Abstract
Recent studies highlight the prominent role played by estrogens in protecting the central nervous system (CNS) against the noxious consequences of a chronic inflammatory reaction. The neurodegenerative process of several CNS diseases, including Multiple Sclerosis, Alzheimer’s and Parkinson’s Diseases, is associated with the activation of microglia cells, which drive the resident inflammatory response. Chronically stimulated during neurodegeneration, microglia cells are thought to provide detrimental effects on surrounding neurons. The inhibitory activity of estrogens on neuroinflammation and specifically on microglia might thus be considered as a beneficial therapeutic opportunity for delaying the onset or progression of neurodegenerative diseases; in addition, understanding the peculiar activity of this female hormone on inflammatory signalling pathways will possibly lead to the development of selected anti-inflammatory molecules. This review summarises the evidence for the involvement of microglia in neuroinflammation and the anti-inflammatory activity played by estrogens specifically in microglia.
Keywords: Estrogen, microglia, neuroinflammation, Multiple Sclerosis, Alzheimer’s disease, Parkinson’s disease
1. Introduction
Substantial evidence supports the role of neuroinflammation in the pathogenesis and progression of neurodegenerative diseases; microglia cells are the resident inflammatory cells of the brain that are primarily involved in promoting brain inflammation in response to both acute or chronic stimuli. Improving our understanding of microglia cells regulation represents a major advancement for future strategies aimed at controlling pathologies, such as multiple Sclerosis (MS), Alzheimer’s disease (AD) and Parkinson’s disease (PD), that are associated with a relevant neuroinflammatory process. The estrogen hormone has a well-known neuroprotective activity, which could be related with the gender prevalence of selected neurodegenerative diseases. Accordingly, the strong effects on the incidence and symptomatology of certain neurodegenerative pathologies observed during pregnancy or at menopause or triggered by the administration of estrogenic drugs have been ascribed to estrogen action in brain cells. Given that a large body of evidence now indicates that estrogens exert an anti-inflammatory activity, we propose that part of its neuroprotective effects may be linked to the inhibition of microglia activation. The aim of the present review is to provide the state-of-the-art knowledge on estrogen action in microglia in selected disorders, such as MS, AD and PD, and to support the hypothesis that the use of estrogens in preventive therapies might delay the onset of neurodegeneration.
2. Inflammation and neurodegeneration
Role of microglia in neurodegeneration
Over the past two decades evidence accumulated from both experimental and post-mortem studies suggested that a sustained inflammatory reaction is present in chronic neurodegenerative states. In addition, a number of proinflammatory mediators, such as cytokines, and inflammatory-associated factors such as cyclooxygenase-2 (COX-2) and inducible-nitric oxide synthase (iNOS) are elevated in the CNS or cerebrospinal fluid of neurodegenerative disease patients. Furthermore, recent epidemiological studies indicate that the chronic use of nonsteroidal anti-inflammatory agents (NSAIDs) reduces the risk of PD and AD ([39], [153], [156] and [227]) and inheritance of polymorphisms resulting in enhanced expression of various inflammatory mediators was reported to increase the risk of these two pathologies ([32] and [248]). Importantly, activated microglia were found at the histopathological sites of several brain disorders, including neurodegenerative diseases such as AD, MS, PD, ALS (amyotrophic lateral sclerosis) and AIDS-associated dementia ([57], [94], [117], [155] and [194]).
Microglia are resident immunocompetent and phagocytic cells of the CNS thought to mediate the innate immune defence and to act as scavenger cells in the event of infection, inflammation, trauma, ischaemia and neurodegeneration in the CNS [129]. Resident microglia in the healthy brain display a “resting state” appearing in a downgraded phenotype, with highly ramified morphology and a low expression of membrane receptors that serve immunological functions [124]; even in this resting state microglia are highly active as shown by the high motility of their processes and protrusions observed by two-photon imaging analysis of brain in vivo ([54] and[174]). Microglia are the first cell type that sense any form of disturbance of the brain and rapidly activate through a well-characterized and graded response; the inner cytoskeleton changes and the cell body becomes enlarged, bearing shorter and thicker cytoplasmic processes ([175] and [220]). This activated, macrophage-like appearance associates with phagocytic activity, as activated microglia engulf toxic molecules and cellular debris [14]. In case of a persistent state of activation, as described in several neurodegenerative diseases, the beneficial activity of microglia may however become detrimental and contribute to neuronal dysfunction and progression of the disease.
As a consequence, inflammatory cells and mediators once thought to be involved only in peripheral immune responses are now considered as key factors also in the pathogenesis of neurodegenerative diseases ([148] and [167]). The identification of the mechanisms underlying microglia persistent activation and the key inflammatory mediators involved in neurotoxicity is currently believed to be instrumental to the development of effective inhibitors able to combat neuroinflammation and provide efficacious treatments for neurodegenerative diseases.
Estrogens and microglia
Detecting the expression of the two estrogen receptors (ERs), ERα and ERβ, in cells of the monocyte-macrophage lineage [245] first suggested that estrogens may play a role in inflammatory diseases and several laboratories showed that these hormones act in a variety of macrophage-like cells blunting the inflammatory response triggered by diverse inflammatory stimuli. The last decade witnessed increasing confidence on the anti-inflammatory effect of estrogens to the point that several Pharmaceutical Companies are presently developing estrogen receptor ligands as anti-inflammatory agents [88]. With regard to microglia, in the recent years our studies showed a major anti-inflammatory activity of estradiol in microglia activated by strong inflammatory stimuli such as lipopolysaccharide (LPS) [244]. This effect was antagonized by ICI182,780, an estrogen receptor antagonist, suggesting a receptor-mediated effect of the hormone and ERα appeared to be selectively involved in estradiol anti-inflammatory activity in brain macrophages ([83] and [242]). Other authors further confirmed these observations using primary cultures of microglia as well as cell lines and assaying estrogen-dependent attenuation of microglia activation in terms of reduced phagocytic activity, production of reactive oxigen and nitrogen species and other factors of the inflammatory cascade ([26], [44], [62], [142] and [264]). Meanwhile, a better evaluation of neurological and neurodegenerative diseases has also pointed to a potential role of estrogens in the pathogenesis and progression of several neuroinflammatory and neurodegenerative diseases, thus providing a new strength to the hypothesis of the potential benefits of the use of estrogenic compounds in the treatment of these disorders. We here review some of the current evidence of the anti-inflammatory role played by estrogens in the manifestation of three major neural diseases: Multiple Sclerosis, Alzheimer’s and Parkinson’s disease.
3. Neuroinflammation, microglia and neurodegenerative disorders
3.1 Multiple sclerosis and neuroinflammation
Multiple sclerosis (MS) is the most common cause of neurological disability in young adults in the Western world that begins with relapsing/remitting episodes and eventually evolves into uninterrupted progression ([45] and [176]). Symptoms are associated with a pathogenic CNS-targeted autoimmune response sustained by leukocytes that invade brain and spinal cord parenchyma and accumulate in multifocal sclerotic plaques, the pathological hallmark of MS from which the disease gets its name [127]. After CNS entry, immune cells destroy myelin and oligodendrocytes and lead to axon degeneration and neuron loss. To date, diverse hypotheses have been raised to explain MS etiology, including genetic predisposition, activation of autoreactive immune cells, environmental factors and neuropathological conditions [191]. Immune-related molecules and lymphocytes are barely detectable in healthy brain, as the architectural and biological composition of the blood-brain barrier (BBB) protects the CNS from peripheral inflammation. The critical event in MS pathogenesis is instead represented by recruitment of lymphocytes into the CNS, which gain access across endothelial cells of the BBB through the activity of inflammatory molecules, including cytokines and adhesion molecules, synthesized by CNS inflammatory cells. The significant progress made in elucidating the molecules involved in immunopathogenesis of MS dramatically changed the therapeutic approach of this disease: molecules that specifically block adhesion receptors and thus inhibit leukocyte extravasation are now used in clinical trials ([204] and [219]). Thus, among the putative mechanisms that trigger the activation of the autoimmune reaction, inflammation is considered pivotal and microglia are thought to play a major role by up-regulating MHC class II molecules, inflammatory cytokines, reactive oxygen and nitrogen species; on the other hand, beneficial effects of microglia activation must be taken into account, such as myelin debris fagocytosis. This led us to propose inflammation as a candidate therapeutic target for MS within selected phases of the disease [150].
The EAE animal model
Major progress in the understanding of MS is due to the development of animal models where the biology of the disease as well as the response to specific pharmacological treatments can be observed. Experimental autoimmune encephalomyelitis (EAE) is the model system which mostly contributed to the understanding of the pathogenesis and the immune inflammatory mechanisms of human MS. EAE is induced by the immunization of susceptible mice with myelin proteins or peptides, such as myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG) in complete Freund’s adjuvant. After 1–2 weeks, immunization induces activation of a delayed-type hypersensitivity, a type of reaction that is mediated by the activation of CD4+-Th1 and Th17 cells, that cross the BBB and secrete cytokines that both activate microglia and recruit circulating leukocytes ([12] and [75]).
Several studies highlighted the prominent role played by microglia activation in EAE. A study by Ponomarev et al. [188] recently investigated the timing of microglia activation in EAE by generating bone marrow chimera mice using MHC-mismatched donors, a model that allows to distinguish between resident microglial cells and monocyte-derived macrophages. These authors observed that the activation of microglia occurs before the onset of disease symptoms and infiltration of macrophages into the CNS. In addition, resting microglia were shown to undergo bystander activation, characterized by the upregulation of MHC II molecules, and were localized to the inflammatory lesions, which suggested a detrimental effect mainly ascribed to the secretion of neurotoxic molecules and self-antigens ([18], [114], [132], [229] and [235]).
Using transgenic mice carrying the selective ablation of proliferating microglia and undergoing experimental MS, it has been recently shown that the inhibition of microglia cells prevents the development and maintenance of the inflammatory CNS lesions [95]. Finally, a direct association between the intensity of microglia activation and EAE symptom gravity has been reported [2].
In both EAE and MS, inflammatory lesions classically occur in white matter, resulting in demyelination and axonal transection [236]. However, neuroimaging studies revealed microglia activation with minimal inflammatory cell infiltrates in proximity of cortical axonal transections [19]; this gray matter abnormality occurs surprisingly early and correlates much better with permanent disability, demonstrating that microglia activation in gray matter correlates with neuron loss and MS onset ([37], [52], [203] and [232]).
Estrogens and multiple sclerosis
Similar to other autoimmune diseases, MS is sexually dimorphic in that it occurs two times more frequently in women than in men [252]. This sexual dimorphism may be due to multiple factors; certainly gender-related differences in immune responsiveness are part of the cause, but sex hormones are likely to play a significant role ([65] and [111]) as indicated by a series of observations: a.) the first clinical symptoms of MS develop post-puberty; b.) increased levels of sex hormones produced during pregnancy are associated with a significant reduction in the severity of MS; c.) MS clinical symptoms are often exacerbated postpartum, a time characterized by significant alternations in sex hormone levels; d.) MS symptoms are altered also during the menstrual cycle ([1], [16], [46], [128] and [190]).
Although MS is more common in women, it affects men with a generally more rapid progression [255]. Also in the case of men, the male sex hormone, testosterone, was shown to affect microglial activation and to inhibit the development of EAE, inducing a Th2 bias in myelin basic protein-specific T-cells [51]. In view of the fact that the enzyme converting testosterone into estradiol, aromatase, is present in several cells and tissues it remains to be shown whether testosterone exerts its anti-inflammatory effect directly or through conversion in the female sex hormone. Gender differences in susceptibility to and severity of EAE have also been known for many years ([72], [163] and [246]).
The apparent conflict that women are more susceptible to the pathology than men, in spite of their capability to synthesize beneficial hormones like estrogens, might be ascribed to the fact that estrogens might be less potent natural inhibitors then testosterone. In addition, estrogen effect on immune function might be biphasic: specifically, low doses of estrogens promote Th1 responses and increase cell-mediated immunity, while high doses result in increased Th-2 responses ([28] and [130]). Accordingly, women are more likely to develop a Th1 response to infective agents than men, so they are more susceptible to autoimmune diseases, except during pregnancy where women exhibit a pronounced Th2 response ([5], [184] and [252]).
Physiological or pharmacological fluctuations in estrogen levels have been recognised since a long time to play a regulatory role in EAE ([13], [109], [112], [121] and [151]). Oral administration of low doses of estrogenic hormones, 17beta-estradiol and ethinyl estradiol, drastically reduce the severity of EAE and this effect was reconciled with a decrease in the production of inflammatory Th1 cytokines (such as inteferon-γ (IFN-γ), Tumor Necrosis Factor-α (TNF-α), Interleukin (IL)-1 and IL-6), chemokines/receptors, while increasing the expression of anti-inflammatory Th2 cytokines (including IL-4, IL-5 and Transforming Growth factor-β3 (TGF-β3)) ([13], [109], [151] and [225]). This observation together with the fact that no infiltrating lymphocytes were found in the hormone-treated animals, led to the conclusion that estrogens protects mice from EAE by inhibiting the recruitment of T cells and macrophages into the CNS.
A further demonstration of estrogens as neuroprotective agents in EAE comes from the use of selective modulators of estrogen receptor (SERMs). Morales et al. [171] demonstrated that treatment with an ERα ligand is sufficient to recapitulate the estrogen-mediated protection in EAE, consistent with a report by Elloso et al. [70] that demonstrated that treatment with an ERα, but not an ERβ, ligand could reduce acute EAE disease severity. The degree of preservation of neuronal integrity in the gray matter of estradiol and ERα ligand-treated mice with EAE in this study was striking, and this has major implications for neurodegenerative changes that occur "beyond the lesion" in EAE and possibly MS.
Two cellular targets have been proposed to mediate estrogen protective activity in MS and EAE, namely T-cells and brain inflammatory cells. Estrogens are able to shift the T-cell population towards a Th2 phenotype, an activity also confirmed in pilot clinical studies ([48], [84], [213] and [216]) and to influence a subpopulation of Th cells, named T-regulatory cells ([186] and [228]). On the other hand, microglia and endothelial cells have probably a more significant role in estrogen action; using irradiation bone marrow chimeras it has been recently shown that the effect of estradiol on clinical EAE and CNS inflammation was not dependent on ERα expression in the peripheral immune system but was conferred by ERα expression on CNS resident cells, namely endothelial and microglial cells ([81] and [187]). It is thus important to fill the gap in the characterization of estrogen signalling in these resident brain cells and in assessing its relevance in estrogen-mediated neuroprotective activity in EAE.
3.2 Alzheimer’s disease and neuroinflammation
Alzheimer's disease (AD) is a progressive neurodegenerative disorder; its pathological hallmarks include extracellular senile plaques mainly made of the amyloid β (Aβ) peptide, and neuronal anomalies including neurofibrillary tangles composed of hyperphosphorylated forms of the microtubule associated protein tau (MAPT); these specific pathologic features are also associated with dystrophic neurites and by reactive astrocytes and activated microglia ([22] and [199]). Although the mechanisms leading to progressive neuronal death in brain are still under investigation, several lines of evidence sustain the amyloid hypothesis, which postulates that abnormal cellular production and deposition of Aβ peptide is a relevant trigger of neurodegeneration in AD [249]. In fact, genetic studies in hereditary AD identified mutations in the genes encoding APP, presenilin (PSEN1) and PSEN2, each associated with increased production of Aβ. Indeed, the well known risk factor apolipoprotein E ε4 allele is shown to control the clearance of Aβ in brain ([9] and [222]).
The Aβ peptide is derived from the two-step enzymatic processing of amyloid precursor protein [APP] in which β-secretase [BACE] cleaves APP to release the N-terminus of Aβ, followed by the cleavage by γ-secretase protein complex to release the C-terminus of Aβ ([87] and [241]). Thus, the initial cleavage of APP by BACE is critical for the formation of the 40 or 42 aminoacids-long Aβ peptides (Aβ1–40 and Aβ1–42), which deposit as fibrillar amyloid in the senile plaques; it has been shown that BACE activity increases with age and it is elevated in AD brains ([100] and [138]). Amyloidogenic peptides were shown to play a major role in brain neurotoxicity, however the molecular mechanisms remain to be clearly defined.
Recent evidence suggests that inflammatory processes play an active role in AD; epidemiological studies reported that the use of nonsteroidal anti-inflammatory drugs is associated with marked reduction in the risk of AD ([107], [156], [157], [221] and [227]). Following this initial observation, additional reports were published that witnessed the involvement of neuroinflammation in AD pathogenesis and progression. In fact, a series of proinflammatory molecules, including proteins of the complement system, cytokines and chemokines and their receptors, were found to be increased in the brain and cerebrospinal fluid (CSF) of AD patients ([76] and [257]). Polymorphisms in inflammatory genes were also found in association with AD [32].
The involvement of microglia in neuroinflammation associated with AD has also been described [116]. Resting microglial cells can be activated by Aβ in brain, they migrate and surround the region of compact Aβ deposits, where they help removing Aβ ([7], [66], [74], [145], [154] and [250]). These data argue in favour of an essential role of microglia cells. In chronic inflammatory processes that may ultimately lead to neuronal degeneration.
Animal models of Alzheimer’s disease
The identification of genetic polymorphisms associated to hereditary forms of AD led to the generation of transgenic animals modelling the human disorder. These transgenic mice produce high levels of human Aβ40 and Aβ42 peptides and develop amyloid deposits in the brain which are very similar to those seen in the human AD brain and thus represent a unique tool in the study of this condition. The first animal models that developed amyloid plaques were generated by integrating in the mouse genome the gene encoding human APP containing mutations associated with early-onset AD ([77], [102] and [217]). These mice develop amyloid plaque pathology and selective cognitive deficits, pathologic features that dramatically accelerate in the next generation of AD animal models generated by crossing APP mutant animals with mice carrying the mutated PSEN1 gene ([20], [64] and [99]). Plaque pathology in these models is associated with microgliosis and astrogliosis. Despite the robust amyloid deposition observed in APP and PSAPP transgenic mice and evidence for progressive synaptic degeneration and dysfunction, none of these models show neuron loss or formation of intraneuronal fibrillary tangles. On the other hand, mouse models expressing wild type or mutant MAPT gene have been generated that recapitulate most of the features of human neurofibrillary pathology and significant neuronal loss ([4], [137], [207] and [265]). Further crossings among AD transgenic models have allowed to reach the conclusion that, although a mouse model that recapitulates all aspects of AD has yet to be obtained, amyloid deposition can accelerate or initiate the formation of neurofibrillary tangles while MAPT accumulation or other secondary events initiate neurodegeneration ([159], [177] and [178]).
The observation that activated microglia are present at amyloid deposits in human AD and in animal models of this disease suggested that it might play a pathogenic role as a result of their chronic activation, although the presence of cytoplasmic Aβ granules in plaque-associated glia and microglia suggest that these cells participate in the clearance of Aβ ([7], [43], [66], [74], [126], [145], [154], [181], [217], [250], [256], [259] and [261]). However, this hypothesis is hard to prove using experimental models of this disease in which many pathological features occur, namely amyloid deposition, neurofibrillary tangle formation, inflammation, neuritic and neuronal loss, synaptic and neuronal dysfunction, vascular alterations [198].
As an example of AD models, the APP23 transgenic mice overexpress the human APP751 with the familial Swedish AD double mutations at positions 670/671 [223]; in this model, amyloid plaques are first observed at 6 months of age and then plaque size and number increase dramatically with aging. The congophilic, dense-core Aβ deposits show many characteristics of human AD plaques such as enlarged dystrophic neurites [30]. Similar to AD, vascular amyloid is also present in aged APP23 animals [29]. Compact amyloid deposits are associated with microglia cells showing a characteristic activated morphology [217] and with reactive astrocytes [223].
A step forward in understanding the role of microglia in amyloid pathology derived from the comprehension of the molecular details of microglia activation by Aβ. The so-called pattern recognition receptors (PRRs) are a heterogenous class of proteins that are constitutively expressed by macrophages/phagocytes to monitor the extracellular environment. Activation of PRRs leads to microglia reactivity, a process that could be both beneficial in removing toxic signals as well as deleterious in producing and enhancing toxicity. Brain and microglial up-regulation of PRRs members has been observed in human and experimental AD ([3], [68], [21] and [260]). The Aβ peptide was shown to activate microglia cells through the interaction with specific PRR: a) scavenger receptors, including scavenger receptor class-A (SR-A), SR-B1 and CD36, that mediate Aβ endocytosis and induce ROS production ([47] and [67]); b) macrophage receptor with collagenous domain (MARCO), a scavenger receptor that mediates adhesion of Aβ to microglia and the cytoskeleton rearrangements induced by this peptide [85]; c) the receptor for advanced glycation endproducts (RAGE), a member of the immunoglobulin superfamily, responsible for the induction of the inflammatory response stimulated by Aβ ([6], [144] and [260]).
Intensive studies further addressed the role of neuroinflammation in AD pathogenesis and progression. In addition to resident microglia, mononuclear cells that are recruited from the blood are key players in AD pathogenesis; in fact, depletion of this cell pool or ablation of the receptor protein that recruits monocytes into the brain, accelerated Aβ accumulation and animal mortality ([69] and [214]). On the other hand, anti-inflammatory agents such as COX-1 inhibitors induced a dose-dependent reduction in pathology in humans and transgenic mouse model of AD [156]. Interestingly, a contribution of T and B cell-mediated immune responses to the inflammatory processes and to the plaque pathology seems unlikely in both human and experimental AD.
Estrogens and Alzheimer’s disease
The hypothesis of a potential role of estrogens in AD has been put forward by a number of epidemiological, retrospective studies that have demonstrated an inverse correlation between estrogen replacement therapy and incidence of AD ([15], [92], [118], [179], [180] and [230]). These observations have been challenged by a recent randomised clinical trial that showed increased risk of dementia in hormone therapy (HT)-assigned women participating at the Women Health Initiative Study ([71] and [195]). Despite these initial claims, a more in depth analysis of the WHI data, taking under consideration the time between menopause onset and HT assumption, showed beneficial effects of estrogens when therapy is initiated early after menopause, while the detrimental effects were associated with a treatment started several years after menopause [196]. Supporting this view, a well focused study carried out on women that underwent surgical removal of the ovaries before menopause clearly demonstrated that oophorectomy was associated with an increased risk of cognitive impairment and dementia [197]. This suggests that more and more well aimed studies are necessary in order to be able to reach a consensus on the effects of estrogens on brain health.
Indeed, most of the well controlled studies carried out in experimental animals are supportive of a protective effect of estrogens against neuronal loss. For instance, aromatase gene knockout mice in which estrogen synthesis is absent, showed enhanced hippocampal neuronal loss in response to neurotoxins compared with WT mice [8]. Interestingly, brain estradiol levels and aromatase expression are significantly reduced in the brains of women with AD [262]. The view of brain estrogen deficiency as a risk factor for developing AD pathology is consistent with genetic studies showing an association between variants of aromatase gene and the risk for several diseases, including AD ([106] and [215]).
A large body of experimental evidence demonstrates that estrogens protect against Aβ neurotoxicity. Estradiol increases APP expression in neuronal cells ([35], [110] and [258]) and reduces Aβ peptide production while enhancing its clearance ([36], [139] and[258]). A modification of Aβ levels was induced by estradiol treatment in the Tg2576 AD model [269]. Recent data from a transgenic animal model developed by Yue et al. that overexpresses APP and lacks the aromatase enzyme, the APP23/Ar+/− mice, show an earlier onset of plaque formation compared to ovariectomized APP23 mice [262]. In this model it was also found that BACE protein expression and activity, as well as Aβ40 and Aβ42 levels, were elevated in the brains of APP23/Ar+/− mice as young as 6 months. Thus, the early-onset AD neuropathology in APP23/Ar+/− mice associated with brain estrogen deficiency may be mediated by increased BACE activity and accelerated Aβ production. In a very recent study, Carroll et al used the triple transgenic mouse model of AD to investigate the individual and combined effects of estrogens and progesterone on different pathological features. Ovariectomy significantly increased Aβ accumulation and worsened memory performance, while chronic estradiol treatment prevented these effects. In addition, progesterone administration reduced tau phosphorylation, while when added in combination with estradiol prevented the effect of estrogen on Aβ accumulation but not on behavioural performance [33].
Taken together, these results suggest that brain estrogen deficiency accelerates AD pathologic features and that the estrogenic therapy may be beneficial in reducing the risk of AD.
Our laboratory provided evidence to demonstrate the ability of estradiol to control brain inflammatory cells reactivity ([242] and [244]). We analysed the effects of the deprivation of endogenous estrogens or of HT on microglia reactivity in the APP23 mice [243]. We first observed that the number of plaques that were associated with reactive microglia increased with age, suggesting that the inflammatory reaction was indeed progressing in parallel with the disease. Interestingly, ovariectomy clearly accelerated microglia activation surrounding Aβ plaques, whereas estradiol replacement delayed this process, thus indicating that estradiol influences the neuroinflammatory process that is associated with the APP genetic defect. In parallel, we showed that estradiol is able to down-regulate inflammatory genes expression in brain: the increase in the mRNA for macrophage/monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-2 (MIP-2) and TNF-α induced by LPS injection in the cerebral ventricles was clearly restricted by hormone administration. Most interestingly, SR-A expression induced by Aβ in macrophage cells was inhibited by estradiol pre-treatment, providing a potential mechanism for hormone inhibitory activity on microglia responsiveness observed in the APP23 mouse model [243].
These data clearly support a role of estrogen anti-inflammaotry action as contributing factor to estrogen therapy prevention of AD through direct regulation of resident microglia to inhibit chronic inflammation associated with AD. However, other cellular targets may underscore estrogen neuroprotective activity in the CNS: i) neurons, through hormone anti-apoptotic and neurotrophic actions; ii) neural stem cells, by inducing their proliferation; iii) astroglial cells, by increasing their potential for secreting neuroprotective molecules or decreasing the production of neurotoxic agents; iv) endothelial cells, on which estrogens act to reduce adhesion molecule expression and other factors that recruit circulating leukocytes ([146] and [189]).
3.3 Parkinson’s disease and neuroinflammation
Parkinson’s disease is a neurodegenerative disease characterized by a progressive loss of dopaminergic neurons in the substantia nigra (SN) and by intracellular inclusions of aggregated α-synuclein, known as Lewy bodies. It is believed that a combination of environmental and genetic factors predisposes to disease onset and severity. The genetic defects associated with PD are due to mutations in proteins that serve disparate functions yet converging on impaired α-synuclein signalling and clearance [73]; on the other hand, several toxins are known to specifically damage dopaminergic (DA) neurons and lead to PD-like symptomatology through recently characterised signalling pathways [17].
The selective loss of DA neurons is likely due to the increased vulnerability of these cells to oxidative stress, as these neurons have lower levels of glutathione compared with other cell types, and are thus more responsive to the effects of mitochondrial dysfunction ([97] and [143]). In addition to oxidative stress also other mechanisms have been proposed to be involved in selective DA neuron degeneration in PD, including excitotoxicity, intracellular calcium and metal ion rise, neurofibrillary tangle formation and disruption of the cytoskeletal transport [113]. More recently, neuroinflammation and microglial activation have been implicated in the neurodegenerative process in PD, as initially suggested by McGeer et al. [154] and then by several authors ([79], [90], [96], [98], [104], [105], [108], [149] and [169]). In fact, studies accumulated over the last two decades have clearly indicated the presence of an abnormal glial response in postmortem nervous system of PD patients. The positive correlation between antecedent brain injuries, such as trauma or exposure to infectious agents, and PD development implies that the brain inflammatory response to these noxious events, and specifically microglial activation, may play a critical role in PD pathogenesis [141]. Accordingly, other authors detected the expression of pro-inflammatory molecules, such as TNF-α, IL-1β and IFN-γ, as well as iNOS and Cox-2 and the accumulation of reactive oxygen and nitrogen species in the nervous system of PD patients ([98], [103] and [125]), further supporting the theory that PD is associated with the chronic activation of the brain inflammatory response. These inflammatory molecules, along with factors released from the dying dopaminergic cells, seem to amplify and sustain neuroinflammation as well as neural cell toxicity leading to a slow and irreversible destruction of SN dopaminergic neurons. In agreement with McGeer’s hypothesis on the role of activated glial cells and brain inflammation in PD, administration of nonsteroidal anti-inflammatory drugs were shown to reduce the risk of PD ([40], [209] and [247]), suggesting that inhibitors of inflammation are promising therapeutics for PD.
Animal models of Parkinson’s disease
The dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine [MPTP] is known to induce parkinsonism in humans, primates, and mice ([27], [91], [133] and [135]). MPTP is converted by astrocytes to the metabolite 1 methyl-4-phenylpyridinium [MPP+], a substrate of the DA transporter ([41] and [42]). MPP+ thus accumulates in dopaminergic neurons where it inhibits the mitochondrial complex I of the electron transport chain, resulting in ATP depletion and subsequent inability to release sufficient amounts of dopamine and ultimately leading to apoptosis [233]. However, Lewy bodies were not observed in the brain of PD patients exposed to MPTP, revealing a significant difference between MPTP-induced neurotoxicity and PD itself despite the considerable clinical similarities [134].
A precise understanding of how MPTP induces DA neuron degeneration is still lacking; in addition to what described above, it has been shown that pre-treatment with N-methyl-D-aspartate [NMDA] receptor antagonists can protect SN neurons, thus linking the effect of MPTP with excitotoxicity [240].
Recently, the induction of neuroinflammation received much attention as a key pathway involved in MPTP-induced pathogenesis and progression ([10], [165] and[182]). Langston et al. found that in post-mortem examination of brains from humans exposed to MPTP activated microglia were present up to 16 years after exposure, indicating a protracted inflammatory response [134]. These observations are strongly indicative of a process by which an ongoing stimulus could lead to disease progression long after the initial toxic insult. These findings are supported by studies in the nervous system of primates, which show that activated microglia and dopaminergic cell loss continue to occur years after exposure to MPTP [158].
Further studies showed that chronic activation of microglia by MPTP leads to their clustering around DA neurons and transformation in phagocytic cells ([11] and [23]).
How does MPTP-stimulated neurons activate microglia? The combination of released factors and expression of surface adhesion molecules by MPTP-treated DA neurons recruits and activates surrounding microglia and leads to a progressive and irreversible neuronal cell death, which is worsened by the release of chemoattractants by the dying neurons to induce even greater infiltration of the region by activated microglia. Recent studies provided molecular details to the understanding of the neuroinflammaotry component in PD. It has been shown that the products secreted by damaged neurons ([253] and [120]) aggregated alpha-synuclein or environmental toxins such as LPS are recognised by the macrophage antigen complex 1, Mac-1, a PRR expressed on microglia cells; in turn, Mac-1 activation is crucial for activation of NADPH oxidase, a membrane-bound enzyme that catalyses the production of superoxide anions, which are released in the extracellular space, and generates intracellular ROS, well known triggers of the inflammatory response ([18], [123], [224] and [267]). NADPH oxidase is associated with neurodegenerative disorders and neuronal damage; it is activated in the brains of patients with PD [254].
Through these evidence it appears that microglia activation has a detrimental role, as it results in the release of ROS by microglia in the extracellular space. Particular attention received the molecular mechanism of action of LPS on dopaminergic neurodegeneration. LPS has no known direct toxic effects on neurons but is a powerful tool for inducing the release of a host of neurotoxic factors through the direct activation of microglia. The substantia nigra is reported to be particularly susceptible to LPS-induced injury because it is rich in microglia [122]. LPS induces a rapid activation of microglia and a delayed, progressive and selective destruction of nigral dopaminergic neurons both in vivo and in vitro ([78], [79], [80]). Finally, using mice carrying a deletion in the gene coding for NADPH oxidase, one major source of intracellular ROS, or in Mac-1 it has been possible to clearly demonstrate that the production of ROS by LPS-activated microglia is directly toxic to neurons as well as the secretion of proinflammatory molecules, which foster neurodegeneration as well ([183], [192], [254], [266] and [267]).
Finally, anti-inflammatory drugs, such as cyclooxygenase inhibitors, statins or pioglitazone, were shown to protect neurons against degeneration induced by MPTP ([55], [210] and [231]), further providing evidence to indicate the role of neuroinflammation in PD neurodegeneration.
Estrogens and Parkinson’s disease
A number of epidemiological studies reported that the incidence and prevalence of PD is higher in men than in women ([56] and [131]). Post-menopausal estrogen deficiency has been reported to cause a worsening of Parkinson-related symptoms, whereas the severity of symptoms in women with early PD is diminished by the use of estradiol [206]. Furthermore, it has been shown that estrogen replacement therapy lowers symptom severity in women with early PD not yet taking L-Dopa [208] and improves motor disability in parkinsonian postmenopausal women with motor fluctuations [238]. Association between ER gene polymorphism and PD has been reported [251]. Rocca et al. recently reported that both unilateral and bilateral oophorectomy performed prior to menopause may be associated with an increased risk of parkinsonism and the effect may be age-dependent [197]. These data are suggestive of a beneficial protective role of estrogens.
Many experimental studies examined the responsiveness of DA neurons to estrogens and showed that estradiol levels induces DA synthesis and release, as well as DA neurons differentiation ([59], [60], [82], [152] and [172]). Importantly, MPTP caused greater DA depletion in male compared with female mice [58]; moreover, treatment with estradiol prevented the reduction in DA concentrations and the activation of glia in the striatum of animals treated with MPTP ([31], [58], [170] and [237]). The essential role of estrogens in maintaining the integrity of DA system in the CNS has also been extended to primates [136]. This evidence suggested a new treatment strategy for patients with PD and encouraging results are being obtained from pilot clinical studies assessing HT safety and effectiveness in PD [140]. Although a direct evidence for a role of estrogen-microglia signalling in PD models is still lacking, clear evidence suggests that estrogen signalling in these cells prevent microglia activaton induced by a number of endogenous or environmental factors ([26], [242] and [244]). It is presumed that the complementary action of estradiol on neurons, astrocyte and microglia may provide beneficial outcome and represents a potential pharmacological target for delaying or preventing PD symptoms.
4. The mechanism of estrogen action in neuroinflammation
As previously described, estrogens are believed to act in microglia via the activation of the endogenous estrogen receptors (ER). The two ER proteins recognised so far, ERα and ERβ, are intracellular proteins which activate genomic as well as nongenomic effectors in neural cells [146]. Through the use of the estrogen receptor antagonist ICI 182780 we and others initially ascribed hormone action in microglia to the activation of endogenous ERs, since this molecule was able to block the effect of estradiol ([24], [25], [142] and [244]). Using ER-null mice several reports described the selective involvement of ERα in the anti-inflammatory and neuroprotective activity of estradiol against neuroinflammatory and vascular pathologies of the brain ([63], [81], [187] and [242]). Despite the fact that ERβ has been shown to be expressed widely in the CNS in adult mice ([162] and [168]), it appears that this receptor isoform is not involved in mediating the protective effect of estrogens in neuroinflammatory diseases. Yet, both ERα and ERβ are involved in the control of inflammation by estradiol, depending on the tissue involved and on the signal utilized [89]. Whether one of the two receptors prevails, reduces, potentiates or does not influence the other isoform in inflammatory cells still remains to be defined.
Basic research in estrogen signalling also provided another relevant key point in estrogen action, that is cell-specificity. Divergent effects of estrogens have been reported for T cell activation [49] as compared with microglia and astroglia based on different hormone concentrations ([61] and [83]). Additionally, expression of iNOS is reduced in certain cell types, including microglia ([26], [50], [205], [244] and [263]) while hormone actually increases the expression of endothelial and neuronal subtypes of NOS ([193] and [205]). Thus, it is important to fully characterize the signal transduction mechanisms of estrogen signaling in different cell types of the CNS and the relative contributions of each of the two isoforms of estrogen receptor involved, so that appropriate therapeutic agents can be devised.
Selective ER modulators
Since their original description ([164] and [218]) ERα and ERβ selective modulators (SERMs) have been employed to dissect the relative contribution of each ER subtype and to obtain more potent responses devoid of toxic effects. The ERα selective ligand propyl pyrazole triol (PPT) provides neuroprotection and anti-inflammatory activities in brain in experimental models of neurodegenerative diseases, including ischemia [166], MPTP-induced neurotoxicity [53] and EAE ([70] and [171]). The in vitro activity of PPT has been studied in neuronal cells [268], while our preliminary data demonstrate a direct anti-inflammatory activity of this ligand in microglia (unpublished results). On the other hand, ERβ selective agonists produced neuroprotective effects in global ischemia ([34] and [166]) and EAE [234], while being inactive in the MPTP-induced dopamine depletion model [53]. Recent work by Tiwari-Woodruff et al. demonstrated that the ERβ selective agonist is also able to protect neurons in advanced stages of the EAE model through a strong neuroprotective action without altering the inflammatory component [234], suggesting that selective activation of ERs can trigger distinct, beneficial effects in the CNS. Future research in this field will lead to the experimental exploitation and possibly to the therapeutic application of SERMs in selected human diseases, in that they would permit to exploit only the benefits deriving from hormone therapy while reducing the undesired side effects.
Molecular aspects of estrogen action in microglia
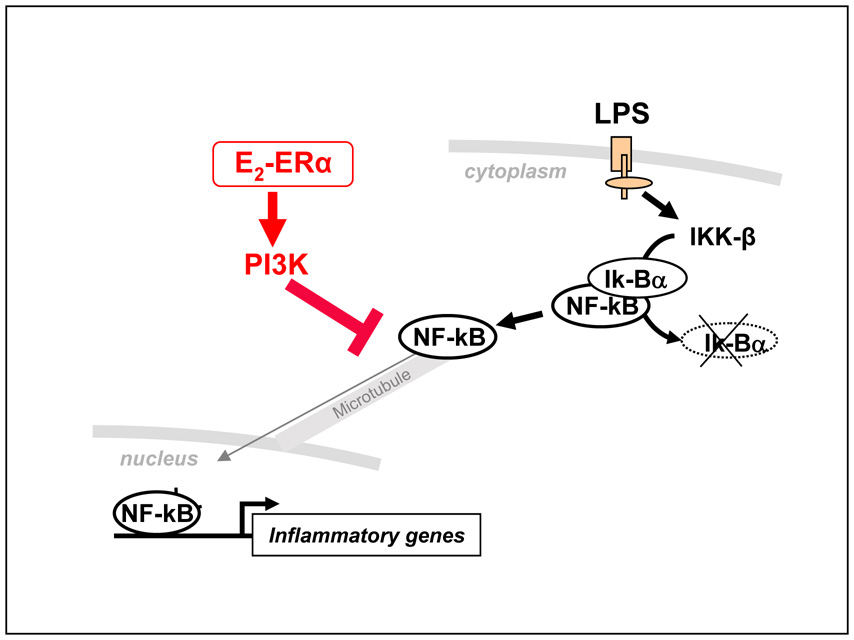
In an attempt to examine the specific mechanism of estrogen action in brain inflammatory cells we recently identified a novel mechanism of estrogen action that results in the inhibition of inflammatory gene transcription. We demonstrated that the intracellular machinery that drives the transport of proteins, and in particular the transcription factor p65, from the cytoplasm to the nucleus is a target for estrogen activity in microglia and macrophage cells [83]. p65 is a member of the NF-κB family of transcription factors, which are confined to the cytoplasm of unstimulated cells and move from this compartment to the nucleus upon inflammatory stimuli, such as LPS, with a rapid and massive translocation; in the nucleus, these factors induce inflammatory gene transcription through the binding to DNA responsive elements in the promoter of target genes. In our study we observed that microglia and macrophage cells treated with estradiol before LPS resulted in the persistent cytoplasmic localization of p65. We ascribed this effect to the interference with the microtubule-dependent intracellular transport of NF-κB ([115] and [200]), which results in reduced nuclear availability of NK-FB and thus in decreased transcription of NF-κB target genes, such as MIP-2 or Ik-Bα. Estrogen action was mediated specifically by ERα and through the activation of the PI3K. Of particular notice is the fact that estradiol does not modify IKKβ activity nor Iκ-Bα degradation, which are known targets of anti-inflammatory drugs, suggesting a unique mechanism of action of this female hormone among anti-inflammatory endogenous signals and drugs [Figure 2] [83]. We believe that these data provide a novel background for the identification of innovative targets and pharmacological interventions aimed at preventing inflammation.
Figure 2. Estrogen anti-inflammatory action.
Schematic representation of the mechanism of action of estradiol in microglia proposed by our lab. The cytoplasmic activity of estrogen-activated ERα, including PI3K induction, inhibits the intracellular transport of NF-kB that is induced by inflammatory stimuli, such as LPS; this leads to reduced synthesis of inflammatory mediators and microglia activation.
The timing hypothesis
As discussed above, post-menopausal estrogen deficiency is a key event in the pathogenesis of a number of neurodegenerative diseases in women. However, the potent activity of exogenous estrogens in experimental models is paralleled by controversial results in humans ([211] and [212]). Secondary analyses of recent randomized clinical trials, that originally raised controversies among the scientific community as to the risk/benefit ratio of HT ([86], [160] and [239]), helped to consolidate a novel hypothesis on the efficacy of hormone therapy [202]; it is now hypothesized that HT should be started in early menopause, as a preventative treatment of relatively healthy women, in order to avoid the negative consequences of hypoestrogenicity per se [93]. In fact, observational and randomized clinical trials show that HT does not improve memory or intellectual functions in women already affected by mild to moderate AD ([71], [173] and [201]), whereas it delays disease onset when administered in healthy perimenopausal women ([101], [118], [202] and [230]). Accordingly, a very recent study demonstrated that surgical menopause is associated with an increased risk of cognitive impairment and dementia in women [197]; novel directions in correctly evaluating specific cognitive functions have also been formulated, since the effect of female steroid hormones on cognitive activities varies across cognitive domains [196]. Thus, it appears that full benefits from HT are achieved through correct timing of hormone assumption. Based on this knowledge, trials are now being designed that will consider the disease duration and menopausal status of the subjects ([147] and [161]).
Substantial experimental data support the hypothesis that hormone anti-inflammatory activity is beneficial if estrogen administration precedes the inflammatory burst and when it is given shortly after ovary removal. In fact, estradiol does not alter the inflammatory signaling cascade in microglia if it is administered after inflammatory stimuli ([26], [83] and [242]); in addition, a prolonged hormonal deprivation affects estrogen protective activity in ischemia and causes a null or even opposite response to exogenous hormone administration [226]. Collectively, the experimental evidence indicate that the efficacy of estrogenic molecules as anti-inflammatory agents is also confined in a therapeutic window and that their use should be considered only as preventive pharmacological strategies.
5. Conclusions
The evidence thus far clearly indicates a prominent role of estrogen anti-inflammatory action in protecting the CNS against neurotoxic stimuli. Experimental data show that receptor selectivity, time frame and concentration of hormone, as well as cell-specific molecular partners are key features in the efficacy of estrogens to control microglia and brain inflammation. It is worth underscoring that microglia activation associated with neurodegenerative processes is also endowed with beneficial effects, as microglia cells were shown to produce trophic and survival factors and to eliminate through phagocytosis the noxious material accumulated in the extracellular space ([119] and [148]). Identifying the molecular mechanisms of estrogen action will elucidate the conditions regulating the beneficial vs detrimental pathways can be separately activated, leading to the design of more selective regulatory agents that inhibit the deleterious effects while maintaining the protective role played by these immune cells in the neural tissue. Finally, future research could lead to identification of the most appropriate and selective estrogenic drugs to be used in post-menopausal as well as fertile female patients eligible for HT.
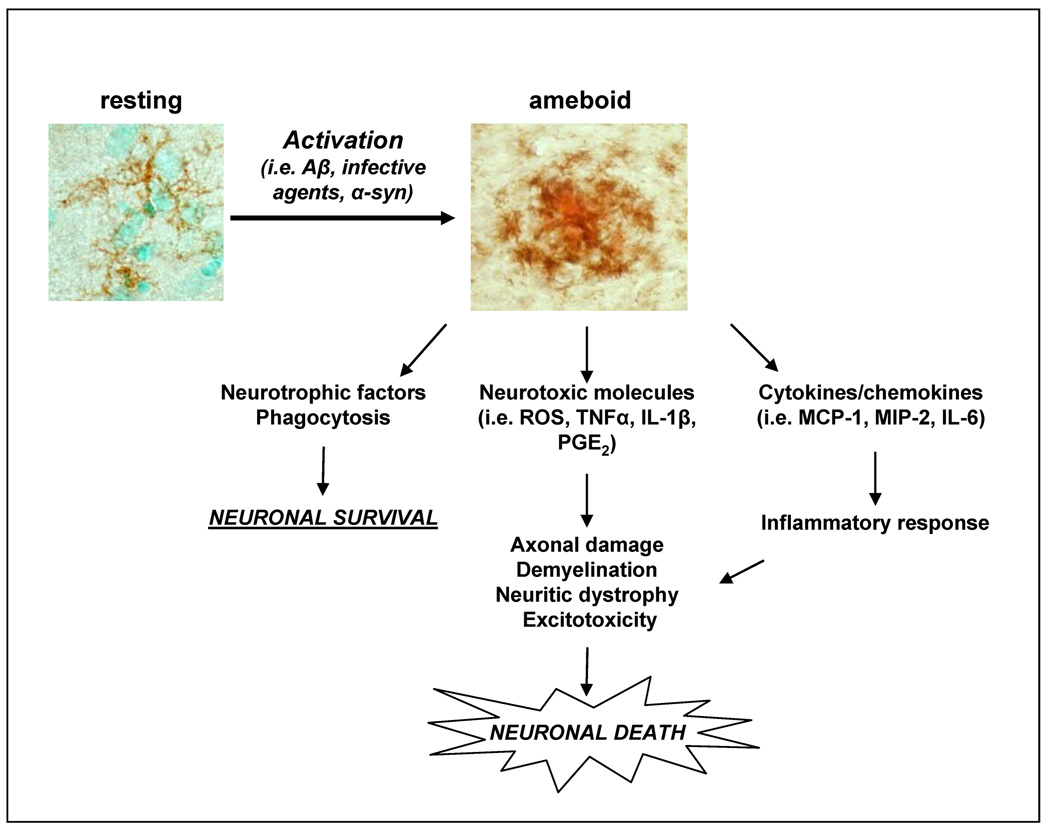
Figure 1. Microglia activation and neural cell loss.
Activation of microglia causes the secretion of short-lived diffusible molecules, such as reactive oxygen species (ROS), that produce an oxidative burst that is directly toxic to surrounding cells. In addition, peptides and inflammatory mediators are produced by activated microglia to communicate the ongoing local reaction to the periphery; circulating inflammatory cells are attracted by these molecules to the injured site and further sustain the local inflammatory reaction. Secretion of neurotrophic factors, as well as elimination of noxious material from the extracellular space through phagocytosis are also key features of microglia activation, which have instead beneficial consequences for brain health.
Acknowledgments
This work was supported by grants from the European Community (“EWA” LSHM-CT-2005-518245 to AM and EV; “DIMI” LSHB-CT-2005-52146 to AM), the NIH (RO1-AG027713-01 to AM) and the Italian Ministry for University and Scientific Research (COFIN 2006065483_003 to EV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abramsky O. Pregnancy and multiple sclerosis. Ann. Neurol. 1994;36 Suppl:S38–S41. doi: 10.1002/ana.410360712. [DOI] [PubMed] [Google Scholar]
- 2.Aharoni R, Arnon R, Eilam R. Neurogenesis and neuroprotection induced by peripheral immunomodulatory treatment of experimental autoimmune encephalomyelitis. J. Neurosci. 2005;25:8217–8228. doi: 10.1523/JNEUROSCI.1859-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akiyama H, McGeer PL. Brain microglia constitutively express beta-2 integrins. J Neuroimmunol. 1990;30:81–93. doi: 10.1016/0165-5728(90)90055-r. [DOI] [PubMed] [Google Scholar]
- 4.Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, Yoshida H, Holzer M, Craxton M, Emson PC, Atzori C, Migheli A, Crowther RA, Ghetti B, Spillantini MG, Goedert M. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 2002;22:9340–9351. doi: 10.1523/JNEUROSCI.22-21-09340.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Shammri S, Rawoot P, Azizieh F, AbuQoora A, Hanna M, Saminathan TR, Raghupathy R. Th1/Th2 cytokine patterns and clinical profiles during and after pregnancy in women with multiple sclerosis. J. Neurol. Sci. 2004;222:21–27. doi: 10.1016/j.jns.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 6.Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, Liu S, Hegde A, Yan SF, Stern A, Luddy JS, Lue LF, Walker DG, Roher A, Buttini M, Mucke L, Li W, Schmidt AM, Kindy M, Hyslop PA, Stern DM, Yan SSD. RAGE potentiates Ab-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ard MD, Cole GM, Wei J, Mehrle AP, Frantkin JD. Scavenging of Alzheimer's amyloid β protein by microglia in culture. J. Neurosci. Res. 1996;43:190–202. doi: 10.1002/(SICI)1097-4547(19960115)43:2<190::AID-JNR7>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.Azcoitia I, Sierra A, Veiga S, Honda S, Harada N, Garcia-Segura LM. Brain aromatase is neuroprotective. J. Neurobiol. 2001;47:318–329. doi: 10.1002/neu.1038. [DOI] [PubMed] [Google Scholar]
- 9.Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat. Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 10.Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J. Neurosci. 2001;21:6480–6491. doi: 10.1523/JNEUROSCI.21-17-06480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Movement Dis. 1998;13:221–227. doi: 10.1002/mds.870130205. [DOI] [PubMed] [Google Scholar]
- 12.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat. Rev. Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 13.Bebo BF, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J. Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 14.Beyer ML, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Phagocytosis of neuronal or glial debris by microglial cells: upregulation of MHC class II expression and multinuclear giant cell formation in vitro. Glia. 2000;31:262–266. doi: 10.1002/1098-1136(200009)31:3<262::aid-glia70>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Birge SJ, Mortel KF. Estrogen and the treatment of Alzheimer's disease. Am. J. Med. 1997;103:36S–45S. doi: 10.1016/s0002-9343(97)00258-1. [DOI] [PubMed] [Google Scholar]
- 16.Birk K, Ford C, Meltzer S. The clinical course of multiple sclerosis during pregnancy and puerperium. Arch. Neurol. 1990;47:738–742. doi: 10.1001/archneur.1990.00530070026007. [DOI] [PubMed] [Google Scholar]
- 17.Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, Wilson B, Yang J, Hong JS, Veronesi B. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. FASEB J. 2004;18:1618–1620. doi: 10.1096/fj.04-1945fje. [DOI] [PubMed] [Google Scholar]
- 18.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 19.Bo L, Vedeler CA, Nyland H, Trapp BD, Mork SJ. Intracortical multiple sclerosis lesions are not associated with increased lymphocyte infiltration. Mult. Scler. 2003;9:323–331. doi: 10.1191/1352458503ms917oa. [DOI] [PubMed] [Google Scholar]
- 20.Borchelt DR, Ratovitski T, van Lare J, Lee MK, Gonzales V, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Nature. 1997;19:939–945. doi: 10.1016/s0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- 21.Bornemann KD, Wiederhold KH, Pauli C, Ermini F, Stalder M, Schnell L, Sommer B, Jucker M, Staufenbiel M. Abeta-induced inflammatory processes in microglia cells of APP23 transgenic mice. Am. J. Pathol. 2001;158:63–73. doi: 10.1016/s0002-9440(10)63945-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. (Berl.) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 23.Bronstein DM, Perez-Otano I, Sun V, Mullis-Sawin SB, Chan J, Wu GC. Glia-dependent neurotoxicity and neuroprotection in mesecephalic cultures. Brain Res. 1995;704:112–116. doi: 10.1016/0006-8993(95)01189-7. [DOI] [PubMed] [Google Scholar]
- 24.Brown CM, Choi E, Xu Q, Vitek MP, Colton CA. The APOE4 genotype alters the response of microglia and macrophages to 17beta-estradiol. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.018. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruce-Keller AJ. Microglial–Neuronal Interactions in Synaptic Damage and Recovery. J. Neurosci. Res. 1999;58:191–201. doi: 10.1002/(sici)1097-4547(19991001)58:1<191::aid-jnr17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 26.Bruce-Keller AJ, Keeling JL, Keller JN, Huang FF, Camondola S, Mattson MP. Antiinflammatory effects of estrogen on microglia activation. Endocrinology. 2000;141:3456–3646. doi: 10.1210/endo.141.10.7693. [DOI] [PubMed] [Google Scholar]
- 27.Burns RS, Chiueh CC, Markey SP, Ebert MH, Jacobowitz DM, Kopin IJ. A primate model of parkinsonism: selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Proc. Natl. Acad. Sci. U.S.A. 1983;80:4546–4550. doi: 10.1073/pnas.80.14.4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buyon JP. The effects of pregnancy on autoimmune diseases. J Leukoc Biol. 1998;63:281–287. doi: 10.1002/jlb.63.3.281. [DOI] [PubMed] [Google Scholar]
- 29.Calhoun ME, Burgermeister P, Phinney AL, Stalder M, Tolnay M, Wiederhold KH, Abramowski D, Sturchler-Pierrat C, Sommer B, Staufenbiel M, Jucker M. Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proc. Natl. Acad. Sci. U.S.A. 1999;96:14088–14093. doi: 10.1073/pnas.96.24.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- 31.Callier S, Morissette M, Grandbois M, Di Paolo T. Stereospecific prevention by 17beta-estradiol of MPTP-induced dopamine depletion in mice. Synapse. 2000;37:245–251. doi: 10.1002/1098-2396(20000915)37:4<245::AID-SYN1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Candore G, Balistreri CR, Grimaldi MP, Listì F, Vasto S, Chiappelli M, Licastro F, Colonna-Romano G, Lio D, Caruso C. Polymorphisms of pro-inflammatory genes and Alzheimer’s disease risk: A pharmacolgenomic approach. Mech. Ageing Dev. 2007;128:67–75. doi: 10.1016/j.mad.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J. Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carswell HV, Macrae IM, Gallagher L, Harrop E, Horsburgh KJ. Neuroprotection by a selective estrogen receptor beta agonist in a mouse model of global ischemia. Am. J. Physiol. 2004;287:H1501–H1504. doi: 10.1152/ajpheart.00227.2004. [DOI] [PubMed] [Google Scholar]
- 35.Chang D, Kwan J, Timiras PS. Estrogens influence growth, maturation, and amyloid beta-peptide production in neuroblastoma cells and in a beta-APP transfected kidney 293 cell line. Adv. Exp. Med. Biol. 1997;429:261–271. doi: 10.1007/978-1-4757-9551-6_19. [DOI] [PubMed] [Google Scholar]
- 36.Chao H, Spencer R, Frankfort M, McEwen B. The effects of aging and hormonal manipulation on amyloid precursor protein APP695 mRNA expression in the rat hippocampus. J. Neuroendocrinol. 1994;6:517–521. doi: 10.1111/j.1365-2826.1994.tb00614.x. [DOI] [PubMed] [Google Scholar]
- 37.Chard DT, Griffin CM, Parker GJ, Kapoor R, Thompson AJ, Miller DH. Brain atrophy in clinically early relapsing-remitting multiple sclerosis. Brain. 2002;125:327–337. doi: 10.1093/brain/awf025. [DOI] [PubMed] [Google Scholar]
- 38.Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- 39.Chen H, Jacobs E, Schwarzschild MA, McCullough ML, Calle EE, Thun JM, Ascherio A. Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann. Neurol. 2005;58:963–967. doi: 10.1002/ana.20682. [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Zhang SM, Herman MA, Schwarzschild MA, Willett WC, Colditz GA. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch. Neurol. 2003;60:1059–1064. doi: 10.1001/archneur.60.8.1059. [DOI] [PubMed] [Google Scholar]
- 41.Chiba K, Trevor AJ, Castagnoli N., Jr Metabolism of the neurotoxic tertiary amine, MPTP, by brain monoamine oxidase. Biochem. Biophys. Res. Commun. 1984;120:574–578. doi: 10.1016/0006-291x(84)91293-2. [DOI] [PubMed] [Google Scholar]
- 42.Chiba K, Trevor A, Castagnoli N., Jr Active uptake of MPP1, a metabolite of MPTP, by brain synaptosomes. Biochem. Biophys. Res. Commun. 1985;120:1228–1232. doi: 10.1016/0006-291x(85)91071-x. [DOI] [PubMed] [Google Scholar]
- 43.Chung H, Brazil MI, Soe TT, Maxfield FR. Uptake, degradation, and release of fibrillar and soluble forms of Alzheimer's amyloid beta-peptide by microglial cells. J. Biol. Chem. 1999;274:32301–32308. doi: 10.1074/jbc.274.45.32301. [DOI] [PubMed] [Google Scholar]
- 44.Colton CA, Brown CM, Vitek MP. Sex steroids, APOE genotype and the innate immune system. Neurobiol. Aging. 2005;26:363–372. doi: 10.1016/j.neurobiolaging.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- 46.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N. Engl. J. Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 47.Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El Khoury JB. CD36, a Class B Scavenger Receptor, Is Expressed on Microglia in Alzheimer’s Disease Brains and Can Mediate Production of Reactive Oxygen Species in Response to β-Amyloid Fibrils. Am. J. Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Correale J, Rojany M, Weiner LP. Steroid hormone regulation of cytokine secretion by proteolipid protein-specific CD4+ T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol. 1998;161:3365–3374. [PubMed] [Google Scholar]
- 49.Cutolo M, Sulli A, Seriolo B, Accardo S, Masi AT. Estrogens, the immune response, and autoimmunity. Clin. Exp. Rheum. 1995;13:216–226. [PubMed] [Google Scholar]
- 50.Cuzzocrea S, Santagati S, Sautebin L, Mazzon E, Calabro G, Serraino I, Caputi AP, Maggi A. 17β-Estradiol antiinflammatory activity in carrageenan-induced pleurisy. Endocrinology. 2000;141:1455–1463. doi: 10.1210/endo.141.4.7404. [DOI] [PubMed] [Google Scholar]
- 51.Dalal M, Kim S, Voskuhl RR. Testosterone therapy ameliorates experimental autoimmune encephalomyelitis and induces a T helper 2 bias in the antigen-specific T lymphocyte response. J. Immunol. 1997;159:3–6. [PubMed] [Google Scholar]
- 52.Dalton CM, Chard DT, Davies GR, Miszkiel KA, Altmann DR, Fernando K, Plant GT, Thompson AJ, Miller DH. Early development of multiple sclerosis is associated with progressive grey matter atrophy in patients presenting with clinically isolated syndromes. Brain. 2004;127:1101–1107. doi: 10.1093/brain/awh126. [DOI] [PubMed] [Google Scholar]
- 53.D'Astous M, Morissette M, Di Paolo T. Effect of estrogen receptor agonists treatment in MPTP mice: evidence of neuroprotection by an ERα agonist. Neuropharmacology. 2004;47:1180–1188. doi: 10.1016/j.neuropharm.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 54.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid mciroglial response to local brain inyury in vivo. Nat. Rev. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 55.Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB. Protection by pioglitazone in the MPTP model of Parkinson's disease correlates with I kappa B alpha induction and block of NF kappa B and iNOS activation. J. Neurochem. 2004;88:494–501. doi: 10.1046/j.1471-4159.2003.02210.x. [DOI] [PubMed] [Google Scholar]
- 56.Diamond SG, Markham CH, Hoelm MM, McDowell FH, Muenter MD. An examination of male-female differences in progression and mortality of Parkinson’s disease. Neurology. 1990;40:763–766. doi: 10.1212/wnl.40.5.763. [DOI] [PubMed] [Google Scholar]
- 57.Dickson DW, Lee SC, Mattiace LA, Yen SH, Brosnan C. Microglia and cytokines in neurological disease, with special reference to AIDS and Alzheimer's disease. Glia. 1993;7:75–83. doi: 10.1002/glia.440070113. [DOI] [PubMed] [Google Scholar]
- 58.Dluzen DE, McDermott JL, Liu B. Estrogen as a neuroprotectant against MPTP-induced neurotoxicity in C57/Bl Mice. Neurotoxicol. And Teratol. 1996;18:603–606. doi: 10.1016/0892-0362(96)00086-4. [DOI] [PubMed] [Google Scholar]
- 59.Dluzen D, Ramirez VD. In vitro dopamine release from the rat striatum: diurnal rhythm and its modification by the estrous cycle. Neuroendocrinology. 1985;41:97–100. doi: 10.1159/000124160. [DOI] [PubMed] [Google Scholar]
- 60.Dluzen DE, Ramirez VD. In vivo changes in responsiveness of the caudate nucleus to L-dopa infusion as a function of the estrous cycle. Brain Res. 1990;536:163–168. doi: 10.1016/0006-8993(90)90021-3. [DOI] [PubMed] [Google Scholar]
- 61.Dodel RC, Du Y, Bales KR, Gao F, Paul SM. Sodium salicylate and 17beta-estradiol attenuate nuclear transcription factor NF-kappaB translocation in cultured rat astroglial cultures following exposure to amyloid A beta(1–40) and lipopolysaccharides. J. Neurochem. 1999;73:1453–1460. doi: 10.1046/j.1471-4159.1999.0731453.x. [DOI] [PubMed] [Google Scholar]
- 62.Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J. Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- 63.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor α, not β, is a critical link in estradiol-mediated protection against brain injury. Proc. Natl. Acad. Sci. U.S.A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 65.Duquette P, Girard M. Hormonal factors in susceptibility to multiple sclerosis. Curr. Opin. Neurol. Neurosurg. 1993;6:195–201. [PubMed] [Google Scholar]
- 66.Eikelenboom P, Zhan SS, Van Gool WA, Allsop D. Inflammatory mechanisms in Alzheimer’s disease. Trends Pharmacol. Sci. 1994;15:447–450. doi: 10.1016/0165-6147(94)90057-4. [DOI] [PubMed] [Google Scholar]
- 67.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to β-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 68.El Khoury J, Hickman SE, Thomas CA, Loike JD, Silverstein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol. Aging. 1998;19:S81–S84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 69.El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- 70.Elloso MM, Phiel K, Henderson RA, Harris HA, Adelman SJ. Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J. Endocrinol. 2005;185:243–252. doi: 10.1677/joe.1.06063. [DOI] [PubMed] [Google Scholar]
- 71.Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J. Women's Health Initiative Memory Study. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 72.Evron S, Brenner T, Abramsky O. Suppressive effect of pregnancy on the development of experimental allergic encephalomyelitis in rabbits. Am. J. Reprod. Immunol. 1984;5:109–113. doi: 10.1111/j.1600-0897.1984.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 73.Farrer MJ. Genetics of Parkinson disease: paradigm shifts and future prospects. Nat. Rev. Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- 74.Frautschy SA, Cole GM, Baird A. Phagocytosis and deposition of vascular beta-amyloid in rat brains injected with Alzheimer beta-amyloid. Am. J. Pathol. 1992;140:1389–1399. [PMC free article] [PubMed] [Google Scholar]
- 75.Furuzawa-Carballeda J, Vargas-Rojas MI, Cabral AR. Autoimmune inflammation from the Th17 perspective. Autoimmun. Rev. 2007;6:169–175. doi: 10.1016/j.autrev.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Galimberti D, Schoonenboom N, Scheltens P, Fenoglio C, Venturelli E, Pijnenburg YA, Bresolin N, Scarpini E. Intrathecal chemokine levels in Alzheimer disease and frontotemporal lobar degeneration. Neurology. 2006;66:146–147. doi: 10.1212/01.wnl.0000191324.08289.9d. [DOI] [PubMed] [Google Scholar]
- 77.Games D, Adams D, Alessandrini R, Barbour R, Borthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue I, Montoya-Zavala M, Mucke I, Paganini I, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressig V717F β-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 78.Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J. Neurosci. 2003b;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J. Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- 80.Gao HM, Liu B, Zhang W, Hong JS. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. FASEB J. 2003a;17:1957–1959. doi: 10.1096/fj.03-0203fje. [DOI] [PubMed] [Google Scholar]
- 81.Garidou L, Laffont S, Douin-Echinard V, Coureau C, Krust A, Chambon P, Guery JC. Estrogen receptor alpha signaling in inflammatory leukocytes is dispensable for 17beta-estradiol-mediated inhibition of experimental autoimmune encephalomyelitis. J. Immunol. 2004;173:2435–2442. doi: 10.4049/jimmunol.173.4.2435. [DOI] [PubMed] [Google Scholar]
- 82.Garnier M, Di Lorenzo D, Albertini A, Maggi A. Identification of Estrogen-Responsive Genes in Neuroblastoma SK-ER3 Cells. J. Neurosci. 1997;17:4591–4599. doi: 10.1523/JNEUROSCI.17-12-04591.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghisletti S, Meda C, Maggi A, Vegeto E. 17beta Estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol. Cell. Biol. 2005;25:2957–2968. doi: 10.1128/MCB.25.8.2957-2968.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gilmore W, Weiner LP, Correale J. Effect of estradiol on cytokine secretion by proteolipid protein-specific T cell clones isolated from multiple sclerosis patients and normal control subjects. J. Immunol. 1997;158:446–451. [PubMed] [Google Scholar]
- 85.Granucci F, Petralia F, Urbano M, Citterio S, Di Tota F, Santambrogio L, Ricciardi-Castagnoli P. The scavenger receptor MARCO mediates cytoskeleton rearrangements in dendritic cells and microglia. Blood. 2003;102:2940–2947. doi: 10.1182/blood-2002-12-3651. [DOI] [PubMed] [Google Scholar]
- 86.Grodstein F, Clarkson TB, Manson JE. Understanding the Divergent Data on Postmenopausal Hormone Therapy. N. Engl. J. Med. 2003;348:645–650. doi: 10.1056/NEJMsb022365. [DOI] [PubMed] [Google Scholar]
- 87.Haass C, De Strooper B. The presenilins in Alzheimer's disease--proteolysis holds the key. Science. 1999;286:916–919. doi: 10.1126/science.286.5441.916. [DOI] [PubMed] [Google Scholar]
- 88.Harris HA. Estrogen receptor-β: recent lessons form in vivo studies. Molecular Endocrinology. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- 89.Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 90.Hartmann A, Hunot S, Hirsch EC. Inflammation and dopaminergic neuronal loss in Parkinson's disease: a complex matter. Exp. Neurol. 2003;184:561–564. doi: 10.1016/j.expneurol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 91.Heikkila RE, Hess A, Duvoisin RC. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Science. 1984;224:1451–1453. doi: 10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- 92.Henderson VW. Estrogen, cognition, and a woman’s risk of Alzheimer’s disease. Am. J. Med. 1997;103:11S–18S. doi: 10.1016/s0002-9343(97)00261-1. [DOI] [PubMed] [Google Scholar]
- 93.Henderson VW. Estrogen-containing hormone therapy and alzheimer’s disease risk: understanding discrepant inferences from observational and experimental research. Neuroscience. 2006;138:1031–1039. doi: 10.1016/j.neuroscience.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 94.Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T. Presence of dendritic cells, MCP-1 and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann. Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 95.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, Waisman A, Rülicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 96.Herrera AJ, Castano A, Venero JL, Machado A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on an opaminergic system. Neurobiol. Dis. 2000;7:429–447. doi: 10.1006/nbdi.2000.0289. [DOI] [PubMed] [Google Scholar]
- 97.Hirsch EC. In: Parkinsonism and cell vulnerability in neurodegenerative disease. Jolles, Stutzmann JM, editors. London: Academic Press; 1994. pp. 155–168. [Google Scholar]
- 98.Hirsch EC, Hunot S, Damier P, Faucheux B. Glial cells and inflammation in Parkinson's disease: a role in neurodegeneration? Ann. Neurol. 1998;44 Suppl. 1:5115–5120. doi: 10.1002/ana.410440717. [DOI] [PubMed] [Google Scholar]
- 99.Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Jantzen P, Wright K, Saad I, Mueller R, Morgan D, Sanders S, Zehr C, O'Campo K, Hardy J, Prada CM, Eckman C, Younkin S, Hsiao K, Duff K. Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat. Med. 1998;4:97–100. doi: 10.1038/nm0198-097. [DOI] [PubMed] [Google Scholar]
- 100.Holsinger RM, McLean CA, Beyreuther K, Masters CL, Evin G. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann. Neurol. 2002;51:783–786. doi: 10.1002/ana.10208. [DOI] [PubMed] [Google Scholar]
- 101.Honjo H, Tanaka K, Kashiwagi T, Urabe M, Okada H, Hayashi M, Hayashi K. Senile dementia-Alzheimer's type and estrogen. Hormone Metab. Res. 1995;27:204–207. doi: 10.1055/s-2007-979941. [DOI] [PubMed] [Google Scholar]
- 102.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 103.Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996;72:355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 104.Hunot S, Dugas N, Faucheux B, Hartmann A, Tardieu M, Debré P, Agid Y, Dugas B, Hirsch EC. Fc epsilonRII/CD23 is expressed in Parkinson's disease and induces, in vitro, the production of nitric oxide and tumor necrosis factor-alpha in glial cells. J. Neurosci. 1999;19:3440–3447. doi: 10.1523/JNEUROSCI.19-09-03440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson’s disease. Ann. Neurol. 2003;53:S49–S60. doi: 10.1002/ana.10481. [DOI] [PubMed] [Google Scholar]
- 106.Iivonen S, Corder E, Lehtovirta M, Helisalmi S, Mannermaa A, Vepsäläinen S, Hänninen T, Soininen H, Hiltunen M. Polymorphisms in the CYP19 gene confer increased risk for Alzheimer disease. Neurology. 2004;62:1170–1176. doi: 10.1212/01.wnl.0000118208.16939.60. [DOI] [PubMed] [Google Scholar]
- 107.in t’ Veld BA, Ruitenberg A, Hofman A, Launer LJ, van Duijn CM, Stijnen T, Breteler MM, Stricker BH. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer's disease. N. Engl. J. Med. 2001;345:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 108.Iravani MM, Kashefi K, Mander P, Rose S, Jenner P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience. 2002;110:49–58. doi: 10.1016/s0306-4522(01)00562-0. [DOI] [PubMed] [Google Scholar]
- 109.Ito A, Bebo BF, matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-α production and reducees the severity of experimental autoimmune encephalomyelitis in cytokine deficient knockout mice. J. Immunol. 2001;167:542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- 110.Jaffe AB, Dominique Toran-Allerand C, Greengard P, Gandy SE. Estrogen Regulates Metabolism of Alzheimer Amyloid p Precursor Protein. 1994;269:13065–13068. [PubMed] [Google Scholar]
- 111.Jansson L, Holmdahl R. Estrogen-mediated immunosuppression in autoimmune diseases. Inflamm. Res. 1998;48:290–295. doi: 10.1007/s000110050332. [DOI] [PubMed] [Google Scholar]
- 112.Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen induced arthritis in mice. J. Neuroimmunol. 1994;53:203–207. doi: 10.1016/0165-5728(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 113.Jenner P, Olanow CW. The pathogenesis of cell death in Parkinson’s disease. Neurology. 2006;66:S24–S36. doi: 10.1212/wnl.66.10_suppl_4.s24. [DOI] [PubMed] [Google Scholar]
- 114.Juedes AE, Ruddle NH. Resident and infiltrating central nervous system APCs regulate the emergence and resolution of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2001;166:5168–5175. doi: 10.4049/jimmunol.166.8.5168. [DOI] [PubMed] [Google Scholar]
- 115.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. Microtubule disruption utilizes an NFkappa B-dependent pathway to stabilize HIF-1alpha protein. J. Biol. Chem. 2003;278:7445–7452. doi: 10.1074/jbc.M209804200. [DOI] [PubMed] [Google Scholar]
- 116.Kalaria RN. Microglia and Alzheimer's disease. Curr. Opin. Hematol. 1999;6:15–24. doi: 10.1097/00062752-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 117.Kawamata H, Akiyama H, Yamada T, McGeer PL. Immunologic reactions in amyotrophic lateral sclerosis brain and spinal cord tissue. Am. J. Pathol. 1992;140:691–707. [PMC free article] [PubMed] [Google Scholar]
- 118.Kawas C, Resnick S, Morrison A, Brookmeyer R, Corrada M, Zonderman A, Bacal C, Lingle DD, Metter E. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997;48:1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 119.Kerschensteiner M, Stadelmann C, Dechant G, Wekerle H, Hohlfeld R. Neurotrophic Cross-talk between the Nervous and Immune Systems: Implications for Neurological Diseases. Ann. Neurol. 2003;53:292–304. doi: 10.1002/ana.10446. [DOI] [PubMed] [Google Scholar]
- 120.Kim YS, Choi DH, Block ML, Lorenzl S, Yang L, Kim YI, Sugama S, Cho BP, Hwang O, Browne SE, Kim SY, Hong JS, Beal MF, Joh TH. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007;21:179–187. doi: 10.1096/fj.06-5865com. [DOI] [PubMed] [Google Scholar]
- 121.Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52:1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- 122.Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 2000;20:6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Klegeris A, Pelech S, Giasson BI, Maguire J, Zhang H, McGeer EG, McGeer PL. α-Synuclein activates stress signaling protein kinases in THP-1 cells and microglia. Neurobiol. Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.11.013. In press. [DOI] [PubMed] [Google Scholar]
- 124.Kloss CUA, Bohatschek M, Kreutzberg GW, Raivich G. Effect of Lipopolysaccharide on the Morphology and Integrin Immunoreactivity of Ramified Microglia in the Mouse Brain and in Cell Culture. Exp. Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- 125.Knott C, Stern G, Wilkin GP. Inflammatory regulators in Parkinson's disease: i NOS, Lipocortin-1, and cyclooxygenase-1 and-2. Mol. Cell. Neurosci. 2000;16:724–739. doi: 10.1006/mcne.2000.0914. [DOI] [PubMed] [Google Scholar]
- 126.Kopec KK, Carroll RT. Alzheimer's β-amyloid peptide 1–42 induces a phagocytic response in murine microglia. J. Neurochem. 1998;71:2123–2131. doi: 10.1046/j.1471-4159.1998.71052123.x. [DOI] [PubMed] [Google Scholar]
- 127.Kornek B, Lassmann H. Axonal pathology in multiple sclerosis. A historical note. Brain Pathol. 1999;9:651–656. doi: 10.1111/j.1750-3639.1999.tb00547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Korn-Lubetzki I, Kahana G, Cooper G, Abramsky O. Activity of multiple sclerosis during pregnancy and uerperium. Ann. Neurol. 1984;16:229–231. doi: 10.1002/ana.410160211. [DOI] [PubMed] [Google Scholar]
- 129.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 130.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J. Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- 131.Kurtzke JF, Goldberg JD. Parkinson death rates by race, sex and geography. Neurology. 1988;38:1558–1561. doi: 10.1212/wnl.38.10.1558. [DOI] [PubMed] [Google Scholar]
- 132.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Brück W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–2712. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- 133.Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 134.Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 135.Langston JW, Langston EB, Irwin I. MPTP-induced parkinsonism in human and non-human primates--clinical and experimental aspects. Acta Neurol. Scand. Suppl. 1984;100:49–54. [PubMed] [Google Scholar]
- 136.Leranth C, Roth RH, Elswoth JD, Naftolin F, Horvath TL, Redmond DE., Jr Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for Parkinson’s disease and memory. J. Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant(P301L) tau protein. Nat. Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 138.Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer's disease patients. Proc. Natl. Acad. Sci. U.S.A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li R, Shen Y, Yang LB, Lue LF, Finch C, Rogers J. Estrogen enhances uptake of amyloid beta-protein by microglia derived from the human cortex. J. Neurochem. 2000;75:1447–1454. doi: 10.1046/j.1471-4159.2000.0751447.x. [DOI] [PubMed] [Google Scholar]
- 140.Liu B, Dluzen DE. Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson’s disease. Clin. Exp. Pharmacol. Physiol. 2007;34:555–565. doi: 10.1111/j.1440-1681.2007.04616.x. [DOI] [PubMed] [Google Scholar]
- 141.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J. Pharmacol. Exp. Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 142.Liu X, Fan XL, Zhao Y, Luo GR, Li XP, Li R, Le WD. Estrogen provides neuroprotection against activated microglia-induced dopaminergic neuronal injury through both estrogen receptor-alpha and estrogen receptor-beta in microglia. J. Neurosci. Res. 2005;81:653–665. doi: 10.1002/jnr.20583. [DOI] [PubMed] [Google Scholar]
- 143.Loeffler DA, DeMaggio AJ, Juneau PL, Havaich MK, LeWitt PA. Effects of enhanced striatal dopamine turnover in vivo on glutathione oxidation. Clin. Neuropharmacol. 1994;17:370–379. doi: 10.1097/00002826-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 144.Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, Stern DM, Yan SD. Involvement of Microglial Receptor for Advanced Glycation Endproducts (RAGE) in Alzheimer’s Disease: Identification of a Cellular Activation Mechanism. Exp. Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- 145.Mackenzie IR, Hao C, Munoz DG. Role of microglia in senile plaque formation. Neurobiol. Aging. 1995;16:797–804. doi: 10.1016/0197-4580(95)00092-s. [DOI] [PubMed] [Google Scholar]
- 146.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and non-reproductive functions. Annu. Rev. Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 147.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML. WHI and WHI-CACS Investigators, Estrogen therapy and coronary-artery calcification. N. Engl. J. Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 148.Marchetti B, Abbracchio MP. To be or not to be “inflamed”—is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol. Sci. 2005;26:515–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 149.Marchetti B, Serra PA, Tirolo C, L’Episcopo F, Caniglia S, Gennuso F, Testa N, Miele E, Desole MS, Barden N, Morale MC. Glucocorticoid receptor-nitric oxide crosstalk and vulnerability to experimental Parkinsonism pivotal role for glia-neuron interactions. Brain Res. Rev. 2005a;48:302–321. doi: 10.1016/j.brainresrev.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 150.Martino G, Adorini L, Rieckmann P, Hillert J, Kallmann B, Comi G, Filippi M. Inflammation in multiple sclerosis: the good, the bad, and the complex. Lancet Neurol. 2002;1:499–509. doi: 10.1016/s1474-4422(02)00223-5. [DOI] [PubMed] [Google Scholar]
- 151.Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H H. 17β-estradiol inhibits cytokine, chemokines and chemokines receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2001;65:529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- 152.McDermott JL, Liu B, Dluzen DE. Sex differences and effects of estrogen on dopamine and DOPAC release from the striatum of male and female CD-1 mice. Exp Neurol. 1994;125:306–311. doi: 10.1006/exnr.1994.1034. [DOI] [PubMed] [Google Scholar]
- 153.McGeer PL. NSAIDs and other antiinflammatory agents in the treatment of neurodegenerative and vascular diseases. Neurobiol. Aging. 2004a;25:S18. [Google Scholar]
- 154.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology. 1988;38:1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 155.McGeer PL, McGeer EG. Glial cell reactions in neurodegenerative diseases: pathophysiology and therapeutic interventions. Alzheimer Dis. Assoc. Disord. 1998;2:S1–S6. [PubMed] [Google Scholar]
- 156.McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neurobiol. Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 157.McGeer PL, Schulzer M, McGeer EG. Arthritis and anti-inflammatory agents as possible protective factors for Alzheimer’s disease: a review of 17 epidemiologic studies. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 158.McGeer PL, Schwab C, Parent A, Doudet D. Presence of reactive microglia in monkey substantia nigra years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration. Ann. Neurol. 2003;54:599–604. doi: 10.1002/ana.10728. [DOI] [PubMed] [Google Scholar]
- 159.McGowan E, Eriksen J, Hutton M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 2006;22:281–289. doi: 10.1016/j.tig.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 160.Mendelsohn ME, Karas RH. Molecular and Cellular Basis of Cardiovascular Gender Differences. Science. 2005;308:1583–1587. doi: 10.1126/science.1112062. [DOI] [PubMed] [Google Scholar]
- 161.Mendelsohn ME, Karas RH. HRT and the Young at Heartn. N. Engl J. Med. 2007;356:2639–2641. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- 162.Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- 163.Mertin LA, Rumjanek VM. Pregnancy and the susceptibility of Lewis rats to experimental allergic encephalomyelitis. J. Neurol. Sci. 1985;68:15–24. doi: 10.1016/0022-510x(85)90046-2. [DOI] [PubMed] [Google Scholar]
- 164.Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen Receptor-α Potency-Selective Ligands: Structure-Activity Relationship Studies of Diarylpropionitriles and Their Acetylene and Polar Analogues. J. Med. Chem. 2001;44:4230–4251. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 165.Miklossy J, Doudet DD, Schwab C, Yu S, McGeer EG, McGeer PL. Role of ICAM-1 in persisting inflammation in Parkinson disease and MPTP monkeys. Exp. Neurol. 2006;197:275–283. doi: 10.1016/j.expneurol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 166.Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen Can Act via Estrogen Receptor α and β to Protect Hippocampal Neurons against Global Ischemia-Induced Cell Death. Endocrinology. 2005;146:3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- 167.Minghetti L. Role of inflammation in neurodegenerative diseases. Curr. Opin. Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- 168.Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of Estrogen Receptor β in the Mouse Brain: Comparison with Estrogen Receptor α. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 169.Morale C, Serra PA, Delogu MR, Migheli R, Rocchitta G, Tirolo C, Caniglia S, Testa N, L’Episcopo F, Gennuso F, Scoto GM, Barden N, Miele E, Desole MS, Marchetti B. Glucocorticoid receptor deficiency increases vulnerability of the nigrostriatal dopaminergic system: critical role of glial nitric oxide. FASEB J. 2004;18:164–166. doi: 10.1096/fj.03-0501fje. [DOI] [PubMed] [Google Scholar]
- 170.Morale MC, Serra PA, L’Episcopo F, Tirolo C, Caniglia S, Testa N, Gennuso F, Giaquinta G, Rocchitta G, Desole MS, Miele E, Marchetti B. Estrogen, neuroinflammation and neuroprotection in Parkinson’s disease: glia dictates resistance versus vulnerability to neurodegeneration. Neuroscience. 2006;138:869–878. doi: 10.1016/j.neuroscience.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 171.Morales LBJ, Loo KK, Liu HB, Peterson C, Tiwari-Woodruff S, Voskuhl RR. Treatment with an Estrogen Receptor α Ligand Is Neuroprotective in Experimental Autoimmune Encephalomyelitis. J. Neurosci. 2006;26:6823–6833. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Morissette M, Di Paolo T. Effect of chronic estradiol and progesterone treatments of ovariectomized rats on brain dopamine uptake sites. J. Neurochem. 1993;60:1876–1883. doi: 10.1111/j.1471-4159.1993.tb13415.x. [DOI] [PubMed] [Google Scholar]
- 173.Mulnard RA, Cotman CW, Kawas C, van Dyck CH, Sano M, Doody R, Koss E, Pfeiffer E, Jin S, Gamst A, Grundman M, Thomas R, Thal LJ. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. JAMA. 2000;283:1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 174.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 175.Nolte C, Moller T, Walter T, Kettenmann H. Complement 5a controls motility of murine microglial cells in vitro via activation of an inhibitory G-protein and the rearrangement of the actin cytoskeleton. Neuroscience. 1996;73:1091–1107. doi: 10.1016/0306-4522(96)00106-6. [DOI] [PubMed] [Google Scholar]
- 176.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Medical progress: multiple sclerosis. N. Engl. J. Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 177.Oddo S, Billings L, Kesslak JP, Cribbs DH, LaFerla FM. Abeta immunotherapy leads to clearance of early, but not late, hyperphosphorylated tau aggregates via the proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 178.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol. Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 179.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease. Am. J. Epidemiol. 1994;140:251–256. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 180.Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch. Intern. Med. 1996;156:2213–2217. [PubMed] [Google Scholar]
- 181.Paresce DM, Chung H, Maxfield FR. Slow degradation of aggregates of the Alzheimer's disease amyloid β-protein by microglia cells. J. Biol. Chem. 1997;272:29390–29397. doi: 10.1074/jbc.272.46.29390. [DOI] [PubMed] [Google Scholar]
- 182.Pattarini R, Smeyne RJ, Morgan JL. Temporal mRNA profiles of inflammatory mediators in the murine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine model of Parkinson's disease. Neuroscience. 2007;145:654–668. doi: 10.1016/j.neuroscience.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pei Z, Zhong P, Pang H, Qian L, Yang S, Wang T, Zhang W, Wu X, Dallas S, Wilson B, Reece JM, Miller DS, Hong JS, Block ML. MAC1 mediates LPS-induced production of superoxide by microglia: The role of pattern recognition receptors in dopaminergic neurotoxicity. Glia. 2007;55:1362–1373. doi: 10.1002/glia.20545. [DOI] [PubMed] [Google Scholar]
- 184.Pernis AB. Estrogen and CD4+ Tcells. Curr. Opin. Rheumatol. 2007;19:414–420. doi: 10.1097/BOR.0b013e328277ef2a. [DOI] [PubMed] [Google Scholar]
- 185.Pike CJ. Estrogen modulates neuronal Bcl-xL expression and β-amyloid-induced apoptosis: relevance to Alzheimer's disease. J. Neurochem. 1999;72:1552–1563. doi: 10.1046/j.1471-4159.1999.721552.x. [DOI] [PubMed] [Google Scholar]
- 186.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J. Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 187.Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J. Neuroimmunol. 2005;170:85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 188.Ponomarev ED, Shriver LP, Maresz K, Dittell BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J. Neurosci. Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 189.Pozzi S, Benedusi V, Maggi A, Vegeto E. Estrogen action in neuroprotection and brain inflammation. Ann. N. Y. Acad. Sci. 2006;1089:302–323. doi: 10.1196/annals.1386.035. [DOI] [PubMed] [Google Scholar]
- 190.Pozzilli C, Falaschi P, Mainero C, Martocchia A, D’Urso R, Proietti A, Frontoni M, Bastianello S, Filippi M. MRI in multiple sclerosis during the menstrual cycle: relationship with sex hormone patterns. Neurology. 1999;53:622–624. doi: 10.1212/wnl.53.3.622. [DOI] [PubMed] [Google Scholar]
- 191.Prat A, Antel J. Pathogenesis of multiple sclerosis. Cur. Opin. Neurol. 2005;18:225–230. doi: 10.1097/01.wco.0000169737.99040.31. [DOI] [PubMed] [Google Scholar]
- 192.Qin L, Liu Y, Wang T, Wei SJ, Block ML, Wilson B, Liu B, Hong JS. NADPH oxidase mediates lipopolysaccharide-induced neurotoxicity and proinflammatory gene expression in activated microglia. J. Biol. Chem. 2004;279:1415–1421. doi: 10.1074/jbc.M307657200. [DOI] [PubMed] [Google Scholar]
- 193.Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS. Regulation of neuronal nitric oxide synthase mRNA in lordosis-relevant neurons of the ventromedial hypothalamus following short-term estrogen treatment. Brain Res. Mol. Brain Res. 1998;59:105–108. doi: 10.1016/s0169-328x(98)00131-4. [DOI] [PubMed] [Google Scholar]
- 194.Raine CS. Multiple sclerosis: immune system molecule expression in the central nervous system. J. Neuropathol. Exp. Neurol. 1994;53:328–337. doi: 10.1097/00005072-199407000-00002. [DOI] [PubMed] [Google Scholar]
- 195.Rapp S, Espeland M, Shumaker S, Henderson V, Brunner R, Manson J, Gass M, Stefanick M, Lane D, Hays J, Johnson K, Coker L, Dailey M, Bowen D. Effect of Estrogen Plus Progestin on Global Cognitive Function in Postmenopausal Women: The Women's Health Initiative Memory Study: A Randomized Controlled Trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 196.Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA. Women's Health Initiative Study of Cognitive Aging Investigators. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J. Clin. Endocrinol. Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 197.Rocca WA, Bower JH, Maraganore DM, Ahlskog JE, Grossardt BR, de Andrade M, Melton LJ. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology. 2007;69:1074–1083. doi: 10.1212/01.wnl.0000276984.19542.e6. [DOI] [PubMed] [Google Scholar]
- 198.Rogers J, Mastroeni D, Leonard B, Joyce J, Grover A. Neuroinflammation in Alzheimer's disease and Parkinson's disease: are microglia pathogenic in either disorder? Int. Rev. Neurobiol. 2007;82:235–246. doi: 10.1016/S0074-7742(07)82012-5. [DOI] [PubMed] [Google Scholar]
- 199.Rogers J, Webster S, Lue LF, Brachova L, Civin WH, Emmerling M, Shivers B, Walker D, McGeer P. Inflammation and Alzheimer's disease pathogenesis. Neurobiol. Aging. 1996;17:681–686. doi: 10.1016/0197-4580(96)00115-7. [DOI] [PubMed] [Google Scholar]
- 200.Rosette C, Karin M. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. J. Cell Biol. 1995;128:1111–1119. doi: 10.1083/jcb.128.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 202.Rossouw JE, Prentice RL, Manson JAE, Wu LL, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML. Postmenopausal Hormone Therapy and Risk of Cardiovascular Disease by Age and Years Since Menopause. JAMA. 2007;297:1465–1477. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 203.Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS, Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53:1698–1704. doi: 10.1212/wnl.53.8.1698. [DOI] [PubMed] [Google Scholar]
- 204.Rychly J, Nebe B. Therapeutic strategies in autoimmune diseases by interfering with leukocyte endothelium interaction. Curr. Pharm. Des. 2006;12:3799–3806. doi: 10.2174/138161206778559696. [DOI] [PubMed] [Google Scholar]
- 205.Saito S, Aras RS, Lou H, Ramwell PW, Foegh ML. Effects of estrogen on nitric oxide synthase expression in rat aorta allograft and smooth muscle cells. J. Heart. Lung. Transplant. 1999;18:937–945. doi: 10.1016/s1053-2498(99)00060-1. [DOI] [PubMed] [Google Scholar]
- 206.Sandyk R. Estrogen and the pathophysiology of Parkinson’s disease. Int. J. Neurosci. 1989;45:119–122. doi: 10.3109/00207458908986223. [DOI] [PubMed] [Google Scholar]
- 207.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Saunders-Pullman R, Gordon-Elliot J, Parides M, Fahn S, Saunders HR, Bressman S. The effect of estrogen replacement on early replacement on early Parkinson’s disease. Neurology. 1999;52:1417–1421. doi: 10.1212/wnl.52.7.1417. [DOI] [PubMed] [Google Scholar]
- 209.Schiess M. Nonsteroidal anti-inflammatory drugs protect against Parkinson neurodegeneration can an NSAID a day keep Parkinson disease away? Arch. Neurol. 2003;60:1043–1044. doi: 10.1001/archneur.60.8.1043. [DOI] [PubMed] [Google Scholar]
- 210.Selley ML. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 2005;1037:1–6. doi: 10.1016/j.brainres.2004.02.083. [DOI] [PubMed] [Google Scholar]
- 211.Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH. Women's Health Initiative Memory Study, Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women's Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 212.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, Assaf AR, Jackson RD. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 213.Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S, Wu TC, Voskuhl RR. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann. Neurol. 2002;52:421–428. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 214.Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone Marrow-Derived Microglia Play a Critical Role in Restricting Senile Plaque Formation in Alzheimer’s Disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 215.Simpson ER. Sources of estrogen and their importance. J. Steroid Biochem. Mol. Biol. 2003;86:225–230. doi: 10.1016/s0960-0760(03)00360-1. [DOI] [PubMed] [Google Scholar]
- 216.Soldan SS, Retuerto AI, Sicotte NL, Voskuhl RR. Immune modulation in multiple sclerosis patients treated with the pregnancy hormone estriol. J. Immunol. 2003;171:6267–6274. doi: 10.4049/jimmunol.171.11.6267. [DOI] [PubMed] [Google Scholar]
- 217.Stalder M, Phinney A, Probst A, Sommer B, Staufenbiel M, Jucker M. Association of microglia with amyloid plaques in brains of APP23 transgenic mice. Am. J. Pathol. 1999;154:1673–1684. doi: 10.1016/S0002-9440(10)65423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Stauffer SR, Coletta CJ, Tedesco R, Nishiguchi G, Carlson K, Sun J, Katzenellenbogen BS, Katzenellenbogen JA. Pyrazole Ligands: Structure-Affinity/Activity Relationships and Estrogen Receptor-α-Selective Agonists. J. Med. Chem. 2000;43:4934–4947. doi: 10.1021/jm000170m. [DOI] [PubMed] [Google Scholar]
- 219.Steinman L. Blocking adhesion molecules as therapy for multiple sclerosis: natalizumab. Nat. Rev. Drug Discov. 2005;4:510–518. doi: 10.1038/nrd1752. [DOI] [PubMed] [Google Scholar]
- 220.Stence N, Waite M, Dailey ME. Dynamics of microglial activation: a confocal time-lapse anlaysis in hippocampal slices. Glia. 2001;33:256–266. [PubMed] [Google Scholar]
- 221.Stewart WF, Kawas C, Corrada M, Meter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurol. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 222.Strittmatter WJ, Roses AD. Apolipoprotein E and Alzheimer disease. 1995;92:4725–4727. doi: 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Bürki K, Frey P, Paganetti P, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc. Natl. Acad. Sci. U.S.A. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Su X, Maguire-Zeiss KA, Giuliano R, Prifti L, Venkatesh K, Federoff HJ. Synuclein activates microglia in a model of Parkinson’s disease. Neurobiol. Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol. 2003;170:1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- 226.Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc. Natl. Acad. Sci. U.S.A. 2007;104:6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Szekely CA, Thorne JE, Zandi PP. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease: a systematic review. Neuroepidemiol. 2004;23:159–169. doi: 10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- 228.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. Induction of regulatory T cells by physiological level estrogen. J. Cell. Physiol. 2008;214:456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 229.Takeuchi H, Wang J, Kawanokuchi J, Mitsuma N, Mizuno T, Suzumura A. Interferon-gamma induces microglial-activation-induced cell death: a hypothetical mechanism of relapse and remission in multiple sclerosis. Neurobiol. Dis. 2006;22:33–39. doi: 10.1016/j.nbd.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 230.Tang MX, Jacobs D, Stern Y, Marder K, Schofield P, Gurland B, Andrews H, Mayeux R. Effect of estrogen during menopause on risk and age at onset of Alzheimer's disease. Lancet. 1996;348:429–432. doi: 10.1016/S0140-6736(96)03356-9. [DOI] [PubMed] [Google Scholar]
- 231.Teismann P, Ferger B. Inhibition of the Cyclooxygenase Isoenzymes COX-1 and COX-2 Provide Neuroprotection in the MPTP-Mouse Model of Parkinson’s Disease. Synapse. 2001;39:167–174. doi: 10.1002/1098-2396(200102)39:2<167::AID-SYN8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 232.Tiberio M, Chard DT, Altmann DR, Davies G, Griffin CM, Rashid W, Sastre-Garriga J, Thompson AJ, Miller DH. Gray and white matter volume changes in early RRMS: a 2-year longitudinal study. Neurology. 2005;64:1001–1007. doi: 10.1212/01.WNL.0000154526.22878.30. [DOI] [PubMed] [Google Scholar]
- 233.Tipton KF, Singer TP. Advances in our understanding of the mechanisms of the neurotoxicity of MPTP and related compounds. J. Neurochem. 1993;61:1191–1206. doi: 10.1111/j.1471-4159.1993.tb13610.x. [DOI] [PubMed] [Google Scholar]
- 234.Tiwari-Woodruff S, Morales LBJ, Lee R, Voskuhl R. Differential neuroprotective and anti-inflammatory effects of estrogen receptor (ER)α and ERβ ligand treatment. Proc. Nat. Acad. Sci. U.S.A. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tran EH, Hoekstra K, van Rooijen N, Dijkstra CD, Owens T. Immune Invasion of the Central Nervous System Parenchyma and Experimental Allergic Encephalomyelitis, But Not Leukocyte Extravasation from Blood, Are Prevented in Macrophage-Depleted Mice. J. Immunol. 1998;161:3767–3775. [PubMed] [Google Scholar]
- 236.Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mörk S, Bö L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 237.Tripanichkul W, Sripanichkulchai K, Finkelstein S. Estrogen down- regulates glial activation in male mice following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication. Brain Res. 2006;1084:28–37. doi: 10.1016/j.brainres.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 238.Tsang KL, Ho SL, Lo SK. Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology. 2000;54:2292–2298. doi: 10.1212/wnl.54.12.2292. [DOI] [PubMed] [Google Scholar]
- 239.Turgeon JL, McDonnell DP, Martin KA, Wise PM. Hormone Therapy: Physiological Complexity Belies Therapeutic Simplicity. Science. 2004;304:1269–1273. doi: 10.1126/science.1096725. [DOI] [PubMed] [Google Scholar]
- 240.Turski L, Bressler K, Rettig KJ, Loschmann PA, Wachtel H. Protection of substantia nigra from MPP1 neurotoxicity by N-methyl D-aspartate antagonists. Nature. 1991;349:414–418. doi: 10.1038/349414a0. [DOI] [PubMed] [Google Scholar]
- 241.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 242.Vegeto E, Belcredito S, Etteri S, Ghisletti S, Brusadelli A, Meda C, Krust A, Dupont S, Ciana P, Chambon P, Maggi A. Estrogen receptor-alpha mediates the brain antiinflammatory activity of estradiol. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9614–9619. doi: 10.1073/pnas.1531957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Vegeto E, Belcredito S, Ghisletti S, Meda C, Etteri S, Maggi A. The endogenous estrogen status regulates microglia reactivity in animal models of neuroinflammation. Endocrinol. 2006;147:2263–2272. doi: 10.1210/en.2005-1330. [DOI] [PubMed] [Google Scholar]
- 244.Vegeto E, Bonincontro C, Pollio G, Sala A, Viappiani S, Nardi F, Brusadelli A, Viviani B, Ciana P, Maggi A. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. J. Neurosci. 2001;21:1809–1818. doi: 10.1523/JNEUROSCI.21-06-01809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vegeto E, Pollio G, Pellicciari C, Maggi A. Estrogen and progesterone induction of survival of monoblastoid cells undergoing TNF-α-induced apoptosis. FASEB J. 1999;13:793–803. doi: 10.1096/fasebj.13.8.793. [DOI] [PubMed] [Google Scholar]
- 246.Voskuhl RR, Goldstein AM, Simonis T, Davey RJ, McFarland HF. DR2/DQw1 inheritance and haplotype sharing in affected siblings from multiple sclerosis families. Ann. Neurol. 1996;39:804–807. doi: 10.1002/ana.410390618. [DOI] [PubMed] [Google Scholar]
- 247.Wahner AD, Bronstein JM, Bordelon YM, Ritz B. Nonsteroidal anti-inflammatory drugs may protect against Parkinson disease. Neurology. 2007;69:1836–1842. doi: 10.1212/01.wnl.0000279519.99344.ad. [DOI] [PubMed] [Google Scholar]
- 248.Wahner AD, Sinsheimer JS, Bronstein JM, Ritz B. Inflammatory cytokine gene polymorphisms and increased risk of Parkinson disease. Arch. Neurol. 2007;64:836–840. doi: 10.1001/archneur.64.6.836. [DOI] [PubMed] [Google Scholar]
- 249.Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer's disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 250.Wegiel J, Wisniewski HM. The complex of microglial cells and amyloid star in three-dimensional reconstruction. Acta Neuropathol. (Berl.) 1990;81:116–124. doi: 10.1007/BF00334499. [DOI] [PubMed] [Google Scholar]
- 251.Westberg A, Hakansson J, Melke J, Shabi HN, Nilsson S, Buervenich S, Carmine A, Ahlberg J, Grundel MB, Klingborg K, Holmberg B, Sydow O, Olson LL, Johnels EB, Ericksson E, Nissbrandt H. Association between the estrogen receptor beta gene and age onset of Parkinson’s disease. Psychoneuroendocrinology. 2004;29:993–998. doi: 10.1016/j.psyneuen.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 252.Whitacre CC, Reingold SC, O’Looney PA, Blankenhorn E, Brinley F, Collier E, Duquette P, Fox H, Gilmore W, Lahita R, Nelson JL, Reiss C, Riskind P, Voskuhl RA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- 253.Wilms H, Rosenstiel P, Sievers J, Deuschl G, Zecca L, Lucius R. Activation of microglia by human neuromelanin is NF-κBdependent and involves p38 mitogen-activated protein kinase: implications for Parkinson’s disease. FASEB J. 2003;17:500–502. doi: 10.1096/fj.02-0314fje. [DOI] [PubMed] [Google Scholar]
- 254.Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wynn DR, Rodriguez M, O’Fallon WM, Kurland LT. A reappraisal of the epidemiology of multiple sclerosis in Olmsted County, Minnesota. Neurology. 1990;40:780–786. doi: 10.1212/wnl.40.5.780. [DOI] [PubMed] [Google Scholar]
- 256.Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Nat. Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- 257.Xia MQ, Hyman BT. Chemokines/chemokine receptors in the central nervous system and Alzheimer's disease. J. Neurovirol. 1999;5:32–41. doi: 10.3109/13550289909029743. [DOI] [PubMed] [Google Scholar]
- 258.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat. Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 259.Yamaguchi H, Sugihara S, Ogawa A, Saido TC, Ihara Y. Diffuse plaques associated with astroglial amyloid beta protein, possibly showing a disappearing stage of senile plaques. Acta Neuropathol. 1998;95:217–222. doi: 10.1007/s004010050790. [DOI] [PubMed] [Google Scholar]
- 260.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, Slattery T, Zhao L, Nagashima M, Morser J, Migheli A, Nawroth P, Stern D, Schmidt AM. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 261.Yang LB, Lindholm K, Yan R, Citron M, Xia W, Yang XL, Beach T, Sue L, Wong P, Price D, Li R, Shen Y. Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 262.Yue X, Lu M, Lavcaster T, Cao P, Hnda SI, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer’s disease animal model. Proc. Natl. Acad. Sci. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Zancan V, Santagati S, Bolego C, Vegeto E, Maggi A, Pugliesi L. 17β-estradiol decreases NOS II synthesis in vascular smooth muscle cells. Endocrinology. 1999;140:2204–2209. doi: 10.1210/endo.140.5.6694. [DOI] [PubMed] [Google Scholar]
- 264.Zemlyak I, Brooke S, Sapolsky R. Estrogen protection against gp120 neurotoxicity: role of microglia. Brain Res. 2005;1046:130–136. doi: 10.1016/j.brainres.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 265.Zhang B, Higuchi M, Yoshiyama Y, Ishihara T, Forman MS, Martinez D, Joyce S, Trojanowski JQ, Lee VM. Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy. J. Neurosci. 2004;24:4657–4667. doi: 10.1523/JNEUROSCI.0797-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhang W, Dallas S, Zhang D, Guo JP, Pang H, Wilson B, Miller DS, Chen B, Zhang W, McGeer PL, Hong JS, Zhang J. Microglial PHOX and Mac-1 are essential to the enhanced dopaminergic neurodegeneration elicited by A30P and A53T mutant alpha-synuclein. Glia. 2007;55:1178–1188. doi: 10.1002/glia.20532. [DOI] [PubMed] [Google Scholar]
- 267.Zhang W, Wang T, Pei Z, Miller DS, Wu X, Block ML, Wilson B, Zhang W, Zhou Y, Hong JS, Zhang J. Aggregated α-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19:533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 268.Zhao L, Wu TW, Brinton RD. Estrogen receptor subtypes alpha and beta contribute to neuroprotection and increased Bcl-2 expression in primary hippocampal neurons. Brain Res. 2004;1010:22–34. doi: 10.1016/j.brainres.2004.02.066. [DOI] [PubMed] [Google Scholar]
- 269.Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, Refolo LM, Petanceska S, Wang R, Duff K. Modulation of A(beta) peptides by estrogen in mouse models. J. Neurochem. 2002;80:191–196. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]