Abstract
The structure and organization of chromatin have attracted a great deal of attention recently because of their implications for the field of epigenetics. DNA methylation and the post-translational modifications that occur on histones can specify transcriptional competency. During cancer development, tumor suppressor genes become silenced by DNA hypermethylation and chromatin modifiers no longer perform in their usual manner. Current epigenetic therapy has been able to take advantage of the reversibility of these epimutations. Progress has been made in the treatment of hematological malignancies and some solid tumors. As the knowledge of how chromatin regulates gene expression is enhanced, improvements in the treatment of cancer can be made.
Keywords: Epigenetics, Chromatin, Cancer, DNA methylation inhibitors, HDAC inhibitors, Epigenetic Therapy
Introduction to Chromatin Structure
The organization of chromatin has been intensely studied and now the focus in the field of epigenetics has shifted to understanding the biological relevance and function of chromatin structure. Epigenetics, which is defined as the study of heritable changes in gene expression that occur without a change in the DNA sequence, provides insight into the extent by which chromatin structure exerts control on transcriptional regulation. Interpreting the patterns of post-translational histone modifications as well as DNA methylation and how these epigenetic mechanisms contribute to gene expression in a normal state and in cancer are key to developing drugs that can reverse abnormalities that occur during tumorigenesis.
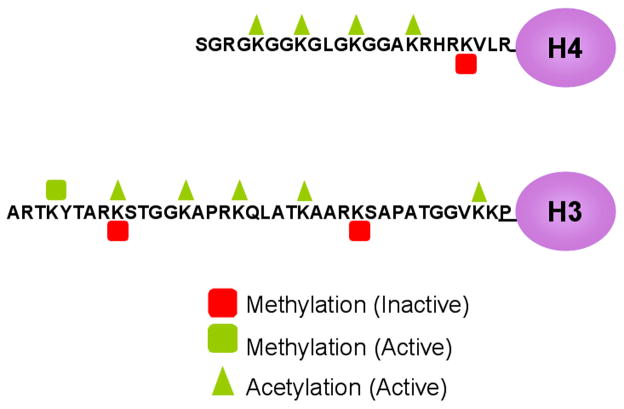
Chromatin is comprised of DNA, histone proteins and non-histone proteins. The fundamental repeating unit of chromatin is the nucleosome, which consists an octamer of histones with two each of the four small and highly basic histones (H3, H4, H2A, and H2B) [1]. Approximately 146 bp of DNA are wrapped twice around each histone core providing a means for higher order packaging of DNA in the nucleus. The histone amino terminal tails that project out of the nucleosome core are subject to many post-translational modifications such as phosphorylation, ubiquitination, sumoylation, acetylation and methylation on specific amino acid residues [2],[3]. This review will highlight the most highly studied modifications, acetylation and methylation of histones H3 and H4 (Fig. 1).
Fig. 1. Post-translational histone modifications on histone tails.
Modifications made on the N-terminal tails of histones are important in establishing the activity state of chromatin. Many modifications are possible however only acetylation and methylation of a subset of lysine residues are depicted here. Active acetylation or methylation marks (green triangles or squares), can act to “loosen” chromatin to allow for access of transcriptional machinery while also serving as docking points for nucleosome remodeling complexes. Conversely, inactive marks such as methylation of specific residues can cause an inactive conformation of chromatin and can recruit repressive complexes.
Acetylation by histone acetyl transferases (HATs) occurs on the lysine residues of histone tails and is strongly correlated with active gene expression. The basic charges of the histone tails become neutralized upon acetylation. This causes increased accessibility for further modifications or access to the DNA for binding factors and transcriptional machinery [4–6]. Unlike acetylation, methylation of histones does not change the charge of the histone tails [7]. Lysine residues can accept up to three methyl groups, which are added by various histone methyltransferases (HMTs). The degree of methylation is informative for both the state of gene activity as well as which proteins/complexes might bind and read the message displayed by those marks [8],[9]. Methylated lysine residues may constitute either active or inactive marks. Active marks include histone H3 lysine 4 (H3K4), lysine 36 (H3K36) and lysine 79 (H3K79) [10–12]. The methylation marks on lysines 9 and 27 on histone H3 and lysine 20 of histone H4 are associated with an inactive chromatin state [13–15]. Interestingly, there is cross regulation between different marks such as the competition for lysine 9 on histone H3 between an inactive methyl mark and acetylation [16,17]. The distinct patterns of post-translational modifications make up the “histone code” and the precise combinations determine how the chromatin is read [18].
The position of nucleosomes on the DNA further adds to the complexity of chromatin structure. Nucleosome positioning and occupancy can also play a key role in regulating gene expression and the presence of a nucleosome at the transcription start site is commonly seen in inactive genes [19]. Studies have shown evidence of the loss of a nucleosome directly upstream of the transcription start site upon gene activation. This may allow greater access for binding of transcription complexes or factors [20]. It has been shown that a promoter of a gene with a basal level of transcription can already be depleted of nucleosomes which allows for quick induction upon stimulation [21]. Also, the reactivation of a completely silenced gene is associated with nucleosome loss [22]. These studies demonstrate the importance of nucleosomes in gene regulation.
DNA methylation influences gene regulation in concert with histone modifications and nucleosome positioning [23]. DNA methylation at the transcriptional start sites of genes is associated with inactivity and is important in imprinting, X inactivation and the silencing of retrotransposons. The 5 carbon on the cytosine ring in DNA can be modified by the placement of a methyl group by DNA methyltransferases (DNMTs). DNMT1 is referred to as the “maintenance” methylase due to its preference for hemimethylated CpG sites in DNA [24]. DNMT3a and DNMT3b are considered to be de novo methylases because they can methylate unmethylated DNA [24,25]. However, all three DNMTs have been shown to act cooperatively and the functional differences between the methylases may to a large extent be due to the genomic regions that they act upon [26,27]. Methylation occurs in the context of CpG dinucleotides, which are underrepresented in the genome possibly due to evolutionary depletion [28]. Regions of high CpG content are termed “CpG islands” and are found at the promoters of more than 50% of genes in the genome. CpG islands are often located at the promoter regions of housekeeping genes in an unmethylated state [29]. The DNA methylation mark can act both directly and indirectly to silence a gene by either inhibiting the binding of transcription factors or by possibly recruiting methyl-binding domain proteins (MBDs), which further recruit histone deacetylases (HDACs) [30].
MicroRNAs (miRs) are another mechanism used by the cell to regulate the expression of genes involved in differentiation, cell proliferation and apoptosis [31]. They are short RNAs 19–24 nucleotides in length that often bind to the 3′UTR of their target mRNA to either inhibit that mRNA’s translation or cause its degradation [32]. MiR expression profiles differ depending on cell type and like DNA methylation, they help to establish the cells identity. Currently more than 400 human miRs have been experimentally identified and are proposed to regulate more than 30% of all mRNAs post-transcriptionally [32,33].
Epigenetic Changes in Cancer
Epimutations in cancer can result in the activation of oncogenes, the silencing of tumor suppressors, and ultimately in the cell’s ability to proliferate uncontrollably. These changes are often linked to the presence of altered levels of chromatin modifying enzymes and a shift in the genome-wide distribution of DNA methylation. Changes in histone marks work together with DNA methylation or independently to silence gene expression depending on the region of chromatin and the type of gene. Advances in our understanding of how these abnormalities occur will help in designing and improving drugs to target the factors that cause these changes during tumorigenesis.
Altered activity of the histone lysine methyltransferases can contribute to the deviant histone methylation patterns found in cancer. For example, histone lysine methylation on histone H3K9 and H3K27 are normally present at transcriptionally inactive or heterochromatic regions, yet they can be found at genes that are aberrantly repressed in cancer cells [34,35]. The methyltransferase MLL, which methylates H3K4, is involved in translocations that lead to the inappropriate expression of various homeotic (Hox) genes, which contributes to leukemic progression [36]. Methyltransferases within complexes well known for their suppressive activities are also up-regulated in cancer.
The Polycomb group (PcG) complexes are chromatin modifiers that are crucial to development, and have been implicated in the development of cancer [37]. These negative regulators of gene expression are very important in sustaining the repressive state of their target genes through the cell cycle [38]. Two of the PcG repressive complexes (PRC1 and PRC2) have both been shown to be involved in various cancers. Enhancer of zeste homologue 2 (EZH2), a component of PRC2 with H3K27 methyltransferase activity, is upregulated in mantle cell lymphoma, breast and prostate cancer [39–41]. RING1, a component of PRC1 that aids in the ubiquitylation of histone H2A lysine 119, is upregulated in prostate cancer [42].
The demethylation of histones is important in transcriptional regulation. Histone lysine methylation had been previously thought to be a very stable mark. However, the discovery of LSD1, a demethylase of mono- and dimethylated histone H3K4, showed that these chromatin marks are reversible [43]. LSD1’s mechanism of action is through the amine oxidation of the methylated histone H3K4. Several histone lysine demethylases have been found since LSD1, including the Jumonji C domain (JmjC) proteins, which can specifically demethylate mono-, di-and trimethylated lysines [44]. Histone demethylases have been found to play a role in cancer progression as seen with JMJD2A, JMJD2B, and JMJD2C, which are expressed at high levels in prostate cancer [45].
Interestingly, LSD1 has been found to associate with HDACs, therefore HDAC inhibitors can potentially affect the function of demethylases [46]. Currently, histone demethylases have been identified which demethylate both active and inactive marks thereby functioning as both co-repressors and co-activators [47]. Therapeutic inhibition of specific demethylases may be a possible direction for the treatment of cancer, however there is still much to uncover about the precise functions and associations of histone demethylases.
The acetylation of histones is held at equilibrium by the action of HATs and HDACs and an imbalance of one or the other enzyme can lead to phenotypic changes in the cell. Alterations in HAT activity have been found in cancer, stressing the importance of strict regulation of histone acetylation. For example, a translocation involving MOZ and p300, both with HAT activity, results in a fusion protein and is associated in leukemogenesis [48]. Errant HAT complexes such as these disturb normal epigenetic processes through inappropriate acetylation, altering chromatin organization and gene expression patterns. Inappropriate deacetylation can also contribute to cancer progression. HDACs are upregulated in various types of cancer, such as gastric, prostate, oral squamous cell, and lung [49–52]. Over-expression of HDACs can also lead to the transcriptional inactivation of tumor suppressors, such as p53 [53].
DNA methylation patterns are altered in the progression of cancer. Both the hypomethylation and hypermethylation of different regions of the genome play roles in contributing to tumorigenesis. During tumorigenesis, a genome-wide demethylation occurs and this can promote genomic instability possibly by activating silenced retrotransposons [54]. Global demethylation of repetitive sequences such as satellite DNAs can lead to increased chromosomal rearrangements further adding to genomic instability [55]. It is also possible that demethylation could lead to the activation proto-oncogenes, such as the R-Ras activation in gastric cancer [56,57].
Focal hypermethylation of CpG islands has been intensively studied in cancer. The list of genes found to have increased levels of methylation in their promoter regions accompanied by decreased expression in cancer has grown rapidly. Nearly all types of cancers have transcriptional inactivation of tumor suppressor genes due to DNA hypermethylation [58]. However, the exact mechanism responsible for the appearance of DNA methylation in a given promoter is not fully understood. Cancer cells can attain a growth advantage through the hypermethylation and silencing of genes that are involved in cell cycle regulation, DNA repair, cell signaling, and apoptosis. Additionally, the levels of DNMTs may also be important [59]. Over-expression of the DNA methyltransferases 1 and 3A was found in the bone marrow of patients with myelodysplastic syndrome (MDS) [60]. Upregulation of DNMTs has also been shown in prostate cancer cell lines and tissues [61].
MiRs have different expression profiles in cancer [62]. If a miR is found to be downregulated in cancer then its potential function as a tumor suppressor has been lost. Conversely, if it is upregulated the miR may be acting as an oncogene by downregulating a tumor suppressor gene. Little is known about the regulation of miRs, however it has now been shown that miRs can be regulated by DNA methylation and histone modifications as shown with miR 127 [63]. In this study miR 127 was reactivated in a cancer cell line upon treatment of both a DNA methylation and HDAC inhibitor. This demonstrates that miRs can be potential targets for epigenetic therapy.
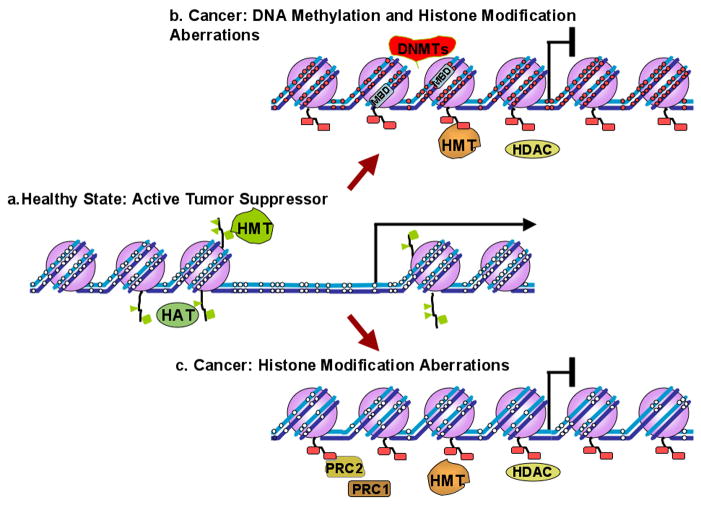
As mentioned earlier, DNA methylation and histone modifications are interconnected and together can influence the regulation of genes [64]. However, it has been shown in some cases that changes in post-translational modifications play a greater role in gene expression than DNA promoter methylation [65] [59]. When there are few CpG sites within a promoter the impact of this methylation on gene expression may be less evident. Although there are examples of non-CpG island promoters where methylation does play a role in gene regulation [66], it is also possible that transcriptional regulation is not always influenced by DNA methylation, but rather is dictated by the surrounding chromatin configuration. Figure 2 depicts the two situations of many potential mechanisms by which a tumor suppressor may be shut down.
Fig. 2. Examples of epigenetic changes that occur to inactivate a gene. a.
) Active genes are characterized by unmethylated CpG sites in their promoters (white circles), absence of a nucleosome (purple spheres) upstream of the transcription start site (black arrow), and active histone marks (green) placed by HATs and HMTs. b) During carcinogenesis, tumor suppressor genes become aberrantly methylated by DNMTs, and in turn MBDs that attach to the methylated CpG sites can recruit HDACs to aid in transcriptional repression. A nucleosome placed at the transcriptional start site, and histone methyltransferases additionally render the chromatin inaccessible. In this scenario, methylation plays a critical role in inactivating a tumor suppressor. However, in some cases c) methylation may not play a role (such as areas with less CpGs) and repression of the gene is dependent on chromatin remodeling factors such as PRC1 and PRC2.
Cancer and Epigenetic Therapy
Epigenetic errors in cancer, unlike genetic lesions, can be reversed relatively easily through chemotherapeutic intervention, which makes epigenetic therapy promising. The goal of epigenetic therapy is to target the chromatin in rapidly dividing tumor cells and return it to a more “normal state” while only mildly disturbing the epigenome of healthy cells. This section will focus on the epigenetic drugs that are currently used in the clinic (Table 1) and their effects on cancer cells. It is important to note both their benefits and shortcomings so that improvements can be made in the next generation of epigenetic drug therapies.
Table 1.
Epigenetic Drugs Used in the Clinic
| Drug Name | Cancer | References |
|---|---|---|
| DNA Methylation Inhibitors | ||
| 5-azacytidine (FDA approved) | MDS, AML, CML | [67] [70] [71] |
| 5-aza-2′deoxycytidine (FDA approved) | AML, CML, MDS | [72] [73] [76] |
| MG98 | Renal cell carcinoma | [83] [84] [85] |
| RG108 | Colon cancer cell line | [81] [82] |
| Procainamide | Colon cancer cell line | [86] |
|
| ||
| HDAC Inhibitors | ||
| SAHA (FDA approved) | CTCL, various solid tumors | [89] [91] [92] [94] |
| PXD101 | Various solid tumors | [95] [97] |
| LBH589 | CTCL | [96] [97] |
| Depsipeptide | Multiple cancer cell lines, MDS, AML | [98] [99] [100] [101] |
| Phenylbutyrate | MDS | [104] [105] |
| Valproic Acid | Neuroblastoma cells | [102] [103] |
| MS-275 | Prostate cancer cell lines, various sold tumors and lymphoid malignancies | [107] [108] [109] |
| CI-994 | Various solid tumors | [110] [111] |
The nucleoside analogues 5-azacytidine (5-Aza-CR) and 5-aza-2′-deoxycytidine (5-Aza-CdR), known clinically as azacitadine (Vidaza®) and decitabine (Dacogen®), respectively, are FDA approved demethylating agents used to treat myelodysplastic syndrome (MDS) [67]. They differ from cytosine by a nitrogen substitution at the 5-carbon position. During replication these drugs are incorporated into DNA and their modified cytosine rings inhibit methylation by trapping DNMTs, thereby depleting the cell of these enzymes and resulting in the reduced methylation of cytosines in DNA synthesized after drug treatment [68,69]. In the study leading to the FDA approval of 5-Aza-CR, there was a 60% response rate of the patients with MDS [70]. It was also shown that 5-Aza-CR prolongs survival rate in high risk MDS patients [71]. Encouraging results were also obtained when MDS patients were treated with 5-Aza-CdR and there was a 70% overall response rate [72]. In addition to MDS, these drugs have proven to be useful in other hematological malignancies such as acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) [73].
These drugs potentially act by restoring normal cellular functions by allowing aberrantly hypermethylated tumor suppressor genes to become re-expressed, although the relationship between therapeutic activity and DNA methylation inhibition has not been formally proven. They have shown promising response rates in patients with MDS and CML along with a reversal of p15 hypermethylation in bone marrow [74–76]. Interestingly, 5-aza-CdR can restore drug sensitivity to cells that have become unresponsive to chemotherapy. For example, treatment of melanoma cell lines with 5-Aza-CdR restores sensitivity to cells that are unresponsive to chemotherapy by aiding in the re-expression of a crucial player in the apoptotic pathway, APAF-1 [77].
The use of these drugs raises questions regarding their potential to affect non-cancerous cells epigenetically. However, normal cells divide at a slower rate than malignant cells and incorporate less of these drugs into their DNA resulting in less of an effect on DNA methylation. Also, long-term negative effects of DNA methylation inhibitors in patients have not been found to date [78]. Drawbacks to these drugs are their chemical and in vivo labilities as well as their acute hematological toxicities. A next generation DNA methylation inhibitor, such as zebularine, might possibly overcome these problems [79,80].
Other small molecule inhibitors such as RG108 or MG98 are not incorporated into DNA but instead bind to the catalytic site of DNMTs thereby causing inhibition of DNA methylation. RG108 (N-Phthalyl-1-tryptophan) has been shown to be minimally toxic in colon cancer cell lines and was successful at inhibiting DNMTs [81,82]. The antisense oligonucleotide MG98 (2′-O-CH3-substituted phosphorothioate oligo deoxynucleotide) targets the 3′ UTR of DNMT1 [83] and can cause a methylation decrease in cell lines and animal models. Phase I trials with MG98 did show DNA demethylation in patients, however the Phase II trials had no effect on methylation reduction in patients with renal cell carcinoma [84,85]. Although procainamide (4-Amino-N-(2-diethylaminoethyl)benzamide hydrochloride) is FDA approved for the use of cardiac arrhythmias, it can also reduce DNMT1’s affinity for both DNA and S-adenosyl-methionine in a colon cancer cell line causing a decrease in DNA methylation [46]. Generally, these non-nucleoside analogue inhibitors are not are not as potent as the nucleoside analogues and therefore the need for improvement for these drugs still exists [86].
DNA methylation inhibitors are successful in affecting one epigenetic pathway that leads to the progression of cancer. HDAC inhibitors have also been proven to be useful in cancer treatment by allowing the re-establishment of acetylation to reactivate silenced genes [87]. HDAC inhibitors are divided into 4 groups based on their structures: hydroxamic acids, cyclic peptides, short chain fatty acids, and benzamides. There are 18 HDAC isoenzymes that have been categorized into 4 classes. The challenge is in designing HDAC isoform-specific inhibitors and determining their potential clinical advantages over general inhibitors [82]. The specificities of the HDAC inhibitors used in the clinic vary from one to three classes of HDACs [85]. HDAC inhibitors have pleiotropic effects including inhibition of angiogenesis, induction of apoptosis and cell cycle arrest [88].
The hydroxamic acid HDAC inhibitors have been successful in treating both hematologic malignancies and solid tumors. X-ray crystallography has shown that the catalytic site of HDACs contains a zinc atom. The hydroxamic acid moiety of these HDAC inhibitors can fit into the catalytic site and bind to the zinc atom thereby inhibiting the HDAC [89]. Suberoylanilide hydroxamic acid (SAHA; vorinostat), a general inhibitor, targets HDACs from Class I and Class II by binding to the active site of the enzyme [90]. SAHA can be administered orally, is minimally toxic and has been FDA approved for the treatment of cutaneous T-cell lymphoma (CTCL). The overall response rate in a recent CTCL Phase IIb trial was 30% and those that did not respond still benefited from relief of pruritus early in the trial [91]. SAHA also is in phase II trials to treat solid tumors [85,92,93]. A recent use of SAHA in women with a recurrence of ovarian cancer showed a progression-free survival over 6 months [94]. Other hydroxamic acids, PXD101 ((E)-N-hydroxy-3-[3-(phenylsulfamoyl)phenyl]prop-2-enamide) and LBH589 ((E)-N-hydroxy-3-[4-[[2-(2-methyl-1H-indol-3-yl)ethylamino]methyl]phenyl]pro p-2-enamide), have also been evaluated in clinical trials. PXD101 treatment of patients with advanced refractory solid tumors was shown to cause an increase in acetylation in their peripheral blood mononuclear cells, stabilize their disease, and was well tolerated [95]. LBH589 is best known for its role in hyperacetylation of histones H3, H4 and the protein Hsp90 [96]. LBH589 has shown clinical activity in cutaneous T-cell lymphoma (CTCL) and soon will be studied in chronic myeloid leukemia, and multiple myeloma [97].
Depsipeptide (FK228), an example of a cyclic peptide HDAC inhibitor, is more specific in that it exerts its effect on three of the Class I HDACs [85]. Depsipeptide has been recently shown to cause a decrease in methylation of DNA while increasing acetylation in lung, pancreatic, and colon cancer cell lines, however the mechanism is not well understood [98]. Depsipeptide has been shown to inhibit growth of human prostate cancer cells [99]. Concerns have arisen with this drug’s potential cardiac toxicity [100], however none was observed in a phase I clinical trial to treat patients with MDS or AML [101].
Short chain fatty acids such as butyrate and valproic acid (2-Propylpentanoic acid) have the longest history of being used as HDAC inhibitors [59]. Valproic acid (VPA), originally used to treat epilepsy, has been used for the last decade as an anti-cancer drug since it can inhibit proliferation and induces differentiation in human neuroblastoma cells [102]. VPA is well tolerated, has low toxicity in adults, and is relatively stable [103]. Phenylbutyrate is in Phase I trials for MDS and was shown to be safe for treatment of solid tumors [104,105]. The shortcoming of these HDAC inhibitors is that a high concentration of drug is required for efficacy resulting in limited use in the clinic [106].
MS-275 (N-(2-aminophenyl)4-[N-(pyridine-3-yl-methoxycarbonyl)aminomethyl] benzamide) and CI-994 (N-(2-aminophenyl)-4-acetylaminobenzamide) are two of the most well known synthetic HDAC inhibitors of the benzamide group. MS-275 can induce p21 expression and increase acetylation in prostate cancer cell lines and inhibit tumor growth in mouse xenograft models [107,108]. In Phase I trials patients with a variety of solid tumors and lymphoid malignancies showed increased levels of acetylation in peripheral blood mononuclear cells and the drugs were well tolerated [109]. CI-994 has undergone Phase I trails and can be used alone or in combination with other chemotherapeutic drugs to treat solid tumors in patients [82,110,111]. Neither of these drugs is as potent as the other classes of HDAC inhibitors and seems to have the greatest effect when used in a combinatorial treatment [112]. The future directions for the development of epigenetic drugs will rely on the elucidation of their mechanisms and the downstream effects of treatment.
Many clinical trials are now studying the combination of either two epigenetic drugs or a non-epigenetic chemotherapeutic and an epigenetic drug in an effort increase response rates and maximize the efficacy of these drugs. Since HDAC inhibitors work primarily to increase acetylation, they may have a limited effect on genes that have been silenced by DNA methylation. However, HDAC inhibitors and DNA methylation inhibitors in combination can work synergistically to cause the re-expression of such genes. A study on colon cancer cell lines showed genes that were only expressed when the HDAC inhibitor and 5-Aza-CdR were coupled [113]. It was also found that DNA methyltransferase inhibitors could enhance the anti-tumor effects of depsipeptide in leukemic cells with the AML/ETO fusion protein [114]. Also, phenylbutyrate and 5-Aza-CdR have synergistic effects on reducing lung tumor formation in mice by more than 50% than with 5-Aza-CdR alone [115]. A similar study in xenograft hepatoma models only showed a decrease in tumor formation when treated with both SAHA and 5-Aza-CdR [116]. DNA methylation inhibitors and HDAC inhibitors are now used together in the clinic after garnering encouraging results in vitro.
For example, in humans a phase I trial involving MDS and AML patients that were treated with both sodium phenylbutyrate and Aza-CR showed reduced promoter methylation and increased global histone acetylation. The results from this trial suggest an increased response rate and it is hypothesized that in a phase II trial, using a longer exposure and lower dose of Aza-CR and HDAC inhibitor could further increase the response rate [117].
Often cells undergoing treatment with one epigenetic drug can have increased sensitivity to an additional drug. Pretreatment with an HDAC inhibitor can greatly increase cytotoxicity in various cell lines when followed by subsequent treatment of a chemotherapeutic drug [118]. This particular study suggests that pre-treatment causes the chromatin structure to become more open therefore increasing the efficiency of the drug to follow. Likewise, cisplatin resistant cells from head and neck cancer cell lines can be reprogrammed to become responsive after treatment with phenylbutyrate [119]. A phase I study in patients with solid tumors showed that CI-994 can be safely administered with paclitaxel and carboplatin and can cause a partial or complete response [111]. The increased sensitivity to other drugs after use of an epigenetic drug is encouraging since drug resistance does present a challenge in effective cancer treatment.
Additionally, it is important to target other enzymes that can disturb the epigenetic balance during carcinogenesis such as histone methyltransferases. Reagents that inhibit S-adenosylhomocysteine (SAH) hydrolase lead to an increase in SAH levels in the cell which inhibit methyltransferases, including histone methyltransferases [120,121]. While SAH hydrolase inhibitors have been used as anti-viral compounds, how they may be effective in cancer is in need of exploration [122].
The Future of Epigenetic Therapy
As the field of epigenetics advances, a better understanding is developing of the precise mechanisms by which DNA methylation and post-translational histone modifications play central roles in gene regulation. The therapeutics designed thus far have had encouraging results in counteracting the epimutations that occur during tumorigenesis. With the FDA approval of 5-Aza-CR, 5-Aza-CdR and SAHA, the use of epigenetic drugs has gained momentum and has proven useful in hematological malignancies and some solid tumors. Additionally, the combinatorial use of DNA methylation inhibitors and HDAC inhibitors in the clinic is gaining traction due to their synergistic effects in re-establishing the expression of tumor suppressor genes.
However, much work remains in designing drugs that will be more stable, less toxic and more specific in their enzyme inhibition. Expanding the use of these drugs to treat more types of solid tumors should also be possible. Broadening combinatorial drug therapies to include different permutations of the DNA methylation inhibitors, HDAC inhibitors and non-epigenetic chemotherapies will also be key in better cancer treatment. Fortunately, advancements in technology will help to further elucidate the understanding of epigenetic mechanisms. As a result, drugs which can better target chromatin modifiers that improperly function during carcinogenesis will be developed. Future epigenetic drugs can also be designed to target histone methyltransferases, histone demethylases or other chromatin modifiers not yet discovered. The rising interest in epigenetics research should therefore lead to improved cancer treatment.
Acknowledgments
We would like to thank Erika Wolff, Gangning Liang, Terry Kelly, Allen Yang, Christine Yoo, Tina Miranda and Jeffrey Friedman for their thoughtful input and time given to reading this review. Financial support is made possible by NIH grant RO1 CA83867.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kornberg RD. Chromatin structure: a repeating unit of histones and DNA. Science. 1974;184:868–871. doi: 10.1126/science.184.4139.868. [DOI] [PubMed] [Google Scholar]
- 2.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 3.Berger SL. Histone modifications in transcriptional regulation. Curr Opin Genet Dev. 2002;12:142–148. doi: 10.1016/s0959-437x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 4.Turner BM. Decoding the nucleosome. Cell. 1993;75:5–8. [PubMed] [Google Scholar]
- 5.Hebbes TR, Thorne AW, Clayton AL, Crane-Robinson C. Histone acetylation and globin gene switching. Nucleic Acids Res. 1992;20:1017–1022. doi: 10.1093/nar/20.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Rice JC, Allis CD. Histone methylation versus histone acetylation: new insights into epigenetic regulation. Curr Opin Cell Biol. 2001;13:263–273. doi: 10.1016/s0955-0674(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 8.Santos-Rosa H, Caldas C. Chromatin modifier enzymes, the histone code and cancer. Eur J Cancer. 2005;41:2381–2402. doi: 10.1016/j.ejca.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Fischle W, Wang Y, Allis CD. Binary switches and modification cassettes in histone biology and beyond. Nature. 2003;425:475–479. doi: 10.1038/nature02017. [DOI] [PubMed] [Google Scholar]
- 10.Liang G, Lin JC, Wei V, Yoo C, Cheng JC, Nguyen CT, Weisenberger DJ, Egger G, Takai D, Gonzales FA, Jones PA. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci U S A. 2004;101:7357–7362. doi: 10.1073/pnas.0401866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannister AJ, Schneider R, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. Spatial distribution of di- and tri-methyl lysine 36 of histone H3 at active genes. J Biol Chem. 2005;280:17732–17736. doi: 10.1074/jbc.M500796200. [DOI] [PubMed] [Google Scholar]
- 12.Shilatifard A. Chromatin modifications by methylation and ubiquitination: implications in the regulation of gene expression. Annu Rev Biochem. 2006;75:243–269. doi: 10.1146/annurev.biochem.75.103004.142422. [DOI] [PubMed] [Google Scholar]
- 13.Peters AH, Mermoud JE, O’Carroll D, Pagani M, Schweizer D, Brockdorff N, Jenuwein T. Histone H3 lysine 9 methylation is an epigenetic imprint of facultative heterochromatin. Nat Genet. 2002;30:77–80. doi: 10.1038/ng789. [DOI] [PubMed] [Google Scholar]
- 14.Peters AH, Kubicek S, Mechtler K, O’Sullivan RJ, Derijck AA, Perez-Burgos L, Kohlmaier A, Opravil S, Tachibana M, Shinkai Y, Martens JH, Jenuwein T. Partitioning and plasticity of repressive histone methylation states in mammalian chromatin. Mol Cell. 2003;12:1577–1589. doi: 10.1016/s1097-2765(03)00477-5. [DOI] [PubMed] [Google Scholar]
- 15.Sims JK, Houston SI, Magazinnik T, Rice JC. A trans-tail histone code defined by monomethylated H4 Lys-20 and H3 Lys-9 demarcates distinct regions of silent chromatin. J Biol Chem. 2006;281:12760–12766. doi: 10.1074/jbc.M513462200. [DOI] [PubMed] [Google Scholar]
- 16.Nicolas E, Roumillac C, Trouche D. Balance between acetylation and methylation of histone H3 lysine 9 on the E2F-responsive dihydrofolate reductase promoter. Mol Cell Biol. 2003;23:1614–1622. doi: 10.1128/MCB.23.5.1614-1622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latham JA, Dent SY. Cross-regulation of histone modifications. Nat Struct Mol Biol. 2007;14:1017–1024. doi: 10.1038/nsmb1307. [DOI] [PubMed] [Google Scholar]
- 18.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 19.Schones DE, Cui K, Cuddapah S, Roh TY, Barski A, Wang Z, Wei G, Zhao K. Dynamic regulation of nucleosome positioning in the human genome. Cell. 2008;132:887–898. doi: 10.1016/j.cell.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shivaswamy S, Bhinge A, Zhao Y, Jones S, Hirst M, Iyer VR. Dynamic remodeling of individual nucleosomes across a eukaryotic genome in response to transcriptional perturbation. PLoS Biol. 2008;6:e65. doi: 10.1371/journal.pbio.0060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gal-Yam EN, Jeong S, Tanay A, Egger G, Lee AS, Jones PA. Constitutive nucleosome depletion and ordered factor assembly at the GRP78 promoter revealed by single molecule footprinting. PLoS Genet. 2006;2:e160. doi: 10.1371/journal.pgen.0020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin JC, Jeong S, Liang G, Takai D, Fatemi M, Tsai YC, Egger G, Gal-Yam EN, Jones PA. Role of nucleosomal occupancy in the epigenetic silencing of the MLH1 CpG island. Cancer Cell. 2007;12:432–444. doi: 10.1016/j.ccr.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaissiere T, Sawan C, Herceg Z. Epigenetic interplay between histone modifications and DNA methylation in gene silencing. Mutat Res. 2008 doi: 10.1016/j.mrrev.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Pradhan S, Bacolla A, Wells RD, Roberts RJ. Recombinant human DNA (cytosine-5) methyltransferase. I. Expression, purification, and comparison of de novo and maintenance methylation. J Biol Chem. 1999;274:33002–33010. doi: 10.1074/jbc.274.46.33002. [DOI] [PubMed] [Google Scholar]
- 25.Okano M, Bell DW, Haber DA, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99:247–257. doi: 10.1016/s0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 26.Liang G, Chan MF, Tomigahara Y, Tsai YC, Gonzales FA, Li E, Laird PW, Jones PA. Cooperativity between DNA methyltransferases in the maintenance methylation of repetitive elements. Mol Cell Biol. 2002;22:480–491. doi: 10.1128/MCB.22.2.480-491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Osta A. DNMT cooperativity--the developing links between methylation, chromatin structure and cancer. Bioessays. 2003;25:1071–1084. doi: 10.1002/bies.10345. [DOI] [PubMed] [Google Scholar]
- 28.Ehrlich M, Wang RY. 5-Methylcytosine in eukaryotic DNA. Science. 1981;212:1350–1357. doi: 10.1126/science.6262918. [DOI] [PubMed] [Google Scholar]
- 29.Razin A, Shemer R. DNA methylation in early development. Hum Mol Genet. 1995;4:1751–1755. doi: 10.1093/hmg/4.suppl_1.1751. Spec No. [DOI] [PubMed] [Google Scholar]
- 30.Wade PA, Jones PL, Vermaak D, Veenstra GJ, Imhof A, Sera T, Tse C, Ge H, Shi YB, Hansen JC, Wolffe AP. Histone deacetylase directs the dominant silencing of transcription in chromatin: association with MeCP2 and the Mi-2 chromodomain SWI/SNF ATPase. Cold Spring Harb Symp Quant Biol. 1998;63:435–445. doi: 10.1101/sqb.1998.63.435. [DOI] [PubMed] [Google Scholar]
- 31.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 32.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 33.Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Res. 2006;34:D140–144. doi: 10.1093/nar/gkj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen CT, Weisenberger DJ, Velicescu M, Gonzales FA, Lin JC, Liang G, Jones PA. Histone H3-lysine 9 methylation is associated with aberrant gene silencing in cancer cells and is rapidly reversed by 5-aza-2′-deoxycytidine. Cancer Res. 2002;62:6456–6461. [PubMed] [Google Scholar]
- 35.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, Jones RS, Zhang Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 36.Krivtsov AV, Armstrong SA. MLL translocations histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7:823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 37.Tonini T, D’Andrilli G, Fucito A, Gaspa L, Bagella L. Importance of Ezh2 polycomb protein in tumorigenesis process interfering with the pathway of growth suppressive key elements. J Cell Physiol. 2008;214:295–300. doi: 10.1002/jcp.21241. [DOI] [PubMed] [Google Scholar]
- 38.Kingston RE, Bunker CA, Imbalzano AN. Repression and activation by multiprotein complexes that alter chromatin structure. Genes Dev. 1996;10:905–920. doi: 10.1101/gad.10.8.905. [DOI] [PubMed] [Google Scholar]
- 39.Visser HP, Gunster MJ, Kluin-Nelemans HC, Manders EM, Raaphorst FM, Meijer CJ, Willemze R, Otte AP. The Polycomb group protein EZH2 is upregulated in proliferating, cultured human mantle cell lymphoma. Br J Haematol. 2001;112:950–958. doi: 10.1046/j.1365-2141.2001.02641.x. [DOI] [PubMed] [Google Scholar]
- 40.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11611. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodes DR, Sanda MG, Otte AP, Chinnaiyan AM, Rubin MA. Multiplex biomarker approach for determining risk of prostate-specific antigen-defined recurrence of prostate cancer. J Natl Cancer Inst. 2003;95:661–668. doi: 10.1093/jnci/95.9.661. [DOI] [PubMed] [Google Scholar]
- 42.van Leenders GJ, Dukers D, Hessels D, van den Kieboom SW, Hulsbergen CA, Witjes JA, Otte AP, Meijer CJ, Raaphorst FM. Polycomb-group oncogenes EZH2, BMI1, and RING1 are overexpressed in prostate cancer with adverse pathologic and clinical features. Eur Urol. 2007;52:455–463. doi: 10.1016/j.eururo.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 43.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 44.Wissmann M, Yin N, Muller JM, Greschik H, Fodor BD, Jenuwein T, Vogler C, Schneider R, Gunther T, Buettner R, Metzger E, Schule R. Cooperative demethylation by JMJD2C and LSD1 promotes androgen receptor-dependent gene expression. Nat Cell Biol. 2007;9:347–353. doi: 10.1038/ncb1546. [DOI] [PubMed] [Google Scholar]
- 45.Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442:307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- 46.Lee MG, Wynder C, Bochar DA, Hakimi MA, Cooch N, Shiekhattar R. Functional interplay between histone demethylase and deacetylase enzymes. Mol Cell Biol. 2006;26:6395–6402. doi: 10.1128/MCB.00723-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agger K, Christensen J, Cloos PA, Helin K. The emerging functions of histone demethylases. Curr Opin Genet Dev. 2008 doi: 10.1016/j.gde.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Kitabayashi I, Aikawa Y, Yokoyama A, Hosoda F, Nagai M, Kakazu N, Abe T, Ohki M. Fusion of MOZ and p300 histone acetyltransferases in acute monocytic leukemia with a t(8;22)(p11;q13) chromosome translocation. Leukemia. 2001;15:89–94. doi: 10.1038/sj.leu.2401983. [DOI] [PubMed] [Google Scholar]
- 49.Song J, Noh JH, Lee JH, Eun JW, Ahn YM, Kim SY, Lee SH, Park WS, Yoo NJ, Lee JY, Nam SW. Increased expression of histone deacetylase 2 is found in human gastric cancer. Apmis. 2005;113:264–268. doi: 10.1111/j.1600-0463.2005.apm_04.x. [DOI] [PubMed] [Google Scholar]
- 50.Halkidou K, Gaughan L, Cook S, Leung HY, Neal DE, Robson CN. Upregulation and nuclear recruitment of HDAC1 in hormone refractory prostate cancer. Prostate. 2004;59:177–189. doi: 10.1002/pros.20022. [DOI] [PubMed] [Google Scholar]
- 51.Sakuma T, Uzawa K, Onda T, Shiiba M, Yokoe H, Shibahara T, Tanzawa H. Aberrant expression of histone deacetylase 6 in oral squamous cell carcinoma. Int J Oncol. 2006;29:117–124. [PubMed] [Google Scholar]
- 52.Bartling B, Hofmann HS, Boettger T, Hansen G, Burdach S, Silber RE, Simm A. Comparative application of antibody and gene array for expression profiling in human squamous cell lung carcinoma. Lung Cancer. 2005;49:145–154. doi: 10.1016/j.lungcan.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 53.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, Moon EJ, Kim HS, Lee SK, Chung HY, Kim CW, Kim KW. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 54.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–5413. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 55.Dunn BK. Hypomethylation: one side of a larger picture. Ann N Y Acad Sci. 2003;983:28–42. doi: 10.1111/j.1749-6632.2003.tb05960.x. [DOI] [PubMed] [Google Scholar]
- 56.Fruhwald MC, Plass C. Global and gene-specific methylation patterns in cancer: aspects of tumor biology and clinical potential. Mol Genet Metab. 2002;75:1–16. doi: 10.1006/mgme.2001.3265. [DOI] [PubMed] [Google Scholar]
- 57.Nishigaki M, Aoyagi K, Danjoh I, Fukaya M, Yanagihara K, Sakamoto H, Yoshida T, Sasaki H. Discovery of aberrant expression of R-RAS by cancer-linked DNA hypomethylation in gastric cancer using microarrays. Cancer Res. 2005;65:2115–2124. doi: 10.1158/0008-5472.CAN-04-3340. [DOI] [PubMed] [Google Scholar]
- 58.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sigalotti L, Fratta E, Coral S, Cortini E, Covre A, Nicolay HJ, Anzalone L, Pezzani L, Di Giacomo AM, Fonsatti E, Colizzi F, Altomonte M, Calabro L, Maio M. Epigenetic drugs as pleiotropic agents in cancer treatment: biomolecular aspects and clinical applications. J Cell Physiol. 2007;212:330–344. doi: 10.1002/jcp.21066. [DOI] [PubMed] [Google Scholar]
- 60.Langer F, Dingemann J, Kreipe H, Lehmann U. Up-regulation of DNA methyltransferases DNMT1, 3A, and 3B in myelodysplastic syndrome. Leuk Res. 2005;29:325–329. doi: 10.1016/j.leukres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Patra SK, Patra A, Zhao H, Dahiya R. DNA methyltransferase and demethylase in human prostate cancer. Mol Carcinog. 2002;33:163–171. doi: 10.1002/mc.10033. [DOI] [PubMed] [Google Scholar]
- 62.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 63.Saito Y, Liang G, Egger G, Friedman JM, Chuang JC, Coetzee GA, Jones PA. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006;9:435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 64.Egger G, Aparicio AM, Escobar SG, Jones PA. Inhibition of histone deacetylation does not block resilencing of p16 after 5-aza-2′-deoxycytidine treatment. Cancer Res. 2007;67:346–353. doi: 10.1158/0008-5472.CAN-06-2845. [DOI] [PubMed] [Google Scholar]
- 65.Richon VM, Sandhoff TW, Rifkind RA, Marks PA. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci U S A. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol. 2003;4:235–240. doi: 10.1038/ni887. [DOI] [PubMed] [Google Scholar]
- 67.Ghoshal K, Bai S. DNA methyltransferases as targets for cancer therapy. Drugs Today (Barc) 2007;43:395–422. doi: 10.1358/dot.2007.43.6.1062666. [DOI] [PubMed] [Google Scholar]
- 68.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 69.Momparler RL. Epigenetic therapy of cancer with 5-aza-2′-deoxycytidine (decitabine) Semin Oncol. 2005;32:443–451. doi: 10.1053/j.seminoncol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 71.Itzykson R, Gardin C, Fenaux P. Meeting report: myelodysplastic syndromes at ASH 2007. Leukemia. 2008;22:893–897. doi: 10.1038/leu.2008.45. [DOI] [PubMed] [Google Scholar]
- 72.Kantarjian HM, O’Brien S, Shan J, Aribi A, Garcia-Manero G, Jabbour E, Ravandi F, Cortes J, Davisson J, Issa JP. Update of the decitabine experience in higher risk myelodysplastic syndrome and analysis of prognostic factors associated with outcome. Cancer. 2007;109:265–273. doi: 10.1002/cncr.22376. [DOI] [PubMed] [Google Scholar]
- 73.Plimack ER, Kantarjian HM, Issa JP. Decitabine and its role in the treatment of hematopoietic malignancies. Leuk Lymphoma. 2007;48:1472–1481. doi: 10.1080/10428190701471981. [DOI] [PubMed] [Google Scholar]
- 74.Daskalakis M, Nguyen TT, Nguyen C, Guldberg P, Kohler G, Wijermans P, Jones PA, Lubbert M. Demethylation of a hypermethylated P15/INK4B gene in patients with myelodysplastic syndrome by 5-Aza-2′-deoxycytidine (decitabine) treatment. Blood. 2002;100:2957–2964. doi: 10.1182/blood.V100.8.2957. [DOI] [PubMed] [Google Scholar]
- 75.Yang AS, Doshi KD, Choi SW, Mason JB, Mannari RK, Gharybian V, Luna R, Rashid A, Shen L, Estecio MR, Kantarjian HM, Garcia-Manero G, Issa JP. DNA methylation changes after 5-aza-2′-deoxycytidine therapy in patients with leukemia. Cancer Res. 2006;66:5495–5503. doi: 10.1158/0008-5472.CAN-05-2385. [DOI] [PubMed] [Google Scholar]
- 76.Aribi A, Borthakur G, Ravandi F, Shan J, Davisson J, Cortes J, Kantarjian H. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer. 2007;109:713–717. doi: 10.1002/cncr.22457. [DOI] [PubMed] [Google Scholar]
- 77.Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL, Lazebnik YA, Cordon-Cardo C, Lowe SW. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001;409:207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 78.Yang AS, Estecio MR, Garcia-Manero G, Kantarjian HM, Issa JP. Comment on "Chromosomal instability and tumors promoted by DNA hypomethylation" and "Induction of tumors in nice by genomic hypomethylation". Science. 2003;302:1153. doi: 10.1126/science.302.5648.1153a. author reply 1153. [DOI] [PubMed] [Google Scholar]
- 79.Marquez VE, Barchi JJ, Jr, Kelley JA, Rao KV, Agbaria R, Ben-Kasus T, Cheng JC, Yoo CB, Jones PA. Zebularine: a unique molecule for an epigenetically based strategy in cancer chemotherapy. The magic of its chemistry and biology. Nucleosides Nucleotides Nucleic Acids. 2005;24:305–318. doi: 10.1081/ncn-200059765. [DOI] [PubMed] [Google Scholar]
- 80.Yoo CB, Chuang JC, Byun H, Egger G, Yang AS, Dubeau L, Long T, Laird PW, Marquez VE, Jones PA. Long-term Epigenetic Therapy with Oral Zebularine Has Minimal Side Effects and Prevents Intestinal Tumors in Mice. Cancer Prev Res 0. 2008 doi: 10.1158/1940-6207.CAPR-07-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brueckner B, Boy RG, Siedlecki P, Musch T, Kliem HC, Zielenkiewicz P, Suhai S, Wiessler M, Lyko F. Epigenetic reactivation of tumor suppressor genes by a novel small-molecule inhibitor of human DNA methyltransferases. Cancer Res. 2005;65:6305–6311. doi: 10.1158/0008-5472.CAN-04-2957. [DOI] [PubMed] [Google Scholar]
- 82.Zheng YG, Wu J, Chen Z, Goodman M. Chemical regulation of epigenetic modifications: Opportunities for new cancer therapy. Med Res Rev. 2008 doi: 10.1002/med.20120. [DOI] [PubMed] [Google Scholar]
- 83.Klisovic RB, Stock W, Cataland S, Klisovic MI, Liu S, Blum W, Green M, Odenike O, Godley L, Burgt JV, Van Laar E, Cullen M, Macleod AR, Besterman JM, Reid GK, Byrd JC, Marcucci G. A phase I biological study of MG98, an oligodeoxynucleotide antisense to DNA methyltransferase 1, in patients with high-risk myelodysplasia and acute myeloid leukemia. Clin Cancer Res. 2008;14:2444–2449. doi: 10.1158/1078-0432.CCR-07-1320. [DOI] [PubMed] [Google Scholar]
- 84.Winquist E, Knox J, Ayoub JP, Wood L, Wainman N, Reid GK, Pearce L, Shah A, Eisenhauer E. Phase II trial of DNA methyltransferase 1 inhibition with the antisense oligonucleotide MG98 in patients with metastatic renal carcinoma: a National Cancer Institute of Canada Clinical Trials Group investigational new drug study. Invest New Drugs. 2006;24:159–167. doi: 10.1007/s10637-006-5938-1. [DOI] [PubMed] [Google Scholar]
- 85.Gronbaek K, Hother C, Jones PA. Epigenetic changes in cancer. Apmis. 2007;115:1039–1059. doi: 10.1111/j.1600-0463.2007.apm_636.xml.x. [DOI] [PubMed] [Google Scholar]
- 86.Chuang JC, Yoo CB, Kwan JM, Li TW, Liang G, Yang AS, Jones PA. Comparison of biological effects of non-nucleoside DNA methylation inhibitors versus 5-aza-2′-deoxycytidine. Mol Cancer Ther. 2005;4:1515–1520. doi: 10.1158/1535-7163.MCT-05-0172. [DOI] [PubMed] [Google Scholar]
- 87.Karagiannis TC, El-Osta A. Clinical potential of histone deacetylase inhibitors as stand alone therapeutics and in combination with other chemotherapeutics or radiotherapy for cancer. Epigenetics. 2006;1:121–126. doi: 10.4161/epi.1.3.3328. [DOI] [PubMed] [Google Scholar]
- 88.Stearns V, Zhou Q, Davidson NE. Epigenetic regulation as a new target for breast cancer therapy. Cancer Invest. 2007;25:659–665. doi: 10.1080/07357900701719234. [DOI] [PubMed] [Google Scholar]
- 89.Marks PA, Richon VM, Rifkind RA. Histone deacetylase inhibitors: inducers of differentiation or apoptosis of transformed cells. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 90.Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, Breslow R, Pavletich NP. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 91.Olsen EA, Kim YH, Kuzel TM, Pacheco TR, Foss FM, Parker S, Frankel SR, Chen C, Ricker JL, Arduino JM, Duvic M. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25:3109–3115. doi: 10.1200/JCO.2006.10.2434. [DOI] [PubMed] [Google Scholar]
- 92.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26:5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 93.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 94.Modesitt SC, Sill M, Hoffman JS, Bender DP. A phase II study of vorinostat in the treatment of persistent or recurrent epithelial ovarian or primary peritoneal carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2008;109:182–186. doi: 10.1016/j.ygyno.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 95.Steele NL, Plumb JA, Vidal L, Tjornelund J, Knoblauch P, Rasmussen A, Ooi CE, Buhl-Jensen P, Brown R, Evans TR, DeBono JS. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14:804–810. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 96.George P, Bali P, Annavarapu S, Scuto A, Fiskus W, Guo F, Sigua C, Sondarva G, Moscinski L, Atadja P, Bhalla K. Combination of the histone deacetylase inhibitor LBH589 and the hsp90 inhibitor 17-AAG is highly active against human CML-BC cells and AML cells with activating mutation of FLT-3. Blood. 2005;105:1768–1776. doi: 10.1182/blood-2004-09-3413. [DOI] [PubMed] [Google Scholar]
- 97.Glaser KB. HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol. 2007;74:659–671. doi: 10.1016/j.bcp.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 98.Wu LP, Wang X, Li L, Zhao Y, Lu S, Yu Y, Zhou W, Liu X, Yang J, Zheng Z, Zhang H, Feng J, Yang Y, Wang H, Zhu WG. Histone deacetylase inhibitor depsipeptide activates silenced genes through decreasing both CpG and H3K9 methylation on the promoter. Mol Cell Biol. 2008;28:3219–3235. doi: 10.1128/MCB.01516-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai MT, Yang CC, Lin TY, Tsai FJ, Chen WC. Depsipeptide (FK228) inhibits growth of human prostate cancer cells. Urol Oncol. 2008;26:182–189. doi: 10.1016/j.urolonc.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 100.Shah MH, Binkley P, Chan K, Xiao J, Arbogast D, Collamore M, Farra Y, Young D, Grever M. Cardiotoxicity of histone deacetylase inhibitor depsipeptide in patients with metastatic neuroendocrine tumors. Clin Cancer Res. 2006;12:3997–4003. doi: 10.1158/1078-0432.CCR-05-2689. [DOI] [PubMed] [Google Scholar]
- 101.Klimek VM, Fircanis S, Maslak P, Guernah I, Baum M, Wu N, Panageas K, Wright JJ, Pandolfi PP, Nimer SD. Tolerability, pharmacodynamics, and pharmacokinetics studies of depsipeptide (romidepsin) in patients with acute myelogenous leukemia or advanced myelodysplastic syndromes. Clin Cancer Res. 2008;14:826–832. doi: 10.1158/1078-0432.CCR-07-0318. [DOI] [PubMed] [Google Scholar]
- 102.Cinatl J, Jr, Kotchetkov R, Blaheta R, Driever PH, Vogel JU, Cinatl J. Induction of differentiation and suppression of malignant phenotype of human neuroblastoma BE(2)-C cells by valproic acid: enhancement by combination with interferon-alpha. Int J Oncol. 2002;20:97–106. [PubMed] [Google Scholar]
- 103.Blaheta RA, Michaelis M, Driever PH, Cinatl J., Jr Evolving anticancer drug valproic acid: insights into the mechanism and clinical studies. Med Res Rev. 2005;25:383–397. doi: 10.1002/med.20027. [DOI] [PubMed] [Google Scholar]
- 104.Gore SD, Weng LJ, Figg WD, Zhai S, Donehower RC, Dover G, Grever MR, Griffin C, Grochow LB, Hawkins A, Burks K, Zabelena Y, Miller CB. Impact of prolonged infusions of the putative differentiating agent sodium phenylbutyrate on myelodysplastic syndromes and acute myeloid leukemia. Clin Cancer Res. 2002;8:963–970. [PubMed] [Google Scholar]
- 105.Camacho LH, Olson J, Tong WP, Young CW, Spriggs DR, Malkin MG. Phase I dose escalation clinical trial of phenylbutyrate sodium administered twice daily to patients with advanced solid tumors. Invest New Drugs. 2007;25:131–138. doi: 10.1007/s10637-006-9017-4. [DOI] [PubMed] [Google Scholar]
- 106.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov. 2002;1:287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 107.Qian DZ, Wei YF, Wang X, Kato Y, Cheng L, Pili R. Antitumor activity of the histone deacetylase inhibitor MS-275 in prostate cancer models. Prostate. 2007;67:1182–1193. doi: 10.1002/pros.20611. [DOI] [PubMed] [Google Scholar]
- 108.Camphausen K, Scott T, Sproull M, Tofilon PJ. Enhancement of xenograft tumor radiosensitivity by the histone deacetylase inhibitor MS-275 and correlation with histone hyperacetylation. Clin Cancer Res. 2004;10:6066–6071. doi: 10.1158/1078-0432.CCR-04-0537. [DOI] [PubMed] [Google Scholar]
- 109.Kummar S, Gutierrez M, Gardner ER, Donovan E, Hwang K, Chung EJ, Lee MJ, Maynard K, Kalnitskiy M, Chen A, Melillo G, Ryan QC, Conley B, Figg WD, Trepel JB, Zwiebel J, Doroshow JH, Murgo AJ. Phase I trial of MS-275, a histone deacetylase inhibitor, administered weekly in refractory solid tumors and lymphoid malignancies. Clin Cancer Res. 2007;13:5411–5417. doi: 10.1158/1078-0432.CCR-07-0791. [DOI] [PubMed] [Google Scholar]
- 110.Prakash S, Foster BJ, Meyer M, Wozniak A, Heilbrun LK, Flaherty L, Zalupski M, Radulovic L, Valdivieso M, LoRusso PM. Chronic oral administration of CI-994: a phase 1 study. Invest New Drugs. 2001;19:1–11. doi: 10.1023/a:1006489328324. [DOI] [PubMed] [Google Scholar]
- 111.Pauer LR, Olivares J, Cunningham C, Williams A, Grove W, Kraker A, Olson S, Nemunaitis J. Phase I study of oral CI-994 in combination with carboplatin and paclitaxel in the treatment of patients with advanced solid tumors. Cancer Invest. 2004;22:886–896. doi: 10.1081/cnv-200039852. [DOI] [PubMed] [Google Scholar]
- 112.Kouraklis G, Theocharis S. Histone deacetylase inhibitors: a novel target of anticancer therapy (review) Oncol Rep. 2006;15:489–494. [PubMed] [Google Scholar]
- 113.Cameron EE, Bachman KE, Myohanen S, Herman JG, Baylin SB. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet. 1999;21:103–107. doi: 10.1038/5047. [DOI] [PubMed] [Google Scholar]
- 114.Klisovic MI, Maghraby EA, Parthun MR, Guimond M, Sklenar AR, Whitman SP, Chan KK, Murphy T, Anon J, Archer KJ, Rush LJ, Plass C, Grever MR, Byrd JC, Marcucci G. Depsipeptide (FR 901228) promotes histone acetylation, gene transcription, apoptosis and its activity is enhanced by DNA methyltransferase inhibitors in AML1/ETO-positive leukemic cells. Leukemia. 2003;17:350–358. doi: 10.1038/sj.leu.2402776. [DOI] [PubMed] [Google Scholar]
- 115.Belinsky SA, Klinge DM, Stidley CA, Issa JP, Herman JG, March TH, Baylin SB. Inhibition of DNA methylation and histone deacetylation prevents murine lung cancer. Cancer Res. 2003;63:7089–7093. [PubMed] [Google Scholar]
- 116.Venturelli S, Armeanu S, Pathil A, Hsieh CJ, Weiss TS, Vonthein R, Wehrmann M, Gregor M, Lauer UM, Bitzer M. Epigenetic combination therapy as a tumor-selective treatment approach for hepatocellular carcinoma. Cancer. 2007;109:2132–2141. doi: 10.1002/cncr.22652. [DOI] [PubMed] [Google Scholar]
- 117.Gore SD, Baylin S, Sugar E, Carraway H, Miller CB, Carducci M, Grever M, Galm O, Dauses T, Karp JE, Rudek MA, Zhao M, Smith BD, Manning J, Jiemjit A, Dover G, Mays A, Zwiebel J, Murgo A, Weng LJ, Herman JG. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 118.Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–7300. [PubMed] [Google Scholar]
- 119.Burkitt K, Ljungman M. Phenylbutyrate interferes with the Fanconi anemia and BRCA pathway and sensitizes head and neck cancer cells to cisplatin. Mol Cancer. 2008;7:24. doi: 10.1186/1476-4598-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chiang PK, Cantoni GL. Perturbation of biochemical transmethylations by 3-deazaadenosine in vivo. Biochem Pharmacol. 1979;28:1897–1902. doi: 10.1016/0006-2952(79)90642-7. [DOI] [PubMed] [Google Scholar]
- 121.Cools M, De Clercq E. Influence of S-adenosylhomocysteine hydrolase inhibitors on S-adenosylhomocysteine and S-adenosylmethionine pool levels in L929 cells. Biochem Pharmacol. 1990;40:2259–2264. doi: 10.1016/0006-2952(90)90720-6. [DOI] [PubMed] [Google Scholar]
- 122.Huggins J, Zhang ZX, Bray M. Antiviral drug therapy of filovirus infections: S-adenosylhomocysteine hydrolase inhibitors inhibit Ebola virus in vitro and in a lethal mouse model. J Infect Dis. 1999;179(Suppl 1):S240–247. doi: 10.1086/514316. [DOI] [PubMed] [Google Scholar]