Abstract
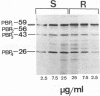
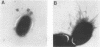
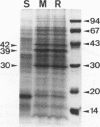
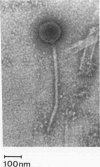
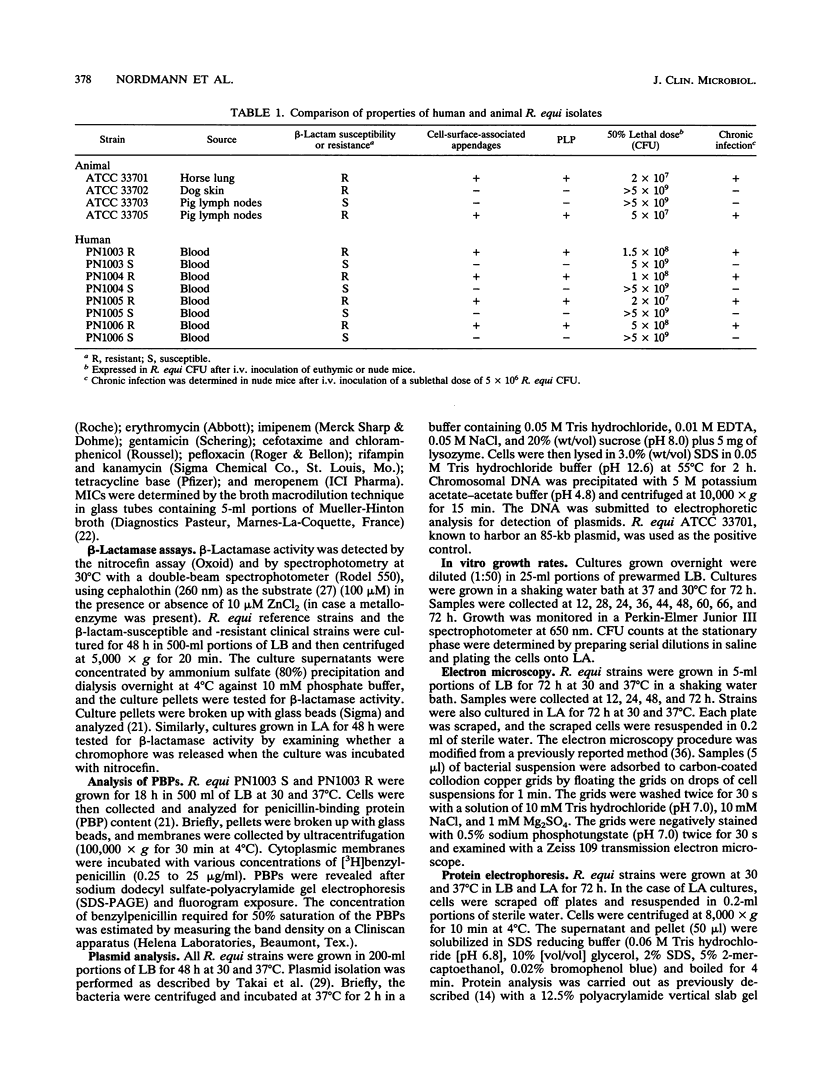
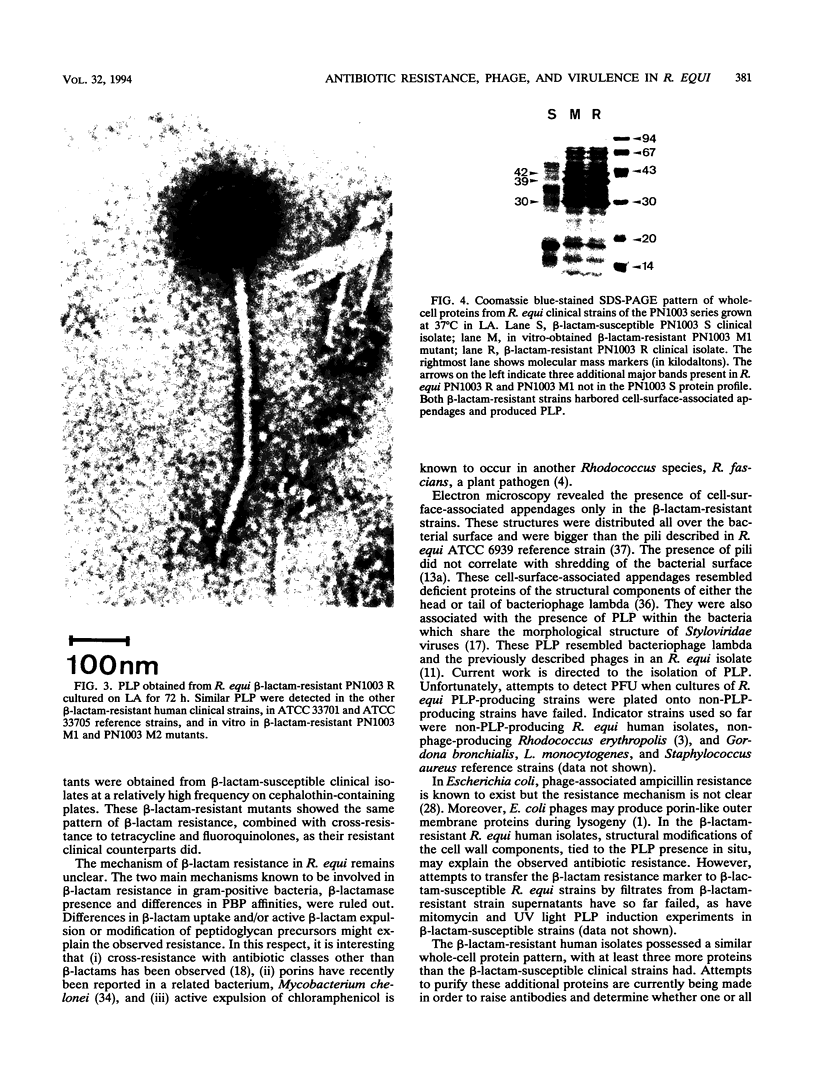
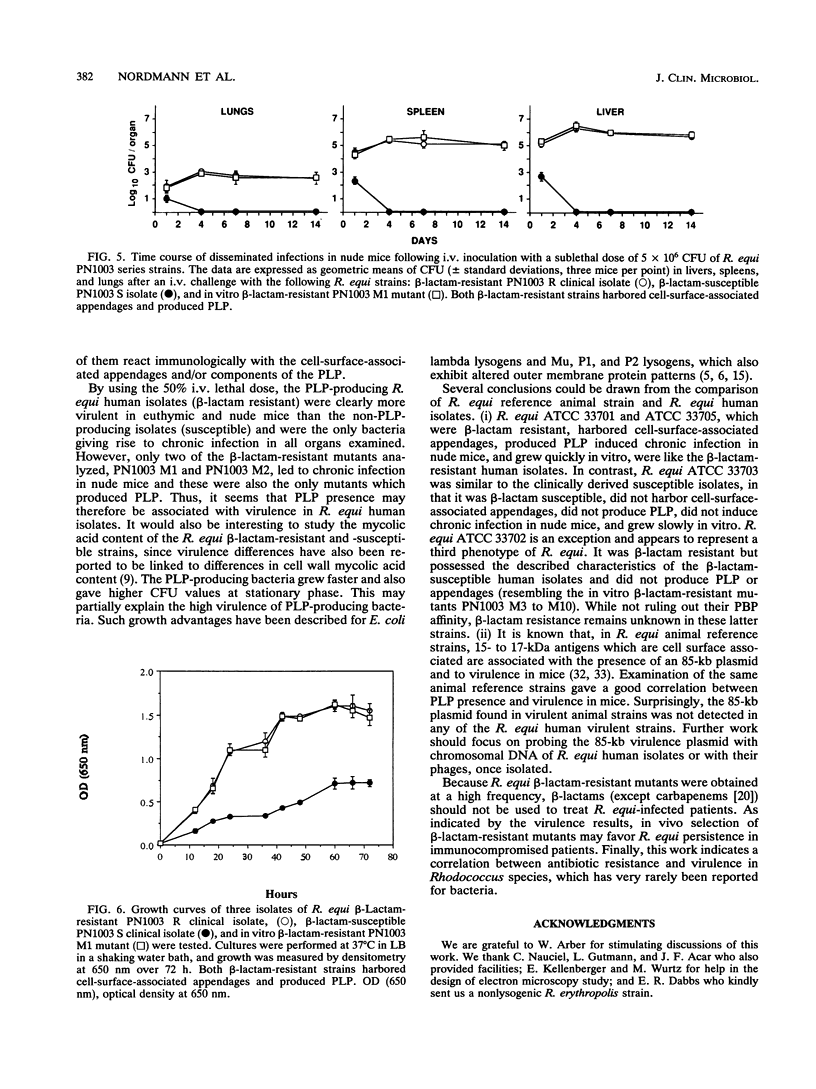
Rhodococcus equi is a gram-positive coccobacillus that appears to be emerging as a pulmonary pathogen in AIDS patients. In four human clinical isolates, two antibiotic resistance phenotypes were found to coexist: one beta-lactam resistant and the other beta-lactam susceptible. In vitro, beta-lactam-resistant mutants were obtained at a frequency of 1 x 10(-5) to 5 x 10(-5) from beta-lactam-susceptible strains on cephalothin-containing plates. Neither beta-lactamase nor plasmid DNA was detected in beta-lactam-resistant or -susceptible strains. The penicillin-binding protein patterns for the two types of strains were identical. Electron microscopy revealed that the beta-lactam-resistant strains possessed cell-surface-associated appendages and produced phage-like particles. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of total cell protein showed at least three additional bands of 42, 39, and 30 kDa found only in the beta-lactam-resistant strains. Testing for virulence in Swiss mice revealed that (i) phage-like-particle-producing strains had lower 50% lethal doses when injected intravenously in euthymic and nude mice than the non-phage-like-particle-producing strains did and (ii) intravenous inoculation of a sublethal dose (5 x 10(6) CFU) in nude mice led to chronic infection by the phage-like-particle-producing bacteria only. Finally, in vitro growth curves indicated that the phage-like-particle-producing strains possessed an ecological selection advantage. These results suggest that, among R. equi human isolates, the antibiotic resistance phenotype is associated with virulence and may be phage mediated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondess J. J., Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature. 1990 Aug 30;346(6287):871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- Classification and nomenclature of viruses. Fourth report of the International Committee on Taxonomy of Viruses. Intervirology. 1982;17(1-3):1–199. doi: 10.1159/000149278. [DOI] [PubMed] [Google Scholar]
- Desomer J., Vereecke D., Crespi M., Van Montagu M. The plasmid-encoded chloramphenicol-resistance protein of Rhodococcus fascians is homologous to the transmembrane tetracycline efflux proteins. Mol Microbiol. 1992 Aug;6(16):2377–2385. doi: 10.1111/j.1365-2958.1992.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Edlin G., Lin L., Bitner R. Reproductive fitness of P1, P2, and Mu lysogens of Escherichia coli. J Virol. 1977 Feb;21(2):560–564. doi: 10.1128/jvi.21.2.560-564.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin G., Lin L., Kudrna R. Lambda lysogens of E. coli reproduce more rapidly than non-lysogens. Nature. 1975 Jun 26;255(5511):735–737. doi: 10.1038/255735a0. [DOI] [PubMed] [Google Scholar]
- Emmons W., Reichwein B., Winslow D. L. Rhodococcus equi infection in the patient with AIDS: literature review and report of an unusual case. Rev Infect Dis. 1991 Jan-Feb;13(1):91–96. doi: 10.1093/clinids/13.1.91. [DOI] [PubMed] [Google Scholar]
- Goodfellow M. The taxonomic status of Rhodococcus equi. Vet Microbiol. 1987 Aug;14(3):205–209. doi: 10.1016/0378-1135(87)90106-4. [DOI] [PubMed] [Google Scholar]
- Gotoh K., Mitsuyama M., Imaizumi S., Kawamura I., Yano I. Mycolic acid-containing glycolipid as a possible virulence factor of Rhodococcus equi for mice. Microbiol Immunol. 1991;35(3):175–185. doi: 10.1111/j.1348-0421.1991.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Harvey R. L., Sunstrum J. C. Rhodococcus equi infection in patients with and without human immunodeficiency virus infection. Rev Infect Dis. 1991 Jan-Feb;13(1):139–145. doi: 10.1093/clinids/13.1.139. [DOI] [PubMed] [Google Scholar]
- Hiddema R., Curran M. D., Ferreira N. P., Coetzee J. N., Lecatsas G. Characterization of phages derived from strains of Rhodococcus australis and R. equii. Intervirology. 1985;23(2):109–111. doi: 10.1159/000149592. [DOI] [PubMed] [Google Scholar]
- Hietala S. K., Ardans A. A. Interaction of Rhodococcus equi with phagocytic cells from R. equi-exposed and non-exposed foals. Vet Microbiol. 1987 Aug;14(3):307–320. doi: 10.1016/0378-1135(87)90118-0. [DOI] [PubMed] [Google Scholar]
- Kanaly S. T., Hines S. A., Palmer G. H. Failure of pulmonary clearance of Rhodococcus equi infection in CD4+ T-lymphocyte-deficient transgenic mice. Infect Immun. 1993 Nov;61(11):4929–4932. doi: 10.1128/iai.61.11.4929-4932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin L., Bitner R., Edlin G. Increased reproductive fitness of Escherichia coli lambda lysogens. J Virol. 1977 Feb;21(2):554–559. doi: 10.1128/jvi.21.2.554-559.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Kerestedjian J. J., Ronco E. Therapy of Rhodococcus equi disseminated infections in nude mice. Antimicrob Agents Chemother. 1992 Jun;36(6):1244–1248. doi: 10.1128/aac.36.6.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Nicolas M. H., Gutmann L. Penicillin-binding proteins of Rhodococcus equi: potential role in resistance to imipenem. Antimicrob Agents Chemother. 1993 Jul;37(7):1406–1409. doi: 10.1128/aac.37.7.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Ronco E. In-vitro antimicrobial susceptibility of Rhodococcus equi. J Antimicrob Chemother. 1992 Apr;29(4):383–393. doi: 10.1093/jac/29.4.383. [DOI] [PubMed] [Google Scholar]
- Nordmann P., Ronco E., Nauciel C. Role of T-lymphocyte subsets in Rhodococcus equi infection. Infect Immun. 1992 Jul;60(7):2748–2752. doi: 10.1128/iai.60.7.2748-2752.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordmann P., Rouveix E., Guenounou M., Nicolas M. H. Pulmonary abscess due to a rifampin and fluoroquinolone resistant Rhodococcus equi strain in a HIV infected patient. Eur J Clin Microbiol Infect Dis. 1992 Jun;11(6):557–558. doi: 10.1007/BF01960815. [DOI] [PubMed] [Google Scholar]
- Prescott J. F. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991 Jan;4(1):20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samies J. H., Hathaway B. N., Echols R. M., Veazey J. M., Jr, Pilon V. A. Lung abscess due to Corynebacterium equi. Report of the first case in a patient with acquired immune deficiency syndrome. Am J Med. 1986 Apr;80(4):685–688. doi: 10.1016/0002-9343(86)90825-9. [DOI] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Iie M., Watanabe Y., Tsubaki S., Sekizaki T. Virulence-associated 15- to 17-kilodalton antigens in Rhodococcus equi: temperature-dependent expression and location of the antigens. Infect Immun. 1992 Jul;60(7):2995–2997. doi: 10.1128/iai.60.7.2995-2997.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Koike K., Ohbushi S., Izumi C., Tsubaki S. Identification of 15- to 17-kilodalton antigens associated with virulent Rhodococcus equi. J Clin Microbiol. 1991 Mar;29(3):439–443. doi: 10.1128/jcm.29.3.439-443.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Michizoe T., Matsumura K., Nagai M., Sato H., Tsubaki S. Correlation of in vitro properties of Rhodococcus (Corynebacterium) equi with virulence for mice. Microbiol Immunol. 1985;29(12):1175–1184. doi: 10.1111/j.1348-0421.1985.tb00907.x. [DOI] [PubMed] [Google Scholar]
- Takai S., Sekizaki T., Ozawa T., Sugawara T., Watanabe Y., Tsubaki S. Association between a large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect Immun. 1991 Nov;59(11):4056–4060. doi: 10.1128/iai.59.11.4056-4060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachuk-Saad O., Prescott J. Rhodococcus equi plasmids: isolation and partial characterization. J Clin Microbiol. 1991 Dec;29(12):2696–2700. doi: 10.1128/jcm.29.12.2696-2700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Jarlier V., Benz R. Porins in the cell wall of mycobacteria. Science. 1992 Nov 27;258(5087):1479–1481. doi: 10.1126/science.1279810. [DOI] [PubMed] [Google Scholar]
- Van Etta L. L., Filice G. A., Ferguson R. M., Gerding D. N. Corynebacterium equi: a review of 12 cases of human infection. Rev Infect Dis. 1983 Nov-Dec;5(6):1012–1018. doi: 10.1093/clinids/5.6.1012. [DOI] [PubMed] [Google Scholar]
- Wurtz M., Kistler J., Hohn T. Surface structure of in vitro assembled bacteriophage lambda polyheads. J Mol Biol. 1976 Feb 15;101(1):39–56. doi: 10.1016/0022-2836(76)90065-6. [DOI] [PubMed] [Google Scholar]
- Yanagawa R., Honda E. Presence of pili in species of human and animal parasites and pathogens of the genuscorynebacterium. Infect Immun. 1976 Apr;13(4):1293–1295. doi: 10.1128/iai.13.4.1293-1295.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]