Abstract
We investigated age and sex effects and determined whether androgen replacement in elderly individuals (≥60 yr) could augment protein synthesis. Thirty young men and 32 young women (18–31 yr) were studied once, whereas 87 elderly men were studied before and after 1 yr of treatment with 5 mg/day testosterone (T), 75 mg/day dehydroepiandrosterone (DHEA), or placebo (P); and 57 elderly women were studied before and after 1 yr of treatment with 50 mg/day DHEA or P. [15N]Phenylalanine and [2H4]tyrosine tracers were infused, with measurements in plasma and vastus lateralis muscle. Whole-body protein synthesis per fat-free mass and muscle protein fractional synthesis rate (FSR) were lower in elderly than in young individuals (P<0.001), not significantly affected by hormone treatments, and higher in women than in men (P<0.0001), with no sex × age interaction. In regression analyses, peak O2 consumption (VO2peak), resting energy expenditure (REE), and sex were independently associated with muscle FSR, as were VO2peak, REE, and interactions of sex with insulin-like growth factor-II and insulin for whole-body protein synthesis. Women maintain higher protein synthesis than men across the lifespan as rates decline in both sexes, and neither full replacement of DHEA (in elderly men and women) nor partial replacement of bioavailable T (in elderly men) is able to amend the age-related declines.—Henderson, G. C., Dhatariya, K., Ford, G. C., Klaus, K. A., Basu, R., Rizza, R. A., Jensen, M. D., Khosla, S., O'Brien, P., Nair, K. S. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements.
Keywords: proteolysis, remodeling, aging, sexual dimorphism
Protein synthesis within skeletal muscle and other tissues is important for maintenance and repair as well as for carrying out cellular functions. The skeletal muscle protein synthesis rate has been shown to decline across the lifespan (1,2,3,4,5,6,7), which may contribute to susceptibility for sarcopenia and corresponds to increasing incidence of comorbidities, such as metabolic syndrome and frailty. With a rapidly expanding aging population, it is of substantial interest to develop strategies to prevent age-related declines in skeletal muscle protein synthesis rates to promote muscle maintenance and repair and to prevent the development of sarcopenia and related metabolic disorders. If it is presumed that alterations in protein turnover across the lifespan occur in response to changing hormone levels, then one strategy could be to replace hormones that decline with age.
It is generally known that men have higher serum testosterone (T) and higher skeletal muscle mass than women, and T has been reported to enhance muscle mass in a dose-dependent manner (8,9,10). Therefore, one might hypothesize that men would have higher rates of skeletal muscle protein synthesis than women. It was reported that T replacement to physiological levels via biweekly injections in hypogonadal men for 6 months substantially increased muscle mass and skeletal muscle protein synthesis (11), and another research group (12) reported similar results for young healthy men in which T was elevated ∼3-fold via weekly injection for 3 months. It has even been shown that 5 days after a single large T injection in young healthy men, skeletal muscle protein synthesis is elevated substantially (13). In men, the circulating total T concentration and especially bioavailable T declines during the aging process (14,15,16). However, 3 yr of T replacement in elderly men that raised circulating T to the middle of the range seen in young individuals was reported to have no effect on muscle strength and only increased truncal lean tissue mass (17), and in a separate study, similarly raising T to normal levels of young people for 6 months was also unable to elevate muscle protein synthesis in elderly men (18). Furthermore, administering low-dose T via a patch for 6 months in elderly men did not increase total fat-free mass (FFM) or thigh muscle (19). Yet, in a study of much briefer duration (1 month) in which administration of a weekly injection of T to elderly men raised circulating T by ∼2-fold, which is still within the physiological range for normal serum T concentrations in young people, there was a substantial increase in skeletal muscle protein synthesis and strength (20). It is apparent that short-term administration of large T doses via injection in young men increases muscle protein synthesis. However, uncertainly remains regarding the efficacy of mild doses, delivery via transdermal patch, responsiveness in elderly individuals, and sustainability of effects of T administration over a long duration. Although large doses would presumably have the most substantial impact on protein synthesis, for therapeutic attempts to raise muscle protein synthesis rates, the T dose would need to be low enough to avoid detrimental effects in the long term, such as prostate cancer and benign prostatic hyperplasia (21). Furthermore, lessons learned from replacement of ovarian hormones in postmenopausal women indicate that full replacement of sex steroids may lead to adverse effects but that low doses could be beneficial (22).
Another steroid hormone that shows a particular decline with age is dehydroepiandrosterone (DHEA), which is considered to be a weaker androgen. Administration of DHEA may modestly increase both T and estradiol (E), because synthesis of these three hormones shares a common pathway (23). It has been reported that the sulfated form of DHEA (DHEAS), the primary form in circulation, progressively declines with age both in men and women (24, 25). Although early short-term studies showed that DHEA replacement may increase muscle mass and strength (26), in another study, 4 wk of daily high-dose DHEA in healthy young men did not affect FFM, whole-body amino acid kinetics, or skeletal muscle protein synthesis (27). Furthermore, it has also been shown that replacement of DHEA for approximately 1 wk in women with adrenal failure (and therefore low androgen levels) does not affect whole-body amino acid kinetics or skeletal muscle protein synthesis (28). Clearly, there are continuing controversies regarding the effect of DHEA administration on protein anabolism, which could be partly related to the doses used, potential sex effects, and duration of administration.
In the current study we investigated whether men, who inherently have higher levels of T and DHEAS than women (23,24,25), may have higher protein synthesis rates as well. We also sought to determine whether age-related changes in protein synthesis are related to changes in hormone levels. We hypothesized that long-term (1 yr) administration of low-dose T in elderly men and of DHEA in elderly individuals of both sexes would elevate skeletal muscle protein synthesis. The results showed some unexpected and novel findings regarding the effects of age, sex, and androgen administration.
MATERIALS AND METHODS
Study participants
After approval by the Mayo Clinic Institutional Review Board and receipt of written, informed consent, 62 young healthy individuals (30 men and 32 women), and 144 elderly individuals with low serum androgen concentrations (87 men and 57 women) participated in the study. Young men and women were between the ages of 18 and 31 yr, and elderly subjects were at least 60 yr of age. Inclusion criteria for elderly men were serum bioavailable T < 103 ng/dl (3.6 nM) and serum DHEAS < 1.57 μg/ml (4.3 μM) and for elderly women serum DHEAS <0.95 μg/ml (2.6 μM). These hormone concentrations were chosen as representing the 15th percentile for normal young individuals of the respective sex (29). Before studies were conducted, potential participants underwent a physical examination to determine whether they were in good health and screening laboratory tests to rule out any chronic medical conditions. All participants had a body mass index (BMI) between 20 and 34 kg/m2 and were not taking any hormone replacement therapy. Participants enrolled in the study were not taking part in vigorous exercise training programs, which were defined as exercising more than 45 min on 3 to 4 occasions per week.
Experimental design
In a randomized and double-blinded fashion, elderly men were assigned to one of three groups: 1) 75 mg/day DHEA in tablet form and transdermal placebo patch (n=29), 2) 5 mg/day T as transdermal patch and daily placebo tablet (n=26), or 3) daily placebo tablet and placebo transdermal patch (n=32); and elderly women were assigned to one of two groups: 1) 50 mg/day DHEA in tablet form (n=27), or 2) daily placebo tablet (n=30). DHEA doses were for full replacement of DHEAS to levels of young individuals. T doses were for full replacement of total T, which led to partial replacement of bioavailable T (30). Body composition was assessed by dual-energy X-ray absorptiometry (Lunar DPX-IQ densitometer; GE Healthcare, Chalfont St. Giles, UK) and thigh muscle area by computed tomography (30). With use of stable isotope tracer methodology, whole-body amino acid kinetics and skeletal muscle protein synthesis were measured at baseline and after 1 yr of treatment or placebo.
A weight-maintaining diet for the 3 days before the tracer infusion trials was individualized for each study participant, and macronutrient composition was made similar between individuals for carbohydrate (55%), lipid (30%), and protein (15%). On the day before tracer infusion trials, resting energy expenditure (REE) was measured using open-circuit indirect calorimetry, and these results have been reported separately (30). The arm vein catheter to be used for tracer infusion was placed on the evening before tracer infusion trials and was kept patent with 0.9% saline infusion. Participants stayed overnight in the General Clinical Research Center, and on the morning of tracer infusion trials, after collection of background blood samples, primed-continuous infusions of [15N]phenylalanine and [2H4]tyrosine were begun at 4:30 AM. Priming doses of [15N]phenylalanine (0.75 mg/kg), [15N]tyrosine (0.30 mg/kg), and [2H4]tyrosine (0.35 mg/kg) were given immediately before continuous infusion of [15N]phenylalanine (0.75 mg/kg/h) and [2H4]tyrosine (0.35 mg/kg/h).
Blood and muscle sampling
On the day of tracer infusion trials, arterialized blood samples were drawn from a retrograde intravenous catheter in a hand vein, with the hand placed in a hot box (55°C), from 7:30 AM to 12:30 PM for a total of 6 sampling time points for tracer analysis and were drawn at 11:30 AM for the endocrine measurements. In addition, blood was drawn at ∼4:30 AM immediately before administration of tracer priming boluses and commencement of tracer infusion to obtain background isotopic enrichments (IEs). Blood for free and total insulin-like growth factor-I (IGF-I), total IGF-II, IGF binding protein-1 (IGFBP-1), and IGFBP-3 was collected as serum. Blood for insulin was collected as plasma in tubes with EDTA (30). Blood for tracer analyses was collected as plasma in tubes containing heparin. Muscle biopsies of the vastus lateralis were performed at 7:30 AM and 12:30 PM on opposite legs.
Sample analyses
Free IGF-I, total IGF-I, IGF-II, IGFBP-1, and IGFBP-3 were determined in serum by RIA. Mixed muscle protein (MMP) and muscle tissue fluid were isolated from biopsy samples, and MMP was subsequently hydrolyzed to liberate the protein-bound amino acids for subsequent IE analysis as described previously (2). IEs of [15N]phenylalanine, [15N]tyrosine, and [2H4]tyrosine were measured in plasma as their tertiary butyl dimethylsilyl ester derivatives by gas chromatography (GC)/mass spectrometry (MS) under electron impact (EI) ionization conditions. Tissue fluid [15N]phenylalanine was also measured by GC/MS under EI conditions. MMP [15N]phenylalanine IEs were measured by GC/combustion/isotope ratio mass spectrometry. Selected ion abundances were compared against external standard curves for calculation of IE.
Calculations
With use of a single-pool steady-state model, whole-body amino acid flux rates were calculated using the average IEs from the final 3 h of tracer infusion, a time at which isotopic steady state had been achieved. Thus, rate of appearance (Ra) was considered to be equal to rate of disappearance (Rd), with both representing the flux rate:
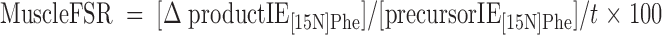 |
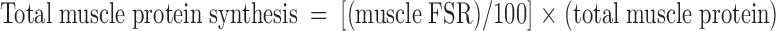 |
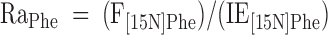 |
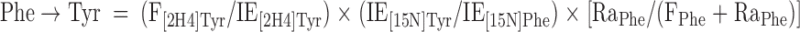 |
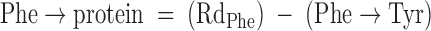 |
where FSR is fractional synthesis rate, Ra is rate of appearance, Rd is rate of disappearance, Phe → Tyr represents the conversion rate of phenylalanine to tyrosine (hydroxylation), Phe → protein represents the incorporation rate of phenylalanine into protein (whole-body protein synthesis), F represents tracer infusion rate, subscript notation represents identity of the amino acid or tracer, and [Δ product IE[15N]Phe] is the incremental increase in muscle protein-bound amino acid IE between the two sequential biopsies. The precursor IE[15N]Phe was the average from the plasma samples drawn over 5 h between the two biopsy time points, and secondarily, in repeated calculations was also represented by that measured in muscle tissue fluid. IEs were corrected for background enrichments in blood samples collected before tracer infusion and in these equations are in units of moles percent excess divided by 100 to express as a decimal. To calculate the total muscle protein synthesis rate, total muscle mass was calculated as appendicular muscle mass divided by 0.75 (31), and 20% of muscle was assumed to comprise protein (2). To calculate total body protein synthesis, we assumed that protein contains 295 μmol phenylalanine/g, which we based on the calculations of Matthews et al. (32) for leucine composition (590 μmol/g) and on reports that leucine Ra is ∼2 times higher than phenylalanine Ra at the level of the whole-body (1, 33, 34), leg (34), and splanchnic bed (34). Nonmuscle protein synthesis was calculated as the difference between whole-body and total muscle protein synthesis and was expressed per unit of nonmuscle FFM.
Statistical analyses
Data were transformed to improve normality for variables for which data were skewed. Results are presented as medians with interquartile ranges or means with ses. Sample sizes were justified previously (30). With use of unpaired t tests to test specific planned comparisons (three comparisons total), within sexes the change from baseline to 1 yr for treatment groups (DHEA for men and women and T for men) was compared with the change from baseline to 1 yr in the P group. In addition, sex and age effects on baseline data (preintervention) were analyzed, using two-way (sex×age) ANOVA followed by Tukey’s honestly significant difference (HSD) post hoc test; t tests and ANOVAs were performed using JMP 7.0 software (SAS, Inc., Cary, NC, USA). Values with a significant Pearson correlation coefficient (R) were included in a stepwise multiple regression analysis, stepping up, to which age category and sex were also added. To arrive at the final multiple regression model, subsequently two-way interactions were added to the model, and multiple regression analysis was continued, stepping down, obeying the hierarchical principle. Regressions were performed with SAS 9.1.3 software. Statistical significance was set at α = 0.05.
RESULTS
Characteristics of study participants
Selected characteristics of study participants are reported in Table 1, and these parameters were not affected by treatments, except that FFM significantly increased with T treatment in EM, as reported separately (30). Men had greater body weights than women (P<0.0001), but there was no significant sex difference in BMI. Elderly individuals had higher body weights than young individuals (P<0.01) as well as higher BMIs (P<0.0001). FFM was higher in men than in women (P<0.0001) and was lower in elderly than in young individuals (P<0.05). Peak O2 consumption (VO2peak) per unit of FFM, a marker of physical fitness, was significantly higher in men than in women (P<0.01) and was lower in elderly than in young individuals (P<0.0001).
TABLE 1.
Characteristics of study participants
| Characteristic | Young men | Young women | Elderly men | Elderly women |
|---|---|---|---|---|
| Age (yr)‡# | 23 (20–26) | 21 (20–23) | 66 (63–71) | 68 (65–73) |
| Height (cm)*§ | 179 (176–184) | 163 (162–166) | 176 (172–181) | 162 (159–165) |
| Weight (kg)*‖ | 80.8 (74.6–88.0) | 61.3 (57.8–72.9) | 85.2 (77.5–93.8) | 70.6 (59.9–79.3) |
| BMI (kg/m2)‡ | 25.1 (22.9–27.4) | 23.2 (22.2–26.1) | 27.6 (26.1–29.4) | 26.1 (24.5–29.9) |
| Body fat (%)*‡ | 29.0 (22.3–31.6) | 38.2 (35.7–41.3) | 33.1 (29.0–36.4) | 46.7 (43.0–51.0) |
| FFM (kg)*¶ | 58.7 (55.6–61.9) | 38.8 (36.9–41.8) | 56.8 (54.0–60.5) | 37.2 (34.4–39.4) |
| VO2peak (ml/kg FFM/min)†‡ | 58.2 (51.4–66.1) | 54.8 (51.4–58.7) | 41.2 (37.1–44.3) | 37.5 (33.1–41.6) |
Baseline characteristics, before chronic administration of hormones or placebo. Values are medians (interquartile ranges). Young men, n = 30; young women, n = 32; elderly men, n = 87, elderly women, n = 57. Main effect of sex:
P < 0.0001;
P < 0.01. Main effect of age:
P < 0.0001;
P < 0.001;
P < 0.01;
P < 0.05.
Sex × age interaction: elderly men significantly different from elderly women, P < 0.05. Statistical analysis by ANOVA with Tukey’s HSD post hoc test.
Hormones and binding proteins
Results for serum DHEAS, T, and E have been reported previously (30), but salient details are reiterated here for the convenience of the reader. DHEA supplementation was reported to significantly increase E and DHEAS in both sexes and to increase total T in elderly women. T administration in elderly men increased total and bioavailable T in circulation but did not increase E (30). Baseline IGF and related binding proteins (IGFBPs) results are reported in Table 2. There were main effects of age indicating age-related declines in free IGF-I (P<0.0001), total IGF-I (P<0.0001), IGF-II (P<0.01), and IGFBP-3 (P<0.0001). There was a main effect of sex for IGFBP-1, reflecting higher levels in women than men (P<0.0001). In addition, some sex × age interactions for IGFs and IGFBPs were noted and are described in Table 2. Although treatment with DHEA or T did not affect protein metabolism (described in Table 3 and text below) compared with the change in the P group, effects on IGF hormones were noted (Table 3). Compared with changes in the elderly women P group, DHEA administration led to a relative decline of serum IGF-II in elderly women by ∼10% (P<0.05) and T administration in elderly men led to a relative elevation in free IGF-I by ∼45% (P<0.05) (Table 3). Compared with the changes in P groups over 1 yr, there were no significant effects of DHEA in elderly men and women and of T in elderly men on IGFBPs (data not shown).
TABLE 2.
IGF hormones and binding proteins
| Characteristic | Young men | Young women | Elderly men | Elderly women |
|---|---|---|---|---|
| Free IGF-I (ng/ml)†§ | 1.6 (1.2–2.3)123 | 1.0 (0.7–2.3)23 | 0.7 (0.5–1.1) | 0.9 (0.6–1.1) |
| Total IGF-I (ng/ml)† | 335 (255–429) | 321 (264–425) | 167 (120–213) | 115 (85–168) |
| Total IGF-II (ng/ml)‡§ | 714 (592–919)2 | 627 (509–885)2 | 567 (478–655)3 | 666 (522–795) |
| IGFBP-1 (ng/ml)*§ | 18 (11–25)123 | 30 (21–67) | 30 (20–38) | 37 (24–49) |
| IGFBP-3 (ng/ml)† | 4425 (3574–4963) | 4129 (3511–4872) | 3071 (2582–3562) | 3444 (2832–4093) |
Baseline IGF and IGFBP concentrations in serum before chronic administration of hormones or placebo. Values are medians (interquartile ranges). Young men, n = 30; young women, n = 32; elderly men, n = 87, elderly women, n = 57. Main effect of sex:
P < 0.0001. Main effect of age:
P < 0.0001;
P < 0.01. Sex × age interaction:
P < 0.05 vs.
young women,
elderly men,
elderly women. Statistical analysis by ANOVA with Tukey’s HSD post hoc test.
TABLE 3.
Change from baseline to 1 year in placebo and treatment groups
| Characteristic
|
Elderly women
|
Elderly men
|
|||
|---|---|---|---|---|---|
| Placebo | DHEA | Placebo | DHEA | Testosterone | |
| Phe Ra (μmol/kg FFM/h) | −2.0 (−3.7 to 0.9) | −2.0 (−2.8 to 1.2) | −0.7 (−3.3 to 1.2) | −0.3 (−2.8 to 2.0) | −0.9 (−3.0 to 1.1) |
| Phe → Tyr (μmol/kg FFM/h) | −0.6 (−0.9 to 0.3) | −0.5 (−1.2 to −0.1) | −0.8 (−1.0 to 0.2) | −0.3 (−1.2 to 0.1) | −0.7 (−1.3 to 0.0) |
| Phe → protein (μmol/kg FFM/h) | −0.8 (−4.7 to 0.4) | −0.9 (−3.6 to 2.8) | −0.1 (−2.7 to 2.2) | 0.6 (−2.5 to 2.2) | −0.8 (−3.1 to 1.3) |
| Muscle protein FSR (%/h) | 0.0017 (−0.0056 to 0.0064) | 0.0006 (−0.0089 to 0.0127) | 0.0035 (0.0000 to 0.0092) | 0.0027 (−0.0036 to 0.0073) | 0.0006 (−0.0032 to 0.0042) |
| Free IGF-I (ng/ml) | 0.20 (−0.30 to 0.50) | −0.05 (−0.38 to 0.28) | −0.10 (−0.55 to 0.20) | −0.10 (−0.45 to 0.35) | 0.20 (−0.10 to 0.65)* |
| Total IGF-I (ng/ml) | 2 (−21 to 24) | 22 (−2 to 51) | −1 (−47 to 23) | 0 (−32 to 18) | 23 (−17 to 53) |
| Total IGF-II (ng/ml) | 93 (21 to 185) | 49 (−81 to 148)* | 123 (34 to 193) | 92 (−5 to 184) | 49 (−28 to 125) |
Difference between repeated measurements made at baseline and 1 yr. Values are medians (interquartile ranges). Elderly women: placebo, n = 30; DHEA, n = 27. Elderly men: placebo, n = 32; DHEA, n = 29; testosterone, n = 26.
P < 0.05 vs. corresponding placebo group. Statistical analysis by unpaired t test for DHEA vs. placebo in elderly women and for DHEA vs. placebo and testosterone vs. placebo in elderly men.
Whole-body amino acid kinetics
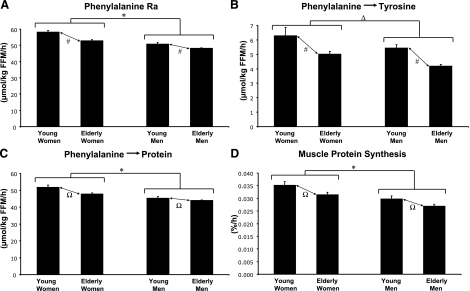
We assumed whole-body FFM to be proportional to total body protein content, and so amino acid kinetics were expressed per unit of FFM. Whole-body proteolysis, represented by phenylalanine Ra (Fig. 1A), was significantly higher in women than in men (P<0.0001) and significantly higher in young than elderly individuals (P<0.0001). These sex and age effects for phenylalanine Ra were also present for each mode of phenylalanine disposal, both conversion to tyrosine (sex and age, P<0.0001) (Fig. 1B) and disposal via whole-body protein synthesis (sex, P<0.0001; age, P<0.001) (Fig. 1C). There were no significant sex × age interactions for these parameters of whole-body amino acid kinetics, and, thus, the sex and age effects reported above were main effects. Compared with the change in P groups over 1 yr, treatment with DHEA in elderly men and women and with T in elderly men did not have any significant impact on whole-body amino acid kinetics (Table 3).
Figure 1.
Baseline protein metabolism before chronic administration of hormones or placebo. A) Phenylalanine Ra. B) Conversion of phenylalanine to tyrosine. C) Disposal of phenylalanine via protein synthesis. D) skeletal muscle protein fractional synthesis rate. Values are means ± se. Young men, n = 30; young women, n = 32; elderly men, n = 87, elderly women, n = 57. Main effect of sex: *P < 0.0001; ΔP < 0.001. Main effect of age: #P < 0.0001; ΩP < 0.001. No significant sex × age interactions. Statistical analysis by ANOVA with Tukey’s HSD post hoc test.
To confirm the validity of expressing data per unit of FFM, we repeated the analysis of sex and age effects on Phe → protein with fat mass as a covariate. In this analysis of covariance (ANCOVA), there were significant effects of sex (P<0.0001) and age (P<0.0001), just as was described in the ANOVA results reported above. This consistency between analyses confirmed that the apparent sex and age effects were not due to an underestimation of the whole-body protein pool size by using FFM as its marker. Also, the ANCOVA revealed a main effect of fat mass (P<0.0001), which did not interact with sex and age effects.
Skeletal muscle protein synthesis
We focused on the muscle protein synthesis rates calculated from the plasma precursor pool. Although a tissue fluid precursor (also reported in the text below) represents a closer IE to aminoacyl tRNA (35), use of the plasma precursor in calculations allows for whole-body and muscle protein synthesis to be calculated from a common data set, which would provide for more appropriate integration of the two results for comparing the whole-body and muscle rates. Furthermore, an advantage of using the plasma precursor is that a 5-h weighted precursor enrichment (6 samples, drawn hourly) can be used as the precursor rather than the average of only two tissue fluid samples. Skeletal muscle FSR in the vastus lateralis (Fig. 1D) was significantly higher in women than in men (P<0.0001) and in young than in elderly individuals (P<0.001). Aside from these main effects of sex and age, there was no significant sex × age interaction. Compared with changes in P groups over 1 yr, treatment with DHEA in elderly men and women and treatment with T in elderly men did not have any significant impact on muscle protein FSR (Table 3). In repeating the calculation of FSR with the tissue fluid precursor, similar results were obtained, demonstrating a lack of effect of DHEA and T treatment (data not shown). Furthermore, with use of tissue fluid precursor, women still displayed significantly higher FSR than men (P<0.01), and there was still a trend for higher FSR in young than in elderly individuals. This age effect approached but did not reach statistical significance (P=0.1).
Nonmuscle protein synthesis
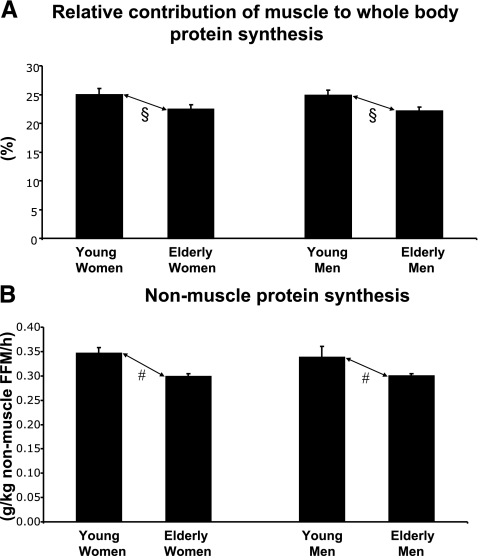
We estimated the percent contribution that skeletal muscle made to whole-body protein synthesis (Fig. 2A), under the assumption that the vastus lateralis is a representative sample of the whole-body pool of muscle. The relative contribution of skeletal muscle to whole-body protein synthesis declined significantly from young to old age (P<0.005), although no significant sex effect or sex × age interaction existed. With use of the difference between total FFM and estimated muscle mass and the difference between whole-body and muscle protein synthesis, nonmuscle protein synthesis was estimated (Fig. 2B). Nonmuscle protein synthesis declined from young to old age (P<0.0001), whereas no significant sex effect or sex × age interaction existed.
Figure 2.
Baseline protein metabolism before chronic administration of hormones or placebo. Relative contribution of muscle to whole-body protein synthesis (A) and nonmuscle protein synthesis (B). Values are means ± se. Young men, n = 30; young women, n = 32; elderly men, n = 87, elderly women, n = 57. Main effect of age: #P < 0.0001; §P < 0.005. No significant main effect of sex or sex × age interactions. Statistical analysis by ANOVA with Tukey’s HSD post hoc test.
Regression analyses
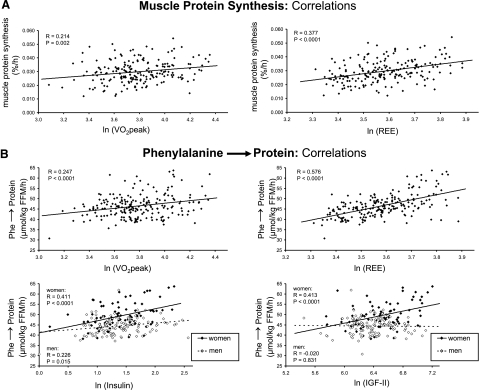
Analyses were performed for each of three dependent variables: phenylalanine Ra, Phe → protein, and skeletal muscle FSR. VO2peak, strength, REE, free IGF-I, total IGF-I, IGF-II, IGFBP-1, IGFBP-3, fasting plasma insulin, and SI* (an index of insulin action; ref. 36) were included in regression analyses. IGF and IGFBP data have not been reported previously, but other parameters included in the analyses were reported previously (30, 37). Various significant univariate correlations existed (Table 4), although not all relationships remained significant in multivariate analyses (Table 5). To exemplify the relationships, univariate correlations are shown in Fig. 3A for muscle FSR and in Fig. 3B for Phe → protein for relationships that remained significant in the multiple regression analysis. To conserve space, correlations for phenylalanine Ra are not shown, but the data appear in Table 5. In the multiple regression analysis (Table 5), when controlling for the other independent variables, age was no longer significantly associated with muscle protein synthesis or whole- body amino acid kinetics. That is, although we report an association of age with protein metabolism (Fig. 1), age appears to act indirectly through other factors in the model (VO2peak and REE). However, sex remained significantly associated with muscle protein synthesis, even when controlling for the physiological and endocrine factors in the model. Sex interacted with insulin and IGF-II in the relationship with whole-body protein synthesis (Table 5), and therefore for these, each sex is plotted separately in Fig. 3B. Sex had a significant impact on whole-body protein synthesis for which the magnitude of the sex effect depended on the level of insulin and especially IGF-II (Fig. 3B). The REE × insulin interaction for phenylalanine Ra was investigated by dividing subjects into two groups (those with lower REE and those with higher REE) based on splitting at the median value for REE, and the interaction was quite subtle with a slightly steeper slope for the relationship between Phe → protein and insulin in those with lower REE than those with higher REE. Although the above relationships between fasting insulin concentration and protein metabolism existed, SI* was not significantly related to phenylalanine Ra, Phe → protein, or skeletal muscle FSR (Table 4). The sex × IGF-II interaction was actually qualitative in nature in that the directions of the relationships between the independent and dependent variables were not similar between sexes; there was a significant positive correlation in women between Phe → protein and IGF-II (P<0.0001) but no significant correlation in men (P=0.83) (Fig. 3B).
TABLE 4.
Univariate regression analyses
| Characteristic | Phe Ra
|
Phe → protein
|
Muscle protein FSR
|
|||
|---|---|---|---|---|---|---|
| P value | Pearson’s R | P value | Pearson’s R | P value | Pearson’s R | |
| VO2peaka | <0.0001 | 0.326 | <0.0001 | 0.274 | 0.0024 | 0.214 |
| Strength | 0.0282 | 0.154 | 0.0919 | 0.118 | 0.1038 | 0.116 |
| REEa | <0.0001 | 0.658 | <0.0001 | 0.576 | <0.0001 | 0.377 |
| Free IGF-Ia | 0.7978 | 0.018 | 0.7982 | 0.018 | 0.3062 | 0.073 |
| Total IGF-Ia | 0.0079 | 0.187 | 0.0254 | 0.158 | 0.4464 | 0.055 |
| IGF-IIa | 0.0029 | 0.210 | <0.001 | 0.255 | 0.9045 | 0.009 |
| IGFBP-1b | 0.6787 | 0.029 | 0.2760 | 0.077 | 0.2027 | −0.091 |
| IGFBP-3 | 0.0031 | 0.208 | <0.001 | 0.235 | 0.6376 | 0.034 |
| Insulina | <0.0001 | 0.302 | <0.0001 | 0.290 | 0.0633 | 0.133 |
| SI* a | 0.4291 | −0.057 | 0.4373 | −0.056 | 0.4573 | 0.055 |
Baseline data, before chronic administration of hormones or placebo. Young men, n = 30; young women, n = 32; elderly men, n = 87, elderly women, n = 57. VO2peak, REE, and SI* are expressed per unit of FFM, and strength is expressed per leg FFM. Hormones and binding proteins were expressed as concentrations in serum or plasma. Italic indicates P < 0.05. Statistical analysis by univariate regression.
Logarithmic transformation (ln).
Square root transformation of data.
TABLE 5.
Multivariate regression analyses
| Characteristic
|
Phe Ra
|
Phe → protein
|
Muscle protein FSR
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter estimate | P value | Partial R | Parameter estimate | P value | Partial R | Parameter estimate | P value | Partial R | |
| VO2peak | 6.3647 | <0.001 | 0.342 | 4.7157 | <0.001 | 0.261 | 0.006776 | 0.003 | 0.216 |
| REE | 20.337 | <0.001 | 0.402 | 34.903 | 0.002 | 0.228 | 0.013854 | 0.016 | 0.174 |
| Sexa | −2.8071 | 0.199 | −0.093 | −47.199 | <0.001 | −0.270 | 0.002885 | 0.048 | 0.143 |
| Insulin | 1.5527 | 0.106 | 0.328 | 53.630 | 0.036 | 0.157 | |||
| Sex × insulin | 3.7715 | 0.010 | 0.186 | 4.7073 | 0.017 | 0.173 | |||
| REE × insulin | −14.807 | 0.042 | −0.147 | ||||||
| IGF-II | −1.2063 | 0.365 | −0.277 | ||||||
| Sex × IGF-II | 6.6832 | <0.001 | 0.247 | ||||||
Baseline data before chronic administration of hormones or placebo. Young men, n = 30; young women, n = 32; elderly men, n = 87, elderly women, n = 57. VO2peak and REE were expressed per unit of FFM. Hormones and binding proteins were expressed as concentrations in serum or plasma. P and R values are omitted for independent variables not significant in the multivariate model unless the independent variable participated in a subsequent interaction.
Model set women to 1 and men to 0; positive parameter estimate indicates women > men. Skewed data were transformed as noted in Table 4. Partial R represents the partial correlation coefficient derived from the multiple regression model. Statistical analysis by stepwise multiple regression.
Figure 3.
Baseline protein metabolism before chronic administration of hormones or placebo. Univariate regressions of mixed muscle protein fractional synthesis rate with variables that were significant in the multiple regression analysis (A) and of whole-body protein synthesis and variables that were significant in the multiple regression analysis (B). Young men, n = 30; young women, n = 32; elderly men, n = 87, elderly women, n = 57. FSR using plasma as precursor pool; Phe → protein, phenylalanine incorporation into protein (whole-body protein synthesis); VO2peak, peak O2 consumption. R represents Pearson product-moment correlation coefficient. Statistical analysis by linear regression.
DISCUSSION
We provide data on whole-body amino acid kinetics and skeletal muscle protein synthesis in young and elderly men and women. The results confirm previous findings of a difference between young and elderly individuals for muscle and whole-body protein metabolism, and it has become apparent that the effect may be due to age-related changes in metabolic rate and physical fitness. Furthermore, we present results of androgen replacement in the elderly individuals, demonstrating that there was no effect of low-dose T in men and no effect of DHEA in either men or women. In the current study, an unexpected sex difference in protein metabolism was revealed. These findings strongly suggest that factors aside from the circulating concentrations of the androgens DHEA and T have substantial effects on protein metabolism. Furthermore, muscle and whole-body protein synthesis were positively associated with VO2peak, REE, and possibly unidentified factors intrinsic to the female sex, and at the whole-body level sex-specific associations of protein synthesis with insulin and IGF-II were noted.
Most investigative teams studying the issue have found no differences between men and women for muscle protein synthesis, based on relatively small sample sizes per subject grouping (1, 2, 38,39,40,41), although intuition could still suggest that men would have higher muscle protein synthesis than women on the basis of having more total muscle mass and higher concentrations of T in serum. However, it was recently reported that obese elderly women display higher muscle protein synthesis than obese elderly men (42), showing that this counterintuitive sex difference can be present at least in certain populations. Our present results extend the recent findings of a sex difference in elderly obese individuals (42) to include normal, healthy individuals of both young and old age, and we report these data within the context of simultaneously determined whole-body amino acid kinetics showing similar sex-related differences for whole-body proteolysis and protein synthesis. These results demonstrate that women, irrespective of their BMI and age, have higher FSR of muscle proteins and higher whole-body protein turnover than men. This sex difference in muscle protein synthesis persists into old age, at which point women would have undergone menopause, and, thus, ovarian hormones do not appear to be the source of the sex-based difference in protein metabolism. Furthermore, as the sex difference is not significant in nonmuscle tissue (Fig. 2B) and because women have elevated muscle protein FSR, we conclude that differences between men and women are based primarily within skeletal muscle. The sex-based difference in muscle FSR would be paradoxical if one were to consider the sole function of protein synthesis to be that of determining muscle size, considering that women have a smaller muscle mass. Therefore, as was the case at the whole-body level, women probably had higher proteolysis in muscle compared with men such that continuous net muscle anabolism would not occur. In addition, as the FSR measurement in the present study was that of mixed muscle protein, proteins contributing to the measurement would include metabolic enzymes not adding greatly to the total muscle mass but yet still important for function. The higher muscle protein turnover in women probably represents a greater rate of replacing old proteins with newer proteins, which could protect against the effects of aging on skeletal muscle over the lifespan, although this remains to be determined.
We report a significant difference between young and old individuals of both sexes for muscle protein synthesis rates, consistent with previous reports (1,2,3,4,5,6,7). Although the differences between age groups for muscle (and whole body) protein synthesis are subtle in the postabsorptive state, we believe that lower rates of synthesis may lead to poorer tissue quality due to slower remodeling. In addition to lower protein synthesis rates, we also found elderly individuals to have lower rates of whole-body proteolysis, a finding that is consistent with the view of slower tissue remodeling. Although various research groups have reported the age-related decline in muscle protein synthesis (1,2,3,4,5,6,7), not all reports have presented significant age effects (43,44,45,46). As we consider the age-related decline in protein synthesis reported here and elsewhere (1,2,3,4,5,6,7) to potentially make an important contribution to age-related decrements in well-being, it is of interest to consider why different research teams attained different results. The discrepancies could be due to statistical power differences between studies based on subject sample sizes, control of prestudy dietary habits, precision of different analytical approaches to measuring tracer IE, or cultural differences between different geographical regions that affect the magnitude of age-related changes. Although all of these issues may play a role, of utmost importance may be the fact that weight-maintaining standardized diets were administered for ≥3 days before experimentation in studies finding an age effect (1,2,3,4,5,6,7), whereas this practice was not used in those reporting no significant effect (43,44,45,46).
Considering the different measurements in this study, that of skeletal muscle FSR is especially strong, not being related to any estimate of FFM but rather being independent of body composition and expressed as a fractional synthesis rate of protein in the biopsy samples. For example, it is possible that FFM and muscle protein mass could be overestimated in elderly individuals (47), perhaps due to potential elevation of hydration status and lipid content of muscle, which would affect our estimate of total protein synthesis rates but would not affect the muscle FSR results. However, even so, the results for whole-body protein synthesis per FFM show a pattern similar to that of the muscle FSR data, which may further enhance one’s level of confidence in the whole-body data. Furthermore, our present results indicate that the synthesis rate of nonmuscle tissue also declines with age and, thus, there seems to be a general decrease in the anabolic character of the environment within the body during the aging process that affects a variety of tissues. Despite possibilities for subtle differences between groups for tissue densities, we consider FFM to be a reasonable marker of whole-body protein content, and so we expressed whole-body protein turnover per FFM. Even when fat mass was used as a covariate in the analysis of whole-body protein synthesis per FFM, there was no interaction of fat mass with the sex and age differences that were significant whether or not the fat mass was considered. Therefore, it does not appear that the conclusions drawn from whole-body amino acid kinetics are artifacts of our use of FFM as a marker of the whole-body protein content. Moreover, because there was a significant association of fat mass with protein synthesis within groups, future studies of protein turnover in adipose tissue and effects of adipose tissue mass on protein turnover in the lean tissue may be of interest.
In agreement with previous reports (27, 28, 48), the present results demonstrate that DHEAS is not a regulator of protein synthesis in vivo. The dose of DHEA used in this study raised the low DHEAS levels of the elderly individuals to the high end of the range seen in young individuals (30). The lack of any effect on muscle and whole-body protein synthesis suggests that declining DHEAS is not the reason for the age-related decline in protein synthesis. Unlike the substantial dose of DHEA used in this study, the T dose was modest. We chose this dose to test a dose that could be used long term therapeutically, with minimal risk, and as previously reported, participants tolerated these doses for 2 yr without significant deleterious effects (30). In studies that have shown an effect of T administration on muscle protein synthesis (11,12,13, 20), a higher dose administered via injection and over a shorter duration was used. Therefore, in this study, administration of a lower T dose via a patch, which would cause a more mild and steady release than injections of larger doses, along with the longer term administration that may lead to habituation, may explain why the partial replacement of bioavailable T led to no anabolic response in the vastus lateralis muscle. It is particularly likely that DHEA simply has no effect on protein synthesis in humans and that the dose of T chosen in this study was below a threshold at which its effects on protein synthesis in axial muscles become apparent. Nonetheless, this dose of T was enough to raise total T to levels seen in young individuals and to raise bioavailable T by ∼50%, which would be a partial restoration toward levels of young individuals. This dose of T did have some physiological effects, as it did raise total FFM slightly (but not leg FFM or thigh muscle area) and it did slightly improve bone mineral density (30). Thus, it is not that the T dose could not be sensed physiologically but simply that it did not impact vastus lateralis protein synthesis or whole-body amino acid kinetics. The results lead one to consider the possibility that factors aside from DHEA and T are dominant in modulating protein metabolism in vivo. Furthermore, as mentioned above, the fact that the sex difference persists after menopause suggests no involvement of ovarian hormones, and so we excluded DHEAS, T, and E from regression analyses. Factors related to energy metabolism (1), hormones and binding proteins of the IGF system (49), and insulin (35) are all known to regulate muscle protein turnover, and so we included such factors in analyses to determine which may explain some of the interindividual variation in skeletal muscle and whole-body protein synthesis and whole-body rates of proteolysis.
The multiple regression analyses allowed us to sift through the significant correlations from univariate analyses (Table 4) and determine which variables had an independent association with protein metabolism, rather than acting through other variables considered in the model. The multiple regression analyses indicated that VO2peak and REE act independently of one another in their associations with whole-body and muscle protein metabolism. The association between VO2peak and muscle mitochondrial enzyme activity is known to be strong (50), and, thus, VO2peak may partly represent muscle oxidative capacity in the analysis, in addition to other factors probably associated with VO2peak such as maximal muscle perfusion rate and physical activity level. Furthermore, a novel and unexpected sexual dimorphism in the responsiveness to IGF-II may have been discovered as IGF-II was significantly correlated with whole-body protein synthesis in women but not in men. As there was no significant relationship between muscle protein FSR and IGF-II, the association appears to be between the turnover rate of nonmuscle tissue and IGF-II in women. At present we are unable to explain this association with IGF-II, but future studies regarding sexual dimorphism in the tissue responsiveness to IGF-II appear to be warranted. On the other hand, the interaction between sex and insulin appears to be far more subtle, with a positive correlation between insulin and whole-body protein synthesis in both sexes. Although we chose a priori to use muscle insulin action in the regression analyses rather than whole-body insulin sensitivity, it is possible that lower insulin sensitivity, rather than elevated insulin concentration, was the factor underlying the association between insulin and whole-body protein turnover, as elevated fasting insulin typically indicates insulin resistance.
An important question in the multiple regression analyses was whether age and sex would remain as significant predictors of rates of protein metabolism even when controlling for physical fitness, energy turnover, hormones, and hormone binding proteins. Age per se was not associated with muscle protein synthesis or whole-body proteolysis and protein synthesis after consideration of these other variables. This finding suggests that the age-related decline in protein metabolism may be due to changes in physical fitness (VO2peak) and metabolic rate (REE). Importantly, the age-related decline may be at least partially treatable by lifestyle intervention, as exercise training can increase VO2peak and muscle protein FSR (1). Unlike the case for the apparent age effect, the effect of sex per se persisted even after consideration of the other variables in the regression model for muscle protein synthesis, and effects of sex persisted for whole-body protein synthesis as well, depending on insulin and IGF-II. The higher rates of muscle and whole-body protein synthesis in women portray sex as a fundamental biological determinant of protein metabolism.
In conclusion, the current study confirmed association of age with protein turnover rates, but neither the declining rates of whole-body protein turnover nor skeletal muscle protein synthesis were amended by 1-yr administration of DHEA in men and women or low-dose T in elderly men, and, thus, the relationship between changes in androgens and protein metabolism during aging does not appear to be causal. Although it is likely that significantly higher doses of T would alter protein metabolism, which would have implications for the treatment of sarcopenia, the long-term safety and the sustainability of efficacy over years in duration at higher doses will need to be determined if this treatment path is further investigated. In addition, we discovered higher rates of whole-body protein turnover and skeletal muscle protein synthesis in women than in men, at both young and old age, which represents a novel and important biological determinant of protein metabolism in vivo. At present, we propose that factors related to age and sex, aside from androgens and ovarian hormones, may be of supreme importance for tuning rates of protein synthesis and degradation in healthy individuals.
Acknowledgments
We thank the study participants for their time and compliance with the protocol. We also thank Traci Hammer for coordinating studies and Barbara Norby, Jean Feehan, and Laurie Wahlstrom for nursing support. We thank Peggy Helwig, Dawn Morse, and members of the Chemical Core Laboratory for technical assistance, Scott Przybelski for statistical support, and members of the General Clinical Research Center nursing and dietetic staff. This work was supported by National Institute of Health grants P01 AG14283, R01 AG09531, and UL1-RR-024150-01. The authors had no conflicts of interest.
References
- Short K R, Vittone J L, Bigelow M L, Proctor D N, Nair K S. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers O E, Adey D B, Ades P A, Nair K S. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol Endocrinol Metab. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Jozefowicz R, Statt M. Myofibrillar protein synthesis in young and old men. Am J Physiol Endocrinol Metab. 1993;264:E693–E698. doi: 10.1152/ajpendo.1993.264.5.E693. [DOI] [PubMed] [Google Scholar]
- Welle S, Thornton C, Statt M. Myofibrillar protein synthesis in young and old human subjects after three months of resistance training. Am J Physiol Endocrinol Metab. 1995;268:E422–E427. doi: 10.1152/ajpendo.1995.268.3.E422. [DOI] [PubMed] [Google Scholar]
- Hasten D L, Pak-Loduka J, Obert K A, Yarasheski K E. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- Yarasheski K E, Zachwieja J J, Bier D M. Acute effects of resistance exercise on muscle protein synthesis rate in young and elderly men and women. Am J Physiol Endocrinol Metab. 1993;265:E210–E214. doi: 10.1152/ajpendo.1993.265.2.E210. [DOI] [PubMed] [Google Scholar]
- Guillet C, Prod'homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. FASEB J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- Forbes G B. The effect of anabolic steroids on lean body mass: the dose response curve. Metabolism. 1985;34:571–573. doi: 10.1016/0026-0495(85)90196-9. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh A B, Bhasin D, Berman N, Chen X, Yarasheski K E, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer T W. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh A B, Mac R P, Lee M, Yarasheski K E, Sinha-Hikim I, Dzekov C, Dzekov J, Magliano L, Storer T W. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- Brodsky I G, Balagopal P, Nair K S. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- Griggs R C, Kingston W, Jozefowicz R F, Herr B E, Forbes G, Halliday D. Effect of testosterone on muscle mass and muscle protein synthesis. J Appl Physiol. 1989;66:498–503. doi: 10.1152/jappl.1989.66.1.498. [DOI] [PubMed] [Google Scholar]
- Ferrando A A, Tipton K D, Doyle D, Phillips S M, Cortiella J, Wolfe R R. Testosterone injection stimulates net protein synthesis but not tissue amino acid transport. Am J Physiol Endocrinol Metab. 1998;275:E864–E871. doi: 10.1152/ajpendo.1998.275.5.E864. [DOI] [PubMed] [Google Scholar]
- Ferrini R L, Barrett-Connor E. Sex hormones and age: A cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. doi: 10.1093/oxfordjournals.aje.a009519. [DOI] [PubMed] [Google Scholar]
- Pirke K M, Doerr P. Age related changes in interrelationships between plasma testosterone, oestradiol and testosterone-binding globulin in normal adult males. Acta Endocrinol. 1973;74:792–800. doi: 10.1530/acta.0.0740792. [DOI] [PubMed] [Google Scholar]
- Harman S M, Metter E J, Tobin J D, Pearson J, Blackman M R. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Snyder P J, Peachey H, Hannoush P, Berlin J A, Loh L, Lenrow D A, Holmes J H, Dlewati A, Santanna J, Rosen C J, Strom B L. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- Ferrando A A, Sheffield-Moore M, Paddon-Jones D, Wolfe R R, Urban R J. Differential anabolic effects of testosterone and amino acid feeding in older men. J Clin Endocrinol Metab. 2003;88:358–362. doi: 10.1210/jc.2002-021041. [DOI] [PubMed] [Google Scholar]
- Giannoulis M G, Jackson N, Shojaee-Moradie F, Nair K S, Sonksen P H, Martin F, Umpleby A M. The effects of growth hormone and/or testosterone on whole body protein kinetics and skeletal muscle gene expression in healthy elderly men: A randomized controlled trial. J Clin Endocrinol Metab. 2008;93:3066–3074. doi: 10.1210/jc.2007-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban R J, Bodenburg Y H, Gilkison C, Foxworth J, Coggan A R, Wolfe R R, Ferrando A A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am J Physiol Endocrinol Metab. 1995;269:E820–E826. doi: 10.1152/ajpendo.1995.269.5.E820. [DOI] [PubMed] [Google Scholar]
- Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy Executive summary. Liverman CT, Blazer DG, editors. Washington, DC, USA: National Academy Press; Testosterone and AgingClinical Research Directions. 2004:1–10. [Google Scholar]
- Prestwood K M, Kenny A M, Kleppinger A, Kulldorff M. Ultralow-dose micronized 17β-estradiol and done density and bone metabolism in older women: a randomized controlled trial. JAMA. 2003;290:1042–1048. doi: 10.1001/jama.290.8.1042. [DOI] [PubMed] [Google Scholar]
- Dhatariya K, Nair K S. Dehydroepiandrosterone: is there a role for replacement? Mayo Clin Proc. 2003;78:1257–1273. doi: 10.4065/78.10.1257. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind J L, Rizer R L, Vogelman J H. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Carlström K, Brody S, Lunell N-O, Lagrelius A, Möllerström G, Pousette A, Rannevik G, Stege R, von Schoultz B. Dehydroepiandrosterone sulfate and dehydroepiandrosterone in serum: differences related to age and sex. Maturitas. 1988;10:297–306. doi: 10.1016/0378-5122(88)90065-5. [DOI] [PubMed] [Google Scholar]
- Yen S S, Morales A J, Khorram O. Replacement of DHEA in aging men and women: potential remedial effects. Ann N Y Acad Sci. 1995;774:128–142. doi: 10.1111/j.1749-6632.1995.tb17377.x. [DOI] [PubMed] [Google Scholar]
- Welle S, Jozefowicz R, Statt M. Failure of dehydroepiandrosterone to influence energy and protein metabolism in humans. J Clin Endocrinol Metab. 1990;71:1259–1264. doi: 10.1210/jcem-71-5-1259. [DOI] [PubMed] [Google Scholar]
- Christiansen J J, Gravholt C H, Fisker S, Møller N, Andersen M, Svenstrup B, Bennett P, Ivarsen P, Christiansen J S, Jørgensen J O L. Very short term dehydroepiandrosterone treatment in female adrenal failure: impact on carbohydrate, lipid, and protein metabolism. Eur J Endocrinol. 2005;152:77–85. doi: 10.1530/eje.1.01810. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton L J, Atkinson E J, O'Fallon W M, Klee G G, Riggs B L. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- Nair K S, Rizza R A, O'Brien P C, Dhatariya K, Short K R, Nehra A, Vittone J L, Klee G G, Basu A, Basu R, Cobelli C, Toffolo G, Man C D, Tindall D, Melton L J, Smith G E, Khosla S, Jensen M D. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355:1647–1659. doi: 10.1056/NEJMoa054629. [DOI] [PubMed] [Google Scholar]
- Heymsfield S B, Smith R, Aulet M, Bensen B, Lichtman S, Wang J, Pierson R N J. Appendicular skeletal muscle mass: measurement by dual-photon absorptiometry. Am J Clin Nutr. 1990;52:214–218. doi: 10.1093/ajcn/52.2.214. [DOI] [PubMed] [Google Scholar]
- Matthews D E, Motil K J, Rohrbaugh D K, Burke J F, Young V R, Bier D M. Measurement of leucine metabolism in man from a primed, continuous infusion of l-[1-13C]leucine. Am J Physiol Endocrinol Metab. 1980;238:E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- Meek S E, Persson M, Ford G C, Nair K S. Differential regulation of amino acid exchange and protein dynamics across the splanchnic and skeletal muscle beds by insulin in healthy human subjects. Diabetes. 1988;47:1824–1835. doi: 10.2337/diabetes.47.12.1824. [DOI] [PubMed] [Google Scholar]
- Nair K S, Ford G C, Ekberg K, Fernqvist-Forbes E, Wahren J. Protein dynamics in whole body and in splanchnic and leg tissues in type 1 diabetes patients. J Clin Invest. 1995;95:2926–2937. doi: 10.1172/JCI118000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L S, Albright R C, Bigelow M L, Toffolo G, Cobelli C, Nair K S. Mechanism of insulin’s anabolic effect on muscle: measurements of muscle protein synthesis and breakdown using aminoacyl-tRNA and other surrogate measures. Am J Physiol Endocrinol Metab. 2006;291:E729–E736. doi: 10.1152/ajpendo.00003.2006. [DOI] [PubMed] [Google Scholar]
- Dalla Man C, Yarasheski K E, Caumo A, Robertson H, Toffolo G, Polonsky K S, Cobelli C. Insulin sensitivity by oral glucose minimal models: validation against clamp. Am J Physiol Endocrinol Metab. 2005;289:E954–E959. doi: 10.1152/ajpendo.00076.2005. [DOI] [PubMed] [Google Scholar]
- Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza R A. Effects of age and sex on postprandial glucose metabolism. Diabetes. 2006;55:2001–2014. doi: 10.2337/db05-1692. [DOI] [PubMed] [Google Scholar]
- Fujita S, Rasmussen B B, Bell J A, Cadenas J G, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Am J Physiol Endocrinol Metab. 2007;292:E77–E83. doi: 10.1152/ajpendo.00173.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn L A, Barrett E J, Genco M L, Wei L, Spraggins T A, Fryburg D A. Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. 1999;84:1007–1010. doi: 10.1210/jcem.84.3.5522. [DOI] [PubMed] [Google Scholar]
- Parise G, MacLennan D, Yarasheski K E, Tarnopolsky M A. Effects of acute creatine monohydrate supplementation on leucine kinetics and mixed-muscle protein synthesis. J Appl Physiol. 2001;91:1041–1047. doi: 10.1152/jappl.2001.91.3.1041. [DOI] [PubMed] [Google Scholar]
- Yarasheski K E, Pak-Loduka J, Hasten D L, Obert K A, Brown M B, Sinacore D R. Resistance exercise training increases mixed muscle protein synthesis rate in frail women and men >76 yr old. Am J Physiol Endocrinol Metab. 1999;277:E118–E125. doi: 10.1152/ajpendo.1999.277.1.E118. [DOI] [PubMed] [Google Scholar]
- Smith G I, Atherton P, Villareal D T, Frimel T N, Rankin D, Rennie M J, Mittendorfer B. Differences in muscle protein synthesis and anabolic signaling in the postabsorptive state and in response to food in 65–80 year old men and women. PLoS One. 2008;3:e1875. doi: 10.1371/journal.pone.0001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Sheffield-Moore P, Rasmussen B B, Wolfe R R. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpi E, Mittendorfer B, Wolfe S E, Wolfe R R. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- Rasmussen B B, Fujita S, Wolfe R R, Mittendorfer B, Roy M, Rowe V L, Volpi E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons T B, Schutzler S E, Cocke T L, Chinkes D L, Wolfe R R, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- Proctor D N, O'Brien P C, Atkinson E J, Nair K S. Comparison of techniques to estimate total body skeletal muscle mass in people of different age groups. Am J Physiol Endocrinol Metab. 1999;277:E489–E495. doi: 10.1152/ajpendo.1999.277.3.E489. [DOI] [PubMed] [Google Scholar]
- Burt M G, Johannsson G, Umpleby A M, Chrsholm D J, Ho K K. Impact of growth hormone and dehydroepiandrosterone on protein metabolism in glucocorticoid-treated patients. J Clin Endocrinol Metab. 2008;93:688–695. doi: 10.1210/jc.2007-2333. [DOI] [PubMed] [Google Scholar]
- Lang C H, Vary T C, Frost R A. Acute in vivo elevation of insulin-like growth factor (IGF) binding protein-1 decreases plasma free IGF-1 and muscle protein synthesis. Endocrinology. 2003;144:3922–3933. doi: 10.1210/en.2002-0192. [DOI] [PubMed] [Google Scholar]
- Short K R, Bigelow M L, Kahl J, Singh R, Coenen-Schimke J M, Raghavakaimal S, Nair K S. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623. doi: 10.1073/pnas.0501559102. [DOI] [PMC free article] [PubMed] [Google Scholar]