Abstract
Progesterone (P4) antagonizes estradiol (E2) in synaptic remodeling in the hippocampus during the rat estrous cycle. To further understand how P4 modulates synaptic plasticity, we used entorhinal cortex lesions, which induce E2-dependent neurite sprouting in the hippocampus. In young ovariectomized rats, the E2-dependent entorhinal cortex lesion-induced sprouting was attenuated by concurrent treatment with P4 and E2. Microglial activation also showed the E2-P4 antagonism. These findings extend reports on the estrous cycle synaptic remodeling without lesions by showing the P4-E2 antagonism during simultaneous treatment with both E2 and P4. Glial mechanisms were analyzed with the wounding-in-a-dish model of cocultured glia and embryonic d-18 cortical neurons from rat. In cocultures of mixed glia (astrocytes plus 30% microglia), P4 antagonized the E2-dependent neurite outgrowth (number and length) and neuron viability in the presence of E2, as observed in vivo. However, removal of microglia (astrocyte-neuron coculture) abolished the antagonism of E2 by P4 on neuron sprouting. The P4 receptor antagonists ORG-31710 and RU-486 blocked the antagonism of P4 on E2-dependent sprouting. These findings suggest a new role for microglia in P4 antagonism of E2 in neuronal plasticity and show its dependence on progesterone receptors. These findings are also relevant to the inclusion of progestins in hormone therapy, which is controversial in relation to cognitive declines during aging and in Alzheimer’s disease.
Brain glia mediate progesterone–estradiol cross-talk in neuronal sprouting after axotomy in rat models.
In brain, as in uterus, cell growth and regression during ovulatory cycles is regulated by the antagonism of progesterone (P4) on estradiol (E2)-induced growth. A beautiful example is synaptic remodeling during the rodent ovulatory cycle. The CA1 region of the hippocampus (Fig. 1) shows increased neuronal spine and synapse density during the preovulatory rise of plasma E2 up to proestrus, followed by rapid regression after ovulation when E2 falls and P4 rises (1). Similarly, in the CA1 region of ovariectomized macaques, the induction by E2 of the synaptic proteins syntaxin, synaptophysin, and spinophilin was antagonized by P4 (2).
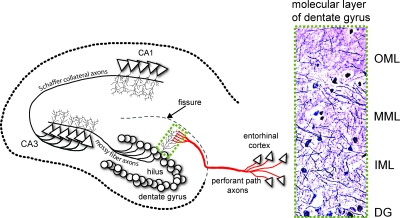
Figure 1.
ECL in the rodent brain are a model for the perforant path degeneration in AD, which damages a major input from the cerebral cortex to the hippocampus, a key center of declarative memory. Perforant path axons arise from large neurons in layers II and III of the lateral entorhinal cortex (EC) and project to dendrites of granule cell neurons in the outer molecular layer (OML) of the dentate gyrus (DG). The hippocampal fissure is an embryonic remnant that physically separates the dentate gyrus from the dendritic field of CA1 neurons. The granule cell axons (mossy fibers) project to CA3 pyramidal neurons, which in turn send Schaffer collateral axons to CA1 pyramidal neurons. These three links comprise the trisynaptic circuit of the hippocampal memory module. Degeneration of EC neurons and perforant path axons arises early in AD, whereas the granule and CA3 neurons are relatively spared throughout AD. After unilateral ECL, compensatory sprouting (reinnervation) within the molecular layer of the dentate gyrus arises from multiple pathways; the commissural/associational axons innervate the inner molecular layer (IML), and axons from the septohippocampal pathway, from the contralateral EC, and from local interneurons sprout to innervate the outer molecular layer (OML). Inset shows Holmes axonal fiber stain.
These synaptic variations are implicated in changes of spatial memory and other cognitive functions seated in the hippocampus that are damaged during Alzheimer’s disease (AD) and that may be hormonally sensitive. Animal and cell models consistently show that E2 is neuroprotective (3,4). In AD transgenic mice, P4 antagonized benefits from E2 that slowed extracellular accumulations of the amyloid β-peptide (5). P4 also antagonized the E2 neuroprotection of hippocampal neurons to excitotoxicity (6,7). However, P4 enhanced memory in aged intact male and female mice (8). Although there are no corresponding subcellular data on humans, exogenous P4 can impair some aspects of memory in healthy young women given a single dose (9,10).
Microglia are also implicated in neuroprotection. In a model of brain traumatic injury with male rats, P4 treatment reduced edema and enhanced neuronal survival in association with greater microglial activity but decreased expression of inflammatory cytokines (11,12,13). Moreover, microglia can secrete brain-derived neurotrophic factor and other neurotrophic factors, depending on the degree and types of neuronal injury (14,15). These findings suggest microglia may have neurotrophic roles under some conditions.
We examined E2-P4 interactions with a rodent model of AD lacking vascular pathology and amyloid deposits. The entorhinal cortex lesion (ECL) model for degeneration of the perforant path in AD shows extensive compensatory axonal sprouting (16) (Fig. 1), which is E2 dependent in animal models (17,18,19). Although astrocytic responses to ECL were shown to be responsive to E2, the hormonal sensitivity of microglia is not known in this paradigm. Perforant path efferents to the hippocampus, a seat of declarative memory, degenerate early in AD, with variable compensatory sprouting (16,20). Because the level of axonal sprouting can blunt memory loss from ECL in rat models (21,22), benefits of E2 in hormone therapy (HT) could include perforant path sprouting. However, progestins in HT could counteract possible benefits of E2. As a precedent, in cultures of neonatal hippocampal slices severed from entorhinal cortex innervation, P4 inhibited E2-dependent compensatory neurite sprouting (23). However, because neonatal brain slices involve other neurons besides the perforant path and because the P4 receptor expression differs markedly between neonatal and adult rat hippocampus (24), these findings may not represent P4-E2 interactions of adult brains. Therefore, we further examined the in vivo ECL model for effects of P4 on the adult rat brain. We also used the wounding-in-a-dish glial-neuron coculture model to analyze glial mechanisms underlying E2-P4 interactions, which show an unexpected role of microglia.
Materials and Methods
Ovariectomy, steroid replacement, and ECL
Animal experiments were conducted in accord with accepted standards of humane animal care, as outlined in the National Institutes of Health Ethical Guidelines. Sprague Dawley rats (Harlan, Indianapolis, IN) were ovariectomized and 2 wk later implanted sc with slow-release E2 (0.72 mg/pellet; 30-d release) or sham vehicle pellets (Innovative Research of America, Sarasota, FL). After 2 wk, rats were given ipsilateral (left-side)ECL (Fig. 1) (16).
P4 (50 mg/pellet; 15-d release) or sham vehicle pellets were inserted sc immediately after lesioning. After 14 d, rats were perfused (left myocardial ventricle) with saline. Brains were fixed in 4% paraformaldehyde at 4 C for 1 d, cryoprotected in 30% sucrose for 3 d, and sectioned at 18 μm. In a pilot study of hormone pellets, serum E2 and P4 was assayed by RIA after solvent extraction and column purification (F. Stanczyk, Reproductive Endocrine Research Laboratory of University of Southern California) (5). Serum E2 and P4 in this pilot showed elevations above placebo with pellet replacement at the 14-d measurement, consistent with implant product description (Table 1). Uterine wet weight in the experimental animals responded to E2, but not P4 alone (Table 2). Body weights were stable; rats had healthy appearance and activity.
Table 1.
Plasma E2 and P4
| Treatment | E2 (pg/ml) | P4 (ng/ml) |
|---|---|---|
| Placebo | 12 ± 5 | 2.5 ± 0.9 |
| E2 | 47 ± 11a | 2.3 ± 0.7 |
| P4 | 3.8 ± 1 | 16 ± 4.3b |
| E2+P4 | 29 ± 6a | 15 ± 2.7b |
Hormones assayed in a pilot study used the same lot of pellets and treatment paradigm with eight rats per group. The RIA sensitivity (Materials and Methods) does not allow statistical distinction of E2 levels between the E2 and P4 replacement groups. The pilot also showed that the initial surge of P4 from the implants subsided after 3 d, consistent with another study (44).
P < 0.05, different from placebo and P4.
P < 0.05, different from placebo and E2.
Table 2.
Effects of E2 and P4 on uterine and body weights
| Treatment | Uterus (g) | Body (g) |
|---|---|---|
| Placebo | 39 ± 2 | 290 ± 4 |
| E2 | 360 ± 33a | 227 ± 3a |
| P4 | 45 ± 3 | 292 ± 3 |
| E2+P4 | 370 ± 35a | 224 ± 3a |
Uteri were blotted for wet weight. Body weights were measured before killing. There were eight rats per group.
P < 0.0001 vs. other groups.
Holmes fiber staining and immunocytochemistry
Neurons were stained by the Holmes fiber method using silver for contrast (25). For immunocytochemistry, sections were permeabilized with Nonidet P-40 and treated with H2O2 to quench endogenous peroxidase. After treatment with normal goat sera, tissues were incubated 16 h at 4 C with anti-glial fibrillary acidic protein (anti-GFAP) (Dako Corp., Carpinteria, CA) or anti-major histocompatibility complex (MHC) class II (anti-MhcII) (clone OX6; Serotec, Oxford, UK). After secondary antibody treatment, signals were visualized by diaminobenzidine (Vectastain ABC Kit; Vector Laboratories, Burlingame, CA); specificity was confirmed by omitting primary or secondary antibody.
Cell culture and wounding-in-a-dish coculture
Primary mixed glia were obtained by standard procedures from cerebral cortex of 2-d-old rats and maintained in DMEM/F12 media (Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), 100 U/ml penicillin, 50 U/ml streptomycin, and 2 mm l-glutamine at 37 C, 5% CO2 (26). Astrocytes were purified from mixed glia by shaking off microglia and oligodendrocytes (27). Monolayer astrocyte or mixed glial cultures were replated onto glass chamber slides (Lab-Tek II, Nalge Nunc International; ThermoFisher Scientific, Rochester, NY) coated with poly-d-lysine 2 d before seeding with embryonic d 18 (E18) cortical neurons and kept 3 d before wounding and hormone treatments.
Glial-neuron cocultures were scratch-wounded with a pipette tip (28), followed immediately by addition of steroids. E2 (0.1 nm; Sigma Chemical Co., St. Louis, MO) and/or P4 (100 nm; Steraloids, Newport, RI) were added in DMEM high-glucose medium without phenol red (Invitrogen, Carlsbad, CA) supplemented with penicillin/streptomycin, 15 mm HEPES (Sigma), 1 mm sodium pyruvate (Sigma), and B27 supplement without antioxidants (Invitrogen). Vehicle control was 0.08% ethanol. After 48 h, cells were fixed in ice-cold methanol.
Immunocytochemistry
Neurite outgrowth was measured by immunostaining for monoclonal microtubule-associated protein-5 (MAP-5, 1:50; Sigma) and secondary goat-antimouse conjugated to Alexa 488 (Molecular Probes Invitrogen; 1:400). MAP-5-positive neurites extending into the wound zone were measured by IPLab Spectrum image analysis software on 10 random areas (0.4 mm2). Neuron numbers were assessed after immunostaining with neuron-specific nuclear protein (NeuN) (monoclonal, 1:50; Chemicon International, Inc., Temecula, CA) or neuron-specific enolase (NSE) (monoclonal, 1:100; Dako) and secondary goat antimouse Alexa 488. Immunolabeled cells were counted from six 0.2-mm2 areas adjacent to wound zones. Microglia were immunostained for complement receptor 3 (CR3) (clone OX42, 1:50; Serotec). Astrocytes were immunostained for GFAP (1:500; Dako). Secondary goat antibodies conjugated to Alexa 488 and 594 (described above) were used to detect CR3 and GFAP. Numbers of cells positive for CR3 (microglia) or GFAP (astrocytes) were counted from 0.2-mm2 (n = 6) areas adjacent to wound sites. Statistics used ANOVA and Fisher post hoc analysis.
Results
P4 antagonizes E2-dependent sprouting after ECL
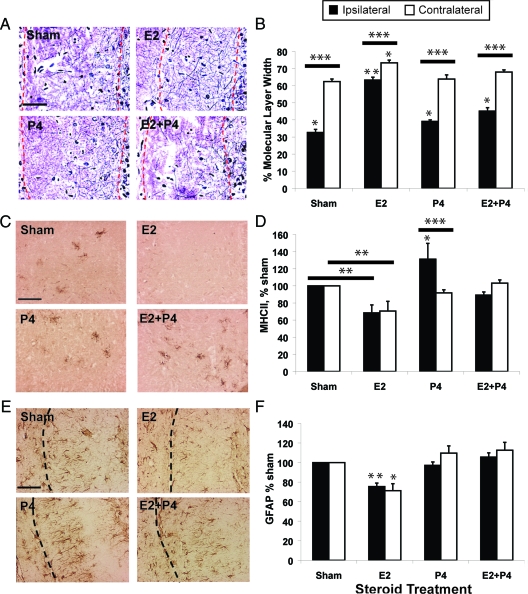
After ECL, compensatory sprouting was assessed at 14 d, when axonal sprouting from the commissural-associational fibers (Fig. 1) is well established (29). In ovariectomized young adult rats given E2 replacement only, sprouting ipsilateral to the ECL increased 2-fold, from 30% (sham) to 60% (E2) of total width of the molecular layer; contralateral sprouting increased modestly (60% for sham to 70% for E2) (Fig. 2, A and B), confirming previous results (17,30). Replacement of both P4 and E2 blocked 70% of the E2-dependent sprouting after lesioning. P4 alone only slightly increased ipsilateral sprouting. These findings extend the antagonism of E2 by P4 shown in physiological cycles of CA1 hippocampal synapses in the absence of lesions, whereby the rapid removal of synapses after proestrous E2 elevations depends on subsequent P4 elevations (31). In the ECL model, P4 antagonism was observed concurrently with E2 treatment.
Figure 2.
P4 attenuates E2-induced compensatory sprouting after ipsilateral ECL (see Fig. 1). All rats were ovariectomized before ECL and killed 14 d after ECL. Controls received sham pellets without steroids. Scale bars, 100 μm. n = 8 rats per group, 3 sections per brain. Results are means ± sem. A, Axonal fibers (Holmes stained) in the ipsilateral molecular layer of the dentate gyrus (borders outlined by red dashed lines). B, Average fiber band width in molecular layers of the ipsilateral (lesioned) and contralateral (unlesioned) sides, expressed as percent total molecular layer width (granule neuron layer edge to fissure). *, P < 0.05 and **, P < 0.001 from other groups in respective hippocampal side; ***, P < 0.0001, ipsi- vs. contralateral side. C, Microglial activation in the hippocampal hilus by OX6 immunostaining (epitope for MhcII 1a peptides). D, OX6-immunostained area in ipsi- and contralateral hilus, as percentage of sham. *, P < 0.05 and **, P < 0.001 from other treatments on respective side; *** P < 0.01, ipsi- vs. contralateral side. E, Astrocyte GFAP immunostaining in the ipsilateral molecular layer of the dentate gyrus. Dashed line shows the hippocampal fissure. F, GFAP-immunostained area in ipsi- and contralateral hippocampus, as percentage of sham. *, P < 0.01 and **, P < 0.005 vs. other treatments on respective hippocampal side.
E2-dependent sprouting does not depend on microglia
To develop a simpler experimental system for these complex in vivo responses, we used the in vitro wounding-in-a-dish model of primary cultures of neurons on a glial layer, in which scratch-wounding induces neurite outgrowth. This model with primary cultures of astrocytes from neonatal cortex (32,33) also shows E2-dependent sprouting (28) and is extended here to include microglia (mixed glia). The glia were either enriched in astrocytes (>95% astrocytes with <3% microglia) or contained 30% microglia (70% astrocytes, mixed-glia) and seeded with E18 cortical neurons at confluence.
In both glial models, E2 (100 pm) stimulated equal numbers of growing neurites (Fig. 3A) of similar length (Fig. 3B). These findings are contrary to expectations that the presence of microglia in mixed cultures might enhance E2 responses. Previously, we showed that induction of the neurotrophic cholesterol carrier apolipoprotein E (apoE) by E2 occurs in mixed glia but not in monotypic astrocyte cultures (34). The ECL model with apoE−/− mice showed that apoE was required for full E2-dependent neurite outgrowth (18). In the present studies, apoE levels in media were increased by wounding but did not differ with E2 treatment (not shown). Secretion of apoJ, which is also implicated in sprouting (18,35), was not altered by E2 and P4 (not shown).
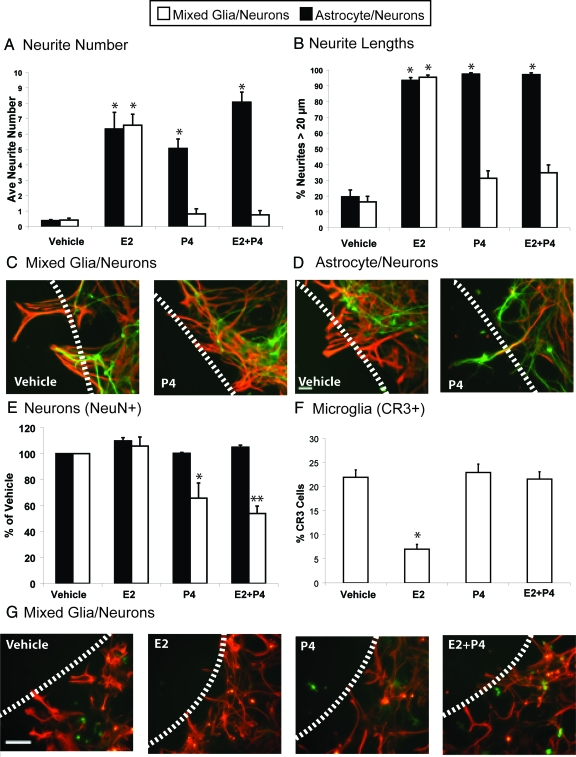
Figure 3.
Wounding-in-a-dish model of lesions to evaluate whether the presence of microglia influences responses of neurite outgrowth in the presence of E2 and P4 in culture media. Glia were cocultured with E18 neurons for 3 d and subjected to scratch wounding, with subsequent analysis 48 h later. Green, neurite MAP-5 immunostaining; red, astrocytic GFAP immunostaining; scale bar, 100 μm; dashed line, scratch wound zone. Results show the average of three experiments ± sem. A, Numbers of neurites extended into the wound zone per 0.5 mm2 in cultures of astrocytes (black bars) or mixed glia (white bars). *, P < 0.001. B, Neurite outgrowth, expressed as percentage of neurites bigger than 20 μm (legend as for A). *, P < 0.001. C, Addition of P4 alone did not induce neurite outgrowth in mixed glia-neuron cocultures containing 30% microglia and 70% astrocytes. D, Addition of P4 alone induced neurite outgrowth in astrocyte-neuron cocultures. E, NeuN-positive neurons in 0.250 mm2 adjacent to wound zone. *, P < 0.0005, vehicle and E2 in respective coculture model; **, P < 0.0001, vehicle and E2. F, Histogram of microglia adjacent to wound zone showing percentage of cells immunoreactive for CR3/total glial cells (CR3+GFAP); *, P < 0.0001 from other treatments. G, Glia in mixed glial-neuron cocultures with steroid treatment. Immunostaining for astrocytes GFAP (red) and microglia CR3 (OX42 antibody, green).
P4 antagonism of E2-dependent sprouting depends on microglia
In neurons cocultured with mixed glia, P4 strongly antagonized E2-mediated neurite outgrowth, whereas P4 alone did not support neurite outgrowth (Fig. 3, A–C). In contrast, when microglia were absent (astrocyte-neuron cocultures), P4 (100 nm) did not antagonize E2 induction of neurite outgrowth, whereas P4 alone supported neurite outgrowth (number and length) almost as well as E2 alone (Fig. 3, A, B, and D). The mixed glia/ neuron coculture thus models the in vivo antagonism of E2 by P4 (Fig. 2, A and B).
Stress effects of P4 on neurons in presence of microglia
Another difference between cultures of astrocytes and mixed glia is differential effects on neuronal immunomarkers after wounding: NSE is a lineage marker detected early in differentiation (36), whereas NeuN indicates mature neurons (37). NeuN shows less stability and is rapidly decreased by insults (ischemia, organophosphorus toxins) that may cause delayed cell death (38,39). In wounded mixed glia-neuron cocultures, treatment with P4 alone and with E2 plus P4 decreased NeuN-positive neurons by 50% at areas adjacent to the wound site, whereas E2 alone did not alter NeuN (Fig. 3E). The number of NeuN-positive neurons in astrocyte-neuron cocultures was not affected by P4. Without steroids, wounding decreased NeuN-positive neurons by 30% in mixed glia-neuron cocultures and by 15% in astrocyte neurons (P < 0.0001 vs. vehicle controls, not shown). In unwounded cultures, E2 or P4 alone did not affect NeuN immunostaining (intensity or cell number, not shown). NSE was not responsive to experimental manipulations (not shown). The combination of microglia with astrocytes appears to increase stress effects of P4 on neurons that might cause subsequent neurodegeneration, as observed in organophosphorus neurotoxicity (39).
Microglial markers are sensitive to E2-P4 interactions
To further resolve the microglial role, we examined the wound zone (Fig. 3, F and G). In mixed glial-neuron cocultures, E2 decreased the number of CR3/OX42-positive microglia by 70%. P4 antagonized these E2 effects, whereas P4 alone did not alter CR3 expression. These effects are reciprocal with neurite outgrowth.
In the ECL model, microglia responses to E2 and P4 resembled those in vitro (Fig. 2, C and D). These data were from the hilar hippocampal region, where microglial activation after ECL persists longer than in the dentate molecular layer. E2 attenuated hilar microglia activation (MhcII microglial marker); inclusion of P4 with E2 blocked the attenuation of E2. We also observed that P4 alone enhanced microglial activity ipsilateral to the lesion. Microglia did not show elevated markers of reactivity in the wound zone at 14 d after ECL (not shown), confirming previous results (40,41,42,43). The enhancement of microglial immunoepitopes by P4 is consistent with the enhanced microglial activation by P4 after head trauma (11). Astrocyte GFAP immunoreactivity also showed hormone responses in the molecular layer of the dentate gyrus (Fig. 2, E and F), the zone of neurite sprouting (Figs. 1 and 2A). Treatment with E2 decreased GFAP, whereas P4 plus E2 and P4 alone did not differ from controls. Decreased GFAP levels correlate with increased neurite sprouting in both in vivo and in vitro models (28,30). Thus, there is overall consistency in the attenuation of astrocyte and microglial activities by E2 and the antagonism of this E2 effect by P4 in the ECL model.
NO production was also examined because of a link to CR3, the microglial marker regulated by E2 and P4; CR3 can stimulate NO production (45) and potentially form neurotoxic peroxynitrite by a further reaction of NO with superoxide (46). However, E2 and P4 did not alter NO secretion in wounded cocultures (not shown).
P4-E2 antagonism of neurite outgrowth is mediated by P4 receptors
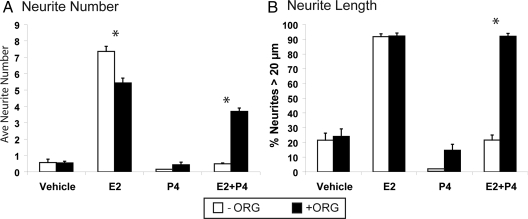
Receptor selectivity was studied with ORG-31710 (ORG), a P4 receptor antagonist type II, which inhibits DNA binding of the liganded receptor and has weak anti-glucocorticoid and antiandrogenic activity (47,48,49). Brain cell specificities of ORG have not been reported. In cocultures with mixed glia, ORG alone (100 nm) did not alter neurite numbers or lengths (Fig. 4, A and B). ORG modestly antagonized (−25%) E2-dependent neurite numbers in mixed glial cocultures (Fig. 4A) but did not alter neurite length relative to E2 alone (Fig. 3B). The P4 antagonism of E2-dependent neurite growth in mixed glia was reversed by ORG. Similarly, in cocultures with astrocytes, ORG (100 nm) completely blocked the outgrowth by P4 alone (not shown).
Figure 4.
The P4 receptor antagonist ORG (100 nm) blocked the antagonism by P4 of E2-induced neurite outgrowth in mixed glial-neuron cocultures. A, Neurite number (total); B, neurites bigger than 20 μm in the wound zone. Results are the average of three experiments ± sem. *, P < 0.0001 for −ORG vs. +ORG.
Moreover, in mixed glia, RU-486 (mifepristone) was similar to ORG in relieving the antagonism of P4 on E2-dependent neurite outgrowth (neurite number and length; not shown). RU-486 is a different type II P4 receptor antagonist with higher anti-glucocorticoid activity than ORG (50,51). These effects of RU-486 resemble its blockade of the P4-dependent dendritic loss after proestrus (1).
Discussion
We report that P4 attenuates E2-dependent lesion-induced neurite sprouting in vivo and in vitro, that the P4 antagonism of E2 on sprouting involves microglia, and that the P4-E2 antagonism is mediated by P4 receptors. These findings are relevant to basic mechanisms in the steroidal regulation of synaptic plasticity and to the clinical optimization of HT for AD.
A new role of microglia in E2-P4 interactions with neurite outgrowth was revealed by coculture of astrocytes or mixed glia with neurons. Only in mixed glial cultures containing 30% microglia and 70% astrocytes did P4 antagonize E2-dependent sprouting. In vivo, microglia responded similarly to E2 and P4 at 14 d after ECL and E2-dependent sprouting was attenuated. The present in vitro model does not include oligodendroglia, which are activated by ECL (52) and which are also important targets of P4 in promoting remyelination (53,54,55). The receptor specificity of the P4 effects was shown with two different synthetic antagonists ORG and RU-486.
The cellular location of the steroid receptors mediating these E2-P4 interactions is unknown in both the in vivo and in vitro models. Microglial sex steroid receptors were reported while this paper was in preparation; with PCR assays, ex vivo adult murine microglia did not have detectable P4 receptors or estrogen receptor-β but did have abundant estrogen receptor-α and glucocorticoid receptor RNA (56). However, we do not yet know the expression of the P4 receptor in the different cells of primary cultures after wounding. The identical activity of ORG and RU-486 in relieving the P4 antagonism suggests that the effect is primarily mediated by the P4 receptor, because RU-486 also shows anti-glucocorticoid activity (50,51). We conclude that the P4 receptor is the main mediator of P4 in antagonizing E2-dependent neurite outgrowth and that the microglial glucocorticoid receptor has a minor role. In the absence of microglial P4 receptor, the known P4 receptors in astrocytes or neurons (57) would appear to modulate the P4-E2 cross talk on sprouting by secondary effects on microglia. Moreover, microglia may possess other P4 receptor isoforms (57) not initially detected (56).
In the ECL model, P4 antagonized the effects of E2 neuronal sprouting and both astrocyte and microglial responses. P4 alone increased microglial activity (MHC II epitope) in the ECL model but did not alter neurite outgrowth or GFAP. An additional factor in the ECL model is a P4 surge during the initial 3 d after implanting P4 (Table 1), which could have down-regulated receptors with consequences persisting to the 14-d time of measurement.
Microglia are proving multifaceted with conditional expression of both neurotoxic and neurotrophic activities depending on the level of neuronal stress or injury (14,15). In a brain trauma model with male rat (introductory section), P4 alone increased microglial activity and enhanced neuron survival (11,12,13). The involvement of microglia in E2-P4 synergies should not be interpreted as neurotoxic because the physiological E2-P4 cross talk mediates normal cycles of synaptic remodeling during the ovulatory cycle in rodents (1,2,3). These complex effects may be better understood when we resolve P4 receptor distributions.
The requirement for astrocytes and microglia in the P4 antagonism recalls that E2 induced apoE expression only in mixed glia but not in monotypic cultures of astrocytes or microglia (30). As a working hypothesis, the P4 activities may depend on primary effects on astrocytes with secondary effects on microglia. Because brain microglia are of bone marrow monocytic lineage, we looked for studies of peripheral leukocytes. Although effects of either E2 or P4 on blood leukocytes have been examined in vitro, we did not find reports on E2-P4 interactions.
Although microglia can have negative roles in neurodegeneration, e.g. as sources of the neurotoxic peroxynitrite (46), our results are the first evidence that microglia mediate P4-E2 antagonism in neuronal plasticity. Microglial markers responded similarly to E2 and P4 lesion models in vivo and in vitro. These findings are broadly consistent with reports that 1) inhibiting microglial activation reversed age-related impairments of hippocampal long-term potentiation, a model for learning and memory (58), and 2) that ECL-induced dendritic retraction was blocked by suppressing microglia activation (59). Because aging is implicated in HT efficacy (60) and because aging (donor age of primary cultures) influences responses of astrocytes and microglia to E2 (61,62), future studies will consider glial aging effects on P4-E2 interactions.
These studies observed cross talk on lesion-induced neurite sprouting from continuous concurrent exposure to E2 and P4, in vivo and in vitro, whereas P4 elevation accelerated the regression of CA1 dendritic spines following declines of E2 after ovulation (1). Because the mechanisms in hippocampal dendritic remodeling during the estrous cycle may differ from lesion-induced sprouting of other neurons (Fig. 1 legend), it would be interesting to examine the CA1 spines during pregnancy, when plasma E2 and P4 are both elevated.
The P4 antagonism of E2 neuroprotection in animal models of AD (these results and Ref. 5) may be relevant to mixed outcomes of HT involving progestins. The Women’s Health Initiative (WHI) Memory Study of Prempro [conjugated equine estrogens (Premarin; low levels of P4) with medroxyprogesterone] observed increased dementia (63,64,65,66). In the WHI Study on Cognitive Aging study ancillary to WHI Memory Study, Prempro had a negative impact on verbal memory (67). By contrast, estrogen-only therapy in premature menopause showed benefits to some cognitive functions (68). Prempro effects cannot be directly compared with the present experiments for many reasons, including the different activities of equine estrogens and the synthetic progestin.
Progestins have been generally incorporated in HT, because unopposed estrogen is well recognized to increase the risk of uterine cancer above estrogens plus progestins. However, if some progestins prove to impair compensatory sprouting or other aspects of plasticity as indicated by the present studies, other approaches need consideration.
Acknowledgments
We thank Wendy Mack, Ph.D. (Department of Preventive Medicine, University of Southern California), for statistical consultation and Bernard Steinman, M.S. (Department of Gerontology, University of Southern California), for preparation of figures.
Footnotes
Present address for A.M.W.: Center for Human Nutrition, David Geffen School of Medicine, University of California, Los Angeles, California 90095.
Present address for Y.D.: Department of Physiology and Biophysics, Georgetown University, Washington, D.C. 20057.
This work was supported by National Institute on Aging: P01 AG14751; P01 AG026572, Project 4; AG13499; and AG00095.
Disclosure Statement: A.M.W., I.R., J.M.A., Y.D., M.W., T.E.M., and C.E.F. have nothing to declare.
First Published Online September 4, 2008
Abbreviations: AD, Alzheimer’s disease; apoE, apolipoprotein E; CR3, complement receptor 3; E18, embryonic d 18; E2, estradiol; ECL, entorhinal cortex lesion; GFAP, glial fibrillary acidic protein; HT, hormone therapy; MAP-5, microtubule-associated protein-5; NeuN, neuron-specific nuclear protein; NSE, neuron-specific enolase; ORG, ORG-31710; P4, progesterone.
References
- Woolley CS, McEwen BS 1993 Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol 336:293–306 [DOI] [PubMed] [Google Scholar]
- Choi JM, Romeo RD, Brake WG, Bethea CL, Rosenwaks Z, McEwen BS 2003 Estradiol increases pre- and post-synaptic proteins in the CA1 regions of the hippocampus in female rhesus macaques (Macaca mulatta). Endocrinology 144:4734–4738 [DOI] [PubMed] [Google Scholar]
- McEwen BS 2002 Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol 91:2785–2801 [DOI] [PubMed] [Google Scholar]
- Chen E, Nilsen J, Brinton RD 2006 Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: therapeutic implications. Endocrinology 147:5303–5313 [DOI] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ 2007 Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci 27:13357–13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario E, Ramsden M, Pike CJ 2006 Progestins inhibit the neuroprotective effects of estrogen in the rat hippocampus. Brain Res 1099:206–210 [DOI] [PubMed] [Google Scholar]
- Nilsen J, Brinton RD 2002 Impact of progestins on estradiol protentiation of the glutamate calcium response. Neuroreport 13:825–830 [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA 2008 Progesterone enhances performance of aged mice in cortical or hippocampal tasks. Neurosci Lett 437:116–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wingen G, van Broekhoven F, Verkes RJ, Petersson KM, Bäckström T, Buitelaar J, Fernández G 2007 How progesterone impairs memory for biologically salient stimuli in healthy young women. J Neurosci 27:11416–11423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman EW, Weinstock L, Rickels K, Sondheimer SJ, Coutifaris C 1992 A placebo-controlled study of effects of oral progesterone on performance and mood. Br J Clin Pharmacol 33:293–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman KJ, Goss CW, Stein DG 2004 Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res 1008:29–39 [DOI] [PubMed] [Google Scholar]
- Pettus EH, Wright DW, Stein DG, Hoffman SW 2005 Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res 1049:112–119 [DOI] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, Salomone JP, Dent LL, Harris OA, Ander DS, Lowery DW, Patel MM, Denson DD, Gordon AB, Wald MM, Gupta S, Hoffman SW, Stein DG 2007 ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med 49:391–402 [DOI] [PubMed] [Google Scholar]
- Lai AY, Todd KG 2008 Differential regulation of trophic and proinflammatory microglial effectors is dependent on severity of neuronal injury. Glia 56:259–270 [DOI] [PubMed] [Google Scholar]
- Shie FS, Woltjer RL 2007 Manipulation of microglial activation as a therapeutic strategy in Alzheimer’s disease. Curr Med Chem 14:2865–2871 [DOI] [PubMed] [Google Scholar]
- Geddes JW, Monaghan DT, Cotman CW, Lott IT, Kim RC, Chui HC 1985 Plasticity of hippocampal circuitry in Alzheimer’s disease. Science 23:1179–1181 [DOI] [PubMed] [Google Scholar]
- Morse JK, DeKosky ST, Scheff SW 1992 Neurotrophic effects of steroids on lesion-induced growth in the hippocampus. II. Hormone replacement. Exp Neurol 118:47–52 [DOI] [PubMed] [Google Scholar]
- Stone DJ, Song Y, Anderson CP, Krohn KK, Finch CE, Rozovsky I 1998 Bidirectional transcription regulation of glial fibrillary acidic protein by estradiol in vivo and in vitro. Endocrinology 139:3202–3209 [DOI] [PubMed] [Google Scholar]
- Kadish I, van Groen T 2002 Low levels of estrogen significantly diminish axonal sprouting after entorhinal cortex lesions in the mouse. J Neurosci 22:4095–4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroub TR, DeToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC 2006 Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci USA 103:10041–10045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JJ, McQuilkin M, Carrigan T, MacDonald K, Kelley MS 1996 Progressive entorhinal cortex lesions accelerate hippocampal sprouting and spare spatial memory in rats. Proc Natl Acad Sci USA 93:15512–15517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JJ, Bulsara K, Moore SC, Ruch K, Abrams W 1999 Progressive unilateral damage of the entorhinal cortex enhances synaptic efficacy of the crossed entorhinal afferent to dentate granule cells. J Neurosci 19:RC42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter B, Harris-White ME, Frautschy SA, Cole GM 1999 Role of apolipoprotein E and estrogen in mossy fiber sprouting in hippocampal slice cultures. Neurosci 91:1009–1016 [DOI] [PubMed] [Google Scholar]
- Quadros PS, Pfau JL, Wagner CK 2007 Distribution of progesterone receptor immunoreactivity in the fetal and neonatal rat forebrain. J Comp Neurol 504:42–56 [DOI] [PubMed] [Google Scholar]
- Holmes W, Young JZ 1942 Nerve regeneration after immediate and delayed suture. J Anat 77:63–96 [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Finch CE, Morgan TE 1998 Age-related activation of microglia and astrocytes: in vitro studies show persistent phenotypes of aging, increased proliferation, and resistance to down-regulation. Neurobiol Aging 19:97–103 [DOI] [PubMed] [Google Scholar]
- McCarthy KD, deVellis J 1980 Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol 85:890–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozovsky I, Wei M, Stone DJ, Zanjani H, Anderson CP, Morgan TE, Finch CE 2002 Estradiol (E2) enhances neurite outgrowth by repressing glial fibrillary acidic protein expression and reorganizing laminin. Endocrinology 143:636–646 [DOI] [PubMed] [Google Scholar]
- Cotman CW, Nieto-Sampedro M 1984 Cell biology of synaptic plasticity. Science 225:1287–1294 [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Lopez LM, Schick J, Finch CE 2000 Effects of age on gene expression during estrogen-induced synaptic sprouting in the female rat. Exp Neurol 165:46–57 [DOI] [PubMed] [Google Scholar]
- McEwen BS, Woolley CS 1994 Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp Gerontol 29:431–436 [DOI] [PubMed] [Google Scholar]
- McMillian MK, Thai Hong JS, O'Callahan JP, Pennypacker KR 1994 Brain injury in a dish: a model for reactive gliosis. Trends Neurosci 17:138–142 [DOI] [PubMed] [Google Scholar]
- Lefrancois T, Fages C, Peschanski M, Tardy M 1997 Neuritic outgrowth associated with astroglial phenotypic changes induced by antisense glial fibrillary acidic protein (GFAP) mRNA in injured neuron-astrocyte cocultures. J Neurosci 17:4121–4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE 1997 Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp Neurol 143:313–318 [DOI] [PubMed] [Google Scholar]
- Kang SW, Shin YJ, Shim YJ, Jeong SY, Park IS, Min BH 2005 Clusterin interacts with SCLIP (SCG10-like protein) and promotes neurite outgrowth of PC12 cells. Exp Cell Res 309:305–315 [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Brighman MW, Marangos PJ 1980 Neurons switch from non-neuronal enolase to neuron-specific enolase during differentiation. Brain Res 190:195–214 [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Nochlin D, Born DE 1998 Neuronal nuclear antigen (NeuN): a marker of neuronal maturation in early human fetal nervous system. Brain Dev 20:88–94 [DOI] [PubMed] [Google Scholar]
- Unal-Cevik I, Kilinc M, Gursoy-Ozdemir Y, Gurer G, Dalkara T 2004 Loss of NeuN immunoreactivity after cerebral ischemia does not indicate neuronal cell loss: a cautionary note. Brain Res 1015:169–174 [DOI] [PubMed] [Google Scholar]
- Collombet JM, Masqueliez C, Four E, Burckhart MF, Bernabe D, Baubichon D, Lallement G 2006 Early reduction of NeuN antigenicity induced by soman poisoning in mice can be used to predict delayed neuronal degeneration in the hippocampus. Neurosci Lett 398:337–342 [DOI] [PubMed] [Google Scholar]
- Hailer NP, Bechmann I, Heizmann S, Nitsch R 1997 Adhesion molecule expression on phagocytic microglial cells following anterograde degeneration of perforant path axons. Hippocampus 7:341–349 [DOI] [PubMed] [Google Scholar]
- Hailer NP, Grampp A, Nitsch R 1999 Proliferation of microglia and astrocytes in the dentate gyrus following entorhinal cortex lesion: a quantitative bromodeoxyuridine-labelling study. Eur J Neurosci 11:3359–3364 [DOI] [PubMed] [Google Scholar]
- Dietrich A, Fulop ZL, Chambers MD, Darrell RS, Stein DG 2000 The effect of Ginkgo biloba extract (EGb 761) on gliotic reactions in the hippocampal formation after unilateral entorhinal cortex lesions. Restorative Neurol Neurosci 16:87–96 [PubMed] [Google Scholar]
- Gall C, Rose G, Lynch G 1979 Proliferative and migratory activity of glial cells in the partially deafferented hippocampus. J Comp Neurol 183:539–549 [DOI] [PubMed] [Google Scholar]
- Singh M, Sumien N, Kyser C, Simpkins JW 2008 Estrogens and P4 as neuroprotectants: what animal models teach us. Front Biosci 13:1083–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien HF, Yeh KY, Jiang-Shieh YF, Wei IH, Chang CY, Chang ML, Wu CH 2005 Signal transduction pathways of nitric oxide release in primary microglial culture challenged with gram-positive bacterial constituent, lipoteichoic acid. Neurosci 133:423–436 [DOI] [PubMed] [Google Scholar]
- Xie Z, Wei M, Morgan TE, Fabrizio P, Han D, Finch CE, Longo VD 2002 Peroxynitrite mediates neurotoxicity of amyloid β-peptide1–42- and lipopolysaccharide-activated microglia. J Neurosci 22:3384–3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloosterboer HJ, Deckers GH, Schoonen WG 1994 Pharmacology of two new very selective antiprogestagens: Org 31710 and Org 31806. Hum Reprod 9(Suppl 1):47–52 [DOI] [PubMed] [Google Scholar]
- Sanchez-Criado JE, Tebar M, Bellido C, De Jong FH 2000 Comparison of the effects of antiprogestins RU38486, ZK98299 and ORG31710 on periovulatory hypophysial, ovarian and adrenal hormone secretion in the rat. J Endocrinol Invest 23:151–157 [DOI] [PubMed] [Google Scholar]
- Thomas CP, Liu KZ, Vats HS 2006 Medroxyprogesterone acetate binds the glucocorticoid receptor to stimulate α-ENaC and sgk1 expression in renal collecting duct epithelia. Am J Physiol Renal Physiol 290:F306–F312 [DOI] [PubMed] [Google Scholar]
- Hodgen GD, van Uem JF, Chillik CF, Danforth DR, Wolf JP, Neulen J, Williams RF, Chwalisz K 1994 Non-competitive anti-oestrogenic activity of progesterone antagonists in primate models. Hum Reprod 9(Suppl 1):77–81 [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Goldman ME 1994 RU486 exerts antiestrogenic activities through a novel progesterone receptor A form-mediated mechanism. J Biol Chem 269:11945–11949 [PubMed] [Google Scholar]
- Drojdahl N, Fenger C, Nielsen HH, Owens T, Finsen B 2004 Dynamics of oligodendrocyte responses to anterograde axonal (Wallerian) and terminal degeneration in normal and TNF-transgenic mice. J Neurosci Res 75:203–217 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Ghoumari A, Massaad C, Robert F, El-Etr M, Akwa Y, Rajkowski K, Baulieu EE 2007 Novel perspectives for progesterone in hormone replacement therapy, with special reference to the nervous system. Endocr Rev 28:387–439 [DOI] [PubMed] [Google Scholar]
- Garcia-Ovejero D, Azcoitia I, Doncarlos LL, Melcangi RC, Garcia-Segura LM 2005 Glia cross-talk in the neuroprotective mechanisms of sex steroid hormones. Brain Res Brain Res Rev 48:273–286 [DOI] [PubMed] [Google Scholar]
- Magnaghi V, Veiga S, Ballabio M, Gonzalez LC, Garcia-Segura LM, Melcangi RC 2006 Sex-dimorphic effects of progesterone and its reduced metabolites on gene expression of myelin proteins by rat Schwann cells. J Peripher Nerv Syst 11:111–118 [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS, Bulloch K 2008 Steroid hormone receptor expression and function in microglia. Glia 56:659–674 [DOI] [PubMed] [Google Scholar]
- Brinton RD, Thompson RF, Foy MR, Baudry M, Wang J, Finch CE, Morgan TE, Pike CJ, Mack WJ, Stanczyk FZ, Nilsen J 2008 Progesterone receptors: form and function in brain. Front Neuroendocrinol 29:313–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA 2006 The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem 99:1263–1272 [DOI] [PubMed] [Google Scholar]
- Eyupoglu IY, Bechmann I, Nitsch R 2003 Modification of microglia function protects from lesion-induced neuronal alterations and promotes sprouting in the hippocampus. FASEB J 17:1110–1111 [DOI] [PubMed] [Google Scholar]
- Sherwin BB, Henry JF 2008 Brain aging modulates the neuroprotective effects of estrogen on selective aspects of cognition in women: a critical review. Front Neuroendocrinol 29:88–113 [DOI] [PubMed] [Google Scholar]
- Rozovsky I, Wei M, Morgan TE, Finch CE 2005 Reversible age impairments in neurite outgrowth by manipulations of astrocytic GFAP. Neurobiol Aging 26:705–715 [DOI] [PubMed] [Google Scholar]
- Johnson AB, Sohrabji F 2005 Estrogen’s effects on central and circulating immune cells vary with reproductive age. Neurobiol Aging 26:1365–1374 [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sheerwin BB, Hsia J, Margolis KL, Hogan PE, Wallace R, Dailey M, Freeman R, Hays J 2004 Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2959–2968 [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, Gass ML, Stefanick ML, LD, Hays J, Johnson KC, Coker LH, Dailey M, Bowen D 2003 Effect of estrogen plus progestin on global cognitive function in postmenopausal women: The Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2663–2672 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones 3rd BN, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J; WHIMS Investigators 2003 Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 289:2651–2662 [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Kuller L, Rapp SR, Thal L, Lane DS, Fillit H, Stefanick ML, Hendrix SL, Lewis CE, Masaki K, Coker LH 2004 Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA 291:2947–2958 [DOI] [PubMed] [Google Scholar]
- Resnick SM, Maki PM, Rapp SR, Espeland MA, Brunner R, Coker LH, Granek IA, Hogan P, Ockene JK, Shumaker SA; Women’s Health Initiative Study of Cognitive Aging Investigators 2006 Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab 91:1802–1810 [DOI] [PubMed] [Google Scholar]
- Henderson VW, Sherwin BB 2007 Surgical versus natural menopause: cognitive issues. Menopause 14:572–579 [DOI] [PubMed] [Google Scholar]
- Grossman KJ, Goss CW, Stein DG 2004 Effects of progesterone on the inflammatory response to brain injury in the rat. Brain Res 1008:29–39 [DOI] [PubMed] [Google Scholar]