Abstract
cAMP-mediated signaling pathways regulate a multitude of important biological processes under both physiological and pathological conditions, including diabetes, heart failure, and cancer. In eukaryotic cells, the effects of cAMP are mediated by two ubiquitously expressed intracellular cAMP receptors, the classic protein kinase A/cAMP-dependent protein kinase (PKA/cAPK) and the recently discovered exchange protein directly activated by cAMP/cAMP-regulated guanine nucleotide exchange factors (Epac/cAMP-GEF). Like PKA, Epac contains an evolutionally conserved cAMP-binding domain that acts as a molecular switch for sensing intracellular second messenger cAMP levels to control diverse biological functions. The existence of two families of cAMP effectors provides a mechanism for a more precise and integrated control of the cAMP signaling pathways in a spatial and temporal manner. Depending upon their relative abundance, distribution and localization, as well as the specific cellular environments, Epac and PKA may act independently, converge synergistically, or oppose each other in regulating a specific cellular function.
Keywords: cyclic AMP, exchange protein directly activated by cAMP (Epac)/cAMP-regulated guanine exchange factor (cAMP-GEF), protein kinase A (PKA)/cAMP-dependent protein kinase (cAPK), signal transduction
Overview of the cAMP second messenger system
Eukaryotic cells respond to a wide range of extracellular signals, including hormones, growth factors, and neurotransmitters, by eliciting the generation of intracellular second messengers. Second messengers in turn trigger a myriad of cellular reactions by orchestrating a network of intracellular signaling events. The discovery of cAMP fifty years ago marked the birth of second messenger theory and the age of signal transduction. cAMP regulates many physiological processes ranging from learning and memory in the brain, contractility and relaxation in the heart, to water uptake in the gut and kidney. At the cellular level, cAMP plays an important role in virtually every known function such as metabolism, gene expression, cell division and growth, cell differentiation and apoptosis, as well as secretion and neurotransmission. In addition to directly regulating many important cellular processes, cAMP is also implicated in an array of cross talks between intracellular signaling pathways. For example, cAMP exerts its growth effects through interactions with the Ras-mediated MAP kinase pathways [1–5]. There is evidence that suggests cAMP cross-talks with the Ca2+-dependent signaling pathway [6,7]. It is also reported that cAMP can potentially modulate cytokine signaling through inhibiting the Jak/STAT pathway [8]. cAMP-signaling is also closely interwoven with the PI3K/PKB pathway [9,10].
For many years, the consensus was that the cAMP-mediated signaling in eukaryotic cells existed as a linear pathway, which involves the sequential activation of a series of signaling molecules, consisting of both plasma membrane and intracellular components. Upon binding of ligand, the G-protein coupled receptor at the cell surface transduces the extracellular signal across the cell membrane via stimulatory or inhibitory heterotrimeric G-proteins that interact with the membrane-bound adenylate cyclase (AC) to regulate cAMP production inside the cell. It was believed until recently that the major effects of cAMP in mammalian cells, with the exception of cyclic nucleotide-gated ion channels in photoreceptor cells, olfactory sensory neurons, and cardiac sinoatrial node cells [11], were mediated intracellularly by protein kinase A (PKA), also known as cAMP-dependent protein kinase (cAPK).
Protein kinase A
PKA was one of the first protein kinases to be discovered [12]. Unlike most of eukaryotic protein kinases, PKA is composed of two separate subunits, the catalytic (C) and regulatory (R) subunits. The C subunit is initially phosphorylated by phosphoinositide-dependent protein kinase at an essential phosphorylation site Threonine 197 (T197) [13,14]. Phosphorylation of T197 in the activation loop is necessary for the maturation and optimal biological activity of PKA [15,16]. However, once phosphorylated, the C subunit of PKA is fully active, and the T197 phosphate does not turn over readily [17]. The C subunit of PKA is then regulated via interaction with the inhibitory R subunit, a major intracellular cAMP receptor that sequesters the C subunit in an inactive heterotetrameric holoenzyme, R2C2. The activating ligand cAMP binds to the R subunit and induces conformational changes that lead to the dissociation of the holoenzyme into its constituent C and R subunits [18]. The free active C subunit can then affect a range of diverse cellular events by phosphorylating an array of cytoplasmic and nuclear protein substrates, including enzymes and transcriptional factors [19].
There are two general classes of PKA, designated as PKA(I) and PKA(II), due exclusively to differences in the R subunits, RI and RII that interact with an identical C subunit [18]. Four different R subunit genes, RIα [20], RIβ [21], RIIα [22], and RIIβ [23] have been identified. Three C subunit genes, Cα, Cβ, and Cγ have also been discovered. However, preferential expression of any of these C subunits with either RI or RII has not been found [24]. While both RI and RII contain two tandem and highly conserved cAMP-binding domains (CBD) at the carboxyl terminus [25], RI and RII differ significantly at their amino terminus, especially at a proteolytically sensitive hinge region that binds to the peptide recognition site of the C subunit. The hinge region of RII subunits contains a serine at the P site that can be autophosphorylated by the C subunit [26], whereas RI contains a pseudo phosphorylation site.
R isoforms are differentially expressed in tissues [27–29], and their subcellular distribution also appears to be distinct [30–33]. The existence of a family of A-kinase anchoring proteins (AKAP) that tether RII subunits to specific subcellular structures has been well documented [34]. The majority of AKAPs preferentially bind RII subunits; however, AKAPs specific for both RI and RII have also been identified recently [35]. These kinase anchoring proteins interact exclusively with the dimerization domain of the R subunits, and only the first 50 N-terminal amino acid residues of the R subunits are required for binding of AKAPs [36]. The extensive sequence diversity at this region between RI and RII may account for the difference in their AKAP binding affinities. Large numbers of AKAPs have been identified. Compartmentalization of PKA molecules to discrete intracellular locations through association with anchoring proteins may ensure specificity in signal transduction by placing the kinase close to its appropriate effectors or substrates [34].
While the ratio of the total R subunits/C subunits in normal tissue was found to be relatively constant at around 1:1, the relative amount of RI and RII varies and depends highly on physiological conditions and the hormonal status of the tissue [28,29,37,38]. One study shows that in knockout mice lacking the gene encoding RIIβ, the loss of RIIβ in brown fat cells is compensated by an increased level of RIα. The switching of PKA isoform from PKA(IIβ) to PK(Iα) results in increased basal level of PKA and increased energy expenditure. The RIIβknockout mice are leaner and protected against diet-induced obesity [39]. These results demonstrate clearly that RIα and RIIβ are functionally distinct. Although many of the physiologic effects of cAMP can be ascribed to the action of one or more of the PKA isoforms, some of the cAMP-dependent effects can not be explained based on the functions of PKA. For example, the ability of cAMP to enhance the secretion of insulin from pancreatic beta cells is not affected by specific inhibitors of PKA [40]. Many similar experimental observations hinted at the existence of “PKA-independent” mechanisms of cAMP action.
Epac, a new intracellular cAMP receptor
Recently, a family of novel cAMP sensor proteins, named Epac (exchange protein directly activated by cAMP) or cAMP-GEF (cAMP-regulated guanine exchange factor), has been identified [41,42]. These proteins contain a CBD that is homologous to that of PKA R subunits and the prokaryotic transcription regulator, cAMP receptor protein (CRP) (Fig. 1). Epac proteins bind to cAMP with high affinity and activate the Ras superfamily small GTPases Rap1 and Rap2. Rap1 was initially identified as an antagonist for the transforming function of Ras [43]. Rap1 can be activated in response to a variety of second messengers including cAMP [45]. Although PKA can phosphorylate Rap1 at its C-terminus, PKA phosphorylation is not required for cAMP-dependent activation of Rap1 [41].
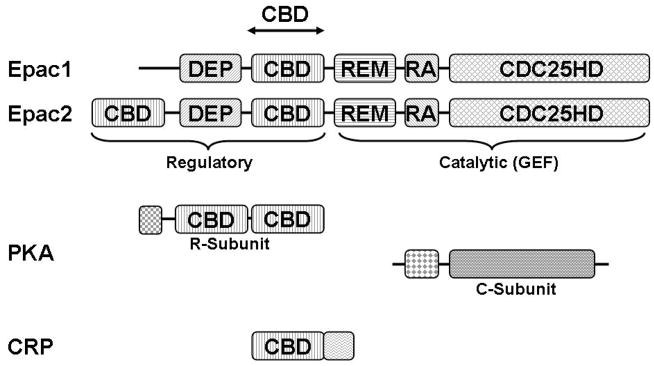
Fig. 1. Domain structure of Epac.
Linear representation of Epac, PKA, and CRP showing the conserved cAMP binding domain (CBD), Dishevelled/Egl-10/pleckstrin (DEP) domain, RAS exchange motif (REM) domain, RAS association (RA) domain, and CDC25-homology domain (CDC25HD).
There are two isoforms of Epac, Epac1 and Epac2, products of independent genes in mammals. While Epac1 is ubiquitously expressed in all tissues, Epac2 is more limited in its tissue distribution [41,42]. Epac1 and Epac2 share extensive sequence homology and both contain an N-terminal regulatory region and a C-terminal catalytic region. The catalytic region of Epac1 consists of a RAS exchange motif (REM) domain, RAS association (RA) domain and a classic CDC25-homology domain (CDC25HD) responsible for nucleotide exchange activity. Whereas the regulatory region of Epac1 and Epac2 shares a Dishevelled/Egl-10/pleckstrin (DEP) domain followed by a CBD domain that is evolutionally conserved to the CBD of PKA and the bacterial transcriptional factor cAMP receptor protein (CRP), an additional CBD N-terminal to the DEP domain is presented in Epac2 (Fig. 1). The function of this extra CBD domain (CBD-A) is not clear as it binds cAMP with low affinity and does not seem to be essential for Epac2 regulation by cAMP [45].
Cellular functions regulated by Epac
The discovery of Epac proteins as a new family of intracellular cAMP receptors suggests that the cAMP-mediated signaling mechanism is much more complex than what was believed earlier. Many cAMP-mediated effects that were previously thought to act through PKA alone may also be transduced by Epac. Extensive studies so far have established that Epac proteins are involved in a host of cAMP-related cellular functions such as cell adhesion [46,47], cell-cell junction [48,49], exocytosis/secretion [50–53], cell differentiation [54] and proliferation, gene expression, apoptosis, cardiac hypertrophy and phagecytosis. With the exception of a few preliminary reports of Epac knockout in fly and worm, so far no detailed in vivo genetic and function analyses of either Epac isoform in an animal model system have been reported. Our discussion of Epac’s biological functions will mainly be based on ex vivo studies in cell culture models.
Epac and cell adhesion
One of the first cellular functions attributed to Epac is its ability to enhance cell adhesion. When Epac is ectopically overexpressed in HEK293 cells, it induces flattened cell morphology and increases cell adhesion [55]. This is not surprising since one of the major functions of Rap1, a down-stream effector of Epac, is control of cell morphology/adhesion [56,57]. A study using a Epac-selective cAMP analog, 8-(4-chlorophenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate [58], suggests that activation of Epac induces Rap-dependent integrin-mediated cell adhesion to fibronectin in Ovcar3, a human ovarian carcinoma cell line [46]. Subsequent analysis further reveals that cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the α3β1 integrin but not the α6β4 integrin [47]. Interaction between Epac1 and light chain 2 (LC2) of the microtubule-associated protein MAP1A enhances Rap1-dependent cell adhesion to laminin [59]. Activation of Epac1 increases the β2-integrin–dependent adhesion of human endothelial progenitor cells (EPCs) to endothelial cell monolayers and to ICAM-1, as well as the β1-integrin-dependent adhesion of EPCs and mesenchymal stem cells to the matrix protein fibronectin [60]. These results demonstrate Epac’s therapeutic potential via enhancing integrin-dependent homing functions of progenitor cells.
Interestingly, cAMP-Epac1-Rap1 signaling also stimulates sickle red blood cells adhesion to lammin. However, the adhesion of sickle red blood cells to lammin promoted by Epac-Rap1 is not dependent on integrin, but mediated by the cell adhesion molecule/Lutheran receptor, a member of the Ig superfamily of receptors [61]. Consistent with the stimulatory effect of Epac1-Rap1 on cell adhesion, activation of Epac1 inhibits epithelial cell migration, which requires the disruption of cell-cell adhesion, in response to both hepatocyte growth factor (HGF) and transforming growth factor β (TGFβ) [62]. Direct interaction between Epac1 and type I TGFβ receptor has been reported and may be responsible for the observed inhibitory effect of Epac1 on TGFβ-mediated cell migration [63].
While the aforementioned effects of cAMP on cell adhesion are reported to be PKA-independent, cAMP regulated integrin-dependent adhesions of vascular endothelial cells to extracellular matrix proteins are coordinated by both PKA and Epac [64]. In human primary monocytes and in monocytic U937 cells, Epac1-Rap1 has been shown to regulate β1-integrin-dependent cell adhesion, cell polarization, and chemotaxis [65]. However, a similar study shows that although Epac1 is expressed in human peripheral monocytes and activates Rap1, cAMP modulates most monocyte immune functions through PKA and not Epac1-Rap1 [66]. Therefore, it appears that the roles of Epac1 and PKA in monocytes also remain unsettled.
Epac and cell junctions
In addition to its effects on integrin-mediated adhesion, Epac1/Rap1 signaling has also shown to contribute to E-cadherin-mediated adhesion [67]. This is consistent with the fact that Rap1 plays an important role in the formation of cell-cell junctions [68]. Stable cell-cell contacts are critical for the barrier function of epithelial and endothelial cells. Endothelial cell junctions are of central importance for regulating vascular permeability. It is well established that cAMP enhances the formation of cell junctions and endothelial barrier function. cAMP decreases basal permeability and reverse vascular leakage induced by inflammatory mediators. Previously, it was believed that cAMP exerts its effect through activation of PKA. However, inhibition of PKA activity does not block cAMP-enhanced endothelial cell barrier function, suggesting the existence of PKA-independent pathways.
Several studies now show that in human umbilical vein endothelial cells (HUVECs) Epac1 induces junction formation and actin remodeling and reduces endothelial permeability through activating Rap1, which is enriched at endothelial cell-cell contacts [48,49,69]. Activation of Epac leads to enhanced basal endothelial barrier function by increasing cortical actin and redistribution of adherens and tight junctional molecules to cell-cell contacts. Moreover, activation of Epac also offsets thrombin-induced hyperpermeability through down-regulation of Rho GTPase activation [48]. Using VE-cadherin null mouse cells immortalized with polyoma mT, Kooistra et al. demonstrate that regulation of endothelial permeability by Epac1 requires VE-cadherin and that Epac specific cAMP analog induced actin rearrangements are independent of cell junction formation [49]. Recently, it has been shown that Epac1 can directly promote microtubule (MT) growth independent of Rap1 activation [70] and that Epac activation reverses MT-dependent increases in vascular permeability induced by tumor necrosis factor-α and TGFβ. Therefore, it appears that Epac1 promotes endothelial barrier function through a two-leg strategy: a Rap1-depedent increase in cortical actin and a Rap-independent regulation of MTs [71].
Studies using human pulmonary artery endothelial cells (HPAEC) show that barrier-protective effects of cAMP, down-stream of Prostaglandin E2 (PGE2), prostacyclin and atrial natriuretic peptide (ANP), on pulmonary endothelial cells are mediated by both PKA and Epac pathways. Activation of PKA and Epac/Rap1 converges on Rac activation via stimulation of Rac-specific GEFs Tiam1 and Vav2 and leads to enhancement of peripheral actin cytoskeleton and adherens junctions [72,73]. In rat venular microvessels, activation of Epac/Rap1 pathway significantly attenuates the platelet-activating factor (PAF)-induced increase in microvessel permeability as measured by hydraulic conductivity and completely prevents the PAF-induced rearrangement of VE-cadherin [74]. Collectively, these results suggest that Epac/Rap1 signaling plays an important role in maintaining endothelial barrier function and vascular integrity.
Epac and secretion
While regulated exocytoses are mainly triggered by the elevation of intracellular Ca2+, second messenger cAMP also plays a role in modulating exocytosis in a variety of secretory cells. Epac has been implicated in stimulating numerous secretory pathways, including insulin secretion in pancreaticβ cells [75,76], the release of the non-amyloidogenic soluble form of amyloid precursor protein [53,77,78], progesterone secretion by luteinizing human granulosa cells [79], secretory activity in mouse melanotrophs [80] and rat chromaffin cells [81,82], neurotensin secretion in human endocrine cells [51], acrosomal exocytosis in sperm [83], and apical exocytotic insertion of aquaporin-2 in inner medullary collecting duct [84]. As cAMP-regulated exocytosis has been reviewed in detail [52], we will focus on some of the most recent advances in the area of Epac-mediated insulin secretion.
A recent study investigates the effect of PKA and Epac on two types of secretory vesicles, namely large dense-core vesicles (LVs) and small vesicles (SVs) in mouse pancreatic β-cells. By directly visualizing Ca2+-dependent exocytosis of both LVs and SVs with two-photon imaging, it is revealed that Epac and PKA selectively regulate exocytosis of SVs and LVs, respectively [85]. In a similar study, FM1-43 epifluorescence imaging was used to dissect the distinct contributions of Epac and PKA in regulating the number of plasma membrane (PM) exocytic sites and insulin secretory granule (SG)-to-granule fusionsthese exocytic events. Again, Epac and PKA modulate both distinct and common exocytic steps to potentiate insulin exocytosis. Whereas Epac activation mobilizes SGs to fuse at the PM, thereby increasing number of PM exocytic sites, PKA and EPAC activation synergistically increases both the number of exocytic sites at the PM and SG-SG fusions [86]. Lastly, a study using primary cultured pancreatic β-cells isolated from wild-type and mutant mice lacking Epac2 suggests that although activation of cAMP signaling alone does not cause either significant docking or fusion events of insulin granules, it substantially potentiates both the first phase (a prompt, marked, and transient increase) and the second phase (a moderate and sustained increase) of glucose-induced fusion events. Moreover, cAMP-potentiated fusion events in the first phase of glucose-induced exocytosis are markedly reduced in Epac2−/− mice. The data indicates that Epac2 signaling is important in cAMP-regulated insulin secretion by controlling insulin granule density near the plasma membrane [87].
Epac and differentiation
cAMP has been implicated in regulating differentiation in a variety of cell systems such as, neurite outgrowth in the neuroendocrine model cell line PC12 [88] and adipocyte formation from mouse 3T3-L1 fibroblasts [89]. The role that PKA plays in these processes is controversial and it has been speculated that a PKA-independent cAMP signaling component may be involved. Indeed, several studies have revealed that Epac plays an important role in mediating the effects of pituitary adenylate cyclase-activating polypeptide (PACAP) in inducing neurite outgrowths in PC12 cells [54,90] and human neuroblastoma SH-SY5Y cells [91]. However, as summarized in a recent Science STKE perspective [92], the detailed signal transduction pathways that mediate the neurotrophic effects of cAMP are not clear and the involvement of PKA remains contentious [93].
Intracellular second messenger cAMP is essential for the induction of adipocyte differentiation in mouse 3T3-L1 preadipocyte cell line. Again, it is generally believed that cAMP exerts its effect through activation of PKA. However, our recent studies suggest that PKA catalytic activity is not required for cAMP-mediated adipocyte differentiation in 3T3-L1 preadipocyte cells as IBMX or forskolin induced 3T3-L1 adipocyte differentiation is not sensitive to two mechanistically distinct PKA inhibitors, H89 and PKI. On the other hand, selectively suppressing Epac1 expression using shRNAs substantially reduces the efficiency of IBMX or forskolin induced 3T3-L1 adipocyte differentiation. Interestingly, while Epac1 is required for cAMP-mediated 3T3-L1 adipocyte differentiation, Epac-selctive cAMP analog, 8-CPT-2’-O-Me-cAMP, is not sufficient to replace IBMX or forskolin to induce 3T3-L1 adipocyte differentiation, nor are cAMP analogs selective for PKA RI or RII. 3T3-L1 adipocyte differentiation requires the combination treatment of cAMP analogs selective for Epac, PKA RI and RII (Cheng et al, unpublished results). We are currently investigating the signaling mechanism of cAMP/Epac-mediated adipocyte differentiation.
Epac and cardiomyocyte hypertrophy
cAMP is the main second messenger in cardiomyocytes which can be activated by the sympathetic and parasympathetic systems and also by cardioactive hormones and drugs [94]. cAMP regulates many important processes such as contractility and relaxation in both normal and failing hearts [95]. Traditionally, these effects have been attributed to act mainly through the classic intracellular cAMP receptor, PKA [96]. For example, PKA has been shown to phosphorylate key Ca2+-handling proteins such as voltage gated L-type Ca2+ channel [97], ryanodine receptor (RyR) [98], and phospholamban [99,100]. The net result in increase in the sarcoplasmic reticulum Ca2+ release via RyR2 and enhanced uptake by SR Ca2+ pump (SERCA2a) results in larger intracellular Ca2+ transients. Increased Ca2+ transients significantly enhance contractility. However, emerging evidences suggest that Epac may also play an important role in many cellular functions particularly, cardiac hypertrophy, as a new mediator of cAMP signaling in the cardiovascular system [101].
Recent studies have shown that the expression of both Epac1 and Epac2 are developmentally increased in the heart from neonatal stages to adulthood, and Epac levels is significantly upregulated in mouse heart with myocardial hypertrophy induced by chronic isoproterenol infusion or pressure overload by transverse aortic banding [102]. In cardiomyocytes, Epac is involved in the formation of gap junctions, which are essential for gating ions and small molecules to coordinate cardiac contractions [103]. Epac also enhances intracellular Ca2+ release during cardiac excitation-contraction (EC) coupling in cardiac myocytes by activation of calcium-calmodulin-dependent protein kinase II [104] or activation of phospholipase Cε [105], which is known to associate with cardiac hypertrophy. Interestingly, activation of EPAC leads to induction of hypertrophic program based on morphological changes, cytoskeletal reorganization, increase in protein synthesis and induction of cardiac hypertrophic markers. This effect is mediated by a Ca2+-dependent activation of Rac, calcineurin, and its primary downstream effector, NFAT [106]. It is reported that Epac1 is the major Epac isoform expressed in human heart and its level is increased in heart failure. Knockdown of Epac1 strongly suppresses beta-adrenergic receptor-induced hypertrophic program. Surprisingly, Epac1’s hypertrophic effects are mediated by the small GTPase Ras, the phosphatase calcineurin, and Ca(2+)/calmodulin-dependent protein kinase II, independent of Rap1, a canonical Epac effector [107].
Cross-talk between Epac and PKA
The discovery of second intracellular cAMP receptor raises many questions regarding the mechanism of cAMP-mediated signaling. The existence of two highly coordinated cAMP effectors provides a mechanism for a more precise and integrated control of the cAMP signaling pathways in a spatial and temporal manner. Since both PKA and Epac are ubiquitously expressed in all tissues, an increase in intracellular cAMP levels will lead to the activation of both PKA and Epac, and possibly other potential cAMP effector(s) as well. Therefore, the net cellular effects of cAMP are not just dictated by PKA or Epac alone, but by the sum of all the relevant pathways. Critical questions include: which cAMP effects are mediated by Epac, which by PKA? Are there cross-talks between Epac and PKA?
Our earlier studies demonstrate that although PKA and Epac are activated by the same second messenger cAMP, they can exert opposing effects on regulation of the PKB/AKT pathway. While PKA suppresses PKB phosphorylation and activity, activation of Epac leads to increased PKB phosphorylation [108]. Since our initial report, many studies have shown that Epac and PKA can act antagonistically in controlling various cellular functions such as insulin-stimulated PKB phosphorylation [109], proliferation and differentiation [54], myelin phagocytosis [110], regulation of hedgehog signaling and glucocorticoid sensitivity in acute lymphoblastic leukemia cells [111], and expression of high affinity choline transporter and the cholinergic locus [112]. On the other hand, depending upon the specific cellular context, we and others have also shown that Epac and PKA can exert synergistic effects on downstream signaling such as stimulation of neurotensin secretion [51], promoting PC-12 cell neurite extension [93], regulation of sodium-proton exchanger isoform 3 [113], and attenuation of cAMP signaling through phosphodiesterases [114]. While a model of synergistic activation of Rap1 by Epac and PKA has been proposed by Stork and colleagues [44], the origin and causes of antagonism between Epac and PKA is not understood. It is very likely that antagonism between Epac and PKA involves complex mechanisms and understanding the basis of Epac and PKA cross-talk may represent a major research interest for future cAMP-mediated signaling studies.
Mechanism of cAMP-mediated Activation
Both Epac and PKA are regulated by a CBD, which is a compact and evolutionally conserved structural/signaling motif that controls a set of diverse functionalities when linked to other structural domains [115,116]. CBD, the only common structural module between PKA and Epac, acts as a molecular switch for sensing intracellular second messenger cAMP levels. X-ray crystal structures and in-depth biochemical/biophysical analyses of PKA holoenzyme complex and individual subunits reveal a molecular mechanism for cAMP-mediated activation of PKA [117–120]. The R and C subunits form a large interface in the PKA holoenzyme complex with several key residues (Y247 and W196) of the C subunit binding directly to the phosphate binding cassette (PBC) of the first CBD in the R subunit [119]. cAMP not only competes directly with the C subunit for these interactions but also induces major conformational changes in the R subunit, particularly the helical subdomain of CBD, the inhibitor sequence and the linker region [118–120]. Binding of the cAMP results in the retraction of the PBC in the direction of cAMP binding pocket and global reorientation of the sub-helical domain of CBD. The pivot motion around the hydrophobic hinge dislodges the single extended B/C helix, and subsequently the inhibitor sequence, from the docking site on the C-subunit. In the absence of stabilization/anchoring effects of the C subunit, the B/C helix bends in the middle to form two individual helices with the C-helix portion folds back onto the β barrel to form the “lid” of the cAMP-binding pocket. These extensive cAMP-induced conformation changes eventually cause the dissociation of the PKA holoenzyme.
On the other hand, the CBD in Epac is covalently connected to the catalytic GEF domain as a single polypeptide chain and the intramolecular interaction between the CBD and GEF domains sterically blocks the access of downstream effector, Rap. Recently, the crystal structural of Epac2 is solved in the absence of cAMP [121]. In this auto-inhibited Epac2 structure, the second CBD of Epac2, which is common in Epac1 and Epac2, is anchored to the catalytic core indirectly by the REM domain through the so called “switchboard”. One major structural difference of the CBD between Epac and PKA is located at the lid region. The lid in CBD of PKA is a helix that covers the cAMP binding packet whereas the corresponding region in Epac points away from the cAMP binding packet as a two-strand β-sheet that forms the first part of the five-strand β-sheet like “switchboard” structure. In addition, unlike the extensive interface between the R and C subunits of PKA holoenzyme, the intramolecular interaction between the regulatory and catalytic regions in Epac2 is surprisingly brief. There is only one direct contact point between the CBD and catalytic core of Epac, described as the “ionic latch”. These major differences suggest that although it is likely that Epac and PKA activations share the same underlying principal, the detailed mechanisms of PKA and Epac activation by cAMP will most likely be different at the structural level.
Since the crystal structure of cAMP-bound Epac in its active state is not currently available, the mechanism of Epac activation is not clear. Extensive biochemical and structural studies by Bos and Wittinghofer groups suggest that the lid region of the C-terminus of CBD in Epac plays an important role in the communication between the regulatory and catalytic domains and is pivotal for the activation of Epac by cAMP [45,122–125]. To further probe the mechanism of Epac activation, we examined the conformation and structural dynamics of Epac1 in the presence and absence of cAMP using amide H/D exchange coupled with Fourier transform infrared spectroscopy (FT-IR) and mass spectrometry, which monitor the global and local protein conformation and dynamics, respectively. Our studies show that unlike that of PKA, the classic intracellular cAMP receptor, binding of cAMP to Epac1 does not induce significant changes in overall secondary structure and structural dynamics, suggesting that conformational changes induced by cAMP in Epac1 are most likely local motion, such as hinge movements [126,127]. Hinge prediction based on GNM (Gaussian Network Model) first normal model displacement analysis revealed a major hinge in Epac1 between residues 310–345 [127]. Indeed, our amide hydrogen/deuterium exchange mass spectrometry study reveals that the solvent accessibility of this hinge region decreases upon cAMP-binding, indicating conformational changes [128]. Based on the cAMP-free Epac2 structure and our in-depth H/D exchange and comparative sequence/structure analyses of Epac and PKA, we propose a model of Epac activation (Fig. 2). In this model, Binding of cAMP induces an allosteric switch manifested by a hinge motion that bends the extended C-helix/lid toward the β-barrel of the CBD. This hinge movement pulls the β-strands S1 and S2 away from the five-strand like β-sheet of the switchboard to form the base of the cAMP-binding pocket. The conformational changes induced upon cAMP binding result in a closed CBD conformation and reorientation of the CBD/DEP domains relative to the rest of the molecule, which releases the catalytic core from the inhibitory contact imposed by the CBD. This structural transition allows Epac, albeit with a completely different lid conformation in the inactive Epac structure, to utilize the same underlying principal to bind cAMP in almost exactly the same manner as PKA and other CBD-containing proteins [118,119,129,130]. Although final validation of the model requires the three dimensional structure of a Epac-cAMP complex, our earlier studies using Epac-based fluorescence resonance energy transfer indicators suggest that binding of cAMP leads to a more extended Epac conformation [131], an observation in agreement with our model.
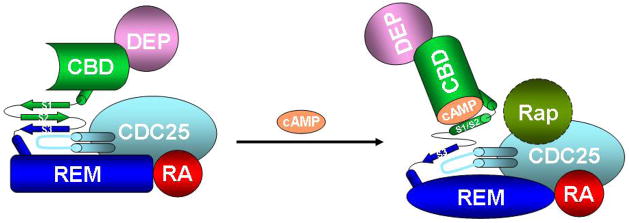
Fig. 2. Mechanism of Epac activation, a model.
Schematic representation of cAMP-induced Epac activation with the DEP, CBD, REM, RA, and CDC25HD domains colored in pink, green, blue, red and cyan, respectively. In the cAMP-free, inactive Epac state, the regulatory region is anchored by the switchboard to keep the CBD in close proximity of the catalytic core to prevent the binding of Rap. Upon binding of cAMP, the β strands S1 and S2 break away from the switchboard, fold back towards the β barrel and interact directly with cAMP to form the base of the cAMP-binding pocket. This localized hinge motion reorients the CDC25HD homology domain relative to CBD, and ultimately leads to an open Epac conformation and the exposure of the catalytic core of Epac for the access of Rap GTPase.
Conclusion
Since the discovery of Epac proteins a decade ago, the cAMP research area has undergone a renaissance. It is now well-recognized that eukaryotic cAMP signaling is much more complex than it was initially believed and that the classic PKA pathway is only part of the story. Net physiological effects of cAMP necessitate the integration of Epac- and PKA-dependent pathways in a spatial and temporal manner, which dramatically increases the complexity and consequently possible readouts of cAMP signaling. Depending upon their relative abundance, distribution and localization, as well as the precise cellular environment, the two intracellular cAMP receptors may act independently, converge synergistically, or oppose each other in regulating a specific cellular function. Therefore, careful dissections of the individual role and relative contribution of Epac and PKA within the overall cAMP signaling in various model systems will continue to be an important part of the future research activity. In addition, although we have learned a great deal about the structure and functions of Epac, much remains to be discovered. Important future researches in the area include but not limited to: understanding the physiological roles of Epac isoforms using animal models, elucidating the mechanism of cross-talk between Epac and PKA, and mapping the conformational changes induced by cAMP during Epac activation.
Acknowledgments
The authors wish to apologize to the investigators whose outstanding work was not cited here because of space limitations.
This work is supported by grants from the National Institute Health (GM061770) and the American Heart Association (0755049Y)
References
- 1.Frodin M, Peraldi P, Van Obberghen E. Cyclic AMP activates the mitogen-activated protein kinase cascade in PC12 cells. J Biol Chem. 1994;269:6207–6214. [PubMed] [Google Scholar]
- 2.Burgering BM, Pronk GJ, van Weeren PC, Chardin P, Bos JL. cAMP antagonizes p21ras-directed activation of extracellular signal-regulated kinase 2 and phosphorylation of mSos nucleotide exchange factor. EMBO J. 1993;12:4211–4220. doi: 10.1002/j.1460-2075.1993.tb06105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu J, Dent P, Jelinek T, Wolfman A, Weber MJ, Sturgill TW. Inhibition of the EGF-activated MAP kinase signaling pathway by adenosine 3′,5′-monophosphate. Science. 1993;262:1065–1069. doi: 10.1126/science.7694366. [DOI] [PubMed] [Google Scholar]
- 4.Graves LM, Bornfeldt KE, Raines EW, Potts BC, Macdonald SG, Ross R, Krebs EG. Protein kinase A antagonizes platelet-derived growth factor-induced signaling by mitogen-activated protein kinase in human arterial smooth muscle cells. Proc Natl Acad Sci USA. 1993;90:10300–10304. doi: 10.1073/pnas.90.21.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cook SJ, McCormick F. Inhibition by cAMP of Ras-dependent activation of Raf. Science. 1993;262:1069–1072. doi: 10.1126/science.7694367. [DOI] [PubMed] [Google Scholar]
- 6.DeBernardi MA, Brooker G. Single cell Ca2+/cAMP cross-talk monitored by simultaneous Ca2+/cAMP fluorescence ratio imaging. Proc Natl Acad Sci USA. 1996;93:4577–4582. doi: 10.1073/pnas.93.10.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogue PJ, Humbert JP, Meyer A, Freyermuth S, Krady MM, Malviya AN. cAMP-dependent protein kinase phosphorylates and activates nuclear Ca2+-ATPase. Proc Natl Acad Sci USA. 1998;95:9178–9183. doi: 10.1073/pnas.95.16.9178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.David M, Petricoin E, III, Larner AC. Activation of protein kinase A inhibits interferon induction of the Jak/Stat pathway in U266 cells. J Biol Chem. 1996;271:4585–4588. doi: 10.1074/jbc.271.9.4585. [DOI] [PubMed] [Google Scholar]
- 9.Monfar M, Lemon KP, Grammer TC, Cheatham L, Chung J, Vlahos CJ, Blenis J. Activation of pp70/85 S6 kinases in interleukin-2-responsive lymphoid cells is mediated by phosphatidylinositol 3-kinase and inhibited by cyclic AMP. Mol Cell Biol. 1995;15:326–337. doi: 10.1128/mcb.15.1.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273:32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 11.Zufall F, Shepherd GM, Barnstable CJ. Cyclic nucleotide gated channels as regulators of CNS development and plasticity. Curr Opin Neurobiol. 1997;7:404–412. doi: 10.1016/s0959-4388(97)80070-0. [DOI] [PubMed] [Google Scholar]
- 12.Walsh DA, Perkins JP, Krebs EG. An adenosine 3′,5′-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968;243:3763–3765. [PubMed] [Google Scholar]
- 13.Cheng X, Ma Y, Moore M, Hemmings BA, Taylor SS. Phosphorylation and activation of cAMP-dependent protein kinase by phosphoinositide-dependent protein kinase. Proc Natl Acad Sci USA. 1998;95:9849–9854. doi: 10.1073/pnas.95.17.9849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cauthron RD, Carter KB, Liauw S, Steinberg RA. Physiological phosphorylation of protein kinase A at Thr-197 is by a protein kinase A kinase. Mol Cell Biol. 1998;18:1416–1423. doi: 10.1128/mcb.18.3.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steinberg RA, Cauthron RD, Symcox MM, Shuntoh H. Autoactivation of catalytic (C alpha) subunit of cyclic AMP-dependent protein kinase by phosphorylation of threonine 197. Mol Cell Biol. 1993;13:2332–2341. doi: 10.1128/mcb.13.4.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams JA, McGlone ML, Gibson R, Taylor SS. Phosphorylation modulates catalytic function and regulation in the cAMP- dependent protein kinase. Biochemistry. 1995;34:2447–2454. doi: 10.1021/bi00008a007. [DOI] [PubMed] [Google Scholar]
- 17.Shoji S, Titani K, Demaille JG, Fischer EH. Sequence of two phosphorylated sites in the catalytic subunit of bovine cardiac muscle adenosine 3′,5′-monophosphate-dependent protein kinase. J Biol Chem. 1979;254:6211–6214. [PubMed] [Google Scholar]
- 18.Taylor SS, Buechler JA, Yonemoto W. cAMP-dependent protein kinase: framework for a diverse family of regulatory enzymes. Annu Rev Biochem. 1990;59:971–1005. doi: 10.1146/annurev.bi.59.070190.004543. [DOI] [PubMed] [Google Scholar]
- 19.Zetterqvist OZ, Ragnarsson U, Engstrom L. In: Peptides and protein phosphorylation. Kemp BE, editor. Boca Raton: CRC Press Inc; 1991. [Google Scholar]
- 20.Lee DC, Carmichael DF, Krebs EG, McKnight GS. Isolation of a cDNA clone for the type I regulatory subunit of bovine cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1983;80:3608–3612. doi: 10.1073/pnas.80.12.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clegg CH, Cadd GG, McKnight GS. Genetic characterization of a brain-specific form of the type I regulatory subunit of cAMP-dependent protein kinase. Proc Natl Acad Sci USA. 1988;85:3703–3707. doi: 10.1073/pnas.85.11.3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott JD, Glaccum MB, Zoller MJ, Uhler MD, Helfman DM, McKnight GS, Krebs EG. The molecular cloning of a type II regulatory subunit of the cAMP-dependent protein kinase from rat skeletal muscle and mouse brain. Proc Natl Acad Sci USA. 1987;84:5192–5196. doi: 10.1073/pnas.84.15.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jahnsen T, Hedin L, Kidd VJ, Beattie WG, Lohmann SM, Walter U, Durica J, et al. Molecular cloning, cDNA structure, and regulation of the regulatory subunit of type II cAMP-dependent protein kinase from rat ovarian granulosa cells. J Biol Chem. 1986;261:12352–12361. [PubMed] [Google Scholar]
- 24.Beebe SJ, Oyen O, Sandberg M, Froysa A, Hansson V, Jahnsen T. Molecular cloning of a tissue-specific protein kinase (C gamma) from human testis--representing a third isoform for the catalytic subunit of cAMP-dependent protein kinase. Mol Endocrinol. 1990;4:465–475. doi: 10.1210/mend-4-3-465. [DOI] [PubMed] [Google Scholar]
- 25.Weber IT, Steitz TA, Bubis J, Taylor SS. Predicted structures of cAMP binding domains of type I and II regulatory subunits of cAMP-dependent protein kinase. Biochemistry. 1987;26:343–351. doi: 10.1021/bi00376a003. [DOI] [PubMed] [Google Scholar]
- 26.Rosen OM, Erlichman J. Reversible autophosphorylation of a cyclic 3′,5′-AMP-dependent protein kinase from bovine cardiac muscle. J Biol Chem. 1975;250:7788–7794. [PubMed] [Google Scholar]
- 27.Corbin JD, Keely SL, Park CR. The distribution and dissociation of cyclic adenosine 3′,5′-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975;250:218–225. [PubMed] [Google Scholar]
- 28.Hofmann F, Bechtel PJ, Krebs EG. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977;252:1441–1447. [PubMed] [Google Scholar]
- 29.Doskeland SO, Maronde E, Gjertsen BT. The genetic subtypes of cAMP-dependent protein kinase––functionally different or redundant? Biochim Biophys Acta. 1993;1178:249–258. doi: 10.1016/0167-4889(93)90201-y. [DOI] [PubMed] [Google Scholar]
- 30.Joachim S, Schwoch G. Localization of cAMP-dependent protein kinase subunits along the secretory pathway in pancreatic and parotid acinar cells and accumulation of the catalytic subunit in parotid secretory granules following beta-adrenergic stimulation. Eur J Cell Biol. 1990;51:76–84. [PubMed] [Google Scholar]
- 31.Pariset C, Feinberg J, Dacheux JL, Oyen O, Jahnsen T, Weinman S. Differential expression and subcellular localization for subunits of cAMP-dependent protein kinase during ram spermatogenesis. J Cell Biol. 1989;109:1195–1205. doi: 10.1083/jcb.109.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Camilli P, Moretti M, Donini SD, Walter U, Lohmann SM. Heterogeneous distribution of the cAMP receptor protein RII in the nervous system: evidence for its intracellular accumulation on microtubules, microtubule-organizing centers, and in the area of the Golgi complex. J Cell Biol. 1986;103:189–203. doi: 10.1083/jcb.103.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skalhegg BS, Tasken K, Hansson V, Huitfeldt HS, Jahnsen T, Lea T. Location of cAMP-dependent protein kinase type I with the TCR-CD3 complex. Science. 1994;263:84–87. doi: 10.1126/science.8272870. [DOI] [PubMed] [Google Scholar]
- 34.Scott JD, McCartney S. Localization of A-kinase through anchoring proteins. Mol Endocrinol. 1994;8:5–11. doi: 10.1210/mend.8.1.8152430. [DOI] [PubMed] [Google Scholar]
- 35.Huang LJ, Durick K, Weiner JA, Chun J, Taylor SS. Identification of a novel protein kinase A anchoring protein that binds both type I and type II regulatory subunits. J Biol Chem. 1997;272:8057–8064. doi: 10.1074/jbc.272.12.8057. [DOI] [PubMed] [Google Scholar]
- 36.Newlon MG, Roy M, Morikis D, Hausken ZE, Coghlan V, Scott JD, Jennings PA. The molecular basis for protein kinase A anchoring revealed by solution NMR. Nat Struct Biol. 1999;6:222–227. doi: 10.1038/6663. [DOI] [PubMed] [Google Scholar]
- 37.Lohmann SM, Walter U. Regulation of the cellular and subcellular concentrations and distribution of cyclic nucleotide-dependent protein kinases. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;18:63–117. [PubMed] [Google Scholar]
- 38.Sugden PH, Corbin JD. Adenosine 3′,5′-cyclic monophosphate-binding proteins in bovine and rat tissues. Biochem J. 1976;159:423–427. doi: 10.1042/bj1590423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RII beta subunit of protein kinase A. Nature. 1996;382:622–626. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 40.Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 42.Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- 43.Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras-related gene with transformation suppressor activity. Cell. 1989;56:77–84. doi: 10.1016/0092-8674(89)90985-9. [DOI] [PubMed] [Google Scholar]
- 44.Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, Stork PJ. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol. 2006;26:2130–2145. doi: 10.1128/MCB.26.6.2130-2145.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Rooij J, Rehmann H, van Triest M, Cool RH, Wittinghofer A, Bos JL. Mechanism of regulation of the Epac family of cAMP-dependent RapGEFs. J Biol Chem. 2000;275:20829–20836. doi: 10.1074/jbc.M001113200. [DOI] [PubMed] [Google Scholar]
- 46.Rangarajan S, Enserink JM, Kuiperij HB, de Rooij J, Price LS, Schwede F, Bos JL. Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2-adrenergic receptor. J Cell Biol. 2003;160:487–493. doi: 10.1083/jcb.200209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Enserink JM, Price LS, Methi T, Mahic M, Sonnenberg A, Bos JL, Taskén K. The cAMP-Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J Biol Chem. 2004;279:44889–44896. doi: 10.1074/jbc.M404599200. [DOI] [PubMed] [Google Scholar]
- 48.Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- 49.Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- 50.Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, et al. cAMP-GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- 51.Li J, O'Connor KL, Cheng X, Mei FC, Uchida T, Townsend CM, Jr, Evers BM. Cyclic adenosine 5′-monophosphate-stimulated neurotensin secretion is mediated through Rap1 downstream of both Epac and protein kinase A signaling pathways. Mol Endocrinol. 2007;21:159–171. doi: 10.1210/me.2006-0340. [DOI] [PubMed] [Google Scholar]
- 52.Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- 53.Maillet M, Robert SJ, Cacquevel M, Gastineau M, Vivien D, Bertoglio J, Zugaza JL, et al. Crosstalk between Rap1 and Rac regulates secretion of sAPPalpha. Nat Cell Biol. 2003;5:633–639. doi: 10.1038/ncb1007. [DOI] [PubMed] [Google Scholar]
- 54.Kiermayer S, Biondi RM, Imig J, Plotz G, Haupenthal J, Zeuzem S, Piiper A. Epac activation converts cAMP from a proliferative into a differentiation signal in PC12 cells. Mol Biol Cell. 2005;16:5639–5648. doi: 10.1091/mbc.E05-05-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Qiao J, Mei FC, Popov VL, Vergara LA, Cheng X. Cell cycle-dependent subcellular localization of exchange factor directly activated by cAMP. J Biol Chem. 2002;277:26581–26586. doi: 10.1074/jbc.M203571200. [DOI] [PubMed] [Google Scholar]
- 56.Zwartkruis FJ, Bos JL. Ras and Rap1: two highly related small GTPases with distinct function. Exp Cell Res. 1999;253:157–165. doi: 10.1006/excr.1999.4695. [DOI] [PubMed] [Google Scholar]
- 57.Bos JL, de Bruyn K, Enserink J, Kuiperij B, Rangarajan S, Rehmann H, Riedl J, et al. The role of Rap1 in integrin-mediated cell adhesion. Biochem Soc Trans. 2003;31:83–86. doi: 10.1042/bst0310083. [DOI] [PubMed] [Google Scholar]
- 58.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Døskeland SO, et al. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 59.Gupta M, Yarwood SJ. MAP1A light chain 2 interacts with exchange protein activated by cyclic AMP 1 (EPAC1) to enhance Rap1 GTPase activity and cell adhesion. J Biol Chem. 2005;280:8109–8116. doi: 10.1074/jbc.M413697200. [DOI] [PubMed] [Google Scholar]
- 60.Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood. 2008;111:2640–2646. doi: 10.1182/blood-2007-04-086231. [DOI] [PubMed] [Google Scholar]
- 61.Murphy MM, Zayed MA, Evans A, Parker CE, Ataga KI, Telen MJ, Parise LV. Role of Rap1 in promoting sickle red blood cell adhesion to laminin via BCAM/LU. Blood. 2005;105:3322–3329. doi: 10.1182/blood-2004-07-2881. [DOI] [PubMed] [Google Scholar]
- 62.Lyle KS, Raaijmakers JH, Bruinsma W, Bos JL, de Rooij J. cAMP-induced Epac-Rap activation inhibits epithelial cell migration by modulating focal adhesion and leading edge dynamics. Cell Signal. 2008;20:1104–1116. doi: 10.1016/j.cellsig.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 63.Conrotto P, Yakymovych I, Yakymovych M, Souchelnytskyi S. Interactome of transforming growth factor-beta type I receptor (TbetaRI): inhibition of TGFbeta signaling by Epac1. J Proteome Res. 2007;6:287–297. doi: 10.1021/pr060427q. [DOI] [PubMed] [Google Scholar]
- 64.Netherton SJ, Sutton JA, Wilson LS, Carter RL, Maurice DH. Both protein kinase A and exchange protein activated by cAMP coordinate adhesion of human vascular endothelial cells. Circ Res. 2007;101:768–776. doi: 10.1161/CIRCRESAHA.106.146159. [DOI] [PubMed] [Google Scholar]
- 65.Lorenowicz MJ, van Gils J, de Boer M, Hordijk PL, Fernandez-Borja M. Epac1-Rap1 signaling regulates monocyte adhesion and chemotaxis. J Leukoc Biol. 2006;80:1542–1552. doi: 10.1189/jlb.0506357. [DOI] [PubMed] [Google Scholar]
- 66.Bryn T, Mahic M, Enserink JM, Schwede F, Aandahl EM, Tasken K. The cyclic AMP-Epac1-Rap1 pathway is dissociated from regulation of effector functions in monocytes but acquires immunoregulatory function in mature macrophages. J Immunol. 2006;176:7361–7370. doi: 10.4049/jimmunol.176.12.7361. [DOI] [PubMed] [Google Scholar]
- 67.Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJ, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–35132. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- 68.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120:17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 69.Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mei FC, Cheng XD. Interplay between exchange protein directly activated by cAMP (Epac) and microtubule cytoskeleton. Molecular Biosystems. 2005;1:325–331. doi: 10.1039/b511267b. [DOI] [PubMed] [Google Scholar]
- 71.Sehrawat S, Cullere X, Patel S, Italiano J, Jr, Mayadas TN. Role of epac1, an exchange factor for rap GTPases, in endothelial microtubule dynamics and barrier function. Mol Biol Cell. 2008;19:1261–1270. doi: 10.1091/mbc.E06-10-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Birukova AA, Zagranichnaya T, Fu P, Alekseeva E, Chen W, Jacobson JR, Birukov KG. Prostaglandins PGE(2) and PGI(2) promote endothelial barrier enhancement via PKA- and Epac1/Rap1-dependent Rac activation. Exp Cell Res. 2007;313:2504–2520. doi: 10.1016/j.yexcr.2007.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol. 2008;215:715–724. doi: 10.1002/jcp.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol. 2008;294:H1188–H1196. doi: 10.1152/ajpheart.00937.2007. [DOI] [PubMed] [Google Scholar]
- 75.Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP-GEFII--Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–4653. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- 76.Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells. J Physiol. 2001;536:375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Robert S, Maillet M, Morel E, Launay JM, Fischmeister R, Mercken L, Lezoualc'h F. Regulation of the amyloid precursor protein ectodomain shedding by the 5-HT4 receptor and Epac. FEBS Lett. 2005;579:1136–1142. doi: 10.1016/j.febslet.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 78.Zaldua N, Gastineau M, Hoshino M, Lezoualc'h F, Zugaza JL. Epac signaling pathway involves STEF, a guanine nucleotide exchange factor for Rac, to regulate APP processing. FEBS Lett. 2007;581:5814–5818. doi: 10.1016/j.febslet.2007.11.053. [DOI] [PubMed] [Google Scholar]
- 79.Chin EC, Abayasekara DR. Progesterone secretion by luteinizing human granulosa cells: a possible cAMP-dependent but PKA-independent mechanism involved in its regulation. J Endocrinol. 2004;183:51–60. doi: 10.1677/joe.1.05550. [DOI] [PubMed] [Google Scholar]
- 80.Sedej S, Rose T, Rupnik M. cAMP increases Ca2+-dependent exocytosis through both PKA and Epac2 in mouse melanotrophs from pituitary tissue slices. J Physiol. 2005;567:799–813. doi: 10.1113/jphysiol.2005.090381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Novara M, Baldelli P, Cavallari D, Carabelli V, Giancippoli A, Carbone E. Exposure to cAMP and beta-adrenergic stimulation recruits Ca(V)3 T-type channels in rat chromaffin cells through Epac cAMP-receptor proteins. J Physiol. 2004;558:433–449. doi: 10.1113/jphysiol.2004.061184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giancippoli A, Novara M, de Luca A, Baldelli P, Marcantoni A, Carbone E, Carabelli V. Low-threshold exocytosis induced by cAMP-recruited CaV3.2 (alpha1H) channels in rat chromaffin cells. Biophys J. 2006;90:1830–1841. doi: 10.1529/biophysj.105.071647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Branham MT, Mayorga LS, Tomes CN. Calcium induced acrosomal exocytosis requires cAMP acting through a PKA-independent, EPAC-mediated pathway. J Biol Chem. 2006;281:8656–8666. doi: 10.1074/jbc.M508854200. [DOI] [PubMed] [Google Scholar]
- 84.Yip KP. Epac-mediated Ca(2+) mobilization and exocytosis in inner medullary collecting duct. Am J Physiol Renal Physiol. 2006;291:F882–F890. doi: 10.1152/ajprenal.00411.2005. [DOI] [PubMed] [Google Scholar]
- 85.Hatakeyama H, Takahashi N, Kishimoto T, Nemoto T, Kasai H. Two cAMP-dependent pathways differentially regulate exocytosis of large dense-core and small vesicles in mouse beta-cells. J Physiol. 2007;582:1087–1098. doi: 10.1113/jphysiol.2007.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwan EP, Gao X, Leung YM, Gaisano HY. Activation of exchange protein directly activated by cyclic adenosine monophosphate and protein kinase A regulate common and distinct steps in promoting plasma membrane exocytic and granule-to-granule fusions in rat islet beta cells. Pancreas. 2007;35:e45–e54. doi: 10.1097/mpa.0b013e318073d1c9. [DOI] [PubMed] [Google Scholar]
- 87.Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, et al. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gunning PW, Landreth GE, Bothwell MA, Shooter EM. Differential and synergistic actions of nerve growth factor and cyclic AMP in PC12 cells. J Cell Biol. 1981;89:240–245. doi: 10.1083/jcb.89.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williams IH, Polakis SE. Differentiation of 3T3-L1 fibroblasts to adipocytes. The effect of indomethacin, prostaglandin E1 and cyclic AMP on the process of differentiation. Biochem Biophys Res Commun. 1977;77:175–186. doi: 10.1016/s0006-291x(77)80180-0. [DOI] [PubMed] [Google Scholar]
- 90.Shi GX, Rehmann H, Andres DA. A novel cyclic AMP-dependent Epac-Rit signaling pathway contributes to PACAP38-mediated neuronal differentiation. Mol Cell Biol. 2006;26:9136–9147. doi: 10.1128/MCB.00332-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monaghan TK, Mackenzie CJ, Plevin R, Lutz EM. PACAP-38 induces neuronal differentiation of human SH-SY5Y neuroblastoma cells via cAMP-mediated activation of ERK and p38 MAP kinases. J Neurochem. 2008;104:74–88. doi: 10.1111/j.1471-4159.2007.05018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gerdin MJ, Eiden LE. Regulation of PC12 cell differentiation by cAMP signaling to ERK independent of PKA: do all the connections add up? Sci STKE 2007. 2007:pe15. doi: 10.1126/stke.3822007pe15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Christensen AE, Selheim F, Rooij JJ, Dremier S, Schwede F, Dao KK, Martinez A, et al. cAMP analog mapping of Epac1 and cAMP-kinase. Discriminating analogs demonstrate that Epac and cAMP-kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem. 2003;278:35394–35402. doi: 10.1074/jbc.M302179200. [DOI] [PubMed] [Google Scholar]
- 94.Vandecasteele G, Rochais F, Abi-Gerges A, Fischmeister R. Functional localization of cAMP signalling in cardiac myocytes. Biochem Soc Trans. 2006;34:484–488. doi: 10.1042/BST0340484. [DOI] [PubMed] [Google Scholar]
- 95.Hunter JJ, Chien KR. Signaling pathways for cardiac hypertrophy and failure. N Engl J Med. 1999;341:1276–1283. doi: 10.1056/NEJM199910213411706. [DOI] [PubMed] [Google Scholar]
- 96.Kopperud R, Krakstad C, Selheim F, Doskeland SO. cAMP effector mechanisms. Novel twists for an 'old' signaling system. FEBS Lett. 2003;546:121–126. doi: 10.1016/s0014-5793(03)00563-5. [DOI] [PubMed] [Google Scholar]
- 97.Kamp TJ, Hell JW. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- 98.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 99.Kiss E, Edes I, Sato Y, Luo W, Liggett SB, Kranias EG. beta-Adrenergic regulation of cAMP and protein phosphorylation in phospholamban-knockout mouse hearts. Am J Physiol. 1997;272:H785–H790. doi: 10.1152/ajpheart.1997.272.2.H785. [DOI] [PubMed] [Google Scholar]
- 100.Simmerman HK, Jones LR. Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- 101.Schmidt M, Sand C, Jakobs KH, Michel MC, Weernink PA. Epac and the cardiovascular system. Curr Opin Pharmacol. 2007;7:193–200. doi: 10.1016/j.coph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Ulucan C, Wang X, Baljinnyam E, Bai Y, Okumura S, Sato M, Minamisawa S, et al. Developmental changes in gene expression of Epac and its upregulation in myocardial hypertrophy. Am J Physiol Heart Circ Physiol. 2007;293:H1662–H1672. doi: 10.1152/ajpheart.00159.2007. [DOI] [PubMed] [Google Scholar]
- 103.Somekawa S, Fukuhara S, Nakaoka Y, Fujita H, Saito Y, Mochizuki N. Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ Res. 2005;97:655–662. doi: 10.1161/01.RES.0000183880.49270.f9. [DOI] [PubMed] [Google Scholar]
- 104.Pereira L, Métrich M, Fernández-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, et al. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oestreich EA, Wang H, Malik S, Kaproth-Joslin KA, Blaxall BC, Kelley GG, Dirksen RT, et al. Epac-mediated activation of phospholipase C(epsilon) plays a critical role in beta-adrenergic receptor-dependent enhancement of Ca2+ mobilization in cardiac myocytes. J Biol Chem. 2007;282:5488–5495. doi: 10.1074/jbc.M608495200. [DOI] [PubMed] [Google Scholar]
- 106.Morel E, Marcantoni A, Gastineau M, Birkedal R, Rochais F, Garnier A, Lompré AM, et al. cAMP-binding protein Epac induces cardiomyocyte hypertrophy. Circ Res. 2005;97:1296–1304. doi: 10.1161/01.RES.0000194325.31359.86. [DOI] [PubMed] [Google Scholar]
- 107.Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc'h F. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 108.Mei FC, Qiao J, Tsygankova OM, Meinkoth JL, Quilliam LA, Cheng X. Differential signaling of cyclic AMP: opposing effects of exchange protein directly activated by cyclic AMP and cAMP-dependent protein kinase on protein kinase B activation. J Biol Chem. 2002;277:11497–11504. doi: 10.1074/jbc.M110856200. [DOI] [PubMed] [Google Scholar]
- 109.Brennesvik EO, Ktori C, Ruzzin J, Jebens E, Shepherd PR, Jensen J. Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell Signal. 2005;17:1551–1559. doi: 10.1016/j.cellsig.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 110.Makranz C, Cohen G, Reichert F, Kodama T, Rotshenker S. cAMP cascade (PKA, Epac, adenylyl cyclase, Gi, and phosphodiesterases) regulates myelin phagocytosis mediated by complement receptor-3 and scavenger receptor-AI/II in microglia and macrophages. Glia. 2006;53:441–448. doi: 10.1002/glia.20303. [DOI] [PubMed] [Google Scholar]
- 111.Ji Z, Mei FC, Johnson BH, Thompson EB, Cheng X. Protein kinase A, not Epac, suppresses hedgehog activity and regulates glucocorticoid sensitivity in acute lymphoblastic leukemia cells. J Biol Chem. 2007;282:37370–37377. doi: 10.1074/jbc.M703697200. [DOI] [PubMed] [Google Scholar]
- 112.Brock M, Nickel AC, Madziar B, Blusztajn JK, Berse B. Differential regulation of the high affinity choline transporter and the cholinergic locus by cAMP signaling pathways. Brain Res. 2007;1145:1–10. doi: 10.1016/j.brainres.2007.01.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Honegger KJ, Capuano P, Winter C, Bacic D, Stange G, Wagner CA, Biber J, et al. Regulation of sodium-proton exchanger isoform 3 (NHE3) by PKA and exchange protein directly activated by cAMP (EPAC) Proc Natl Acad Sci USA. 2006;103:803–808. doi: 10.1073/pnas.0503562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dodge-Kafka KL, Soughayer J, Pare GC, Carlisle Michel JJ, Langeberg LK, Kapiloff MS, Scott JD. The protein kinase A anchoring protein mAKAP coordinates two integrated cAMP effector pathways. Nature. 2005;437:574–578. doi: 10.1038/nature03966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Berman HM, Ten Eyck LF, Goodsell DS, Haste NM, Kornev A, Taylor SS. The cAMP binding domain: an ancient signaling module. Proc Natl Acad Sci USA. 2005;102:45–50. doi: 10.1073/pnas.0408579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kannan N, Wu J, Anand GS, Yooseph S, Neuwald AF, Venter JC, Taylor SS. Evolution of allostery in the cyclic nucleotide binding module. Genome Biol. 2007;8:R264. doi: 10.1186/gb-2007-8-12-r264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Knighton DR, Zheng JH, Ten Eyck LF, Ashford VA, Xuong NH, Taylor SS, Sowadski JM. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991;253:407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- 118.Su Y, Dostmann WR, Herberg FW, Durick K, Xuong NH, Ten Eyck L, Taylor SS, et al. Regulatory subunit of protein kinase A: structure of deletion mutant with cAMP binding domains. Science. 1995;269:807–813. doi: 10.1126/science.7638597. [DOI] [PubMed] [Google Scholar]
- 119.Kim C, Xuong NH, Taylor SS. Crystal structure of a complex between the catalytic and regulatory (RIalpha) subunits of PKA. Science. 2005;307:690–696. doi: 10.1126/science.1104607. [DOI] [PubMed] [Google Scholar]
- 120.Wu J, Brown SH, von Daake S, Taylor SS. PKA type IIalpha holoenzyme reveals a combinatorial strategy for isoform diversity. Science. 2007;318:274–279. doi: 10.1126/science.1146447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rehmann H, Das J, Knipscheer P, Wittinghofer A, Bos JL. Structure of the cyclic-AMP-responsive exchange factor Epac2 in its auto-inhibited state. Nature. 2006;439:625–628. doi: 10.1038/nature04468. [DOI] [PubMed] [Google Scholar]
- 122.Rehmann H, Rueppel A, Bos JL, Wittinghofer A. Communication between the regulatory and the catalytic region of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem. 2003;278:23508–23514. doi: 10.1074/jbc.M301680200. [DOI] [PubMed] [Google Scholar]
- 123.Kraemer A, Rehmann HR, Cool RH, Theiss C, de Rooij J, Bos JL, Wittinghofer A. Dynamic interaction of cAMP with the Rap guanine-nucleotide exchange factor Epac1. J Mol Biol. 2001;306:1167–1177. doi: 10.1006/jmbi.2001.4444. [DOI] [PubMed] [Google Scholar]
- 124.Rehmann H, Schwede F, Doskeland SO, Wittinghofer A, Bos JL. Ligand-mediated activation of the cAMP-responsive guanine nucleotide exchange factor Epac. J Biol Chem. 2003;278:38548–38556. doi: 10.1074/jbc.M306292200. [DOI] [PubMed] [Google Scholar]
- 125.Rehmann H, Prakash B, Wolf E, Rueppel A, de Rooij J, Bos JL, Wittinghofer A. Structure and regulation of the cAMP-binding domains of Epac2. Nat Struct Biol. 2003;10:26–32. doi: 10.1038/nsb878. [DOI] [PubMed] [Google Scholar]
- 126.Yu S, Mei FC, Lee JC, Cheng X. Probing cAMP-dependent protein kinase holoenzyme complexes I alpha and II beta by FT-IR and chemical protein footprinting. Biochemistry. 2004;43:1908–1920. doi: 10.1021/bi0354435. [DOI] [PubMed] [Google Scholar]
- 127.Yu S, Fan F, Flores SC, Mei F, Cheng X. Dissecting the mechanism of Epac activation via hydrogen-deuterium exchange FT-IR and structural modeling. Biochemistry. 2006;45:15318–15326. doi: 10.1021/bi061701x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brock M, Fan F, Mei FC, Li S, Gessner C, Woods VL, Jr, Cheng X. Conformational analysis of Epac activation using amide hydrogen/deuterium exchange mass spectrometry. J Biol Chem. 2007;282:32256–32263. doi: 10.1074/jbc.M706231200. [DOI] [PubMed] [Google Scholar]
- 129.McKay DB, Steitz TA. Structure of catabolite gene activator protein at 2.9 Å resolution suggests binding to left-handed B-DNA. Nature. 1981;290:744–749. doi: 10.1038/290744a0. [DOI] [PubMed] [Google Scholar]
- 130.Clayton GM, Silverman WR, Heginbotham L, Morais-Cabral JH. Structural basis of igand activation in a cyclic nucleotide regulated potassium channel. Cell. 2004 Nov 24;119(5):615–27. doi: 10.1016/j.cell.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 131.DiPilato LM, Cheng X, Zhang J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc Natl Acad Sci USA. 2004;23;101(47):16513–8. doi: 10.1073/pnas.0405973101. [DOI] [PMC free article] [PubMed] [Google Scholar]