Abstract
The pharmacologic effects of (S)-N-(4-cyano-3-trifluoromethyl-phenyl)-3-(3-fluoro, 4-chlorophenoxy)-2-hydroxy-2-methyl-propanamide (S-23) were characterized in male rats as an animal model of hormonal male contraception. S-23 showed high binding affinity (inhibitory constant = 1.7 ± 0.2 nm) and was identified as a full agonist in vitro. In castrated male rats, the ED50 of S-23 in the prostate and levator ani muscle was 0.43 and 0.079 mg/d, respectively. In intact male rats treated for 14 d, S-23 alone suppressed LH levels by greater than 50% at doses greater than 0.1 mg/d, with corresponding decreases in the size of the prostate but increases in the size of levator ani muscle. In intact male rats treated for up to 10 wk with S-23 and estradiol benzoate (EB; necessary to maintain sexual behavior in rats), S-23 showed biphasic effects on androgenic tissues and spermatogenesis by suppressing serum concentrations of LH and FSH. EB alone showed no effect on spermatogenesis. In the EB + S-23 (0.1 mg/d) group, four of six animals showed no sperm in the testis and zero pregnancies (none of six) in mating trials. After termination of treatment, infertility was fully reversible, with a 100% pregnancy rate observed after 100 d of recovery. S-23 increased bone mineral density and lean mass but reduced fat mass in a dose-dependent manner. This is the first study to show that a selective androgen receptor modulator combined with EB is an effective and reversible regimen for hormonal male contraception in rats. The beneficial effects of S-23 on the muscle, tissue selectivity, and favorable pharmacokinetic properties make it a strong candidate for use in oral male contraception.
An aryl-propionamide selective androgen receptor modulator (S-23) is an effective and reversible agent for hormonal male contraception in rats.
The negative feedback loop existing between testosterone synthesized in the testis and gonadotropins released from the pituitary was first discovered in 1932 (1). Since then, numerous studies further proved that endogenous testosterone synthesis could be inhibited by using endogenous and synthetic androgens (2,3,4). The goal of hormonal male contraception is to suppress LH and FSH, resulting in depletion of intratesticular testosterone and suppression of spermatogenesis, and replacing the physiologic need for androgens by an androgen preparation (5). Although 90–100% of Asian men receiving testosterone-alone regimens achieved azoospermia, this regimen was not highly effective in non-Asian men (∼60% azoospermia) (6,7). As such, clinical trials using testosterone in combination with progestins or GnRH analogs are widely viewed as the most promising approach to hormonal male contraception (8,9). Identification of orally bioavailable androgens with the ability to mimic the beneficial anabolic effects of testosterone at doses that do not support spermatogenesis would be a significant step toward achieving this goal. The first studies with a promising selective androgen receptor modulator (SARM) that achieves these goals are described herein.
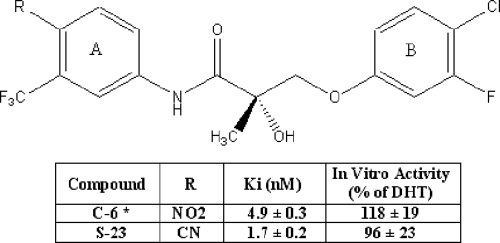
We previously identified two potent SARMs (denoted S-1 and S-4) (10) and demonstrated their potential use for androgen-related diseases, including muscle wasting, osteoporosis (11), and benign prostate hyperplasia in animal models (12). Another novel SARM (C-6), which bears a para-nitro substituent in the A-ring, a para-chloro group and a meta-fluoro group in the B-ring (Fig. 1), was identified as the first aryl propionamide SARM that exhibited potent ability to regulate gonadotropin and testosterone levels in castrated and intact male rats, respectively (13). Pharmacokinetic studies showed that C-6 is orally bioavailable (76%) with a moderate terminal half-life (6.3 h) at a dose of 10 mg/kg in male rats. Additional pharmacologic studies of C-6 demonstrated its profound effects on spermatogenesis in intact male rats after 70 d treatment at 1 mg/d by sc daily injections (13). However, in vivo metabolism studies revealed that the major metabolites arose from the step-wise reduction of the nitro group in the A-ring of the aryl propionamide SARM backbone (Fig. 1). Although structure-activity relationship studies showed that changing the para-nitro group on the A-ring to a cyano group slightly decreased the androgen receptor (AR) binding affinity and in vitro functional activity (14), this modification significantly improved the in vivo pharmacokinetic properties and efficacy of closely related aryl propionamide SARMs (15). We show herein that the optimized molecule with the B-ring of C-6 and a cyano-group in the A-ring (S-23, Fig. 1) is a promising candidate for hormonal male contraception.
Figure 1.
Chemical structures, AR binding affinity, and in vitro transcriptional activation of C-6 and S-23. AR binding affinity was determined using a radioligand competitive assay. The ability of compound of interest to induce AR-mediated transcriptional activation was determined using a cotransfection assay in CV-1 cells. The transcriptional activity induced by each compound at a concentration of 10 nm was reported as the percentage of that observed for 1 nm DHT. Each value represents the mean ± sd of three replicates. *, Data were reported by Chen et al. (13).
In the current studies, we examined the in vitro and in vivo characteristics of S-23, including in vitro AR binding affinity, AR-mediated transcriptional activation, and pharmacologic activity in castrated and intact male rats. Although such an agent is most likely to be used in combination with a progestin for oral male contraception in humans, we first examined the effects of S-23 in male rats when coadministered with a low dose of estradiol shown previously to be sufficient to support sexual behavior when administered with dihydrotestosterone (DHT) (16,17,18). Male rats require estrogen to maintain normal sexual behavior, whereas androgen alone appears to be the major determinant of human male sexual behavior (17,19,20,21). As such, we assessed the endocrine, pharmacological, and reproductive effects of S-23 at doses ranging from 0.05 to 0.75 mg/d when combined with estradiol benzoate (EB; 5 μg/d) by daily sc injections for 70 d. The antireproductive activity of S-23 was evaluated by its ability to suppress spermatogenesis and prevent pregnancy in mating trials. The effects of S-23 on body weight, androgen-dependent organ weights, body composition, and endocrine hormones were also determined. These studies are the first to demonstrate that an aryl propionamide SARM can be used as a component of an oral male contraceptive regimen.
Materials and Methods
Chemicals and experimental animals
S-23 was synthesized using the methods described by Marhefka et al. (22). Chemical purity was confirmed using elemental analysis, mass spectrometry, and proton nuclear magnetic resonance. Unlabeled mibolerone and 17α-methyl-[3H]mibolerone (84 Ci/mmol) were purchased from PerkinElmer Life Sciences (Boston, MA). Ethanol was purchased from Pharmco Products (Brookfield, CT). All other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). All male and female Sprague Dawley rats were purchased from Harlan Bioproducts for Science (Indianapolis, IN). Animals were maintained on a 12-h light, 12-h dark cycle with food and water available ad libitum. The animal protocol was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University.
In vitro AR binding affinity and transcriptional activation of S-23
Cytosolic AR was prepared from the ventral prostates of male Sprague Dawley rats 24 h after castration. The AR binding affinity and the in vitro functional activity of S-23 were determined using a radiolabeled competitive binding assay and a cotransfection assay, as previously described (23). Previous experiments in our laboratories determined that the lowest concentration of DHT that produced maximal AR-mediated transcriptional activation was 1 nm (24). Therefore, in the present study, the transcriptional activation induced by this concentration of DHT was set as 100% and used as the reference for quantifying the agonist activity of S-23.
Androgenic activity and anabolic activity of S-23 in castrated male rats
The in vivo pharmacologic activity of S-23 was examined in 30 (n = 5/group) male Sprague Dawley rats weighing approximately 200 g. Animals were castrated via scrotal incision under anesthesia (87 mg/kg ketamine and 13 mg/kg xylazine in saline, ip) 24 h before drug treatment and received daily sc injections of S-23 at dose rates of 0.01, 0.05, 0.1, 0.5, 1, and 3 mg/d for 14 d. S-23 was freshly dissolved in vehicle containing dimethylsulfoxide [5% (vol/vol)] in polyethylene glycol 300 (PEG 300) before daily administration. An additional two groups of animals with or without castration received vehicle only and served as castrated or intact control groups, respectively. Animals were killed at the end of the treatment. Plasma samples were collected and stored at −80 C until use. The ventral prostate, seminal vesicles, and levator ani muscle were removed, cleared of extraneous tissue, and weighed. All organ weights were normalized to body weight and compared. The weights of prostate and seminal vesicles were used to evaluate androgenic activity, whereas the levator ani muscle weight was used as a measure of anabolic activity. Nonlinear regression analysis was performed to obtain the maximal response (Emax) induced by S-23 and the dose rate of S-23 that induced ED50, using the sigmoid Emax model with a baseline effect parameter and WinNonlin software (Pharsight Corp., Mountain View, CA). No upper or lower boundary was used for this modeling.
Pharmacokinetics of S-23 in male rats
The pharmacokinetics of S-23 were examined in male Sprague Dawley rats weighing approximately 250 g after a dose of 10 mg/kg by iv injection or oral gavage. A catheter was implanted into the right external jugular vein of each animal 24 h before drug administration. S-23 was dissolved in a vehicle containing dimethylsulfoxide [10% (vol/vol)] in PEG 300 and administered to animals through the jugular vein catheter (iv group) or oral gavage [per os (p.o.) group] at a volume of 100 μl/dose. For animals in the iv group, the catheter was flushed three times with saline (3 times the volume of the dosing solution) before blood sampling. Blood samples (∼200 μl) were then withdrawn through the jugular vein catheter at 5, 10, 20, 30, 60, 120, 240, 360, 480, 720, 1440, 1800, 2160, and 2880 min after the iv dose and at 20, 30, 60, 90, 120, 180, 240, 360, 480, 720, 1440, 1800, 2160, and 2880 min after the p.o. dose. An aliquot of saline equal in amount to the volume of blood withdrawn was administered to each animal after each sample collection. Blood samples were immediately centrifuged at 1000 × g, 4 C for 10 min. Plasma samples were prepared and stored at −20 C until HPLC analysis. Stability of S-23 in plasma at room temperature for 48 h and at −20 C for 30 d was determined to be within 10% of a fresh analytical sample.
An aliquot (90 μl) of each plasma sample from the pharmacokinetic study was spiked with 10 μl of an internal standard (C-3; a structural analog of S-23) and mixed well with 1 ml acetonitrile. After centrifugation at 16,000 × g, 4 C for 10 min, the supernatant was collected and evaporated. The residues were reconstituted in 200 μl of mobile phase. An aliquot of each sample was injected into a Nova-pak C18 column (3.9 × 150 mm, 4 μm particle size) purchased from Waters Corp. (Milford, MA). The HPLC system consisted of a solvent pump, a degasser, an autosampler, and a UV detector (model 1100; Agilent Technologies, Palo Alto, CA). HPLC separation was performed using an isocratic mobile phase [H2O/acetonitrile: 50/50 (vol/vol)] at a flow rate of 1 ml/min. The UV absorbance of eluents was monitored at 296 nm. Calibration standards were prepared in blank rat plasma with S-23 concentrations ranging from 0.1 to about 50 μg/ml with the limit of quantitation at 0.1 μg/ml.
The plasma concentration-time data were analyzed using noncompartmental methods and WinNonlin software (Pharsight). The terminal half-life (t½) was calculated as t½ = 0.693/λ, where λ was the terminal elimination constant. The area under the plasma concentration-time curve (AUC0-∞) was calculated using the trapezoidal method with extrapolation to time infinity. The plasma clearance (CL) was calculated as CL = doseiv/AUC0-∞, iv, where the doseiv and AUC0-∞, iv were the intravascular dose and the corresponding area under the plasma concentration-time curve from time 0 to infinity, respectively. The apparent steady-state volume of distribution at equilibrium (Vdss) was calculated as Vdss = CL · MRT, where the MRT was the mean residence time after the iv bolus dose. The peak plasma concentration and time to reach the peak concentration after a p.o. dose were obtained directly from the plasma concentration-time curves. Oral bioavailability (Fp.o.) was defined as Fp.o. = (AUC0-∞, p.o. · doseiv)/(AUC0-∞, iv · dosep.o.), where the dosep.o. andAUC0-∞, p.o. were the oral dose and the corresponding AUC from time 0 to infinity after oral administration, respectively.
Effects of S-23 on spermatogenesis and endocrine physiology in intact male rats
Animals and treatment
Forty-two male Sprague Dawley rats (90 d old) were randomly assigned to seven groups (n = 6/group). All doses were administered via sc injection (100 μl/d). Group 1 was the intact control group and received vehicle alone (5% dimethylsulfoxide + 95% PEG 300). Group 2 was the EB control group and received EB alone at a dose of 5 μg/d. In addition to EB (5 μg/d), animals in groups 3–7 were treated with S-23 at dose rates of 0.05, 0.1, 0.3, 0.5, and 0.75 mg/d, respectively. A pilot study with a structural similar analog (C-6) alone was tested for hormonal male contraception at a single dose of 1 mg/d. In that study, partial but marked suppression of spermatogenesis was observed after 10 wk of treatment with C-6 in intact male rats (13). However, the effects of C-6 alone on fertility could not be evaluated due to the fact that the presence of estrogen is essential to maintain normal sexual behavior in the rat. Thus, S-23 was coadministered with a low dose of EB to evaluate its contraceptive efficacy and avoid potential confounding effects related to libido.
Compounds were freshly prepared immediately before dosing and delivered to animals daily for 70 d. Although S-23 shows high oral bioavailability sc dosing was used for these studies to lessen animal handling, stress, and morbidity during long-term treatment. Mating trials were conducted during the last week of treatment for animals in groups 1, 2, 4, and 5 to ensure that the animals in each group demonstrated appropriate mounting and copulatory behavior. Total body composition of each animal was determined using dual-energy x-ray absorptiometry (DEXA; Lunar Prodigy; GE, Milwaukee, WI) 1 d before the end of treatment.
An additional study was conducted in separate animals to determine the reversibility of S-23. Forty-two male rats were treated identically as that stated above and then mated. However, after the mating trial, the rats were maintained for an additional 70 d without treatment, after which a second (d 140) mating trial was performed. A third and final mating trial was performed on d 168 for seven animals that were deemed infertile on d 140.
Tissue and blood sample collection and preparation
At the end of treatment, animals were killed under anesthesia. Body weight was recorded at autopsy. Prostate, seminal vesicles, right testis, and right epididymis (divided in the middle of corpus epididymis to caput and cauda epididymis) were collected and weighed. All organ weights were normalized with body weights and compared. Blood samples were collected in Falcon tubes, maintained at room temperature for 40 min, and centrifuged at 3000 × g for 10 min to prepare serum samples. Serum samples were aliquoted and stored at −80 C until analysis. One testis from each rat was removed and weighed. After decapsulation, testicular parenchyma was homogenized in a volume of PBS that was equivalent to its weight (assuming a density of 1 g/ml). An aliquot of each testicular homogenate was used to count advanced spermatids (step 17–19) in each testis using a hemacytometer. Results were expressed as million sperm per testis. The remaining testicular homogenates were centrifuged at 3000 × g for 10 min. The supernatant of each sample was collected and used to determine the intratesticular testosterone concentrations.
Hormone assays
Serum levels of LH and FSH were measured by the Center for Research in Reproduction (University of Virginia, Charlottesville, VA) using RIA kits validated for use in rodent samples. The lower limit of quantification for LH and FSH were 0.040 and 1.0 ng/ml, respectively.
Whole-body DEXA analysis
One day before the end of treatment, body weight, total body bone mineral density (BMD), percent fat mass (FM), and fat-free mass were determined by DEXA (GE, Lunar Prodigy) using the small animal software (Lunar enCORE, version 6.60.041). For scanning, animals were anesthetized with ketamine-xylazine (87:13 mg/kg) and positioned in a prone position. The total body data were obtained by selecting an area encompassing the entire animal as the region of interest during data processing.
Mating trials
Mating and behavior studies were conducted during the last week of treatment for animals in groups 1, 2, 4, and 5 to assure that the animals in each group demonstrated appropriate mounting and copulatory behavior and examine their fertility. During the last week of treatment, individual male rats from groups 1, 2, 4, and 5 were cohabitated with two sexually active female rats. The number of mounts, mount latency, intromissions, and intromission latency were recorded. Female rats were individually housed after mating trials with free access to water and standard food chow. The female rats were allowed to bring the pregnancy to term. Upon delivery, the number of successful pregnancies was counted. A male rat was defined as fertile if it impregnated either one or two female partners. For the additional reversibility study that was conducted, male rats from all groups were cohabitated with two sexually active females, first, at the end of treatment to assess infertility, and again 70 d later (d 140) without treatment to establish fertility rates. A third and final mating trial was performed on d 168 for seven animals that were deemed infertile on d 140 in the reversibility study.
Statistical analyses
All statistical analyses were performed using single-factor ANOVA followed by Dunnett’s multiple comparison test. Tests in which P < 0.05 were considered as statistically significant differences.
Results
In vitro AR binding affinity and transcriptional activation of S-23
AR binding affinity was determined using a competitive binding assay with DHT as the positive control in each assay. The inhibitory constant values of DHT and S-23 were 0.45 ± 0.2 and 1.7 ± 0.2 nm, respectively. As shown in Fig. 1, S-23 was structurally modified from C-6 by changing the para-nitro group of C-6 to a cyano group, resulting in about 2-fold higher binding affinity than that of C-6 (inhibitory constant = 4.9 ± 0.3 nm). This result was inconsistent with previous structure-activity relationships for the aryl propionamide SARMs, in which five pairs of compounds with cyano-substituents at the para-position of the A-ring exhibited lower AR binding affinity than their corresponding nitro-substituted counterparts (23) but suggested that S-23 would demonstrate promising in vivo pharmacologic activity.
The ability of S-23 to induce AR-mediated transcriptional activation was determined using a cotransfection assay in CV-1 cells. Upon binding to the AR, S-23 induced a concentration-dependent increase in AR-mediated transcriptional activation (data not shown). Data listed in Fig. 1 represents the transcriptional activity induced by each compound at 10 nm and reported as the percentage of activity observed for 1 nm of DHT. Both C-6 and S-23 were identified as full AR agonists in vitro. Although the transcriptional activation induced by C-6 was higher than that of S-23, the difference was not statistically significant.
Androgenic activity and anabolic activity of S-23 in castrated male rats
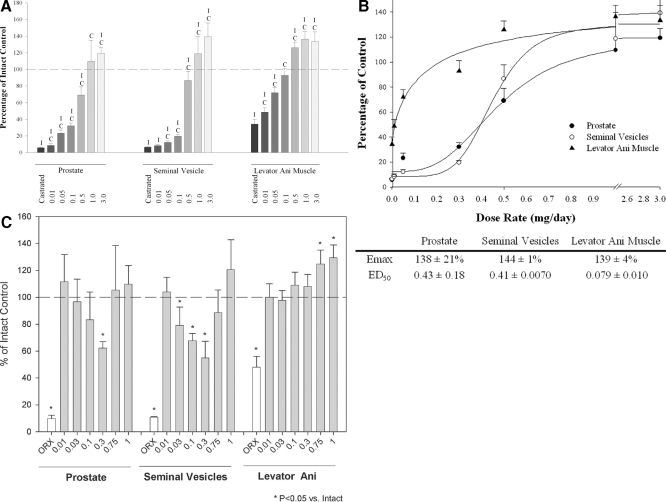
The in vivo pharmacologic activity of S-23 was initially evaluated in castrated male rats after 14 d treatment. Figure 2A shows the androgenic activity (prostate and seminal vesicles) and anabolic activity (levator ani muscle) of S-23 in castrated male rats at doses ranging from 0.01 to 3 mg/d. After castration, the weight of prostate, seminal vesicles, and levator ani muscle decreased significantly to 5.7, 6.5, and 35% of control values in intact animals, respectively. By administration of S-23 to castrated animals, androgen-dependent organ weights increased in a dose-dependent manner. At a dose rate of 1.0 mg/d, S-23 was able to maintain the prostate and seminal vesicles at weights equal to or greater than that observed in intact animals. Notably, at a dose rate as low as 0.1 mg/d, S-23 was able to selectively maintain the weight of the levator ani muscle at the intact control level, whereas its effects on the prostate and seminal vesicles were lower than 30% of those observed in intact controls. As shown in Fig. 2B, the Emax of S-23 in the prostate, seminal vesicles, and levator ani muscle was 138 ± 21, 144 ± 1, and 129 ± 4% of those observed in intact controls, respectively. Accordingly, the ED50 of S-23, calculated by nonlinear regression of the dose-response relationship, in the prostate, seminal vesicles, and levator ani muscle was 0.43 ± 0.18, 0.41 ± 0.0070, and 0.079 ± 0.010 mg/d, respectively. In comparison with C-6 (13), S-23 demonstrated a 2-fold higher potency (ED50) in all three organs without significantly increasing the efficacy (Emax) or changing the androgenic/anabolic activity in vivo. The increase in potency of S-23 in vivo was possibly due to its higher AR binding affinity compared with C-6. Another possibility was the improved in vivo drug exposure after administration, which would be verified by results of pharmacokinetic studies.
Figure 2.
A, The androgenic activity and anabolic activity of S-23 in castrated male rats. S-23 was administrated to animals via daily sc injections for 14 d with doses ranging from 0.01 to 3 mg/d. All organ weights were normalized with body weight and presented as the percentage of intact control. Each bar represents the mean ± sd of five rats. I and C, Significant difference from intact control and castrated control groups analyzed by single-factor ANOVA with P < 0.05 followed by Dunnett’s multiple comparison test. B, Dose-response curves of S-23 in castrated male rats. Emax and ED50 in this figure were obtained by nonlinear least-square regression analysis. Each value represents the mean ± sd of five rats. C, The androgenic activity and anabolic activity of S-23 in intact male rats. S-23 was administrated to animals via daily sc injections for 14 d with doses ranging from 0.01 to 1 mg/d. All organ weights were normalized with body weight and presented as the percentage of intact control. Each bar represents the mean ± sd of five rats. *, Significant difference from the vehicle-treated, intact control group analyzed by single-factor ANOVA with P < 0.05 followed by Dunnett’s multiple comparison test. ORX, Castrated.
Pharmacokinetics of S-23 in male rats
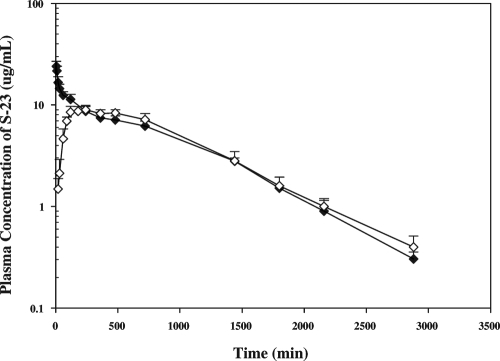
The plasma concentrations of S-23 after iv and p.o. administration are shown in Fig. 3, and the pharmacokinetic parameters are listed in Table 1. After iv administration, S-23 concentrations were high initially, then declined, and remained detectable until 48 h after the dose. The mean terminal t½ of S-23 in male rats was 10.9 h. The systemic CL and Vdss were 0.87 ml/min · kg and 655 ml/kg, respectively. S-23 appeared rapidly in the systemic circulation after oral administration. Oral absorption was prolonged with maximum plasma concentrations, forming a plateau over 6–10 h after dose. Plasma concentrations of S-23 diminished with a mean terminal t½ of 11.9 h. Statistical analysis revealed no significant difference between the two t½ values observed after iv and p.o. administration. The oral bioavailability of S-23 after oral administration was 96%, indicating its near to complete absorption after oral dose.
Figure 3.
Mean plasma concentration-time profile of S-23 in male rats. Plasma concentrations of S-23 were measured using HPLC. Data were expressed as microgram per milliliter, and each point represents the mean ± sd (n = 4 or 5/group).
Table 1.
Mean (±sd) pharmacokinetic parameters of S-23 in male rats
| Parameter | 10 mg/kg, iv (n = 4) | 10 mg/kg, p.o. (n = 5) |
|---|---|---|
| AUC0→∞ (min,μg/liter) | 11.3 ± 0.33 | 10.8 ± 0.66 |
| λz (min−1) | 0.00153 ± 0.00017 | 0.00140 ± 0.000156 |
| MRT (min) | 751 ± 67 | 879 ± 61 |
| CL (ml/min · kg) | 0.87 ± 0.02 | |
| Vss (ml/kg) | 655 ± 78 | |
| F p.o. (%) | 95.9 | |
| Tmax (min) | 252 ± 137 | |
| Cmax (mg/ml) | 9.4 ± 0.7 | |
| CL/F (ml/min · kg) | 0.90 ± 0.06 | |
| Vss/F (ml/kg) | 649 ± 68 |
AUC0-∞, iv, Area under the plasma concentration-time curve from time 0 to infinity; λ, terminal elimination constant; Vss, apparent volume of distribution at equilibrium; Tmax, time to reach the peak concentration (Cmax).
Effects of S-23 on spermatogenesis and endocrine physiology in intact male rats
Effects of treatments on spermatogenesis and hormonal regulation
After identification of S-23 as a more potent SARM than C-6, the in vivo contraceptive activity of this compound was immediately investigated in intact male rats. To examine the efficacy of male contraception, mating trials were designed at the end of treatment. Previous studies of the effects of C-6 on masculine mating behavior showed that it maintains mating behavior in sexually experienced rats but failed to stimulate any mating behavior in sexually naïve male rats after long-term castration when administered alone (our unpublished data). A small amount of EB in combination with a SARM is effective and sufficient to fully restore mating behavior in long-term castrated male rats. Animals in group 1 served as vehicle controls. Animals in groups 2–7 were treated with EB at a dose of 5 μg/d in combination with S-23 at doses of 0, 0.05, 0.1, 0.3, 0.5, and 0.75 mg/d, respectively. Maintenance of libido plays a key role in the success of mating. EB was included to ensure that any differences in reproductive ability of rats were not due to deficiencies in sexual libido. The doses of S-23 were chosen based on the pharmacological activity of this compound (administered alone) in castrated male rats, which covered the whole range of the therapeutic window (Table 2).
Table 2.
Effects of S-23 on LH and FSH in intact and castrated male rats
| Dose (mg/d) | LH (ng/ml)
|
FSH (ng/ml)
|
||
|---|---|---|---|---|
| Intact | Castrated | Intact | Castrated | |
| 0.58 ± 0.14C | 11.0 ± 1.71I | 5.6 ± 1.1C | 43.4 ± 8.6I | |
| 0.01 | 0.58 ± 0.15C | 10.9 ± 2.28I | 5.5 ± 1.9C | 42.0 ± 4.7I |
| 0.03 | 0.37 ± 0.13I,C | 11.45 ± 2.46I | 6.0 ± 1.1C | 52.0 ± 17.9I |
| 0.1 | 0.25 ± 0.16I,C | 2.29 ± 2.06C | 4.8 ± 0.8C | 24.3 ± 14.5I,C |
| 0.3 | 0.08 ± 0.04I,C | 0.06 ± 0.04I,C | 4.0 ± 0.6I,C | 6.1 ± 1.4C |
| 0.75 | < 0.04I,C | < 0.04I,C | 4.2 ± 1.1C | 6.0 ± 0.3C |
| 1 | < 0.04I,C | < 0.04I,C | 3.3 ± 0.5I,C | 6.6 ± 1.1C |
Rats were treated for 14 d via sc injection and serum LH and FSH concentrations measured. Data presented as mean ± se (n = 5/group). I and C, Significant difference between the group and intact control group or castrated control group, respectively, as analyzed by single-factor ANOVA with P < 0.05.
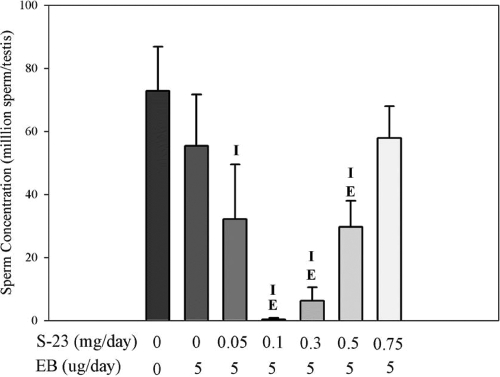
Spermatogenesis was evaluated by counting the number of homogenization-resistant advanced (steps 17–19) spermatids in the testis from control and drug-treated rats. As shown in Fig. 4, mean sperm counts were expressed as the number of spermatids per testis. No differences in the mean sperm counts were observed between EB-treated and intact control animals. Coadministration of S-23 with EB caused a biphasic effect on sperm counts in the testis, which was consistent with the androgenic activity of drug treatment in the peripheral tissues and sexual organs. The greatest inhibition of spermatogenesis was observed in animals that received 0.1 mg/d of S-23 plus 5 μg/d of EB. Four of six animals in this group showed no sperm in the testis with barely detectable sperm counts in the remaining two animals. Higher doses of S-23 actually supported spermatogenesis in a dose-dependent manner, with the 0.75 mg dose resulting in a similar mean sperm count as those in the EB alone group and intact control group.
Figure 4.
Testicular sperm concentrations. Each bar represents the mean ± sd (n = 5 or 6/group). The letters I and E above each error bar represent a significant difference between the group and intact control group and estrogen-treated group, respectively, as analyzed by single-factor ANOVA followed by Dunnett’s multiple comparison test with P < 0.05.
Serum concentrations of LH and FSH were determined using RIA and ELISA and summarized in Table 3. The average serum concentration of LH in the intact control group was 0.21 ± 0.050 ng/ml. Drug treatments caused marked suppression of serum LH levels that were lower than the assay detection limit (0.04 ng/ml). The average serum concentration of FSH in the intact control group was 5.6 ± 1.2 ng/ml. FSH levels were significantly suppressed at the higher doses of S-23 (i.e. groups 6 and 7). The most remarkable suppression of spermatogenesis was observed in animals treated with 0.1 mg/d of S-23 plus EB. Sixty-seven percent of animals in this group achieved no sperm in the testis (four of six), whereas the remaining animals achieved very low sperm counts in the testis (two of six).
Table 3.
Effects of S-23 on hormones and fertility
| Group | EB (μg/d) | S-23 (mg/d) | Animal numbers | LH (ng/ml) | FSH (ng/ml) | Number of pregnancies |
|---|---|---|---|---|---|---|
| 1 | 6 | 0.21 ± 0.050 | 5.6 ± 1.2 | 12/12 | ||
| 2 | 5 | 5 | < 0.04I | 5.2 ± 0.43 | 8/10 | |
| 3 | 5 | 0.05 | 6 | < 0.04I | 4.8 ± 1.4 | ND |
| 4 | 5 | 0.1 | 6 | < 0.04I | 4.8 ± 0.90 | 0/12 |
| 5 | 5 | 0.3 | 6 | < 0.04I | 4.3 ± 1.5 | 2/12 |
| 6 | 5 | 0.5 | 6 | < 0.04I | 3.4 ± 0.30I, E | ND |
| 7 | 5 | 0.75 | 6 | < 0.04I | 3.6 ± 0.46I, E | ND |
Rats were treated for 70 d via sc injection and serum LH and FSH concentrations were measured. Data presented as mean ± sd. I and E, Significant difference between the group and intact control group or EB control group, respectively, as analyzed by single-factor ANOVA with P < 0.05; ND, not determined.
Effects of treatments on body composition and androgen-dependent organ weights
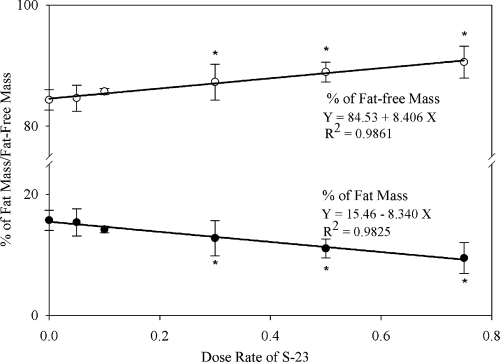
As shown in Table 4, the body weight of animals after treatment was recorded and compared. The body weight and prostate weight in all EB-treated groups was significantly less, whereas the total body BMD was slightly greater than that observed in the intact group treated with vehicle alone, indicative of the effects of estrogen in the male body. Although a statistically significant but small difference was noted at one dose level in each case, coadministration of S-23 did not result in dose-dependent effects on body weight or BMD, similar to previous results that we observed when a structurally related SARM (i.e. S-4) was administered alone (i.e. without EB) to rats (11). Of note, the weights of the testis were significantly less in all of the animals that received S-23, whereas the weights of the levator ani muscle were unaffected. For direct comparison, all parameters of body composition and organ weights were normalized to body weight in the current studies. Body composition was also analyzed using DEXA. The effect of treatments on the percentage of FM is shown in Fig. 5. Treatment with EB alone did not significantly alter FM compared with the intact control. Coadministration of S-23 with EB decreased FM in a dose-dependent manner. FM was significantly less than that of EB controls at doses of S-23 at 0.3 mg/d or higher and was significantly less than that of intact controls at the highest dose (0.75 mg/d) used. In addition, FM was linearly correlated to the dose of S-23, with R2 = 0.9825. Correspondingly, the fat-free mass also exhibited a dose-dependent increase after EB + S-23 treatment (Fig. 5).
Table 4.
Effects of treatment on body weight, BMD, and androgen-dependent organ weights
| Group | EB (μg/d) | S-23 (mg/d) | Animal numbers | Body weight (g) | BMD (g/cm2) | Prostate (percent of intact control) | Seminal vesicles (percent of intact control) | Levator ani muscle (percent of intact control) | Testis (percent of intact control) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 423 ± 29 | 0.172 ± 0.0057 | 100 ± 20 | 100 ± 7.5 | 100 ± 15 | 100 ± 10 | ||
| 2 | 5 | 5 | 351 ± 8.0I | 0.181 ± 0.0036I | 46.9 ± 15I | 28.1 ± 12I | 62 ± 5.6I | 94 ± 10 | |
| 3 | 5 | 0.05 | 6 | 379 ± 20I | 0.188 ± 0.0036I,E | 34.4 ± 7.6I | 23.9 ± 7.6I | 90 ± 11E | 69 ± 14I,E |
| 4 | 5 | 0.1 | 6 | 376 ± 26I | 0.183 ± 0.0040I | 31.1 ± 6.6I | 21.8 ± 5.3I | 97 ± 15E | 34 ± 3.8I,E |
| 5 | 5 | 0.3 | 6 | 387 ± 25I,E | 0.188 ± 0.0085I | 45.1 ± 5.3I | 52 ± 8.7I,E | 100 ± 3.8E | 45 ± 3.9I,E |
| 6 | 5 | 0.5 | 6 | 351 ± 9.0I | 0.184 ± 0.0037I | 78.2 ± 5.6I,E | 89 ± 4.8E | 123 ± 8.5I,E | 63 ± 6.3I,E |
| 7 | 5 | 0.75 | 6 | 344 ± 11I | 0.183 ± 0.0036I | 94.7 ± 89.2E | 113 ± 12I,E | 131 ± 8.1I,E | 75 ± 7.2I,E |
Rats were treated for 70 d via sc injection. Data presented as mean ± sd. I and E, Significant difference between the group and intact control group or EB control group, respectively, as analyzed by single-factor ANOVA with P < 0.05.
Figure 5.
The linear correlation between the dose rate of S-23 and body composition (percent of FM and percent of fat free mass). Each value represents the mean ± sd (n = 5 or 6/group). *, Significant difference between the group and estrogen-treated group, as analyzed by single-factor ANOVA followed by Dunnett’s multiple comparison test with P < 0.05.
Mating trials
Our pharmacology study showed that the levator ani muscle in the castrated rats was maintained at the intact control level for animals that received S-23 at a dose between 0.1 and 0.3 mg/d for 14 d. As such, we conducted mating studies using doses of 0.1 and 0.3 mg/d of S-23 (i.e. the lowest doses of S-23 that were capable of fully maintaining levator ani muscle mass). Mating and behavior trials were performed during the last week of treatment for animals in groups 1, 2, 4, and 5, receiving vehicle, EB alone, EB plus 0.1 mg/d S-23, and EB plus 0.3 mg/d S-23, respectively. There were no differences in the number of mounts, mount latency, number of intromissions, or intromission latency between males in groups 1, 2, 4, and 5, indicating that EB alone and EB+S-23 maintained normal sexual behavior. The efficacy of hormonal male contraception was evaluated by the fertility rate of male rats in these mating trials. One hundred percent of the animals in the vehicle control group were fertile. Four of five male rats (80%) were fertile after treatment with EB alone. All male rats, treated with EB plus 0.1 mg of S-23, were infertile. Whereas only one of the six male rats treated with EB plus 0.3 mg of S-23 was fertile.
Reversibility
As shown in Table 5, the body weights as well as androgenic- and anabolic-dependent tissues were recorded and compared. The body weights and the weights of the seminal vesicles and testis were restored to that of control. In addition, muscle weights were maintained; however, prostate weights remained below that of control. As shown in Table 6, serum hormone levels as well as mean intratesticular sperm counts returned to that of control. As a result, an 83% fertility rate was achieved 70 d after termination of treatment; however, infertility was fully reversible after 100 d, with a 100% pregnancy rate.
Table 5.
Reversible effects on body weight and androgen-dependent organ weights
| Group | EB (μg/d) | S-23 (mg/d) | Animals | Body weight (g) | Prostate (percent of intact control) | Seminal vesicles (percent of intact control) | Testis (percent of intact control) | Levator ani muscle (percent of intact control) |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 511.3 ± 24.4 | 100 ± 12.4 | 100 ± 8.9 | 100 ± 8.4 | 100 ± 8.2 | ||
| 2 | 5 | 5 | 487.8 ± 29.1 | 99.2 ± 4.1 | 95.6 ± 18.7 | 104.8 ± 6.6 | 102.6 ± 3.3 | |
| 3 | 5 | 0.05 | 6 | 503.8 ± 44.2 | 88.2 ± 12.3 | 95.4 ± 8.4 | 106.5 ± 5.2 | 96.5 ± 8.7 |
| 4 | 5 | 0.1 | 6 | 500.2 ± 35.4 | 79.4 ± 12.3I,E | 87.7 ± 11.6 | 99.8 ± 8.6 | 103.9 ± 14.3 |
| 5 | 5 | 0.3 | 6 | 506.7 ± 19.2 | 68.5 ± 7.7I,E | 92.8 ± 9.5 | 100.3 ± 6.0 | 85.8 ± 8.3I,E |
| 6 | 5 | 0.5 | 6 | 491.0 ± 19.8 | 73.7 ± 9.1I,E | 96.0 ± 10.3 | 100.4 ± 7.9 | 95.6 ± 8.3 |
| 7 | 5 | 0.75 | 6 | 478.2 ± 16.1I | 86.3 ± 9.4E | 105.2 ± 10.9 | 100.5 ± 6.0 | 98.7 ± 11.0 |
Rats were treated for 70 d via sc injection and then maintained without treatment for an additional 100 d. Data presented as mean ± sd. I and E, Significant difference between the group and intact control group or EB control group, respectively, as analyzed by single-factor ANOVA with P < 0.05.
Table 6.
Reversible effects on serum hormones, intratesticular sperm counts and fertility
| Group | EB (μg/d) | S-23 (mg/d) | Animals | LH (ng/ml) | FSH (ng/ml) | Sperm (million sperm/testis) | Number of pregnancies at 70 d | Number of pregnancies at 140 d |
|---|---|---|---|---|---|---|---|---|
| 1 | 6 | 0.64 ± 0.47 | 4.47 ± 1.15 | 72.42 ± 7.74 | 12/12 | 12/12 | ||
| 2 | 5 | 6a | 0.39 ± 0.16 | 3.76 ± 0.62 | 65.9 ± 9.52 | 12/12 | 10/12 | |
| 3 | 5 | 0.05 | 6b | 0.44 ± 0.24 | 3.92 ± 0.34 | 69.08 ± 8.39 | 2/12 | 8/12 |
| 4 | 5 | 0.1 | 6b | 0.41 ± 0.15 | 3.54 ± 0.84 | 67.58 ± 5.73 | 0/12 | 10/12 |
| 5 | 5 | 0.3 | 6 | 0.41 ± 0.1 | 3.37 ± 0.65 | 65.75 ± 5.68 | 2/12 | 10/12 |
| 6 | 5 | 0.5 | 6 | 0.42 ± 0.18 | 3.22 ± 0.8 | 65.42 ± 4.89 | 2/12 | 10/12 |
| 7 | 5 | 0.75 | 6 | 0.39 ± 0.22 | 3.51 ± 1.01 | 65.42 ± 4.19 | 6/12 | 10/12 |
Rats were treated for 70 d via sc injection and then maintained without treatment for an additional 100 d. Data presented as mean ± sd. I and E, Significant difference between the group and intact control group or EB control group, respectively, as analyzed by single-factor ANOVA with P < 0.05.
Serum was not available for analyses for two animals in this group (n = 4 for hormone values).
Serum was not available for analyses for one animal in this group (n = 5 for hormone values).
Discussion
Among the current experimental methods for male contraception, the most promising approach is the hormonal method. Spermatogenesis is tightly regulated by hormones through the hypothalamus-pituitary-testis axis. The rationale of hormonal male contraception is to suppress spermatogenesis by interrupting the action of one or more hormones involved in the hypothalamus-pituitary-testis axis, including GnRH, LH, FSH, and testosterone. Testosterone is a logical choice for hormonal contraception because it not only inhibits LH, FSH, and intratesticular testosterone levels via its negative feedback mechanism but also provides androgens for peripheral, extragenital tissues. Although testosterone-alone regimens showed an overall high efficacy in an Asian population, the rate of azoospermia in a Caucasian population was only two thirds of that observed in Asians (6,7,25). Other antigonadotropin substances, such as progestins and GnRH analogs, were used in combination with testosterone to achieve more rapid and complete suppression of spermatogenesis (26). For hormonal male contraception, the disadvantages of using testosterone include inconvenient routes of administration (e.g. im injection or implantation) and unwanted effects on the prostate, hematologic system, and lipid metabolism.
In our current studies, multiple in vitro and in vivo assays were used to characterize the feasibility of S-23 for hormonal male contraception. First, S-23 was identified as a potent AR agonist with high AR binding affinity and specificity in vitro. In the following in vivo study using castrated male rats, S-23 revealed the most potent and efficacious anabolic activity that we observed among the aryl propionamide SARMs with multiple substituents in the B-ring (23). The levator ani muscle weight was maintained at the intact control level by S-23 at doses as low as 0.1–0.3 mg/d in castrated male rats. Previous masculine behavior studies showed that C-6 alone could not fully maintain or induce mating behavior in castrated rats, but that inclusion of 5 μg/d of EB in the dosing regimen with C-6 fully restored mating behavior in long-term castrated rats to the same extent as that observed in intact control animals (our unpublished data). Baum and Vreeburg (17) previously showed that male rats are dependent on estrogen for normal sexual behavior, whereas it appears that estrogens are not essential for human male sexual behavior (27,28,29,30). Likewise, Miner et al. (31) recently reported an oral bioavailable SARM that was efficacious in a sexual behavior model using rats concurrently treated with estrogen.
In our current contraception studies, S-23 and EB combination regimens were used in intact male rats to maintain the libido of animals during or after treatment. The pharmacological end points included androgen-dependent organ weights, body composition, total body BMD, spermatogenesis, and serum levels of LH and FSH. Consistent with previous literature (32,33), EB alone significantly decreased serum LH levels. Consequently, EB alone caused a significant decrease in body weight, reproductive organs, and lean muscle weights. In animals that received EB and SARM combination regimens, their physiological needs for androgen were replaced by S-23 as evidenced by the fully maintained levator ani muscle and partially maintained prostate and seminal vesicles. Furthermore, animals treated with EB and S-23 were leaner than intact controls with lower average body weight and significantly less percentage of fat mass. A high inverse correlation existed between the dose rate of S-23 and percentage of fat mass in rats. Androgen levels were negatively associated with leptin concentrations in both man (34) and animals (35,36). Our previous findings of S-1 showed that administration of a SARM significantly decreased leptin levels in castrated male rats (37). The findings from our current studies and previous studies (38) support the hypothesis that SARMs may mimic the effects of testosterone on body weight and body composition.
In adult animals, intratesticular testosterone concentration is an important determinant of testicular size, which is approximately 30- and 100-fold higher than its serum level in rats and men, respectively (39,40). It is unclear why such a high local concentration of testosterone is physiologically needed. Nevertheless, Zirkin et al. (41) have shown experimentally that an 80% reduction of intratesticular testosterone concentration to 20 ng/ml, only 10-fold higher than its serum level (∼2 ng/ml), was sufficient to quantitatively maintain spermatogenesis in adult rats. In the current studies, no significant inhibition in the weights of testis was observed after EB treatment alone, presumably implying that the intratesticular concentration of testosterone was still sufficient to maintain normal spermatogenesis and testicular size. Indeed, the sperm counts of animals treated with EB alone were similar to those of intact animals. In combination with S-23, the overall effects on spermatogenesis and testis weights were biphasic. In the absence of testosterone, EB plus higher doses of S-23 fully maintained spermatogenesis, suggesting that S-23 achieved sufficiently high intratesticular concentrations to support spermatogenesis at these higher doses. Unfortunately, we were unable to develop analytical methods sufficient to measure intratesticular concentrations of S-23 to confirm this hypothesis. However, similar biphasic phenomena in spermatogenesis observed using testosterone-based hormonal male contraception corroborate this conclusion (42). At the highest dose (0.75 mg/d) we studied herein, S-23 demonstrated quantitative maintenance of spermatogenesis with an average subnormal testicular weight of 75 ± 7% of that observed in vehicle-treated animals. This finding is consistent with a number of studies using testosterone to restore spermatogenesis in rats made azoospermic either by hypophysectomy or gonadotropin withdrawal (41,43,44,46).
A possible explanation is that measurement of homogenization-resistant sperm head overestimates the true sperm counts caused by abnormal retention or failed release of step 19 spermatids beyond stage VIII, the most subtle abnormal changes of spermatogenesis associated with various experimental conditions (47,48). Histological analysis of testis and epididymides from animals after drug treatment is ongoing in our laboratory. Decreases in testis size are commonly observed during treatment with exogenous androgens and appear to be directly related to decreases in sperm count (49). Animals that received EB and 0.1 mg/d of S-23 were infertile as hoped, and testis weights in these animals were indeed decreased to 34% of that observed in intact males. Doses of EB + 0.05 or 0.3 mg/d S-23 resulted in very low sperm counts in the testis and unacceptable rates of infertility, demonstrating that a very narrow therapeutic dose range to achieve no sperm in the testis and infertility exists when using a hormonal contraceptive based on androgen alone. Drug development issues with regard to targeting this narrow therapeutic window (i.e. high enough to stop LH production but not high enough to support spermatogenesis) in men of differing age, weight, medication compliance, and ethnicity represent a significant challenge toward development of an androgen-only male pill. Our studies suggest that the use of an androgen alone (orally or parenterally) for male contraception may be problematic, but that combination of an orally available SARM (like S-23) with an orally available progestin (to suppress LH and thus widen the therapeutic window for SARM action) represents a logical and likely feasible way to achieve oral male contraception.
Furthermore, treatment with EB plus 0.75 mg S-23 (group 7) not only suppressed LH levels but also significantly suppressed FSH concentrations. FSH plays a key role in spermatogenesis by maintaining the amount of sperm produced in adult male rats (50). It is likely that suppression of FSH contributes to the reduction of sperm counts as evidenced by S-23 alone or in combination with EB. Additionally, synergistic effects of testosterone and estradiol on suppression of spermatogenesis were reported both in the rat (51) and man (45). It remains unknown whether SARM and estradiol have a similar synergistic and inhibitory effect on spermatogenesis.
The effect of S-23 on androgen-dependent tissue weights and suppression of spermatogenesis was reversible as evidenced by the ability of these tissues to return to that of control in the absence of exogenous androgen administration. Restoring serum hormones and therefore mean sperm counts to that of control indicates that normal spermatogenesis ensued. Therefore, as expected, all male rats were fertile after termination of treatment with S-23 plus EB.
In summary, S-23 was identified as a potent and efficacious SARM in castrated male rats with significantly higher anabolic activity than androgenic activity. In intact male rats, S-23 plus EB demonstrated potential use for hormonal male contraception. Either no sperm or very low sperm counts in the testis was successfully achieved in animals that received combination treatment with EB plus 0.1 mg/d S-23, and a 100% infertility rate was observed in the efficacy study. Unlike testosterone, which showed biphasic effects in the testis and eipdidymides and nonselective increases in peripheral reproductive tracts (e.g. prostate and seminal vesicles), S-23 displayed only biphasic effects in the organs mentioned above. Additionally, S-23 selectively increased levator ani muscle weight and decreased the percentage of FM. After termination of treatment, serum hormones and mean sperm counts returned to that of control, resulting in 100% pregnancy rates in all treatment groups. These results indicate that the effect of S-23 plus EB on spermatogenesis is completely reversible. In conclusion, this is the first study to show that no sperm in the testis can be induced using an EB-SARM combination therapy, in which, EB was used to maintain libido without affecting quantitative spermatogenesis. This effective therapy selectively decreased weights of the prostate, seminal vesicles, testis, and epdidymides, retained muscle weight, percent of fat and fat-free mass, and increased BMD. In an additional PK study, S-23 demonstrated high oral bioavailability (96%). The identification of a SARM with these promising pharmacologic and pharmacokinetic characteristics represents an important step toward the male pill, but much remains to be done.
Acknowledgments
We thank Dr. Mitch A. Phelps for help in carrying out the contraception experiments. Also, we thank the Center for Research in Reproduction (University of Virginia, Charlottesville, VA) for measuring serum concentrations of LH and FSH.
Footnotes
This work was supported by a grant from National Institute of Diabetes and Digestive and Kidney Diseases Grant 1 R01 DK59800-05. J.C. was partially supported by a Presidential Fellowship from The Ohio State University.
Disclosure Summary: A.J. has stock options with GTx, Inc.; J.C. is an inventor on U.S. patent 6492554 and has received patent royalties; D.J.H., D.D.M., and J.T.D. are employed by and have stock options with GTx, Inc., are inventors on U.S. Patent 6492554, and have received patent royalties.
First Published Online September 4, 2008
Abbreviations: AR, Androgen receptor; AUC, area under the curve; BMD, bone mineral density; CL, clearance; DEXA, dual-energy x-ray absorptiometry; DHT, dihydrotestosterone; EB, estradiol benzoate; Emax, maximal response; FM, fat mass; Fp.o., oral bioavailability; MRT, mean residence time after the iv bolus dose; PEG 300, polyethylene glycol 300; p.o., per os; SARM, selective androgen receptor modulator; T½, half-life; Vdss, steady-state volume of distribution at equilibrium.
References
- Hohlweg WaJ K 1932 Die hormonal-nervose regulierung der funktion des hypophysenvorderlappens. Klin Wochenschr 11:321 [Google Scholar]
- Dischreit J 1939 Wirkung des testikelhormonpraparates erugon auf den juvenilen rat-tenhoden. Klin Wochenschr 18:1493 [Google Scholar]
- Gaarenstroom JH 1939 Inhibition of the ‘antimasculine effect’ of oestrone by testosterone propionate. Acta Brev Neerl Physiol 9:134 [Google Scholar]
- Ludwig DJ 1950 The effect of androgen on spermatogenesis. Endocrinology 46:453 [DOI] [PubMed] [Google Scholar]
- Nieschlag E, Nieschlag S, Behre HM 1998 Testosterone: action, deficiency, substitution. 2nd ed. New York: Springer [Google Scholar]
- 1990 Contraceptive efficacy of testosterone-induced azoospermia in normal men. World Health Organization Task Force on methods for the regulation of male fertility. Lancet 336:955–959 [PubMed] [Google Scholar]
- 1996 Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil Steril 65:821–829 [PubMed] [Google Scholar]
- Amory JK, Page ST, Bremner WJ 2006 Drug insight: recent advances in male hormonal contraception. Nat Clin Pract Endocrinol Metab 2:32–41 [DOI] [PubMed] [Google Scholar]
- Kamischke A, Nieschlag E 2004 Progress towards hormonal male contraception. Trends Pharmacol Sci 25:49–57 [DOI] [PubMed] [Google Scholar]
- Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT 2003 Pharmacodynamics of selective androgen receptor modulators. J Pharmacol Exp Ther 304:1334–1340 [DOI] [PubMed] [Google Scholar]
- Kearbey JD, Gao W, Narayanan R, Fisher SJ, Wu D, Miller DD, Dalton JT 2007 Selective androgen receptor modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm Res 24:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kim J, Dalton JT 2005 Discovery and therapeutic promise of selective androgen receptor modulators. Mol Interv 5:173–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hwang DJ, Bohl CE, Miller DD, Dalton JT 2005 A selective androgen receptor modulator for hormonal male contraception. J Pharmacol Exp Ther 312:546–553 [DOI] [PubMed] [Google Scholar]
- Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N, Kirkovsky L, Miller DD, Dalton JT 2003 Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol Pharmacol 63:211–223 [DOI] [PubMed] [Google Scholar]
- Kim J, Wu D, Hwang DJ, Miller DD, Dalton JT 2005 The para substituent of S-3-(phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamides is a major structural determinant of in vivo disposition and activity of selective androgen receptor modulators. J Pharmacol Exp Ther 315:230–239 [DOI] [PubMed] [Google Scholar]
- Lodder J, Baum MJ 1977 Facilitation of mounting behavior by dihydrotestosterone propionate in castrated estradiol benzoate-treated male rats following pudendectomy. Behav Biol 20:141–148 [DOI] [PubMed] [Google Scholar]
- Baum MJ, Vreeburg JT 1973 Copulation in castrated male rats following combined treatment with estradiol and dihydrotestosterone. Science 182:283–285 [DOI] [PubMed] [Google Scholar]
- Feder HH 1971 The comparative actions of testosterone propionate and 5-androstan-17-ol-3-one propionate on the reproductive behaviour, physiology and morphology of male rats. J Endocrinol 51:241–252 [DOI] [PubMed] [Google Scholar]
- McDonald P, Beyer C, Newton F, Brien B, Baker R, Tan HS, Sampson C, Kitching P, Greenhill R, Pritchard D 1970 Failure of 5α-dihydrotestosterone to initiate sexual behaviour in the castrated male rat. Nature 227:964–965 [DOI] [PubMed] [Google Scholar]
- Jones ME, Boon WC, Proietto J, Simpson ER 2006 Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab 17:55–64 [DOI] [PubMed] [Google Scholar]
- Carani C, Rochira V, Faustini-Fustini M, Balestrieri A, Granata AR 1999 Role of oestrogen in male sexual behaviour: insights from the natural model of aromatase deficiency. Clin Endocrinol (Oxf) 51:517–524 [DOI] [PubMed] [Google Scholar]
- Marhefka CA, Gao W, Chung K, Kim J, He Y, Yin D, Bohl C, Dalton JT, Miller DD 2004 Design, synthesis, and biological characterization of metabolically stable selective androgen receptor modulators. J Med Chem 47:993–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hwang DJ, Chung K, Bohl CE, Fisher SJ, Miller DD, Dalton JT 2005 In vitro and in vivo structure-activity relationships of novel androgen receptor ligands with multiple substituents in the B-ring. Endocrinology 146:5444–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton JT, Mukherjee A, Zhu Z, Kirkovsky L, Miller DD 1998 Discovery of nonsteroidal androgens. Biochem Biophys Res Commun 244:1–4 [DOI] [PubMed] [Google Scholar]
- Gu YQ, Wang XH, Xu D, Peng L, Cheng LF, Huang MK, Huang ZJ, Zhang GY 2003 A multicenter contraceptive efficacy study of injectable testosterone undecanoate in healthy Chinese men. J Clin Endocrinol Metab 88:562–568 [DOI] [PubMed] [Google Scholar]
- Anderson RA, Baird DT 2002 Male contraception. Endocr Rev 23:735–762 [DOI] [PubMed] [Google Scholar]
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER 1997 Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med 337:91–95 [DOI] [PubMed] [Google Scholar]
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C 2004 Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab 89:61–70 [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K 1995 Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698 [DOI] [PubMed] [Google Scholar]
- Herrmann BL, Saller B, Janssen OE, Gocke P, Bockisch A, Sperling H, Mann K, Broecker M 2002 Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. J Clin Endocrinol Metab 87:5476–5484 [DOI] [PubMed] [Google Scholar]
- Miner JN, Chang W, Chapman MS, Finn PD, Hong MH, Lopez FJ, Marschke KB, Rosen J, Schrader W, Turner R, van Oeveren A, Viveros H, Zhi L, Negro-Vilar A 2007 An orally active selective androgen receptor modulator is efficacious on bone, muscle, and sex function with reduced impact on prostate. Endocrinology 148:363–373 [DOI] [PubMed] [Google Scholar]
- Oshima H, Wakabayashi K, Tamaoki BI 1967 The effect of synthetic estrogen upon the biosynthesis in vitro of androgen and luteinizing hormone in the rat. Biochim Biophys Acta 137:356–366 [DOI] [PubMed] [Google Scholar]
- Verjans HL, de Jong FH, Cooke BA, van der Molen HJ, Eik-Nes KB 1974 Effect of oestradiol benzoate on pituitary and testis function in the normal adult male rat. Acta Endocrinol (Copenh) 77:636–642 [DOI] [PubMed] [Google Scholar]
- Hislop MS, Ratanjee BD, Soule SG, Marais AD 1999 Effects of anabolic-androgenic steroid use or gonadal testosterone suppression on serum leptin concentration in men. Eur J Endocrinol 141:40–46 [DOI] [PubMed] [Google Scholar]
- Watanobe H, Suda T 1999 A detailed study on the role of sex steroid milieu in determining plasma leptin concentrations in adult male and female rats. Biochem Biophys Res Commun 259:56–59 [DOI] [PubMed] [Google Scholar]
- Pinilla L, Seoane LM, Gonzalez L, Carro E, Aguilar E, Casanueva FF, Dieguez C 1999 Regulation of serum leptin levels by gonadal function in rats. Eur J Endocrinol 140:468–473 [DOI] [PubMed] [Google Scholar]
- Chen J, Guan N, Chung K, Miller DD, Dalton JT 2002 Modulation of hormonal biomarkers and target organ weights in vivo by selective androgen receptor modulators (SARMs). AAPS Pharm Sci 4:W5251 (Abstract) [Google Scholar]
- Gao W, Kearbey JD, Nair VA, Chung K, Parlow AF, Miller DD, Dalton JT 2004 Comparison of the pharmacological effects of a novel selective androgen receptor modulator, the 5α-reductase inhibitor finasteride, and the antiandrogen hydroxyflutamide in intact rats: new approach for benign prostate hyperplasia. Endocrinology 145:5420–5428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner TT, Jones CE, Howards SS, Ewing LL, Zegeye B, Gunsalus GL 1984 On the androgen microenvironment of maturing spermatozoa. Endocrinology 115:1925–1932 [DOI] [PubMed] [Google Scholar]
- Jarow JP, Zirkin BR 2005 The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann NY Acad Sci 1061:208–220 [DOI] [PubMed] [Google Scholar]
- Zirkin BR, Santulli R, Awoniyi CA, Ewing LL 1989 Maintenance of advanced spermatogenic cells in the adult rat testis: quantitative relationship to testosterone concentration within the testis. Endocrinology 124:3043–3049 [DOI] [PubMed] [Google Scholar]
- Walsh PC, Swerdloff RS 1973 Biphasic effect of testosterone on spermatogenesis in the rat. Invest Urol 11:190–193 [PubMed] [Google Scholar]
- Awoniyi C, Santulli R, Chandrashekar V, Schanbacher BD, Zirkin BR 1989 Quantitative restoration of advanced spermatogenic cells in adult male rats made azoospermic by active immunization against LH or GnRH. Endocrinology 125:1303–1309 [DOI] [PubMed] [Google Scholar]
- Awoniyi C, Zirkin BR, Chandrashekar V, Schlaff WD 1992 Exogenously administered testosterone maintains spermatogenesis quantitatively in adult rats actively immunized against GnRH. Endocrinology 130:3283–3288 [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Wishart S, Conway AJ 2000 Oestradiol enhances testosterone-induced suppression of human spermatogenesis. Hum Reprod 15:672–679 [DOI] [PubMed] [Google Scholar]
- Awoniyi C, Sprando RL, Santulli RL, Chandrashekar V, Ewing LL, Zirkin BR 1990 Restoration of spermatogenesis by exogenously administered testosterone in rats made azoospermic by hypophysectomy or withdrawal of luteinizing hormone alone. Endocrinology 127:177–184 [DOI] [PubMed] [Google Scholar]
- Saito K, O'Donnell L, McLachlan RI, Robertson DM 2000 Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone withdrawal in adult rats. Endocrinology 141:2779–2785 [DOI] [PubMed] [Google Scholar]
- Beardsley A, O'Donnell L 2003 Characterization of normal spermiation and spermiation failure induced by hormone suppression in adult rats. Biol Reprod 68:1299–1307 [DOI] [PubMed] [Google Scholar]
- Palacios A, McClure RD, Campfield A, Swerdloff RS 1981 Effect of testosterone enanthate on testis size. J Urol 126:46–48 [DOI] [PubMed] [Google Scholar]
- Meachem SJ, Wreford NG, Stanton PG, Robertson DM, McLachlan RI 1998 Follicle-simulating hormone is required for the initial phase of spermatogenic restoration in adult rats following gonadotropin suppression. J Androl 19:725–735 [PubMed] [Google Scholar]
- Ewing LL, Desjardins C, Irby DC, Robaire B 1977 Synergistic interaction of testosterone and oestradiol inhibits spermatogenesis in rats. Nature 269:409–411 [DOI] [PubMed] [Google Scholar]