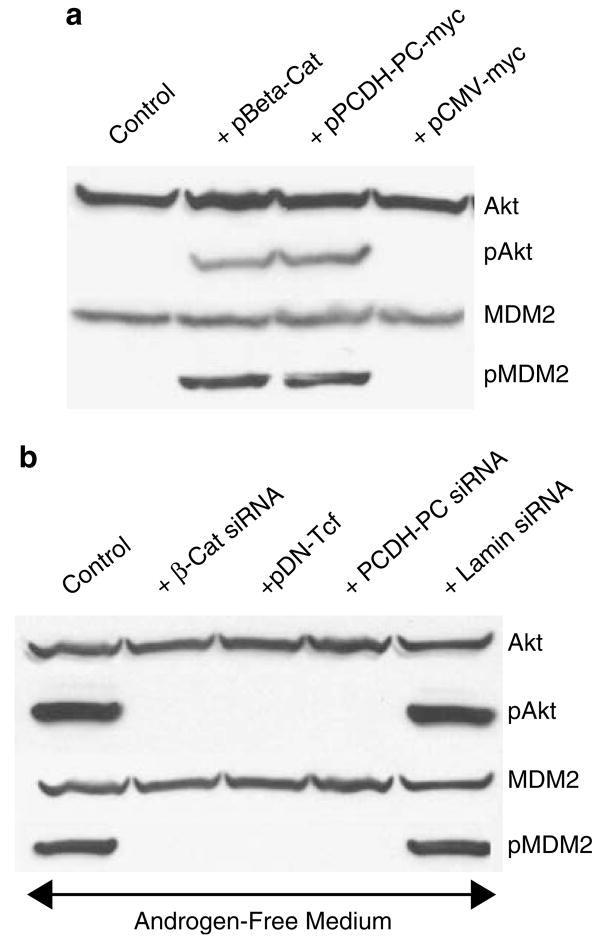
Figure 4.
Phosphorylation of Akt and its downstream target, MDM2, is increased in LNCaP cells with increased Wnt signaling. (a) Western blot shows that transfection of LNCaP cells with β-catenin or PCDH-PC increases pAKT and pMDM2 levels approximately 50-fold compared to control cells (untransfected or transfected with empty vector). (b) Western blot shows that agents that suppress Wnt signaling in LNCaP cells grown in androgen-free medium (transfection with siRNA against β-catenin or PCDH-PC or dominant-negative (DN-) TCF suppress pAkt and pMDM2 levels compared to control (untransfected or transfected with siRNA against lamin) LNCaP cells.
The role of Akt and its downstream target MDM2 in hAR protein degradation under Wnt-stimulated conditions
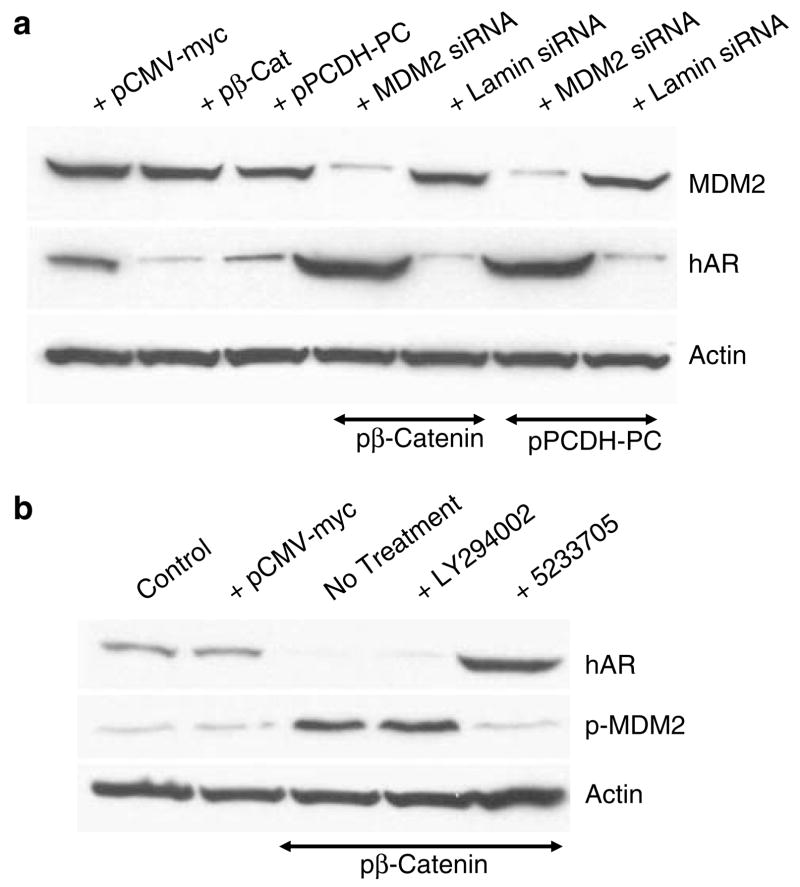
Prior evidence that activated (phosphorylated) Akt mediates an MDM2-directed ubiquitinylation and degradation of hAR (Lin et al., 2002) led to an evaluation of the effects of Wnt signaling on Akt and MDM2 in LNCaP cells tested by assessing the effects of β-catenin or PCDH-PC transfection on phospho-Akt (ser 473) levels and, as shown in Figure 4a, phospho-Akt levels are greater than 50 fold enhanced by transfection with either of these molecules. The activation of Akt signaling was consistent with a similar increase in the phosphorylation (at ser 166) of the Akt downstream target, MDM2 (Ashcroft et al., 2002). Further evidence that Wnt mediates activation of Akt signaling is shown in the results of Figure 4b wherein siRNAs against PCDH-PC or β-catenin or dominant negative TCF-4 strongly suppressed Akt (and MDM2) phosphorylation in LNCaP cells maintained in androgen-free medium. The critical participation of the MDM2 protein in the AR degradation process was shown in an experiment in which MDM2 expression was suppressed by an siRNA revealing that hAR levels, again were upregulated to higher than control levels in β-catenin transfected cells when MDM2 expression was suppressed (Figure 5a). Whereas a recent report suggested that Wnt signaling influences Akt signaling in PCa cells (Ohigashi et al., 2005), there was no prior evidence of the mechanism of this cross-talk. As is shown in Figure 5b, an inhibitor of PI3-kinase, LY2294002, was not able to suppress upregulation of MDM2 phosphorylation when LNCaP cells were transfected by β-catenin nor did this affect the downregulation of hAR protein expression. However, a direct inhibitor of Akt action (compound 5233705) (26) was able to suppress downstream phosphorylation of MDM2 in β-catenin-transfected cells and this resulted in a significant elevation in the levels of hAR protein, similar to effects of proteasomal inhibitors or MDM2 knockout. These result suggest that the effects of Wnt on Akt signaling are not mediated by stimulation of PI3-kinase activity. One potential indication of the mechanistic link between increased Wnt signaling and increased phosphorylation of Akt was found when protein extracts of β-catenin transfected or androgen-free LNCaP cells were reanalysed for phosphorylated MDM2 levels (Figure 6). Here, we found that proteasome inhibitors suppressed phosphorylation of MDM2, which implies that some activity associated with Wnt signaling may stimulate proteolytic degradation of an endogenous inhibitor of Akt or MDM2 activation. Indeed, when we evaluated these same protein extracts for expression of the Akt signaling inhibitor, protein phosphatase-2A (PP2A) (Stack et al., 2004), we found that the B catalytic subunit of this complex enzyme was reduced by approximately 87% in Wnt-stimulated cells and this loss was blocked by the proteasome inhibitors (Figure 6). There was no effect of Wnt-stimulation or proteasome inhibiton on expression of the catalytic C subunit of PP2A. Since the PP2A B subunit is known to bind to the β-catenin degradation complex that controls the canonical Wnt signaling pathway (Ratcliffe et al., 2000), our results suggest that Wnt crosstalk to Akt is mediated, at least partially, by proteasome-mediated destruction of the PP2A B subunit when Wnt signaling is activated.
Figure 5.
Suppression of MDM2 expression or direct Akt activity relieves Wnt-mediated suppression of hAR protein expression. (a) Western blot (top) shows that MDM2 protein expression is suppressed by greater than 88% by an siRNA that targets the gene and this siRNA relieves the Wnt-mediated suppression of hAR expression induced by transfection with β-catenin or PCDH-PC. (middle). Actin control (bottom). (b) Western blot shows that direct suppression of Akt signaling by inhibitor 5233705 but not by PI3-kinase inhibitor LY294002 relieves Wnt-mediated suppression of hAR protein (top) and Wnt-mediated upregulation in phosphorylation of MDM2 (middle) in β-catenin transfected LNCaP cells. Actin control (bottom).
Figure 6.
Proteasome inhibitors block the suppression of MDM2 phosphorylation and suppress degradation of PP2A B subunit protein in Wnt-activated LNCaP cells. Western blots shows that Wnt-activation (by β-catenin transfection or culture of LNCaP cells for 7 days in androgen-free medium) upregulates phosphorylation of MDM2 (top) that is blocked by proteasome inhibitors MG132 or lactacystin and this activity corresponds with loss of the regulatory subunit (b) of PP2A that is blocked by proteasome inhibitors (middle). There was no change in the PP2A catalytic C subunit levels in Wnt-activated or proteasome-inhibitor treated cells (bottom).
Discussion
Although the Wnt signaling pathway is involved in normal embryonic development, tissue differentiation and morphogenetic processes, it also plays an important role in human oncogenesis (Barker and Clevers, 2000; Lustig and Behrens, 2003). Intestinal/colon, breast, skin (melanoma) and oral cancers all show evidence for upregulation of wnt signaling during the natural history of their development and progression. As is best described in colon cancer (Sancho et al., 2004), Wnt signaling becomes dysregulated in association with mutations in the APC gene whose product is required for ubiquitin-mediated degradation of the β-catenin protein before it can activate LEF-1/TCF transcription or by mutations in the β-catenin gene that makes the protein refractory to the degradation process. Increasing evidence also indicates that the Wnt signaling pathway plays a role in PCa, especially in progression to the most aggressive and therapeutic-resistant state (de la Taille et al., 2003; Chen et al., 2004b). Mutations in both APC (Watanabe et al., 1996) and β-catenin (Voeller et al., 1998; Chesire et al., 2000) have been described in human PCa specimens, however, their apparent occurrence is at too low a frequency to account for the evidence for more frequent activation of Wnt signaling in this tumor system. Recently, we provided evidence that a novel member of the protocadherin gene family, protocadher-in-PC (PCDH-PC) is upregulated in apoptosis- and hormone-resistant human PCa cells and that a major effect of this gene product is the upregulation of Wnt signaling (Yang et al., 2005). In our prior study, Wnt signaling mediated by PCDH-PC expression or by expression of mutated β-catenin was shown to confer neuroendocrine-like characteristics on PCa cells and this phenotype is often described in association with aggressive PCa cells in vivo. Evidence presented here shows that PCDH-PC, β-catenin or Wnt-1 drastically increases levels of hAR mRNA and phospho-Akt. Since elevated Akt phosphorylation is also associated with aggressive PCa (Ghosh et al., 2003) the phenotypic transformation of the PCa cell mediated by PCDH-PC expression and Wnt signaling appears to confer many characteristics associated with the most aggressive forms of the disease.
Our findings add to the growing body of literature showing that the Wnt signaling pathway crosstalks with the androgen-signaling pathway. Previous work showing that β-catenin promotes androgen signaling through coactivation of liganded hAR identified a synergistic relationship between Wnt and androgen signaling in PCa cells. Here, we showed that Wnt signaling is also able to significantly upregulate hAR mRNA expression through transcriptional promotion mediated by TCF-binding elements within the promoter of the hAR gene and, if this resulted in similar upregulation of hAR protein, would imply that the upregulation of Wnt signaling alone would be sufficient to confer virtually all the characteristics of the most aggressive form of PCa. Enigmatically, however, increased Wnt signaling appears to have an opposite effect on expression of the hAR protein. Our observations suggest that this effect is likely mediated by the influence of Wnt on the Akt signaling pathway leading to increased phosphorylation of the Akt target, MDM2 and increased proteasomal degradation of hAR protein. Inhibitors of proteasomal activity (MG132 and lacacystin), Akt signaling (by compound 5233705) or MDM2 expression (with siRNA that targets this gene) resulted in hAR levels that were approximately eight to 12-fold higher in β-catenin-transfected cells than in control PCa cells and this increase was consistent with increased hAR mRNA levels in Wnt-stimulated cells. The inability of the PI3-kinase inhibitor LY29004 to suppress Akt phosphorylation subsequent to activation of Wnt signaling indicates that the mechanism of Wnt to Akt cross-talk likely does not involve an effect of Wnt on PI3-kinase activity. However, the evidence that Wnt activation leads to specific degradation of the B subunit of PP2A supports the concept that loss of PP2A activity is involved in this phenomenon since PP2A downregulates Akt signaling.
With regards to the situation in hormone refractory PCa cells found in specimens obtained from patients, there is evidence that these cells have upregulated hAR mRNA (Gil-Diez de Medina et al., 1998; Latil et al., 2001) as well as hAR protein (Ford et al., 2003). Similar findings are also reported for human PCa cell xenografts (Chen et al., 2004a) and cultured PCa cells that are chronically deprived of androgen (Shi et al., 2004). If Wnt signaling is a driving force involved in the generation of hormone refractory PCa, this would imply that there might be a two-step process; one in which the hAR gene is transcriptionally upregulated under conditions of increasing Wnt signaling immediately following androgen deprivation and a second step, which involves suppression of the hAR protein degradative process in the presence of highly active Akt signaling. This two-step progression pathway would be consistent with the natural biology of PCa in which hormonal ablation therapies transiently suppress disease progress for a limited period followed by a breakthrough in which the cancer cells acquire the ability to grow in the absence of androgens as well as with observations in animal models of hormone-dependent PCa (Craft et al., 1999).
Materials and methods
Cell lines, plasmids and siRNAs
LNCaP, CWR22rv-1, PC-3 and DU145 cells were obtained from ATCC and were passaged in medium (for LNCaP, CWR22rv-1 and DU145 RPMI 1640 or for PC-3, F-12k with 10% fetal calf serum and supplements) or androgen-free maintained as previously described (Yang et al., 2005). A defective adenovirus that expresses Wnt-1 protein (Ad-Wnt-1) and control, Lac Z expressing adenovirus (Ad-lac Z) were previously described (Young et al., 1998). These viruses were applied at 20 particles/cell in low serum (2%) medium for 1 h. Expression plasmids containing mutated (stabilized) human β-catenin (Tetsu and McCormick, 1999), dominant negative TCF-4 (Chen et al., 2001) or PCDH-PC cDNA were transfected into cells as previously described (Yang et al., 2005). Small interfering (si) RNAs targeting β-catenin or lamin were purchased from Dharmacon Inc. siRNA targeting human MDM2 was purchased from Qiagen Inc (Valencia, CA). siRNAs were transfected into cells as previously described (20). Proteasome inhibitors MG132 and lactacystin were purchased from Sigma Chemical Co. (St Louis, MO) and were used at 5 (MG132) or 10 (lactacystin) μM for 12 h prior to cell harvesting. PI3-kinase inhibitor LY294002 (Sigma Chemical Co.) and Akt Inhibitor IV (compound 5233705, EMD Biosciences Inc., San Diego, CA) (Kau et al., 2003) were used at 4 and 50 μM concentrations, respectively, for 12 h prior to harvesting cells.
Preparation of cell extracts and western blots
Cells were harvested and protein extracts prepared, quantified and used to prepare Western blots as previously described (Yang et al., 2005). Western blots were probed with mouse monoclonal antibodies against human Akt protein, phospho-MDM2 (ser 166), hAR (Santa Cruz Biotechnology Inc., Santa Cruz, CA), actin (Sigma Chemical Co., St Louis, MO) or with rabbit polyclonal antibodies against phospho-Akt (ser 473) or human MDM2 (Cell Signaling Technology, Beverly, MA). Recombinant Wnt-1 protein (HA-tagged) was detected with anti-HA antibody (Clonetech Inc., Mountain View, CA). Antibody binding to the Western blot was detected as previously described (Yang et al., 2005). Densitometry of films was carried out using a Kodak Image Station 420.
CHIP assay of regions of the hAR gene promoter bound to β-catenin protein
A 2000 bp region immediately upstream of the hAR gene (genbank accession #L14435) was analysed for core LEF-1/TCF binding sites (5′-CTTTG-3′) using the TransFac computer analysis program. PCR primer sets were designed to amplify small regions within this promoter sequence: Primer set #1 (−322 to −218) forward 5′-TTAGATTGGGCTTTG GAACC-3′, reverse 5′-GCTTCCTGAATAGCTCCTGCT-3′; Primer set #2 (−733 to −543) forward 5′-CAAAATTGAGCGC CTATGTG-3′, reverse 5′-TTGCTCTAGGAACCCTCAGC-3′; Primer set #3 (−1082 to −938) forward 5′-GGCAAAAATCT CGGAATGAC-3′, reverse 5′-AAAGGTGGAGATGCAAG TGG-3′; Primer set #4 (−1257 to −1088) forward 5′-ATCC AGTCTTCCTTGCCTTT-3′, reverse 5′-TTCTGGGAGGCTCTCTGTTC-3′; Primer set #5 (−1456 to −1295) forward 5′-CAGGTGAAAGGGTCTTCAGG-3′, reverse 5′-AGGAC ATAATTTGTTCTATGTTCCAC-3′; Primer set #6 (−1795 to −1698) forward 5′-TTTTTCAGGCCTCTTTGTGTC-3′, reverse 5′-TGTGTCTACACACTAACAGTGAAGGA-3′; Primer set #7 (−1902 to −1808) forward 5′-TGGTGATGTG GAAGCAACATA-3′, reverse 5′-AAGGTGAGAAATAATG CTCTGAAGTT-3′. Two additional primer sets were designed to amplify regions within the promoters of the human c-myc (He et al., 1998) (forward 5′-GCTCTCCACTTGCCCCTTT TA-3′, reverse 5′-GTTCCCAATTTCTCAGCC-3′) and cyclin D1 gene (Tetsu and McCormick, 1999) (forward 5′-GGGAG GAATTCACCCTGAAA-3′, reverse 5′-CCTGCCCCAAAT TAAGAAAA-3′) that contain known LEF-1/TCF binding sites. CHIP assays were then performed on LNCaP cells that were transfected by empty vector (pCMV-myc), β-catenin or PCDH-PC expression plasmids for 48 h using the CHIP-IT kit of Active Motif Inc. (Carlsbad, CA) using the manufacturer’s protocol. A specimen of formalin-fixed sheared chromatin from empty vector transfected LNCaP cells was used as ‘input DNA’ for control amplifications. Fixed chromatin was immunoprecipitated using monoclonal mouse anti-β-catenin antibody (Santa Cruz Biotechnology Inc.) and DNA was extracted from the immunoprecipitate and amplified using the primer sets described above. Amplification products on 1.2% agarose gels were visualized under UV light after ethidium bromide staining and sized according to molecular weight markers in adjacent lanes. Control immunoprecipitations was carried out using nonimmune mouse IgG (Santa Cruz Biotechnology Inc.) from each of the specimens did not yield any reaction products for any of the primer sets.
Construction of hAR promoter-luciferase reporter vectors and test for Wnt-responsiveness
A series of PCR primers were designed to amplify increasing regions of the hAR promoter region, each anchored at the 3′ termini at base −528 upstream the transcription start site (reverse primer 5′-GCGAAGCTTGTGGCATTGTGC CATTTG-3′). The various upstream (forward) primers utilized were: 5′ position −2129, 5′-GCGCTCGAGTCAAAATCCAA ATAAAGTATATGGCC-3′; 5′ position −1628, 5′-GCGCTCG AGAGCCCACTCAATTCCTATTGAG-3′; 5′ position −1228, 5′-CTCGAGACCTTCTTTGGTCAAGGTAAGTAAA-3′; 5′ position −1128, 5′-CTCGAGACCTTCTTTGGTCAAGGT AAGTAAA-3′ and; 5′ position −828, 5′-CTCGAGCCTTG-GATAGTTCCAGTTGTAAAG-3′. Primers were utilized to amplify DNA extracted from human LNCaP cells using thermocycles of 94°C for 20 s for one cycle, 94°C for 3 min, 56°C for 30 s and 72°C for 30 s for 32 cycles and finished by a 10 min cycle at 72°C. DNA fragments from the various amplifications were inserted into the pGEM-T Easy vector (Promega Life Sciences Inc., Madison, WI). Inserted fragment were removed using HindIII and XhoI restriction endonucleases and were purified using the Nucleo Trap Nucleic Acid Purification Kit (BD Biological Science Inc., Palo Alto, CA) and ligated into HindIII, XhoI cleaved pGL3 vector (Promega) using the Rapid DNA Ligation Kit (Roche Applied Science, Indianapolis, IN). Reporter vectors (3 μg) were co-transfected with 3 μg of pCDNA3 (empty vector) or β-catenin along with 0.3 μg of a β-galactosidase vector (Promega). After 48 h, luciferase and β-gal activity was measured using the Luciferase Assay System and β-galactosidase Assay Systems of Promega Inc. Normalized luciferase activity is calculated as Light Units normalized to β-gal activity present in each specimen. Each assay was performed in triplicate.
Semiquantitative and real time RT–PCR analysis of AR mRNA expression
RNA was extracted from control or transfected cells using the Rneasy Kit from Qiagen Inc. and RNA was quantified by spectrophotometry at 260 nm. RNA (1 μg) was converted to cDNA using oligo-dT primer and reverse transcriptase (Superscript III, Invitrogen Life Technologies). For semiquantitative evaluation of hAR and G3PDH mRNA expression, 1/50 reverse transcription reaction product was amplified with the hAR primer set (forward, 5′-GGACTTCACCGCACCTG ATG-3′; reverse, 5′-CTGGCAGTCTCCAAACGCAT) or the G3PDH primer set (forward, 5′-GGATTTGGTCGTATTGG GCGC-3′; reverse, 5′-GTTCTCAGCCTTGACGGTGC-3′) using Amplitaq Gold Taq polymerase (Invitrogen Life Sciences) for 5 min at 90°C followed by 35 cycles of 92°C for 1 min, 57°C for 1 min and 72°C for 1 min and finished by 10 min at 72°C. Ethidium bromide-stained amplification products were visualized after electrophoresis under UV light. For semiquantitative (real time) RT–PCR, 1/50 reverse transcription reaction product was amplified using hAR (forward, 5′CGGAAGCTGAAGAAACTTGG-3′; reverse 5′-CGTGTCCAGCACACACTACA-3′) or actin (forward, 5′-ATGGATGATGATATCGCCGC-3′; reverse, 5′-AAGCA TTTGCGGTGGACGAT-3′) primer sets in triplicate for each specimen using the reagents of the Roche Applied Biosystems LightCycler® FastStart reaction mix that monitors amplification products based upon SYBR Green I fluoresence on a LightCycler 2.0 instrument (Roche Diagnostics Inc.). Data was analysed using the LightCycler® software that calculates the crossing point of each sample on the quantification curve. The specificity of each reaction was demonstrated by conducting a melting curve analysis of the PCR product at the end of each run.
Acknowledgments
This work was supported by funding from the TJ Martell Foundation, New York, NCI (RO1 CA111618), Department of Defense (PC050402) and from l’Association pour la Recherche sur les Tumeurs de la Prostate, France. Xuezhen Yang is the White Fellow for Prostate Cancer Research. Debra L Bemis is the Charles Royce Fellow.
References
- Ashcroft M, Ludwig RL, Woods DB, Copeland TD, Weber HO, MacRae EJ, et al. Oncogene. 2002;21:1955–1962. doi: 10.1038/sj.onc.1205276. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Bioessays. 2000;22:961–965. doi: 10.1002/1521-1878(200011)22:11<961::AID-BIES1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee H-J, et al. Crit Rev Eukaryot Gene Exp. 1995;5:97–125. doi: 10.1615/critreveukargeneexpr.v5.i2.10. [DOI] [PubMed] [Google Scholar]
- Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Nature Med. 2004a;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- Chen G, Shukeir N, Potti A, Sircare K, Aprikian A, Goltzman D, et al. Cancer. 2004b;10:1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, et al. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Prostate. 2000;45:323–334. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON, et al. Cancer Res. 1999;59:5030–5036. [PubMed] [Google Scholar]
- de la Taille A, Rubin MA, Chen M-W, Vacherot F, Gil-Diez de Medina S, Burchardt M, et al. Clin Cancer Res. 2003;9:1804–1807. [PubMed] [Google Scholar]
- Ford OH, Gregory CW, Kim D, Smitherman AB, Mohler JL. J Urol. 2003;170:1817–1821. doi: 10.1097/01.ju.0000091873.09677.f4. [DOI] [PubMed] [Google Scholar]
- Ghosh PM, Malik S, Bedolla R, Kreisberg JI. Curr Drug Metabol. 2003;4:487–496. doi: 10.2174/1389200033489226. [DOI] [PubMed] [Google Scholar]
- Gil-Diez de Medina S, Salomon L, Colombel M, Abbou CC, Bellot J, Thiery JP, et al. Hum Pathol. 1998;29:1005–1012. doi: 10.1016/s0046-8177(98)90208-8. [DOI] [PubMed] [Google Scholar]
- Grossmann ME, Huang H, Tindall DJ. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Kau TR, Schroeder F, Ramaswamy S, Wojciechowski CL, Zhao JJ, Roberts TM, et al. Cancer Cell. 2003;4:463–476. doi: 10.1016/s1535-6108(03)00303-9. [DOI] [PubMed] [Google Scholar]
- Koh SS, Li H, Lee YH, Widelitz RB, Chuong CM, Stallcup MR. J Biol Chem. 2002;277:26031–26035. doi: 10.1074/jbc.M110865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latil A, Bieche I, Vadaud D, Lidereau R, Berthon P, Cussonet O, et al. Cancer Res. 2001;61:1919–1926. [PubMed] [Google Scholar]
- Li H, Kim JH, Koh SS, Stallcup MR. J Biol Chem. 2004;279:4212–4220. doi: 10.1074/jbc.M311374200. [DOI] [PubMed] [Google Scholar]
- Lin H-K, Wang L, Hu Y-C, Altuwaijri S, Chang C. EMBO J. 2002;21:4037–4048. doi: 10.1093/emboj/cdf406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig B, Behrens J. J Cancer Res Clin Oncol. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]
- Miyamoto H, Messing EM, Chang C. Prostate. 2005;61:332–353. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- Ohigashi T, Mizuno R, Nakashima J, Marumo K, Murai M. Prostate. 2005;62:61–68. doi: 10.1002/pros.20117. [DOI] [PubMed] [Google Scholar]
- Rahman M, Miyamoto H, Chang C. Clin Cancer Res. 2004;10:2208–2219. doi: 10.1158/1078-0432.ccr-0746-3. [DOI] [PubMed] [Google Scholar]
- Ratcliffe MJ, Itoh K, Sokol SY. J Biol Chem. 2000;275:35680–35683. doi: 10.1074/jbc.C000639200. [DOI] [PubMed] [Google Scholar]
- Sancho E, Batlle E, Clevers H. Ann Rev Cell Develop Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- Shi XB, Ma AH, Tepper CG, Xia L, Gregg JP, Gandour-Edwards R, et al. Prostate. 2004;60:257–271. doi: 10.1002/pros.20039. [DOI] [PubMed] [Google Scholar]
- Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP. Mol Cell Biol. 2003;23:1674–1687. doi: 10.1128/MCB.23.5.1674-1687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack S, Cribbs JT, Gomez L. J Biol Chem. 2004;279:47732–47739. doi: 10.1074/jbc.M408015200. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tilley WD, Buchanan G, Hickey TE, Bentel JM. Clin Cancer Res. 1996;2:277–285. [PubMed] [Google Scholar]
- Truica CI, Byers S, Gelmann EP. Cancer Res. 2000;60:4709–4713. [PubMed] [Google Scholar]
- Verras M, Brown J, Li X, Nusse R, Sun Z. Cancer Res. 2004;64:8860–8866. doi: 10.1158/0008-5472.CAN-04-2370. [DOI] [PubMed] [Google Scholar]
- Voeller HJ, Truica CI, Gelmann EP. Cancer Res. 1998;58:2520–2523. [PubMed] [Google Scholar]
- Watanabe M, Kakiuchi H, Kato H, Shraishi T, Yatani R, Sugimura T, et al. Jap J Clin Oncol. 1996;26:77–81. doi: 10.1093/oxfordjournals.jjco.a023188. [DOI] [PubMed] [Google Scholar]
- Yang F, Li X, Sharma M, Sasaki CY, Longo DL, Lim B, et al. J Biol Chem. 2002;277:11336–11344. doi: 10.1074/jbc.M111962200. [DOI] [PubMed] [Google Scholar]
- Yang X, Chen M-W, Terry S, Vacherot F, Chopin DK, Bemis DL, et al. Cancer Res. 2005;65:5263–5271. doi: 10.1158/0008-5472.CAN-05-0162. [DOI] [PubMed] [Google Scholar]
- Young CS, Kitamura M, Hardy S, Kitajewski J. Mol Cell Biol. 1998;18:2474–2485. doi: 10.1128/mcb.18.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]