Abstract
Chronic low-grade inflammation has emerged as a key contributor to the cardiovascular complications of diabetes, however, the mechanisms by which diabetes increases inflammation remain poorly understood. Here, we report that exposure to high glucose (HG) stimulates ectodomain shedding of TNF-α from rat aortic smooth muscle cells in culture. Our results show that exposure to HG decreases membrane-associated TNF-α. This decrease in unprocessed TNF-α was prevented by the aldose reductase (AR) inhibitor sorbinil and AR small interference RNA. Treatment with HG, but not equimolar mannitol or 3-O-methyl glucose, resulted in phosphorylation and activation of TNF-α converting enzyme (TACE) (ADAM17), which were attenuated by sorbinil or AR-specific small interference RNA. HG-induced TACE phosphorylation and TNF-α processing were also prevented by TNF-α protease inhibitor-1, an inhibitor of TACE. Inhibition of protein kinase C (PKC)-δ by rottlerin prevented HG-induced TACE activation and the accumulation of unprocessed TNF-α. Treatment with sorbinil decreased elevated levels of circulating TNF-α in streptozotocin-treated diabetic rats. Sorbinil treatment also decreased the expression of TNF-α, matrix metalloproteinase-2, matrix metalloproteinase-9, and increased tissue inhibitor of metalloproteinase-3 in vascular smooth muscle cells treated with HG and in balloon-injured carotid arteries of diabetic rats. These results indicate that HG-induced TNF-α shedding could be attributed to TACE activation, which is regulated, in part, by PKC-δ and AR. Therefore, inhibition of TACE by TNF-α protease inhibitor-1, or pharmacological inhibition of PKC-δ or AR may represent useful strategies for treating vascular inflammation associated with diabetes.
In adrenocortical cells high versus low levels of steroidogenic factor 1 (SF-1) differentially regulates the expression of aldosterone synthase and aldosterone production.
Diabetes is associated with the development of chronic low-grade inflammation (1,2). Extensive clinical evidence demonstrates that both type 1 (3,4) and type 2 diabetes (5,6,7,8) are associated with an increase in circulating markers of inflammation, and that high levels of inflammation increase cardiovascular mortality risk in diabetics (9,10,11). An increase in inflammation has also been reported in tissues of animals with diabetes (12,13). Extant data suggest that inflammation plays a circuitous role in diabetes. An increase in inflammation is a significant feature of the prediabetic states of obesity (1,14), and metabolic syndrome (15), indicating that inflammation may have a causal role in the development of insulin resistance and early pathogenesis of diabetes (7,16). In established diabetes, inflammation is associated with microcirculatory abnormalities (3), vascular dysfunction (5), and atherogenesis (17), suggesting that it is a symptom or a manifestation of diabetic tissue injury.
Mechanisms that link inflammation to the development of diabetes and diabetic complications are poorly understood. Although inflammatory cytokines such as TNF-α have induced insulin resistance by stimulating serine phosphorylation of IRS-1 (18,19), the processes by which diabetes induces and sustains inflammation are less clear. Previous studies show that hyperglycemia is an important contributor to inflammation. In cell culture experiments, high glucose (HG) stimulates inflammatory signaling that activates nuclear factor-κB (NF-κB) (20,21,22). In humans, an increase in plasma glucose leads to an acute increase in the levels of circulating cytokines, even in healthy nondiabetic individuals (23). Our studies show that vascular smooth muscle cells (VSMCs), when exposed to high levels of glucose, secrete TNF-α, which then binds to the TNF-α receptor, leading to stimulation of NF-κB-mediated gene transcription (24). HG-induced release of preformed TNF-α to TNF-α-mediated NF-κB activation could be prevented by soluble TNF receptors, indicating the presence of an autocrine/paracrine loop linking TNF-α release to subsequent NF-κB activation. Nevertheless, the mechanisms by which HG stimulates TNF-α release remain unknown.
TNF-α is initially produced as a membrane-bound precursor that is processed by TNF-α converting enzyme (TACE), a recently identified type I membrane-bound metalloproteinase (ADAM-17) (25,26). TACE is activated by phosphorylation, and activated phospho-TACE cleaves membrane-bound pro-TNF-α, which has a molecular mass of 27 kDa, into an active, soluble form of 19 kDa (27,28). Recent studies show that inhibition of TACE prevents several inflammatory conditions, including those that are associated with hyperglycemia (29,30,31). However, it is unclear whether TACE is expressed in vascular tissues and whether activation of TACE is an essential step in the processing of TNF-α in these tissues when they encounter high levels of glucose. Therefore, the current study was designed to examine the effects of HG on TACE and the role of TACE in regulating TNF-α release. Based on the results of our previous studies on VSMCs, which showed that HG-induced release of TNF-α is prevented by inhibiting the aldose reductase (AR)-mediated activation of protein kinase C (PKC), we investigated the role of AR in regulating HG-induced TACE activation and TNF-α processing. The results presented here suggest that AR is an obligatory mediator of HG-induced TACE activation, and point toward a new link between cardiovascular inflammation and activation of the rate-limiting enzyme of the polyol pathway.
Materials and Methods
Materials
PBS, trypsin, penicillin/streptomycin solution, fetal bovine serum (FBS), DMEM, and Opti-MEM were purchased from Life Technologies, Inc. (Gaithersburg, MD). Antibodies against rat TNF-α were purchased from Calbiochem (San Diego, CA). Antibodies against TACE and tissue inhibitor of metalloproteinase (TIMP)-3 were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-matrix metalloproteinase (MMP)-2 and MMP-9 antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA). The fluorescein isothiocyanate-TNF-α and fluorescein isothiocyanate-IgG were purchased from eBioscience (San Diego, CA). The 7-amino-actinomycin D staining solution was purchased from BD PharMingen (San Diego, CA). Sorbinil was a gift from Pfizer Inc. (New York, NY). The TNF-α protease inhibitor-1 (TAPI-1) and TACE-FRET substrate peptide were purchased from Peptides International Inc. (Louisville, KY). The PKC-δ inhibitor, Rottlerin, was purchased from EMD Biosciences, Inc. (Gibbstown, NJ). AR inhibitors (ARIs) sorbinil and zopolrestat were gifts from Pfizer, and tolrestat was obtained from American Home Products (Wyeth Pharmaceuticals Inc., Philadelphia, PA). All other reagents were of the highest purity available.
Cell culture
Rat VSMCs were isolated from adult rat aorta and characterized by smooth muscle cell-specific α-actin expression. The VSMCs were maintained and grown in endotoxin-free DMEM supplemented with 10% FBS and 1% penicillin/streptomycin at 37 C in a humidified atmosphere of 5% CO2 as described before (32). For glucose-stimulation experiments, the cells were serum starved in medium containing 0.1% serum and 5.5 mm glucose. After 24 h the medium was replaced with fresh medium containing 25 mm glucose (HG) or 5.5 mm glucose [normal glucose (NG)] with ARIs or vehicle (25% dimethylsulfoxide). The high-glucose medium was prepared fresh before use by adding 19.5 mm glucose to DMEM containing 5.5 mm glucose.
RNA interference ablation of AR
Small interference RNA (siRNA) specific to AR was designed, synthesized, and used to ablate AR in VSMCs as described by us earlier (24). Briefly, VSMCs grown in DMEM supplemented with 10% FBS were washed with Opti-MEM and incubated for 60 min with Opti-MEM media before transfection with siRNA oligonucleotides. The cells were incubated with 1 μm AR siRNA or scrambled control oligonucleotides using Lipofectamine Plus (15 μg/ml; Invitrogen Corp., Carlsbad, CA) as the transfection reagent. After 12 h the medium was replaced with fresh DMEM (containing 10% FBS), and the samples were incubated for another 12 h. Using this procedure a VSMC transfection efficacy of approximately 90% was obtained, and the levels of AR protein and activity were reduced by more than 95% (24).
Determination of TACE phosphorylation
Confluent (75–80%) VSMCs were serum starved overnight in DMEM containing 0.1% FBS. The medium was then replaced with fresh phosphate-free DMEM (Invitrogen) containing 0.1% FBS with or without ARIs and 0.5 mCi [33P]orthophosphate (GE Healthcare Bio-Sciences Corp., Piscataway, NJ), and incubated for 30 min before treatment with HG. Cells were washed twice with ice-cold PBS, disrupted in cold Nonidet P-40 (NP-40) lysis buffer containing 50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% NP-40, and 1× protease inhibitor cocktail (Sigma Chemical Co., St. Louis, MO). The lysates were cleared by centrifugation. TACE was immunoprecipitated using 50 μg cell lysate protein incubated overnight with 5 μg anti-TACE at 4 C, and the immune complexes were collected using an immunoprecipitation kit (Sigma Chemical). The immune complexes that were eluted were resolved on SDS-PAGE, followed by autoradiography and densitometric analysis.
Measurement of TACE activity
TACE activity in lysates of VSMCs was measured using a fluorescence-quenching substrate for ADAM17/TNF-α (Peptide International) as described earlier (33). Briefly, VSMCs were treated with HG or HG plus ARI for 5 d, and the cells were collected in TACE-activity buffer containing 50 mm Tris-HCl (pH 7.4), 25 mm ZnCl2, 4% glycerol, and 0.5% NP-40. The cells were lysed by freeze-thaw, and the supernatants were collected. Equal amounts of cell lysates were incubated with 5 μm TACE substrate for 30 min at 37 C, and changes in fluorescence were monitored at excitation wavelength (λex) = 320 nm and emission wavelength (λem) = 420 nm using a fluorescence plate reader. Fluorescence quenching was used to calculate percent activity using appropriate control and blank values.
Western blot analysis
To examine the effect of AR inhibition on HG-induced expression of TIMP-3, TACE, MMP-2, MMP-9, and TNF-α, growth-arrested VSMCs were treated with HG in the absence and presence of ARIs for indicated times. Extracts containing cytoplasmic and membrane-bound proteins were prepared as described earlier (34). Equal amounts of cytosolic or membrane-bound protein extracts were subjected to Western analyses using antibodies directed against TIMP-3, TACE, MMP-2, MMP-9, TNF-α, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and actin. The antigen-antibody complexes were detected by enhanced chemiluminescence (Pierce, Rockford, IL). All blots were probed with either GAPDH or actin as a loading control, and densitometric analysis was performed using Kodak Image station 2000R (Eastman Kodak Co., Rochester, NY).
Balloon injury of carotid arteries
Sprague Dawley rats (250–300 g) were made diabetic by a single injection of streptozotocin (65 mg/kg ip) as described earlier (35). Blood glucose was monitored after 2 wk, and only the rats with blood glucose more than 16 mmol/liter entered the study. Control rats were treated with the vehicle only. Their blood glucose was less than 6 mmol/liter. Four weeks after streptozotocin injection, the carotid arteries were injured by a balloon as described before (21,25). One day before injury and throughout the observation time, 10 rats in each group were gavage fed either tolrestat (15 mg/kg · d) or vehicle (2.5 mm sodium bicarbonate). The injured and uninjured arteries were perfusion fixed with 4% paraformaldehyde and stored in 70% ethanol. All animal protocols were approved by the Institutional Animal Care Committee.
Immunohistochemical studies
For analysis, paraffin sections of the arteries were warmed at 60 C for 1 h and then deparaffinized in xylene. The sections were then rehydrated by passing through 100, 95, 80, and 70% ethanol, and finally washed in deionized water. After blocking the peroxidase activity with 3% H2O2, the slides were rinsed in PBS twice and incubated overnight in the blocking solution (2% BSA, 0.1% Triton X-100, 2% normal rabbit IgG, and 2% normal goat serum) at 4 C. The slides were incubated with antibodies directed against TNF-α, MMP-2, MMP-9, TIMP-3, and TACE for 1 h at room temperature. The slides were stained using universal LSAB+ System-horseradish peroxidase (DakoCytomation, Carpinteria, CA) and counter stained with hematoxylin. The sections were examined under bright-field light microscopy (EPI-800 microscope; Nikon Corp., Tokyo, Japan) and photographed with a Nikon camera fitted to the microscope.
Quantification of TNF-α in rat serum
After indicated treatment, the rats were euthanized, and their blood was collected in heparin-coated Vacutainers (BD, Franklin Lakes, NJ) by heart puncture. The blood was incubated for 1 h at 37 C and centrifuged at 1000 rpm for 20 min. The supernatant (plasma) was collected. To measure TNF-α levels in cultured cell supernatants, after the treatments, collected the cell culture medium and used directly for the assay. Levels of TNF-α were measured according to the manufacturer’s instructions using a rat TNF-α sandwich ELISA kit obtained from BD Biosciences (San Jose, CA). Known concentrations of rat TNF-α were used to generate a standard curve. The assay was linear between 5 and 1000 pg/ml. This was well within the range of TNF-α levels measured in the present study. The reaction was initiated by the addition of 0.1 ml of the serum and read at 450 nm on an ELISA plate reader. The results obtained are expressed as pg/ml plasma.
Statistical analysis
Data are presented as mean ± sem, and the P values were determined using the one-way ANOVA and the unpaired Student’s t test.
Results
HG-induced TNF-α shedding
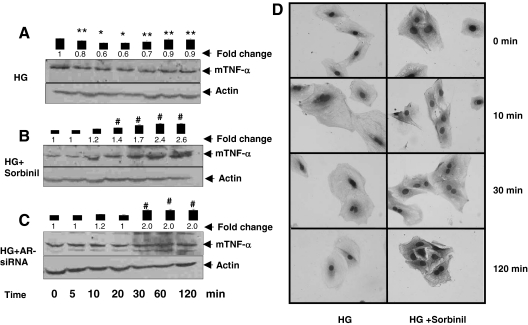
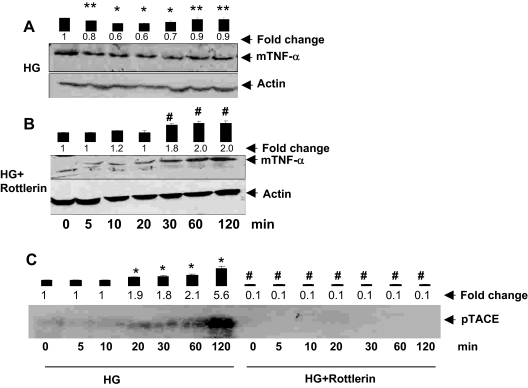
To understand how HG causes TNF-α release, we examined the mechanism of TNF-α processing and shedding. As shown in Fig. 1A, addition of HG to the medium caused a time-dependent decrease in the level of unprocessed TNF-α associated with the cell membrane. Time course studies showed a 30–40% decrease in membrane-associated TNF-α within 20–30 min of HG exposure. However, when the duration of high-glucose exposure was more than 60 min, a partial recovery in the levels of membrane bound TNF-α (mTNF-α) was observed (Fig. 1A). These results indicate that HG rapidly stimulates the shedding of processed TNF-α from the membrane and that this process results in the appearance of TNF-α in the culture media.
Figure 1.
Role of AR in HG-induced shedding of mTNF-α by VSMCs. Serum-starved VSMCs were either left untreated (A) or preincubated (B) with sorbinil (10 μm), and AR-siRNA and control siRNA transfected cells in 5.5 mm glucose (NG) medium were treated with 19.5 mm glucose (HG) for the indicated times (C). Time point 0 min represents NG. Cell extracts containing membranes were subjected to Western analysis as described in Materials and Methods. Western blots were developed using anti-TNF-α and antiactin antibodies. Bars represent mean ± sem; (n = 3) values of normalized intensity measured by densitometric scanning. *, P < 0.001 and **, P < 0.05 vs. 0 min HG; #, P < 0.001 vs. HG. D, Serum-starved VSMCs cultured in NG were treated with sorbinil, followed by incubation with HG for the indicated time points. Cells were fixed and stained with anti-TNF-α antibodies, followed by counterstaining with nuclear stain and were photographed at the indicated times after exposure to HG.
To assess the role of AR in this process, we designed loss-of-function experiments in which AR was inhibited pharmacologically (by sorbinil), or the expression of the AR gene was suppressed by RNA interference (siRNA). As shown in Fig. 1B, preincubation of cells with sorbinil prevented the loss of membrane-associated unprocessed TNF-α in cells cultured in HG. No decrease in membrane-associated TNF-α levels was observed at any of the time points examined. Indeed, there was a significant increase in the abundance of membrane-associated TNF-α when the cells were cultured in HG and ARI, sorbinil for more than 20 min. After 120 min treatment with HG and sorbinil, a 2.6-fold increase in the membrane level of TNF-α was observed. Similar results were observed in cells treated with HG and AR siRNA, which also showed a 2-fold increase in membrane-associated TNF-α after 120 min high-glucose treatment (Fig. 1C). Results of these experiments were further validated by immunocytochemical analysis in which the TNF-α protein was stained in VSMCs using specific anti-TNF-α antibodies. As shown in Fig. 1D, there was a gradual time-dependent decrease in the intensity of cell-associated staining, suggesting that HG stimulates the release of TNF-α from the membrane into the medium. In contrast, cells treated with sorbinil, when exposed to HG, showed a persistent increase in cell-associated staining, which seemed to progressively increase up to a maximal observation time of 120 min. Based on this evidence, we conclude that inhibition of AR prevents HG-induced TNF-α shedding.
Inhibition of AR prevents HG-induced TACE phosphorylation
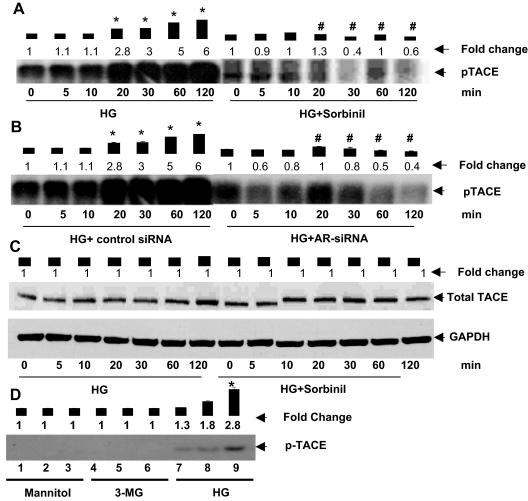
To elucidate further the mechanism of TNF-α shedding, we next examined the role of TACE. TACE is a matrix metalloprotease, which has previously mediated the processing of membrane bound pro-TNF-α to an active soluble form (27,28). Based on our observation that inhibition of AR prevents HG-induced decrease in membrane-associated TNF-α, we tested whether inhibition of AR would also affect phosphorylation and activation of TACE. As shown in Fig. 2A, treatment with HG led to a time-dependent increase in the abundance of phospho-TACE in the cells. A significant increase in TACE phosphorylation was observed within 20 min high-glucose exposure. Between 60 and 120 min exposure, a 5- to 6-fold increase in TACE phosphorylation was observed. These observations suggest that HG stimulates TACE phosphorylation, and that the resultant increase in TACE activity may be responsible for greater cleavage and release of TNF-α from the membrane. To probe the role of AR in this process, the cells were treated with the sorbinil. As shown in Fig. 2A, treatment with sorbinil led to nearly complete inhibition of TACE phosphorylation, and no increase in phospho-TACE was observed for 120 min. Similar results were obtained from cells treated with AR siRNA (Fig. 2B). In these cells no significant increase in HG-induced TACE phosphorylation was observed at any time during observation, although the cells that were treated with control siRNA showed, as expected, a 5- to 6-fold increase in TACE phosphorylation. Under these conditions no changes in the levels of TACE protein were observed when the cells were treated either with HG alone or HG and sorbinil (Fig. 2C). These data indicate that within the observation time, the levels of TACE protein were unaffected by any of the treatment protocols. Furthermore, TACE phosphorylation was not affected by the addition of equimolar concentrations of either mannitol or a metabolically inactive analog of glucose [3-methyl glucose (3-MG)] to the medium (Fig. 2D). Together, these results indicate that inhibition of AR prevents HG-induced TACE phosphorylation and that this effect is not due to osmotic changes induced by the addition of HG to the medium.
Figure 2.
Inhibition of AR prevents HG-induced TACE phosphorylation. Serum-starved VSMCs in 5.5 mm glucose (NG) medium incubated with sorbinil (10 μm) (A) or AR-siRNA transfected cells (B) were treated with 0.5 mCi [33P]orthophosphate for 30 min, followed by stimulation with 19.5 mm glucose (total glucose 25 mm, HG) for the indicated times. Time point 0 min represents NG. The cells were immunoprecipitated with anti-TACE antibodies, and the eluted immune complexes were resolved on SDS-PAGE, followed by autoradiography. C, Growth-arrested VSMCs were treated with HG plus sorbinil for the indicated time periods, and total cell extracts were subjected to Western blot analysis using antibodies against total-TACE and GAPDH. D, Autoradiograph showing phospho-TACE 30 min after treatment in lanes 1, 4, and 7 with 5.5 mm, lanes 2, 5, and 8 with 15 mm, and lanes 3, 6, and 9 with 25 mm d-mannitol, 3-O-methyl glucose, and glucose, respectively. Bars represent values of mean ± sem (n = 3) band intensity normalized to the intensity of the band in cells before the addition of HG. Average fold changes are listed for each treatment. *, P < 0.001 vs. 0 min HG; #, P < 0.001 vs. HG.
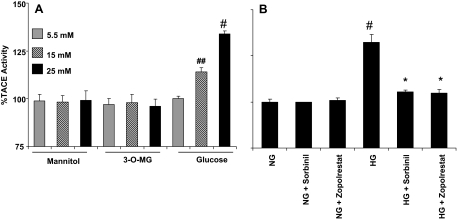
To determine whether the change in phosphorylation corresponds to a change in the protease activity of TACE, we conducted additional experiments in which TACE activity was measured directly using a TACE-specific fluorescent substrate. As shown in Fig. 3A, 15 or 25 mm glucose, but not mannitol or 3-MG, increased TACE activity by approximately 15 and 40%, respectively. In addition, HG (25 mm)-induced increase in TACE activity was prevented by treating the cells with two structurally different ARIs, zopolrestat or sorbinil (Fig. 3B). Similar results obtained with two structurally unrelated inhibitors indicate that the effect is unlikely to be due to a nonspecific effect of a single drug. No change in TACE activity was observed in cells treated with either zopolrestat or sorbinil alone in NG. Together, results of this series of experiments suggest that exposure to HG increases TACE phosphorylation, which increases its catalytic activity.
Figure 3.
Inhibition of AR prevents HG-induced TACE activity. A, VSMCs incubated with medium containing 5.5. 15, and 25 mm glucose, mannitol, and 3-MG were harvested, and TACE activity was determined by incubating equal amounts of cell extracts with 5 μm TACE substrate for 30 min at 37 C. Fluorescence quenching was monitored at excitation wavelength (λex) = 320 nm and emission wavelength (λem) = 420 nm using a fluorescence plate reader. B, VSMCs incubated with NG (5.5 mm) and HG (25 mm) without or with ARIs, zopolrestat and sorbinil, were harvested and measured TACE activity as described in Materials and Methods. Bars represent mean ± sem (n = 4). *, P < 0.001 vs. HG; #, P < 0.001 and ##, P < 0.01 vs. NG.
TAPI-1 inhibits HG-induced TNF-α processing
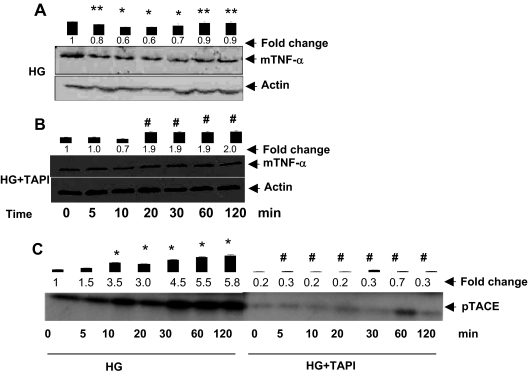
Our experiments so far indicated that exposure to HG leads to an increase in TACE phosphorylation and TNF-α processing. To determine whether TACE phosphorylation and activation are required for increasing TNF-α shedding, we examined the effects of TAPI-1 on HG-induced TNF-α processing (Fig. 4, A and B). Previous studies have shown that TAPI-1 inhibits TACE and that incubation of cells with TAPI-1 prevents TNF-α processing by TACE (36). As before, exposure to HG led to an increase in TACE phosphorylation, however, this increase in phosphorylation was completely prevented in cells treated with TAPI-1 (Fig. 4C), indicating that TAPI-1 is a potent inhibitor of TACE phosphorylation. To examine whether this would affect the processing of TNF-α, the cells were treated with HG in the presence of TAPI-1. Pretreatment with TAPI-1 resulted in the accumulation of unprocessed TNF-α in the membrane of cells treated with HG (Fig. 4, A and B). Similar results were obtained when TNF-α levels were measured by immunocytochemistry (data not shown). Treatment with TAPI-1 led to a progressive increase in TNF-α in the presence of HG (Fig. 4B), indicating that treatment with TAPI-1 prevents HG-induced processing of TNF-α. Together, the results of these experiments suggest that phosphorylation of TACE is required for TNF-α shedding, and that TACE activation and TNF-α shedding are related events in cells exposed to HG. Thus, inhibition of TNF-α shedding by ARIs may be related to inhibition of HG-induced TACE activation.
Figure 4.
TAPI inhibits HG-induced TNF-α shedding and TACE phosphorylation: Serum-starved VSMCs in 5.5 mm glucose (NG) medium incubated without or with TAPI-1 for 30 min, followed by stimulation with HG for the indicated time periods. The cells were treated with HG (A) or HG plus sorbinil (B) and harvested; cell membrane extracts were prepared, and an equal amount of membrane protein was subjected to Western analysis using anti-TNF-α antibodies. C, The VSMCs were treated with 0.5 mCi [33P]orthophosphate for 30 min, followed by stimulation with HG for the indicated times. Time point 0 min represents NG. The cells were immunoprecipitated with anti-TACE antibodies, and the eluted immune complexes were resolved on SDS-PAGE, followed by autoradiography. Bars represent normalized values of mean ± sem (n = 3) intensity measured by densitometric analysis. *, P < 0.001 and **, P < 0.05 vs. 0 min HG; #, P < 0.001 vs. HG.
HG-induced TACE activation is mediated by PKC-δ
Our previous results show that HG stimulates the release of TNF-α from VSMCs and that this increase in TNF-α release is prevented by inhibiting AR, which also inhibits HG-induced PKC-δ activation (24). Therefore, we next examined whether PKC-δ mediates HG-mediated TACE activation. For this, VSMCs were treated with HG in the presence and absence of the PKC-δ inhibitor rottlerin. As shown in the Fig. 5, A and B, pretreatment with rottlerin prevented HG-induced accumulation of unprocessed TNF-α in cell membranes of VSMCs. Similar results were obtained when the cell surface expression of TNF-α in VSMCs was measured immunocytochemically (data not shown). Significantly, pretreatment with rottlerin significantly blunted the HG-induced TACE phosphorylation (Fig. 5C), suggesting that HG-induced processing of TNF-α by TACE is mediated by PKC-δ and that inhibition of PKC-δ prevents HG-induced TACE phosphorylation.
Figure 5.
Inhibition of PKC-δ prevents HG-induced TACE phosphorylation: Serum-starved VSMCs in 5.5 mm glucose (NG) medium incubated without or with rottlerin (2 μm) for 30 min, followed by stimulation with HG for the indicated time. The cells were treated with HG (A) and HG plus rottlerin (B) and harvested; cell membrane extracts were prepared, and an equal amount of membrane protein was subjected to Western analysis using anti-TNF-α antibodies. C, VSMCs were treated with 0.5 mCi [33P]orthophosphate for 30 min, followed by stimulation with HG for the indicated time. Time point 0 min represents NG. The cells were immunoprecipitated with anti-TACE antibodies, and the eluted immune complexes were resolved on SDS-PAGE, followed by autoradiography. Bars represent normalized values of mean ± sem (n = 3) intensity measured by densitometry analysis. *, P < 0.001 and **, P < 0.05 vs. 0 min HG; #, P < 0.001 vs. HG.
Effect of AR inhibition on HG-induced changes in TIMP-3 and MMP expression
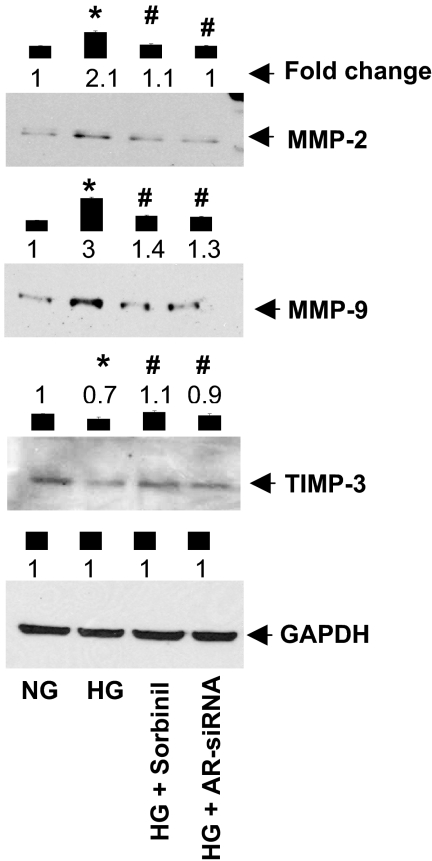
In addition to phosphorylation, TACE is also regulated by the TIMP-3. TIMP-3 is a natural inhibitor of MMPs, including TACE, and plays a key role in maintaining homeostasis of the tissue extracellular matrix (31). The expression of several MMPs and TIMPs is significantly altered during diabetes, which dysregulates the mitogenic, genetic, and metabolic effects of cytokines such as TNF-α (37,38). Therefore, we next examined the effect of AR inhibition or ablation on HG-induced expression of MMP2, MMP9, and TIMP-3 in VSMCs. As shown in Fig. 6, the expression of MMP2 and MMP9 was significantly elevated, whereas TIMP-3 expression was decreased in cells treated with HG. This was prevented by inhibiting AR activity by sorbinil or by treating cell with AR siRNA. These observations suggest that AR maintains the homeostasis in the expression of MMPs and TIMPs, and thereby controls HG-induced TNF-α signaling.
Figure 6.
Inhibition of AR prevents HG-induced expression of matrix metalloproteases. Serum-starved VSMCs, in 5.5 mm glucose (NG) medium, incubated with sorbinil (10 μm) or AR-siRNA transfected cells were treated with HG for 24 h. The cells were then harvested, and whole cell extracts were prepared. Equal amounts of protein were subjected to Western analysis using antibodies directed against MMP-2, MMP-9, TIMP-3, and GAPDH. Bars represent normalized values of mean ± sem (n = 3) intensity measured by densitometry analysis. *, P < 0.001 vs. NG; #, P < 0.001 vs. HG.
Inhibition of AR diminishes TNF-α levels during diabetes
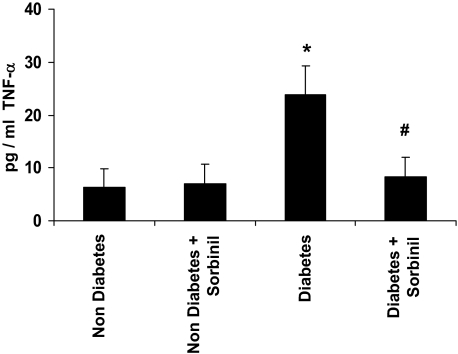
Based on our in vitro data suggesting that AR regulates HG-induced activation of TNF-α by TACE, we next examined whether inhibition of AR would affect TNF-α levels during diabetes. For this we used a streptozotocin model of diabetes in rats that resembles human type 1 diabetes (T1D). We chose a T1D model because it is simpler, and the changes in cytokines in T1D models, unlike type 2 diabetes models, do not affect the extent of hyperglycemia due to changes in pancreatic secretion of insulin (i.e. there is no feed-forward loop). Therefore, in T1D models the strength of the hyperglycemic insults remains unchanged, even if the cytokine production is decreased. Using adult rats with 4 wk diabetes (blood sugar 19 ± 3 mmol/liter), we evaluated the effect of AR inhibition on plasma and tissue levels of TNF-α. Plasma from diabetic rats showed a nearly 3-fold higher TNF-α content as measured by ELISA (Fig. 7). In contrast, the TNF-α levels of diabetic rats treated with sorbinil were similar to those observed in nondiabetic rats. Treatment with sorbinil did not affect TNF-α levels in nondiabetic rats. These observations suggest that inhibition of AR decreases systemic inflammation in streptozotocin-diabetic rats.
Figure 7.
Inhibition of AR prevents TNF-α secretion in diabetic rats. Diabetes was induced in adult Sprague Dawley rats by injection with a single dose of streptozotocin (65 mg/kg body weight ip). After 4 wk diabetes, the mice were treated either with vehicle or the ARI sorbinil at a dose of 25 mg/kg/body weight · d ip for 4 wk. The levels of TNF-α were measured in the serum by ELISA. Data represent mean ± sd (n = 4). *, P < 0.001 vs. nondiabetic and #, P < 0.001 vs. diabetic rats.
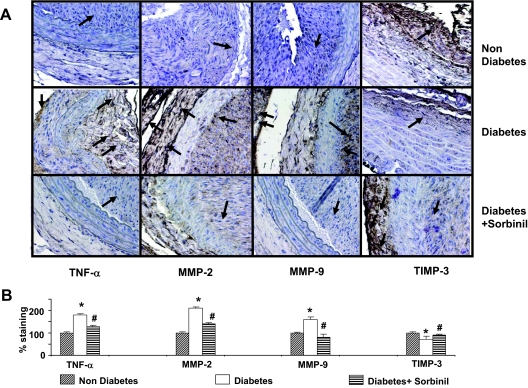
To examine how inhibition of AR affects diabetes-induced changes in arterial lesions, we used the balloon-injury model of carotid arteries. Previous studies show that diabetes accelerates and exaggerates neointima formation in response to injury (39). Our previous studies using this model have shown that inhibition of AR prevents neointimal hyperplasia (32). Immunohistochemical analysis of arterial lesion 10 d after injury revealed higher staining with anti-TNF-α antibody in diabetic vessels when compared with nondiabetic vessels (Fig. 8, A and B), indicating that diabetes increases the tissue levels of TNF-α. In addition to TNF-α, diabetic lesions showed greater levels of MMP-2 and MMP-9, and decreased expression of TIMP-3 as evidenced by immunostaining of arterial lesions with anti-MMP-2, anti-MMP-9, and TIMP-3 (Fig. 8, A and B). Interestingly, even though TNF-α was diffusely distributed in the media and neointima, both MMP-2 and TIMP3 were largely associated with the adventitia. MMP-9 on the other hand was localized to the neointima and the adventitia but was absent from the media (Fig. 8A). When the rats were treated 1 d before and then for 10 d after balloon injury with sorbinil (10 mg/kg · d), the intensity of staining with anti-TNF-α antibody was significantly decreased. A similar decrease in the MMP-2, MMP-9, and higher staining compared with diabetes animals for TIMP-3 was observed in sorbinil-treated animals. Together, these observations suggest that diabetes leads to an increase in vascular inflammation as evidenced by an increase in TNF-α, MMP-2, and MMP-9, and a decrease in TIMP-3. These changes are prevented in animals treated with ARIs.
Figure 8.
Inhibition of AR prevents the expression of MMPs and TIMP-3 in balloon-injured rat carotid arteries. The carotid artery of nondiabetic and diabetic rats was injured by a balloon as described in Materials and Methods. A, Ten days after injury, the uninjured (control) and injured arteries were harvested, fixed by paraformaldehyde treatment, and stained with antibodies raised against TNF-α, MMP-2, MMP-9, and TIMP-3 using the LSAB+ System-horseradish peroxidase. Photomicrographs of the stained sections were acquired using an EPI-800 microscope (bright-field) connected to a Nikon camera. Percent staining was determined by measuring positive immunoreactivity per unit area. Arrows represent the area for positive staining for an antigen. B, The intensity of antigen staining was quantified by digital image analysis. The bar graphs show means ± sd (n = 3). *, P < 0.01 vs. nondiabetic and #, P < 0.01 vs. diabetic rats.
Discussion
Cardiovascular complications are the leading cause of morbidity and mortality in patients with diabetes (40). Although the mechanisms by which diabetes affects cardiovascular function and health remain unclear, it is currently believed that HG induces low-grade vascular inflammation (1,2). This increase in inflammation has been linked to accelerated atherogenesis (41), an increased incidence of myocardial infarction (42,43) and cardiomyopathy (44,45), and a greater propensity for restenosis after balloon angioplasty (46). Extensive evidence suggests that exposure to HG increases the activity of the transcription factor NF-κB in tissues in which glucose uptake is not regulated by insulin (47). A similar increase in NF-κB activity has been reported in tissues and mononuclear cells in animal models of diabetes (20,48) and in diabetic subjects (49). HG and diabetes have also been associated with an increase in tissue and plasma levels of cytokines such as TNF-α (23). Nevertheless, mechanisms by which HG increases inflammation are not well understood.
High levels of glucose induce a wide range of metabolic derangements that occur from several biochemical abnormalities. The major biochemical abnormalities induced by HG are thought to be due to: 1) excessive flux of glucose through the polyol pathway, which reduces glucose to sorbitol; 2) activation of PKC isoforms; and 3) increased accumulation of advanced glycosylation end products (50). Although multiple studies present convincing data showing that these pathways are activated in tissues exposed to HG (22,24,32,50), the mechanisms by which they induce and increase inflammation remain unclear. Previous studies show that inhibition of advanced glycosylation end product formation decreases atherosclerotic lesion formation in diabetic mice, and that inhibition of PKC ameliorates diabetic nephropathy and neuropathy (51). Studies in our laboratory have shown that HG-induced activation of PKC and NF-κB could be prevented by inhibiting AR (24,32,52,53), indicating that HG-induced increase in inflammation is in part due to activation of the polyol pathway. In addition, we have found that when VSMCs are exposed to HG, they secrete TNF-α into the media (24). This TNF-α then acts in a paracrine/autocrine fashion to stimulate the activity of NF-κB, which in turn increases the transcription of cytokine genes and stimulates cell growth (24,50). Consistent with a central role of early release of TNF-α in HG-induced inflammation, we have found that treatment with soluble TNF-α receptors or anti-TNF-α antibodies prevents HG-induced mitogenesis and NF-κB activation (24). Our current data extend these observations and demonstrate that HG stimulates the processing of preformed TNF-α by a mechanism that depends upon the activation of PKC and the polyol pathway.
TNF-α is a central mediator of inflammatory responses. Its synthesis in most cells is stimulated by NF-κB, which binds to the promoter of the TNF-α gene (54). TNF-α is synthesized in a pro-form, and has to be processed to an active form for secretion and binding to the TNF-α receptor (55,56). Processing mechanisms of TNF resemble those of other cytokines and growth factors such as TGF-β and epidermal growth factor (57). The protease responsible for TNF-α cleavage has been identified to be TACE or ADAM17 (58). TACE is a zinc-dependent membrane-bound disintegrin metalloproteinase that is responsible for ectodomain shedding of cytokines and growth factors such as TNF-α or TGF-α (57,58). In agreement with its role in TNF-α processing, we found that an increase in TNF-α shedding in cells exposed to HG (Fig. 1) was associated with a corresponding increase in TACE phosphorylation (Fig. 2). That TACE activation is essential for HG-induced TNF-α ectodomain shedding is indicated by the observation that treatment with TAP1–1 prevented TACE phosphorylation and the release of TNF-α from membrane-associated sites (Fig. 4).
Our results also indicate that the mechanisms that regulate HG-induced TNF shedding relate to the activation of the polyol pathway and PKC-δ. The central role of the polyol pathway in regulating TACE activation and TNF-α shedding is consistent with several lines of evidence showing that inhibition of AR prevents: 1) TNF-α shedding in VSMCs exposed to HG, 2) phosphorylation and activation of TACE in these cells, and 3) the increase in TNF-α in the plasma and arterial lesion of diabetic animals. Collectively, these data suggest that AR is an obligatory mediator of HG-induced TNF-shedding because it is required for the activation and phosphorylation of TACE. Moreover, the observation that treatment with ARIs decreases TNF-α levels in diabetic rats provides new and compelling evidence supporting a proinflammatory role of this enzyme in diabetes. Previous studies show that obesity and diabetes increase the expression of inflammatory cytokines such as TNF-α in adipose tissue, liver, as well as skeletal muscle, and that these cytokines contribute to insulin resistance (1). However, in the context of hyperglycemia, the increase in intracellular glucose in tissues with insulin-insensitive glucose transport, such as endothelial, smooth muscle, or renal cells or neurons, are likely to be significant sources of inflammatory cytokines. This is consistent with our observation that inhibition of AR, which prevents HG-induced cytokine production, decreases circulating levels of cytokines in diabetic rats. Therefore, even though the tissue sources of cytokine generation during diabetes remain unclear, it is likely that inhibition of AR by preventing glucose-induced changes in noninsulin-dependent tissues could decrease the overall cytokine load during diabetes. Because cytokine production is regenerative, inhibition of these sources could also decrease inflammation in insulin-sensitive tissues such as heart and skeletal muscle, which become hypoglycemic, and those such as the liver that do not express much AR (59).
Although the mechanisms by which inhibition of AR prevents inflammation and hyperglycemic injury remain unclear, our studies suggest that the beneficial effects of AR inhibition could be, in part, attributable to inhibition of PKC isoforms. Both cytokines and HG activate several PKC isoforms. In VSMCs, exposure to HG results in the activation of several PKC isoforms, including, PKCβ1, β2, ε, and δ (52). Simultaneous activation of several PKC isoforms is due to an increase in the synthesis of diacylglycerol (DAG), which is an essential cofactor and activator of classical as well as novel PKC isoforms (60,61). Our studies show that inhibition of AR prevents DAG formation, and thereby simultaneously affects the activation of both classical and novel PKCs (52). The present study showing that treatment with the PKC-δ inhibitor rottlerin prevents TACE phosphorylation and TNF-α shedding (Fig. 5) suggests that HG-induced TNF-α shedding is mediated by PKC-δ-dependent activation of TACE and that inhibition of AR diminishes TNF-α shedding by preventing PKC-δ activation. Although several previous studies show that ectodomain shedding of growth factors and cytokines is regulated by PKC (62), to the best of our knowledge, the present study is the first to show that PKC-δ is required for TACE activation.
PKC-δ is strongly activated by HG and diabetes along with PKC-β1 and β2 (52,63), and has been linked to endothelial cell apoptosis in HG (64), nonetheless, its role as a mediator of the cardiovascular complications of diabetes is unclear. Moreover, even though significant attention has been divested in studying and treating diabetic activation of PKC-β, and PKC-β inhibitors have been effective in attenuating diabetic retinopathy and nephropathy (65,66), an essential role of PKC-δ in regulating TACE activation and TNF-α shedding demonstrated here suggests that PKC-δ inhibitors could also be useful in preventing or treating diabetic complications. Alternatively, it may be even more beneficial to prevent the activation of all PKC isoforms simultaneously by preventing glucose-induced DAG accumulation. This could be accomplished by either increasing DAG kinase activity, e.g. by peroxisome proliferator activated receptor-γ activation (67), or by preventing DAG formation by inhibiting AR (52). In either case, it appears that interrupting signaling events upstream to DAG formation is likely to provide more extensive protection than inhibition of individual PKC isoforms.
Several studies show that PKC regulates TACE phosphorylation and activation. For example, TNF-α release and TACE phosphorylation in ionomycin-stimulated RBL-2H3 cells have required PKC (68). Similarly, phorbol ester-activated TACE-induced release of meprin-β is prevented by PKC inhibition (69). Despite these observations, the molecular mechanisms by which PKC regulates TACE have not been fully elucidated. In human airway epithelial cells, TACE is activated by reactive oxygen species (ROS), and PKC inhibitors prevent TACE activation by preventing ROS generation (70), indicating that PKC is an indirect regulator of TACE and that this regulation is mediated by ROS. In contrast, PKC-δ has been shown to bind directly to ADAM (MDC9) to stimulate the shedding of heparin-binding epidermal growth factor-like growth factor (71). PKC-δ could also induce TACE phosphorylation by activating ERKs, which have phosphorylated Thr-735 of TACE (72). Clearly, additional studies are required to elucidate the specific mechanism by which PKC-δ regulates HG-induced TACE phosphorylation and activation. Finally, because TACE-mediated shedding is not limited to TNF-α, and TACE has been involved in cleaving the membrane-anchored forms of several other proteins such as TGF-α (73), L-selectin (74), TNF-receptor-II (75), and fractalkine (76), inhibition of TACE, by TACE-specific inhibitors, or by inhibiting PKC-δ or AR, could potentially, at the same time, prevent several aspects of HG-induced inflammation, cell adhesion, and abnormal cell growth.
Footnotes
This work was supported by National Institutes of Health Grants DK36118 (to S.K.S.), ES11860, HL55477 (to A.B.), and GM71036 (to K.V.R.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online September 4, 2008
Abbreviations: AR, Aldose reductase; ARI, aldose reductase inhibitor; DAG, diacylglycerol; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HG, high glucose (25 mm); MMP, matrix metalloproteinase; mTNF-α, membrane bound TNF-α; 3-MG, 3-methyl glucose; NF-κB, nuclear factor-κB; NG, normal glucose (5.5 mM); NP-40, Nonidet P-40; PKC, protein kinase C; ROS, reactive oxygen species; siRNA, small interference RNA; TACE, TNF-α converting enzyme; TAPI-1, TNF-α protease inhibitor-1; TIMP, tissue inhibitor of metalloproteinase; T1D, type 1 diabetes; VSMC, vascular smooth muscle cell.
References
- Wallen KE, Hotamisligil GS 2005 Inflammation, stress, and diabetes. J Clin Invest 115:1111–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM 2003 Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 41:1071–1077 [DOI] [PubMed] [Google Scholar]
- Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glaser N, Aoki T 2007 Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes 56:2790–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaraj S, Glaser N, Griffen S, Wang-Polagruto J, Miguelino E, Jialal I 2006 Increased monocytic activity and biomarkers of inflammation in patients with type 1 diabetes. Diabetes 55:774–779 [DOI] [PubMed] [Google Scholar]
- Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, Ferrannini E 2006 Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes 55:1133–1140 [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Williams K, Gonzalez C, D'Agostino Jr RB, Wagenknecht LE, Stern MP, Haffner SM, San Antonio Heart Study, Mexico City Diabetes Study, Insulin Resistance Atherosclerosis Study 2004 Prediction of type 2 diabetes using simple measures of insulin resistance: combined results from the San Antonio Heart Study, the Mexico City Diabetes Study, and the Insulin Resistance Atherosclerosis Study. Diabetes [Erratum (2003) 52:1306] 52:463–469 [DOI] [PubMed] [Google Scholar]
- Helmsersson J, Vessby B, Larsson A, Basu S 2004 Association of type 2 diabetes with cyclooxygenase-mediated inflammation and oxidative stress in an elderly population. Circulation 109:1729–1734 [DOI] [PubMed] [Google Scholar]
- Duncan BB, Schmidt MI, Pankow JS, Ballantyne CM, Couper D, Vigo A, Hoogeveen R, Folsom AR, Heiss G, Atherosclerosis Risk in Communities Study 2003 Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes 52:1799–1805 [DOI] [PubMed] [Google Scholar]
- De Jager J, Dekker JM, Kooy A, Kostense PJ, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD 2006 Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler Thromb Vasc Biol 26:1086–1093 [DOI] [PubMed] [Google Scholar]
- Engström G, Stavenow L, Hedblad B, Lind P, Eriksson K, Janzon L, Lindgärde F 2003 Inflammation-sensitive plasma proteins, diabetes, and mortality and incidence of myocardial infarction and stroke: a population-based study. Diabetes 52:442–447 [DOI] [PubMed] [Google Scholar]
- Stehouwer CD, Gall M, Twisk JW, Knudsen E, Emeis JJ, Parving HH 2002 Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes 51:1157–1165 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y 2003 Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation 108:472–478 [DOI] [PubMed] [Google Scholar]
- Takeda R, Suzuki E, Satonaka H, Oba S, Nishimatsu H, Omata M, Fujita T, Nagai R, Hirata Y 2005 Blockade of endogenous cytokines mitigates neointimal formation in obese Zucker rats. Circulation 111:1398–1406 [DOI] [PubMed] [Google Scholar]
- Dandona P, Aljada A, Bandyopadhyay A 2004 Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25:4–7 [DOI] [PubMed] [Google Scholar]
- Hanley AJ, Festa A, D'Agostino RB, Wagenknecht LE, Savage PJ, Tracy RP, Saad MF, Haffner SM 2004 Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes 53:1773–1781 [DOI] [PubMed] [Google Scholar]
- Ferrannini E 1986 Insulin resistance, insulin deficiency and the pathogenesis of diabetes mellitus. Clin Physiol 6:311–317 [DOI] [PubMed] [Google Scholar]
- Nigro J, Osman N, Dart AM, Little PJ 2006 Insulin resistance and atherosclerosis. Endocr Rev 27:242–259 [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM 1994 Tumor necrosis factor-α inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA 91:4854–4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB 2006 Inflammation and insulin resistance. J Clin Invest 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerneni KK, Bai W, Khan BV, Medford RM, Natarajan R 1999 Diabetes hyperglycemia-induced activation of nuclear transcription factor κB in vascular smooth muscle cells. Diabetes 48:855–864 [DOI] [PubMed] [Google Scholar]
- Chen S, Mukherjee S, Chakraborty C, Chakrabarti S 2003 High glucose-induced, endothelin-dependent fibronectin synthesis is mediated via NF-κ B and AP-1. Am J Physiol Cell Physiol 284:C263–C272 [DOI] [PubMed] [Google Scholar]
- Ramana KV Chandra D, Srivastava S, Bhatnagar A, Srivastava SK 2003 Nitric oxide regulates the polyol pathway of glucose metabolism in vascular smooth muscle cells. FASEB J 17:417–425 [DOI] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D 2002 Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation 106:2067–2072 [DOI] [PubMed] [Google Scholar]
- Ramana KV, Ramana KV, Tammali R, Reddy AB, Bhatnagar A, Srivastava SK 2007 Aldose reductase-regulated tumor necrosis factor-α production is essential for high glucose-induced vascular smooth muscle cell growth. Endocrinology 148:4371–4384 [DOI] [PubMed] [Google Scholar]
- Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP 1997 A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 385:729–733 [DOI] [PubMed] [Google Scholar]
- Moss ML, Jin SL, Milla ME, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassler D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Becherer JD 1997 Cloning of a disintegrin metalloproteinase that processes precursor tumour necrosis factor-α. Nature 385:733–736 [DOI] [PubMed] [Google Scholar]
- Rosendahl MS, Ko SC, Long DL, Brewer MT, Rosenzweig B, Hedl E, Anderson L, Pyle SM, Moreland J, Meyers MA, Kohno T, Lyons D, Lichtenstein HS 1997 Identification and characterization of a pro-tumor necrosis factor-α-processing enzyme from the ADAM family of zinc metalloproteases. J Biol Chem 272:24588–24593 [DOI] [PubMed] [Google Scholar]
- Lunn CA, Fan X, Dalie B, Miller K, Zavodny PJ, Narula SK, Lundell D 1997 Purification of ADAM10 from bovine spleen as a TNF-α convertase. FEBS Lett 400:333–335 [DOI] [PubMed] [Google Scholar]
- Patel IR, Attur MG, Patel RN, Stuchin SA, Abagyan RA, Abramson SB, Amin AR 1998 TNF-α convertase enzyme from human arthritis-affected cartilage: isolation of cDNA by differential display, expression of the active enzyme, and regulation of TNF-α. J Immunol 160:4570–4579 [PubMed] [Google Scholar]
- Doggrell SA 2002 TACE inhibition: a new approach to treating inflammation. Expert Opin Investig Drugs 11:1003–1006 [DOI] [PubMed] [Google Scholar]
- Federici M, Hribal ML, Menghini R, Kanno H, Marchetti V, Porzio O, Sunnarborg SW, Rizza S, Serino M, Cunsolo V, Lauro D, Mauriello A, Smookler DS, Sbraccia P, Sesti G, Lee DC, Khokha R, Accili D, Lauro R 2005 Timp3 deficiency in insulin receptor—haploinsufficient mice promotes diabetes and vascular inflammation via increased TNF-α. J Clin Invest 115:3494–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Ramana KV, Tammali R, Srivastava SK, Bhatnagar A 2006 Contribution of aldose reductase to diabetic hyperproliferation of vascular smooth muscle cells. Diabetes 55:901–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin G, Huang X, Black R, Wolfson M, Rauch C, McGregor H, Ellestad G, Cowling R 2002 A continuous fluorimetric assay for tumor necrosis factor-α converting enzyme. Anal Biochem 302:269–275 [DOI] [PubMed] [Google Scholar]
- Nilsson P, Hillered L, Ponten U, Ungerstedt U 1990 Changes in cortical extracellular levels of energy-related metabolites and amino acids following concussive brain injury in rats. J Cereb Blood Flow Metab 10:631–637 [DOI] [PubMed] [Google Scholar]
- Chandra D, Jackson EB, Ramana KV, Kelley R, Srivastava SK, Bhatnagar A 2002 Nitric oxide prevents aldose reductase activation and sorbitol accumulation during diabetes. Diabetes. 51:3095–3101 [DOI] [PubMed] [Google Scholar]
- Gómez MI, Sokol SH, Muir AB, Soong G, Bastien J, Prince AS 2005 Bacterial induction of TNF-α converting enzyme expression and IL-6 receptor α shedding regulates airway inflammatory signaling. J Immunol 175:1930–1936 [DOI] [PubMed] [Google Scholar]
- Amour A, Slocombe PM, Webster A, Butler M, Knight CG, Smith B J, Stephens PE, Shelley C, Hutton M, Knauper V, Docherty AJ, Murphy G 1998 TNF-α converting enzyme (TACE) is inhibited by TIMP-3. FEBS Lett 435:39–44 [DOI] [PubMed] [Google Scholar]
- Kadoglou NP, Daskalopoulou SS, Perrea D, Liapis CD 2005 Matrix metalloproteinases and diabetic vascular complications. Angiology 56:173–189 [DOI] [PubMed] [Google Scholar]
- Phillips JW, Barringhaus KG, Sanders JM, Yang Z, Chen M, Hesselbacher S, Czarnik AC, Ley K, Nadler J, Sarembock IJ 2003 Rosiglitazone reduces the accelerated neointima formation after arterial injury in a mouse injury model of type 2 diabetes. Circulation 108:1994–1999 [DOI] [PubMed] [Google Scholar]
- Reusch JE 2003 Diabetes, microvascular complications, and cardiovascular complications: what is it about glucose? J Clin Invest 112:986–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuand JT, Wu LL 2006 Linking inflammation and atherogenesis: soluble markers identified for the detection of risk factors and for early risk assessment. Clin Chim Acta 366:74–80 [DOI] [PubMed] [Google Scholar]
- Oswald G, Smith C, Betteridge J, Yudkin J 1986 Determinants and importance of stress hyperglycaemia in non-diabetic patients with myocardial infarction. Br Med J (Clin Res Ed) 293:917–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norhammar A, Tenerz A, Nilsson G, Hamsten A, Efendíc P, Rydén L, Malmberg K 2002 Glucose metabolism in patients with acute myocardial infarction and no previous diagnosis of diabetes mellitus: a prospective study. Lancet 359:2140–2144 [DOI] [PubMed] [Google Scholar]
- Galderisi M, Anderson KM, Wilson PWF, Levy D 1991 Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study). Am J Cardiol 68:85–89 [DOI] [PubMed] [Google Scholar]
- Westermann D, Van Linthout S, Dhayat S, Dhayat N, Escher F, Bücker-Gärtner C, Spillmann F, Noutsias M, Riad A, Schultheiss HP, Tschöpe C 2007 Cardioprotective and anti-inflammatory effects of interleukin converting enzyme inhibition in experimental diabetic cardiomyopathy. Diabetes 56:1834–1841 [DOI] [PubMed] [Google Scholar]
- Stein B, Weintraub WS, Gebhart SSP Cohen-Bernstein CL, Grosswald R, Liberman HA, Douglas JS, Morris DC, King SB 1995 Influence of diabetes mellitus on early and late outcome after percutaneous transluminal coronary angioplasty. Circulation 91:979–989 [DOI] [PubMed] [Google Scholar]
- Wei Y, Chen K, Whaley-Connell AT, Stump CS, Ibdah JA, Sowers JR 2008 Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol 294:R673–R680 [DOI] [PubMed] [Google Scholar]
- Nam JS, Cho MH, Lee GT, Park JS, Ahn CW, Cha BS, Lim SK, Kim KR, Ha HJ, Lee HC 2008 The activation of NF-κB and AP-1 in peripheral blood mononuclear cells isolated from patients with diabetic nephropathy. Diabetes Res Clin Pract 81:25–32 [DOI] [PubMed] [Google Scholar]
- Yang B, Hodgkinson A, Oates PJ, Milward BA, Demaine AG 2008 High glucose induction of DNA-binding activity of the transcription factor NF-κB in patients with diabetic nephropathy. Biochim Biophys Acta 1782:295–302 [DOI] [PubMed] [Google Scholar]
- Srivastava SK, Ramana KV, Bhatnagar A 2005 Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev 26:380–392 [DOI] [PubMed] [Google Scholar]
- Kim H, Sasaki T, Maeda K, Koya D, Kashiwagi A, Yasuda H 2003 Protein kinase Cβ selective inhibitor LY333531 attenuates diabetic hyperalgesia through ameliorating cGMP level of dorsal root ganglion neurons. Diabetes 52:2102–2109 [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK 2005 Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes 54:818–829 [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK 2004 Activation of nuclear factor-κB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes 53:910–920 [DOI] [PubMed] [Google Scholar]
- Liu H, Sidiropoulos P, Song G, Pagliari LJ, Birrer MJ, Stein B, Anrather J, Pope RM 2000 TNF-α gene expression in macrophages: regulation by NF-κ B is independent of c-Jun or C/EBP β. J Immunol 164: 4277–4285 [DOI] [PubMed] [Google Scholar]
- Kriegler M, Perez C, DeFay K, Albert I, Lu SD 1988 A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell 53:45–53 [DOI] [PubMed] [Google Scholar]
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woolley K 1994 Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 370:555–557 [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, Boyce RW, Nelson N, Kozlosky CJ, Wolfson MF, Rauch CT, Cerretti DP, Paxton RJ, March CJ, Black RA 1998 An essential role for ectodomain shedding in mammalian development. Science 282:1281–1284 [DOI] [PubMed] [Google Scholar]
- Edwards DR, Handsley MM, Pennington CJ 2008 The ADAM metalloproteinases. Mol Aspects Med 29: 258–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A, Stevens VJ, Monnier VM 1979 Role of non-enzymatic glycosylation in the development of the sequelae of diabetes mellitus. Metabolism 38:431–438 [DOI] [PubMed] [Google Scholar]
- Mellor H, Parker PJ 1998 The extended protein kinase C superfamily. Biochem J 332(Pt 2):281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y 1992 Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 258:607–614 [DOI] [PubMed] [Google Scholar]
- Arribas J, Borroto A 2002 Protein ectodomain shedding. Chem Rev 102:4627–4638 [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL 1992 Preferential elevation of protein kinase C isoform β II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA 89:11059–11063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H 2000 High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes 49:1939–1945 [DOI] [PubMed] [Google Scholar]
- Tuttle KR, Bakris GL, Toto RD, McGill JB, Hu K, Anderson PW 2005 The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care 28:2686–2690 [DOI] [PubMed] [Google Scholar]
- Aiello LP, Davis MD, Girach A, Kles KA, Milton RC, Sheetz MJ, Arora V, Vignati L 2006 Effect of ruboxistaurin on visual loss in patients with diabetic retinopathy. Ophthalmology 113:2221–2230 [DOI] [PubMed] [Google Scholar]
- Panchapakesan U, Chen X, Carol A 2005 Drug insight: thiazolidinediones and diabetic nephropathy—relevance to renoprotection. Nat Clin Pract Nephrol 1:33–43 [DOI] [PubMed] [Google Scholar]
- Abdel-Raheem IT, Hide I, Yanase Y, Shigemoto-Mogami Y, Sakai N, Shirai Y, Saito N, Hamada FM, El-Mahdy NA, Elsisy AE, Sokar SS, Nakata Y 2005 Protein kinase C-α mediates TNF release process in RBL-2H3 mast cells. Br J Pharmacol 145:415–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn D, Pischitzis A, Roesmann S, Hansen SK, Leuenberger B, Luginbuehl U, Sterchi EE 2003 Phorbol 12-myristate 13-acetate-induced ectodomain shedding and phosphorylation of the human meprinβ metalloprotease. J Biol Chem 278:42829–42839 [DOI] [PubMed] [Google Scholar]
- Nade JA 2007 Innate immune mucin production via epithelial cell surface signaling: relationship to allergic disease. Curr Opin Allergy Clin Immunol 7:57–62 [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hirata M, Hasuwa H, Iwamoto R, Umata T, Miyado K, Tamai Y, Kurisaki T, Sehara-Fujisawa A, Ohno S Mekada E 1998 A metalloprotease-disintegrin, MDC9/meltrin-γ/ADAM9 and PKCδ are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. EMBO J 17:7260–7272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soond SM, Everson B, Riches DW, Murphy G 2005 ERK-mediated phosphorylation of Thr735 in TNFα-converting enzyme and its potential role in TACE protein trafficking. J Cell Sci 118(Pt 11):2371–2380 [DOI] [PubMed] [Google Scholar]
- Arribas J, Lâopez-Casillas F, Massaguâe J 1997 Role of the juxtamembrane domains of the transforming growth factor-α precursor and the β-amyloid precursor protein in regulated ectodomain shedding. J Biol Chem 272:17160–17165 [DOI] [PubMed] [Google Scholar]
- Borland G, Murphy G, Ager A 1999 Tissue inhibitor of metalloproteinases-3 inhibits shedding of L-selectin from leukocytes. J Biol Chem 274:2810–2815 [DOI] [PubMed] [Google Scholar]
- Reddy P, Slack JL, Davis R, Cerretti DP, Kozlosky CJ, Blanton RA, Shows D, Peschon JJ, Black RA 2000 Functional analysis of the domain structure of tumor necrosis factor-α converting enzyme. J Biol Chem 275:14608–14614 [DOI] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, Raines EW 2001 Tumor necrosis factor-α converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem 276: 37993–38001 [DOI] [PubMed] [Google Scholar]