Abstract
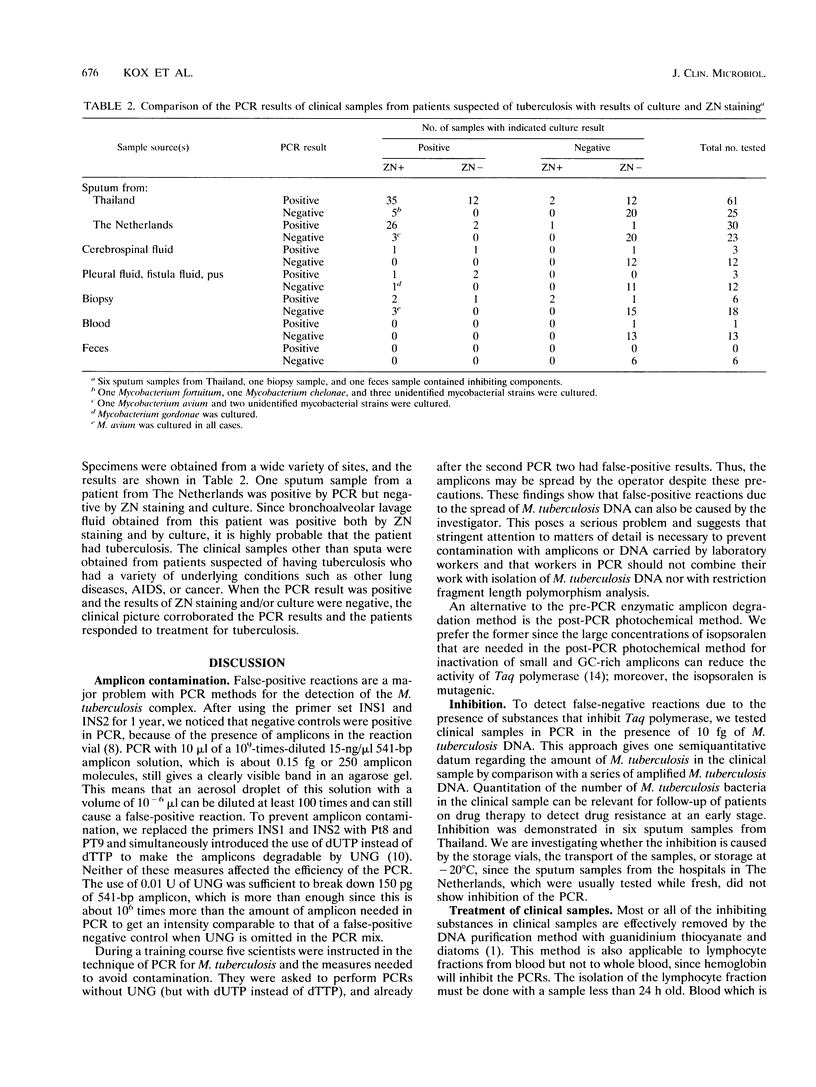
Diagnostic techniques based on PCR have two major problems: false-positive reactions due to contamination with DNA fragments from previous PCRs (amplicons) and false-negative reactions caused by inhibitors that interfere with the PCR. We have improved our previously reported PCR based on the amplification of a fragment of the Mycobacterium tuberculosis complex-specific insertion element IS6110 with respect to both problems. False-positive reactions caused by amplicon contamination were prevented by the use of uracil-N-glycosylase and dUTP instead of dTTP. We selected a new set of primers outside the region spanned by the formerly used primers to avoid false-positive reactions caused by dTTP-containing amplicons still present in the laboratory. With this new primer set, 16 copies of the IS6110 insertion element, the equivalent of two bacteria, could be amplified 10(10) times in 40 cycles, resulting in a mean efficiency of 77% per cycle. To detect the presence of inhibitors of the Taq polymerase, which may cause false-negative reactions, part of each sample was spiked with M. tuberculosis DNA. The DNA purification method using guanidinium thiocyanate and diatoms effectively removed most or all inhibitors of the PCR. However, this was not suitable for blood samples, for which we developed a proteinase K treatment followed by phenol-chloroform extraction. This method permitted detection of 20 M. tuberculosis bacteria per ml of whole blood. Various laboratory procedures were introduced to reduce failure or inhibition of PCR and avoid DNA cross contamination. We have tested 218 different clinical specimens obtained from patients suspected of having tuberculosis. The samples included sputum (n=145), tissue biopsy samples (n=25), cerebrospinal fluid (n=15), blood (n=14), pleural fluid (n=9), feces, (n=7), fluid from fistulae (n=2), and pus from a wound (n=1). The results obtained by PCR were consistent with those obtained with culture, which is the "gold standard." We demonstrate that PCR is a useful technique for the rapid diagnosis of tuberculosis at various sites.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boom R., Sol C. J., Salimans M. M., Jansen C. L., Wertheim-van Dillen P. M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990 Mar;28(3):495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Noel A., Aznar C., Chureau C., Nguyen S., Pierre C., Bartoli M., Bonete R., Pialoux G., Gicquel B., Garrigue G. Diagnosis of tuberculosis by DNA amplification in clinical practice evaluation. Lancet. 1991 Aug 10;338(8763):364–366. doi: 10.1016/0140-6736(91)90492-8. [DOI] [PubMed] [Google Scholar]
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Cousins D. V., Wilton S. D., Francis B. R., Gow B. L. Use of polymerase chain reaction for rapid diagnosis of tuberculosis. J Clin Microbiol. 1992 Jan;30(1):255–258. doi: 10.1128/jcm.30.1.255-258.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Sifford M. D., Cave M. D., Bates J. H., Crawford J. T. Detection of Mycobacterium tuberculosis in sputum samples using a polymerase chain reaction. Am Rev Respir Dis. 1991 Nov;144(5):1160–1163. doi: 10.1164/ajrccm/144.5.1160. [DOI] [PubMed] [Google Scholar]
- Hermans P. W., Schuitema A. R., Van Soolingen D., Verstynen C. P., Bik E. M., Thole J. E., Kolk A. H., van Embden J. D. Specific detection of Mycobacterium tuberculosis complex strains by polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1204–1213. doi: 10.1128/jcm.28.6.1204-1213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBICA G. P., DYE W. E., COHN M. L., MIDDLEBROOK G. Sputum digestion and decontamination with N-acetyl-L-cysteine-sodium hydroxide for culture of mycobacteria. Am Rev Respir Dis. 1963 May;87:775–779. doi: 10.1164/arrd.1963.87.5.775. [DOI] [PubMed] [Google Scholar]
- Kolk A. H., Schuitema A. R., Kuijper S., van Leeuwen J., Hermans P. W., van Embden J. D., Hartskeerl R. A. Detection of Mycobacterium tuberculosis in clinical samples by using polymerase chain reaction and a nonradioactive detection system. J Clin Microbiol. 1992 Oct;30(10):2567–2575. doi: 10.1128/jcm.30.10.2567-2575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo M. C., Berninger M. S., Hartley J. L. Use of uracil DNA glycosylase to control carry-over contamination in polymerase chain reactions. Gene. 1990 Sep 1;93(1):125–128. doi: 10.1016/0378-1119(90)90145-h. [DOI] [PubMed] [Google Scholar]
- McAdam R. A., Hermans P. W., van Soolingen D., Zainuddin Z. F., Catty D., van Embden J. D., Dale J. W. Characterization of a Mycobacterium tuberculosis insertion sequence belonging to the IS3 family. Mol Microbiol. 1990 Sep;4(9):1607–1613. doi: 10.1111/j.1365-2958.1990.tb02073.x. [DOI] [PubMed] [Google Scholar]
- Pao C. C., Yen T. S., You J. B., Maa J. S., Fiss E. H., Chang C. H. Detection and identification of Mycobacterium tuberculosis by DNA amplification. J Clin Microbiol. 1990 Sep;28(9):1877–1880. doi: 10.1128/jcm.28.9.1877-1880.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persing D. H. Polymerase chain reaction: trenches to benches. J Clin Microbiol. 1991 Jul;29(7):1281–1285. doi: 10.1128/jcm.29.7.1281-1285.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince A. M., Andrus L. PCR: how to kill unwanted DNA. Biotechniques. 1992 Mar;12(3):358–360. [PubMed] [Google Scholar]
- Schmitz G. G., Walter T., Seibl R., Kessler C. Nonradioactive labeling of oligonucleotides in vitro with the hapten digoxigenin by tailing with terminal transferase. Anal Biochem. 1991 Jan;192(1):222–231. doi: 10.1016/0003-2697(91)90212-c. [DOI] [PubMed] [Google Scholar]
- Shawar R. M., el-Zaatari F. A., Nataraj A., Clarridge J. E. Detection of Mycobacterium tuberculosis in clinical samples by two-step polymerase chain reaction and nonisotopic hybridization methods. J Clin Microbiol. 1993 Jan;31(1):61–65. doi: 10.1128/jcm.31.1.61-65.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sritharan V., Barker R. H., Jr A simple method for diagnosing M. tuberculosis infection in clinical samples using PCR. Mol Cell Probes. 1991 Oct;5(5):385–395. doi: 10.1016/s0890-8508(06)80011-3. [DOI] [PubMed] [Google Scholar]
- Thierry D., Brisson-Noël A., Vincent-Lévy-Frébault V., Nguyen S., Guesdon J. L., Gicquel B. Characterization of a Mycobacterium tuberculosis insertion sequence, IS6110, and its application in diagnosis. J Clin Microbiol. 1990 Dec;28(12):2668–2673. doi: 10.1128/jcm.28.12.2668-2673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. M., McNerney R., Nye P. M., Godfrey-Faussett P. D., Stoker N. G., Voller A. Progress toward a simplified polymerase chain reaction and its application to diagnosis of tuberculosis. J Clin Microbiol. 1993 Apr;31(4):776–782. doi: 10.1128/jcm.31.4.776-782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]