Abstract
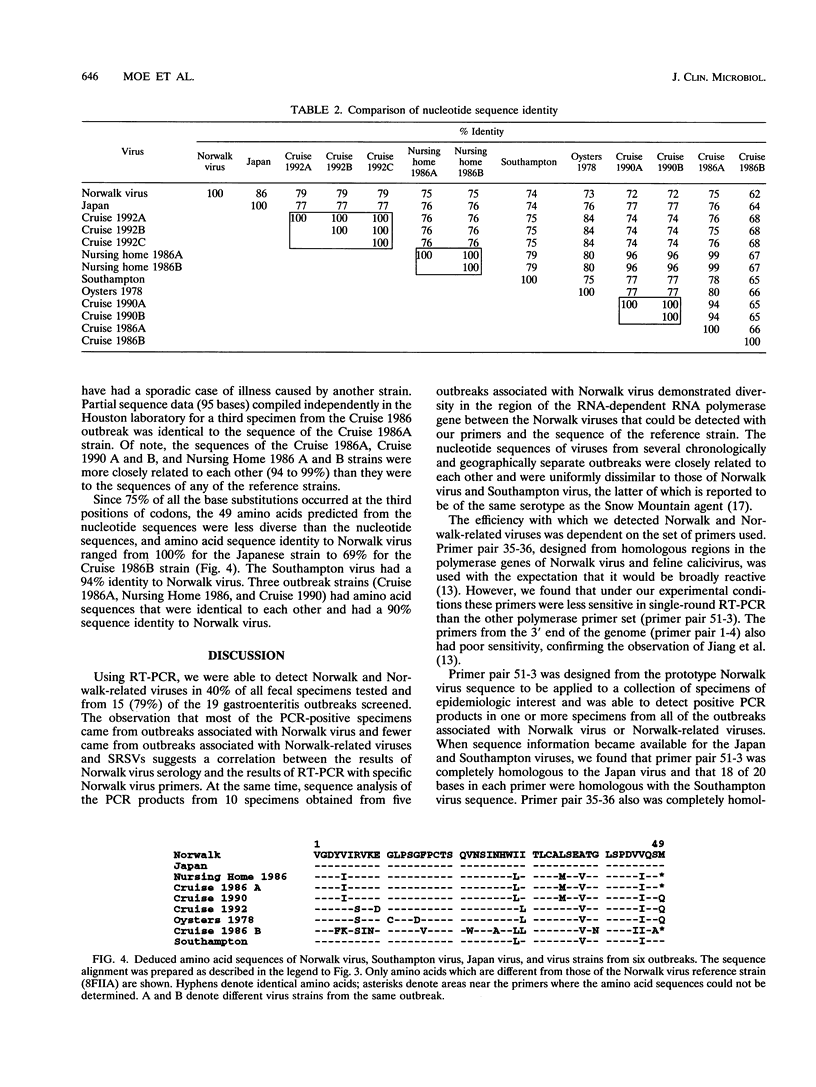
Norwalk virus (NV) and other small round-structured viruses (SRSVs) are frequent causes of gastroenteritis outbreaks. The recent cloning and sequencing of the NV genome has made it possible to detect NV and Norwalk-related viruses from fecal specimens by reverse transcription (RT)-PCR. We applied this technique to the examination of a total of 139 fecal specimens from 19 outbreaks characterized by NV serology, including 56 samples from 7 NV outbreaks, 36 from 6 Norwalk-related virus outbreaks, and 47 from 6 outbreaks with SRSVs visualized by electron microscopy that were serologically unrelated to NV. Three primer pairs were evaluated: two pairs in the polymerase region of NV and one pair near the 3' end of the genome. When one set of primers (primer pair 51-3) from the polymerase region was used, 40% of all samples were positive by RT-PCR and specimens from the NV outbreaks were more likely to be positive (64%) than those from outbreaks associated with Norwalk-related viruses (44%) or SRSVs (8%). To determine the relationship of the outbreak strains to NV, we compared the sequences of a 145-base portion of the polymerase gene from 10 specimens obtained from five different outbreaks characterized as NV by serology. No two outbreak strains had the same sequence in this 145-base portion of the polymerase gene, and the identities of the nucleotide and amino acid sequences of these products compared with the sequences of the corresponding region of NV ranged from 62 to 79% and 69 to 90%, respectively. Because of sequence diversity in the polymerase region, the successful application of RT-PCR to investigations of outbreaks of suspected NV-associated gastroenteritis will depend on the use of either multiple primer pairs or primers made against regions of the genome that are more conserved.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. L., Zickl R. Winter vomiting disease. J Infect Dis. 1969 Jun;119(6):668–673. doi: 10.1093/infdis/119.6.668. [DOI] [PubMed] [Google Scholar]
- Cristiano K., Di Bisceglie A. M., Hoofnagle J. H., Feinstone S. M. Hepatitis C viral RNA in serum of patients with chronic non-A, non-B hepatitis: detection by the polymerase chain reaction using multiple primer sets. Hepatology. 1991 Jul;14(1):51–55. doi: 10.1002/hep.1840140109. [DOI] [PubMed] [Google Scholar]
- Cubitt W. D., Blacklow N. R., Herrmann J. E., Nowak N. A., Nakata S., Chiba S. Antigenic relationships between human caliciviruses and Norwalk virus. J Infect Dis. 1987 Nov;156(5):806–814. doi: 10.1093/infdis/156.5.806. [DOI] [PubMed] [Google Scholar]
- De Leon R., Matsui S. M., Baric R. S., Herrmann J. E., Blacklow N. R., Greenberg H. B., Sobsey M. D. Detection of Norwalk virus in stool specimens by reverse transcriptase-polymerase chain reaction and nonradioactive oligoprobes. J Clin Microbiol. 1992 Dec;30(12):3151–3157. doi: 10.1128/jcm.30.12.3151-3157.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary G. W., Anderson L. J., Keswick B. H., Johnson P. C., DuPont H. L., Stine S. E., Bartlett A. V. Norwalk virus antigen and antibody response in an adult volunteer study. J Clin Microbiol. 1987 Oct;25(10):2001–2003. doi: 10.1128/jcm.25.10.2001-2003.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gary G. W., Jr, Kaplan J. E., Stine S. E., Anderson L. J. Detection of Norwalk virus antibodies and antigen with a biotin-avidin immunoassay. J Clin Microbiol. 1985 Aug;22(2):274–278. doi: 10.1128/jcm.22.2.274-278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grohmann G., Glass R. I., Gold J., James M., Edwards P., Borg T., Stine S. E., Goldsmith C., Monroe S. S. Outbreak of human calicivirus gastroenteritis in a day-care center in Sydney, Australia. J Clin Microbiol. 1991 Mar;29(3):544–550. doi: 10.1128/jcm.29.3.544-550.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang J., Graham D. Y., Estes M. K. Detection of Norwalk virus in stool by polymerase chain reaction. J Clin Microbiol. 1992 Oct;30(10):2529–2534. doi: 10.1128/jcm.30.10.2529-2534.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang M., Graham D. Y., Estes M. K. Expression, self-assembly, and antigenicity of the Norwalk virus capsid protein. J Virol. 1992 Nov;66(11):6527–6532. doi: 10.1128/jvi.66.11.6527-6532.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X., Wang M., Wang K., Estes M. K. Sequence and genomic organization of Norwalk virus. Virology. 1993 Jul;195(1):51–61. doi: 10.1006/viro.1993.1345. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Dolin R., Thornhill T. S., Kalica A. R., Chanock R. M. Visualization by immune electron microscopy of a 27-nm particle associated with acute infectious nonbacterial gastroenteritis. J Virol. 1972 Nov;10(5):1075–1081. doi: 10.1128/jvi.10.5.1075-1081.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. S., Moe C. L., Glass R. I., Monroe S. S., Estes M. K., Chapman L. E., Jiang X., Humphrey C., Pon E., Iskander J. K. Norwalk virus-associated gastroenteritis traced to ice consumption aboard a cruise ship in Hawaii: comparison and application of molecular method-based assays. J Clin Microbiol. 1994 Feb;32(2):318–322. doi: 10.1128/jcm.32.2.318-322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden P. R., Caul E. O., Ashley C. R., Clarke I. N. Sequence and genome organization of a human small round-structured (Norwalk-like) virus. Science. 1993 Jan 22;259(5094):516–519. doi: 10.1126/science.8380940. [DOI] [PubMed] [Google Scholar]
- Lewis D. C., Lightfoot N. F., Pether J. V. Solid-phase immune electron microscopy with human immunoglobulin M for serotyping of Norwalk-like viruses. J Clin Microbiol. 1988 May;26(5):938–942. doi: 10.1128/jcm.26.5.938-942.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. Norwalk agent and other small-round structured viruses in the U.K. J Infect. 1991 Sep;23(2):220–222. doi: 10.1016/0163-4453(91)92567-o. [DOI] [PubMed] [Google Scholar]
- Matson D. O., Estes M. K., Glass R. I., Bartlett A. V., Penaranda M., Calomeni E., Tanaka T., Nakata S., Chiba S. Human calicivirus-associated diarrhea in children attending day care centers. J Infect Dis. 1989 Jan;159(1):71–78. doi: 10.1093/infdis/159.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui S. M., Kim J. P., Greenberg H. B., Su W., Sun Q., Johnson P. C., DuPont H. L., Oshiro L. S., Reyes G. R. The isolation and characterization of a Norwalk virus-specific cDNA. J Clin Invest. 1991 Apr;87(4):1456–1461. doi: 10.1172/JCI115152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. S., Stine S. E., Jiang X., Estes M. K., Glass R. I. Detection of antibody to recombinant Norwalk virus antigen in specimens from outbreaks of gastroenteritis. J Clin Microbiol. 1993 Nov;31(11):2866–2872. doi: 10.1128/jcm.31.11.2866-2872.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. M., Grohmann G. S., Christopher P. J., Lopez W. A., Davey G. R., Millsom R. H. An Australia-wide outbreak of gastroenteritis from oysters caused by Norwalk virus. Med J Aust. 1979 Oct 6;2(7):329–333. doi: 10.5694/j.1326-5377.1979.tb104133.x. [DOI] [PubMed] [Google Scholar]
- Okada S., Sekine S., Ando T., Hayashi Y., Murao M., Yabuuchi K., Miki T., Ohashi M. Antigenic characterization of small, round-structured viruses by immune electron microscopy. J Clin Microbiol. 1990 Jun;28(6):1244–1248. doi: 10.1128/jcm.28.6.1244-1248.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde J., Eiden J., Yolken R. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J Clin Microbiol. 1990 Jun;28(6):1300–1307. doi: 10.1128/jcm.28.6.1300-1307.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi J. N., Graham D. Y., Wang K. N., Estes M. K. Norwalk virus genome cloning and characterization. Science. 1990 Dec 14;250(4987):1580–1583. doi: 10.1126/science.2177224. [DOI] [PubMed] [Google Scholar]