Abstract
A synthesis of the novel tyrosine analogue (2S)-2-methyl-3-(2,6-dimethyl-4-carbamoylphenyl)propanoic acid [(2S)-Mdcp] (15) was developed. In (2S)-Mdcp the amino- and hydroxyl groups of 2',6'-dimethyltyrosine are replaced by a methyl- and a carbamoyl group, respectively, and its substitution for Tyr1 in opioid agonist peptides resulted in compounds showing antagonism at all three opioid receptors. The cyclic peptide (2S)-Mdcp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 (1) was a potent and selective μ antagonist, whereas (2S)-Mdcp-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OH (3) showed subnanomolar δ antagonist activity and extraordinary δ selectivity.
Introduction
Substitution of 2',6'-dimethyltyrosine (Dmt) for the Tyr1 residue in opioid peptides generally increases agonist potency by 1-2 orders of magnitude,1 presumably due to additional hydrophobic binding interactions of the two methyl groups with the receptor. Deletion of the N-terminal amino group in Dmt1-opioid peptide analogues or its replacement with a methyl group produced potent opioid antagonists.2-4 This was achieved through substitution of 3-(2,6-dimethyl-4-hydroxyphenyl)propanoic acid (Dhp)a or (2S)-2-methyl-3-(2,6-dimethyl-4-hydroxyphenyl)propanoic acid [(2S)-Mdp, Figure 1] for Dmt1. Replacement of the Tyr1 hydroxyl group in opioid peptides with a carbamoyl (-CONH) group resulted in compounds that retained high opioid agonist potency.5,6 This interesting observation prompted the replacement of the hydroxyl group of Dhp1 in opioid peptide antagonists with a carbamoyl group, as achieved by substitution of 3-(2,6-dimethyl-4-carbamoylphenyl)propanoic acid (Dcp) for Dhp.7 The Dcp1-analogue of the cyclic enkephalin agonist peptide H-Tyr-c[D-Cys-Gly-Phe(pNO)-D-Cys]NH28 showed high μ opioid antagonist activity, whereas [Dcp1]dynorphin A(1-11)-NH2 was a moderately potent κ opioid antagonist.7
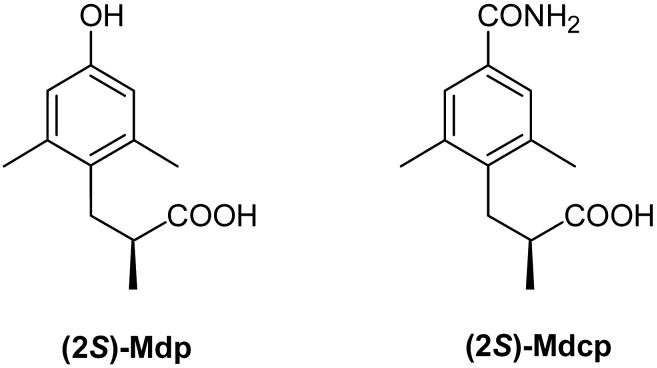
Figure 1.
Chemical structures of (2S)-Mdp and (2S)-Mdcp.
Since (2S)-Mdp1-analogues of opioid peptides are more potent antagonists than their corresponding Dhp1-analogues,3 it is of interest to determine how the replacement of the (2S)-Mdp hydroxyl group in (2S)-Mdp1-containing opioid peptide antagonists with a-CONH2 group would affect the in vitro opioid activity profile. This requires the substitution of (2S)-2-methyl-3-(2,6-dimethyl-4-carbamoylphenyl)propanoic acid [(2S)-Mdcp] for (2S)-Mdp (Figure 1). Here, we describe the stereoselective synthesis of (2S)-Mdcp. The (2S)-Mdcp1-analogues of the nonselective cyclic opioid peptide H-Tyr-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2, the P-selective enkephalin analogue H-Tyr-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OH9 and dynorphin A(1-11)-NH2 (κ-selective) were prepared and pharmacologically characterized in vitro.
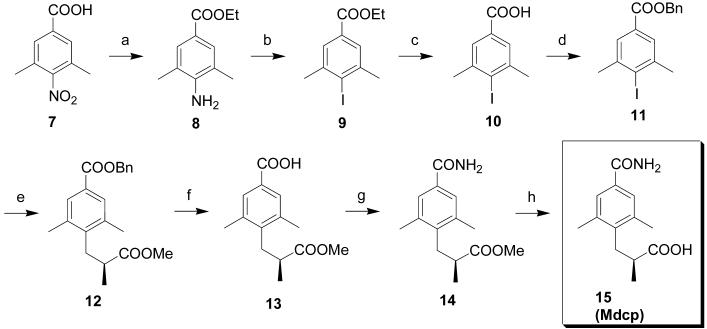
The preparation of Mdcp (15) is illustrated in Scheme 1. 3,5-Dimethyl-4-nitrobenzoic acid (7) was synthesized from mesitylene in two steps following literature procedures.10,11 Subsequent esterification and reduction afforded ethyl 4-amino-3,5-dimethyl benzoate (8)12, which was next converted to ethyl 4-iodo-3,5-dimethylbenzoate (9) via a Sandmeyer reaction.13 Basic hydrolysis and re-esterification provided the advanced intermediate benzyl 4-iodo-3,5-dimethyl benzoate (11). The organozinc reagent, prepared by sonicating a mixture of (R)-methyl 3-iodo-2-methylpropanoate and Zn-Cu couple, underwent palladium-mediated coupling reaction with 11 to afford diester (12).14 Catalytic hydrogenolysis of 12 yielded free benzoic acid 13, which was converted to amide 14. Final basic hydrolysis gave the desired (2S)-Mdcp (15). Peptides were prepared by standard solid-phase and solution synthesis techniques.
Scheme 1.
Reagents and conditions: (a) EtOH, dry HCl (g), reflux 6 h, followed by Sn powder, 40 °C, 4 h, 82%; (b) NaNO2, conc. HCl, KI, acetone, 83%; (c) LiOH, THF-MeOH-H2O, 0 °C to rt, 95%; (d) K2CO3, BnBr, DMF, rt, 6 h, 92%; (e) Zn-Cu couple, (R)-methyl 3-iodo-2-methylpropanoate, PdCl2[P(o-Tol)3]2, Benzene, 52%; (f) Pd/C, H2(g), MeOH, rt, overnight, 96%; (g) (COCl)2, CH2Cl2, rt, 2 h then NH4OH (25%), 0 °C to rt, 2 h, 87%; (h) LiOH (1N) - THF (1 : 1), 0 °C, 2.5 h, 78%.
Results and Discussion
The cyclic pentapeptide analogue (2S)-Mdcp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 (1) displayed antagonist activity at all three opioid receptors (μ, δ, κ), as determined in the functional in vitro assays (Table 1). In the GPI assay it showed very high μ opioid antagonist activity (Keμ = 1.24 ± 0.23 nM) and considerably lower antagonist potency at the κ opioid receptor (Keκ = 18.9 ± 0.3 nM). Its δ antagonist activity determined in the MVD assay (Keκ = 9.78 ± 1.16 nM) was also weaker. As indicated by the calculated Ke ratios (Table 1), compound 1 turned out to be a quite selective μ opioid antagonist. In agreement with its high μ opioid antagonist activity determined in the GPI assay, cyclic peptide 1 showed high μ opioid receptor binding affinity in the rat brain membrane binding assay (Kiμ = 1.02 ± 0.15 nM) (Table 2). The μ receptor selectivity of this compound was confirmed by its relatively weak δ and κ receptor affinities determined in the binding assays. These results indicate that (2S)-Mdcp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 (1) is a slightly more potent μ antagonist than (2S)-Mdp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 (2) (Tables 1 and 2). Furthermore, the (2S)-Mdcp1-analogue (1) is μ-selective, whereas the (2S)-Mdp1-analogue has almost no μ vs. δ selectivity.
Table 1.
Angatonist Potencies (Ke values) of (2S)-Mdcp1 - and (2S)-Mdp1 -Analogues of Opioid Peptidesa
| GPI |
GPI |
MVD |
Ke ratio |
|||
|---|---|---|---|---|---|---|
| no. | antagonist | Keμ (nM)b | Keκ (nM)c | Keδ (nM)d | μ/δ/κ | δ/μ/κ |
| 1 | (2S)-Mdcp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 | 1.24 ± 0.23 | 18.9 ± 0.3 | 9.78 ± 1.16 | 1/8/15 | |
| 2 | (2S)-Mdp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2e | 1.48 ± 0.18 | 6.41 ± 0.18 | 3.48 ± 0.28 | 1/2/4 | |
| 3 | (2S)-Mdcp-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OH | 1170 ± 100 | 9980 ± 1650 | 0.326 ± 0.030 | 1/3590/30600 | |
| 4 | (2S)-Mdp-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OHf | 668 ± 40 | 4370 ± 860 | 0.785 ± 0.135 | 1/851/5570 | |
| 5 | [(2S)-Mdcp1]Dyn A(1-11)-NH2 | 401 ± 62 | 5.96 ± 0.80 | 843 ± 88 | 1/2/0.015 | |
| 6 | [(2S)-Mdp1]Dyn A(1-11)-NH2g | 925 ± 94 | 3.92 ± 0.65 | 3320 ± 520 | 1/4/0.004 | |
Table 2.
Opioid Receptor Binding Affinities of (2S)-Mdcp1 - and (2S)-Mdp1 -Analogues of Opioid Peptidesa
| Ki ratio |
||||||
|---|---|---|---|---|---|---|
| no. | antagonist | Kiμ (nM) | Kiκ (nM) | Kiδ (nM) | μ/δ/κ | δ/μ/κ |
| 1 | (2S)-Mdcp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 | 1.02 ± 0.15 | 185 ± 32 | 19.0 ± 1.5 | 1/19/181 | |
| 2 | (2S)-Mdp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2b | 2.23 ± 0.08 | 92.8 ± 5.3 | 3.61 ± 0.41 | 1/2/42 | |
| 3 | (2S)-Mdcp-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OH | 5850 ± 300 | 3650 ± 650 | 2.92 ± 0.23 | 1/2000/1250 | |
| 4 | (2S)-Mdp-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OHc | 406 ± 21 | 3710 ± 400 | 2.32 ± 0.40 | 1/175/1600 | |
| 5 | [(2S)-Mdcp1]Dyn A(1-11)-NH2 | 29.6 ± 6.8 | 26.0 ± 4.1 | 27.2 ± 1.0 | 1/1/1 | |
| 6 | [(2S)-Mdp1]Dyn A(1-11)-NH2d | 213 ± 50 | 0.823 ± 0.162 | 163 ± 15 | 1/1/0.004 | |
The cyclic hexapeptide analogue (2S)-Mdcp-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OH (3) showed subnanomolar δ opioid antagonist activity (Keδ = 0.326 ± 0.30 nM) in the MVD assay (Table 1). It displayed very weak μ and κ antagonist activity in the GPI assay. As indicated by the selectivity ratios based on the Ke values, it is an extraordinarily selective δ opioid antagonist with higher δ selectivity than the corresponding (2S)-Mdp1 analogue (4).4 In agreement with these results, peptide 3 showed very high δ receptor binding affinity and extraordinary δ receptor selectivity in the opioid receptor binding assays (Table 2). The selectivity ratios based on the receptor binding constants (Ki values) indicate that the (2S)-Mdcp1-analogue (3) has higher δ selectivity than the (2S)-Mdp1-analogue (4), in confirmation of its higher δ selectivity established in the functional assays (Ke value ratios).
In the functional assays, [(2S)-Mdcp1]Dyn A(1-11)-NH2 (5) showed slightly lower κ antagonist potency than [(2S)-Mdp1]Dyn A(1-11)-NH2 (6) and somewhat lower κ receptor selectivity, as determined from the Ke selectivity ratios (Table 1). In the opioid receptor binding assays, compound 5 displayed about 30-fold lower κ receptor binding affinity than 6 and, unlike 6, was non-selective. The opioid receptor binding affinities of [(2S)-Mdcp]Dyn A(1-11)-NH2 (5) are similar to those of [Dcp1]Dyn A(1-11)-NH2 which also lacks opioid receptor binding selectivity.7 The discrepancies between the antagonist activities (Ke values) of [(2S)-Mdcp1]Dyn A(1-11)-NH2 determined in the functional assays and its receptor affinities measured in the binding assays may be due to differences in structural requirements for ligand binding between opioid receptors in the peripheral tissue preparations and brain opioid receptors. Such discrepancies have previously been observed on several occasions.
In conclusion, replacement of Tyr1 with (2S)-Mdcp in three opioid agonist peptides with different opioid receptor selectivity resulted in compounds with antagonist activity at all three opioid receptors (μ, δ, κ). It is noteworthy that (2S)-Mdcp substitution produced opioid receptor selectivity profiles that were distinct among the three peptides investigated and, in some cases, were also distinct from the profiles shown by the corresponding (2S)-Mdp1-analogues. This may be due to differences between the -OH and -CONH2 substituent of the 1-position residue with regard to steric bulk or the H-bonding pattern with receptor moieties. The cyclic peptide (2S)-Mdcp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 (1) is another example of an opioid peptide-derived μ antagonist. It shows high μ antagonist potency and marked μ receptor binding selectivity (Table 2), but is less μ-selective than the somatostatin-derived μ opioid antagonists (e.g. CTOP; IC50μ =2.8 nM, IC50δ = 13500 nM15). It has 2-3-fold higher μ antagonist activity than Dcp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH27 and similar μ receptor binding selectivity, and is a more potent and more selective μ antagonist than Dhp-c[Nε,Nβ-carbonyl-D-Lys2,Dap5]enkephalinamide (Kiμ = 15.5 nM, Kiδ = 273 nM).4 Furthermore, compound 1 has higher μ antagonist potency and higher μ receptor binding selectivity than the endomorphin-derived μ antagonists antanal-1 ([Dmt1,D-2-Nal4]endomorphin-1; Kiμ = 2.38 nM, Kiδ = 17.4 nM) and antanal-2 ([Dmt1,D-2-Nal4]endomorphin-2; Kiμ = 1.52 nM, Kiδ = 7.74 nM)16, and higher μ antagonist potency but lower μ-selectivity than the tetrapeptide H-Dmt-Sar-Phe-D-Nal-NH2 (Keμ = 2.34 nM, Keδ = 305 nM).17 These various opioid peptide-derived μ antagonists are of interest because their mode of binding to the μ opioid receptor is likely to be different from that of the somatostatin-derived μ antagonists. The cyclic hexapeptide (2S)-Mdcp-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OH (3) is a δ opioid antagonist with very high δ antagonist potency and extraordinary δ opioid receptor selectivity. Together with some of the Tic2-containing opioid antagonists (for a review, see ref. 18) and naltrindole19, it ranks among the most potent and most selective δ opioid antagonists reported to date. Finally it should be pointed out that the novel opioid peptide antagonists described here are more lipophilic than their corresponding parent peptides because they contain a methyl group in place of the positively charged N-terminal amino group. For this reason, they can be expected to have an improved ability to cross the blood-brain barrier.
Experimental Section
Chemistry. Synthesis of Mdcp (15)
Ethyl 4-amino-3,5-dimethylbenzoate (8)
3,5-Dimethyl-4-nitrobenzoic acid (7) (12.3 g, 63.1 mmol) was dissolved in absolute ethanol (75 mL), and dry HCl gas was passed into the solution for 20 min. The resulting mixture was refluxed for 5 h, then cooled and passed with HCl gas for another 20 min. and refluxed for 5 h. After the reaction mixture was cooled down to room temperature, tin (22.5 g, 189.4 mmol) was added slowly, maintaining the temperature of the mixture between 35 - 40 °C. The mixture was then allowed to stand for 4 h, and basified with aqueous NaOH (30 %), and extracted with Et2O (3 × 300 mL). The combined ether layers were washed with brine, dried, and concentrated. The residue was purified by flash column chromatography to give 8 as a light yellow solid (10 g, 82 %); mp 67.3-68.4 °C. 1H NMR (300 MHz, CDCl3) δ 7.65 (s, 2H), 4.27-4.34 (q, 2H, J = 7.2 Hz), 2.18 (s, 6H), 1.33-1.38 (t, 3H, J = 7.08 Hz). 13C NMR (75 MHz, CDCl3) δ167.1, 147.2, 129.9, 120.3, 119.1, 60.1, 17.3, 14.3. HMRS (ESI) m/e cacld for C11H14O2N [M-H]- 192.1024; obsd, 192.1026.
Ethyl 4-iodo-3,5-dimethylbenzoate (9)
To a solution of 8 (7.65 g, 39.6 mmol) in acetone (50 mL) was added concentrated HCl (250 mL) at room temperature. The resulting solution was cooled to 0 °C, and a solution of NaNO2 (3.58 g, 51.9 mmol) in H2O (25 mL) was added dropwise. The mixture was stirred at 0 °C for 2 h, then a solution of KI (19.72 g, 118.8 mmol) in H2O (50 mL) was added. After 6 h, the solution was extracted with Et2O (3 × 300 mL). The combined ether layers were washed with brine, dried, and concentrated. The residue was purified by flash column chromatography to afford 9 as a yellowish solid (10 g, 83 %); mp 41.2-43.2 °C. 1H NMR (300 MHz, CDCl3) δ 7.68 (s, 2H), 4.32-4.39 (q, 2H, J = 7.05 Hz), 2.51 (s, 6H), 1.36-1.41 (t, 3H, J = 7.05 Hz). 13C NMR (75 MHz, CDCl3) δ 166.3, 142.4, 129.6, 127.3, 114.3, 61.0, 29.6, 14.2. HRMS (ESI) m/e cacld for C11H14O2I [M+H]+ 305.0039; obsd, 305.0033.
4-Iodo-3,5-dimethylbenzoic acid (10)
To a solution of 9 (9.39 g, 30.9 mmol) in a mixture of THF (45 mL) and MeOH (30 mL) at 0 °C was added LiOH (2.22 g, 92.6 mmol) dissolved in H2O (30 mL). The resultant was allowed to warm up to room temperature. After stirring for 4 h, the organic solvents were removed and the aqueous phase was neutralized with pre-cooled aqueous HCl (1 N) at 0 °C, and extracted with EtOAc (2 × 100 mL). The combined EtOAc extracts were washed with brine, dried over Na2SO4, and concentrated to yield 10 as a white solid (8.1 g), which was directly used for the next step without further purification.
Benzyl 4-iodo-3,5-dimethylbenzoate (11)
To a solution of crude 10 obtained from the previous step in dry DMF (40 mL) was added K2CO3 (6.0 g, 44.0 mmol), followed by benzyl bromide (3.6 ml, 29.9 mmol) at room temperature under nitrogen atmosphere. The resulting mixture was stirred for 6 h. Water (350 mL) was then added, and the mixture was extracted with EtOAc (2 × 200 mL). The combined EtOAc extracts were washed with brine, dried and concentrated. The crude product was purified by flash column chromatography to afford 11 as a light yellow solid (9.8 g, 87 % for two steps); mp 56.8-57.9 °C. 1H NMR (300 MHz, CDCl3) δ 7.76 (s, 2H), 7.30-7.48 (m, 5H), 5.39 (s, 2H), 2.55 (s, 6H). 13C NMR (75 MHz, CDCl3) δ 166.2, 142.5, 135.9, 129.3, 128.5, 128.3, 128.2, 127.4, 114.7, 66.7, 29.6. HMRS (ESI) m/e cacld for C16H15IO2 [M+] 366.0117; obsd, 366.0127.
(S)-Benzyl 4-(3-methoxy-2-methyl-3-oxopropyl)-3,5-dimethylbenzoate (12)
Preparation of (R)-methyl 3-iodo-2-methylpropanoate To a solution of (S)-methyl 2-(hydroxymethyl)propanoate (1.18 g, 10 mmol) in dichloromethane (30 mL) at 0 °C were added triphenylphosphine (10.49 g, 40 mmol), imidazole (2.72 g, 40 mmol) and iodine (7.62 g, 30 mmol). The mixture was warmed up to room temperature and stirred for 4 hours. The mixture was then washed with aqueous sodium thiosulfate solution (5%), and extracted with ethyl acetate (2×40 mL). The combined organic extracts were concentrated and the residue was purified by silica gel flash chromatography (5% hexane in ethyl acetate) to afford the desired iodide as a colorless oil (1.80 g, 79%). [α]D = +24.0 ° (c = 2.1, CHCl3). 1H NMR (300 MHz, CDCl3): δ 3.72 (s, 3H), 3.23 - 3.40 (m, 2H), 2.76 - 2.83 (m, 1H), 1.27 (d, 3H); 13C NMR (75 MHz, CDCl3): δ 173.7, 52.0, 42.1, 18.1, 6.8.
A solution of (R)-methyl 3-iodo-2-methylpropanoate (6.60 g, 28.9 mmol) in anhydrous benzene (96 mL) and DMA (6.4 mL) was added to a dry nitrogen-purged flask charged with zinc-copper couple (3.47 g). The resulting mixture was sonicated under nitrogen for 40 min. Bis(tri-o-tolylphosphine) palladium dichloride (1.12 g, 1.28 mmol) was added followed by 11 (8.82 g, 24.1mmol). The resulting mixture was stirred under nitrogen at 55 °C for 2 h and then allowed to cool down to room temperature. Ethyl acetate (400 mL) was added and the mixture filtered into a separating funnel. The mixture was washed with aqueous hydrochloric acid (0.1 N) (400 mL) and distilled water (3 × 100 mL), and dried, filtered and concentrated. The crude product was purified by flash chromatography to afford the desired product 12 as a light yellow oil (4.27 g, 52%). [α]D20 + 39.5 ° (c 0.22, CHCl3). 1H NMR (300 MHz, CDCl3)δ 7.71 (s, 2H), 7.36-7.43 (m, 7H), 5.34 (s, 2H), 3.63 (s, 3H), 3.05-3.12 (a, 1H), 2.71-2.86 (m, 2H), 2.36 (s, 6H), 1.14 (d, 3H, J = 6.9 Hz). 13C NMR (75 MHz, CDCl3) 176.5, 166.6, 141.9, 137.1, 136.2, 129.4, 128.5, 128.19, 129.17, 127.7, 66.5, 51.7, 39.1, 33.2, 20.2, 16.5. HRMS (ESI) m/e cacld for C21H24O4Na [M + Na]+ 363.1573; obsd, 363.1573.
(S)-4-(3-Methoxy-2-methyl-3-oxopropyl)-3,5-dimethylbenzoic acid (13)
To a solution of 12 (4.1 g, 12.1 mmol) in MeOH (20 mL), 10 % Pd/C (240 mg) was added, and the mixture was stirred overnight under atmospheric pressure of H2. The reaction mixture was then filtered through a short pad of celite and the filter cake was washed with EtOAC (2 × 20 mL). The filtrate and washings were combined and concentrated in vacuo to give 13 as a white solid. (2.9 g, 96 %); mp 128.4-129.9 °C. [α]D20 + 57.3 ° (c 0.20, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.78 (s, 2H), 3.68 (s, 3H), 3.11-3.18 (q, 1H), 2.77-2.92 (m, 2H), 2.42 (s, 6H), 1.20 (d, 3H, J = 6.72 Hz). 13C NMR (75 MHz, CDCl3) δ 176.5, 172.1, 142.8, 137.2, 129.9, 126.9, 51.7, 39.0, 33.2, 20.2, 16.5. HRMS (ESI) m/e cacld for C14H17O4 [M - H]- 249.1126; obsd, 258.1121.
(S)-Methyl 3-(4-carbamoyl-2,6-dimethylphenyl)-2-methylpropanoate (14)
Oxalyl chloride (2.76 mL, 31.7 mmol) was added slowly to a solution of 13 (2.64 g, 10.6 mmol) in CH2Cl2 (20 mL) at room temperature under nitrogen atmosphere, and slow gas formation was observed. DMF (20 μL) was then added, accelerating gas evolution considerably. After stirring at room temperature for 2 h, organic solvent was removed, and the brown oily residue was dissolved in THF (20 mL). Aqueous NH4OH (25%) (23 mL) was then added to the reaction mixture at 0 °C and stirred at the same temperature for 30 min. The reaction mixture was then acidified with HCl (1 N) at 0 °C, and extracted with EtOAc (2 × 50 mL). After concentration, the residue was purified by flash chromatography to give 14 as a white solid (2.3 g, 87 %); mp 91.8-93.2 °C. [α]D20 + 58.5 ° (c 0.22, CHCl3); 1H NMR (300 MHz, CDCl3) δ 7.44 (s, 2H), 6.0 (br s, 1H), 5.76 (br s, 1H), 3.63 (s, 3H), 3.05-3.10 (q, 1H), 2.74-2.85 (m, 2H), 2.36 (s, 6H), 1.15 (d, 3H, J = 6.75 Hz). 13C NMR (75 MHz, CDCl3) δ 176.5, 169.5, 140.8, 137.3, 130.8, 127.1, 51.7, 39.1, 33.1, 20.3, 16.5. HRMS (ESI) m/e cacld for C14H19O3NNa [M + Na]+ 272.1263; obsd, 272.1256.
(2S)-2-Methyl-(2,6-dimethylbenzyl-4-carbamoyl)propanoic acid [(2S)-Mdcp] (15)
To a solution of 14 (2.03 g, 8.16 mmol) in THF (50 mL) was added an aqueous solution of LiOH (1N, 50 mL) at 0 °C. After stirring at this temperature for 2.5 h, the organic solvent was removed and the aqueous phase was neutralized with pre-cooled HCl (1 N) at 0 °C, and extracted with EtOAc (2 × 75 mL). The combined EtOAc extracts were washed with brine, dried over Na2SO4, and concentrated to yield 15 as a white solid (1.5 g, 78%); mp 224.1-225.9 °C. [α]D20 + 64.2 ° (c 0.25, MeOH); 1H NMR (300 MHz, DMSO-d6) δ 12.17 (br s, 1H), 7.80 (s, 1H), 7.51 (s, 2H), 7.18 (s, 1H), 2.96-3.03 (q, 1H), 2.50-2.74 (m, 2H), 2.32 (s, 6H), 1.04 (d, 3H, J = 6.72 Hz). 13C NMR (75 MHz, CDCl3) δ 177.0, 167.9, 139.8, 136.3, 131.6, 127.1, 79.1, 32.6, 19.9, 16.6. HRMS (ESI) m/e cacld for C13H17O3NNa [M + Na]+ 258.1106; obsd, 258.1088.
Peptide Synthesis (2S)-Mdcp-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 (1)
The cyclic tetrapeptide H-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 was prepared by the manual solidphase technique as described elsewhere.7 To a solution of (2S)-Mdcp (15) (37.7 mg, 0.16 mmol), HBTU (62.7 mg, 0.16 mmol) and N,N-diisopropylethylamine (DIEA) (91.0 μL, 0.82 mmol) in 5 mL DMF were added H-c[D-Cys-Gly-Phe(pNO2)-D-Cys]NH2 × TFA (90.0 mg, 0.15 mmol) and NMM (16.5 μL, 0.15 mmol). After stirring for 30 min, the solvent was evaporated to dryness in vacuo, and the residue was extracted with 20 mL of AcOEt. After washing with 5% KHSO4, saturated NaHCO3 and brine, the organic phase was dried (MgSO4), filtered, and evaporated to dryness in vacuo. The peptide was purified by reversed-phase HPLC. HPLC K' 3.75; TLC Rf 0.65 (I), Rf 0.86 (II), Rf 0.16 (III); MS [M+H]+ 688.
(2S)-Mdcp-c[D-Pen-Gly-Phe(pF)-Pen]-Phe-OH (3)
The linear precursor peptide was prepared by the manual solid-phase technique using Boc-protection of the α-amino group and Mob protection of the Pen residues, and DIC/HOBt as coupling agents. The peptide was assembled on a polystyrene-divinylbenzene (1%) resin (200-400 mesh) (Boc-Pheresin, 0.65 equiv./g, Bachem Bioscience, King of Prussia, PA) according to a published protocol.3 The peptide was cleaved from the resin and deprotected by HF/anisole treatment in the usual manner. After evaporation of the HF, the resin was extracted three times with Et2O and subsequently three times with glacial AcOH. The peptide was obtained in solid form through lyophilization of the acetic acid extract. For disulfide bond formation, the peptide (150 mg) dissolved in 20 mL MeOH was slowly added to a solution of K3Fe(CN)6 (293 mg) in 750 mL ammonium acetate buffer (0.05N, pH 8.5) over a period of 20h. After lowering the pH to 4.5 by addition of AcOH the solution was treated with Amberlite® IRA-400 (Cl). After subsequent filtration, solvent evaporation and dissolution of the residue in AcOH, the product was obtained in solid form through lyophilization, and was purified by preparative reversed-phase HPLC. HPLC K' 5.83; TLC Rf 0.88 (I), Rf 0.81 (II), Rf 0.30 (III), MS [M+H]+ 865.
[(2S)-Mdcp1]Dyn A(1-11)-NH2 (5)
The peptide was prepared by the manual solid-phase technique by using the protocol described above for the synthesis of the linear precursor peptide of 3. Side chain protection was as follows: tosyl (Arg) and 2-chlorobenzyloxycarbonyl (Lys). HPLC K' 3.78; TLC Rf 0.38 (II), Rf 0.32 (IV); MS [M+H]+ 1414.
Supplementary Material
Acknowledgement
This work was financially supported by the National University of Singapore (to Y.L.) and by a grant from the U.S. National Institutes of Health (to P.W.S).
Footnotes
Abbreviations: Acm, acetamidomethyl; CTOP, H-D-Phe-c[Cys-Tyr-D-Trp-Orn-Thr-Pen]-Thr-NH2; DAMGO, H-Tyr-D-Ala-Gly-NαMePhe-Gly-ol; Dcp, 3-(2,6-dimethyl-4-carbamoylphenyl)propanoic acid; Dhp, 3-(2,6-dimethyl-4-hydroxyphenyl)propanoic acid; DIC, diisopropylcarbodiimide; DIEA, N,N-diisopropylethylamine; Dmt, 2′,6′-dimethyltyrosine; DPDPE, H-Tyr-c[D-Pen-Gly-Phe-D-Pen]OH; DSLET, H-Tyr-D-Ser-Gly-Phe-Leu-Thr-OH; Dyn A, dynorphin A; GPI, guinea pig ileum; HBTU, 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate; HOBt, 1-hydroxybenzotriazole; (2S)-Mdcp, (2S)-2-methyl-3-(2,6-dimethyl-4-carbamoylphenyl)propanoic acid; (2S)-Mdp, (2S)-2-methyl-3-(2,6-dimethyl-4-hydroxyphenyl)propanoic acid; Mob, methoxybenzyl; MVD, mouse vas deferens; NMM, N-methylmorpholine; Pen, penicillamine; TAPP, H-Tyr-D-Ala-Phe-Phe-NH2; TFA, trifluoroacetic acid; Tic, tetrahydroisoquinoline-3-carboxylic acid; U50,488, trans-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]-benzeneacetamide; U69,593, (5α,7α,8β-(—)-N-methyl-N-[7-pyrrolidinyl)-1-oxaspiro[4,5]dec-8-yl]benzeneacetamide.
Supporting Information Available: Experimental details and refs 20-26. This material is available free of charge via the Internet.
References
- 1.Hansen DW, Jr., Stapelfeld A, Savage MA, Reichman M, Hammond DL, Haaseth RC, Mosberg HI. Systemic Analgesic Activity and δ-Opioid Selectivity in [2,6-Dimethyl-Tyr1,D-Pen2,D-Pen5]Enkephalin. J. Med. Chem. 1992;35:684–687. doi: 10.1021/jm00082a008. [DOI] [PubMed] [Google Scholar]
- 2.Schiller PW, Lu Y, Weltrowska G, Berezowska I, Wilkes BC, Nguyen TM-D, Chung NN, Lemieux C. A General New Concept for the Development of Opioid Peptide Derived μ-, δ- and κ Antagonists. In: Lebl M, Houghten RA, editors. Peptides: The Wave of the Future, Proceedings of the 2nd International Peptide Symposium/17th American Peptide Symposium. Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. pp. 676–678. [Google Scholar]
- 3.Lu Y, Nguyen TM-D, Weltrowska G, Berezowska I, Lemieux C, Chung NN, Schiller PW. [2',6'-Dimethyltyrosine]dynorphin A(1-11)-NH2 Analogues Lacking an N-Terminal Amino Group: Potent and Selective κ Opioid Antagonists. J. Med. Chem. 2001;44:3048–3053. doi: 10.1021/jm0101186. [DOI] [PubMed] [Google Scholar]
- 4.Schiller PW, Weltrowska G, Nguyen TM-D, Lemieux C, Chung NN, Lu Y. Conversion of δ-, κ- and μ-Receptor Selective Opioid Peptide Agonists into δ-, κ- and μ-Selective Antagonists. Life Sci. 2003;73:691–698. doi: 10.1016/s0024-3205(03)00389-8. [DOI] [PubMed] [Google Scholar]
- 5.Dolle RE, Machaut M, Martinez-Teipel B, Belanger S, Cassel JA, Stabley GJ, Graczyk TM, DeHaven RN. (4-Carboxamido)phenylalanine is a Surrogate for Tyrosine in Opioid Receptor Peptide Ligands. Bioorg. Med. Chem. Lett. 2004;14:3545–3548. doi: 10.1016/j.bmcl.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 6.Weltrowska G, Lemieux C, Chung NN, Schiller PW. Cyclic Enkephalin Analogues Containing Various para-Substituted Phenylalanine Derivatives in Place of Tyr1 are Potent Opioid Agonists. J. Pept. Res. 2005;65:36–41. doi: 10.1111/j.1399-3011.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- 7.Lu Y, Lum TK, Augustine YWL, Weltrowska G, Nguyen TM-D, Lemieux C, Chung NN, Schiller PW. Replacement of the N-terminal Tyrosine Residue in Opioid Peptides with 3-(2,6-Dimethyl-4-carbamoylphenyl)propanoic Acid (Dcp) Results in Novel Opioid Antagonists. J. Med. Chem. 2006;49:5382–5385. doi: 10.1021/jm060369k. [DOI] [PubMed] [Google Scholar]
- 8.Schiller PW, Nguyen TM, DiMaio J, Lemieux C. Comparison of μ-, δ- and κ-Receptor Binding Sites Through Pharmacologic Evaluation of p-Nitrophenylalanine Analogs of Opioid Peptides. Life Sci. 1983;33(Suppl 1):319–322. doi: 10.1016/0024-3205(83)90507-6. [DOI] [PubMed] [Google Scholar]
- 9.Hruby VJ, Bartosz-Bechowski H, Davis P, Slaninova J, Zalewska T, Stropova D, Porreca F, Yamamura HI. Cyclic Enkephalin Analogues with Exceptional Potency and Selectivity for Delta-Opioid Receptors. J. Med. Chem. 1997;40:3957–3962. doi: 10.1021/jm9704762. [DOI] [PubMed] [Google Scholar]
- 10.Garfield P, Johnson FR. Organic Synthesis, Coll. 1943;Vol. 2:449. [Google Scholar]
- 11.Goldstein SL, McNelis E. Migrations in Oxidations of Mesidine. J. Org. Chem. 1984;49:1613–1620. [Google Scholar]
- 12.Arthur JH, Mildred VC. Some Amidines of the Holocaine Type II. Ester Substituted Amidines. J. Am. Chem. Soc. 1926;48:3214–3219. [Google Scholar]
- 13.Wang L, Wang GT, Wang X, Tong Y, Sullivan G, Park D, Leonard NM, Li Q, Cohen J, Gu W-Z, Zhang H, Bauch JL, Jakob CG, Hutchins CW, Stoll VS, Marsh K, Rosenberg SH, Sham HL, Lin NH. Design, Synthesis, and Biological Activity of 4-[(4-Cyano-2-arylbenzyloxy)-(3-methyl-3H-imidazol-4-yl)methyl]benzonitriles as Potent and Selective Farnesyltransferase Inhibitors. J. Med. Chem. 2004;47:612–626. doi: 10.1021/jm030434f. [DOI] [PubMed] [Google Scholar]
- 14.Jackson RFW, Wishart N, Wood A, James K, Wythes MJ. Preparation of Enantiomerically Pure Protected 4-Oxo-α-Amino Acids and 3-Aryl-α-Amino Acids from Serine. J. Org. Chem. 1992;57:3397–3404. [Google Scholar]
- 15.Pelton JT, Kazmierski W, Gulya K, Yamamura HI, Hruby VJ. Design and Synthesis of Conformationally Constrained Somatostatin Analogues with High Potency and Specificity for Mu Opioid Receptors. J. Med. Chem. 1986;29:2370–2375. doi: 10.1021/jm00161a037. [DOI] [PubMed] [Google Scholar]
- 16.Fichna J, do-Rego J-C, Chung NN, Lemieux C, Schiller PW, Vanden Broeck J, Costentin J, Janecka A. Synthesis and Characterization of Potent and Selective μ-Opioid Receptor Antagonists, [Dmt1,D-2-Nal4]endomorphin-1 (Antanal-1) and [Dmt1,D-2-Nal4]endomorphin-2 (Antanal-2) J. Med. Chem. 2007;50:512–520. doi: 10.1021/jm060998u. [DOI] [PubMed] [Google Scholar]
- 17.Fichna J, do-Rego J-C, Janecki T, Staniszewska R, Poels J, Vanden Broeck J, Costentin J, Schiller PW, Janecka A. Novel Highly Potent μ-Opioid Receptor Antagonist Based on Endomorphin-2 Structure. Bioorg. Med. Chem. Lett. 2008;18:1350–1353. doi: 10.1016/j.bmcl.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Schiller PW, Weltrowska G, Berezowska I, Nguyen TM-D, Wilkes BC, Lemieux C, Chung NN. The TIPP Opioid Peptide Family: Development of δ Antagonists, δ Agonists, and Mixed μ Agonist/δ Antagonists. Biopolymers (Peptide Sci.) 1999;51:411–425. doi: 10.1002/(SICI)1097-0282(1999)51:6<411::AID-BIP4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Portoghese PS, Sultana M, Takemori AE. Design of Peptidomimetic δ Opioid Receptor Antagonists Using the Message-Address Concept. J. Med. Chem. 1990;33:1714–1720. doi: 10.1021/jm00168a028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.