Abstract
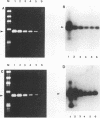
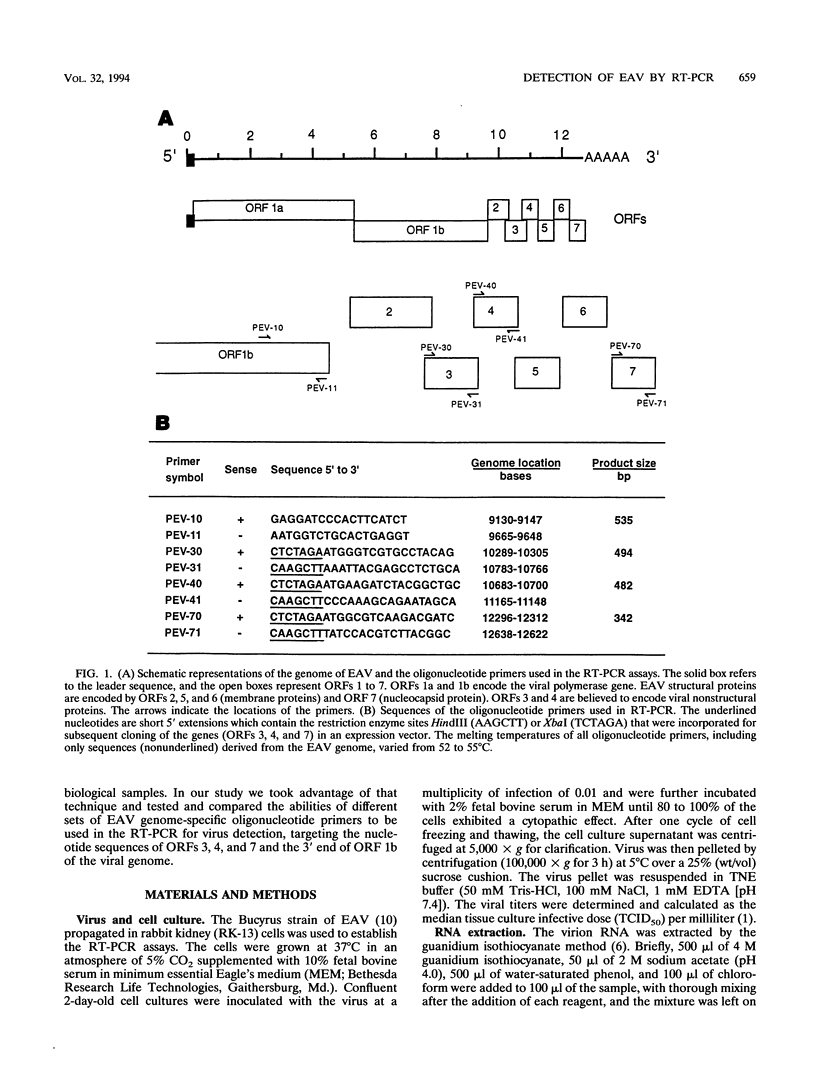
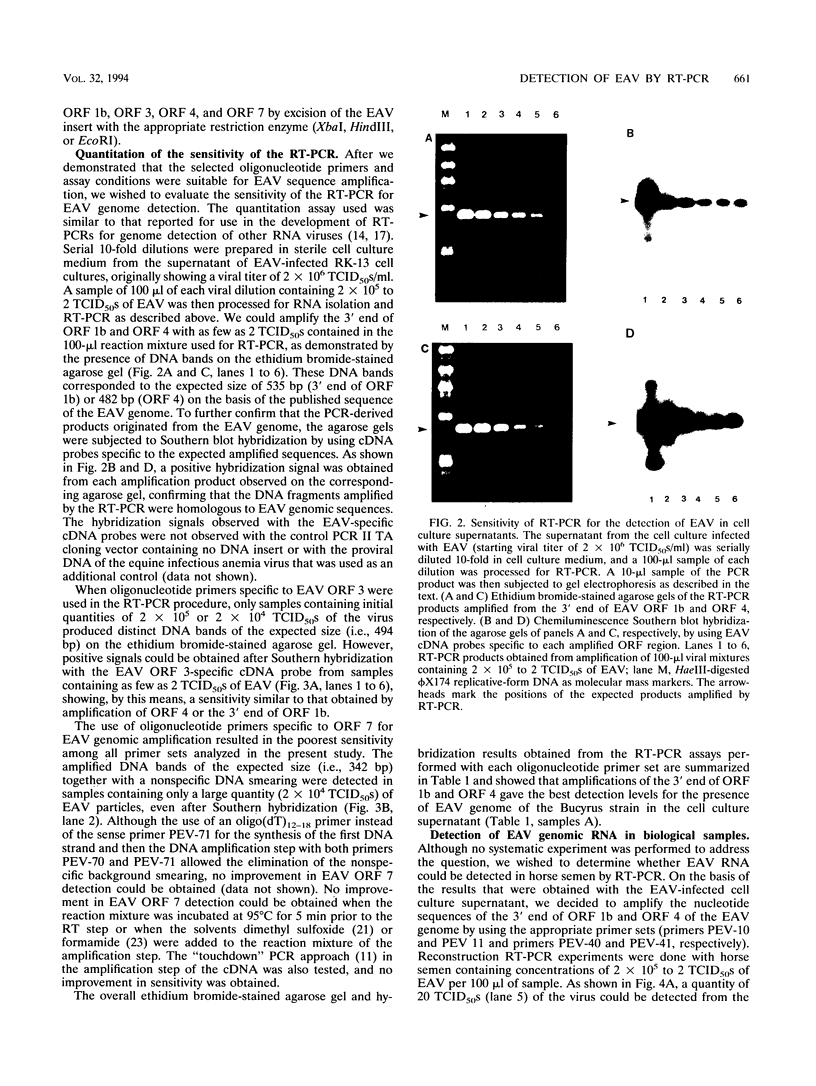
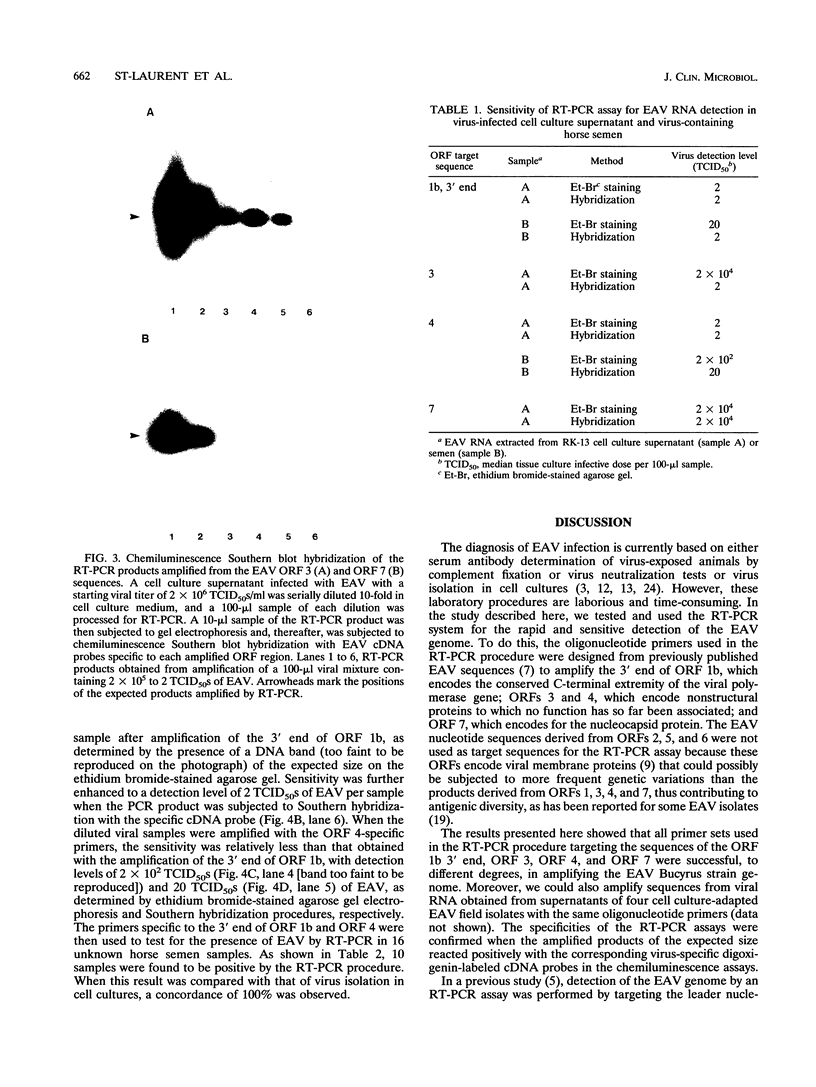
A reverse transcription (RT)-PCR assay was developed for the detection of equine arteritis virus (EAV) in cell culture supernatant and in horse semen. Four different sets of oligonucleotide primers complementary to sequences located in the 3' end of the polymerase gene (open reading frame [ORF] 1b) and to sequences representing the entire ORFs 3, 4, and 7, which encode for nonstructural (ORFs 3 and 4) or viral nucleocapsid (ORF 7) proteins, were compared for their abilities to amplify the targeted EAV sequences by the RT-PCR procedure. The sensitivities of the RT-PCR for amplification of EAV sequences located in the 3' end of ORF 1b and ORF 4 were 2 median tissue culture infective doses (TCID50s) of viral particles in the EAV-infected cell culture supernatant for both ORFs and 20 and 200 TCID50s of viral particles, respectively, in virus-containing horse semen. The sensitivities were much lower when primers complementary to ORFs 3 and 7 were used in the RT-PCR, with a minimum detection limit of only 2 x 10(4) TCID50s of viral particles in virally infected cell culture supernatant, as determined by analyzing the resulting RT-PCR products on ethidium bromide-stained agarose gels. The specificities of the RT-PCR assays for all primer sets tested were confirmed when the amplified cDNA products of the expected size reacted positively with the corresponding virus-specific digoxigenin-labeled cDNA probes in the chemiluminescence assays. Although the sensitivity of the RT-PCR for amplification of ORF 3 and 7 sequences was lower, all sets or primers were capable of amplifying several cell culture-adapted EAV field isolates when the virus was present in high enough quanities in the test sample. When horse semen samples were analyzed for the presence of EAV by the RT-PCR with primers specific to the ORF 1b 3' end and ORF 4 sequences and by virus isolation in cell cultures, there was 100% concordance among the assays. The RT-PCR assay targeting the 3' end of ORF 1b and/or ORF 4 EAV RNA may be an alternative to conventional methods for the diagnosis of EAV infection in horses.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archambault D., Morin G., Elazhary Y., Roy R. S., Joncas J. H. Immune response of pregnant heifers and cows to bovine rotavirus inoculation and passive protection to rotavirus infection in newborn calves fed colostral antibodies or colostral lymphocytes. Am J Vet Res. 1988 Jul;49(7):1084–1091. [PubMed] [Google Scholar]
- Archambault D., Wang Z. M., Lacal J. C., Gazit A., Yaniv A., Dahlberg J. E., Tronick S. R. Development of an enzyme-linked immunosorbent assay for equine infectious anemia virus detection using recombinant Pr55gag. J Clin Microbiol. 1989 Jun;27(6):1167–1173. doi: 10.1128/jcm.27.6.1167-1173.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANS J. T., DOLL E. R., KNAPPENBERGER R. E. An outbreak of abortion caused by the equine arteritis virus. Cornell Vet. 1957 Jan;47(1):69–75. [PubMed] [Google Scholar]
- Bürki F. Eigenschaften des Virus der Equinen Arteritis. Pathol Microbiol (Basel) 1965;28(6):939–949. [PubMed] [Google Scholar]
- Carman S., Nagy E., Caldwell D., van Dreumel T. A. Equine herpesvirus type 1 neurological disease and enterocolitis in mature standardbred horses. J Vet Diagn Invest. 1993 Apr;5(2):261–265. doi: 10.1177/104063879300500221. [DOI] [PubMed] [Google Scholar]
- Chirnside E. D., Spaan W. J. Reverse transcription and cDNA amplification by the polymerase chain reaction of equine arteritis virus (EAV). J Virol Methods. 1990 Nov;30(2):133–140. doi: 10.1016/0166-0934(90)90014-7. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fukunaga Y., McCollum W. H. Complement-fixation reactions in equine viral arteritis. Am J Vet Res. 1977 Dec;38(12):2043–2046. [PubMed] [Google Scholar]
- Golnik W., Michalska Z., Michalak T. Natural equine viral arteritis in foals. Schweiz Arch Tierheilkd. 1981 Oct;123(10):523–533. [PubMed] [Google Scholar]
- Hörling J., Vene S., Franzén C., Niklasson B. Detection of Ockelbo virus RNA in skin biopsies by polymerase chain reaction. J Clin Microbiol. 1993 Aug;31(8):2004–2009. doi: 10.1128/jcm.31.8.2004-2009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugaro G., Campagnari F., Moretti R., Casellato M. M. Inhibition of DNA polymerization and DNA transcription to RNA by seminal plasma peptides. Biochim Biophys Acta. 1988 Sep 7;950(3):420–428. doi: 10.1016/0167-4781(88)90139-x. [DOI] [PubMed] [Google Scholar]
- Muir P., Nicholson F., Jhetam M., Neogi S., Banatvala J. E. Rapid diagnosis of enterovirus infection by magnetic bead extraction and polymerase chain reaction detection of enterovirus RNA in clinical specimens. J Clin Microbiol. 1993 Jan;31(1):31–38. doi: 10.1128/jcm.31.1.31-38.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford J. A. Preparing for equine arteritis. Equine Vet J. 1985 Jan;17(1):6–11. doi: 10.1111/j.2042-3306.1985.tb02026.x. [DOI] [PubMed] [Google Scholar]
- Murphy T. W., McCollum W. H., Timoney P. J., Klingeborn B. W., Hyllseth B., Golnik W., Erasmus B. Genomic variability among globally distributed isolates of equine arteritis virus. Vet Microbiol. 1992 Sep;32(2):101–115. doi: 10.1016/0378-1135(92)90099-f. [DOI] [PubMed] [Google Scholar]
- Panaccio M., Lew A. PCR based diagnosis in the presence of 8% (v/v) blood. Nucleic Acids Res. 1991 Mar 11;19(5):1151–1151. doi: 10.1093/nar/19.5.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomp D., Medrano J. F. Organic solvents as facilitators of polymerase chain reaction. Biotechniques. 1991 Jan;10(1):58–59. [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Strauss E. G. Evolution of RNA viruses. Annu Rev Microbiol. 1988;42:657–683. doi: 10.1146/annurev.mi.42.100188.003301. [DOI] [PubMed] [Google Scholar]
- Timoney P. J., McCollum W. H., Roberts A. W., Murphy T. W. Demonstration of the carrier state in naturally acquired equine arteritis virus infection in the stallion. Res Vet Sci. 1986 Sep;41(2):279–280. [PubMed] [Google Scholar]
- Westaway E. G., Brinton M. A., Gaidamovich SYa, Horzinek M. C., Igarashi A., Käriäinen L., Lvov D. K., Porterfield J. S., Russell P. K., Trent D. W. Togaviridae. Intervirology. 1985;24(3):125–139. doi: 10.1159/000149632. [DOI] [PubMed] [Google Scholar]
- de Vries A. A., Chirnside E. D., Bredenbeek P. J., Gravestein L. A., Horzinek M. C., Spaan W. J. All subgenomic mRNAs of equine arteritis virus contain a common leader sequence. Nucleic Acids Res. 1990 Jun 11;18(11):3241–3247. doi: 10.1093/nar/18.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries A. A., Chirnside E. D., Horzinek M. C., Rottier P. J. Structural proteins of equine arteritis virus. J Virol. 1992 Nov;66(11):6294–6303. doi: 10.1128/jvi.66.11.6294-6303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boon J. A., Snijder E. J., Chirnside E. D., de Vries A. A., Horzinek M. C., Spaan W. J. Equine arteritis virus is not a togavirus but belongs to the coronaviruslike superfamily. J Virol. 1991 Jun;65(6):2910–2920. doi: 10.1128/jvi.65.6.2910-2920.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]